Abstract
Background
On January 20, 2020, a new coronavirus epidemic with human-to-human transmission was officially declared by the Chinese government, which caused significant public panic in China. In light of the coronavirus disease 2019 outbreak, pregnant women may be particularly vulnerable and in special need for preventive mental health strategies. Thus far, no reports exist to investigate the mental health response of pregnant women to the coronavirus disease 2019 outbreak.
Objective
This study aimed to examine the impact of coronavirus disease 2019 outbreak on the prevalence of depressive and anxiety symptoms and the corresponding risk factors among pregnant women across China.
Study Design
A multicenter, cross-sectional study was initiated in early December 2019 to identify mental health concerns in pregnancy using the Edinburgh Postnatal Depression Scale. This study provided a unique opportunity to compare the mental status of pregnant women before and after the declaration of the coronavirus disease 2019 epidemic. A total of 4124 pregnant women during their third trimester from 25 hospitals in 10 provinces across China were examined in this cross-sectional study from January 1, 2020, to February 9, 2020. Of these women, 1285 were assessed after January 20, 2020, when the coronavirus epidemic was publicly declared and 2839 were assessed before this pivotal time point. The internationally recommended Edinburgh Postnatal Depression Scale was used to assess maternal depression and anxiety symptoms. Prevalence rates and risk factors were compared between the pre- and poststudy groups.
Results
Pregnant women assessed after the declaration of coronavirus disease 2019 epidemic had significantly higher rates of depressive symptoms (26.0% vs 29.6%, P=.02) than women assessed before the epidemic declaration. These women were also more likely to have thoughts of self-harm (P=.005). The depressive rates were positively associated with the number of newly confirmed cases of coronavirus disease 2019 (P=.003), suspected infections (P=.004), and deaths per day (P=.001). Pregnant women who were underweight before pregnancy, primiparous, younger than 35 years, employed full time, in middle income category, and had appropriate living space were at increased risk for developing depressive and anxiety symptoms during the outbreak.
Conclusion
Major life-threatening public health events such as the coronavirus disease 2019 outbreak may increase the risk for mental illness among pregnant women, including thoughts of self-harm. Strategies targeting maternal stress and isolation such as effective risk communication and the provision of psychological first aid may be particularly useful to prevent negative outcomes for women and their fetuses.
Key words: COVID-19, Edinburgh Postnatal Depression Scale, perinatal anxiety, perinatal depression
Introduction
Several clusters of individuals with pneumonia of unknown etiology in Wuhan, Hubei province, China, were reported to the Chinese health authorities starting from December 8, 2019.1 The pathogen was identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the World Health Organization declared the virus outbreak a public health emergency of international concern.2 At the beginning of the outbreak, much remained unknown except that it was transmitted by direct exposure at a local animal market.3 Information on person-to-person transmission of the coronavirus disease 2019 (COVID-19) was revealed by the government to the Chinese public on January 20, 2020 and that asymptomatic individuals were potential sources of infection.4 , 5 The number of identified cases continued to increase as did the public panic and flow of misinformation.
AJOG at a Glance.
Why was this study conducted?
A crisis such as the coronavirus disease 2019 outbreak is a major public health event that causes significant uncertainty and isolation. The negative impact may be even greater among pregnant women who have increased stress owing to concerns for their fetus.
Key findings
An increase in the prevalence of depressive and anxiety symptoms was found after the declaration of coronavirus disease 2019 human-to-human transmission. Pregnant women also reported having significantly more thoughts of self-harm. Women at increased risk for depression after the crisis declaration included those who were primiparous, younger than 35 years, underweight before pregnancy, employed full time, in middle income category, and had a per capita living area of ≥20 m2 and decreased physical activity.
What does this add to what is known?
Major public health emergencies may increase the depression and anxiety levels in pregnant women.
To date, epidemiologic data on the mental health problems of the general population during the COVID-19 outbreak have not been available and how to best respond to challenges during the outbreak remains unknown. Those in quarantine often experience boredom, loneliness, and anger, in addition, the virus has been repeatedly described as a killer virus on social media, perpetuating a sense of danger and uncertainty among the public. In the early phase of the 2003 SARS outbreak, a range of psychiatric morbidities, including persistent depression, anxiety, panic attacks, psychotic symptoms, and even suicidality, were reported.6 , 7 Mandatory contact tracing and 14-day quarantine, which form part of the public health responses to the COVID-19 outbreak, increased anxiety, feelings of isolation, and stigma.6 , 7
One particularly vulnerable group during a viral outbreak may be pregnant women. Mental health disorders are a common cause of morbidity during pregnancy with approximately 12% of women experiencing depression and up to 22% experiencing high levels of anxiety in late pregnancy.8 , 9 Pregnant women are more vulnerable to infections because of their naturally suppressed immune system and are generally considered at increased risk for severe complications.10 Moreover, pregnant women may be further vulnerable to anxiety due to increased concern about vertical transmission to their fetus. To date, the mental health response of pregnant women to the COVID-19 outbreak has not been investigated. This study aimed to examine the impact of the COVID-19 outbreak on the prevalence of depression and anxiety and the corresponding risk factors during pregnancy.
Materials and Methods
Study design and data collection
Human-to-human transmission of COVID-19 was first confirmed and reported by the Chinese government on January 20, 2020. In early December 2019 before the COVID-19 outbreak, our team initiated a multicenter cross-sectional study to identify mental health concerns in pregnancy using the Edinburgh Postnatal Depression Scale (EPDS). This study provided a unique opportunity to compare the mental status of pregnant women before and after the declaration of the COVID-19 epidemic. To examine the effect of a major public health emergency on the mental health of pregnant women, data from January 1, 2020, to February 9, 2020, were obtained from the Perinatal and Postpartum Depression Information Collection System we created. For this study, all participants were categorized into 2 groups based on whether human-to-human transmission of COVID-19 had been reported when the study questionnaire was completed (group 1, before the declaration of human-to-human transmission; group 2, after the declaration). Information of the number of suspected, confirmed, and death cases of COVID-19 in China were obtained after the declaration made on January 20, 2020. This study was registered to the Chinese Clinical Trial Registry (ChiCTR1900027020), and the ethical approval was obtained from the institutional review board of International Peace Maternity and Child Health Hospital (GKLW2019-11). Informed consent was obtained from all participants.
Participants
This cross-sectional study was performed in 25 public hospitals from 10 provinces across China, covering the East, the Middle, the Northwest, the Northeast, and the South. Pregnant women in their third trimester of pregnancy were invited to complete a study questionnaire by a trained research assistant during their regular obstetrical clinic visit. Questions pertained to sociodemographic characteristics, lifestyles, reproductive history, history of mental health problems, current depressive symptoms, marital and family support, and pregnancy complications (threatened abortion, gestational diabetes mellitus, hypertensive disorder, placenta previa, intrahepatic cholestasis of pregnancy, oligohydramnios, and intrauterine growth restriction).
Assessment criteria
Depressive symptoms were measured by EPDS, the most frequently used and internationally recommended screening measure for perinatal depression.11 , 12 EPDS was used to evaluate feelings in the last 7 days with a recommended cutoff score of 10 or higher to detect possible depression during community-based screening.13 , 14 In this study, a cutoff score of 10 or higher was used to analyze group differences in depression because this lower cutoff score is recommended for Asian population.12 In addition to depression, EPDS may be used to assess anxiety symptoms. The accumulative score from the items 3, 4, and 5 in EPDS (EPDS-3A) represents the anxiety dimension.15 , 16
Statistical analysis
All analyses were conducted with R statistical software version 3.6.2 (packages rms, ggplot2, ggradar, nCOV2019, recharts). All reported probability values were 2-tailed, and the criterion for significance was set at P=.05. Univariate statistics and distribution were assessed; continuous variables were presented as means and standard deviations or median and interquartile ranges (IQRs). Categorical variables were expressed as frequency and percentage. The crude prevalence of depressive symptoms was displayed from January 1, 2020, to February 9, 2020. The chi-square test was used for categorical variables. Mann-Whitney U tests were applied to continuous variables with a non-normal distribution. We investigated the association between the number of changes per day (confirmed COVID-19 cases, suspected cases, or death cases) and EPDS scores using ordinary least squares linear regression models. We also investigated the risk for depression (EPDS≥10) using logistic regression models. The analyses were adjusted for potential confounders such as age, body mass index (BMI), education levels, occupation, annual household income, parity, investigation site, family support, per capita living area, maternal only-child status, pregnancy complications, and exercise level. Ordinal logistic regression model was used for a response variable with each EPDS items and with explanatory factors. Further model assumptions were assessed by plotting model residuals and evaluating R 2/sum of squared residuals.
We performed a subgroup analysis to investigate whether the increased risk for depressive symptoms was associated with the declaration of the COVID-19 epidemic using radar plot and attributable risk proportion and 95% confidence intervals (CIs). To further examine risk factors, we examined COVID-19–related factors, baseline variables, and pregnancy complications using logistic regression. The parameters included COVID-19 epidemic declaration, education levels, occupation, annual household income, family support, per capita living area, maternal only-child status, placenta previa, and exercise. We also considered investigation sites, history of diseases, use of assisted reproductive technology (ART), and twin pregnancy as potential confounders, but adjustment for these variables did not change the results and therefore were not include in the final models. We did not use imputing data analyses due to no missing data.
Results
Sample characteristics
A total of 4124 pregnant women were included in the analysis, 2839 of whom were investigated before January 21, 2020 (group 1). The distribution of demographic characteristics, including geographic region, was similar between the 2 study groups (Table 1 ). Owing to the small proportion of women with a history of anxiety or depression (<1%), we did not exclude them in the following analyses.
Table 1.
Group baseline characteristics
| Characteristic | Group 1 (n=2839) |
Group 2 (n=1285)a |
|---|---|---|
| Jan. 1–20 |
Jan. 21 to Feb. 9 |
|
| n (%) | n (%) | |
| Age, median (range) y | 30 (27–32) | 30 (27–32) |
| Age groups | ||
| <35 y | 2461 (86.7) | 1097 (85.4) |
| ≥35 y | 378 (13.3) | 188 (14.6) |
| BMI, median (range) (kg/m2) | 20.7 (19.1–22.9) | 20.6 (19.0–22.7) |
| BMI groups | ||
| <18.5 | 468 (16.5) | 231 (18.0) |
| 18.5–23.9 | 1907 (67.2) | 851 (66.2) |
| ≥24 | 464 (16.3) | 203 (15.8) |
| Race | ||
| Han | 2750 (96.9) | 1240 (96.5) |
| Minorities | 89 (3.1) | 45 (3.5) |
| Education | ||
| Primary school or less | 271 (9.5) | 96 (7.5) |
| High school | 376 (13.2) | 165 (12.8) |
| College | 1822 (64.2) | 876 (68.2) |
| Professional or graduate | 370 (13.1) | 148 (11.5) |
| Annual household income | ||
| <$4000 | 101 (3.6) | 54 (4.2) |
| $4001–$10,000 | 408 (14.4) | 195 (15.2) |
| $10,001–$20,000 | 797 (28.0) | 357 (27.8) |
| $20,001–$40,000 | 909 (32.0) | 430 (33.4) |
| >$40,000 | 624 (22.0) | 249 (19.4) |
| Per capita living area, median (range) (m2) | 48 (38–60) | 47 (39–60) |
| Occupation | ||
| Does not work | 531 (18.7) | 228 (17.7) |
| Full-time worker | 1776 (62.6) | 831 (64.7) |
| Part-time worker | 532 (18.7) | 226 (17.6) |
| Marital status | ||
| Married | 2799 (98.6) | 1271 (98.9) |
| Single | 33 (1.2) | 12 (0.9) |
| Divorced | 7 (0.2) | 2 (0.2) |
| Parity | ||
| Primiparous | 1875 (66.0) | 884 (68.8) |
| Multiparous | 964 (34.0) | 401 (31.2) |
| Current smoker | ||
| Yes | 16 (0.6) | 5 (0.4) |
| Current alcohol consumption | ||
| Yes | 41 (1.4) | 18 (1.4) |
| Pregnancy complicationsb | ||
| Yes | 1203 (42.4) | 548 (42.6) |
| ART | ||
| Yes | 184 (6.5) | 68 (5.3) |
| History of anxiety or depressionc | ||
| Anxiety only | 10 (0.4) | 4 (0.3) |
| Depression only | 6 (0.2) | 3 (0.2) |
| Anxiety and depression | 3 (0.1) | 2 (0.2) |
| Confirmed COVID-19 cases in participating Chinese provincesd | ||
| <500 | 1471 (51.8) | 647 (50.4) |
| ≥500 | 1368 (48.2) | 638 (49.6) |
Data are expressed as median (quartiles) or n (%). Group 1: before the COVID-19 epidemic declaration. Group 2: after the COVID-19 epidemic declaration.
ART, assisted reproductive technology; BMI, body mass index; COVID-19, coronavirus disease 2019;
Wu et al. Depressive and anxiety symptoms in pregnancy during COVID-19 outbreak. Am J Obstet Gynecol 2020.
COVID-19 was incorporated as a notifiable disease in the infection law and health and quarantine law in January 20, 2020, and it was the first time that COVID-19 was reported by official media to spread from human to human. Confirmed COVID-19 and suspected cases have rapidly increased since January 20, 2020
Included gestational diabetes mellitus, preeclampsia, gestational hypertension, intrahepatic cholestasis of pregnancy, placenta previa, malposition, fetal growth restriction, and high-risk pregnancy status
Included generalized anxiety disorder, panic, agoraphobia, posttraumatic stress disorder, social phobia, and depression disorder
Participating Chinese provinces with confirmed COVID-19 cases <500 included Shanghai, Shaanxi, Xinjiang, and Hainan. Provinces with confirmed COVID-19 cases ≥500 included Henan, Zhejiang, Anhui, Hunan, and Jiangxi.
Depressive and anxiety symptoms and coronavirus disease 2019
We found that women in group 2 had higher mean EPDS scores (mean±SD, 7.7±4.4 vs 7.4±4.3) and anxiety subscale scores (mean±SD, 3.4±1.7 vs 3.2±1.7) than those in group 1. Furthermore, awareness of the COVID-19 epidemic significantly increased the prevalence of depressive symptoms (EPDS≥10) (adjusted risk ratio [aRR], 1.20; 95% CI, 1.04–1.40; P=.01) and the risk of thoughts of self-harm (aRR, 2.85; 95% CI, 1.70–8.85; P=.005).
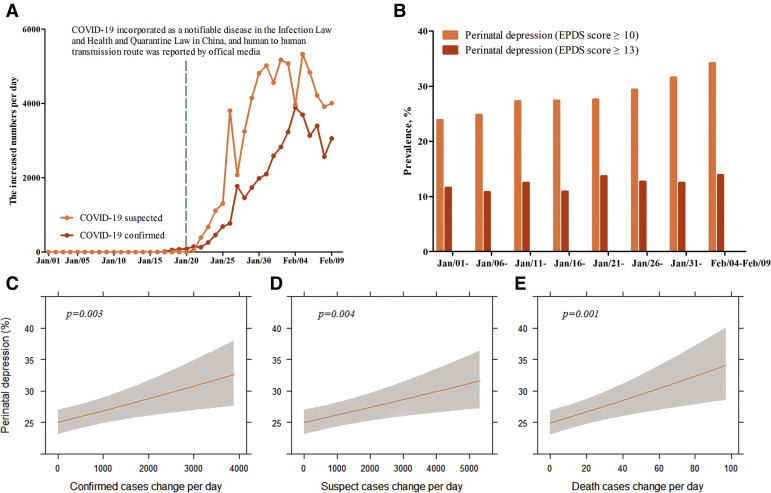
The number of suspected and confirmed COVID-19 cases increased rapidly after human-to-human transmission was first reported on January 20, 2020 (Figure 1 , A and B). Between January 20, 2020, and February 9, 2020, a stable increase in the prevalence of depressive symptoms (EPDS≥10) was found as the number of suspected and confirmed cases increased. Overall, the prevalence of depressive symptoms was 26.0% in group 1 and 29.6% in group 2. Subgroup analysis showed that the frequency of high EPDS scores between January 20 and February 9 was 34.2% (n=81, EPDS≥10) and 13.9% (n=33, EPDS≥13) with the largest increase in symptoms after February 5. To further analyze the adverse effect of the COVID-19 epidemic, standard linear regression models were used to examine the association between depressive symptoms and the number of new infections reported per day. Both number of newly confirmed infections (β=0.09, per 1000 cases increase, P=.003) and suspected infections (β=0.06, per 1000 cases increase, P=.004) per day were positively correlated with the prevalence of depressive symptoms (Figure 1, C and E). A linear positive association was also noted between EPDS scores and the number of newly confirmed infections per day (β=0.09, per 1000 cases increase, P=.04). A significant positive correlation was found between the number of new deaths per day and the prevalence of depression (β=0.05, per 10 cases increase, P=.001) and correlation with EPDS scores (β=0.08, per 10 cases increase, P=.008).
Figure 1.
The COVID-19 epidemic characteristics, prevalence of perinatal depression, and association between perinatal depression and daily cases change of COVID-19 infection
A, The increased number of COVID-19 infection confirmed cases and suspected cases. B, The prevalence of perinatal depression change. C–E, Association between confirmed COVID-19, suspected, or death cases and perinatal depression prevalence as predicted prevalence and 95% confidence interval in the whole population. Analyses were adjusted for potential confounders such as age, body mass index, education levels, occupation, annual household income, parity, investigation sites, family support, per capita living area, maternal only-child status, pregnancy complication, and exercise.
COVID-19, coronavirus disease 2019; EPDS, Edinburgh Postnatal Depression Scale.
Wu et al. Depressive and anxiety symptoms in pregnancy during COVID-19 outbreak. Am J Obstet Gynecol 2020.
Risk factors for depressive symptoms
We explored previously identified meta-analytic risk factors and found that in the overall sample, women were at increased risk if they (1) had below college education, (2) had a low annual household income, (3) worked part time or less, (4) had a per capita living area of ≤20 m2, (5) were an only child, (6) had perceived poor family support, (7) reported less than 7 hours per week of physical exercise, and (8) had a placenta previa (Table 2 ).
Table 2.
Depression risk factors among the entire sample
| Variables | EPDS≥10 n (%) | EPDS<10 n (%) | aRR (95% CIa) | P value |
|---|---|---|---|---|
| COVID-19–related factors | ||||
| Investigation time | ||||
| Jan. 1–20, 2020 | 739 (26.0) | 2100 (74.0) | Ref | |
| Jan. 21–Feb. 9, 2020 | 381 (29.6) | 904 (70.4) | 1.20 (1.04–1.40) | .01 |
| Investigation site | ||||
| Confirmed cases <500 | 546 (25.8) | 1573 (74.2) | Ref. | |
| Confirmed cases ≥500 | 574 (28.6) | 1432 (71.4) | 0.96 (0.82–1.11) | .56 |
| Baseline factors | ||||
| Education | ||||
| Primary school or less | 144 (39.2) | 233 (60.8) | Ref. | |
| High school | 174 (32.2) | 367 (67.8) | 0.82 (0.62–1.09) | .18 |
| College | 694 (25.7) | 2004 (74.3) | 0.76 (0.58–0.98) | .04 |
| Professional or graduate | 108 (20.8) | 410 (79.2) | 0.66 (0.47–0.94) | .02 |
| Annual household income | ||||
| Low (<$10,000) | 280 (36.9) | 478 (63.1) | Ref. | |
| Middle ($10,001–$40,000) | 640 (25.7) | 1853 (74.3) | 0.70 (0.56–0.84) | <.001 |
| High (>$40,000) | 200 (22.9) | 673 (77.1) | 0.67 (0.53–0.85) | .001 |
| Occupation | ||||
| Full-time worker | 607 (23.3) | 2000 (76.7) | Ref. | |
| Does not work | 263 (34.7) | 496 (65.3) | 1.40 (1.15–1.70) | .001 |
| Part-time worker | 250 (33.0) | 508 (67.0) | 1.43 (1.17–1.74) | <.001 |
| Per capita living area | ||||
| ≥20 m2 | 1029 (26.7) | 2832 (73.3) | Ref. | |
| <20 m2 | 91 (34.6) | 172 (65.4) | 1.41 (1.07–1.85) | .01 |
| Exercise per wk | ||||
| ≥7 h | 240 (24.5) | 741 (75.5) | Ref. | |
| <7 h | 880 (28.0) | 2263 (72.0) | 1.23 (1.04–1.46) | .02 |
| Having siblings | ||||
| No | 330 (23.0) | 1106 (77.0) | Ref. | |
| Yes | 790 (29.4) | 1898 (70.6) | 1.22 (1.04–1.42) | .01 |
| Family support | ||||
| Perceived good support from family | 15 (0.5) | 2989 (99.5) | Ref. | |
| Perceived poor support from family | 15 (1.3) | 1105 (98.7) | 2.33 (1.12–4.86) | .02 |
| Pregnancy complication factors | ||||
| Placenta previa | ||||
| No | 1028 (26.5) | 2846 (73.5) | Ref. | |
| Yes | 92 (36.8) | 158 (63.2) | 1.59 (1.21–2.08) | <.001 |
aRR, adjusted risk ratio; CI, confidence interval; COVID-19, coronavirus disease 2019; EPDS, Edinburgh Postnatal Depression Scale.
Wu et al. Depressive and anxiety symptoms in pregnancy during COVID-19 outbreak. Am J Obstet Gynecol 2020.
Data are expressed as multivariable adjusted risk ratio (aRR, 95% CI). Multivariate analyses were adjusted for the effects of COVID-19–related factors, baseline factors, and pregnancy complication factors. Other factors including age, parity, body mass index, assisted reproductive technology, and other pregnancy complication factors (gestational diabetes mellitus, preeclampsia, gestational hypertension, intrahepatic cholestasis of pregnancy, malposition, fetal growth restriction, and high-risk pregnancy status) are not significantly associated with perinatal depression and not shown in the table.
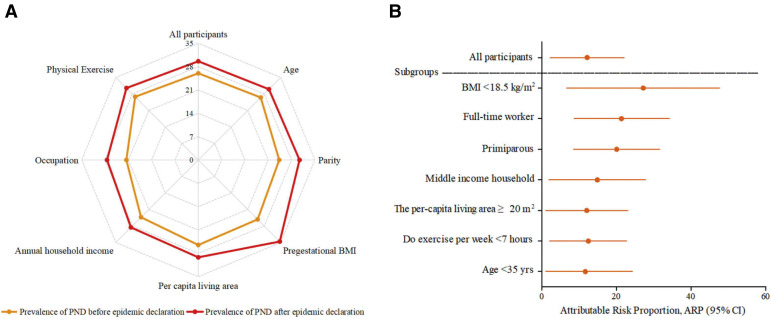
A subgroup analysis was conducted to explore risk factors specifically associated with depressive symptoms during the COVID-19 outbreak from January 20, 2020, to February 9, 2020 (Figure 2 ). The contribution of sociodemographic factors to the increased prevalence during the outbreak is shown in Figure 2, B. Women at higher risk for depressive symptoms include those with a middle annual household income and low levels of physical exercise. Interestingly, pregnant women who were primiparous, younger than 35 years, with a per capita area of ≥ 20 m2, and pregestational BMI of ≤18.5 kg/m2 seemed to be more vulnerable to develop depressive symptoms during the COVID-19 outbreak as were those who were employed full time.
Figure 2.
Prevalence of perinatal depression and attributable risk proportion in subgroups
A, The prevalence of PND before and after the COVID-19 epidemic declaration Filled circles signify significance in which P<.05. BMI, <18.5 kg/m2; age, <35 years; parity, primiparous; per capita living area, ≥20 m2; annual household income, middle ($10,001–$40,000); occupation, full-time worker; exercise, <7 hours per week. B, ARP and 95% CI of COVID-19 epidemic declaration in subgroups.
ARP, attributable risk proportion; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; PND, perinatal depression.
Wu et al. Depressive and anxiety symptoms in pregnancy during COVID-19 outbreak. Am J Obstet Gynecol 2020.
Discussion
Principal findings
This study examined the relationship between a major life-threatening public health event and maternal mental health wherein there is increased stress and fear due to the additional concern for an unborn fetus. After the declaration of human-to-human transmission of the COVID-19 by the Chinese government on January 20, 2020, global concern and uncertainty increased dramatically. A clinically significant rise in the prevalence of depressive symptoms was found among pregnant women increasing from 26% before January 20, 2020, to 34.2% between February 5 and 9, 2020. A significant increase in anxiety symptoms was also found. The COVID-19 outbreak may contribute to a significant increase in mild depressive symptoms instead of severe symptoms, as we found that the percentage of women with EPDS scores between 10 and 12 was higher after the COVID-19 epidemic declaration (data not shown).
Not surprising, the prevalence of depressive symptoms increased as the number of death and newly diagnosed cases also increased. Owing to the sudden outbreak, the features of the COVID-19 virus and symptoms remained unknown for weeks, causing overall heightened fear and concern for vertical mother-to-fetus transmission among pregnant women. Rapid reporting through the news and social media eliminated the regional differences such that increased rates of depressive symptoms were found among pregnant women in the 10 participating provinces independent of the number of cases found locally.
Results in context of what is known
Consistent with previous meta-analyses, we confirmed several risk factors for depressive symptoms in the whole sample such as low socioeconomic status, insufficient social support, and poor health behaviors including a lack of physical activity.17 , 18 In addition, maternal only-child status, a common phenomenon in women of reproductive age due to China’s 1-child policy, was found to be an independent risk factor for depression. In the face of the COVID-19 outbreak and the knowledge of human-to-human transmission, subgroup analyses found pregnant women who were younger than 35 years, underweight, primiparous, had middle-income, were working full time, and residing in a per capita living area of ≥20 m2 were now more likely to experience depressive symptoms. These women are not typically at high risk for perinatal mental illness as we identified in the entire study population of pregnant women, and this may help explain the increased rates found in this study after the pivotal date of January 20, 2020. Concern for the economic fallout and increased unemployment caused by the COVID-19 crisis may explain why middle-class women were at increased risk.19 Conversely, having to leave home to work full time may have increased concern for infection and vertical transmission and thus increased risk for depression and anxiety. A lack of physical activity was continuously associated with depressive symptoms independent of the epidemic. Meta-analytic results suggest physical activity has a protective effect on mental health in young adults. Even small amounts of exercise (eg, walking <150 minutes per week) decreases the incidence of depressive episodes.20 These findings suggest that 1 potentially modifiable factor for the onset of depression is physical activity and that programs to support exercise across the perinatal period are warranted. In an infectious disease pandemic, exercises performed in isolation or at home may help to decrease the depressive symptoms of pregnant women. For example, home-based exercises, such as walking extra steps around the same rooms inside the flat, or exercising on stairs every few hours could be included as an essential part of the self-isolation or protection guidelines.21
Clinical implications
Our present data revealed an increase in the proportion of women with thoughts of self-harm, which could potentially result in death and injury indirectly caused by the COVID-19 outbreak. The interventions for perinatal mental health should be a priority during any public-wide epidemic. Although mental health issues have been acknowledged during pandemics and a guideline of psychological crisis intervention specifically for COVID-19 has been published by the National Health Commission of China, perinatal women have not been highlighted in this guideline as a vulnerable population.22 Screening for perinatal depression and anxiety has been internationally recommended and should be a priority during an international public health crisis. Under the circumstances of population-based social distancing and isolation, psychological hotlines and online counseling would be a safe and feasible strategy to manage perinatal mental illness. With the limited medical resources, women who have previously experienced perinatal depression could be recruited as trained volunteers to provide telephone-based peer support that has been shown to have a preventive effect.23
Research implications
Further intervention studies addressing the screening and management of perinatal mental health through virtual care are warranted. From the SARS experience, we learned that the stressful impact of an infectious disease is qualitatively distinct in 2 ways from the stress of other disasters. First, potential exposure to a contagion brings social isolation. Interpersonal isolation in an infectious disease outbreak results in (1) the required use of personal protective equipment and physical distancing to control the spread of disease, (2) the strong tendency for those who are potentially exposed to cope with significant stress, and (3) the tendency of others to fear, avoid, and stigmatize those who have been potentially exposed.24 , 25 Second, pregnant women have heightened fear for their unborn fetus’s safety. Given the mental health consequences of a dramatic life-threatening outbreak for pregnant women, organizational and personal resilience strategies should be investigated and may include evidence-based approaches to effective risk communication and the provision of psychological first aid.26 Clear communication with regular and accurate updates about the outbreak should be provided as preventive strategy for mental well-being. Emotional and behavioral responses are part of an adaptive response to extraordinary stress and psychotherapy techniques such as those based on stress adaptation may be effective. In any public health emergency, feelings of fear, uncertainty, and stigmatization are common and may act as help-seeking barriers to appropriate mental health interventions.
Strengths and limitations
This study is the first to report the mental health response of pregnant women during the COVID-19 outbreak in China. An ongoing multicenter, cross-sectional study to examine the mental health of pregnant women provided a unique opportunity to compare the prevalence of depression before and after the national declaration of an epidemic. Although this innovative study has numerous strengths, it did not include pregnant women from Wuhan, Hubei province. The severe situation and strict quarantine measures in Wuhan owing to the COVID-19 outbreak prevented us from conducting a further study. Another limitation is the assessment of depressive and anxiety symptoms, which relied on a self-reported measure. Although the EPDS and the anxiety subscale is an internationally recommended screening measure and it has been used in numerous studies with Chinese women, it does not provide a diagnosis of depression or anxiety.
Conclusion
This study examined the mental health of pregnant women during the COVID-19 outbreak. Our findings indicate a clinically significant increase in the prevalence of depressive and anxiety symptoms after the declaration of human-to-human transmission and an increased threat of the COVID-19 epidemic. In addition to the well-documented perinatal mental health risk factors, we found that primiparous women of younger age, of middle-income status, and with full-time employment were at increased risk for developing depressive symptoms during the COVID-19 outbreak. More worryingly, the percentage of women with thoughts of self-harm was significantly higher during the outbreak. Our results suggest that effective interventions to manage the mental trauma that developed and potentially continues after serious life-threatening public health events are warranted.
Acknowledgments
The authors thank the National Natural Science Foundation of China (81661128010) and the Medical Engineering Cross Research Foundation of Shanghai Jiao Tong University (YG2020YQ29) for their support.
Footnotes
The authors report no conflict of interest.
The study was supported by the National Natural Science Foundation of China (81661128010) and the Medical Engineering Cross Research Foundation of Shanghai Jiao Tong University (YG2020YQ29). The funding source was not involved in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This study was registered at the Chinese Clinical Trial Registry (ChiCTR1900027020).
Cite this article as: Wu Y, Zhang C, Liu H, et al. Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. Am J Obstet Gynecol 2020;223:240.e1-9.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Clinical management of severe acute respiratory infection when COVID-19 is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at: Accessed Jan. 20, 2020.
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.People.cn #Novel coronavirus pneumonia latent period. https://s.weibo.com/weibo?q=%23%E6%96%B0%E5%9E%8B%E5%86%A0%E7%8A%B6%E7%97%85%E6%AF%92%E8%82%BA%E7%82%8E%E6%BD%9C%E4%BC%8F%E6%9C%9F%23&from=default Available at: Accessed Jan. 21, 2020.
- 5.Chan J.F.-W., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T.B., Chen X.Y., Miao G.D., et al. Recommendations on diagnostic criteria and prevention of SARS-related mental disorders. J Clin Psychol Med. 2003;13:188–191. [Google Scholar]
- 7.Maunder R., Hunter J., Vincent L., et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003;168:1245–1251. [PMC free article] [PubMed] [Google Scholar]
- 8.Palladino C.L., Singh V., Campbell J., Flynn H., Gold K.J. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056–1063. doi: 10.1097/AOG.0b013e31823294da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woody C.A., Ferrari A.J., Siskind D.J., Whiteford H.A., Harris M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 12.Lee D.T., Yip S.K., Chiu H.F., et al. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1998;172:433–437. doi: 10.1192/bjp.172.5.433. [DOI] [PubMed] [Google Scholar]
- 13.Haga S.M., Drozd F., Lisøy C., Wentzel-Larsen T., Slinning K. Mamma Mia - a randomized controlled trial of an internet-based intervention for perinatal depression. Psychol Med. 2019;49:1850–1858. doi: 10.1017/S0033291718002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisner K.L., Sit D.K., McShea M.C., et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–498. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loyal D., Sutter A.L., Rascle N. Screening beyond postpartum depression: occluded anxiety component in the EPDS (EPDS-3A) in French mothers. Matern Child Health J. 2020;24:369–377. doi: 10.1007/s10995-020-02885-8. [DOI] [PubMed] [Google Scholar]
- 16.Matthey S. Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders. Depress Anxiety. 2008;25:926–931. doi: 10.1002/da.20415. [DOI] [PubMed] [Google Scholar]
- 17.Gelaye B., Rondon M.B., Araya R., Williams M.A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016;3:973–982. doi: 10.1016/S2215-0366(16)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancaster C.A., Gold K.J., Flynn H.A., Yoo H., Marcus S.M., Davis M.M. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202:5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng J., Sham A., Tang P.L., Fung S. SARS: pregnant women’s fears and perceptions. Br J Midwif. 2004;12:698–702. [Google Scholar]
- 20.Mammen G., Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45:649–657. doi: 10.1016/j.amepre.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Shovlin C.L., Moorthy K., Lees C. Covid-19: home based exercise activities could help during self isolation. https://blogs.bmj.com/bmj/2020/03/16/covid-19-home-based-exercise-activities-could-help-during-self-isolation/ Available at: Accessed March 16, 2020.
- 22.National Health Commission of the People’s Republic of China The guideline of psychological crisis intervention for 2019-nCoV pneumonia. http://www.nhc.gov.cn/xcs/zhengcwj/202001/6adc08b966594253b2b791be5c3b9467.shtml Available at: Accessed Jan. 27, 2020.
- 23.Dennis C.L., Hodnett E., Kenton L., et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ. 2009;338:a3064. doi: 10.1136/bmj.a3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maunder R.G., Lancee W.J., Balderson K.E., et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12:1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maunder R. The experience of the 2003 SARS outbreak as a traumatic stress among frontline healthcare workers in Toronto: lessons learned. Philos Trans R Soc Lond B Biol Sci. 2004;359:1117–1125. doi: 10.1098/rstb.2004.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Child Traumatic Stress Network, National Center for PTSD Psychological first aid. Field operations guide. Natl Child Trauma Stress Network 2006. https://www.nctsn.org/sites/default/files/resources//pfa_field_operations_guide.pdf Available at: Accessed July, 2006.