Abstract
Occupational, residential, dietary and environmental exposures to mixtures of synthetic anthropogenic chemicals after World War II have a strong relationship with the increase of chronic diseases, health cost and environmental pollution. The link between environment and immunity is particularly intriguing as it is known that chemicals and drugs can cause immunotoxicity (e.g., allergies and autoimmune diseases). In this review, we emphasize the relationship between long-term exposure to xenobiotic mixtures and immune deficiency inherent to chronic diseases and epidemics/pandemics. We also address the immunotoxicologic risk of vulnerable groups, taking into account biochemical and biophysical properties of SARS-CoV-2 and its immunopathological implications. We particularly underline the common mechanisms by which xenobiotics and SARS-CoV-2 act at the cellular and molecular level. We discuss how long-term exposure to thousand chemicals in mixtures, mostly fossil fuel derivatives, exposure toparticle matters, metals, ultraviolet (UV)–B radiation, ionizing radiation and lifestyle contribute to immunodeficiency observed in the contemporary pandemic, such as COVID-19, and thus threaten global public health, human prosperity and achievements, and global economy. Finally, we propose metrics which are needed to address the diverse health effects of anthropogenic COVID-19 crisis at present and those required to prevent similar future pandemics.
Keywords: Xenobiotics, Pollutants, Immune deficiency, COVID-19, Chronic diseases, Coronavirus, SARS-CoV-2, Epidemic, Pandemic
Graphical abstract
Highlights
-
•
Developmental exposure to environmental factors can disrupt the immune system.
-
•
Long-term low-dose exposure to chemical mixtures is linked to imunodeficiency
-
•
Immunodeficiency contributes to chronic diseases and the current Covid-19 pandemics.
-
•
Environmental chemicals and microorganisms share similar molecular pathomechanisms (AhR pathway).
-
•
Understanding the underlying pathomechanisms helps to improve public health.
1. Introduction
Human and animal health threats from coronaviruses have been present over time. Uncontrolled porcine, bat, mouse, bovine, avian and human coronaviruses dispersal can impact both global public health and economic stability. As early as 2002, beta coronaviruses (CoV) zoonotic outbreaks have been reported (Ou et al., 2020), including severe acute respiratory syndrome coronavirus (SARS)-CoV in 2002–2003 (Ksiazek et al., 2003), Middle East respiratory syndrome (MERS)-CoV in 2012 (Zaki et al., 2012) and, in late 2019, the novel SARS-CoV-2 (Docea et al., 2020). The porcine epidemic diarrhea virus (PEDV) should also be included (Chen et al., 2014). Coronaviruses have been associated with various ailments, including respiratory, gastrointestinal, and central nervous system diseases both in humans and animals, impacting not only human health but also economic stability (Perlman and Netland, 2009). The transmission model, clinical manifestations, pathogenesis and treatment of coronavirus disease have been recently reviewed (Docea et al., 2020).
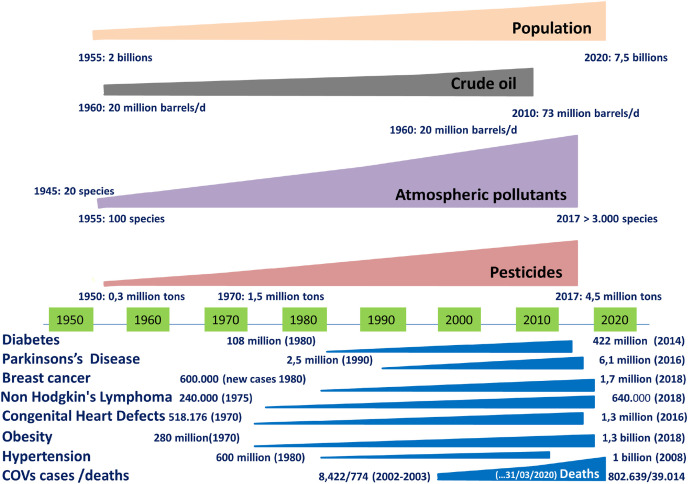
Fig. 1 shows an anthropological and scientific approach for the evaluation of the association of the global production of crude oil, atmospheric pollutants, and pesticides with the population growth and chronic diseases trends (Guillette et al., 1998). We emphasize that long-term exposure to anthropogenic pollutants, among other risk factors, may have a positive association with chronic diseases such as neurodegenerative disorders (e.g., Parkinson's disease) and diabetes (Dimakakou et al., 2018; Roca et al., 2012), breast cancer (DeSantis et al., 2019), non-Hodgkin's lymphoma (Huh, 2012), cancer (Brown, 1992), leukemia (Cocco et al., 1997), obesity (OECD, 2014), congenital heart defects (Liu et al., 2019), hypertension (WHO. Raised blood pressure,” n. c.d.) and increased vulnerability to microbial and viral infections and their mortality (Figà-Talamanca et al., 1993).
Fig. 1.
Association of global crude oil, atmospheric pollutants and pesticide production with population growth and chronic disease trends.
While increases in disease correlate with increases in anthropogenic pollutants, correlation is not causation. For causation, mechanisms need to be identified that link pollutants to eventual diseases credibly. The remainder of this paper presents evidence of the biological mechanisms that link the pollutants to myriad diseases of interest, especially through adverse impacts on the immune system.
2. Influence of environmental risk factors on the immune system
The immune system can be the target of many chemical, biological, and physical agents that may elicit adverse effects on the host's health; however, it is still unknown the extent to which low exposure to environmental toxic stimuli may adversely affect human health. The proinflammatory and immunomodulatory properties of many toxic stimuli may have limited effects on the immune system, unless exposure occurs early in life or concurrently with infections or malignant diseases. Under these circumstances, there is an increased risk of progression from exposure to infection, autoimmune diseases, or even cancer. Nevertheless, the compensatory mechanisms of the immune system limit the potential health impact at the individual level, although minor immunomodulatory effects may have an impact at the population level by contributing to increased burden of diseases (Hartung and Corsini, 2013).
Environmental factors are believed to contribute to the increased prevalence of allergies and autoimmune diseases, many of which are due to the activity of Th17 T cells. These Th17 T cells are a newly-identified helper T-cell subset characterized by production of interleukin (IL)-17 and IL-22, as observed in experimental autoimmune encephalomyelitis (Veldhoen et al., 2008) and more recently in COVID-19 patients in China. The immunologic response of barrier organs (e.g., skin, gut, lung, eyes, and oral and genital mucosal tissues) against pathogenic microbes and protein antigens, including SARS-CoV-2, is disrupted in hematopoietic stem cells, innate immune system cells, as well as in T-cell subsets and B cells.
Chronic exposure to environmental chemicals has been shown to suppress or enhance immune responsiveness, depending on factors such as dose, timing, and route of exposure. For example, environmental immunology has led to the understanding of how xenobiotics (such as polycyclic aromatic hydrocarbons (PAH)) acting via immunomodulatory signaling pathways, and specifically the aryl hydrocarbon receptors (AhR), may adversely affect the developing immune system (which seems to be more sensitive to environmental insults than the adult immune system (Kreitinger et al., 2016)). Immunotoxicology can also be beneficial in investigating whether an epidemic/pandemic, or a chronic disease, is idiopathic or a deterministic event reflecting cumulative adverse effects on the immune system. Understanding the impact of environmental toxic stimuli on the immune response ultimately contributes to improving public health.
Many decades ago, infectious diseases were the main cause of mortality globally. With the increased influx of modern technology and its under-regulated adverse byproducts into the environment and workplace, non-communicable diseases have replaced infectious diseases as the leading cause of mortality world-wide. However, infectious diseases are still amongst the top five causes of global death (Winans et al., 2011).
Xenobiotics, whether natural or anthropogenic, can readily interact with the immune system, leading to a suppressed or enhanced immune responsiveness (Fenga et al., 2017). Of increasing concern is the observed reduced immune response to viral and bacterial infection, as well as myelosuppression and thymic dysfunction. For example, coplanar polychlorinated biphenyls (PCBs) and dioxins have been shown to mediate their effect via the AhR. Such effects include diminished humoral antibody responses, humoral immune suppression (Silkworth and Antrim, 1985), severe thymic atrophy, and reduced cell-mediated immunity, to name a few.
2.1. The AhR signaling pathway in immune system responses against xenobiotics and viruses
The AhR is an evolutionarily ancient protein that can be activated or inhibited by various types of exogenous and endogenous ligands. While AhR canonical signaling has been identified as a primary mediator of biological responsiveness to environmental chemicals, alternative signaling events are increasingly being understood to mediate the physiological effects of AhR (Khazaal et al., 2018). AhR is a cytosolic signal sensor and transcription factor that translocates into the nucleus upon binding to planar aromatic hydrocarbons. In addition, AhR signaling is intricately involved in fine-tuning distinct immune responses, particularly at environmental interfaces where endogenous and exogenous AhR ligands accumulate (Quintana et al., 2008). For example, AhR activity has been documented to be a key determinant of T helper (Th) cell differentiation. Notably, ligands with differential AhR agonist activity may have opposing effects on Th cell differentiation, demonstrating the finely tuned regulation of this transcription factor at the ligand-binding level (Quintana et al., 2008).
The AhR signaling pathway plays a key role in innate and adaptive immune responses, and controls transcription of xenobiotic-metabolizing enzymes like cytochrome P450 family members (e.g., CYP1A1 and CYP1B1) (Stockinger et al., 2014). Exogenous AhR ligands include environmental toxic stimuli (e.g., dioxin), bacterial metabolites, or naturally occurring ligands, such as dietary ligands (e.g., flavonoids and glucosinolates), abundantly found in plants (McIntosh et al., 2010). Starting from infancy, AhR levels in hepatic, lung, and thymic cytosol slowly decline with age. Thus, while innate immune cells in children have high expression of AhR, immune cells in adults have conservative to low AhR expression (Esser and Rannug, 2015), representing a potential important highlighted finding in COVID-19 prevalence in adults.
It is recognized that polyhalogenated aromatic hydrocarbons (PAHs), dioxins, and biphenyls can activate AhR given their structural resemblance to physiologic ligands. This, in turn, may increase the metabolic turnover of physiologic ligands, thus decreasing their half-life. As a result, uncontrolled or persistent activation of the AhR by exogenous small molecules or UVB radiation may alter the tightly controlled and transient AhR-regulated cell functions (Esser and Rannug, 2015).
AhR signaling is dependent of the formation of complexes with other proteins (cross-talk), such as AhR-interacting protein (AIP), estrogen receptor (ER), inducible nitric oxide synthase (iNOS), matrix metalloproteinase (MMP), nuclear factor-κB (NF-κB), prostaglandin synthase (PGHS), retinoblastoma protein (RB), tumor necrosis factor α (TNF-α) and xenobiotic-metabolizing enzymes (XME) (Beischlag et al., 2008).
AhR canonical and noncanonical signaling pathways can regulate master regulatory transcriptional factors, such as the nuclear factor (NF)-κB promoter and signal transducer and activator of transcription (STAT). These transcriptional factors regulate the general transcriptional machinery to induce transcriptional, post-translational and epigenetic expression of selective inflammatory genes (Bhatt and Ghosh, 2014). Additionally, cytosolic AhR and NF-kB regulate calcium-related genes, increasing rapidly the intracellular Ca2+ concentrations that drive cardiac and skeletal muscle pathology. This sequence leads to downstream pro-inflammatory responses mediated by c-src, cyclooxygenase-2 (COX2), and C–C motif chemokine ligand 1 (CCL1) (N'Diaye et al., 2006; Peterson et al., 2018). AhR is also a key component for T cell development and activation mainly through the action of the Wnt/β-catenin pathway (Ma et al., 2012). Xenobiotics induce regulatory T cell (iTreg) deficits, as found in autoimmune diseases such as multiple sclerosis (Wing and Sakaguchi, 2010), through their impact on AhR.
Xenobiotics and viruses affect the immune cells (e.g. hematopoietic, myeloid, lymphoid) as well as the immune system barriers of the lung, gut, mucosal epithelia and the placenta through differential and variable AhR expression levels (Quintana and Sherr, 2013). AhR deficiency and AhR dysregulation can impair the development of the aforementioned tissue barriers as well as the function of the adaptive and innate immune systems (Sherr and Monti, 2013). For example, smokers are more sensitive to viral and bacterial infection due to increased CYP1A1/CYP1B1 and matrix metalloproteinase-1 (MMP-1) expression through AhR activation by PAHs present in tobacco smoke, in a dose-dependent manner (Ono et al., 2013).
The AhR molecular pathway regulates the immune response against bacterial and viral attacks. Therefore, it plays a role in the context of autoimmunity, infection, and cancer. Additionally, AhR modulates the circadian clinical manifestations of infectious diseases and opens potential opportunities for developing targeted therapeutics (Gutiérrez-Vázquez and Quintana, 2018). Hence, the circadian rhythm (evening)- observed exacerbation of clinical symptoms in malaria, bacterial sepsis, or seriously ill COVID-19 patients can be regulated by AhR.
AhR not only affects the immune responses to viral infection, but can also interact with viral proteins and affects viral latency. This suggests that AhR may modulate viral infection beyond influencing innate and adaptive responses (Head and Lawrence, 2009).
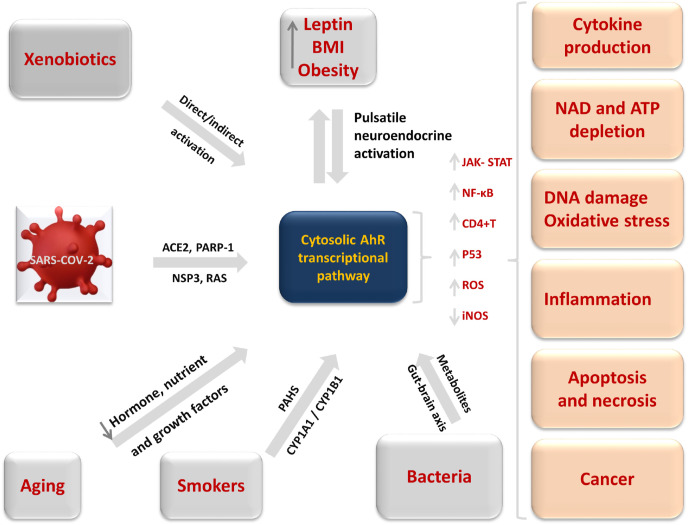
Xenobiotics and viruses acting through AhR can affect the gut-associated immune system and microbiome (Gutiérrez-Vázquez and Quintana, 2018) (Fig. 2 ). The human gut virome, the ‘missing link’ between gut bacteria and host immunity, is a poorly understood component of the gut microbiota (Reyes et al., 2012). Diet, antibiotic use, and geographic variation can substantially affect the gut virome. Synthetic diets or diets with no vegetables and fruits have very low AhR activators, such as polyphenols and glucosinolates. These deficient diets can adversely affect the organogenesis of intestinal lymphoid follicles and innate immune cell homeostasis in the gut (Holtz et al., 2014; Tsiaoussis et al., 2019).
Fig. 2.
Xenobiotics, viruses, bacteria, obesity and aging acting through AhR.
Exposure of the gut microbiota to various antibiotics has led to a substantial enrichment of the gut microbiome with phage-encoded genes that confer resistance to the administered and similarly acting antibiotics. This increased resistance, however, has created rather complicated biofilms promoting persistent infections or chronic inflammation (Modi et al., 2013). Interestingly, microbiome is a highly dynamic ecosystem that evolves with age (Lim et al., 2015). This also reflects the profound interpersonal diversity of the gut virome (Minot et al., 2013). The virus–bacterial-host interactions in the gut play a protective role against gastrointestinal diseases such as necrotizing enterocolitis in preterm infants (Neu and Walker, 2011), ulcerative colitis and Crohn's disease, and highlight the role of viruses in contributing to the initiation of inflammation (Wang et al., 2015).
It is well established that pulmonary tissue is the most sensitive human tissue in COVID-19. Of particular interest is the fact that alveolar cells express the AhR at high levels at the basal matrix underneath the alveoli in which immune cells are found [namely mast cells, lymphocytes, dendritic cells (DCs), and innate lymphoid cells (ILCs)] (Frericks et al., 2007). This part of the respiratory system, however, is vulnerable to the effects of very fine particulate matter (especially matter with a diameter less than 2.5 μm, which is mainly produced by diesel engines, charcoal-burning, wood-burning, and industrial exhausts). This vulnerability is because particles of this size are able to bypass the protective mechanisms of the respiratory tract and accumulate in the alveolar tissue. If the particles are less than 0.1 μm, they can penetrate completely the alveolar cell membrane and enter the bloodstream. However, airborne particulate matter may act as AhR activators and thus trigger immune responses (van Voorhis et al., 2013).
Exposure to airborne particles (urban dust, PAH, diesel exhaust, cigarette smoke) by intranasal administration has been shown to increase pulmonary IL-17 expression (van Voorhis et al., 2013). Th17 cells are associated with autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and psoriasis (Zambrano-Zaragoza et al., 2014), but also glomerulonephritis, severe spontaneous colitis, asthma, dengue virus disease (Jain et al., 2013), and swine flu determined by pandemic H1N1 influenza virus (Bermejo-Martin et al., 2009). Epidemiologic studies have linked ambient particulate matter, nitrogen dioxide and other air pollutants exposure to immunologic diseases of the respiratory system (asthma and chronic obstructive pulmonary disease (COPD)), atopic diseases, allergic sensitization, eczema and polymorphisms of inflammatory cytokines via AhR-dependent mechanisms (Morgenstern et al., 2008). Furthermore, COPD has also been associated with an abnormal pulmonary and systemic immune response to tobacco smoking, although only the so-called “susceptible smokers” develop the disease (Cruz et al., 2019). Smokers and COPD patients are subpopulations with an increased risk of severe COVID-19, likely because of the enhanced airway expression of angiotensin-converting enzyme 2 (ACE2) receptor, the entry receptor for the SARS-CoV-2 virus, in lower airways (Leung et al., 2020). Thus, many COVID-19 patients with COPD develop acute respiratory distress syndrome (ARDS), leading to pulmonary edema and lung failure. They also have liver, heart, and kidney damage, reflecting acute systemic inflammatory reaction syndrome and further multiple organ dysfunction syndrome (Docea et al., 2020).
Cytosolic AhR levels and target genes, as well as AhR levels in the nucleus, might be useful markers of COVID-19 patient prognosis, based on previous studies reporting that AhR levels have been used for the diagnosis and prognosis in several aggressive tumors and multiple sclerosis patients (Wheeler et al., 2017). The contribution of innate versus adaptive immune cells to lung pathology is still under investigation, as many inflammatory mediators are implicated (Esser and Rannug, 2015). Excessive or non-effective host immune responses due to long-term xenobiotic exposures or viral attacks can impair T cells and monocytes functionality, burden lung pathology and immunodeficiency, as observed in critical COVID-19 patients (D Zhou et al., 2020).
Macrophages are critical for the development of inflammatory processes. These cells express the AhR, and several studies have corroborated their activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), to produce and release modulatory chemokines and proinflammatory cytokines (Vorderstrasse et al., 2003). The AhR is a known modulator of antiviral pulmonary immunity. AhR activation by TCDD has been shown to decrease survival after infection with a nonlethal dose of influenza A virus, concomitant with doubling of pulmonary neutrophil influx and suppression of expansion and differentiation of virus-specific effector CD8+ T cells (Vorderstrasse et al., 2003). Thus, exposure to environmentally-derived AhR activators is associated with increased respiratory infections such as bronchial asthma and COPD (Roca et al., 2013). COPD affects approximately 200 million people worldwide and is globally one of the leading causes of death. Smoking, biomass burning, air pollution, and inhalation of fine dust are contributing factors to COPD and atmospheric particulate matter —a known carrier of AhR ligands— can aggravate COPD (Roca et al., 2013). PCBs also act through AhR, causing severe thymic atrophy and humoral immune suppression (Silkworth and Antrim, 1985).
2.2. Xenobiotics affect the developing and adult immune system
Immunological development is dependent on both genetic and environmental influences (Vassilopoulou et al., 2017). Both cellular and humoral immunity are factors that drive inter-individual variation in correlation with age, gender, and season. They all play a major role in shaping the immune profile analogous to lymphoid and myeloid cell level, B cell subsets, and Ig levels, or T cell immune traits as compared to the B cell immune traits. All these parameters are sensitive to toxic environmental exposures, especially in the adult immune system. The developing immune system can also be disrupted by early-life environmental insults, which may adversely affect the health of the exposed offspring later in life. These adverse effects could extend to following generations through epigenetic inheritance, thus weakening future generations’ bodily defenses against infections (Vassilopoulou et al., 2017). The increased risk for injury to the developing immune system has been shown across several categories of drugs, chemicals, as well as heavy metals, some metalloids and mold toxins, through perinatal genome reprogramming, increasing the risk for adverse immune outcomes persisting into later life (Vassilopoulou et al., 2017). In particular, exposure to AhR agonists may cause persistent changes to immune responses that can affect subsequent generations, meaning that the environment of prior generations shapes susceptibility to pathogens and antiviral immunity in later generations (Post et al., 2019).
Developmental exposure to AhR-activating chemicals perturbs immune cell development, resulting in attenuated hematopoietic stem cell (HSC) differentiation and long-term self-renewal (Sakurai et al., 2017). It also results in delicate deviations in the architecture of the crystal structure of AhR, thus contributing to increased incidence of autoimmunity and decreased responsiveness to infectious pathogens in later life, via gradual and long-term repression of AhR-mediated gene transcription (Sakurai et al., 2017). Furthermore, long-term activation of AhR by TCDD has been shown to induce epigenetic changes in lymphocytes, especially T cells, leading to DNA hypermethylation and resulting in decreased CD8+ T cell antiviral immunity (Boule and Winans, 2015). Conversely, early life exposure to TCDD enhances the CD4+ T cell response to viral infection in the lung, resulting in more significant broncho-pulmonary inflammation and reduced antiviral immunity (Boule and Winans, 2015).
Exposure to a wide array of environmental chemicals, such as pesticides, heavy metals, and endocrine disruptors, may also affect the developing immune system. Pesticides, such as organochlorines (chlordane and DDT/DDE) organophosphates (diazinon) and carbamates (carbofuran) have been shown to induce developmental immunotoxicity (Holladay, 1999; Ruszkiewicz et al., 2017). Prenatal exposure of humans to DDE has been associated with reduced levels of IgG and recurrent respiratory infections in infants, enhancing allergic responses via induction of Th2 cytokines (Sunyer et al., 2010). The use of lead as a petrol additive, or more generally as an environmental contaminant, has adverse effects on the immune system, since the immune system appears to be quite sensitive to lead. Lead-induced skewing of immune responses has the potential to alter the incidence of asthma, autoimmunity, infectious diseases, or cancer (Dietert et al., 2004).
Early-life exposure to environmental and anthropogenic chemicals may result in reprogramming of the immune system. Exposure of the developing immune system to heavy metals, specifically lead, has been shown to adversely affect the generation of innate immune cells, such as macrophages and DCs (Fenga et al., 2017; Gao et al., 2007; Kishikawa and Lawrence, 1998). Dysregulated macrophages and DCs effector functions reduce phagocytosis, lysosomal activation, produce excessively reactive oxygen species, and other inflammatory mediators (i.e., TNF-α, PGE2) by activation of the Erk/MAP kinase pathway, contributing thus to persistent Th2-biased immune responses and increasing the risk for inflammatory disorders and disease unmasked later in life (Kim and Lawrence, 2000).
Endocrine disruptors, such as bisphenol A (BPA), have also been shown to alter immune system by interfering with hormone production and activity, and neuroendocrine axes (Karzi et al., 2018; Petrakis et al., 2017). Perinatal exposure to low doses of BPA has been shown to alter the neonatal immune system and render it more susceptible to food intolerance (Nakajima et al., 2012). Low-dose BPA exposure during the juvenile period of development may aggravate allergic airway inflammation by enhancing Th2 responses via disruption of the immune system (Koike et al., 2018).
Long-term exposure to mixtures of anthropogenic pollutants such as nanomaterials (NMs), pesticides, BPA, phthalates, heavy metals, particulate matter, PAH and toxins during critical developmental periods, could hypothetically impact the immune system and increase viral infectivity, morbidity and mortality in children and adults (Kwak et al., 2009). Xenobiotics can impair a protective immune response, especially in organs expressing high ACE2 expressions, such as intestine and kidney.
The degree to which xenobiotics deregulate immune responses depends on cell-type and maturational state, especially in vulnerable population groups. Both inflammatory and regulatory gene expressions via cross-talk between transcriptional (e.g. AhR and NF-κB), xenobiotic response elements activation and other posttranslational and epigenetic alterations, together with viral immunotoxicological engagements, contribute to unwanted complications on macrophages, dendritic cells, the cytolytic activity of murine NK cells, innate lymphoid cells and Foxp3+ regulatory T cells (Qiu et al., 2012).
3. Long-term low-dose exposures to mixtures of anthropogenic chemicals may affect the human health through dysregulation of the immune system
Exposure to single chemicals during development has been shown to impair immune responses. However, under real-life exposure scenarios, humans are widely exposed to mixtures of numerous chemicals and other stressors, such as radiation, sound, biotoxins, etc., present in different sources (environment, diet, workplace). Thus, the relevant exposure framework for evaluating potential impairment of the immune function relies on assessing mixtures of chemicals (and other toxic stressors) rather than single chemicals in our changing environment. According to this approach, exposure to chemical mixtures during development could be more detrimental to the immune system than exposure to single chemicals, depending on the nature of the chemicals and their dosages. While the developmental stage is the most vulnerable period to chemical toxicity, exposure during non-critical periods should also be considered. Furthermore, it should be highlighted that low-dose disruptive effects of chemicals on key biological pathways and mechanisms in certain subpopulations may enhance susceptibility to diseases.
The immune system recognizes engineered nanomaterials (NMs) as foreign bodies, resulting in multilevel responses that can range from acute to chronic. These responses can range from immune-stimulatory to immunosuppressive, but can also vary in host toxicity and/or reduced therapeutic efficacy of conventional pharmaceuticals (Engin et al., 2017; Kendall and Holgat, 2012; Neagu et al., 2017). NMs-induced immune activation may increase the incidence of allergic reactions, inflammatory responses, or autoimmunity. Additionally, NMs-induced suppression may reduce maturation and proliferation of immune cells, resulting in increased susceptibility to infectious diseases or tumor growth.
In nanotoxicology, numerous studies clearly demonstrate that multi-walled carbon nanotubes (MWCNT) and inhaled particulate matter (PM) induce lung injury (Kendall and Holgat, 2012; Piperigkou et al., 2016) through acute and chronic inflammation, granuloma formation, and substantial interstitial lung fibrosis. MWCNT and PM can also exacerbate asthma-like conditions via innate pathways such as TLRs that trigger the generation of IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). MWCNT also induce epithelial damage resulting in IL-33 release, which in turn promotes innate lymphoid cell recruitment and the development of IL-13-dependent inflammatory response (Beamer et al., 2013 ).
A number of studies similarly linked MWCNT-induced oxidative stress, phagolysosomal permeabilization, cathepsin B release, NLRP3 inflammasome assembly, and caspase-1 activation with the secretion of important regulatory cytokines (e.g., IL-1β and IL-18). Titanium dioxide nanomaterials, long carbon nanotubes, asbestos and silica, affect innate immune activation through reactive oxygen species (ROS) generation, cathepsin B activity that activate Nalp3 inflammasome and induce Il-1α and IL-1β secretion by caspase1, autophagy and lung fibrosis (Palomäki et al., 2011). These findings highlight that inflammasome activation and autophagy induction may occur via pathways other than the well-established mTOR pathway (Palomäki et al., 2011).
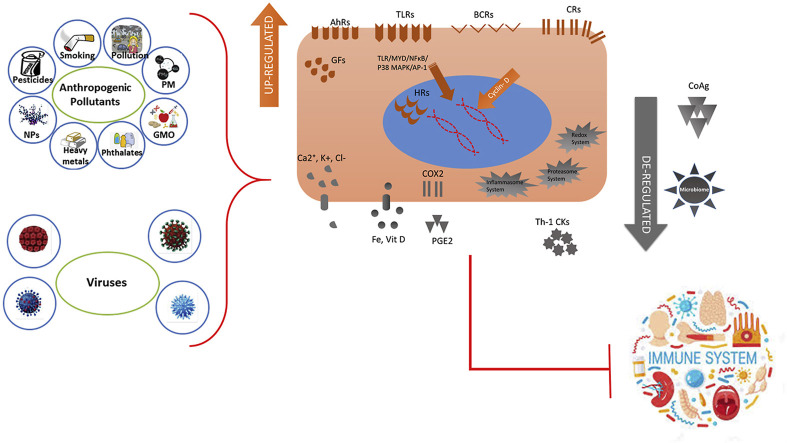
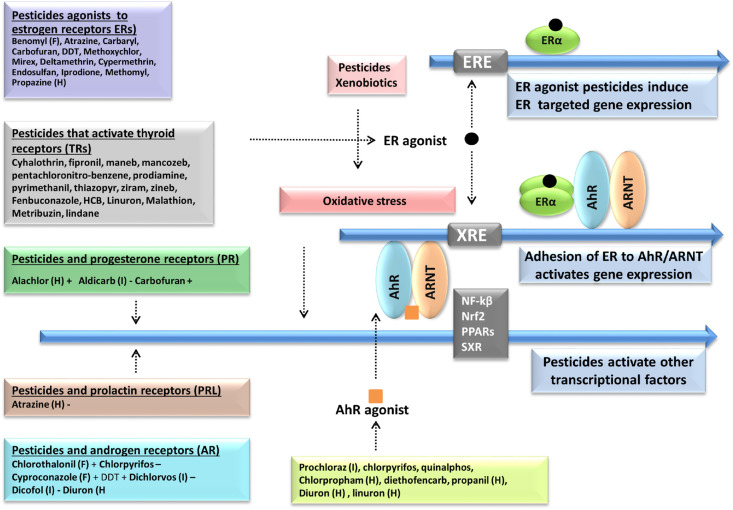
Pesticides can impact human health and the environment adversely through cellular and extracellular, direct and indirect mechanisms in a complex and synergistic mode. Extracellularly, synthetic pesticides activate primarily receptors of cytokines (Gangemi et al., 2016 ) (Fig. 3 ). These key receptors, via secondary signaling pathways such as TLR/MYD/NF-kB, P38 MAPK/AP-1, AhR, ER/FFAs/PPARγ, B-cell receptor or Cyclin D signaling pathways, directly and indirectly, alter transcriptionally and epigenetically global gene expression (Vidya et al., 2018) (Fig. 4 ).
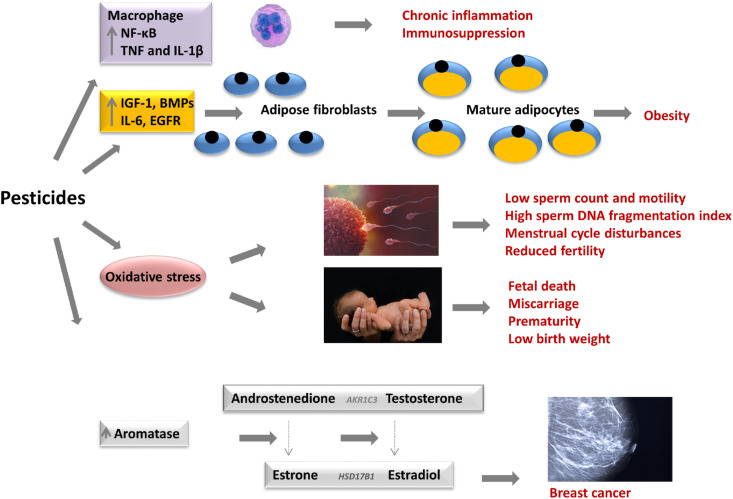
Fig. 3.
Molecular and cellular mechanisms triggered by long term pesticide exposure. Pesticides induced pro-inflammatory mediators (TNF-α, IL-1β, IL-6) of macrophages, aromatase expression, growth factors and oxidative stress, oestrogenicity, carcinogenesis, DNA damage, genomic, epigenetic changes, obesity and abnormal embryo development.
Fig. 4.
Transcriptional alterations induced by selective pesticides.
Pesticides also, affect the metabolism of Ca+2, vitamin D and iron, disrupt redox biology (Ffrench-Constant, 2013), activate proteasome system, alter microbiome (Joly Condette et al., 2015), increase PGE2 via COX2, increase Th 1 cytokines and decrease Th 2 cytokines, leading to T-cell immaturity. The pesticidal loosening of tight junctions increases the intestinal permeability and disrupts the integrity of vasogenic homeostasis and vascular permeability, thus increasing translocation of bacteria to distal organs and resulting in bacterial infectivity (Stanaway et al., 2016). Pesticides also disrupt redox biology, reduce antioxidant enzyme activities, increase reactive oxygen species (ROS) and oxidative stress, which may impact the immune system function because of the cross-talk between oxidative and pro-inflammatory pathways. In addition, pesticides can induce ATP and energy depletion and DNA instability through DNA damage or epigenetic alterations, sister chromatid exchanges, telomere shortening, diverse mutations, and premature cell death, which also impact the immune system function (Demsia et al., 2007; Fountoucidou et al., 2019; Tsatsakis et al., 2019a; Tsiaoussis et al., 2018).
In any case, given the plethora of physiological pathways and functions that can be disrupted by chemical exposures, the current risk assessment approach based on the assessment of individual substances is insufficient to address exposure to chemical mixtures under real-life conditions (Kostoff et al., 2018; Tsatsakis et al., 2019c; Hernández et al., 2020).
4. Molecular and cellular mechanisms of SARS-CoV-2 cell attack
SARS-CoV-2 is a member of the coronavirus family that has a single-stranded RNA genome of positive sense. This gene encodes four structural proteins, namely spike glycoprotein (S), a small envelope protein (E), matrix glycoprotein (M) and nucleocapsid protein (N) (Li et al., 2020). The entry of the virus into cells is mediated by the S protein, in particular by attaching its surface unit (S2) to ACE2, an enzyme present in primarily type II pneumocytes (lung alveolar epithelial cells). Then the S protein is cleaved by cellular proteases and the viral capside is fused with the cellular membrane, thus allowing intracellular translocation of SARS-CoV-2 coupled with ACE2 by endocytosis (Cava et al., 2020). This is followed by massive intracellular replication of SARS-CoV-2, which then undergoes exocytosis where it binds again to ACE2, thus entering into a vicious cycle (Abassi et al., 2020) Furthermore, after endocytosis of the SARS-CoV-2/ACE2 complex, surface ACE2 is down-regulated, resulting in unopposed angiotensin II accumulation (Vaduganathan et al., 2020). The binding affinity of specific receptor binding of S protein is related to virus infectivity and pathogenicity (Li et al., 2020https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7093363)). In this regard, ACE2 genetic polymorphisms and expression levels in different tissues might alter host susceptibility to COVID-19 by affecting the interaction with SARS-CoV-2 S-protein.
ACE2 belongs to the renin-angiotensin-aldosterone system (RAAS), which contributes to the pathophysiology of hypertension and cardiovascular/renal diseases by maintaining homeostasis of blood pressure, electrolyte balance and inflammatory responses (Cava et al., 2020). ACE2 can be considered as a key counterregulatory enzyme that degrades angiotensin II to angiotensin-(1–7), which exerts vasodilatory, natriuretic/diuretic, anti-inflammatory, and antifibrotic effects via Mas receptor (MasR) (Abasi et al., 2020; Vaduganathan et al., 2020).
ACE2 is highly expressed in the lung, where it appears to exert a protective role in acute lung injury as loss of pulmonary ACE2 function has been associated with acute lung injury. Hence, the binding of SARS-CoV-2 with ACE2 neutralizes the advantageous physiological effects of this enzyme. Attenuation of ACE2 catalytic function alters RAAS system activity, resulting in enhanced inflammation and vascular permeability observed in the pathogenesis of inflammatory lung disease, eventually leading to acute lung injury and adult respiratory distress syndrome as observed in patients with severe COVID-19 (Cheng et al., 2020 27). Hence, local activation of the RAAS may mediate lung injury responses to viral insults (Vaduganathan et al., 2020). Indeed, when angiotensin I and angiotensin II receptors (AT1R and AT2R, respectively) are activated, they lead to the increased expression of proinflammatory mediators (e.g., IL-6), triggering an inflammatory process in the lungs and other organs. In particular, AT1R activates the transcription factors NF-kB and activator protein 1 (AP-1), which in turn increases cytokine expression, apoptosis, vasoconstriction, fibroproliferation, retention of Na+, and the enhancement of lung injury. The endogenous angiotensin II inhibits alveolar fluid clearance and dysregulates epithelial Na+ channel (ENaC) expression via AT1R, contributing to alveolar filling and pulmonary edema (Cava et al., 2020).
ACE inhibitors and angiotensin-receptor blockers (ARBs) have shown to up-regulate ACE2 expression in animal studies, thus increasing the availability of target molecules for SARS-CoV-2. These considerations have led to speculation that ACE inhibitors and ARBs might be harmful in patients with Covid-19. However, a number of studies conducted in different populations and with different designs have come to the conclusion that ACE inhibitors and ARBs are unlikely to be harmful in patients with Covid-19 (Jarcho et al., 2020 1). Conversely, because of the protective effects of ACE2 on acute lung injury and chronic diseases, the development of drugs enhancing ACE2 activity might be an approach for the treatment of COVID-19 (Cheng et al., 2020 27). The aforementioned speculations should not be regarded as evidence to prescribe or not these drugs in patients with Covid-19. Clearly, randomized clinical trials are warranted to shed light on this apparent paradox.
The early onset of rapid viral replication may cause massive epithelial and endothelial cell apoptosis and vascular leakage, triggering the release of exuberant pro-inflammatory cytokines and chemokines (Yang, 2020). The excessive immune reaction produced by SARS-CoV-2 infection in the host can lead in some cases to the so-called ‘cytokine storm’ or ‘cytokine release syndrome’. This syndrome can be triggered by a variety of factors, such as infections and certain drugs, and consists of an acute systemic inflammatory syndrome characterized by fever and multiple organ dysfunction resulting in an extensive tissue damage. The main cytokine responsible of this storm is IL-6, which is produced by activated leukocytes and acts on a large number of cells and tissues (Cascella et al., 2020).
The Toll-Like Receptor (TLR) signaling pathway is evolutionarily conserved and functionally linked to innate immunity. Signaling results in nuclear translocation of the transcription factor NF-κB, which regulates the expression of distinct genes in immunity or development (Anthoney et al., 2018). TLR has been implicated in the pathogenesis of airway disease resulting from respiratory virus infections, with TLR signaling leading to the activation of type I interferons (IFN-α and IFN-β), proinflammatory cytokines (IL-6, TNF, IFN-γ, and CCL5), and interferon-stimulated genes (Totura et al., 2015). The binding of SARS-CoV-2 to the TLR activates the its downstream signaling pathway, causing the release of pro-IL-1β (which is cleaved by caspase-1). This is followed by inflammasome activation and production of active mature IL-1β, which is a mediator of lung inflammation, fever and fibrosis (Conti et al., 2020).
5. Xenobiotics and viruses have common and more than one mechanisms of action
Chronic organochlorine, organophosphate, paraquat and pyrethroid exposures have been linked to Parkinson's disease and other neurodegenerative diseases through mitochondrial dysfunction, oxidative stress, and apoptosis via cell signaling pathways (Cao et al., 2019). Some pesticides have been reported as potential risk factors of chronic erectile dysfunctions (Polsky et al., 2007), probably via NADPH oxidase/ROS production and reduced bioavailability of NO (Jin and Burnett, 2008). Experimentally, DDE (a metabolite of DTT) exposure exhibits a diabetogenic potential, with an underlying immunomodulatory mechanism likely via cytokine cascades, or disrupted glucose homeostasis (Cetkovic-Cvrlje et al., 2016). Cardiovascular toxicity has also been reported following exposure of zebrafish to deltamethrin (Meng et al., 2019).
Adjuvants in pesticides formulations are used to increase the pesticidal effect of the active ingredient. However, this is a constant underestimated factor of pesticide mixture chronic toxicity (Mesnage et al., 2013). For instance, it has been shown that animals subjected to concomitant exposure to certain fungicides, such as ergosterol biosynthesis inhibitors, are able to increase the pyrethroid and neonicotinoid insecticides cytotoxicity up to 1000- and 8-fold respectively. This combined exposure may result in enforced lipogenesis and/or oncogenic transcriptional alterations, which may not occur with individual chemicals (Thompson et al., 2014).
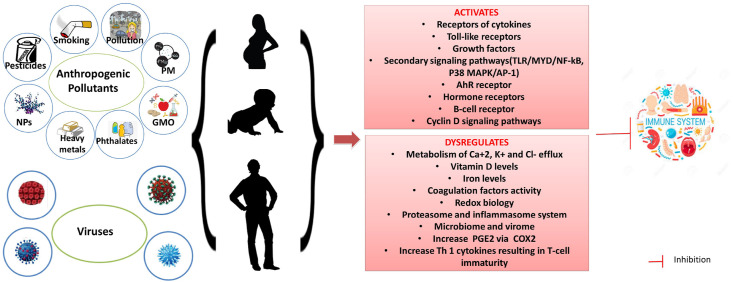
Xenobiotics and viruses activate similar transmembrane receptors of cytokines, toll-like receptors, growth factors, secondary signaling pathways, such as TLR/MYD/NF-kB, P38 MAPK/AP-1, AhR, hormone receptors, B-cell receptor or Cyclin D signaling pathways, directly and indirectly and thus alter the global gene expression transcriptionally and epigenetically (Conti et al., 2020; Gangemi et al., 2016). Xenobiotics and viruses affect the metabolism of Ca+2, K+ and Cl− efflux, vitamin D, iron and coagulation factors, disrupt redox biology, activate proteasome and the inflammasome system, alter microbiome and virome, increase PGE2 via COX2, and increase Th 1 cytokines, resulting in T-cell immaturity (Go and Jones, 2014; Joly Condette et al., 2015).
Xenobiotics and viruses disrupt the redox biology, reduce antioxidant enzyme activities, increase reactive oxygen species (ROS) and oxidative stress, ATP and energy depletion, DNA instability through DNA damage or epigenetic alterations, premature cell death and ageing (Go and Jones, 2014; Joly Condette et al., 2015; Martínez-Valenzuela et al., 2017). Furthermore, both xenobiotics and living microorganisms may contribute to increased resistance to treatments, bacteria or viruses aggressiveness, communicable diseases and decreased vaccination effectiveness.
Viral infections and chemical exposures can produce pro-inflammatory mediators that, if sustained over time, result in chronic inflammation in target organs. This condition often entails tissue damage, increased mutation rate and genomic instability, which represents a cancer-prone microenvironment (Kidane et al., 2014). In cardiovascular morbidity, xenobiotics and viruses have been found to increase chemokines, cytokines, heart mitochondrial GPx activity, DNA damage, cholesterolemia, expression of tissue plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1) and pro-coagulant activity, ROS, TNF-α and TGF-β, protein and lipid oxidation in heart cells, endothelial dysfunction, and decreased mitochondrial GSH levels, resulting in hyper-coagulability and thrombosis (Zafiropoulos et al., 2014).
Both xenobiotic and viral immune morbidity are due to alterations in gene mRNA and protein expression via pro-inflammatory cytokine, chemokine, kinase and neuroendocrine signaling, activation of T-cells, disruption of gut microbiota and immune homeostasis (Go et al., 2015; Jin et al., 2017). These reprogram the cell cycle control in the Golgi region via cycline gene alterations and premature cell death (Chiappini et al., 2014). Crohn's disease, rheumatoid arthritis, autoimmune thyroidopathies, systemic lupus erythematosus, psoriasis, fibromyalgia-osteoarthritis, chronic pain, and multiple chemical hypersensitivity have been associated with pesticide exposure (Fernández-Solà et al., 2005; Langer, 2010; Williams et al., 2018). Global immune deficiency, vaccine and drug ineffectiveness, bacterial and virus virulence, cancer aggressiveness, increased chronic inflammatory pathologies are associated with various pesticide adverse immune effects.
Additionally, pesticide exposure in neonates and children is correlated with alterations in B-cell maturation, rapid cell proliferation, disruptive homeostasis of the pro-oxidant agents, altered fecal microbiota composition, cytokine expression and antioxidant defenses, steroids and genomic alterations (Gonzalez et al., 2018; Ling et al., 2014; Mona et al., 2012). Both NMs and viruses can affect the adaptive immune response by direct activation of antigen-presenting cells (APCs), including B cells, dendritic cells, macrophages, helper and cytotoxic T cells, and can contribute to both intracellular and extracellular immune response deficiency to pathogens but also to increase tissue-specific autoimmunity, observed in autoimmune diseases (Kalkanidis et al., 2006). Both hydrophobic nanoparticles <5 nm and viruses dictate the immune response exhibiting the greatest expression of inflammatory cytokines (Moyano et al., 2012).
Moreover, many nodal points used by viruses and chemicals to generate, develop and disperse pathologies and diseases have been used over the last three decades individually or in combination as targets for modern checkpoint therapies through monoclonal antibodies in order to address these diseases (Peng et al., 2016; Hafeez et al., 2018). Transcription factors, membrane receptor signaling pathways, and protein kinase signaling pathways, are all key players of xenobiotic-induced cancer in adults (Eldakroory et al., 2017) and children ( Hargrave et al., 2006), hematological and lymphoid malignancies (Leon et al., 2019), Alzheimer's disease and Parkinson's disease (Aloizou et al., 2020) and stroke (Tsatsakis et al., 2019b). Furthermore, this knowledge has also been valuable for molecular-oriented anti-cancer therapy (Liu et al., 2018).
ACE2 expression has been shown to be upregulated by infection with SARS-CoV-2 (Wang and Cheng, 2020). However, the relation between ACE2 and SARS-CoV-2 virulence is complex and it is attracting extreme interest currently (Hoffmann et al., 2020), following concern about the potential ability of some anti-hypertensive drugs, such as ACE-inhibitors and angiotensin-receptor blockers (ARBs) to increase Covid-19 severity and case fatality rate (Day, 2020). This is a clear example of environmental toxins laying the groundwork for increased susceptibility to COVID-19 (and other viral diseases), and subsequent treatments exacerbating the severity of COVID-19, and is why elimination of the environmental toxin is the much preferred route.
On the other hand, the relation between ACE2 levels and SARS-CoV-2 virulence might go in both directions, i.e. both high and low ACE2 activity could theoretically increase Covid-19 severity (Guo et al., 2020). Therefore, more research is urgently needed about the interaction between the ACE-inhibitor/ARB use and the susceptibility to increased Covid-19 severity. Observational studies are currently underway on this issue, and need to be expanded. In the meantime, caution has been suggested by scientific and professional societies about any modifications to current anti-hypertenive drug therapy based on ACE-inhibitors and ARBs in people with high blood pressure, in the absence of convincing clinical evidence of their benefits against severity of Covid-19 (Fang et al., 2020; ESC, 2020).
It has been posited that the antimalaric drugs chloroquine and hydroxychloroquine reduce glycosylation of ACE2, thus preventing SARS-CoV-2 from binding to host cells (Devaux et al., 2020). Chloroquine has also been shown to inhibit quinone reductase 2, an enzyme that functions prominently in sialic acids biosynthesis (Olofsson et al., 2005) and critical components of ligand recognition (Kwiek et al., 2004). Many trials have recently been initiated on the real clinical usefulness of these drugs, which have been suggested to be useful in early therapy and even in the prevention of Covid-19. However, based on their established and potentially very serious side effects, these drugs should not be initiated until there is clear evidence of their efficacy for the treatment of Covid-19.
In addition, given that SARS-CoV-2 entry into the host cells is mainly mediated by the endocytic pathway, chloroquine seems effective in blocking endocytosis and/or inducing viral entrapment into the lysosome. The latter can be achieved by neutralizing the endosomal pH, thus favoring virus–endosome fusion, inhibit lysosomal protease activity, and prevent the cleavage of S protein and therefore viral entry into the host cell (Yang et al., 2004). Chloroquine may also thwart viral protein maturation, block the recognition of viral antigens by dendritic cells, and increase cytotoxic CD8+ T-cell activity to viral antigens. In addition, it blocks phosphorylation (activation) of the p38 mitogen-activated protein kinase (MAPK) and caspase-1 (Steiz et al., 2003) in THP-1 cells, both of which are required for SARS-CoV-2 and other viruses replication (Briant et al., 1998). Moreover, chloroquine affects immune system activity by mediating an anti-inflammatory response, which might reduce damage due to the cytokine storm. Chloroquine inhibits IL-1β mRNA expression in THP-1 cells, reduces IL-1β release, IL-1 and IL-6 cytokines in monocytes/macrophages, and IFNα, IFNβ, IFNγ, TNFα, IL-6 and IL-12 gene expression (Devaux et al., 2020). Lysosomotropic agents, ammonia chloride and bafilomycin A, have been found to reduce the transduction on 293/hACE2 cells by SARS-CoV-2 S, and thus inhibit the SARS-CoV-2 entry into cells through endocytosis (Villamil Giraldo et al., 2014; Ou et al., 2020).
Finally, the newly-introduced anti-viral agent Remdesivir was found to be beneficial against SARS and MERS in animal models (Holshue et al., 2020). Remdesivir is an adenosine analogue, antagonizing adenosine for RNA-dependent RNA polymerase, causing their premature viral RNA termination (Sheahan et al., 2017). However, as Remdesivir is a prodrug, it has to be metabolized in order to reach its active form GS-441524. Its clinical significance was proved during the West African Ebola virus epidemic of 2013–2016 and the Kivu Ebola epidemic in 2018 (Warren et al., 2015). Given its promising effectiveness against previous corona-viruses, Remdesivir has also been tested against COVID-19. According to various studies, favorable outcome is to be expected from animal use given the in-vitro efficiency of the drug (Wang et al., 2020). Although Remdesivir has led to quick recoveries in symptoms of Covid-19 patients in a clinical trial, results from its Phase III trial involving severe Covid-19 patients are expected to be available soon.
6. Approaches and challenges to tackle the Covid-19 epidemics/pandemics
It is well understood that COVID-19 outbreak and current policies are based on regulations; the main strategy worldwide mirrors and expands the strategy used in the prior SARS pandemic to limit the spread of the pandemic: ‘Stay at home’/quarantine. This approach primarily does not account for human real-life needs scenarios, lacks realism as a directive for subsequent new viral epidemics or pandemics, does not provide the protections afforded by ‘herd immunity, does not meet current and future needs in health and safety of the global population, and adversely impacts development of production and economy worldwide. The only advantages are 1) flattening the contagion curve through social distancing and lockdowns and 2) avoiding hospitals (and Intensive Care Units) overwhelmed by Covid-19 cases.
The global spending on cancer therapies, cholesterol-lowering drugs, malaria, spina bifida and anencephaly, osteoporosis, vaccine market and diabetes nowadays exceeds $ 5 trillion USD and, according to WHO is expected to grow at a compound annual growth rate of 5% in the next ten years period (WHO, 2019a). According to WHO, the global total 2019 health cost is about 10 trillion USD, 10% of Gross National Product (GDP) and 1080 USD per capita (WHO, 2019b). The total global cost for COVID-19 will be unknown for months, and it will likely have a substantial secondary cost burden for decades.
As health authorities are developing effective measures to stem the health effects of COVID-19, uncertainties remain regarding both the virus-host interaction and the evolution of the epidemic. The spatial-temporal features of the pandemic, the attempts to conceal the origin and the first medical data from Wuhan, the route and the difference of the spread of the virus, the criteria used to assign deaths to the COVID-19 category, the reduced mortality-to-incidence ratio as testing rises and more patients are identified, the non-observance of individual protection measures and treatment of mild Covid-19 cases in new temporary hospitals, the intensified competition and mutual accusations between USA and China, the lack of scientific data on treatment and vaccine, difficulties in verification of alleged or attempted biological attacks by the use of living-biologically modified - viral agents, all raise insecurity and reasonable questions worldwide.
As of mid-March 2020, many countries in the world are on partial lockdown to control the pandemic (COVID-19) spread, and the only effective ‘treatments’ at this time are good hygiene (washing hands), wearing a mask, and quarantine. Although quarantine may reduce the spread of the virus, it may impose the self-medication at home for chronic diseases without medical diagnosis and treatment, increase the risk of adverse complications of cardiovascular, metabolic, autoimmune and psychiatric diseases, shifting thus the health cost from public providers to patients. Scientists in fields of public policy have presented combined tactical and strategic treatment approaches that aimed at preventing and reversing COVID-19 and other diseases, including treatment repurposing as well. Optimally, the tactical and strategic approach components would be implemented in parallel, to provide benefit from the synergies of the combined approach This combined approach would allow the vulnerable to survive the near-term potentially lethal effects of SARS-CoV-2 exposure through tactical treatments, and would allow the most vulnerable and the larger population to be more resistant to future viral attacks of all types through strategic treatments (Kostoff, 2020).
7. Effects of diet on immune system and role of nutrient supplements on infected vulnerable groups
The immune system is remarkably dynamic throughout life in relation to its components’ number and its function. Interestingly, older adults exhibit a decline in immune function -known as immunosenescence-resulting in increased susceptibility to infectious diseases and a higher risk of serious complications than younger people, reflecting altered acquired and innate immunity (Crooke et al., 2019). Aging is associated with altered T cell function, decreased thymic output and thymic involution, and reduced numbers of naive T cells (Berzins et al., 2002) and micronutrient deficiencies. All these elements play key roles in increasing inflammation and its resulting morbidity and mortality (Salanitro et al., 2012). Immune function may be improved in immune competent adults, particularly those over 65 years old, by restoring deficient micronutrients, such as vitamins A, C, D, E, B2, B6, and B12, folic acid, iron, selenium, and zinc to optimal (Maggini et al., 2018). Adequate intake and bodily levels of these micronutrients has been shown to be critical in reducing risks stemming from inflammatory and non-communicable diseases (Miles et al., 2008).
Nutritional modulation of the immune system is also associated with reduction or delayed onset of immune-mediated chronic diseases. Several micronutrients and dietary components have been shown to play specific roles in the development and maintenance of an effective immune system. For example, arginine is necessary for the nitric oxide generation by macrophages, and vitamin A and zinc are known regulators of cell division. Vitamin E is both an antioxidant (Lee and Han, 2018) and protein kinase C activity inhibitor (Childs et al., 2019). Dietary and supplementation interventions enriching the gut microbiome, such as the use of probiotics and prebiotics (Hill et al., 2014), may be needed to help enhancing or restoring a healthy gut microbiome. In addition, diets lacking vegetables may decrease the diversity of nutrients reaching the gut microbiome. This in turn alters epithelial integrity resulting in increased permeability or ‘leaky gut’, allowing immune cells within the gut-associated lymphoid tissue to be directly exposed to intraluminal nutrients or elements of the gut microbiota. A great example of altered gut permeability induced by micronutrient status is that of vitamin D. Specifically, vitamin D-deficient diets are found to be responsible for increased epithelial permeability due to dysfunctioning tight junctions, resulting in both acute and chronic gut inflammation (Bischoff et al., 2014; Sassi et al., 2018). Optimal probiotic bacterial composition has been shown to effectively reduce inflammation, characterized by reduction of proinflammatory Th1 and Th17 cytokines, such as IL-17 and IFN-γ, and concomitant increases in the levels of inflammation-resolving cytokine, such as IL-10 (Santiago-Lopez et al., 2018). On the other hand, prebiotics can be used not only as substrates for bacterial metabolism but also to enhance barrier function. The unparalleled importance of natural and well-balanced diets has been also highlighted vis-à-vis infant formula consumptions. It is well established that breastfeeding affords passive immunity to the infant via transfer of antibodies, growth factors, and cytokines to the offspring (Torres-Castro et al., 2018; Plaza-Diaz et al., 2018). Breastmilk is also enriched with microbiota thatpromote the maturation of gut-associated lymphoid tissue (Donovan and Comstock, 2016), playing a key role in development of the immune system during thymus maturation, particularly for T cell function. In breastfed infants, complementary feeding with prebiotics for optimal microbiome maintenance has been proposed (McKeen et al., 2019).
The Western-type diet (described as a diet rich in processed sugars, trans- and saturated-fats, but low in complex carbohydrates, fibers, micronutrients, and other bioactive molecules such as polyphenols and omega 3 polyunsaturated fatty acids) has been shown to predispose to inflammation, likely by increasing the uptake of lipopolysaccharide (LPS) from gut microbes secondary to altered gut permeability and increased leakiness (Vardavas et al., 2011; Zinöcker and Lindseth, 2018). Although activation of TLR4 by LPS may cause inflammatory response, a number of nutrients, such as long-chain omega 3 polyunsaturated fatty acids, have been shown to thwart TLR4 activation, thus attenuating inflammation (Rogero and Calder, 2018). In contrast, a Mediterranean-type diet, which is rich in certain foods (vegetables, fruit, nuts, legumes, fish, ‘healthy’ dietary fats such as virgin olive oil), has been shown to be associated with lower risk for chronic diseases, including cancer, cardiovascular disease, and neurological disorders (Dinu et al., 2018). These effects have been attributed to immune-modulation and the anti-inflammatory properties of various polyphenols and other bioactive compounds inherent to these foods, especially fruits and vegetables (Yahfoufi et al., 2018). Νutrients from genetically modified organisms (GMOs) affect the adaptive immune response because the cellular ability for specific recognition of a genetically modified protein is lacking. There is no ‘memory’ on repeated exposure, resulting in allergic reactions observed in consumers after consuming modified plants. One example is the “Starlink” maize, which was engineered with genetic information from Bacillus thuringiensis to increase the plant's resistance to various insects (Nawaz et al., 2019; Tsatsakis et al., 2017; Zhang et al., 2016). Except for xenobiotic and GMOs allergenicity, there are genetic hazards arising from the inserted gene, its expressed protein per se, and secondary or pleiotropic effects on other gene expression. This results in disruption of natural genes in the manipulated organism (Bawa and Anilakumar, 2013).
Micronutrients, such as Zn and Selenium, have important catalytic and structural roles as co-factors in numerous proteins involved both in adaptive and innate immune response (Ibs and Rink, 2003). Significantly, selenoproteins regulate immunity in numerous infectious diseases, including human immunodeficiency virus infection (Avery and Hoffmann, 2018).
Under acute infections, such as COVID-19, glutamine consumption rate by immune cells increases, especially in rapidly dividing cells of the immune cells. Thus, glutamine supplementation in critically ill patients is necessary (Cruzat et al., 2018). Finally, the activation of vitamin D nuclear receptor (VDR) by 1,25(OH)2D3 (calcitriol, the active form of vitamin D) is found to enhance both the innate and adaptive immune response (Wang et al., 2004). This can be very important in cases of infectious diseases in the elderly, such as COVID-19, where the patient is probably suffering from exhausted vitamin D.
8. Discussion
Atmospheric pollutants from power plants, industries, transport, fuel combustion of military missiles and aircrafts, weapon of mass destruction such as chemical, nuclear and biological weapons (Petrakis et al., 2016), spacecrafts, electromagnetic fields (Kostoff and Lau, 2017) and irradiation from nuclear weapons, nuclear power plants and modern technology radiation (Kostoff, 2019; Kostoff et al., 2020) are environmental factors that seriously harm health of humans, and other forms of life (Malagoli et al., 2010). Historically, viruses may have been used in the past as biological weapons. Today, despite the ‘Biological and Toxins Weapons Convention’ that entered into force in 1975, it is known that many countries are still working on and stockpiling biological weapons (Christopher et al., 1997). Chemicals, metals, particulate matter, nanoparticles, anthropogenic climate change, and increased UVB radiation disrupt health, ecosystems and environment. These toxins, along with the toxic modern lifestyle and smoking, constitute an ideal man-made environment for the development, and spread, of modern diseases, including the new viral epidemics/pandemics that occurred during the two last decades (Fig. 5 ).
Fig. 5.
The common mechanisms through which exposure to different stressors and viruses leads to inhibition of immune system.
We have described the common intracellular mechanisms that environmental and anthropogenic pollutants, as well as the SARS-CoV-2, use for their immunotoxicity and mechanism-based treatment approaches. Βasic and applied immunotoxicology can enhance public health and provide significant advantages in the prevention and treatment of epidemics/pandemics and chronic diseases.
While several animal species may harbor SARS-CoV-2, the precise animal reservoir has yet to be confirmed. It seems likely that a spike mutation, which probably occurred in late November 2019, triggered transmission of the virus to humans (Cascella et al., 2020). Τhe current anti- SARS-CoV-2 therapies used in some patients have been unable to counteract disease progression and to save patients’ lives. Since the S2 subunit of SARS-CoV-2 is highly conserved, it could be a target for antiviral (anti-S2) drugs; however, the potential for viral mutations may be responsible for future disease relapses (Cascella et al., 2020). In this study, we emphasize that in addition to the urgent measures that are necessary to interrupt the coronavirus transmission from farming animals to humans, there is also a need to improve the current global strategy for energy management and other factors that may adversely affect the immune system of the human, and thereby increase the risk of both infectious and chronic-degenerative diseases. Exposures to large classes of xenobiotics have a triple impact on the immune system. First, xenobiotics promote immunomodulatory effects in immune cells. Second, xenobiotics increase the range of immunotoxicity of human-associated micro-organisms or new viral and microbial attacks. Third, xenobiotics may reduce vaccine efficacy.
Global health cost and COVID-19 effects on the world economy, health systems, and production are massive currently, and will be far more massive when a final accounting is performed. We recommend application of massive COVID-19 screening tests as an important step for properly 1) recording the extent of the COVID-19 outbreak and, through evidence-based corrective actions, 2) delaying the spread of the pandemic. Given the guidance of the health authorities to transfer COVID-19 patients into hospitals only when presenting fever and dyspnea, it appears that for all admitted patients, and especially for ICU patients, the inflammosome is already at an advanced stage precluding effective treatment. Accordingly, novel AhR-mediated immunotherapies for a plethora of immune-associated diseases/disorders should be pursued.
Conclusively, additional common safety factors need to be added when calculating COVID-19 prevalence and infectivity, including the cooperation of healthcare providers and the scientific community experts. The use of a self-managed qualitative and quantitative evaluator for COVID-19 spread is also a realistic suggestion, keeping in mind that the increased incidence in COVID-19 outbreak in Italy, Spain, Iran, Turkey, USA and United Kingdom appears to have a close correlation to probably inadequate and delayed implementation of uniform antivirus measures, despite the socio-economic, racial, demographic, cultural and administrative characteristics of each country. New investigations into both genetic and environmental fields of immunotoxicology will advance our understanding of immune function, provide the foundation for the development of novel immunotherapeutics and, more importantly, decrease the effect of immunological risk factors.
9. Conclusion
This paper highlights that environmental-related diseases (e.g., energy-metabolism-immune mediated obesity, type II diabetes, metabolic syndrome and cancers) and infectious diseases (e.g., parasitic, influenza or coronavirus-related epidemic or pandemic) share the same pathogenic mechanisms at the molecular level, particularly the AhR pathway. Viral epidemics and pandemics, in addition to causing significant morbidity and mortality, can challenge societal structure and healthcare. As novel viruses continue to emerge, novel therapies and preventative measures must be sought. Understanding and optimizing host cell health and viral infectivity parameters are critical steps for any successful cytolytic virology.
Χenobiotics immunotoxicity, and immune deficiency, deviation or dysregulation affect immunological development, and induce immuno-dependent diseases that are transmitted transgenerationally to offspring. The best long term therapy to mitigate the effects of anthropogenic pollutants related to immune deficiency and fatal viral outbreaks is to introduce much more stringent regulation on the emissions resulting from unabated introduction of modern technologies into our environment, our workplace, and our daily life.
In addition, integrated chemical management, economic and political measures to reduce industrial pollution, greenhouse gases and the effects of climate change on human and environmental health (the ‘one health’ goal), new technology systems should continue to provide positive outcomes for immune system function and chronic disease prevention, as well as for restriction of viral and bacterial invasion and aggressiveness. Currently, the public health measures based on social distancing, appropriate quarantine, and increase of COVID-19 diagnostic tests globally are the necessary options for our defense against the SARS-CoV-2 pandemic, although they don't contribute to the potentially protective ‘herd immunity’.
CRediT authorship contribution statement
Aristidis Tsatsakis: Conceptualization, Methodology, Writing - review & editing, Supervision. Demetrious Petrakis: Conceptualization, Investigation, Formal analysis, Writing - original draft. Taxiarchis Konstantinos Nikolouzakis: Conceptualization, Investigation, Formal analysis, Writing - original draft. Anca Oana Docea: Conceptualization, Investigation, Formal analysis, Writing - original draft. Daniela Calina: Investigation, Formal analysis, Writing - original draft. Marco Vinceti: Investigation, Formal analysis, Writing - original draft. Marina Goumenou: Investigation, Formal analysis, Writing - original draft. Ronald N. Kostoff: Conceptualization, Methodology, Writing - review & editing, Supervision. Charalampos Mamoulakis: Investigation, Formal analysis, Writing - original draft. Michael Aschner: Conceptualization, Methodology, Writing - review & editing, Supervision. Antonio F. Hernández: Conceptualization, Methodology, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abassi Z., Assady S., Khoury E.E., Heyman S.N. Letter to the Editor: angiotensin-converting enzyme 2: an ally or a Trojan horse? Implications to SARS-CoV-2-related cardiovascular complications. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H1080–H1083. doi: 10.1152/ajpheart.00215.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloizou A.M., Siokas V., Vogiatzi C., Peristeri E., Docea A.O., Petrakis D., Provatas A., Folia V., Chalkia C., Vinceti M., Wilks M., Izotov B.N., Tsatsakis A., Bogdanos D.P., Dardiotis E. Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. doi: 10.1016/j.toxlet.2020.03.005. [DOI] [PubMed] [Google Scholar]
- Anthoney N., Foldi I., Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145(9) doi: 10.1242/dev.156018. [DOI] [PubMed] [Google Scholar]
- Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa A.S., Anilakumar K.R. Genetically modified foods: safety, risks and public concerns-a review. J. Food Sci. Technol. 2013;50(6):1035–1046. doi: 10.1007/s13197-012-0899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer C.A., Girtsman T.A., Seaver B.P., Finsaas K.J., Migliaccio C.T., Perry V.K., Rottman J.B., Smith D.E., Holian A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2013;7(6):1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag T.V., Luis Morales J., Hollingshead B.D., Perdew G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18(3):207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P., Martin-Loeches I., Varillas D., Gallegos M.C., Serón C., Micheloud D., Gomez J.M., Tenorio-Abreu A., Ramos M.J., Molina M.L., Huidobro S., Sanchez E., Gordón M., Fernández V., Del Castillo A., Marcos M.A., Villanueva B., López C.J., Rodríguez-Domínguez M., Galan J.C., Cantón R., Lietor A., Rojo S., Eiros J.M., Hinojosa C., Gonzalez I., Torner N., Banner D., Leon A., Cuesta P., Rowe T., Kelvin D.J. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care. 2009;13(6):R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins S.P., Uldrich A.P., Sutherland J.S., Gill J., Miller J.F., Godfrey D.I., Boyd R.L. Thymic regeneration: teaching an old immune system new tricks. Trends Mol. Med. 2002;8:469–476. doi: 10.1016/S1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- Bhatt D., Ghosh S. Regulation of the NF-κB-Mediated transcription of inflammatory genes. Front. Immunol. 2014;5:71. doi: 10.3389/fimmu.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule L.A., Winans B. Activation of the aryl hydrocarbon receptor during development enhances the pulmonary CD4+ T-cell response to viral infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L305–L313. doi: 10.1152/ajplung.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant L., Robert-Hebmann V., Acquaviva C., Pelchen-Matthews A., Marsh M., Devaux C. The protein tyrosine kinase p56lck is required for triggering NF-κB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4. J. Virol. 1998;72(7):6207–6214. doi: 10.1128/jvi.72.7.6207-6214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.P. Mortality of workers employed at organochlorine pesticide manufacturing plants - an update. Scand. J. Work. Environ. Health. 1992;18:155–161. doi: 10.5271/sjweh.1593. [DOI] [PubMed] [Google Scholar]
- Cao F., Souders Ii C.L., Perez-Rodriguez V., Martyniuk C.J. Elucidating conserved transcriptional networks underlying pesticide exposure and Parkinson's disease: a focus on chemicals of epidemiological relevance. Front. Genet. 2019;9:701. doi: 10.3389/fgene.2018.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. 2020. Features, Evaluation and Treatment Oronavirus (COVID-19) [Updated 2020 Apr 6]. in: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.https://www.ncbi.nlm.nih.gov/books/NBK554776/ Available from: [PubMed] [Google Scholar]
- Cava C., Bertoli G., Castiglioni I. In silico discovery of candidate drugs against covid-19. Viruses. 2020;12(4) doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M., Olson M., Schindler B., Gong H.K. Exposure to DDT metabolite p,p'-DDE increases autoimmune type 1 diabetes incidence in NOD mouse model. J. Immunot. 2016;13(1):108–118. doi: 10.3109/1547691X.2015.1017060. [DOI] [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020 Mar 27 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini F., Pontillo C., Randi A., Alvarez L., Kleiman de Pisarev D.L. Hexachlorobenzene induces TGF-β1 expression, which is a regulator of p27 and cyclin D1 modifications. Toxicol. Lett. 2014;230(1):1–9. doi: 10.1016/j.toxlet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Childs C.E., Calder P.C., Miles E.A. Diet and immune function. Nutrients. 2019;11(8):1933. doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher G.W., Cieslak T.J., Pavlin J.A., Eitzen E.M. Biological warfare. A historical perspective. J. Am. Med. Assoc. 1997;278(5):412–417. [PubMed] [Google Scholar]
- Cocco P., Blair A., Congia P., Saba G., Ecca A.R., Palmas C. Annals of the New York Academy of Sciences. Blackwell Publishing Inc.; 1997. Long-term health effects of the occupational exposure to DDT. A preliminary report; pp. 246–256. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence: a systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019;124:110632. doi: 10.1016/j.exger.2019.110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz T., López-Giraldo A., Noell G., Casas-Recasens S., Garcia T., Molins L., Juan M., Fernandez M.A., Agustí A., Faner R. Multi-level immune response network in mild-moderate chronic obstructive pulmonary disease (COPD) Respir. Res. 2019;20:152. doi: 10.1186/s12931-019-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10:1654. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368:m1165. doi: 10.1136/bmj.m1165. [DOI] [PubMed] [Google Scholar]
- Demsia G., Vlastos D., Goumenou M., Matthopoulos D.P. Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bone-marrow. Mutat. Res. 2007;634(1–2):32–39. doi: 10.1016/j.mrgentox.2007.05.018. [DOI] [PubMed] [Google Scholar]
- DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., Jemal A., Siegel R.L. Breast cancer statistics, 2019. CA. Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;105938 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert R.R., Lee J.E., Hussain I., Piepenbrink M. Developmental immunotoxicology of lead. Toxicol. Appl. Pharmacol. 2004;198:86–94. doi: 10.1016/j.taap.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Dimakakou E., Johnston H.J., Streftaris G., Cherrie J.W. Exposure to environmental and occupational particulate air pollution as a potential contributor to neurodegeneration and diabetes: a systematic review of epidemiological research. Int. J. Environ. Res. Publ. Health. 2018;15(8) doi: 10.3390/ijerph15081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018;72:30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- Docea A.O., Tsatsakis A., Albulescu D., Cristea O., Zlatian O., Vinceti M., Moschos S.A., Tsoukalas D., Goumenou M., Drakoulis N., Dumanov J.M., Tutelya V.A., Onischenko G.G., Aschner M., Spandidos D.A., Calina D. A new threat from an old enemy: Re-emergence of coronavirus (Review) Int. J. Mol. Med. 2020;45:1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S.M., Comstock S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016;69:42–51. doi: 10.1159/000452818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldakroory S.A., Morsi D.E., Abdel-Rahman R.H., Roshdy S., Gouida M.S., Khashaba E.O. Correlation between toxic organochlorine pesticides and breast cancer. Hum. Exp. Toxicol. 2017;36(12):1326–1334. doi: 10.1177/0960327116685887. [DOI] [PubMed] [Google Scholar]
- Engin A.B., Nikitovic D., Neagu M., Henrich-Noack P., Docea A.O., Shtilman M.I., Golokhvast K., Tsatsakis A.M. Mechanistic understanding of nanoparticles' interactions with extracellular matrix: the cell and immune system. Part. Fibre Toxicol. 2017;14(1):22. doi: 10.1186/s12989-017-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESC 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- Esser C., Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenga C., Gangemi S., Di Salvatore V., Falzone L., Libra M. Immunological effects of occupational exposure to lead (Review) Mol. Med. Rep. 2017;15(5):3355–3360. doi: 10.3892/mmr.2017.6381. [DOI] [PubMed] [Google Scholar]
- Fernández-Solà J., LluísPadierna M., NoguéXarau S., Munné Mas P. [Chronic fatigue syndrome and multiple chemical hypersensitivity after insecticide exposure] Med. Clin. 2005;124(12):451–453. doi: 10.1157/13073217. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant R.H. The molecular genetics of insecticide resistance. Genetics. 2013;194(4):807–815. doi: 10.1534/genetics.112.141895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figà-Talamanca I., Mearelli I., Valente P. Mortality in a cohort of pesticide applicators in an urban setting. Int. J. Epidemiol. 1993;22:674–676. doi: 10.1093/ije/22.4.674. [DOI] [PubMed] [Google Scholar]
- Fountoucidou P., Veskoukis A.S., Kerasioti E., Docea A.O., Taitzoglou I.A., Liesivuori J., Tsatsakis A., Kouretas D. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: the time and dose issue. Toxicol. Lett. 2019;317:24–44. doi: 10.1016/j.toxlet.2019.09.015. [DOI] [PubMed] [Google Scholar]
- Frericks M., Meissner M., Esser C. Microarray analysis of the AHR system: tissue-specific flexibility in signal and target genes. Toxicol. Appl. Pharmacol. 2007;220:320–332. doi: 10.1016/j.taap.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A.M., Wilks M.F., Spandidos D.A. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases. Int. J. Mol. Med. 2016;38:1012–1020. doi: 10.3892/ijmm.2016.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Mondal T.K., Lawrence D.A. Lead effects on development and function of bone marrow-derived dendritic cells promote Th2 immune responses. Toxicol. Appl. Pharmacol. 2007;222(1):69–79. doi: 10.1016/j.taap.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go R.E., Kim C.W., Choi K.C. Effect of fenhexamid and cyprodinil on the expression of cell cycle- and metastasis-related genes via an estrogen receptor-dependent pathway in cellular and xenografted ovarian cancer models. Toxicol. Appl. Pharmacol. 2015;289(1):48–57. doi: 10.1016/j.taap.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Go Y.M., Jones D.P. Redox biology: interface of the exposome with the proteome, epigenome and genome. Redox Biol. 2014;2:358–360. doi: 10.1016/j.redox.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette E.A., Meza M.M., Aquilar M.G., Soto A.D., Garcia I.E. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ. Health Perspect. 1998;106:347–353. doi: 10.1289/ehp.98106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Vázquez C., Quintana F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez U., Gan H.K., Scott A.M. Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr. Opin. Pharmacol. 2018;41:114–121. doi: 10.1016/j.coph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Hargrave D., Bartels U., Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- Hartung T., Corsini E. Immunotoxicology: challenges in the 21st century and in vitro opportunities. ALTEX. 2013;30:411–426. doi: 10.14573/altex.2013.4.411. [DOI] [PubMed] [Google Scholar]
- Head J.L., Lawrence B.P. The aryl hydrocarbon receptor is a modulator of anti-viral immunity. Biochem. Pharmacol. 2009;77:642–653. doi: 10.1016/j.bcp.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A.F., Docea A.O., Goumenou M., Sarigiannis D., Aschner M., Tsatsakis A. Application of novel technologies and mechanistic data for risk assessment under the real-life risk simulation (RLRS) approach. Food Chem. Toxicol. 2020;137:111123. doi: 10.1016/j.fct.2020.111123. [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay S.D. Prenatal immunotoxicant exposure and postnatal autoimmune disease. Environ. Health Perspect. 1999;107(Suppl. 5):687–691. doi: 10.1289/ehp.107-1566498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz L.R., Cao S., Zhao G., Bauer I.K., Denno D.M., Klein E.J., Antonio M., Stine O.C., Snelling T.L., Kirkwood C.D., Wang D. Geographic variation in the eukaryotic virome of human diarrhea. Virology. 2014;468:556–564. doi: 10.1016/j.virol.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J. Epidemiologic overview of malignant lymphoma. Korean J. Hematol. 2012;47:92—104. doi: 10.5045/kjh.2012.47.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibs K.H., Rink L. Zinc-altered immune function. J. Nutr. 2003;133:1452–1456. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- Jain A., Pandey N., Garg R.K., Kumar R. IL-17 level in patients with dengue virus infection & its association with severity of illness. J. Clin. Immunol. 2013;33:613–618. doi: 10.1007/s10875-012-9855-0. [DOI] [PubMed] [Google Scholar]
- Jarcho J.A., Ingelfinger J.R., Hamel M.B., D'Agostino R.B., Harrington D.P. Inhibitors of the renin-angiotensin-aldosterone system and covid-19. N. Engl. J. Med. 2020 May 1 doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Burnett A.L. NADPH oxidase: recent evidence for its role in erectile dysfunction. Asian J. Androl. 2008;10:6–13. doi: 10.1111/j.1745-7262.2008.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wu S., Zeng Z., Fu Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017;222:1–9. doi: 10.1016/j.envpol.2016.11.045. [DOI] [PubMed] [Google Scholar]
- Joly Condette C., Bach V., Mayeur C., Gay-Queheillard J., Khorsi-Cauet H. Chlorpyrifos exposure during perinatal period affects intestinal microbiota associated with delay of maturation of digestive tract in rats. J. Pediatr. Gastroenterol. Nutr. 2015;61:30–40. doi: 10.1097/MPG.0000000000000734. [DOI] [PubMed] [Google Scholar]
- Kalkanidis M., Pietersz G.A., Xiang S.D., Mottram P.L., Crimeen-Irwin B., Ardipradja K., Plebanski M. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods (San Diego, Calif) 2006;40(1):20–29. doi: 10.1016/j.ymeth.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Karzi V., Tzatzarakis M.N., Vakonaki E., Alegakis T., Katsikantami I., Sifakis S., Rizos A., Tsatsakis A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018;38(8):1144–1152. doi: 10.1002/jat.3627. [DOI] [PubMed] [Google Scholar]
- Kendall M., Holgate S. Health impact and toxicological effects of nanomaterials in the lung. Respirology. 2012;17:743–758. doi: 10.1111/j.1440-1843.2012.02171.x. [DOI] [PubMed] [Google Scholar]
- Khazaal A.Q., Jaeger C.D., Bottum K.M., Tischkau S.A. Environmental factors act through aryl hydrocarbon receptor activation and circadian rhythm disruption to regulate energy metabolism. J. Recept. Ligand Channel Res. 2018;10:13–24. doi: 10.2147/JRLCR.S133886. [DOI] [Google Scholar]
- Kidane D., Chae W.J., Czochor J., Eckert K.A., Glazer P.M., Bothwell A.L., Sweasy J.B. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 2014;49:116–139. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lawrence D.A. Immunotoxic effects of inorganic lead on host resistance of mice with different circling behavior preferences. Brain Behav. Immun. 2000;14(4):305–317. doi: 10.1006/brbi.2000.0609. [DOI] [PubMed] [Google Scholar]
- Kishikawa H., Lawrence D.A. Differential production of interleukin-6 in the brain and spleen of mice treated with lipopolysaccharide in the presence and absence of lead. J. Toxicol. Environ. Health. 1998;53(5):357–373. doi: 10.1080/009841098159222. [DOI] [PubMed] [Google Scholar]
- Koike E., Yanagisawa R., Win-Shwe T.T., Takano H. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 2018;32 doi: 10.1177/2058738418774897. 2058738418774897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostoff R.N., Goumenou M., Tsatsakis A. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol Rep. 2018;5:1169–1172. doi: 10.1016/j.toxrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostoff R.N., Heroux P., Aschner M., Tsatsakis A. Adverse health effects of 5G mobile networking technology under real-life conditions. Toxicol. Lett. 2020;323:35–40. doi: 10.1016/j.toxlet.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Kostoff R.N., Lau C.G.Y. Modified health effects of non-ionizing electromagnetic radiation combined with other agents reported in the biomedical literature. In: Geddes C.D., editor. Microwave Effects on DNA and Proteins. Springer International Publishing AG; 2017. [DOI] [Google Scholar]
- Kostoff R.N. PDF; 2019. Adverse Effects of Wireless Radiation.http://hdl.handle.net/1853/61946 [Google Scholar]
- Kostoff R.N. Georgia Institute of Technology. PDF; 2020. Combining Tactical and Strategic Treatments for COVID-19.http://hdl.handle.net/1853/62523 [Google Scholar]
- Kreitinger J.M., Beamer C.A., Shepherd D.M. Environmental immunology: lessons learned from exposure to a select panel of immunotoxicants. J. Immunol. 2016;196:3217–3225. doi: 10.4049/jimmunol.1502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Roca P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kwak E.S., Just A., Whyatt R., Miller R.L. Phthalates, pesticides, and bisphenol-A exposure and the development of nonoccupational asthma and allergies: how valid are the links? Open Allergy J. 2009;2:45–50. doi: 10.2174/1874838400902010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiek J.J., Haystead T.A., Rudolph J. Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the antimalarial quinolines. Biochemistry. 2004;43(2004):4538–4547. doi: 10.1021/bi035923w. [DOI] [PubMed] [Google Scholar]
- Langer P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front. Neuroendocrinol. 2010;31(4):497–518. doi: 10.1016/j.yfrne.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M.E., Schinasi L.H., Lebailly P., Beane Freeman L.E., Nordby K.C., Ferro G., Monnereau A., Brouwer M., Tual S., Baldi I., Kjaerheim K., Hofmann J.N., Kristensen P., Koutros S., Straif K., Kromhout H., Schüz J. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int. J. Epidemiol. 2019;48(5):1519–1535. doi: 10.1093/ije/dyz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., Dorscheid D.R., Sin D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020 doi: 10.1183/13993003.00688-2020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu W. Puzzle of highly pathogenic human coronaviruses (2019-nCoV) Protein Cell. 2020;11:235–238. doi: 10.1007/s13238-020-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E.S., Zhou Y., Zhao G., Bauer I.K., Droit L., Ndao I.M., Warner B.B., Tarr P.I., Wang D., Holtz L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015;21(10):1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z., Li Z., Liu X., Cheng Y., Luo Y., Tong X., Yuan L., Wang Y., Sun J., Li L., Xiang C. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014;80(8):2546–2554. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Yang X., Geng M., Huang M. Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B. 2018;8(4):552–562. doi: 10.1016/j.apsb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen S., Zühlke L., Black G.C., Choy M.K., Li N., Keavney B.D. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019;48:455–463. doi: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Wang R., Fang X., Sun Z. β-catenin/TCF-1 pathway in T cell development and differentiation. J. Neuroimmune Pharmacol. 2012;7:750–762. doi: 10.1007/s11481-012-9367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagoli C., Fabbi S., Teggi S., Calzari M., Poli M., Ballotti E., Notari B., Bruni M., Palazzi G., Paolucci P., Vinceti M. Risk of hematological malignancies associated with magnetic fields exposure from power lines: a case-control study in two municipalities of northern Italy. Environ. Health. 2010;9:16. doi: 10.1186/1476-069X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Valenzuela C., Waliszewski S.M., Amador-Muñoz O., Meza E., Calderón-Segura M.E., Zenteno E., Huichapan-Martínez J., Caba M., Félix-Gastélum R., Longoria-Espinoza R. Aerial pesticide application causes DNA damage in pilots from Sinaloa, Mexico. Environ. Sci. Pollut. Res. Int. 2017;24(3):2412–2420. doi: 10.1007/s11356-016-7974-5. [DOI] [PubMed] [Google Scholar]
- McIntosh B.E., Hogenesch J.B., Bradfield C.A. Mammalian per-arnt-sim proteins in environmental adaptation. Annu. Rev. Physiol. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- McKeen S., Young W., Mullaney J., Fraser K., McNabb W.C., Roy N.C. Infant complementary feeding of prebiotics for theMicrobiome and immunity. Nutrients. 2019;11:364. doi: 10.3390/nu11020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Li, Xing Yu, Liu Xi, Zeng Feng. Cardiovascular toxicity and anxiety-like behavior induced by deltamethrin in zebrafish (Danio rerio) larvae. Chemosphere. 2019;219:155–164. doi: 10.1016/j.chemosphere.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Mesnage R., Bernay B., Séralini G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313(2–3):122–128. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Miles E.A., Rees D., Banerjee T., Cazzola R., Lewis S., Wood R., Oates R., Tallant A., Cestaro B., Yaqoob P., Wahle K.W., Calder P.C. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196(1):298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Minot S., Bryson A., Chehoud C., Wu G.D., Lewis J.D., Bushman F.D. Rapid evolution of the human gut virome. Proc. Natl. Acad. Sci. U. S. A. 2013;110(30):12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S.R., Lee H.H., Spina C.S., Collins J.J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499(7457):219–222. doi: 10.1038/nature12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern V., Zutavern A., Cyrys J., Brockow I., Koletzko S., Krämer U., Behrendt H., Herbarth O., von Berg A., Bauer C.P., Wichmann H.E., Heinrich J., GINI Study Group; LISA Study Group Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med. 2008;177(12):1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Moyano D.F., Goldsmith M., Solfiell D.J., Landesman-Milo D., Miranda O.R., Peer D., Rotello V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012;134(9):3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Diaye M., Le Ferrec E., Lagadic-Gossmann D., Corre S., Gilot D., Lecureur V., Monteiro P., Rauch C., Galibert M.D., Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J. Biol. Chem. 2006;281:19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Goldblum R.M., Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ. Health. 2012;11:8. doi: 10.1186/1476-069X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M.A., Mesnage R., Tsatsakis A.M., Golokhvast K.S., Yang S.H., Antoniou M.N., Chung G. Addressing concerns over the fate of DNA derived from genetically modified food in the human body: a review. Food Chem. Toxicol. 2019;124:423–430. doi: 10.1016/j.fct.2018.12.030. [DOI] [PubMed] [Google Scholar]
- Neagu M., Piperigkou Z., Karamanou K., Engin A.B., Docea A.O., Constantin C., Negrei C., Nikitovic D., Tsatsakis A. Protein bio-corona: critical issue in immune nanotoxicology. Arch. Toxicol. 2017;91(3):1031–1048. doi: 10.1007/s00204-016-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J., Walker W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Obesity update. 2014. https://www.oecd.org/els/health-systems/Obesity-Update-2014.pdf
- Olofsson S., Kumlin U., Dimock K., Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect. Dis. 2005;5:184–188. doi: 10.1016/S1473-3099(05)70026-8. [DOI] [PubMed] [Google Scholar]
- Ono Y., Torii K., Fritsche E., Shintani Y., Nishida E., Nakamura M., Shirakata Y., Haarmann-Stemmann T., Abel J., Krutmann J., Morita A. Role of the aryl hydrocarbon receptor in tobacco smoke extract-induced matrix metalloproteinase-1 expression. Exp. Dermatol. 2013;22:349–353. doi: 10.1111/exd.12148. [DOI] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomäki J., Välimäki E., Sund J., Vippola M., Clausen P.A., Jensen K.A., Savolainen K., Matikainen S., Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5(9):6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Peng H., Brimijoin S., Hrabovska A., Krejci E., Blake T.A., Johnson R.C., Masson P., Lockridge O. Monoclonal antibodies to human butyrylcholinesterase reactive with butyrylcholinesterase in animal plasma. Chem. Biol. Interact. 2016;243:82–90. doi: 10.1016/j.cbi.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J.M., Wang D.J., Shettigar V., Roof S.R., Canan B.D., Bakkar N., Shintaku J., Gu J.M., Little S.C., Ratnam N.M., Londhe P., Lu L., Gaw C.E., Petrosino J.M., Liyanarachchi S., Wang H., Janssen P.M.L., Davis J.P., Ziolo M.T., Sharma S.M., Guttridge D.C. NF-κB inhibition rescues cardiac function by remodeling calcium genes in a Duchenne muscular dystrophy model. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-05910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis D., Vassilopoulou L., Docea A.O., Gofita E., Vucinic S., Rakitskii V.N., Tsatsakis A.M. An overview update in chemical, biological and nuclear weapons and their effects in human health. Health care of the Russian Federation. Russian Journal. 2016;61(2) doi: 10.18821/0044-197X-2017-61-2-103-112. [DOI] [Google Scholar]
- Petrakis D., Vassilopoulou L., Mamoulakis C., Psycharakis C., Anifantaki A., Sifakis S., Docea A.O., Tsiaoussis J., Makrigiannakis A., Tsatsakis A.M. Endocrine disruptors leading to obesity and related diseases. Int. J. Environ. Res. Publ. Health. 2017;14(10) doi: 10.3390/ijerph14101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperigkou Z., Karamanou K., Engin A.B., Gialeli C., Docea A.O., Vynios D.H., Pavão M.S., Golokhvast K.S., Shtilman M.I., Argiris A., Shishatskaya E., Tsatsakis A.M. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem. Toxicol. 2016;91:42–57. doi: 10.1016/j.fct.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J., Fontana L., Gil A. Human milk oligosaccharides and immune system development. Nutrients. 2018;10:1038. doi: 10.3390/nu10081038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky J.Y., Aronson K.J., Heaton J.P., Adams M.A. Pesticides and polychlorinated biphenyls as potential risk factors for erectile dysfunction. J. Androl. 2007;28:28–37. doi: 10.2164/jandrol.106.000851. [DOI] [PubMed] [Google Scholar]
- Post C.M., Boule L.A., Burke C.G., O'Dell C.T., Winans B., Lawrence B.P. The ancestral environment shapes antiviral CD8(+) T cell responses across generations. iScience. 2019;20:168–183. doi: 10.1016/j.isci.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Quintana F.J., Sherr D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013;65:1148–1161. doi: 10.1124/pr.113.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Semenkovich N.P., Whiteson K., Rohwer F., Gordon J.I. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat. Rev. Microbiol. 2012;10(9):607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M., Verduri A., Corbetta L., Clini E., Fabbri L.M., Beghé B. Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur. J. Clin. Invest. 2013;43(5):510–521. doi: 10.1111/eci.12064. [DOI] [PubMed] [Google Scholar]
- Roca M., Manes F., Chade A., Gleichgerrcht E., Gershanik O., Arévalo G.G., Torralva T., Duncan J. The relationship between executive functions and fluid intelligence in Parkinson's disease. Psychol. Med. 2012;42:2445–2452. doi: 10.1017/S0033291712000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero M.M., Calder P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10:432. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszkiewicz J.A., Pinkas A., Ferrer B., Peres T.V., Tsatsakis A., Aschner M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol Rep. 2017;4:245–259. doi: 10.1016/j.toxrep.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai S., Shimizu T., Ohto U. The crystal structure of the AhRR–ARNT heterodimer reveals the structural basis of the repression of AhR-mediated transcription. J. Biol. Chem. 2017;292(43):17609–17616. doi: 10.1074/jbc.M117.812974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro A.H., Ritchie C.S., Hovater M., Roth D.L., Sawyer P., Locher J.L., Bodner E., Brown C.J., Allman R.M. Inflammatory biomarkers as predictors of hospitalization and death in community-dwelling older adults. Arch. Gerontol. Geriatr. 2012;54:387–391. doi: 10.1016/j.archger.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Lopez L., Hernandez-Mendoza A., Mata-Haro V., Vallejo-Cordoba B., Wall-Medrano A., Astiazaran-Garcia H., Estrada-Montoya M.D.C., Gonzalez-Cordova A.F. Effect of milk fermented with lactobacillus fermentum on the inflammatory response in mice. Nutrients. 2018;10:1039. doi: 10.3390/nu10081039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi F., Tamone C., D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr D.H., Monti S. The role of the aryl hydrocarbon receptor in normal and malignant B cell development. Semin. Immunopathol. 2013;35(6):705–716. doi: 10.1007/s00281-013-0390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkworth J.B., Antrim L. Relationship between Ah receptor-mediated polychlorinated biphenyl (PCB)-induced humoral immunosuppression and thymic atrophy. J. Pharmacol. Exp. Therapeut. 1985;235(3):606–611. [PubMed] [Google Scholar]
- Stanaway I.B., Wallace J.C., Shojaie A., Griffith W.C., Hong S., Wilder C.S., Green F.H., Tsai J., Knight M., Workman T., Vigoren E.M., McLean J.S., Thompson B., Faustman E.M. Human oral buccal microbiomes are associated with farmworker status and azinphos-methyl agricultural pesticide exposure. Appl. Environ. Microbiol. 2016;83(2) doi: 10.1128/AEM.02149-16. e02149-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiz M., Valbracht J., Quach J., Lotz M. Gold sodium thiomalate and chloroquine inhibit cytokine production in monocytic THP-1 cells through distinct transcriptional and posttranslational mechanisms. J. Clin. Immunol. 2003;23:477–484. doi: 10.1023/B:JOCI.0000010424.41475.17. [DOI] [PubMed] [Google Scholar]
- Stockinger B., Meglio P., Di, Gialitakis M., Duarte J.H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- Sunyer J., Garcia-Esteban R., Alvarez M., Guxens M., Goñi F., Basterrechea M., Vrijheid M., Guerra S., Antó J.M. DDE in mothers' blood during pregnancy and lower respiratory tract infections in their infants. Epidemiology (Cambridge, Mass) 2010;21(5):729–735. doi: 10.1097/EDE.0b013e3181e5ea96. [DOI] [PubMed] [Google Scholar]
- Thompson H.M., Fryday S.L., Harkin S., Milner S. Potential impacts of synergism in honeybees (Apismellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie. 2014;45:545–553. [Google Scholar]
- Torres-Castro P., Abril-Gil M., Rodriguez-Lagunas M.J., Castell M., Perez-Cano F.J., Franch A. TGF-beta2, EGF, and FGF21 growth factors present in breast milk promote mesenteric lymph node lymphocytes maturation in suckling rats. Nutrients. 2018;10:1171. doi: 10.3390/nu10091171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., Baric R.S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6(3) doi: 10.1128/mBio.00638-15. e00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A., Docea A.O., Calina D., Tsarouhas K., Zamfira L.M., Mitrut R., Sharifi-Rad J., Kovatsi L., Siokas V., Dardiotis E., Drakoulis N., Lazopoulos G., Tsitsimpikou C., Mitsias P., Neagu M. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019;8(9):1295. doi: 10.3390/jcm8091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A., Docea A.O., Constantin C., Calina D., Zlatian O., Nikolouzakis T.K., Stivaktakis P.D., Kalogeraki A., Liesivuori J., Tzanakakis G., Neagu M. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol. Lett. 2019;316:154–170. doi: 10.1016/j.toxlet.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Tsatsakis A., Goumenou M., Liesivuori J., Dekant W., Hernández A.F. Toxicology for real-life risk simulation - editorial preface to this special issue. Toxicol. Lett. 2019;309:33–34. doi: 10.1016/j.toxlet.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Tsatsakis A.M., Nawaz M.A., Tutelyan V.A., Golokhvast K.S., Kalantzi O.I., Chung D.H., Kang S.J., Coleman M.D., Tyshko N., Yang S.H., Chung G. Impact on environment, ecosystem, diversity and health from culturing and using GMOs as feed and food. Food Chem. Toxicol. 2017;107(Pt A):108–121. doi: 10.1016/j.fct.2017.06.033. [DOI] [PubMed] [Google Scholar]
- Tsiaoussis J., Antoniou M.N., Koliarakis I., Mesnage R., Vardavas C.I., Izotov B.N., Psaroulaki A., Tsatsakis A. Effects of single and combined toxic exposures on the gut microbiome: current knowledge and future directions. Toxicol. Lett. 2019;312:72–97. doi: 10.1016/j.toxlet.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Tsiaoussis J., Hatzidaki E., Docea A.O., Nikolouzakis T.K., Petrakis D., Burykina T., Mamoulakis C., Makrigiannakis A., Tsatsakis A. Molecular and clinical aspects of embryotoxicity induced by acetylcholinesterase inhibitors. Toxicology. 2018;409:137–143. doi: 10.1016/j.tox.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Voorhis M., Knopp S., Julliard W., Fechner J.H., Zhang X., Schauer J.J., Mezrich J.D. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas C.I., Flouris A.D., Tsatsakis A., Kafatos A.G., Saris W.H. Does adherence to the Mediterranean diet have a protective effect against active and passive smoking? Publ. Health. 2011;125(3):121–128. doi: 10.1016/j.puhe.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Vassilopoulou L., Psycharakis C., Petrakis D., Tsiaoussis J., Tsatsakis A.M. Obesity, persistent organic pollutants and related health problems. Adv. Exp. Med. Biol. 2017;960:81–110. doi: 10.1007/978-3-319-48382-5_4. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vidya M.K., Kumar V.G., Sejian V., Bagath M., Krishnan G., Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2018;37:20–36. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- Villamil Giraldo A.M., Appelqvist H., Ederth T., Öllinger K. Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem. Soc. Trans. 2014;42(5):1460–1464. doi: 10.1042/BST20140145. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse B.A., Bohn A.A., Lawrence B.P. Examining the relationship between impaired host resistance and altered immune function in mice treated with TCDD. Toxicology. 2003;188:15–28. doi: 10.1016/s0300-483x(02)00749-7. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.H., Cheng Y. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020;2020:2020. doi: 10.1002/jmv.26139. 02.24.963348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., White J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Wang W., Jovel J., Halloran B., Wine E., Patterson J., Ford G., OʼKeefe S., Meng B., Song D., Zhang Y., Tian Z., Wasilenko S.T., Rahbari M., Reza S., Mitchell T., Jordan T., Carpenter E., Madsen K., Fedorak R., Dielemann L.A., Ka-Shu Wong G., Mason A.L. Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm. Bowel Dis. 2015;21(6):1419–1427. doi: 10.1097/MIB.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T., Jordan R., Lo M., Soloveva V., Ray A., Bannister R., Mackman R., Perron M., Stray K., Feng J., Xu Y., Wells J., Stuthman K., Welch L., Doerffler E., Zhang L., Chun K., Hui H., Neville S., Lew W., Park Y., Babusis D., Strickley R., Wong P., Swaminathan S., Lee W., Mayers D., Cihlar T., Bavari S. Nucleotide prodrug GS-5734 is a broad-spectrum filovirus inhibitor that provides complete therapeutic protection against the development of Ebola virus disease (EVD) in infected non-human primates. Open Forum Infectious Diseases. 2015;2(Suppl. l_1) [Google Scholar]
- Wheeler M.A., Rothhammer V., Quintana F.J. Control of immune-mediated pathology via the aryl hydrocarbon receptor. J. Biol. Chem. 2017;292(30):12383–12389. doi: 10.1074/jbc.R116.767723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO. Raised blood pressure [WWW Document], n.d. URL https://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/(accessed 4.6.20).
- WHO . 2019. Countries Are Spending More on Health, but People Are Still Paying Too Much Out of Their Own Pockets.https://www.who.int/news-room/detail/20-02-2019-countries-are-spending-more-on-health-but-people-are-still-paying-too-much-out-of-their-own-pockets 20 February 2019. [Google Scholar]
- WHO . 2019. Global Spending on Health: A World in Transition 2019.https://www.who.int/health_financing/documents/health-expenditure-report-2019.pdf?ua=1 [Google Scholar]
- Williams J.N., Chang S.C., Sinnette C., Malspeis S., Parks C.G., Karlson E.W., Fraser P., Costenbader K. Pesticide exposure and risk of systemic lupus erythematosus in an urban population of predominantly African-American women. Lupus. 2018;27:2129–2134. doi: 10.1177/0961203318805844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans B., Humble M.C., Lawrence B.P. Environmental toxicants and the developing immune system: a missing link in the global battle against infectious disease? Reprod. Toxicol. 2011;31:327–336. doi: 10.1016/j.reprotox.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K., Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. vol. 29. 2020. (Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection). January, 2020. [DOI] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiropoulos A., Tsarouhas K., Tsitsimpikou C., Fragkiadaki P., Germanakis I., Tsardi M., Maravgakis G., Goutzourelas N., Vasilaki F., Kouretas D., Hayes A., Tsatsakis A. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum. Exp. Toxicol. 2014;33(12):1241–1252. doi: 10.1177/0960327114532384. [DOI] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zambrano-Zaragoza J.F., Romo-Martínez E.J., Durán-Avelar Mde J., García-Magallanes N., Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int. J. Inflamm. 2014;2014:651503. doi: 10.1155/2014/651503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wohlhueter R., Zhang H. Genetically modified foods: a critical review of their promise and problems. Food Science and Human Wellness. 2016;5(3):116–123. [Google Scholar]
- Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. pii: dkaa114. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinöcker M.K., Lindseth I.A. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3):365. doi: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]