A 69-year-old man with a 7-day history of fever and cough, was admitted to the University Hospital of Guadeloupe (French indies) for confusion and severe headache. He had no medical history and reported having traveled to the Middle-East (cruise ship) with his wife 15 days before hospitalization. He did not report insect bites. A week after returning home, he presented fever, myalgia, cough, anosmia, ageusia, cervical pain, stiff neck, and diarrhea. At admission, he reported a worsening of his condition for 24 hours with painful and stiff neck and headache, confusion, walking disability with falls, and dyspnea. Noteworthy, his wife had also had isolated cough and ageusia for the past 10 days.
On examination, the patient was febrile (38.5 °C) with diffuse headache, neck stiffness, altered consciousness (Glasgow Coma Scale 14), confusion, swallowing disorders, and right-sided hemiparesis. Respiratory rate was increased (36/min), pulse rate was 95/min, blood pressure 160/89 mmHg, and oxygen saturation was 91% in ambient air.
Laboratory analyses showed increased C-reactive protein at 95 mg/L and creatine kinase level (655 U/L), raised transaminases (aspartate aminotransferase = 85 U/L, alanine aminotransferase = 94 U/L) and lactate dehydrogenase (442 U/L). Arterial partial pressure of oxygen was decreased at 64 mmHg with normal arterial partial pressure of carbon dioxide and pH. Chest computed tomographic scan was highly suggestive of COVID-19 (Fig. 1 ). Cerebrospinal fluid was purely lymphocytic (37 × 106/L) with no red blood cells, an increased protein level at 84 mg/dL and normal glucose level. Brain MRI with gadolinium was normal. Electroencephalogram showed a bilateral slowed activity without seizures. The detection of SARS-CoV-2 by specific real-time reverse PCR (RT-PCR) was negative in nasopharyngeal swab and cerebrospinal fluid (CSF) on days 2 and 4 after admission but was positive in bronchoalveolar lavage on day 5. Using a similar biological tool, the search for Influenza virus was negative. RT-PCR for varicella-zoster virus, herpes simplex virus (HSV), and enterovirus in CSF were all negative. Other tests for endemic infections in our area were performed, but all negative.
Fig. 1.
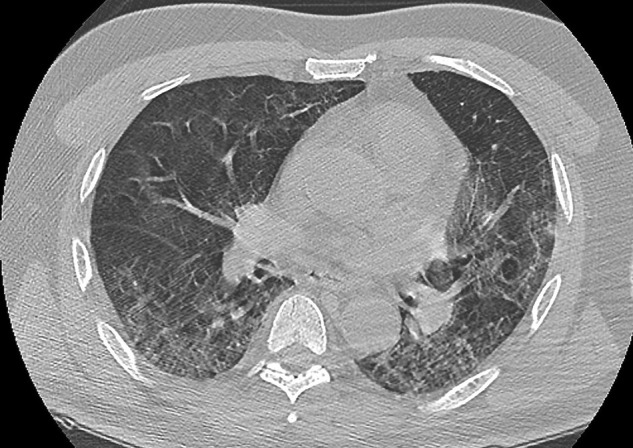
Chest CT scan in a patient with COVID-19 pneumonia and meningoencephalitis.
Transverse thin-section CT scan on day 7 after symptom onset revealed bilateral, peripheral ground-glass opacities associated with crazy-paving pattern and subpleural lesions, predominant in the middle and lower lobes. There were no airway abnormalities, neither mediastinal lymphadenopathy nor pleural effusion. It is a typical image of a severe form (50% lung damage) of COVID-19 pneumonitis.
The patient received nasal oxygen therapy. Acyclovir infusions were performed for 3 days and stopped when RT-PCR for HSV was found negative. We started hydroxychloroquine sulfate 200 mg, three times per day, and azithromycin 250 mg daily for 7 days. On hospital day 4, his neurological condition improved with normal consciousness and abatement of swallowing disorders. At discharged on day 10, mild neuropsychiatric features were still present with an alteration of executive functions. Montreal Cognitive Assessment was decreased to 26/30.
We describe meningoencephalitis one week after the onset of COVID-19 based on the combination of altered mental consciousness, fever, focal neurological defects, and cerebrospinal fluid abnormalities. We suspect the meningoencephalitis was related to COVID-19, possibly through a direct infectious mechanism although the virus was not detected in CSF [1]. Detection of SARS-CoV-2 by RT-PCR in bronchoalveolar lavage at the time of lumbar puncture is rather in favor of a direct infectious than a post-infectious mechanism. Currently, we have no information about the sensitivity of specific RT-PCR in CSF nor the kinetics of the virus in the central nervous system. Manifestations such as impaired consciousness, acute cerebrovascular disease, seizure, diffuse cortical tract signs, dysexecutive syndrome have been reported among COVID-19 patients [2], [3]. In addition, recent case reports described one patient with an acute haemorrhagic encephalopathy [4] and one patient with a meningoencephalitis associated with a COVID-19 infection [5]. Our observation provides further evidence suggesting that SARS-CoV-2 may be responsible for central nervous system damage, including meningoencephalitis.
Funding
Study funded by the French network for REsearch and ACTion targeting emerging infectious diseases (REACTing) of the French National Institute of Health and Medical Research (INSERM).
Consent for publication
Patient's consent has been obtained.
Disclosure of interest
Dr Chaumont reports having received travel grant from PEPS development, Roche and Pfizer. Dr Roze reports served on scientific advisory boards for Orkyn, Aguettant, Merz-Pharma; received honoraria for speeches from Orkyn, Aguettant, Merz-Pharma, Medday-Pharma, Everpharma, International Parkinson and Movement disorders Society; received research support from Merz-Pharma, Orkyn, Aguettant, Elivie, Ipsen, Everpharma, Fondation Desmarest, AMADYS, Fonds de Dotation Brou de Laurière, Agence Nationale de la Recherche; received travel grant from Vitalair, PEPS development, Aguettant, Merz-Pharma, Ipsen, Merck, Orkyn, Elivie, Adelia Medical, Dystonia Medical Research Foundation, International Parkinson and Movement disorders Society, European Academy of Neurology, International Association of Parkinsonism and Related Disorders. Dr Couratier reports having received travel grant from Allergan, Novartis, Esai, UCB, Medtronic, Merck Serono, Biogen, LFB, Teva, Icomed, GSK, Genzyme, Aguettant, Cyberonics and Merz-Pharma. Dr Lannuzel reports having received research support from France Parkinson, PSP France, Agence Nationale de la Recherche, Fonds Européen de DEveloppement Regional, French Ministry of Health, University Hospital of Guadeloupe, received honoraria for a speech from Association des Neurologues du Québec; received travel grant from Vitalair, PEPS development, Merz-Pharma, International Parkinson and Movement disorders Society. Dr Etienne and Dr Roger reports no disclosures.
References
- 1.Steardo L., Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [NEJMc2008597] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated Acute hemorrhagic necrotizing encephalopathy: CT and MRI Features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [201187] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.062. [S1201971220301958]. [DOI] [PMC free article] [PubMed] [Google Scholar]


