Abstract
Background:
Non-adherence to tuberculosis (TB) treatment jeopardizes the individual’s health and contributes to disease transmission and drug resistance. New patient-centered strategies are needed to improve TB treatment outcomes.
Purpose:
To convert and expand a texting-based intervention into a mobile optimized application (app), evaluate the feasibility of an added self-administered paper-based drug metabolite test, and identify needs and preferences to inform their iterative design.
Methods:
Qualitative methods using focus groups and field testing with patients in active TB treatment were used to gather initial input on the converted intervention design, content and issues using at home test strips to report medication adherence. Seven participants were recruited from an outpatient clinic within a regional public reference hospital specialized in respiratory diseases in Argentina. Thematic analyses were conducted on the transcripts and session notes.
Results:
Participants considered interactive communication, access to answers to frequently asked questions, and tracking of progress in treatment as important. Participants reported having many questions and uncertainties at initiation of treatment and emphasized a need for reliable information, assurance and support from both providers and peers. Other suggestions included streamlining the graphical user interface for easier and shorter data entry times and usability.
Conclusions:
Overall feedback from the participants regarding the intervention was positive, reporting that it was useful and relevant, and they were eager to contribute their ideas for improvement and additional functionality. Valuable feedback to improve functionality and meet the needs of end-users were obtained to inform the generation of new design ideas for refinement and testing in a pilot study.
Keywords: mobile application development, mHealth, tuberculosis, treatment adherence, patient-centered design
BACKGROUND AND SIGNIFICANCE
Tuberculosis (TB) remains one of the top ten causes of death globally despite being largely curable.1 Adherence to TB treatment has long been recognized as complex and the consequences of medication non-adherence include continued spread of the disease, morbidity, mortality, and the development of drug resistance. If efforts go unchanged it is projected that by 2050 multidrug-resistant TB could kill as many as 2.5 million people per year and cost the global economy up to US$16.7 trillion.2 In response, new patient-centered strategies to improve treatment outcomes and to evolve to meet challenges are needed.3,4
With the increase in access to mobile phones and network coverage globally, the potential for interventions using mobile devices for TB management has gained popularity.5,6 In particular, mobile applications (apps) can facilitate personalized treatment supervision, patients’ self-management, on-demand or tailored education, and bidirectional communication with healthcare teams.7 Despite their potential usefulness, few mobile apps have been developed or evaluated to support patient self-management of TB treatment 8-10
Another promising digital adherence approach that remains largely unexplored is drug metabolite testing.11 Direct metabolite testing has been highlighted as the most promising, ethical, accurate, and least intrusive and least stigmatizing strategy compared to other mobile solutions (e.g., video observation, medication bottles containing SIM card, ingestible sensors).11 Concerns of privacy, accuracy, and costs of the other solutions have been raised. For example, while video directly observed therapy may avoid issues of stigmatization from attending clinic daily or receiving a daily visit from a treatment observer, patients can feign ingestion. Preliminary reports outside of the peer-reviewed literature highlight the potential for drug metabolite testing,12 however, there is a need for further development of the technology and rigorous research to assess its feasibility and impact on treatment outcomes.
Given the lack of evidence for digital technologies to support TB treatment adherence, the goal of our research was to convert and expand a texting-based intervention developed with end users and experts13-15 into a mobile app with an added drug metabolite monitoring. The intervention is aimed to support individuals with active TB where self-administered therapy is standard. First steps included concept mapping, low- and high-fidelity testing with design and domain experts, and initial reengineering of test strips. Here we report the exploratory research using focus groups and field testing with patients in active TB treatment to inform the design of a fully functioning TB support mobile app and identify issues with using a paper-based direct adherence test. To our knowledge this is the first study to report on findings of home use of the metabolite test.
METHODS AND TOOLS
Qualitative methods using focus groups, individual interviews, and field testing with patients in active TB treatment was conducted with a beta app prototype and initial reengineered direct adherence test strips to gather early feedback on the app content, design and functionalities and usability issues. Field testing was to provide participants a sense of what it would be like using the app and taking the test daily to better inform discussion rather than supply hypothetical situations. Our purpose in using this approach was to allow the data to be used for iterative intervention refinement and to understand patient needs during treatment and challenges related to adherence. Data were summarized into main themes and recommendations to inform the ongoing iterative development.
This study was approved by the Institutional Review Board at the University of Washington and the regional pulmonary reference hospital in Argentina. All participants provided informed consent prior to participating in the research activities.
Description of the intervention evaluated
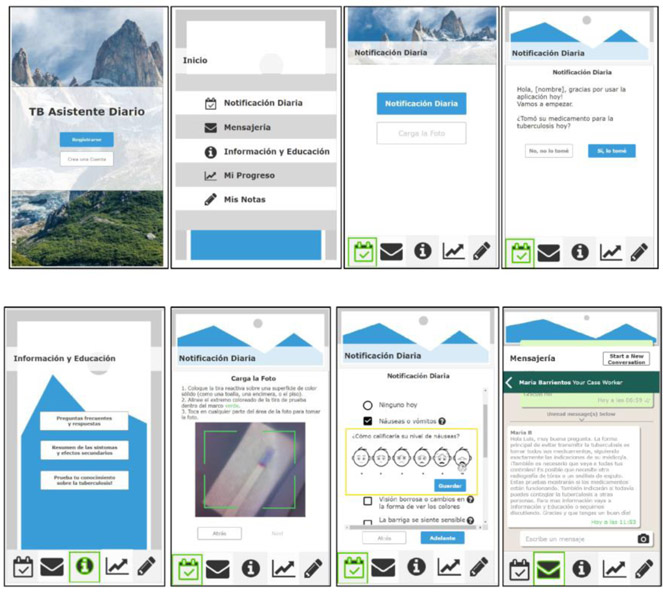
The beta app version requirements were based on prior formative research with endusers with TB15-17 and further concept mapping and low- and high-fidelity testing with design and domain experts. The base functioning features included: education in the form of information and answers to frequently asked questions, side-effect/symptom reporting, and self-administration tracking (Figure 1). Exemplar, nonfunctional functions were notes and a communication tool. The app was developed as a web based app to be accessible on all smartphones, as opposed to “native” applications (e.g., iOS or Android specific) with the added benefit for easier data transfer and potential for being a more cost effective option.18 The drug metabolite test was a new component added for enhanced adherence monitoring. Urine testing for drug adherence was new to patients and not a part of the usual care. This paper-based test strip detects if one of the medications to treat TB has been taken in the past 24 hours. The test required the participant to collect a small amount of urine in a provided specimen cup, place about 7-10 drops of urine onto the test window, wait for results and take a picture in the app of the test result (a color change to blue/purple denotes metabolite identification).
Figure 1.
Screen shots of the beta application
Participants
Eligible participants were at least 18 years of age; in TB treatment for at about 1 month or longer; and having a smartphone and familiarity with smartphone operation. Patients are given a one to two-month supply of medication and asked to return for follow-up appointments or as needed. All patients returning to the clinic for follow-up appointment or picking up their TB medication during the two-week recruitment period of the study were invited to participate by clinic staff and referred to research staff to discuss the study and arrange focus group dates. The goal was to recruit 5-10 individuals. Seven participants attended sessions, two canceled and were unable to be rescheduled, and two were accompanied by a family member who provided insights – one a mother who had previously completed TB treatment twice and another a sister. Participant characteristics are provided in Table 1.
Table 1.
Participant characteristics
| Variable | N (%) |
|---|---|
| Age (average years, range) | 29.7 (23-40) |
| Male | 4 (57.1) |
| Female | 3 (42.8) |
| Months in treatment (average months, range) | 1.57 (1-3) |
| Years having a personal telephone (average years, range) | 8.57 (1-16) |
| Regular Access to the Internet | 7 (100) |
| Access to Internet that is not through phone | 2 (28.6%) |
| Mobile application use | |
| Regular basis | 5 (71.4) |
| On occation | 1 (14.3) |
| Never | 1 (14.3) |
| Nonsmoker | 7 (100) |
Procedures
The following steps were used: 1) focus group discussions; 2) introduction to the beta app and its current functionalities; 3) instruction on how to interact and use the app and test strips at home; and 4) follow-up sessions to discuss experiences and recommendations (Figure 2). Discussions were conducted in a private room at the hospital, lasted for 45-60 minutes, were audio recorded and were led by the lead researcher and a qualitative researcher. On arrival the purpose of the sessions were reviewed and ground rules established. Informed consent was obtained and participants were asked to complete a short questionnaire on demographics and phone use.
Figure 2.
Timeline and participants
A semi-structured topic guide was used to promote discussion of experiences, challenges, and needs during treatment and the content and functionality of the app and the drug metabolite test. Questions covered topics related to types of questions they had after diagnosis and starting/during treatment, side effects experienced, what was helpful to take the medication, and barriers and difficulties to continuing treatment. The beta app and test strips were demonstrated, and feedback was solicited regarding initial impressions. Instructions were provided for field testing the tools for 5-10 days and returning for follow-up sessions. Participants were asked to use and review all components of the app and complete the metabolite test daily. During the follow-up sessions participants were asked to describe what worked well, challenges or problems experienced during field testing, potential barriers, and recommendations.
A sample size of 2-3 focus groups has been found to capture about 80% of themes on a topic using a semi-structured guide.19 Three focus groups were conducted with 2-4 participants (1 initial and 2 following field testing with the same participants), seven individual interviews with participants who canceled the day of, were unable to attend the scheduled sessions, or were recruited later in the recruitment period. Three returned for a second round of follow-up session with additional recommendations for a total of 221 minutes of recording for the initial (pre-field testing) sessions and 319 minutes of recording of follow-up (post-field testing) sessions. The interviewers also took notes and summarized main themes from the sessions immediately following. Participants were given the USD equivalent of $20 to participate in each session.
Theoretical Framework
We used the Information Motivation Behaviors Skills model (IMB) to guide evaluation and organize our findings.17,20 The IMB model asserts that to effectively self-manage treatment appropriate information about the disease, treatment, personal and social motivation, and adherence behavioral skills to achieve sustained adherence behavior and targeted health outcomes are needed. Each construct can work independently of each other to affect behavior change but use together increases the likelihood of success.20 Moderating factors such as culture, class, economics, environment, and life circumstances can influence and vary the specific informational, motivational, and skill factors thus requiring inquiry in to patient populations and the settings of the planned intervention. Although the framework provides general constructs, it does not provide the specific content needed or end user preferences.
Data analysis
Audio recordings of the discussions were transcribed verbatim by a local, native Spanish speaking transcriptionist. We used an iterative thematic approach to analyze the data in its original language.21 First, three of the bilingual members of the research team (SI, LL, YR) independently read the transcripts and took notes. We then held collaborative coding sessions to discuss significance of transcript excerpts and developed an initial coding scheme. Qualitative data analysis software (Dedoose) was used for data management and coding. Reliability was enhanced by double coding and comparing a subset of transcripts and by using training session (a function in Dedoose) to assess and maintain inter-rater reliability. These ‘tests’ provide inter-rater reliability to aid in training and further discussion of code application. Where discrepancies occurred consensus was negotiated. Main themes and recommendations were compiled to inform design iteration and guide programmers. We translated exemplar Spanish quotes to English after coding for final presentation.
RESULTS
Participants expressed general enthusiasm towards receiving support during treatment through a mobile app. Within the broad themes of moderating factors, informational needs, motivation, and behavior skills and recommendations for app and test strip refinement, a number of sub-themes emerged. Salient priority areas focused on challenges from delayed diagnosis, need for reliable non-conflicting information, preventing transmission, and identifying strategies to maintain medication adherence and minimize side effects. Recommendations to improve the app included simplifying the reporting process, making the homepage a calendar with prior days reported highlighted to provide a quick view of history, improving readability of the information/education and adding interactivity with other participants and more detailed instructions. Discussion regarding the metabolite test focused on improving instructions, clarifying the meaning of results, simplifying the test, and improving the photo upload process. The following describes the results in more detail with illustrative examples from participant comments (Table 2).
Table 2.
Informational, Motivational, and Behavior skills priority areas
| Information needs |
|
| Motivation needs |
|
| Behavior skills |
|
1. Themes related to Information, Motivation and Behavioral Skills needs Moderating factors.
All but one participant experienced extended delays for diagnosis of up to six months, multiple rounds of misdiagnoses and courses of antibiotics, and having to travel further from home to other healthcare facilities for services or specialists before being diagnosed with TB and starting treatment. The participant who was rapidly diagnosed had a mother who was a nurse and suspected that it was TB leading to quick testing and early diagnosis. There was consensus that the diagnostic process should be improved, and more awareness of TB symptoms was needed across all health care facility types to increase the speed in which people are diagnosed and initiate treatment. Emotions expressed included relief once the correct diagnosis was made and the appropriate treatment was started.
“Personally, for them to diagnose me I felt relieved because they started the medication and it was immediate that I felt better. Because to take 2 or 3 months of antibiotics for other things and continue feeling bad and lose weight. Everyone told me how skinny I was!” [01_pre_FG1_P1]
Other emotions expressed included fear of the disease or potential diagnosis of other diseases, anxiety, depression and isolation, and not being able to tell others about the disease citing stigma and public misconceptions.
“When you start, you are faced with not knowing what the disease is, you do not have much knowledge, the fear of not knowing if it is something serious or chronic, if it has a cure. It scares you that the treatment is long and the amount of pills. If you're not used to medication, it's very shocking.” [09_pre_IDI_F]
“On top of everything what they mostly first say, because what it first appears is that it comes from AIDS, you see, therefore they always ask [if you have HIV]…”
“In the case of my son he ended up losing his job, it was under the table [work] but he worked there for 5 years. He suffered discrimination from the others because not knowing they would say “you were contaminated by your mom the ‘tuberculosa’”. Stuff like this because the people didn’t know.” [02_post_FG2_P1]
All participants reported being deeply impacted by their diagnosis and experiencing lifestyle changes such as having to stop work, school, or usual activities. The desire to return to normal/pre-diagnosis activities as quickly as possible was expressed.
“I train in my house until I can return to train with my group of friends and I am waiting the possibility to start to work because I am a person who studies, works, this was my life.” [16_pre_IDI_M]
A general lack of knowledge and / or precaution about the disease was observed.
“Something I noticed, although I don’t know if I know that much about the disease but I investigated a lot, was as I look at other people who could have tuberculosis they do not know how to take care of themselves or be careful. For example, I saw a lot of people who started to cough and did not cover their mouth, or were coughing and not using a mask, or were coughing and next to them was their family and I don’t know if they realize that they could be spreading it [TB]. This is a barrier, people’s knowledge.” [09_pre_IDI_F]
While continued stigma surrounding TB was acknowledged, participants agreed that there has been a cultural shift towards being more respectful and open about discussing TB. There was a sentiment that the government should provide more support to patients throughout treatment.
Information needs.
Participants reported having many questions after diagnosis and treatment initiation and a need for reliable information about the disease and what to expect during treatment. Questions reflected their uncertainty about what to expect and how to go about their daily lives such as work, diet and exercise.
“I would like to know what it is [TB] and what is going to happen to me.” [01_pre_FG_P2]
“When you realize it so much has happened, the worst. At first your whole head is filled with everything bad, “what do I do?” and many things cross your mind.” [01_pre_FG_P4]
“I have questions, for example, can I do sports or not, can do exercise in the house, these things.” [01_pre_FG_P3]
There was a desire to know how they were infected in the first place.
“…I ask myself, when could I have got it [TB]. I had an acquaintance who had TB, could say a ‘cousin’ with TB, but many years ago and I did not see him often, it’s the only person I know…because the Internet says that you have to live with the person a certain number of hours to be infected.” [03_post_IDI_F]
A predominant concern was how to prevent the spread of the disease to others, in particular, family members. Similarly, there was uncertainty about when to discontinue mask use or return to work/school.
“What worried me the most was spreading it, because I have two little girls and I was looking for everything online. The doctor told me to see a pediatrician, he did not tell me if I could kiss them, if I could raise then, if eating with them could spread it, no information at all. The only thing that you are told is the procedure, that you take them to the pediatrician, that they get screened, you are not told be careful with this thing [TB].” [01_pre_FG1_P4]
The Internet was reported as the main information source with the caveat that conflicting information was often identified leaving uncertainty.
“All the Information I have is from the Internet.” [01_pre_FG1_P4]
“There are many web pages where one says one thing and another says something different.” [01_pre_FG1_P3]
Conflicting or confusing information was also noted in the healthcare settings.
“They told him here that if his sputum [test] was good he could take off the mask and can’t work for one month. The other doctor said for two months and after the two months we will see if he can return to work.” [02_post_FG2_P2]
“…it made me wonder, when I was in the hospital, in the same room there was a boy with TB and he didn’t have a mask.” [05_pre_IDI_M]
A common information need was to understand potential side effects and how to manage them. Stomach problems (e.g., pain and nausea) was reported as one of the most common and hardest side effects to deal with. Other side effects experienced included confusion, dizziness and feeling melancholy, particularly at the start of treatment. Taking a gastric protection medication was noted to be helpful but an out-of-pocket expense. Participants reported not knowing that drinking alcohol should not be consumed while on TB treatment.
“he walked halfway out of it, lets just say, he left the house to go to the doctor and said “where am I going?”, he didn’t know, he was a bit confused and lost. But after a week he adjusted perfectly, the body adjusted to the medication and he was fine.” [01_FG1_P2]
“At first I became a little melancholic, I did not expect it. But I took the medication. The first week I felt really bad, lousy.” [05_pre_IDI_M]
Two participants indicated they experienced no side effects while others reported that the side effects improved over time.
“I did great with the treatment. The truth is it took away the cough and I never felt sick.” [09_pre_IDI_F]
“The rifampicin made me sick, I felt nauseous. The first week I said, “I don’t know if I will be able to endure this medication.” But it passed now that I am taking everything with omeprazole. When I started taking omeprazole all of the nausea and dizziness went away. Now what I have is my body itching.” [05_pre_IDI_M]
“At first I had to take vitamins. The exhaustion, the body was different until you get used to it [medication]. Also, I didn’t eat much and now I eat everything, I grab whatever. The pills leave you without a sense of taste of anything and now I have a sense of taste and smells. Many things change and now, thank God, much better. I have more strength, I get up early, before I slept a lot.” [01_pre_FGI_P3]
Motivation.
Personal motivators to adhere to treatment were responding to treatment after feeling extremely ill before diagnosis, preventing further spread of the disease to others, and taking personal responsibility for taking the treatment.
“Maybe the rest of the world doesn’t understand it this way, but I took my treatment and felt great. At the beginning when I didn’t know that I had the disease [TB] I was sick, I felt that I was dying.” [18_pre_IDI_M]
“I was afraid that I was contagious to others, the doctors who I saw told me that I was not contagious because I did not have a cough with blood and this is the way it is contagious. From the beginning I used a mask because I knew it was contagious and I was trying not to infect others. It was a concern to infect my family, students or anyone else.” [09_pre_IDI_F]
“…you have to take it on yourself and look at it as it is – I am in treatment and I have to get my act together.” [01_pre_FG1_P4]
Participants emphasized the importance of social motivation during the treatment process to combat isolation. Overall the consensus was that social support from family and others experiencing the same situation is important and could be added as a support group functionality to the intervention. One participant strongly recommended getting a pet to combat the social isolation.
“Yes, this [social support function] would be good to ask “how are you doing? Everything good? You take your pills?” Like reminders because to motivate is also good, we all have the same disease.” [01_pre_FGI_P2]
“…you could say, “look this happened to me did it happen to you?” like you could make a connection and through the application become friends. Therefore you have someone else to say, “no, continue, do not abandon [treatment]” to strengthen and accompany the people who are alone.” [14_post_IDI_M]
“…the best therapy for a person who feels alone are animals, a dog, a cat.” [17_pre_IDI_M]
Participants had a desire to offer encouraging/motivational messages to others who would be starting treatment. Suggested motivational message emphasized the need to ask questions, take charge, and to not miss any doses.
“I would tell people starting with this disease that they come in without fear, without shame to ask everything. They [health care personnel] don’t lie, they will tell you everything, ask and do not feel guilty. Take charge and do the treatment. There is a cure, because if there wasn’t a cure that would be another thing.” [08_post_IDI_M]
“Need to make aware that you can’t skip [taking the medication] even on day because the bacteria become stronger and then you will have to start over, from zero. I believe it is very important to clarify and say constantly to the patient, “if you stop the medication you will not be cured and after you will have to start from zero.” No one wants to start from zero…for example you can add this to the application…” [03_post_IDI_F]
Adherence behavior skills.
Skills needed to adhere to treatment included being able to get questions answered, communicate needs to providers or loved ones, adapt strategies to reduce side effects and integrate treatment into daily life. Participants sought information, in addition to what they received from healthcare professionals, from the Internet or family members who had completed TB treatment. Strategies to integrate treatment into their daily routine included reminder alarms and taking medication first thing in the morning to avoid missing doses.
“it would be good to have [reminders] because it is a disease that needs care and attention.” [01_pre_FGI_P3]
“…the application is good because it makes you participate and remember the treatment that you are living, to be conscious that you are sick, and that you are doing the treatment and you have to complete it to be cured.” [03_post_IDI_F]
Strategies used to manage side effects of the treatment (e.g., upset stomach and dizziness) included using a gastric protector and taking the medication with food.
"I take everything [pills] with omeprazol, before I was not. When I started taking omeprazol all my nausea and dizziness went away.” [05_pre_IDI_M]
“…for example if I take it [medication] without breakfast I feel really bad, even get a little dizzy but after I eat something, I tolerate it good. I take it [medication] with coffee and milk but fasting it is very hard.” [07_post_FGI_P1]
Ongoing Adherence Behavior.
Factors described as influencing ongoing adherence included pill burden, feeling better after initiating treatment, forgetfulness, and out-of-pocket costs of other medication or vitamins used for side effect management.
“There are people who stop their medicine because they [the health system] don’t provide the necessary gastric protectors or vitamins, which are an added expense. Some people cannot buy them…” [07_post_FGI_P2]
An example of a strategy reported to combat pill burden or side effects was altering the medication regimes, such as, taking the medication three times per day rather than all pills at once. One participant noted the temptation to stop adhering to treatment once symptoms of TB started disappearing or once a sputum test comes back negative.
Health Outcomes.
Since participants had been in treatment for approximately 1-3 months, they were able to describe outcomes of maintaining adherence behavior over time. Health outcomes described included weight gain, cessation of cough, and negative sputum results. The negative sputum results were recognized as meaning that the mask was no longer required and patients were able to return to school or work. Another health outcome reported was smoking cessation.
“I am doing quite well with the treatment and one good thing about the treatment was it required me to quit smoking.” [09_pre_IDI_F]
2. Themes related to what to keep, improve, and add to the intervention
Table 3 outlines participant recommendations for the app and test strips. The following provides more detail with illustrative examples.
Table 3.
What worked, needs improvement, and could be added to improve app and test strip intervention
| Application | |
|---|---|
| What worked / liked / keep |
|
| Make functional |
|
| How to improve |
|
| What to add |
|
| Test strip | |
| What worked |
|
| Issues/ Questions |
|
| What to improve |
|
| Add |
|
|
Application
To keep or make functional.
Participants reported that the app was easy to use and endorsed the utility and convenience offered by remote access through the app to healthcare professional support, reliable information about the disease and treatment, and a plan for visualization of tracking of treatment progress.
“…if you didn’t know where to look for information, this way you have it at hand [in application] and you do not have to go look for it in books or elsewhere.” [08_post_IDI_M]
“…to have an application that helps you, informs you, and also explains the symptoms is very good” [10_pre_IDI_F]
Although the interactive communication function was a static image as an exemplar, its utility was endorsed.
“This [messaging function] would be great, this I would like. I saw “Hello, how are you? and I thought “Ah! Hi, you are there!”.” [08_post_IDI_M]
Being able to view changes over time during treatment was noted as potentially helpful.
“…headaches, dizziness, nausea, feeling bad [medication side effects]. It would be nice to know if they are going away.” [09_pre_IDI_F]
“Yes, it is true that it [the medication] causes effects in everyone…I see that I noted here [in the app] that I had nausea.” [08_post_IDI_M]
The language used throughout the app was recommended to be direct and concise and all but one participant suggested an informal over formal tone. The icons for each function were reported as clear and understandable.
“I thought it [the app] was quite easy for whatever age. Yes, because the questions were easy and there were not many…and more or less every icon is understandable. This one should be notifications, messages, information - this is always the icon for this [information].” [14_post_IDI_M]
"The envelope and pencil [icons] are understandable. Yes, there are people who may not understand but once they go into [the function] they will understand.” [09_pre_IDI_F]
The exemplar functions of notes, messaging, and a ‘countdown’ clock (display of days left in treatment) were recommended to move forward with making functional. The notes function was reported as a way to maintain a diary about their treatment course and any situation that might arise.
“The app I would say is very beautiful but what happened is I wanted to use ‘my notes’ and it did not save…notes, for example, ‘today was my fifth day’… ‘today was my first day taking the test’… ‘I am starting to see how I am doing’…but when I wanted to review the notes they didn’t save.” [08_post_IDI_M]
“What is already there [functionalities] should be made functional.” [06_post_FGI_P1]
To modify or improve.
Areas identified to improve included the self-reporting survey flow, visualization of treatment progress, image upload speed, verification of upload, app layout, app icon image, and the format of the information/education. The current self-reporting survey flow was noted as separated and could be simplified by allowing questions to be answered at one time without returning to the home page or combining the reporting with the metabolite test photo upload, as illustrated in the following quotes.
“When I think about it it gave me the impression that the information about taking the medication is very separate from taking the photo [of test]. You have to take a photo and leave and return to enter the photo.” [03_post_IDI_F]
“…for example, I take the medication in the morning, but I report [do survey] when I did the test. At 15:30 I do the test and upload everything.” [08_post_IDI_M]
“There you could with the photo, when you upload the photo, you would have to indicate the time you took it [the medication].” [14_post_IDI_M]
The progress/history page was an exemplar and not yet connected to the actual database to return reported data. Participants wanted to be able to see the photos they uploaded and survey results.
“It would be good if you clicked on the day you did the test and the photo appears and the survey results.” [03_post_IDI_F]
Uploading the test strip image took longer than expected or there was uncertainty if the image was uploaded. Possible suggested causes included poor Internet connection or the photo size. Converting to a native app was suggested as a possible fix to this issue.
“For me it [the app] ran very bad. It was likely because of the Internet.” [02_post_FGI]
“It might be that with another phone it might use more pixels, you take the same picture and it takes longer [to upload]. It is an issue of technology…” [14_post_IDI_M]
“…it seems to me that you make it an app because the [web] page is very slow.” [02_post_FGI]
Overall participants reported that the images/icons within the app were appropriate but the size of the text could be reduced. The look of the app was considered basic and could be improved by better ‘catching the attention’ of users. It was suggested that the app icon that saves to the phone’s home screen be changed and the ‘TB’ abbreviation in the name removed to reduce fear or hesitation with its use. Suggestions for the icon included a figure of a doctor or consultant rather than the university symbol.
“Another recommendation is to change the name [of app]. A lot of people do not know what TB means…it needs to be a word that is not impactful or make you feel bad. For example, tuberculosis sounds strong and people are fearful to find out they have tuberculosis.” [08_post_IDI_M]
The information page could be improved by adding videos or links to reliable information sources and the option to select and expand the information to improve readability.
“…with a video it could be better understood than written form. This [adding video] would be great.” [06_post_FGI_P1]
“In the frequently asked questions part, it would be good to have a list and be able to select what you want. Click and be able to choose, because all of the Information is shown at once.” [06_post_FGI_P2]
Messages that were not translated, remained in English, were identified during field testing.
“…I was going to respond ‘no’ to the question and a question appeared in English. I think there are people here who do not know English.” [13_post_IDI_M]
To add.
Recommended additions to improve customization and adherences support included a communication function with other patients, calendar view as home page, reminders, adding headache and dizziness to the list of the possible medication side effects, list of centers ‘near me’ and a provider progress note. An option to communicate with other patients in treatment was suggested as a potential highly valuable support tool by several participants. WhatsApp was reported as the best option for communication because of its common use and familiarity.
“…everyone operates with WhatsApp groups. You could put together a WhatsApp group to encourage conversations of people with tuberculosis to be able to consult and clear up doubts. With the help of everyone one can come out ahead and help in many ways…to send messages to see how the other is doing or how they feel will give them confidence to confide in one another…” [17_pre_IDI_M]
“The application can provide information and patients can communicate using WhatsApp because there are people who do not have Internet all day and will not be able to communicate but with WhatsApp they are connected 24 hours a day and are in contact with everyone.” [17_pre_IDI_M]
A calendar view of medication tracking as a homepage was recommended to provide a quick view of self-reported history and progress.
“It would also be nice to have a type of calendar with 6 months [duration of treatment].” [01_pre_FGI_P2]
“…the app is missing a calendar, a good way to see progress, when I started.” [02_post_FGI]
“For me I would like the calendar directly [when enter app]. This would catch your attention and you can mark it… For me the ideal would be the calendar. It seems super important. If a person is not using the calendar or not taking the pills there would appear a red mark that catches your attention that you forgot.” [07_post_FGI_P1]
Other ways to improve the app included adding provider progress notes and a list of centers ‘near me’ with the types of services they offer.
“There are lots of tests and follow-up visits. For this reason, it would be nice if the application had a list of the centers that treat tuberculosis because for us we live far away but we can come here [pulmonary reference hospital] because we have a car.” [07_post_FGI_P2]
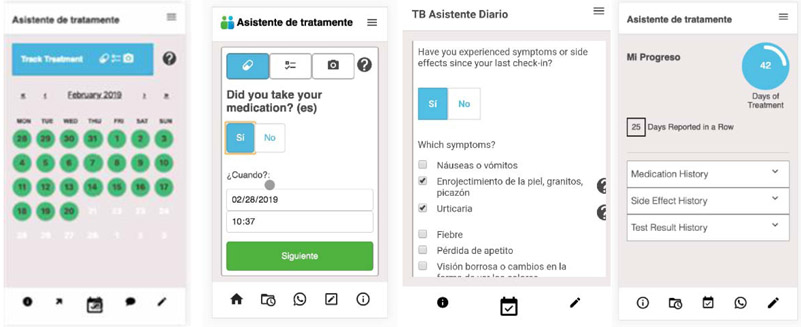
Based on these findings mock of changes were developed (Figure 3).
Figure 3.
Responses to requests to add calendar and simplified reporting flow
Urine metabolite test (direct adherence test)
Overall the test was reported as easy to conduct with some inconveniences with frequency and testing procedure. The test was considered more for the doctors than the patient but could help in particular those who live far from a healthcare center. Questions were raised regarding where the images go, who reviews them, what the test results mean, and how best to conduct the test and take the photo. As previously noted, some participants experienced slow image upload and uncertainty if the image was recorded.
To improve.
Recommendations to improve the test and the associated within app functionality included simplifying the test (e.g., like a pregnancy test) and clarifying the instructions for how to take the test and the photo of the test.
“…for me it's good, but it is a little uncomfortable to do every day, you have to clean it [cup for urine]. It would be good if the test was like a pregnancy test, even if it was a smaller version.” [06_post_FGI_P1]
“What does it [instructions] mean take picture ‘on a solid color background’? That there is contrast?” [04_post_IDI_F]
“I don’t think you use a flash because it can alter whatever type of picture, alters whatever thing. It could even make the color lighter when it is not light.” [04_post_IDI_F]
The results were noted to get better when waiting more time.
“I realized that it [test strip] functions much better when you wait more time” [06_post_FGI_P1]
One participant wanted to see the images of other patients to compare his results with those of others.
“These images are mine [photos of test]. I wanted to see the images of others to be able to see and compare the color and know how they are doing in treatment. Also, I to be able to ask everyone, start a dialog and be able to build friendships.” [08_post_IDI_M]
To add.
The metabolite test page in the app needs to include clearer instructions on how to conduct the test as well as a description of the meaning of the results.
“These are instructions on how to take the picture but not how to take the test.” [04_post_IDI_F]
“…it would be nice for the photo function to have instructions on how to take the test and tells you to wait 5 minutes if the photo does not detect the color, that it [color change] is not immediate, so that the person knows to wait.” [04_post_IDI_4]
A confirmation for the test photo that includes the results of the test should be added.
“Of course, it would be nice if you could open the photo [of test strip] and get an answer if the color is good or not.” [02_post_FGI]
DISCUSSION
Our collaborative inter-disciplinary team is developing tools to customize patient support to increase adherence to drug-susceptible TB treatment using a smartphone app and a direct drug metabolite test reengineered for home use. This iterative process is being conducted with and for individuals in active TB treatment in settings where self-administered therapy is the usual care. Our interdisciplinary team consists of members from the fields of nursing, bioengineering, medicine (pulmonology/tuberculosis management), social and behavioral sciences, human-center design, and computer science to bring unique and integrated perspectives to the challenges of developing a user-centered scalable platform to be used as a potential tool to improve treatment outcomes. This theory guided intervention fits well with the priorities of the WHO and others to promote new patient-centered technology to improve TB treatment outcomes.3,4 Review of past research showed that there is evidence to support that technologies have reduced the burden of the management of TB on healthcare systems allowing systems to deliver more efficient prevention, treatment, and management support.22 However, there remains limited evidence on the impact of digital health technologies on TB treatment outcomes.22,23 Furthermore, while consideration for design features for mobile health apps has been given for chronic conditions, little is known about user preferences for apps to deliver TB treatment support.8 An exploratory research approach using focus groups, field testing of beta prototypes and return discussions was therefore conducted and led to identifying a number of recommendations for further refinement.
In this paper, we identify informational, motivational, and behavior skills needs to incorporate in the content and app functionality preferences. For example, reliable, accurate and personalized information on what to expect during treatment, support from healthcare providers and peers in treatment and skills to maintain adherence, manage side-effects, and prevent transmission to others. We also identify challenges to be diagnosed and potential moderating factors to treatment adherence. It is important to note that our findings are in reference to an early prototype design with functioning and exemplar features and field testing for multiple days to identify preference, acceptability, issues, and attractiveness of features. Preferences and concerns might vary across populations and may depend on other factors such as the framing of the focus group questions and resulting discussions.24 Therefore, we plan to follow up with new patients in a pilot study using a functional version of the app for a longer period and starting at treatment initiation to further refine the intervention prior to testing in a fully powered efficacy trial. Nonetheless, this study provides important first steps in informing the design features relevant for mobile self-administered therapy for active TB.
A major theme to emerge from the discussions was around finding credible or reliable answers to numerous initial questions about TB, its treatment, and what to expect, and, seemingly most important, how to prevent its spread to others. Although participants were successful in obtaining answers to their questions, predominantly from the Internet, they were concerned with identifying conflicting information both from online and occasionally from healthcare professionals. With the infinite access to information on the Internet and social media it is difficult to assess quality which includes dimensions of credibility: trustworthiness (or belief in the integrity of the provider) and expertise (perceived competence of the provider).25 The information within the app was based on current sources from the WHO and the national TB program in Argentina, however, credibility could be improved by adding clearer references and direct links to the sources. The intervention will include a healthcare provider/treatment supporter who is a member of a TB team to provide the necessary expertise. In a systematic review of adherence related apps only two were identified that provided disease-related or medication information.26 These researchers recommend that developers of adherences apps consider features that provide therapy-related information. Thus, one focus area for this app was to provide access to accurate, up-to-date, and relevant information about the disease and its treatment.
Our findings emphasized the importance of including self-management features in the app. Participants were actively engaged in tracking their treatment progress and requested additional features to visualize progress ( e.g., calendar view of their progress and make days in treatment a working function). There was interest in medication reminders within the app even though it was recognized that all phone types had alarms that could be used as reminders. Self-management of treatment, symptoms or side-effects is a central strategy for chronic disease management that is commonly incorporated into technology-based behavior change interventions.27-33 Mobile apps can provide a more convenient way to accurately capture progress, then other methods. The potential effectiveness of digital technologies for self-monitoring is based on the proposed functionality for more comprehensive and precise measurement, allowing for more personalized feedback.7 Surprisingly, in a systematic review of adherence related apps none were identified to have features for side-effect self-management.26 Researchers recommended including features to help patients manage side-effects. Additionally, symptom tracking can assist with identification of concerning symptoms and timely linking with appropriate services.31
Another recommendation was to foster a sense of connection, community and support, both from health professionals and peers. In this study participants were enthusiastic about an option to conveniently communication with healthcare team members to ask questions and resolve doubts or issues as they arise. Communicating with a healthcare professional through an app has the potential to significantly expand access to professional support and reflects ongoing initiatives to promote remote health care delivery and monitoring.34 Only technologies that improved communication with patients demonstrated benefits in improving disease control.35 Additionally, the participants emphasized the importance of social support through family or friends or interacting with others in treatment. Similarly, researchers have found acceptance and strong desire to interact with peers and have topical discussions for disease management support.36,37 Furthermore, social motivation is recognized as an integral component of the IMB framework.17,20
Finally, the app interface should be simple, easy to use, and easy to understand.7,38,39 The participants wanted to quickly input data and see their history and progress in treatment. They were more concerned about access to accurate information and functionality performance and less with the appearance or changing or adding pictures. Providing clear instructions, improving the performance of image upload and verification of successful upload will be addressed based on recommendations. The participants recognized the value of the urine testing but requested increased speed in results or stronger color change to help determine when the testing was complete. Feedback about text size and icons highlight the need for adaptive design to address tailored preferences.
Unlike other technology or app development research, in this study, the issue of privacy was not mentioned. This may be due to the app already having an established password protected login. Additionally, many phones require a pin or fingerprint to enter the phone and will timeout after a period of inactivity. However, participants did raise issues related to this, specifically, the recommendation to remove the abbreviation of TB in the app name. This could help make less obvious to an observer that a user is interacting with a TB related tool since there is recognized stigma associated with TB.40,41 The use of symbol-based, nondescript logos and app icons, use of ‘ambiguous’ terminology for the name of the app, avoiding use of client-identifying data in the interface, and ‘subtle’ language and nomenclature has been recommended to help with this issue of discreet design.42
Unfortunately, the findings of diagnostic delays, misdiagnosis, and multiple healthcare provider visits before being diagnosed with TB are consistent with findings in the literature.15,43-45 In fact, the total mean diagnostic delay in a region of Argentina was identified to be 92 days.45 Such moderating factors can have an impact on treatment initiation and overall experiences with healthcare systems. Participant frustrations with diagnostic delays and with the availability of healthcare services in their areas is not a new finding and has been identified in the same setting.15 Similarly, in other countries a recognized barrier to care is that some clinics offer certain services thus requiring that the individual travel further distances for necessary services.46,47 Historically, access to health care services has been referred to as an attribute of the supply of services, both in terms of availability and capacity to produce the services that meet the needs of the population including considerations of geographic distance and cost.48 Adding a recommended resource of TB treatment facilities and their services could facilitate better access to health services.
Overall, the findings from this study led us to consider and plan for additional user interface needs specific to TB self-administered therapy. The feedback from the participants supported that the tools were useful and relevant, and they were eager to contribute their ideas for improvement and additional functionality. The use of low- and high-fidelity prototypes with content and design experts to guide initial programming of a functional beta app paved the way to better explore further refinement needs and recommendations with endusers rather than using hypothetical scenarios. This type of iterative development process in early stage design helps end users become engaged and willing to assist with future testing and design, solidify high level concepts, identify and solve major usability problems, and minimize cost and time for programming rewriting to design a product more closely representing the final version.49 The theoretical framework was comprehensive in guiding both the assessment and the design. The recommendations proposed here are intended to supplement the more general user interface design guidelines such as the Information Systems Research (ISR) framework to guide the design of mHealth apps.7
Limitations
Although this study provided information content and interface design and functionality, we were limited to one geographic area in Argentina. However, the reference hospital serves a large patient population including rural, and semi-urban settings and the participants were from varied areas within this large region. Sample size was small and while the sample represented a range of ages and geographic location means that the findings may not be fully transferrable to other settings. The recommendations drawn from this work should therefore be considered tentative at this stage and further work is planned to explore and enhance the transferability of our findings to broader settings. Next steps are to build a fully functional app and improve the home metabolite test and assess the extent to which the recommendations proposed impact usability, user acceptance in practice in a new subset of patients initiating treatment in a pilot RCT. We will also identify further refinement needs based on a large sample and longer study duration. Future studies will explore effect on outcomes in a larger population, other countries or settings to assess for cultural design needs.
CONCLUSION
Experiences and needs of patients in active TB treatment were identified and their recommendations summarized for the refinement of the next iteration of a mobile TB support app and drug metabolite test. Participants found the intervention overall useful, relevant, and easy to use and reported issues and further recommendations to meet their needs. They expressed priority needs of reliable information and assurance and support (both provider and peer). Many of their questions may not be readily found in the literature supporting the relevance of a treatment supporter to help answer questions and navigate challenges as they arise. We have generated new design ideas and insights to explore such as a more streamlined graphical user interface which may lead to shorter entry times and easier usability. Development of an app that meets identified information, motivation, and behavioral skill needs and preferences will maximize its utility, thereby augmenting support and optimizing outcomes. Refinements will be assessed in a pilot study with individuals initiating TB treatment.
What was known
New tools, interventions, and strategies are urgently needed to address TB which is recognized as a global health crisis
Few studies have explored mobile apps or home-based direct adherence test or have focused on patients as endusers to support TB management and improve outcomes
What this study added
Needs and specific recommendations to improve the app and the paper-based metabolite test were identified
Streamlining graphical user interface which may lead to shorter entry times and easier usability
ACKNOWLEDGEMENTS
SI is supported by the National Institute of Nursing Research through a mentored career development award K23NR017210 and a Research and Intramural Funding Program (RIFP) grant from the University of Washington. YR is funded as T32 Omics and Symptom Science Trainee. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We want to thank the participants for their time and thorough contributions to the project.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest in the research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah Iribarren, Biobehavioral Nursing and Health Informatics, University of Washington, 1959 NE Pacific Street, Seattle, WA, 98195.
Yvette Rodriguez, University of Washington, 1959 NE Pacific Street, Seattle, WA, 98195.
Lorelei Lin, University of Washington, 1959 NE Pacific Street, Seattle, WA, 98195.
Cristina Chirico, Tuberculosis Control Program of the 5th Health Region, Ministry of Health of the Province of Buenos Aires, Hospital Cetrangolo, Buenos Aires, Argentina.
Vilda Discacciati, Division of Family and Community Medicine, Hospital Italiano de Buenos Aires, Juan D. Per´on 4190, C1181ACH Buenos Aires, Argentina.
Rebecca Schnall, Columbia University, School of Nursing, Columbia University, New York, NY, USA.
George Demiris, University of Pennsylvania, Claire Fagin Hall, Rm 324, 418 Curie Blvd, Philadelphia PA 19104.
References
- 1.World Health Organization. The top 10 causes of death worldwide. Geneva, 2017. [Google Scholar]
- 2.All Party Parliamentary Group on Global TB. The price of a pandemic:counting the cost of MDR-TB, 2015.
- 3.World Health Organization. Global Plan to End TB: The Paradigm Shift 2016–2020. Geneva, Switzerland, 2015. [Google Scholar]
- 4.United Nations. Sustainable Development Goals. 2015. http://www.un.org/sustainabledevelopment/sustainable-development-goals/.
- 5.World Health Organization. Digital Health for the End TB Strategy: An Agenda for Action Geneva, Switzerland, 2015. [Google Scholar]
- 6.World Health Organization. Digital health for the End TB strategy: progress since 2015 and future perspectives. Geneva: World Health Organization, 2017. [Google Scholar]
- 7.Schnall R, Rojas M, Bakken S, et al. A user-centered model for designing consumer mobile health (mHealth) applications (apps). J Biomed Inform 2016; 60: 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iribarren SJ, Schnall R, Stone PW, Carballo-Dieguez A. Smartphone Applications to Support Tuberculosis Prevention and Treatment: Review and Evaluation. JMIR Mhealth Uhealth 2016; 4(2): e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile Phone Apps to Improve Medication Adherence: A Systematic Stepwise Process to Identify High-Quality Apps. JMIR Mhealth Uhealth 2016; 4(4): e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Lee Y, Lee S, Islam SMS, Kim SY. Toward Developing a Standardized Core Set of Outcome Measures in Mobile Health Interventions for Tuberculosis Management: Systematic Review. JMIR Mhealth Uhealth 2019; 7(2): e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiStefano MJ, Schmidt H. mHealth for Tuberculosis Treatment Adherence: A Framework to Guide Ethical Planning, Implementation, and Evaluation. Glob Health Sci Pract 2016; 4(2): 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer E TB Drug Compliance. Paper drug test and text messaging could help thwart the most deadly strains of tuberculosis. 2009. https://www.technologyreview.com/s/412203/tb-drug-compliance/. [Google Scholar]
- 13.Iribarren S, Sward K, Beck S, Pearce PF, Thurston D, & Chirico C Qualitative evaluation of an mHealth intervention to support patients with active tuberculosis: Implementation considerations. JMIR Mhealth Uhealth 2015; 3(1): e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iribarren S, Beck S, Pearce PF, et al. TextTB: A mixed method pilot study evaluating acceptance, feasibility and exploring initial efficacy of a text messaging intervention to support TB treatment adherence. Tuberculosis Research and Treatment 2013; Volume 2013, Article ID 349394(Article ID 349394): 12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iribarren SJ, Rubinstein F, Discacciati V, Pearce PF. Listening to those at the frontline: Patient and healthcare personnel perspectives on tuberculosis treatment barriers and facilitators in high TB burden regions of Argentina. Tuberculosis Research and Treatment 2014: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iribarren S, Sward K, Beck S, Pearce PF, Thurston D, Chirico C. Qualitative Evaluation of a Text Messaging Intervention to Support Patients With Active Tuberculosis: Implementation Considerations. JMIR Mhealth Uhealth 2015; 3(1): e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iribarren SJ, Beck S, Pearce PF, et al. mHealth intervention development to support patients with active tuberculosis. Journal of Mobile Technology in Medicine 2014; 3(2): 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd JM, Louise J, Cramp C, Grivell RM, Moran LJ, Deussen AR. Evaluation of a smartphone nutrition and physical activity application to provide lifestyle advice to pregnant women: The SNAPP randomised trial. Matern Child Nutr 2018; 14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guest G, Namey E, Taylor J, Eley N & McKenna K. Comparing focus groups and individual interviews: findings from a randomized study. International Journal of Social Research Methodology 2017; 20(6): 693–708. [Google Scholar]
- 20.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Current HIV/AIDS reports 2008; 5(4): 193–203. [DOI] [PubMed] [Google Scholar]
- 21.Patton M Qualitative research & evaluation methods: Integrating theory and practice (4th ed.). 4th ed. Thousand Oaks, CA: SAGE Publications, Inc; ; 2015. [Google Scholar]
- 22.Devi BR, Syed-Abdul S, Kumar A, et al. mHealth: An updated systematic review with a focus on HIV/AIDS and tuberculosis long term management using mobile phones. Comput Methods Programs Biomed 2015; 122(2): 257–65. [DOI] [PubMed] [Google Scholar]
- 23.Ngwatu BK, Nsengiyumva NP, Oxlade O, et al. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J 2018; 51(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan N, Swendeman D, Comulada WS, Estrin D, Rotheram-Borus MJ. Identifying preferences for mobile health applications for self-monitoring and self-management: focus group findings from HIV-positive persons and young mothers. International journal of medical informatics 2013; 82(4): e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger M, Flanagin A,. Digital media, youth, and credibility. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 26.Ali EE, Teo AKS, Goh SXL, Chew L, Yap KY. MedAd-AppQ: A quality assessment tool for medication adherence apps on iOS and android platforms. Res Social Adm Pharm 2018; 14(12): 1125–33. [DOI] [PubMed] [Google Scholar]
- 27.Bailey SC, Belter LT, Pandit AU, Carpenter DM, Carlos E, Wolf MS. The availability, functionality, and quality of mobile applications supporting medication self-management. Journal of the American Medical Informatics Association : JAMIA 2014; 21(3): 542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YK, Demiris G, Lin SY, et al. Smartphone Applications to Support Sleep Self-Management: Review and Evaluation. J Clin Sleep Med 2018; 14(10): 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. The Cochrane database of systematic reviews 2012; 12: CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. Journal of the American Medical Informatics Association : JAMIA 2017; 24(3): 619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iribarren S, Siegel K, Hirshfield S, et al. Self-Management Strategies for Coping with Adverse Symptoms in Persons Living with HIV with HIV Associated Non-AIDS Conditions. AIDS and behavior 2018; 22(1): 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnall R, Mosley JP, Iribarren SJ, Bakken S, Carballo-Dieguez A, Brown Iii W. Comparison of a User-Centered Design, Self-Management App to Existing mHealth Apps for Persons Living With HIV. JMIR Mhealth Uhealth 2015; 3(3): e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Yao X, Vespasiani G, et al. Mobile App-Based Interventions to Support Diabetes Self-Management: A Systematic Review of Randomized Controlled Trials to Identify Functions Associated with Glycemic Efficacy. JMIR Mhealth Uhealth 2017; 5(3): e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. Journal of acquired immune deficiency syndromes 2006; 43 Suppl 1: S127–33. [DOI] [PubMed] [Google Scholar]
- 35.Marcolino MS, Oliveira JAQ, D'Agostino M, Ribeiro AL, Alkmim MBM, Novillo-Ortiz D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth 2018; 6(1): e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whittaker R, Maddison R, McRobbie H, et al. A multimedia mobile phone-based youth smoking cessation intervention: findings from content development and piloting studies. Journal of medical Internet research 2008; 10(5): e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henwood R, Patten G, Barnett W, et al. Acceptability and use of a virtual support group for HIV-positive youth in Khayelitsha, Cape Town using the MXit social networking platform. AIDS care 2016; 28(7): 898–903. [DOI] [PubMed] [Google Scholar]
- 38.Brown W 3rd, Yen PY, Rojas M, Schnall R. Assessment of the Health IT Usability Evaluation Model (Health-ITUEM) for evaluating mobile health (mHealth) technology. J Biomed Inform 2013; 46(6): 1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. Management Information Systems 1989; 13(3): 319–40. [Google Scholar]
- 40.Gebremariam MK, Bjune GA, Frich JC. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: A qualitative study. BMC public health 2010. 10: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roba AA, Dasa TT, Weldegebreal F, et al. Tuberculosis patients are physically challenged and socially isolated: A mixed methods case-control study of Health Related Quality of Life in Eastern Ethiopia. PloS one 2018; 13(10): e0204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gkatzidou V, Hone K, Sutcliffe L, et al. User interface design for mobile-based sexual health interventions for young people: design recommendations from a qualitative study on an online Chlamydia clinical care pathway. BMC medical informatics and decision making 2015; 15: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagbakken M, Bjune GA, Frich JC. Experiences of being diagnosed with tuberculosis among immigrants in Norway--factors associated with diagnostic delay: a qualitative study. Scand J Public Health 2010. 38(3): 283–90. [DOI] [PubMed] [Google Scholar]
- 44.Sagbakken M, Frich JC, Bjune GA. Perception and management of tuberculosis symptoms in Addis Ababa, Ethiopia. Qual Health Res 2008; 18(10): 1356–66. [DOI] [PubMed] [Google Scholar]
- 45.Zerbini E, Chirico MC, Salvadores B, Amigot B, Estrada S, Algorry G. Delay in tuberculosis diagnosis and treatment in four provinces of Argentina. The International Journal of Tuberculosis and Lung Disease 2008; 12(1): 63–8. [PubMed] [Google Scholar]
- 46.Hill PC, Stevens W, Hill S, et al. Risk factors for defaulting from tuberculosis treatment: A prospective cohort study of 301 cases in the Gambia. Int J Tuberc Lung Dis 2005; 9(12): 1349–54. [PubMed] [Google Scholar]
- 47.Khan MA, Walley JD, Witter SN, Shah SK, Javeed S. Tuberculosis patient adherence to direct observation: Results of a social study in Pakistan. Health Policy Plan 2005; 20(6): 354–65. [DOI] [PubMed] [Google Scholar]
- 48.Donabedian A The Quality of Care. How Can It Be Assessed? Jama 1988; 260(12): 1743–8. [DOI] [PubMed] [Google Scholar]
- 49.Nelson SD, Del Fiol G, Hanseler H, Crouch BI, Cummins MR. Software Prototyping: A Case Report of Refining User Requirements for a Health Information Exchange Dashboard. Appl Clin Inform 2016; 7(1): 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]