Graphical abstract
Keywords: Efficacy, Safety, Integrated Traditional Chinese and Western Medicine, COVID-19, Meta-analysis
Abbreviations: COVID-19, Corona Virus Disease 2019; NCP, Novel Coronavirus Pneumonia; SARS-CoV-2, Severe Acute Respiratory Syndrome CoronaVirus-2; α-INF, alpha-interferon; TCM, Traditional Chinese Medicine; WBC, White blood cell; CRP, C-Reactive Protein; TNF-a, Tumor Necrosis Factor-α; RCTs, Randomized Controlled Trials; CCSs, Case-Control Studies; RoB, Risk of Bias; NOS, Newcastle-Ottawa Scale; RR, Risk Ratio; WMD, Weighted Mean Difference; CI, Confidence Intervals
Abstract
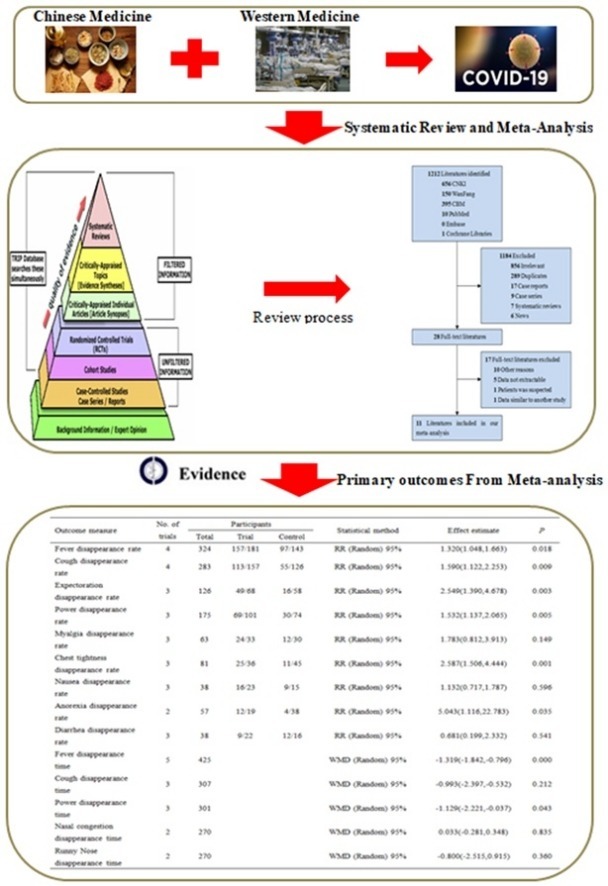
Corona virus disease (COVID-19) has now spread to all parts of the world and almost all countries are battling against it. This study aimed to assess the efficacy and safety of Integrated Traditional Chinese and Western Medicine (Hereinafter referred to as “Integrated Medicine”) to COVID-19. We searched six major Chinese and English databases to identify randomized controlled trials (RCTs) and case-control studies (CCSs) of Integrated Medicine on COVID-19. Two reviewers independently screened, identified studies, and extracted data. Cochrane Risk of Bias tool and the Newcastle-Ottawa Scale were used to assess the quality of included RCTs and CCSs, respectively. Stata (version 13.0; StataCorp) was used to perform meta-analyses with the random-effects model. Risk ratio (RR) was used for dichotomous data while the weighted mean difference (WMD) was adopted for continuous variables as effect size, both of which were demonstrated in effect size and 95% confidence intervals (CI). A total of 11 studies were included. Four were RCTs and seven were CCSs. The sample size of including studies ranged from 42 to 200 (total 982). The traditional Chinese medicine included Chinese medicine compound drugs (QingFei TouXie FuZhengFang) and Chinese patent medicine (e.g. Shufeng Jiedu Capsule, Lianhua Qingwen granules). Compared with the control group, the overall response rate [RR = 1.230, 95%CI (1.113, 1.359), P = 0.000], cure rate [RR = 1.604, 95%CI (1.181, 2.177), P = 0.002], severity illness rate [RR = 0.350, 95%CI (0.154, 0.792), P = 0.012], and hospital stay [WMD = -1.991, 95%CI (-3.278, -0.703), P = 0.002] of the intervention group were better. In addition, Integrated Medicine can improve the disappearance rate of fever, cough, expectoration, fatigue, chest tightness and anorexia and reduce patients’ fever, and fatigue time (P < 0.05). This review found that Integrated Medicine had better effects and did not increase adverse drug reactions for COVID-19. More high-quality RCTs are needed in the future.
1. Introduction
As a member of coronavirus subfamily Coronaviridae, the coronavirus can infect human beings, many kinds of mammals and birds. Some coronavirus can spread between humans, livestock, and poultry. In December 2019, many cases of Novel Coronavirus Pneumonia (NCP) patients have appeared in Wuhan, Hubei, China [1]. The cause is possibly related to contact with a local fish and wild animal market (Huanan Seafood Wholesale Market). WHO named it as Coronavirus Disease 2019 (COVID-19), and the International Classification Committee named the virus as Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) [2], [3]. So far, although transmission in China has been gradually controlled, the rate of infections outside China is rising rapidly, especially in the United States, Italy, and Spain. Till 27 March 2020, about 531,806 cases of COVID-19 and 24,073 deaths have reported [4]. The COVID-19 has posed significant threats to international health. So the effective prevention and treatment of COVID-19 are a very urgent task.
1.1. Western medicine for COVID-19
China has accumulated a lot of experience in prevention, diagnosis, and treatment of COVID-19. So far, China has issued seven versions of COVID-19 clinical guidelines (Trial Version). According to the latest seventh edition, the treatments of COVID-19 still don’t have specific medicine [5]. The treatment of COVID-19 involves multiple disciplines, and the current recommendations are mainly based on Western Medicine including supportive care, respiratory assisted ventilation, anti-infection (mainly antiviral agents), and glucocorticoid therapy [5]. Suggested antiviral agents are alpha-interferon (a-INF), lopinavir, ribavirin, chloroquine phosphate, and abidol. At present, there is no evidence to support the general or routine use of Western Medicine, nor is there any evidence to prove the risks and benefits of Western Medicine for COVID-19.
1.2. Integrated Traditional Chinese and Western Medicine for COVID-19
Traditional Chinese Medicine (TCM) has a history of thousands of years and has saved the Chinese from major infectious diseases on many occasions. Now, TCM has been practiced worldwide. During the SARS epidemic in 2003, TCM played a huge role [6], [7], [8]. COVID-19 belongs to the category of “Pestilence” in TCM. Its main clinical manifestations are fever, fatigue, dry cough, and the disease is situated in the lung and related to the spleen, stomach, and heart. Like the SARS period, TCM played a major role in the “Fight against the Pestilence in China”, saving many people's lives [5], [9], [10]. Existing evidence showed that compared with the simple treatment of Western Medicine; Integrated Traditional Chinese and Western Medicine (Hereinafter referred to as “Integrated Medicine”) for COVID-19 may have better effects [11], [12], [13], [14], [15]. However, these studies have small sample sizes, and no convincing evidence is available to demonstrate the benefits and risks of Integrated Medicine for COVID-19.
This study summarized controlled trials and methods of Integrated Medicine treatment of COVID-19, including the changes of clinical symptoms. The secondary objective is to investigate the changes of laboratory indicators and the safety of Integrated Medicine of COVID-19.
2. Materials and Methods
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16]. In addition, we also performed this study according to some other methodological study on meta-analysis [17], [18], [19]. The protocol for this study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020177097).
2.1. Literature search
A pre-developed search strategy was used to identify all relevant clinical trials, regardless of languages or types of publication (excluded unpublished trials). We searched the following six databases: PubMed, Embase.com, Cochrane Library, CNKI (China National Knowledge Infrastructure), WanFang and CBM (Chinese Biomedical Database). The search time was limited from December 01, 2019 to March 24, 2020. Search terms included “traditional Chinese medicine”, “Western medicine”, “Integrated traditional Chinese and Western medicine”, “novel coronavirus pneumonia”, “2019-nCoV”, “COVID-19”, “SARS-CoV-2” and “NCP”. The search strategy of the PubMed database is presented in Appendix Table 1.
2.2. Inclusion criteria and study selection
Inclusion criteria: (1) Patients: confirm diagnosed COVID-19 patients (Age≥18 years) by laboratory; (2) Intervention: patients in treatment groups were given TCM therapy in addition to the baseline medication similar to the control group (the TCM therapy included Chinese medicine compound drugs, Chinese patent medicine); (3) Comparison: the patients of the control group were given modern Western conventional treatments; (4) Outcomes: a. clinical efficacy (e.g. overall response rate, cure rate, hospital stay), b. clinical symptoms (e.g. fever, cough, expectoration, fatigue, myalgia), c. laboratory indicators (e.g. lymphocyte percentage, white blood cell (WBC) count, C-reactive protein (CRP), tumor necrosis factor-α (TNF-a)), d. adverse drug reactions (e.g. nausea and vomit, diarrhea, liver damage); (5) Study types: randomized controlled trials (RCTs) and case-control studies (CCSs) were included. This followings were excluded: review, abstract, letter, case reports, case series reports, and animal experiments.
Two reviewers independently screened the title/abstract of each record by the inclusion criteria. For the indistinguishable record of the title/abstract, we retrieved the full text for further assessment. Finally, resolve any disagreements through discussion between two reviewers or consultation with a third reviewer.
2.3. Data extraction and quality assessment
A pre-designed data form was used to extract the relevant information, including the author, journal, study type, study location, study time, interventions, the dose of drugs, and outcomes. Primary outcomes including the clinical efficacy and the changes of clinical symptoms, such as cure rate, total effective rate, nausea disappearance rate, fever disappearance rate, and fatigue disappearance rate. Second outcomes including the changes of laboratory indicators and the safety of Integrated Medicine of COVID-19, such as CRP, TNF-α, WBC count, liver damage and diarrhea. The Risk of Bias (RoB) assessment tool from the Cochrane Handbook was used to assess the methodological quality of RCTs [20], and the Newcastle-Ottawa Scale (NOS) was used to assess the quality of CCSs [21]. Each RCT was assessed at low risk, high risk, or unclear risk relating to the following items: sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. The NOS assesses the quality of CCSs with eight questions in three broad categories: (1) patient selection; (2) comparability of study groups; (3) assessment of the outcome. The total score is 9, the higher the score, the better the quality of the study. Two reviewers independently completed the data extraction and quality assessment. Any disagreements between reviewers were resolved by discussion or consultation with a third reviewer.
2.4. Statistical analysis
Stata (version 13.0; StataCorp) was used to perform the statistical analysis. Risk ratio (RR) was used for dichotomous data while weighted mean difference (WMD) was adopted for continuous variables as effect size, both of which were demonstrated with effect size and 95% confidence intervals (CI). Considering heterogeneity of drugs used in different trials, we calculated all results based on the random effect model. We assessed statistical heterogeneity in each pairwise comparison with I2 statistic, and value of < 25%, 25-50%, and > 50% considered as low, moderate, and high level of heterogeneity, respectively [22]. We would perform subgroup analyses and sensitivity analyses to explore sources of heterogeneity if enough data were available. The Egger's test and funnel plots were used to detect the potential publication bias if the number of included trials was larger than ten for an outcome. Statistical significance was set at P < 0.05.
3. Results
3.1. Eligible studies
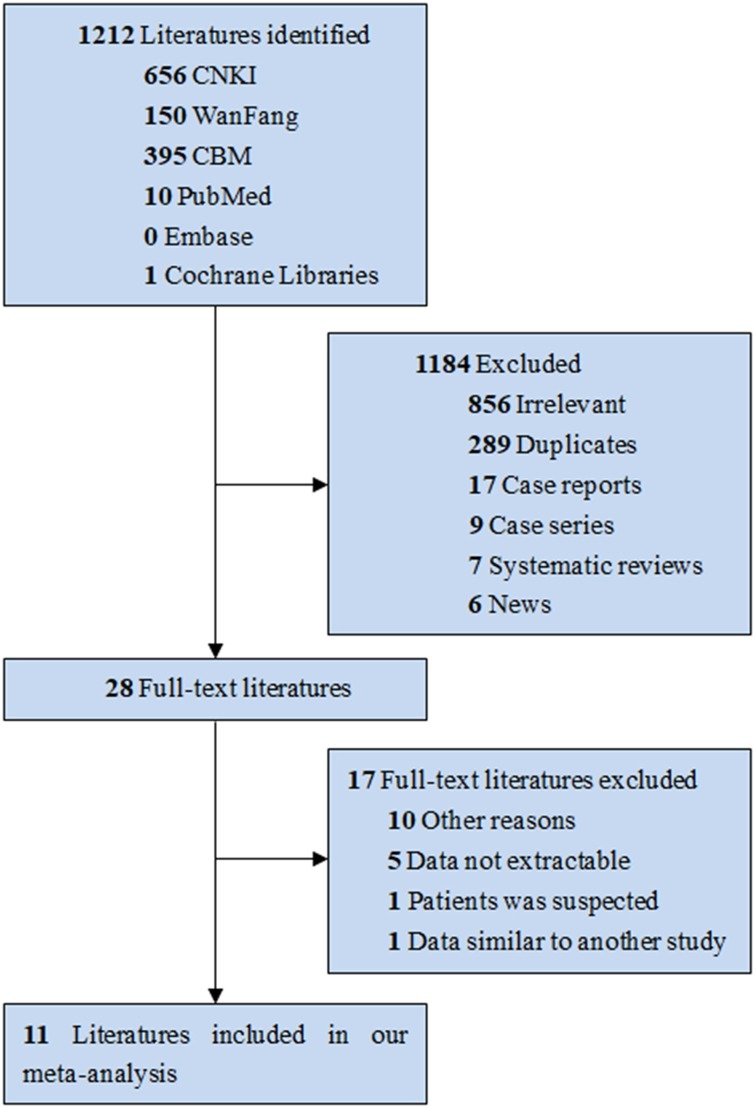
Fig. 1 showed the study selection process. A total of 11 studies were included in our study. All the articles were published by Chinese, among them, four studies were RCTs [12], [23], [24], [25] and seven were CCSs [11], [12], [26], [27], [28], [29], [30].
Fig. 1.
Flow Diagram of Literature Search and Trial Selection.
The detail of included studies is shown in Table 1 . Except for two studies that did not provide the range of study time [23], [24], the study time was from January 01, 2020 to March 02, 2020. The sample size of the included studies ranged from 42 to 200 (total 982). The duration of treatment ranges from 5 to 30 days, with an average of 13.55 days. Two studies did not provide specific Chinese medicine compound drugs and Chinese patent medicine [11], [13]. One studies intervention groups were Chinese medicine compound drugs (QingFei TouXie FuZhengFang) [24]. And the other studies were Chinese patent medicine (such as Shufeng Jiedu Capsule, Lianhua Qingwen granules). The drugs used in the control group were Lopinavir, Ribavirin, Arbidol and et al. In addition, both groups of patients received basic treatment, such as oxygen inhalation and nutritional support.
Table 1.
Characteristics of studies included in the meta-analysis.
| First author | Type of study | Location of study | Rang of time (2020) | Type of disease | Samples(male) |
Treatment |
Duration | ||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||||
| Zhou WM [23] | RCTs | ChangSha | Light | 52(32) | 52(28) | Diammonium glycyrrhizinate enteric coated capsules (150 mg,tid) + Lopinavir tablets(500 mg,bid) | Lopinavir tablets(500 mg,bid) | 14d | |
| Ding XJ [24] | RCTs | WuHan | Light/Common/Severe/Critical | 51(39) | 49(39) | Qingfeitouxie fuzhengfang(150 ml,bid) + ifn-α(5 million U,bid) + Ribavirin(0.5 g,bid) | ifn-α(5 million U,bid) + Ribavirin(0.5 g,bid) | 10d | |
| Qu XK [26] | CCSs | HaoZhou | 01.31∼02.11 | Light/Common | 40(25) | 30(16) | Shufeng Jiedu Capsule(2.08 g,tid) + Arbidol(0.2 g,tid) | Arbidol(0.2 g,tid) | 10d |
| Xia WG [11] | CCSs | WuHan | 01.15∼02.08 | Common/Severe/Critical | 34(17) | 18(6) | Integrated Traditional Chinese and Western Medicine | Western Medicine | 23d |
| Yao KT [27] | CCSs | WuHan | 01.11∼01.30 | Common | 21(16) | 21(12) | Lianhua Qingwen granules(6 g,tid) + Western Medicine | Western Medicine | 19d |
| Xiao Q [28] | CCSs | WuHan | 01.24∼01.30 | Light/Common | 100(64) | 100(66) | Shufeng Jiedu Capsule(2.08 g,tid) + Arbidol(0.2 g,tid) | Arbidol(0.2 g,tid) | 14d |
| Cheng DZ [29] | CCSs | WuHan | 01.01∼01.30 | Light/Common | 51(26) | 51(27) | Lianhua Qingwen granules(6 g,tid) + Western Medicine | Western Medicine | 7d |
| Shi J [13] | CCSs | ShangHai | 01.01∼02.01 | Light/Common/Severe | 49(26) | 18(10) | Traditional Chinese Medicine | Western Medicine | 30d |
| Yang MB [30] | CCSs | GuangZhou | 01.21∼03.02 | Light/Common | 26(16) | 23(9) | Reyanning mixture(10-20 ml,bid) + Lopinavir(100 mg,bid) + ifn-α(5 million U,bid) + abidol(0.2 g,tid) + ribavirin(0.5 g,bid) | Lopinavir(100 mg,bid) + ifn-α(5 million U,bid) + abidol(0.2 g,tid) + ribavirin(0.5 g,bid) | 7d |
| Fu XX [12] | RCTs | GuangZhou | 01.20∼02.23 | Common | 37(19) | 36(19) | Tongjiequwen granule formula(150 ml,bid) + Arbidol(0.2 g,tid) | Arbidol(0.2 g,tid) | 10d |
| Duan C [25] | RCTs | WuHan | 02.01∼02.05 | Light | 82(39) | 41(23) | Jinhua Qinggan granules(10 g,tid) + Western Medicine | Western Medicine | 5d |
3.2. Study quality
The quality of the included RCTs is shown in Table 2 [12], [23], [24], [25]. Four RCTs described the adequate random sequence generation process, but only one RCT [25] described the methods used for allocation concealment. Only one RCT [25] described the blinding of participants and personnel and blinding of outcome assessment (High risk), and none described how the incomplete outcome data were processed and reported selective outcome reporting. Overall, the quality of the included RCTs was low.
Table 2.
The risk of bias of included Randomized Controlled Trials.
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|
| Fu XX [12] | L | U | U | U | U | U | U |
| Zhou WM [23] | L | U | U | U | U | U | U |
| Ding XJ [24] | L | U | U | U | U | U | U |
| Duan C [25] | L | L | L | H | U | U | U |
H: High risk, L: Low risk, U: Unclear risk
Seven CCSs were assessed for quality by the NOS [11], [13], [26], [27], [28], [29], [30]. The maximum quality score is 9 and the range of scores was 3 to 7 (Table 3 ), with a median of 6 (5.4 ± 1.4). Only one study did not report the case definition [13], and all study reported the definition of controls and comparability of cases and controls [11], [13], [26], [27], [28], [29], [30]. None of the studies reported representativeness of the cases and selection of controls [11], [13], [26], [27], [28], [29], [30]. The reporting for exposure was better, but only one study reported non-response rate [30]. These studies showed a moderate quality.
Table 3.
The quality of included Case-Control Studies.
| Study | Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls | Ascertainment of Exposure | Use the same method to determine case and control exposure factors | Non-Response Rate | Total |
|---|---|---|---|---|---|---|---|---|---|
| Qu XK [26] | ★ | ★ | ★★ | ★ | ★ | 6 | |||
| Xia WG [11] | ★ | ★ | ★★ | ★ | ★ | 6 | |||
| Yao KT [27] | ★ | ★ | ★★ | 4 | |||||
| Xiao Q [28] | ★ | ★ | ★★ | ★ | ★ | 6 | |||
| Cheng DZ [29] | ★ | ★ | ★★ | ★ | ★ | 6 | |||
| Shi J [13] | ★ | ★★ | 3 | ||||||
| Yang MB [30] | ★ | ★ | ★★ | ★ | ★ | ★ | 7 |
3.3. Clinical Efficacy
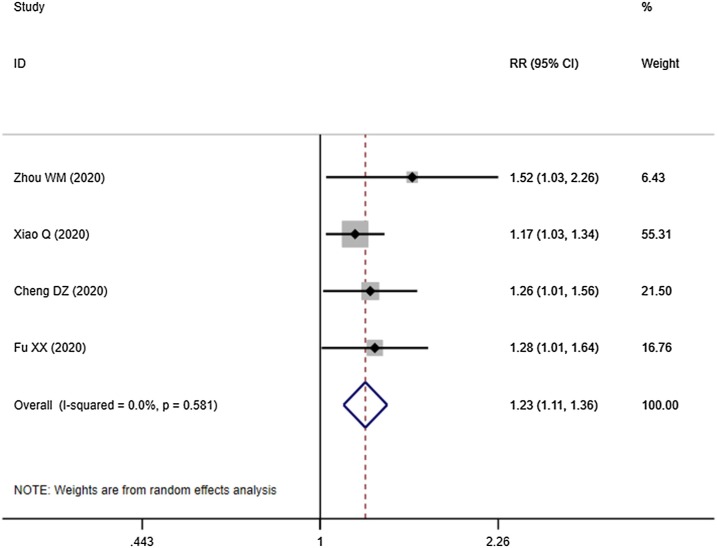
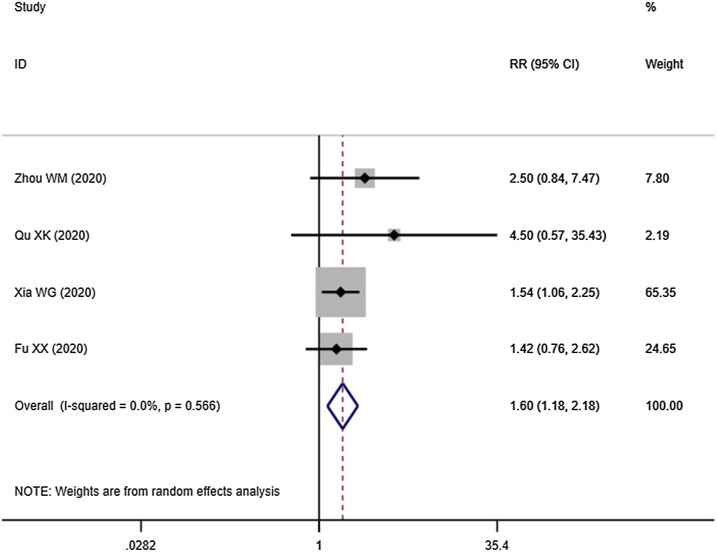
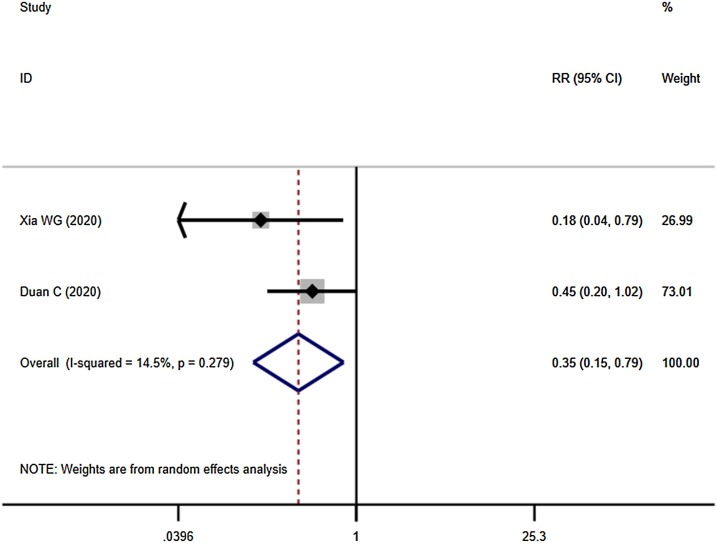
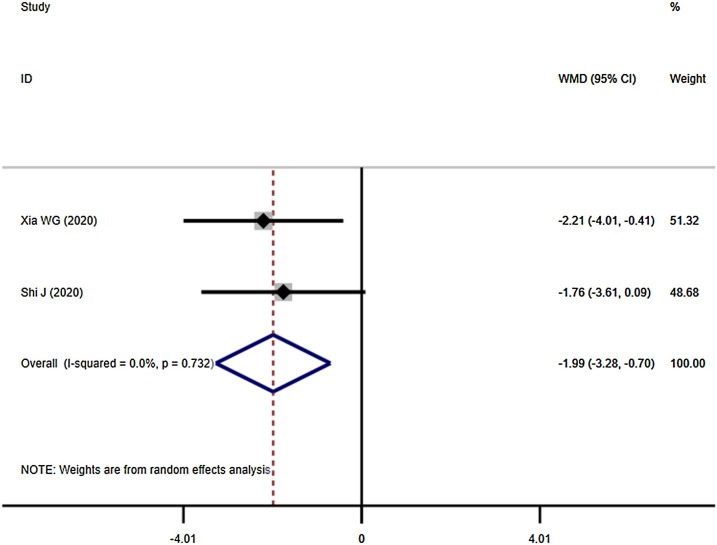
Four studies demonstrated the overall response rate: Integrated Medicine was better than Western Medicine alone [RR = 1.230, 95%CI (1.113, 1.359), P = 0.000] (Fig. 2 ). Four studies compared the cure rate of the COVID-19 between Integrated Medicine and Western Medicine. The outcome indicated the cure rate in Integrated Medicine was higher than Western Medicine (Fig. 3 ). The difference was statistically significant [RR = 1.604, 95%CI (1.181, 2.177), P = 0.002]. Besides, Integrated Medicine can reduce the severity of illness rate [RR = 0.350, 95%CI (0.154, 0.792), P = 0.012] (Fig. 4 ). And compared with Western Medicine treatment, the Integrated Medicine treatment can shorten the hospital stay [WMD = -1.991, 95%CI (-3.278, -0.703), P = 0.002] (Fig. 5 ).
Fig. 2.
Overall response rate of the COVID-19 between Integrated Medicine and Western Medicine.
Fig. 3.
Cure rate of the COVID-19 between Integrated Medicine and Western Medicine.
Fig. 4.
Severity illness rate of the COVID-19 between Integrated Medicine and Western Medicine.
Fig. 5.
Hospital stay of the COVID-19 between Integrated Medicine and Western Medicine.
3.4. Symptoms disappearance rate or time
We compared the effects of Integrated Medicine and Western Medicine on the clinical symptoms. The results showed that Integrated Medicine can better improve the symptoms disappearance rate and reduced the symptoms disappearance time than Western Medicine (Table 4 ). Except for the difference in myalgia and nausea is not statistically significant, Integrated Medicine significantly increased the disappearance rate of fever, cough, expectoration, fatigue, chest tightness and anorexia in patients (P < 0.05). In addition, Integrated Medicine can reduce patients’ fever, and fatigue time (P < 0.05).
Table 4.
Comparison of the symptoms disappearance rate or time between integrated Chinese and Western medicine.
| Outcome measure | No. of studies | Samples |
Statistical method | Effect estimate | P-value | ||
|---|---|---|---|---|---|---|---|
| Total | Events/Intervention | Events/Control | |||||
| Fever disappearance rate | 4 | 324 | 157/181 | 97/143 | RR (Random) 95%CI | 1.320(1.048,1.663) | 0.018 |
| Cough disappearance rate | 4 | 283 | 113/157 | 55/126 | RR (Random) 95%CI | 1.590(1.122,2.253) | 0.009 |
| Expectoration disappearance rate | 3 | 126 | 49/68 | 16/58 | RR (Random) 95%CI | 2.549(1.390,4.678) | 0.003 |
| Fatigue disappearance rate | 3 | 175 | 69/101 | 30/74 | RR (Random) 95%CI | 1.532(1.137,2.065) | 0.005 |
| Myalgia disappearance rate | 3 | 63 | 24/33 | 12/30 | RR (Random) 95%CI | 1.783(0.812,3.913) | 0.149 |
| Chest tightness disappearance rate | 3 | 81 | 25/36 | 11/45 | RR (Random) 95%CI | 2.587(1.506,4.444) | 0.001 |
| Nausea disappearance rate | 3 | 38 | 16/23 | 9/15 | RR (Random) 95%CI | 1.132(0.717,1.787) | 0.596 |
| Anorexia disappearance rate | 2 | 57 | 12/19 | 4/38 | RR (Random) 95%CI | 5.043(1.116,22.783) | 0.035 |
| Diarrhea disappearance rate | 3 | 38 | 9/22 | 12/16 | RR (Random) 95%CI | 0.681(0.199,2.332) | 0.541 |
| Fever disappearance time | 5 | 425 | WMD (Random) 95%CI | −1.319(-1.842,-0.796) | 0.000 | ||
| Cough disappearance time | 3 | 307 | WMD (Random) 95%CI | −0.993(-2.397,-0.532) | 0.212 | ||
| Fatigue disappearance time | 3 | 301 | WMD (Random) 95%CI | −1.129(-2.221,-0.037) | 0.043 | ||
| Nasal congestion disappearance time | 2 | 270 | WMD (Random) 95%CI | 0.033(-0.281,0.348) | 0.835 | ||
| Runny nose disappearance time | 2 | 270 | WMD (Random) 95%CI | −0.800(-2.515,0.915) | 0.360 | ||
3.5. Laboratory indicators
Meta-analyses revealed that the Integrated Medicine was more beneficial to the recovery of laboratory indicators. We found that Integrated Medicine was beneficial for IFN-a [WMD = -3.13, 95%CI (-4.23, -2.04), P = 0.000] and lymphocyte percentage [WMD = 1.59, 95%CI (0.61, 2.58), P = 0.002] to return to normal. And these differences were statistically significant (P < 0.05). Besides, as for the CRP and WBC count, there were no significant differences between Integrated Medicine and Western Medicine (P > 0.05) (Table 5 ).
Table 5.
Comparison of the laboratory indicators between integrated Chinese and Western medicine.
| Outcome measure | No. of studies | Samples | Statistical method | Effect estimate | P-value |
|---|---|---|---|---|---|
| CRP | 4 | 326 | WMD (Random)95%CI | −1.16(-6.96,4.65) | 0.695 |
| TNF-α | 2 | 204 | WMD (Random)95%CI | −3.13(-4.23,-2.04) | 0.000 |
| Lymphocyte percentage | 2 | 273 | WMD (Random)95%CI | 1.59(0.61,2.58) | 0.002 |
| WBC count | 2 | 273 | WMD (Random)95%CI | 0.66(-0.03,1.34) | 0.060 |
CRP, C - reactive protein; TNF-a, Tumor Necrosis Factor-α; WBC, White blood cell; CI, Confidence Intervals
3.6. Adverse Drug Reaction
The common adverse drug reactions of Integrated Medicine were nausea and vomiting, diarrhea, liver damage, and reduced blood cell count. As showed in Table 6 , there was no significant difference in the adverse drug reactions caused by the two different interventions (P > 0.05).
Table 6.
Comparison of the adverse drug reaction between integrated Chinese and Western medicine.
| Outcome measure | No. of studies | Samples |
Statistical method | Effect estimate | P-value | ||
|---|---|---|---|---|---|---|---|
| Total | Events/Intervention | Events/Control | |||||
| Nausea and vomiting | 2 | 172 | 5/92 | 5/80 | RR (Random) 95%CI | 0.915(0.267,3.138) | 0.888 |
| Diarrhea | 2 | 225 | 32/134 | 3/91 | RR (Random) 95%CI | 5.598(0.267,166.774) | 0.320 |
| Liver damage | 2 | 202 | 3/103 | 12/99 | RR (Random) 95%CI | 0.281(0.046,1.706) | 0.168 |
RR, Risk Ratio; CI, Confidence Intervals
3.7. Subgroup analysis of the primary outcomes
Results of subgroup analyses of the Chinese medicine compound drugs and Chinese patent medicine for the primary outcome were shown in Appendix Tables 2 and 3. When the Integrated Medicine included Diammonium glycyrrhizinate enteric coated capsules, it can improve the cure rate compared with Western Medicine (P = 0.036). Lianhua Qingwen granules can improv the total effective rate (P = 0.037), fever disappearance rate (P = 0.003), fatigue disappearance rate (P = 0.032), myalgia disappearance rate (P = 0.025), expectoration disappearance rate (P = 0.004), and chest tightness disappearance rate (P = 0.007). In addition, Lianhua Qingwen granules shorten the fever (P = 0.01), fatigue (P = 0.02), and cough (P = 0.038) time. Shufeng Jiedu Capsule can improvthe total effective rate (P = 0.02) and shorten the fever (P = 0.003) time. Tongjiequwen granule formula can improve the total effective rate (P = 0.044). Jinhua Qinggan granules can improve fever disappearance rate (P = 0.02), cough disappearance rate (P = 0.023), and expectoration disappearance rate (P = 0.003). Qingfeitouxie fuzhengfang can improve cough disappearance rate (p = 0.032) and chest tightness disappearance rate (P = 0.025).
3.8. Publication bias
Since the number of studies in any comparative analysis did not exceed ten, we did not assess the publication bias.
4. Discussion
Our study systematically evaluated the effect of Integrated Medicine for COVID-19. After a comprehensive search of six databases, we included four RCTs and seven CCTs. The study results showed that the Integrated Medicine had better effects and fewer adverse drug reactions compared with Western Medicine. This study is not the first to find that Integrated Medicine has a greater effect on acute infectious diseases. Similar studies have shown that it has positive effects on lung infiltrate absorption in SARS patients [31]. Facing such a severe epidemic situation in the world, the Western countries should pay attention to the therapeutic effect of TCM. We think it is necessary to hire TCM experts to participate in the treatment of COVID-19 in Western countries.
TCM has served the Chinese people since ancient times and has played an important role in today's medical care. And it especially has a very systematic understanding of the etiology and pathogenesis of acute infectious diseases. And in TCM, the dosage, composition, treatment time, withdrawal and follow-up criteria, and treatment plan of the compound Chinese herbal medicine can be adjusted according to the situation of the patient. In the included studies, eight different herbal medicine or Chinese patent medicine were used. This means that in terms of treatment, TCM can make more choices to make the best treatment. In addition, TCM was involved in the treatment of COVID-19 with different severity from light to critical [11], [24]. However, the use of these traditional herbs has been controversial due to unclear composition and lack of scientific evidence [32]. In our study, we found that the quality of these studies was low. In the treatment of many diseases, TCM is only used as adjuvant therapy [33], [34]. So, standard treatment and outcome index need to be developed. In this way, the best evidence can be systematically reviewed, summarized and disseminated to better provide evidence-based TCM decision-making.
TCM is superior to western medicine in improving the symptoms and quality of life of patients. This study found that Integrated Medicine can improve the disappearance rate of fever, cough, expectoration, fatigue, chest tightness and anorexia and reduce patients’ fever, and fatigue time. This is related to TCMs can affect immune cells and cytokine production associated with immune responses [35]. Immune regulation maintains the homeostasis of the immune system, protects the body from sources of infection or other harmful substances, thereby alleviating the clinical symptoms. It is essential for normal health. However, we found that the outcome indicators were not uniform in the included studies. This situation is dangerous and increases the waste of research resources, may cause some ineffective or adverse interventions to be applied clinically [36]. The diversity of outcome indicators also exists in the laboratory indicators and the adverse reaction indicators. Although we found that the Integrated Medicine may change the inflammation index and have fewer adverse drug reactions than western medicine. But these are not enough, we found that many important indicators cannot be analyzed due to outcome indicators were not uniform. Such as erythrocyte sedimentation rate, each interleukin type, macrophage ratio [13], [24].
As a new kind of respiratory disease, COVID-19 has many unknown factors to be solved. We found that included studies had a short duration, the ranges from 5 to 30 days. COVID-19 is likely to require a longer period of follow-up. In this way, the efficacy and possible adverse drug reaction of COVID-19 can be better observed. Besides, adverse events should be monitored through standardized and effective reporting systems, and some serious adverse events should be observed through epidemiological studies [37], [38].
However, this study also has the following limitations. The TCM and Western Medicine used in the intervention group and the control group is different. But we did not perform subgroup analysis or sensitivity analysis. And many merger statistical analysis studies have more heterogeneity. In addition, most of the included trials had flaws in the methodological design, including randomization, concealment of allocation, and inadequate reports on blinding, withdrawal, and sample size estimates. We also tried to contact the authors who participated in the trial for detailed information; however, we did not get a response at the end. And we did not perform a subgroup analysis according to the severity of the disease in patients withCOVID-19. In COVID-19 patients with different syndromes, the treatment effect may be different.
Above all, COVID-19 is a sudden outbreak disease. There are difficulties for clinicians to conduct RCTs, especially in the acute or critical period. So we included both RCTs and CCTs in this study. Therefore, some high-quality RCTs are needed to evaluate the effect of Integrated Medicine for COVID-19.
5. Conclusion
The study results showed that compared with Western Medicine, the Integrated Medicine for COVID-19 has better effects and did not increase adverse drug reactions. However, due to the low number of included studies, low quality, and inadequate methodologies, high-quality RCTs are needed to evaluate the effect of Integrated Medicine for COVID-19 in future.
Ethical approval
Ethical approval and patient consent are not required since this is an overview based on published studies.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Conflicts of Interest
The authors declare that they have no competing interests.
Author Contributions
Jinhui Tian, and Junhua Zhang designed this study; Ming Liu and Ya Gao ran the search strategy; Ming Liu and Yuan Yuan collected data, Shuzhen Shi and Ya Gao re-checked data; Ya Gao performed analysis and Jinhui Tian re-checked; Ming Liu and Kelu Yang assess the quality of studies, Shuzhen Shi and Junhua Zhang re-checked; Ming Liu wrote the manuscript, Ya Gao, Jinhui Tian and Junhua Zhang edited. All listed authors reviewed and revised the manuscript.
Acknowledgement
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phrs.2020.104896.
Contributor Information
Junhua Zhang, Email: zjhtcm@foxmail.com.
Jinhui Tian, Email: tjh996@163.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Hui D.S., Azhar E.I., Madani T.A. The continuing 2019- nCoV epidemic threat of novel coronaviruses to global health-the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W.H.O., Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. https://www.who.int/internal-publications-detail/clinical-management-of-severe-acuterespiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 24 March 2020).
- 3.Nie J.H., Li Q.Q., Wu J.J. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes. Infect. 2020;9:680–685. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus Outbreak, available at: https://www.worldometers.info/coronavirus/. (accessed 27 March 2020).
- 5.General Office of the National Health and Health Commission, Office of the State Administration of Traditional Chinese Medicine. Notice on Issuing a New Coronary Virus Pneumonia Diagnosis and Treatment guidelines (Trial Version 7) [EB/OL], http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml, (accessed 4 March 2020).
- 6.Hai X. Clinical experience of treating SARS in Guangdong hospital of TCM. TianJin ZhongYiYao FeiDian ZhuanJi. 2003;20:24–25. [Google Scholar]
- 7.Xiao P.G., Wang Y.Y., Cheng H.S. Some research clues on Chinese herbal medicine for SARS prevention and treatment. ZhongGuo ZhongYao ZaZhi. 2003;28:481–483. [PubMed] [Google Scholar]
- 8.Xiao X.H., Wang J.B., He C.S. On the rational exertion for the prescriptions and drugs of TCM in prevention and treating SARS. ZhongGuo ZhongYao ZaZhi. 2003;28:664–668. [PubMed] [Google Scholar]
- 9.S.F. Shi, Q.Q. Liu. To explore the value of Chinese medicine in the treatment of COVID-19 from the perspective of “Jiangxia square cabin Chinese Medicine Model”, JiangSu ZhongYiYao http://kns.cnki.net/kcms/detail/32.1630.r.20200325.0908.001.html. (accessed 27 March 2020).
- 10.H. Huang, Y. Zhao, X.H. Zuo, et al., Treatment of COVID-19 by Pneumonia No.1 Prescription and Pneumonia No.2 Prescription, ZhongYi XueBao http://kns.cnki.net/kcms/detail/41.1411.R. 20200323.1016.002.html, (accessed 27 March 2020).
- 11.W.G. Xia, C.Q. An, C.J. Zheng, et al., Clinical study on 34 cases of COVID-19 treated by integrated Chinese and western medicine, ZhongYi ZaZhi http://kns.cnki.net/kcms/detail/11.2166.R. 20200217.1502.004.html, (accessed 27 March 2020).
- 12.X.X. Fu, L.P. Lin, X.H. Tan, Clinical study on 37 cases of COVID-19 treated by integrated Chinese and western medicine, ZhongYao XinYao & LinChuang YaoLi http://kns.cnki.net/kcms/detail/44.1308.R. 20200319.1644.002.html, (accessed 27 March 2020).
- 13.J, Shi, Z.G. Yang, C. Ye, et al., Clinical observation of 49 cases of non-critical COVID - 19 treated by integrated traditional Chinese and western medicine in Shanghai, ShangHai ZhongYi ZaZhi http://kns.cnki.net/kcms/detail/31.1276.R. 20200304.1127.001.html, (accessed 27 March 2020).
- 14.Li R.F., Hou Y.L., Huang J.C. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 20 March:104761. doi: 10.1016/j.phrs.2020.104761. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren J.L., Zhang A.H.X.J., Wang Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020 4 March;55:104743. doi: 10.1016/j.phrs.2020.104743. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Tian J., Zhang J., Ge L. The methodological and reporting quality of systematic reviews from China and the USA are similar. J. Clin. Epidemiol. 2017;85:50–58. doi: 10.1016/j.jclinepi.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Tian J., Tian H. Network meta-analyses could be improved by searching more sources and by involving a librarian. J. Clin. Epidemiol. 2014;67:1001–1007. doi: 10.1016/j.jclinepi.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Li X.X., Zhang Y., Chen Y.L. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. 2014;119:503–510. doi: 10.1016/j.healthpol.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G.A., Shea B., O’Connell D. Ottawa Health Research Institute; Ottawa, Ontario: 2004. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in metaanalysis. [Google Scholar]
- 22.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.W.M. Zhou, F.M. Zhao, B.L. Li, et al., Clinical value of glycyrrhizinate in the treatment of patients with common new coronavirus pneumonia, BingDu XueBao http://kns.cnki.net/kcms/detail/11.1865.r.20200228.1135.001.html, (accessed 27 March 2020).
- 24.X.J. Ding, Y. Zhang, D.C. He, et al., Clinical Effect and Mechanism of Qingfei Touxie Fuzheng Recipe in the Treatment of Novel Coronavirus Pneumonia. YiXue DaoBao http://kns.cnki.net/kcms/detail/42.1293.R. 20200302.1615.002.html, (accessed 27 March 2020).
- 25.C. Duan, W.G. Xia, C.J. Zheng, et al., Clinical Observation of Jinhua Qinggan Granule in Treating Pneumonia Infected by New Coronavirus, ZhongYi ZaZhi http://kns.cnki.net/kcms/detail/11.2166.R. 20200323.0853.002.html, (accessed 27 March 2020).
- 26.X.K. Qun, S.L. Hao, J.H. Ma, et al., Observation on the clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsules in the treatment of COVID-19, ZhongCaoYao http://kns.cnki.net/kcms/detail/12.1108.r.20200225.1549.008.html, (accessed 27 March 2020).
- 27.K.T. Yao, M.Y. Liu, X. Li, et al., Retrospective Clinical Analysis on Treatment of Novel Coronavirus-infected Pneumonia with Traditional Chinese Medicine Lianhua Qingwen, ZhongGuo ShiYan FangJiXue http://kns.cnki.net/kcms/detail/11.3495.R. 20200206.1500.004.html, (accessed 27 March 2020).
- 28.Q. Xiao, Y.J. Jiang, S.S. Wu, et al., Analysis of the value of Shufeng Jiedu capsules combined with Abidol in the treatment of mild new type of coronary toxin pneumonia, ZhongGuo ZhongYi JiZheng http://kns.cnki.net/kcms/detail/50.1102.R. 20200309.1528.004.html, (accessed 27 March 2020).
- 29.D.Z. Cheng, W.J. Wang, Y. Li, et al., Analysis of 51 cases of new coronavirus pneumonia treated with traditional Chinese medicine Lianhua Qingwen: a multicenter retrospective study, TianJin ZhongYiYao http://kns.cnki.net/kcms/detail/12.1349.R. 20200310.1024.004.html, (accessed 27 March 2020).
- 30.M.B. Yang, S.S. Dang, S. Huang, et al., Multi-center Clinical Observation of Reyanning Mixture in Treatment of Novel Coronavirus Pneumonia, ZhongGuo ShiYan FangJiXue ZaZhi http://kns.cnki.net/kcms/detail/11.3495.R. 20200318.1327.001.html, (accessed 27 March 2020).
- 31.Zhang M.M., Liu X.M., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J. Gastroenterol. 2004;10(23):3500–3505. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao L., Chen W.Q. Atheroprotective Effects and Molecular Targets of Bioactive Compounds from Traditional Chinese Medicine. Pharmacol. Res. 2018;135:212–229. doi: 10.1016/j.phrs.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Y., Menon M.C., Deng Y. Recent Advances in Traditional Chinese Medicine for Kidney Disease. Am. J Kidney. Dis. 2015;66(3):513–522. doi: 10.1053/j.ajkd.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Shen C.Y., Jiang J.G., Yang L. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br. J Pharmaco. 2017;174(11):1395–1425. doi: 10.1111/bph.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C.F., Lin S.S., Liao P.H. The immunopharmaceutical effects and mechanisms of herb medicine. Cell. Mol. Immunol. 2008;5:23–31. doi: 10.1038/cmi.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials. 2007;8:39. doi: 10.1186/1745-6215-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cochrane . 2018. Adverse event: cochrane. Available: http://community.cochrane.org/glossary, (accessed 24 March 2020) [Google Scholar]
- 38.Altman D.G. The revised consort statement for reporting randomized trials: explanation and elaboration. Ann. Intern. Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.