Highlights
-
•
Chest CT patterns in COVID-19 may be divided into three main phenotypes with different characteristics o In phenotype 1, respiratory mechanics are consistent with high pulmonary compliance and severe hypoxemia.
-
•
In phenotype 2, moderate to high PEEP as well as lateral and/or prone positioning may help recruit collapsed areas.
-
•
Phenotype 3 resembles typical ARDS and should be managed as such.
-
•
Attention should be paid to the risk of pulmonary embolism, regardless of phenotype.
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; HFNO, high flow nasal oxygen; HME, heat-moisture exchanger; HR, hazard ratio; IBW, ideal body weight; ICU, intensive care unit; iNO, inhaled nitric oxide; nCoV, novel coronavirus; NIV, non-invasive ventilation; NMBA, neuromuscular blocking agents; PaO2, partial pressure of oxygen; PBW, predicted body weight; PEEP, positive end-expiratory pressure; Ppl, pleural pressure; RM, recruitment maneuver; RSI, rapid sequence intubation; SaO2, arterial saturation of oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SBT, spontaneous breathing trial; SpO2, peripheral saturation of oxygen; SvO2, venous saturation of oxygen; TBW, total body weight; VILI, ventilator-induced lung injury; VT, tidal volume; VV, veno-venous
Keywords: COVID-19, SARS-CoV-2, Mechanical ventilation, Prone position, Positive end expiratory pressure, Non-Invasive ventilation
Abstract
Coronavirus disease 2019 (COVID-19) can cause severe respiratory failure requiring mechanical ventilation. The abnormalities observed on chest computed tomography (CT) and the clinical presentation of COVID-19 patients are not always like those of typical acute respiratory distress syndrome (ARDS) and can change over time. This manuscript aimed to provide brief guidance for respiratory management of COVID-19 patients before, during, and after mechanical ventilation, based on the recent literature and on our direct experience with this population. We identify that chest CT patterns in COVID-19 may be divided into three main phenotypes: 1) multiple, focal, possibly overperfused ground-glass opacities; 2) inhomogeneously distributed atelectasis; and 3) a patchy, ARDS-like pattern. Each phenotype can benefit from different treatments and ventilator settings. Also, peripheral macro- and microemboli are common, and attention should be paid to the risk of pulmonary embolism. We suggest use of personalized mechanical ventilation strategies based on respiratory mechanics and chest CT patterns. Further research is warranted to confirm our hypothesis.
1. Introduction
A novel human coronavirus, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was identified in Wuhan, China, in late 2019 (Huang et al., 2020). Within few weeks, outbreaks of so-called 2019 novel coronavirus (nCoV) infection had spread worldwide. On February 11, 2020, the World Health Organization announced coronavirus disease 2019 (COVID-19) as the name of this new disease, and exactly one month later, declared the situation a pandemic [2–10]. As of April 2020, the pandemic is ongoing in the majority of countries [1, 11]. Data from China suggest that 40 % of patients who require intensive care unit (ICU) admission share similar comorbidities, such as diabetes and preexisting heart disease (Wang et al., 2020).
Although an extensive literature is available to guide management of the acute respiratory distress syndrome (ARDS), COVID-19 is a new viral infection of the lower respiratory tract whose pathophysiology and treatment are still poorly understood. More than 80 % of confirmed COVID-19 cases present as a mild febrile illness. However, a small proportion of patients will experience critical illness, with many of these requiring mechanical ventilation (Chen et al., 2020). In one study conducted in Wuhan, 41.8 % of patients hospitalized for COVID-19 develop acute respiratory failure, with a fatality rate of 52.4 % (Wu et al., 2020). The median time from symptom onset to respiratory failure was 8 days (Wang et al., 2020). At ICU admission, most COVID-19 patients present acute onset of hypoxemic respiratory failure, with oxygen saturation (SpO2) levels below 93 % (Zhang et al., 2020). Risk factors associated with respiratory failure and death include older age, neutrophilia, coagulation dysfunction, organ failure, and elevated D-dimer (Wu et al., 2020). At autopsy, the lungs of patients with confirmed SARS-CoV-19 infection exhibit slurry within the alveolar cavity, fibrinous exudation, and proliferation of Type II alveolar epithelial cells and macrophages. Moreover, alveolar septal vascular congestion, edema, vascular thrombi with focal intraparenchymal hemorrhage, and hemorrhagic infarction are common, suggesting an important role of the vascular compartment and lung perfusion in the pathophysiology of COVID-19 (Yao et al., 2020).
To date, the respiratory management of COVID-19 has relied on the general principles of ARDS management; however, computed tomography of the chest may provide interesting insights into the pathophysiology and individualization of mechanical ventilation in these patients. The aim of this manuscript is to provide brief guidance for respiratory management before, during, and after mechanical ventilation in COVID-19 patients, based on the literature and our direct experience with this population. Furthermore, we describe three distinct phenotypes of COVID-19 pneumonia, represented by distinct patterns of chest CT findings, their pathophysiological correlations, and their implications for management.
1.1. Chest CT findings in COVID-19
Computed tomography (CT) of the chest is essential to understand the diversity of pathological findings and optimize and individualize therapy for COVID-19 patients (Fig. 1 ) [18–22].
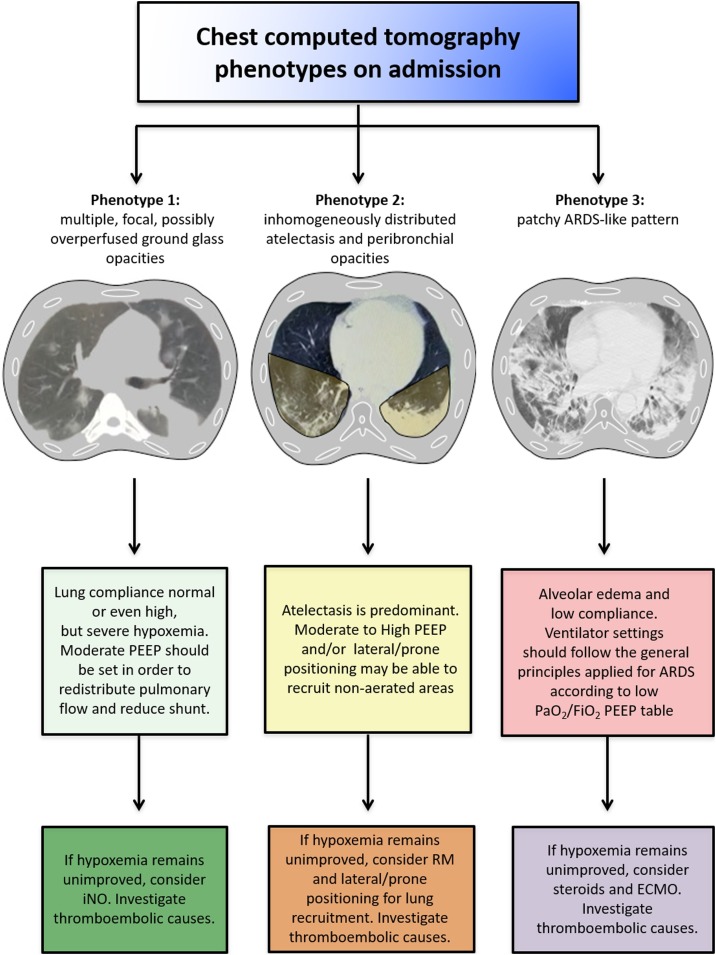
Fig. 1.
Summary of the key points for respiratory management of COVID-19 patients according to the three distinct phenotypes.
Phenotype 1: good compliance, but severe hypoxemia. PEEP should be set with the aim to redistribute pulmonary flow and reduce shunting. In this case, using the principles generally applied in ARDS, and thus setting the PEEP according to the best driving pressure, will probably lead to use of lower PEEP (as the compliance is good), resulting in less oxygenation. iNO could be considered in these cases, and prone positioning can redistribute perfusion, but is generally not very useful at this stage.
Phenotype 2: atelectasis and derecruitment are predominant. In this case, high PEEP and prone positioning can recruit non-aerated areas of the lung. Recruitment maneuvers (RMs) may play a role in these cases, whereas iNO is less useful.
Phenotype 3: typical CT pattern of moderate-to-severe ARDS, with alveolar edema and low compliance. Respiratory settings should follow the general principles applied for ARDS. PEEP should be set according to the best driving pressure; eventually, RMs, prone positioning, and ECMO may be considered.
Typical chest CT findings in COVID-19 include bilateral infiltrates with multiple ground-glass opacities or consolidation, but no edema (Zhang et al., 2020). Some patients exhibit asymmetrical edematous lesions and atelectasis, or scattered fibrosis (Zhang et al., 2020). Given the low resolution of plain radiography, we recommend that chest CT be performed in all severe patients. However, unfortunately, CT scanning is not available in all emergency departments, and may require transfer of the patient to a radiology suite. Lung ultrasound is a developing technique which has been used extensively in ARDS patients over the last decades (Peng et al., 2020) and may be useful for safe, noninvasive bedside diagnosis of COVID-19 pneumonia; specific lung ultrasound patterns have been described (Peng et al., 2020). Nevertheless, this technique has several limitations, such as the need for formal training, interobserver variability, and limited accuracy (particularly in obese patients and in the presence of subcutaneous emphysema).
To date, there have been few reports of chest CT findings in COVID-19 [17–21]. CT imaging demonstrates five stages according to the time since onset and disease progression: 1) Very early stage (asymptomatic, positive nasopharyngeal swab): single, double, or scattered focal ground-glass opacity, nodules located in central lobule surrounded by patchy ground-glass opacities, patchy consolidation and air bronchogram sign; 2) Early phase: (1–3 days after clinical manifestations): dilatation and congestion of alveolar septal capillaries, exudation of fluid in alveolar cavity, interlobular interstitial edema; 3) Rapid progression phase (3–7 days after clinical manifestations): massive accumulation of cell-rich exudates in the alveolar cavity, vascular expansion and exudation in the interstitium, large-scale light consolidation with air bronchogram sign; 4) Consolidation stage (7–14 days after clinical manifestations): fibrous exudation of the alveolar cavity with multiple patchy consolidations; and 5) Dissipation stage (2–3 weeks after clinical manifestations): grid-like thickening of interlobular septum, thickening and strip-like twisting of bronchial walls, and a few scattered patchy consolidations.
Monitoring of chest CT features is of extreme importance in these patients to personalize treatment strategies and mechanical ventilator settings (Fig. 1). In particular, chest CT scan can help in the assessment of areas of atelectasis or overperfusion and shunting, as well as evaluation of the risk of pulmonary embolism. We have identified three main chest CT patterns in COVID-19 patients, representing three different phenotypes: 1) multiple, focal, possibly overperfused ground-glass opacities mainly in the subpleural region; 2) inhomogeneously distributed atelectasis and peribronchial opacities; and 3) a patchy ARDS-like pattern. These differing phenotypes are attributable to different pathophysiological mechanisms, and therefore require different ventilatory strategies; however, the phenotypes we propose seem to be in agreement with Gattinoni et al.(Gattinoni et al., 2020a;Gattinoni et al., 2020b), who proposed a phenotype L (low elastance, low ventilation to perfusion ratio, and low lung reclutability) compatible with our phenotype 1, a phenotype H (high elastance, and simil ARDS pattern), compatible with our phenotype 3, and a transitioning phenotype, which reflects the evolution of the disease.
1.2. Oxygen therapy and noninvasive ventilation
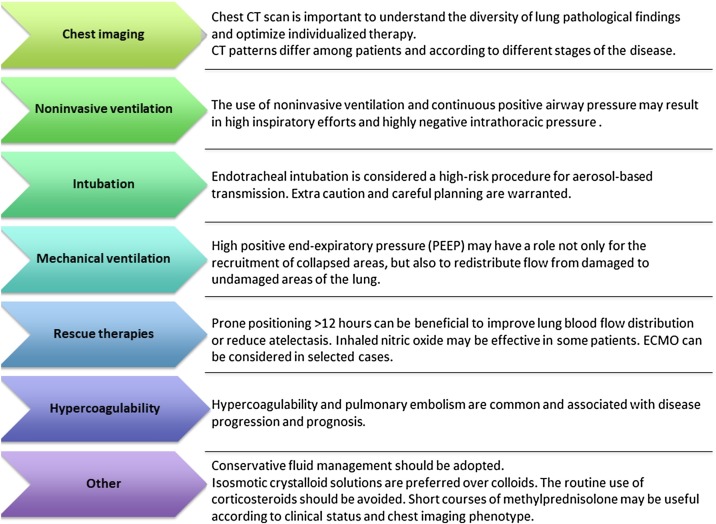
Supplemental oxygen is recommended in COVID-19 patients if the peripheral saturation of oxygen (SpO2) is below 93 %, and SpO2 should be maintained no higher than 96 % (Alhazzani et al., 2020). Few data are available on the efficacy of noninvasive support—which includes continuous positive airway pressure (CPAP), noninvasive ventilation (NIV), and high flow nasal oxygen (HFNO)—in COVID-19 pneumonia. In patients with influenza A(H1N1) infection, noninvasive positive-pressure ventilation was associated with a high incidence of failure (57–85 %), and patients who failed noninvasive support experienced a higher mortality rate than those treated with early mechanical ventilation (Kumar et al., 2009)24]. The experience in COVID-19 patients from Wuhan was similar, with a high rate of noninvasive support failure and need for intubation and mechanical ventilation in 76 % of cases; nevertheless, there was no difference in mortality between patients receiving noninvasive vs invasive ventilation (Yang et al., 2020). In general, noninvasive ventilatory management carries a high risk of generating negative (and unmeasured) intrathoracic pressures, and is thus potentially counterproductive (Brochard et al., 2017). Noninvasive support also presents a higher risk of viral spread through mask leaks, with increased risk of transmission (Fig. 2 ). Furthermore, delayed intubation increases the risk of clinical deterioration and the need for emergency airway management. In general ICU patients, HFNO has been shown to decrease the need for tracheal intubation in acute hypoxemic respiratory failure when compared to conventional oxygen therapy, without impacting mortality (Rochwerg et al., 2019). A randomized controlled trial comparing NIV and HFNO in patients with hypoxic respiratory failure showed that HFNO was able to reduce mortality at 90 days without affecting the need for intubation (50 % vs 47 %, p = 0.18) (Frat et al., 2015), while a meta-analysis comparing HFNO vs NIV demonstrated that HFNO is able to significantly decrease the need for intubation (Ni et al., 2018). Therefore, in adults with COVID-19 and acute respiratory failure, HFNO should be preferred over NIV. Both NIV and HFNO are being used extensively in COVID-19 patients, especially in cases of milder disease or to buy time before invasive ventilation is commenced. However, the potential advantages of using NIV or HFNO in these circumstances have to be balanced against their risks. When a patient presents with severe respiratory failure or on a downward spiral that suggests intubation will be inevitable, noninvasive respiratory support should not be attempted [22].
Fig. 2.
Key points summarizing our recommendations for the respiratory management of COVID-19 patients.
1.3. Airway management and tracheal intubation
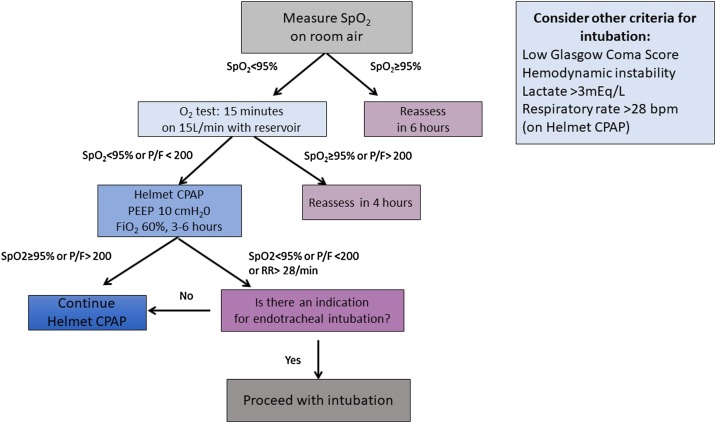
When hypoxemia and respiratory failure persist or worsen after oxygen therapy or within a short time (1 h) of placement of HFNO or NIV support, or in case of persistent hypercapnia, organ failure, coma, risk or aspiration, or hemodynamic instability, invasive mechanical ventilation should be implemented as soon as possible (Fig. 2) (Jin et al., 2020). Hypoxemic respiratory failure and need for invasive ventilation should be considered when patients receiving standard oxygen therapy exhibit tachypnea (> 30 bpm) and hypoxemia (SpO2 < 90 % or partial pressure of oxygen (PaO2) < 60 mmHg) with oxygen administered via a facemask and reservoir bag (gas flow of 10∼15 L/min, fraction of inspired oxygen (FiO2) 0.60–0.95). Similarly, when a patient under HFNO support with FiO2 >70 % and gas flow >50 L/min or NIV experiences persistent respiratory failure or deteriorates acutely, mechanical ventilation should be promptly initiated (Jin et al., 2020). Fig. 3 presents our algorithm for trialing CPAP and indications for centralization and intubation of patients with COVID-19 respiratory failure. According to our experience, phenotype 1 is likely to be found when patients are promptly intubated and receive only brief or no noninvasive respiratory support. Patients who receive prolonged noninvasive ventilation are likely to develop phenotype 2 or 3, thus becoming more difficult to ventilate, with lower compliance and worse deterioration of oxygenation.
Fig. 3.
Genoa algorithm for the advanced respiratory management of patients with COVID-19-related respiratory failure. This algorithm establishes objective tests which can be performed at bedside to determine whether a patient can be managed on oxygen alone, thus rationalizing ventilator and PPE use, and provides clear steps for escalation to CPAP and intubation.
The process of endotracheal intubation is considered to pose a high risk of aerosol-based transmission [30, 31]. Some hospitals have created dedicated spaces for planned airway management of COVID-19 patients (such as airborne infection isolation rooms); negative pressure ventilation rooms with an antechamber are ideal to minimize exposure during the procedure, whereas positive-pressure areas must be avoided (Zuo et al., 2020).
The intubation team should start the maneuver only after appropriate airborne/droplet protections are in place and all team members are wearing adequate personal protective equipment (PPE). Airway devices, venous access devices, anesthetics, suction, ventilators, and basic monitoring should be guaranteed and readily available before starting. A rapid difficult-airway assessment should be done to recognize those at risk for difficult airway management. Intubation should be performed by the most experienced clinician, with the help of another doctor, and the most familiar airway device should be the first choice for intubating (Zuo et al., 2020). Preoxygenation with 100 % FiO2 for 5 min before induction of anesthesia could be useful. Pre-oxygenation should be performed, using a well-fitting occlusive face mask attached to a manual ventilation device with an oxygen source (Zuo et al., 2020). A viral filter must be inserted between the facemask and manual ventilation device to minimize aerosolization. The viral filter should be applied directly to the face mask, since the greater the number of connections between the facemask and filter, the greater the risk of disconnection on the patient side and subsequent aerosolization of the virus (Zuo et al., 2020).
Rapid sequence induction should be used as the default technique, unless concerns with airway difficulty make this inappropriate. The literature suggests administration of rocuronium (>1.5 mg/kg ideal body weight) or suxamethonium (1.5 mg/kg total body weight) to obtain rapid onset of deep neuromuscular blockade and minimize the risk of the coughing during airway instrumentation [31]. Routine use of a videolaryngoscope is recommended for the first attempt at intubation to maximize the distance between the airway operator’s face and the patient. The choice of videolaryngoscope should be made according to the skillset and clinical judgement of the airway operator (Brewster et al., 2020). In case of failed intubation, a second-generation laryngeal mask should be placed to guarantee tracheal intubation passage through the mask with the aid of a fiberscope (Zuo et al., 2020).
After intubation, if using a humidified ventilator circuit, the viral filter used for intubation will need to be removed promptly; if a dry circuit is used, a combined heat-moisture exchanger (HME) and viral filter can be left in place, but this means that nebulization cannot be administered without breaking the circuit (to place a nebulizer between the patient and the HME). If the viral filter has been removed, the ventilator should be placed on standby for all circuit disconnections. Each disconnection from the ventilator should occur with the tube clamped to minimize the risk of aerosolization.
1.4. Ventilatory strategies
The cornerstone of management in respiratory failure is mechanical ventilation, always with the aim of minimizing ventilator-induced lung injury (VILI) (Fan et al., 2018). Early in the outbreak, all patients with severe COVID-19 were considered to have ARDS and consequently ventilated with low tidal volumes—6 mL/kg of predicted body weight (PBW)—and plateau pressure, as well as high positive end-expiratory pressure (PEEP) (Fan et al., 2017). The Surviving Sepsis Campaign Guidelines on the Management of Critically Ill Adults with Coronavirus Disease, 2020 (Alhazzani et al., 2020) recommend using low-tidal volume (VT) ventilation (VT 4−8 mL/kg of PBW) instead of higher tidal volumes (VT >8 mL/kg PBW). Additionally, a higher PEEP (>10 cm H2O) strategy should be preferred over a lower PEEP, and PEEP should be titrated according to FiO2 to maintain an appropriate SpO2 in order to reduce atelectasis and alveolar hyperinflation as well as pulmonary vascular resistance [23]. Even though COVID-19 patients fulfill the Berlin criteria for ARDS (The ARDS Definition Task Force et al., 2012), the clinical and chest CT features of mechanically ventilated COVID-19 patients do not always resemble ARDS. In fact, these patients may present with relatively well-maintained lung mechanics (good compliance) but severe hypoxemia, which could be consequent to impaired lung perfusion. Moreover, chest CT patterns differ among patients and over time. As noted above, we have found that chest CT findings in COVID-19 fall into three different phenotypes, each warranting unique mechanical ventilation settings and management strategies, which should thus be individualized based on clinical and CT features (Fig. 1, Additional File 1, Fig. S1).
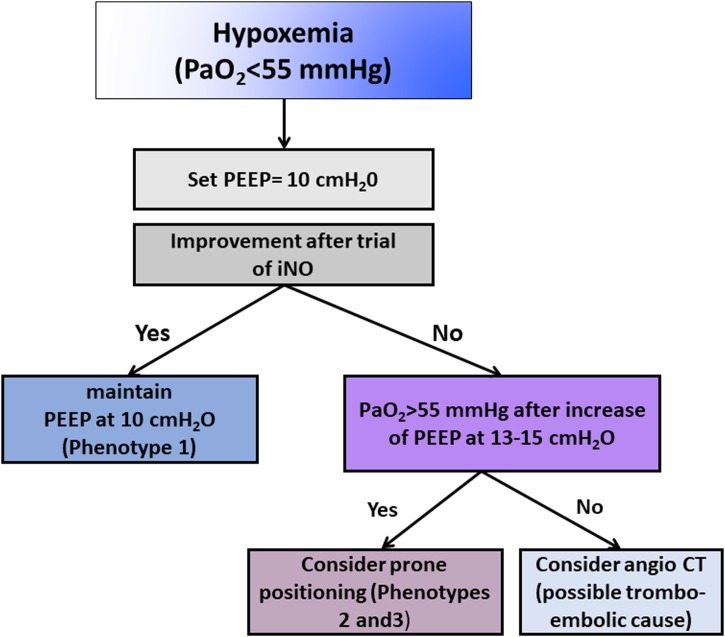
In phenotype 1, lung compliance is preserved or even elevated; chest CT shows no or few alveolar areas to recruit, but rather high-perfusion areas (Fig. 1, Additional File 1, Fig. S1A). In these cases, the main cause of hypoxemia seems to be not atelectasis, but impaired distribution of lung perfusion and shunting. Moderate PEEP levels may therefore be able to redistribute pulmonary blood flow from damaged to non-damaged lung areas; however, higher PEEP levels can impair cardiac function, thus increasing the need for fluids and vasoconstrictor drugs without having important effects on oxygenation. Tidal volumes >6 mL/kg should also be considered.
In phenotype 2, atelectasis is inhomogenously distributed. Moderate to high PEEP can be therefore useful to improve lung recruitment, as well as lateral or prone positioning (Fig. 1, Additional File 1, Fig. S1B).
In phenotype 3, general principles applied to ARDS management should be used, including low tidal volume (<6 mL/kg) and PEEP titration according to PEEP/FiO2 table and respiratory mechanics [32, 33] (Fig. 1, Additional File 1, Fig. S1C).
1.5. Rescue therapies
Prone positioning can have an important role in severe ARDS to redistribute pulmonary blood flow, reduce atelectasis, and improve oxygenation (Fig. 2) (Guérin et al., 2013). In a meta-analysis including more than 2000 patients with moderate to severe ARDS, prone ventilation for at least 12 h had a beneficial effect on mortality (five randomized controlled trials; relative risk 0.74, 95 % confidence interval 0.56 to 0.99); however, no effect on mortality was detected when prone ventilation was used for less than 12 h (Sud et al., 2014). A recent study showed that prone ventilation has been frequently used in COVID-19 patients (11.5 %) (Yang et al., 2020). However, based on the foregoing, we do not recommend prone positioning of COVID-19 patients with phenotype 1. It should be reserved for phenotypes 2 and 3, to redistribute pulmonary blood flow and reduce atelectasis.
In short, the recommendation to use prone positioning is associated with chest CT features and should be individualized and reevaluated in each patient over time. It is fundamental that a protocol for prone positioning by available and that proning be performed by specifically trained personnel to avoid risks of infection or accidental endotracheal tube disconnection from the ventilator (Alhazzani et al., 2020).
Inhaled nitric oxide (iNO) can theoretically have an important role as rescue therapy to improve lung perfusion, but the effect of iNO is balanced by the lung anatomical pattern as well as by regional perfusion. Recently published guidelines (Alhazzani et al., 2020) did not recommend routine use of iNO in COVID-19 patients with respiratory failure. In phenotype 1, iNO may potentially improve oxygenation by acting on lung perfusion (Fig. 4 ), but no data are available from this population.
Fig. 4.
Individualized strategies and pathophysiological features according to chest CT findings.
Continuous infusion of neuromuscular blocking agents (NMBA) should be reserved for COVID-19 patients in which intermittent dosing may not suffice, such as: patients undergoing prone positioning, persistent ventilator asynchrony, and those with high plateau pressures (Alhazzani et al., 2013).
Recruitment maneuvers (RMs) are not routinely recommended in COVID-19 patients [43, 44]. In a systematic review and meta-analysis including 1423 patients, traditional RMs significantly reduced mortality, whereas incremental PEEP titration RMs increased death rate. Patients with phenotype 1 are likely not to benefit from RMs, whereas in phenotype 2 and 3 RMs may help improve oxygenation. When needed, traditional RMs along with higher levels of PEEP should be preferred over incremental PEEP-based RMs (Gattinoni et al., 2006).
Finally, in mechanically ventilated COVID-19 patients with refractory hypoxemia despite conventional treatment and prone positioning, veno-venous (VV) extracorporeal membrane oxygenation (ECMO) can be considered as an option. However, given the need for resources, training, and associated risks, it should be used as rescue therapy only, in carefully selected patients.
1.6. Hypercoagulability
Patients with COVID-19 often present in a hypercoagulable state. Thromboembolic events, ranging from microemboli to massive pulmonary embolism, are common and can contribute to respiratory failure, as well as precipitate clinical deterioration (Additional File 2, Fig. S2). In a preprint study [48], decreased platelet counts, increased fibrinogen and d-dimer levels were observed in 1 out of 5 patients, and prolonged prothrombin time was detected in 62.1 %. In this context, early coagulation screening, serial echocardiography, and CT pulmonary angiography can provide important information, particularly in more severe cases with sudden respiratory or hemodynamic deterioration. In a recent retrospective study of 165 patients with COVID-19 [49], d-dimer and fibrinogen correlated linearly with CT imaging score and changed dynamically according to disease progression. Therefore, although the literature is lacking on this specific topic, strict monitoring and early anticoagulation should be considered to mitigate multiorgan damage in severe COVID-19.
1.7. Hemodynamic management
Changes in pleural pressure (Ppl) and transpulmonary pressure can be detrimental to hemodynamics. The increase of Ppl after application of positive airway pressure has the effect of decreasing left ventricular afterload and blood pressure. Of note, if Ppl exceeds pulmonary venous pressure, West zone 2 conditions arise due to microvascular collapse; likewise, if Ppl and interstitial pressures overcome pulmonary arterial pressure, pulmonary blood flow is obstructed (West zone 1). In both conditions, alveolar pressure represents the driver that boosts right ventricle afterload (Vieillard-Baron et al., 2016).
During controlled mechanical ventilation, PEEP and tidal forces increase pulmonary vascular resistance and Ppl, thus influencing mean alveolar pressure (which can be clinically approximated to mean airway pressure). It can also be influenced by longer duty cycles and higher driving pressures (Vieillard-Baron et al., 2016). High mean arterial pressure can increase West zone 2 conditions, thus increasing dead space and shunt fraction. In some cases, elevated right-side pressures may overdistend the right ventricle, thus causing stiffness of the left ventricle in an interdependent manner. Such condition needs to be evaluated by changes in venous saturation of oxygen (SvO2), and arterial saturation (SaO2) (Vieillard-Baron et al., 2016).
1.8. Cardiac injury
Cardiac injury is common after COVID-19, occurring in 20–30 % of cases. It is heralded by increased levels of troponin and NT-proBNP, and is associated with poor outcome (Guo et al., 2020). Patients with a history of cardiovascular conditions, such as hypertension or diabetes, are at higher risk. We suggest close monitoring of cardiac function and serial echocardiography in all patients with COVID-19.
1.9. Sepsis
Although in most cases COVID-19 patients present with isolated viral pneumonia, septic shock may occur and should be promptly recognized and treated. If fluids are needed, isosmotic crystalloids are preferred vs colloids; albumin may be considered as a resuscitation fluid; conservative fluid management should be adopted, and vasopressors should be administered if necessary to improve microcirculation, titrated to a target mean arterial pressure of 65 mmHg with lactate ≥2 mmol/L. Empiric antibiotics targeting any suspected potential bacterial superinfection should be administered as soon as possible. Systemic corticosteroids are controversial in severe ARDS; methylprednisolone can be used as appropriate for patients with rapid disease progression or severe illness. According to severity, 40–80 mg of methylprednisolone per day can be considered, and the total daily dose should not exceed 2 mg/kg. In a recent study (Wu et al., 2020), methylprednisolone therapy decreased the risk of death in COVID-19 patients with respiratory failure. However, due to lack of evidence, the routine use of corticosteroids should be avoided. Short courses (3–5 days) can be considered according to clinical status and chest imaging [23]. Corticosteroids might be especially useful in patients with a heightened inflammatory response; therefore, PCR and interleukin (IL)-6 levels should be considered when deciding whether to start steroids in these patients.
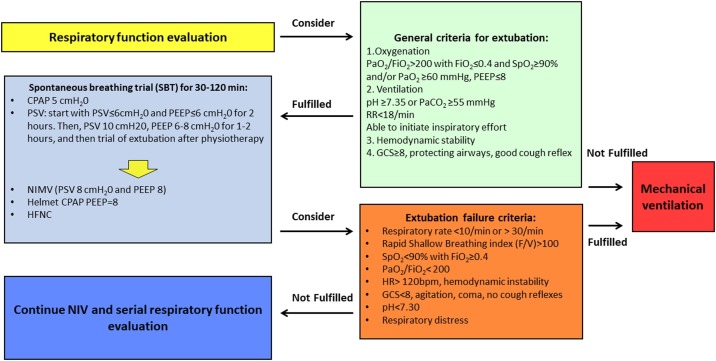
1.10. Weaning and extubation
The weaning process should follow the general criteria for weaning in any type of respiratory failure. As specific criteria for extubation of COVID-19 patients have not yet been established, generic guidelines (Popat et al., 2012) should be followed. Fig. 5 presents our algorithm for weaning and extubation. Patients can be eligible for a trial of extubation once they are well awake, exhibit good cough reflexes, and have stable hemodynamic and ventilatory parameters. At this stage, a spontaneous breathing trial (SBT) is performed before considering endotracheal tube removal. NIV and HFNO can be considered after extubation (Jin et al., 2020). Patients should ideally be non-infective prior to extubation, but this is likely to be unfeasible. When patients are still at risk of transmission, few recommendations are available to reduce the risk of infection; these include placement of a simple oxygen mask on the patient immediately after extubation to minimize aerosolization, the use of high PEEP levels, and avoidance of cough. If feasible, separate beds should be reserved for patients suitable for extubation trials (regardless of whether they are still positive for SARS-CoV-2) to reduce the risk of reagudization and, ultimately, reinfection of other patients.
Fig. 5.
Genoa algorithm for the weaning and extubation of patients with COVID-19.
2. Conclusions
The novel severe acute respiratory syndrome caused by SARS-CoV-2 progresses incredibly quickly and is associated with high fatality rates. Although the pulmonary pattern of critically ill patients with COVID-19 has been defined as ARDS, it does not always represent or even resemble ARDS. Chest CT scan features differ among patients, establishing distinct phenotypes; over time, these and might guide therapy and ventilator settings. Further studies are warranted to provide additional insight on the respiratory management of patients with severe COVID-19.
Authors’ contributions
CR, DB, PRMR and PP participated in the design of the review, and write the manuscript; LB, NP, ML, and IB contributed discussing different parts of the mechanical ventilation strategies. All authors read and approved the final manuscript.
Not applicable
Availability of supporting data
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Funding
Brazilian Council for Scientific and Technological Development (CNPq) and Rio de Janeiro State Research Foundation (FAPERJ).
Declaration of Competing Interest
The authors declare that they have no competing interests
Acknowledgements
The authors thank Mr. Filippe Vasconcellos (São Paulo), Brazil, for his assistance in editing the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resp.2020.103455.
Contributor Information
Chiara Robba, Email: kiarobba@gmail.com.
Denise Battaglini, Email: battaglini.denise@gmail.com.
Lorenzo Ball, Email: lorenzo.loryball@gmail.com.
Nicolo’ Patroniti, Email: npatroniti@gmail.com.
Maurizio Loconte, Email: maurizio.loconte15@gmail.com.
Iole Brunetti, Email: brunettimed@gmail.com.
Antonio Vena, Email: anton.vena@gmail.com.
Daniele Roberto Giacobbe, Email: daniele.roberto.giacobbe@gmail.com.
Matteo Bassetti, Email: matteo.bassetti@hsanmartino.it.
Patricia Rieken Macedo Rocco, Email: prmrocco@gmail.com.
Paolo Pelosi, Email: ppelosi@hotmail.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alhazzani W., Alshahrani M., Jaeschke R., Forel J.M., Papazian L., Sevransky J., Meade M.O. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013:17. doi: 10.1186/cc12557. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzani W., Hylander Møller M., Arabi Y.M., Loeb M., Ng Gong M., Fan E., Oczkowski S., Levy M.M., Maitland K., Alshamsi F., Belley-Cote E., Greco M., Laundy M., Morgan J.S., Kesecioglu J., McGeer A., Mermel L., Mammen M.J., Alexander P.E., Arrington A., Centofanti J., Citerio G., Baw B., Memish Z.A., Hammond N., Hayden F.G., Evans L., Rhodes A. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster D.J., Chrimes N.C., Do T.B.T., Fraser K., Groombridge C.J., Higgs A., Humar M.J., Leeuwenburg T.J., McGloughlin S., Newman F.G., Nickson C.P., Rehak A., Vokes D., Gatward J.J. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med. J. Aust. Med. J. Aust. 2020 doi: 10.5694/mja2.50598. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201605-1081CP. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-19) outbreak [WWW Document], 2020. URL https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 3.18.20).
- Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., Adhikari N.K.J., Amato M., Branson R., Brower R.G., Ferguson N.D., Gajic O., Gattinoni L., Hess D., Mancebo J., Meade M.O., McAuley D.F., Pesenti A., Ranieri M., Rubenfeld G.D., Rubin E., Seckel M., Slutsky A.S., Talmor D., Thompson B.T., Wunsch H., Uleryk E., Brozek J., Brochard L.J., American Thoracic Society, European Society of Intensive Care Medicine, S. of C.C.M An official american thoracic Society/European society of intensive care Medicine/Society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome advances in diagnosis and treatment. JAMA. 2018 doi: 10.1001/jama.2017.21907. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Frat J.-P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., Prat G., Boulain T., Morawiec E., Cottereau A., Devaquet J., Nseir S., Razazi K., Mira J.-P., Argaud L., Chakarian J.-C., Ricard J.-D., Wittebole X., Chevalier S., Herbland A., Fartoukh M., Constantin J.-M., Tonnelier J.-M., Pierrot M., Mathonnet A., Béduneau G., Delétage-Métreau C., Richard J.-C.M., Brochard L., Robert R. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. NEJM. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V.M., Quintel M., Russo S., Patroniti N., Cornejo R., Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. NAJM. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;14:1–4. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin C., Reignier J., Richard J.-C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., Clavel M., Chatellier D., Jaber S., Rosselli S., Mancebo J., Sirodot M., Hilbert G., Bengler C., Richecoeur J., Gainnier M., Bayle F., Bourdin G., Leray V., Girard R., Baboi L., Ayzac L. Prone positioning in severe acute respiratory distress syndrome. NEJM. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q., Han Y., Hu B., Hu F., Li B.H., Li Y.R., Liang K., Lin L.K., Luo L.S., Ma J., Ma L.L., Peng Z.Y., Pan Y.B., Pan Z.Y., Ren X.Q., Sun H.M., Wang Y., Wang Yun Yun, Weng H., Wei C.J., Wu D.F., Xia J., Xiong Y., Xu H.B., Yao X.M., Yuan Y.F., Ye T.S., Zhang X.C., Zhang Y.W., Zhang Y.G., Zhang H.M., Zhao Y., Zhao M.J., Zi H., Zeng X.T., Wang Yong Yan, Wang X.H. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020 doi: 10.1186/s40779-020-0233-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J., Stelfox T., Bagshaw S., Choong K., Lamontagne F., Turgeon A.F., Lapinsky S., Ahern S.P., Smith O., Siddiqui F., Jouvet P., Khwaja K., McIntyre L., Menon K., Hutchison J., Hornstein D., Joffe A., Lauzier F., Singh J., Karachi T., Wiebe K., Olafson K., Ramsey C., Sharma S., Dodek P., Meade M., Hall R., Fowler R.A. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- Ni Y.N., Luo J., Yu H., Liu D., Liang B.M., Liang Z.A. The effect of high-flow nasal cannula in reducing the mortality and the rate of endotracheal intubation when used before mechanical ventilation compared with conventional oxygen therapy and noninvasive positive pressure ventilation. A systematic review and meta-analysis. Am J Emerg Med. 2018;36:226–233. doi: 10.1016/j.ajem.2017.07.083. [DOI] [PubMed] [Google Scholar]
- Peng Q.-Y., Wang X.-T., Zhang L.-N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. Exp. 2020:1–2. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat M., Mitchell V., Dravid R., Patel A., Swampillai C., Higgs A. Difficult airway society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67:318–340. doi: 10.1111/j.1365-2044.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- Rochwerg B., Granton D., Wang D.X., Helviz Y., Einav S., Frat J.P., Mekontso-Dessap A., Schreiber A., Azoulay E., Mercat A., Demoule A., Lemiale V., Pesenti A., Riviello E.D., Mauri T., Mancebo J., Brochard L., Burns K. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019 doi: 10.1007/s00134-019-05590-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sud S., Friedrich J.O., Adhikari N.K.J., Taccone P., Mancebo J., Polli F., Latini R., Pesenti A., Curley M.A.Q., Fernandez R., Chan M.-C., Beuret P., Voggenreiter G., Sud M., Tognoni G., Gattinoni L., Guérin C. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2014;186:E381–90. doi: 10.1503/cmaj.140081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARDS Definition Task Force, Ranieri V., Rubenfeld G., Thompson B., Ferguson N., Caldwell E., Fan E., Camporota L., Slutsky A. ARDS guidelines JAMA 2012-ARDS the Berlin definition. Jama. 2012;307:1. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A., Matthay M., Teboul J.L., Bein T., Schultz M., Magder S., Marini J.J. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med. 2016 doi: 10.1007/s00134-016-4326-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020:S2213–2600. doi: 10.1016/S2213-2600(20)30079-5. 30079–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Li Q.R., Huang X., Cui Y., Li X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.G., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zuo M.Z., Huang Y.G., Ma W.H., Xue Z.G., Zhang J.Q., Gong Y.H., Che L. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin. Med. Sci. J. 2020:10. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable