Abstract
Introduction
Patient education is crucial for improving disease outcomes in atopic dermatitis (AD). This review aims to summarize evidence about the effectiveness of educational programs for parents of pediatric AD patients.
Methods
PubMed and Embase (inception to Feb 2020) were searched and randomized controlled trials (RCTs) in English were included. Risk of bias was assessed using Cochrane risk of bias tools and quality of evidence was assessed by Grading of Recommendations Assessment, Development and Evaluation (GRADE). Pooled standardized mean difference (SMD) and 95% confidence intervals (CIs) were calculated for the disease severity instrument (Scoring of Atopic Dermatitis, SCORAD) and quality of life (QoL) instruments using the random-effects model.
Results
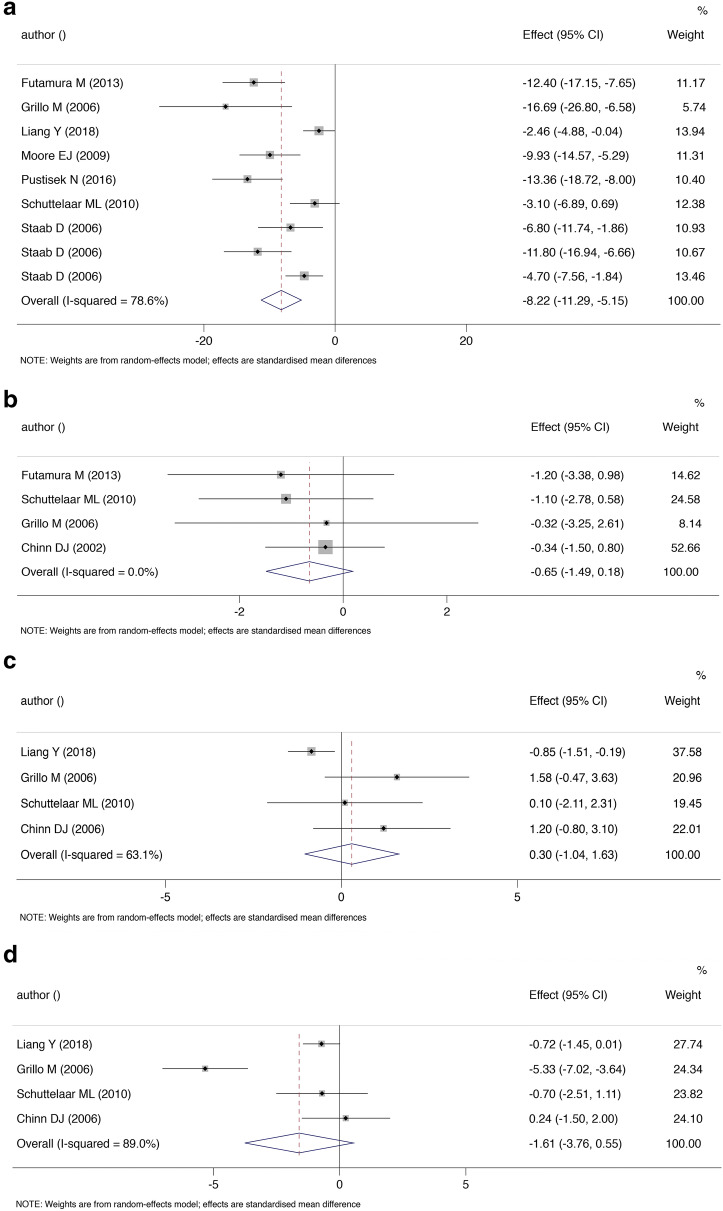
A total of 13 RCTs were included in the systematic review. The meta-analysis of SCORAD contained seven studies with a total of 1853 patients. The reduction in disease severity (SCORAD) was larger in the treatment group (SMD = − 8.22, 95% CI = − 11.29, − 5.15; P < 0.001; I2 = 78.6%). Subgroup analyses revealed that the association was modified by the frequency of sessions (P for Cochran Q < 0.01) and the duration of follow-up (P for Cochran Q < 0.01). No significant effect-modification was observed for disease severity and borderline significance was observed for session delivery (individual vs group session). The pooled effect sizes for QoL measures including Dermatitis Family Index (SMD = − 0.65, 95% CI = − 1.49, 0.18), Children’s Dermatology Life Quality Index (SMD = − 1.61, 95% CI = − 3.76, 0.55; I2= 89.0%) and Infants’ Dermatology Quality of Life Index (SMD = 0.30, 95% CI = − 1.04, 1.63; I2= 63.1%) were not significant.
Conclusions
Structured patient education is beneficial and should be implemented for the management of AD patients. However, an optimal delivery mode needs to be determined.
Electronic supplementary material
The online version of this article (10.1007/s13555-020-00365-z) contains supplementary material, which is available to authorized users.
Keywords: Atopic dermatitis, Education, Meta-analysis, Parental education, Pediatric, Systematic review
Key Summary Points
| Atopic dermatitis parental education of pediatric patients is associated with a significant reduction in disease severity and thus should be implemented into daily clinical practice. |
| Atopic dermatitis educational programs are encouraged to incorporate the following areas of focus: skincare, diet, psychological managements, and topical steroid usage. |
| Follow-up duration modifies the effect with longer follow-up showing inferior results, indicating that iterations of implementation might be promising in providing long-standing benefits. |
| The existing body of evidence was rate as very low to moderate for certainty due to risk for performance and detection bias. |
| Contamination was likely due to the single center design of the studies included, suggesting that the true effect might be even larger. |
Introduction
Atopic dermatitis (AD) is a chronic relapsing disease that affects an increasing population [1]. A significant health burden is inflicted by severe pruritus and the prolonged disease course. Studies have shown that patients with AD have compromised quality of live (QoL), a higher likelihood of anxiety and depression, and increased use of healthcare resources [2–4].
Since 1998, therapeutic patient education (TPE) has been recognized as beneficial in both health and financial terms [5]. It plays a crucial role in the management of chronic diseases including cardiovascular diseases and cancer [6–10]. Recent guidelines on AD also recommended that patient education be provided [11–13]. However, no consensus has been reached on the optimal scope, frequency, and tailoring of delivery. Meanwhile, the initiatives of incorporating digital tools as new ways of education delivery including educational video and on-line resources, have shown promising results [14, 15]. Synthesizing the current literature to better understand the role of TPE is important for decision-making by both clinical practitioners and policy-makers. The present study thus aimed to systematically review the impact of various TPE programs on the disease severity and QoL improvements in the population of pediatric AD patients.
Methods
This review was registered on PROSPERO (CRD42019129832). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Search Strategy
The MEDLINE and Embase databases were searched from inception to Feb 2020 using the following inquiry terms “atopic dermatitis” or “eczema” and “patient education” and “randomized controlled trials” and “pediatric” (Supplementary Table E1). The inquiry was restricted to human subjects in English. A manual review of references cited in existing reviews and meta-analyses was conducted to identify additional studies. Two researchers (Mutong Zhao and Yuan Liang) evaluated the retrieved articles independently using the inclusion criteria described in the subsequent section. Disagreements were resolved by a third reviewer (Chunping Shen).
Eligible Criteria
All published randomized clinical trials (RCTs) that evaluated the effects of parental education on pediatric AD patients were included. Studies published in English were included. An article was excluded when only hand eczema was evaluated as a subject of disease. Interventions that were entirely psychologic without evaluation of AD disease severity changes were excluded from the current analysis and were discussed in a previous review [18]. Studies that evaluated the educational effect of written action plans alone without a concomitant comprehensive educational program were excluded to maintain treatment homogeneity, as action plans were found to have been written at an inappropriately high reading level [19] which might induce heterogeneous interpretations without instruction. Case reports, observational studies, letters to editors and quasi-experimental studies were also excluded. Furthermore, duplicate data were excluded while the study reporting the largest number of participants was retained. Studies that only presented data in graphic form without reporting a numerical value were included in the qualitative but excluded from the quantitative analysis.
Data Extraction
Two researchers independently extracted data on the first author, year, study design, sample sizes, intervention and control treatments, outcome measures, and potential confounders including disease severity, session delivery matrices, and follow-up duration.
Quality Assessment
The risk of bias in individual studies was assessed according to the Cochrane risk of bias guidelines, which included random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of evidence, which has four levels of evidence: high, moderate, low, or very low.
Quantitative Synthesis
The effects were pooled using Stata version 12.0 (StataCorp, College Station, TX, USA). The primary outcome was the Scoring of Atopic Dermatitis (SCORAD) index at the end of the follow-up. The QoL instruments, such as Infants’ Dermatitis Quality of Life Index (IDQOL), Children’s Dermatology Life Quality Index (CDLQI) and Dermatitis Family Impact (DFI) were pooled as the secondary outcome measures. The estimates for standardized mean differences (SMDs) were pooled using the random-effects models. Heterogeneity was assessed using the I2 and the Cochran’s Q statistics and an I2 of > 50% or a P for Cochran’s Q of < 0.1 were regarded as significant. Subgroup analyses were conducted based on curriculum frequency, follow-up duration, disease severity, and delivery methods (i.e., individual vs group sessions). For sensitivity analysis, the objective measure of SCORAD was pooled. Publication bias was assessed by the visual inspection of the funnel plots. All statistical tests were two-sided, and statistical significance was defined as P < 0.05. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Search Results
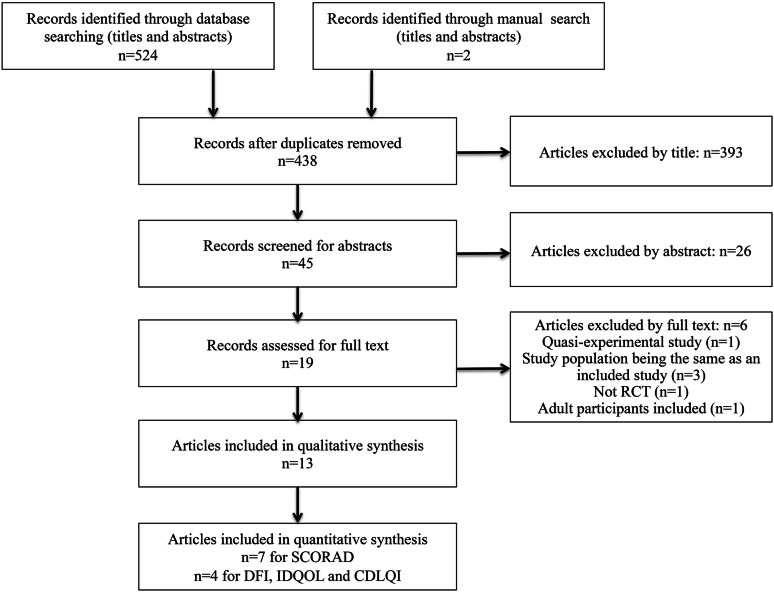
Our search generated 524 citations (Fig. 1). A total of 393 articles were excluded by title or abstract. Nineteen studies were assessed for full text and thirteen RCTs were included in the final systematic review [20–30], which involved 2632 subjects.
Fig. 1.
Flow diagram of study selection. SCORAD Scoring of Atopic Dermatitis, DFI Dermatitis Family Index, IDQOL Infants’ Dermatitis Quality of Life, CDLQI Children’s Dermatology Life Quality Index, RCT randomized controlled trial
Characteristics of Studies
Table 1 outlines the characteristics of the included studies. Pre-intervention disease severity varied across studies, e.g., five and one study exclusively included moderate to severe and mild to moderate AD patients, respectively, and four others recruited participants with unlimited disease severity. Follow-up ranged from 1 week to 24 months post-intervention. The delivery of sessions included individual consultations (n = 2) [23, 26], videotape (n = 2) [31, 32], daily text messages (n = 1) [29] and group sessions (n = 8) [20–22, 24, 25, 27, 28, 30]. Programs focused on the medical delineation of the disease, allergic components, lifestyle recommendations, stress management and practical instructions on skin care and topical drugs. The outcome assessments were divided into five elements: disease severity (n = 10); QoL (n = 10); psychological burden (n = 3); knowledge, including topical steroid phobia (n = 2); and moisturizer consumption (n = 1). Disease severity was measured using the SCORAD (n = 8) [20, 21, 23–28] and Eczema Area and Severity Index (EASI) (n = 3) [29, 31, 32]. Both patient-oriented [21–24, 27, 28, 30] and family [20–22, 24, 25, 28, 30] QoL were delineated.
Table 1.
Characteristics of the studies included in the systematic review
| References | Year | Age (years) | Follow-up | Treatment/control, no. | Treatment | Control | Disease severity | Contents | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|
| Singer [29] | 2018 | 0.3–3.8 | 42 days or until follow-up appointment | 14/16 | Daily educational text messages | Standard care | Unclear | Disease burden; precipitators; skin care | (1) EASI |
| Liang [27] | 2018 | 4–14 | 6 months | 293/249 | Four weekly lectures | Unclear | Moderate to severe | Long-term management, food allergy, psychological management, skin care | (1) SCORAD; (2) IDQOL, CDLQI; (3) Knowledge assessment questionnaire |
| Pustisek [25] | 2016 | 0.25–12 | 2 months | 64/64 | One-time lecture with written material | Standard care | Moderate to severe | Precipitators, diet, diagnosis and treatment, skin care, quality of life impacts and managements, practical session | (1) SCORAD; (2) FDLQI; (3) STAI and PPS |
| Staab [20] | 2006 | 0.25–7 | 12 months | 274/244 | Six weekly group sessions | Standard care | Moderate to severe | Basic medical information, psychological management, food allergy and nutrition, skin care | (1) SCORAD and PO-SCORAD; (2) Parents’ quality of life questionnaire; (3) Itch questionnaires JUCKKI and JUCKJU as age indicated |
| 8–12 | 102/83 | ||||||||
| 13–18 | 70/50 | ||||||||
| Futamura [21] | 2013 | 0.5–6 | 6 months | 29/30 | Two continuous days of group meeting with information booklet | Standard care and information booklet | Moderate to severe | Basic medical information, treatments and adverse effects, allergen and avoidance, skin care | (1) SCORAD and PO-SCORAD; (2) Questionnaire on scores for pruritus, sleeplessness and corticosteroid anxiety; (3) DFI |
| Grillo [28] | 2006 | 0–16 | 12 weeks | 32/29 | One-time workshop | Standard care | Unlimited | Basic medical information, precipitators, skin care, practical session | (1) Objective SCORAD; (2) DFI, CDLQI, and IDQOL |
| Shaw [23] | 2008 | 0–18 | The 1 or 3 month follow-up appointment determined by severity | 51/55 | One-time individualized education | Unclear | Unlimited | Medical treatment, skin care, allergen avoidance, pruritus reliefs, lifestyle managements | (1) SCORAD; (2) CDLQI, and IDQOL |
| Chinn [30] | 2002 | 0.5–4 | 12 weeks | 55/42 | One-time session | Unclear | Unclear | Basic medical information; treatment application; skin care | (1) CDLQI or IDQOL and DFI |
| 4–16 | 50/50 | ||||||||
| Weber [22] | 2008 | 2–16 | 24 months | 16/16 | Fortnightly support group for 6 months | Unclear | Moderate to severe | Overview of the disease and treatment followed by discussion | (1) CDLQI and DFI; (2) McGill pain questionnaire for pruritus assessment |
| Moore [26] | 2009 | < 16 | 4 weeks | 49/50 | One-time individual consultation with information booklets | Standard care | Unlimited | Basic medical information, precipitator avoidance, treatments and information booklet | (1) SCORAD |
| Schuttelaar [24] | 2010 | < 4 | 12 months | 37/34 | One-time group session or education during individual follow-up visits | Standard care | Unlimited | Basic medical information, allergies, skin care, practical session and written action plan | (1) SCORAD and objective SCORAD; (2) CDLQI or IDQOL and DFI; (3) Program satisfaction |
| 4–16 | 35/35 | ||||||||
| Saritha [31] | 2018 | 0–16 | 6 weeks | 5/5 | Video on AD | Video on a placebo topic | Unclear | Unclear | (1) Topical steroid phobia and adherence; (2) EASI |
| Park [32] | 2017 | 0.3–4.3 | 1 week | 10/11 | One-time video tape with tailored leaflet | Same video tape without tailored leaflet | Mild to moderate | Skin care and tailored ideal amount of moisturizer | (1) Moisturizer usage; (2) EASI |
EASI eczema area and severity index, SCORAD Scoring of Atopic Dermatitis, CDLQI Children’s Dermatology Life Quality Index, IDQOL Infants’ Dermatology Quality of Life Index, DLQI Dermatology Life Quality Index, PO-SCORAD Patient Oriented SCORAD; FDLQI Family Dermatitis Life Quality Index, STAI State Trait Anxiety Inventory, PPS Perceived Stress Scale, DLQI Dermatitis Life Quality Index, QoLIAD Quality of Life Index for Atopic Dermatitis, BDI Beck Depression Inventory, EQ-5D EuroQol 5-Dimension, POEM patient-oriented eczema measure, HADS-D Hospital Anxiety and Depression Score, DFI Dermatitis Family Impact, RCT randomized control trial, JUCKKI/JUCKJU Itching cognitions questionnaires
Qualitative Assessment of Studies
Supplementary Figure S1 displays the risk of bias summary. Overall, the risk for performance bias and detection bias was high. The quality of evidence was rated as moderate for SCORAD and DFI, and very low for IDQOL and CDLQI by GRADE (Supplementary Table E2). The funnel plot for publication bias of studies included in the quantitative analyses of SCORAD, DFI, IDQOL, and CDLQI was largely symmetric, Supplementary Figure S2.
Quantitative Analyses of SCORAD
A trial that failed to adequately randomize baseline characteristics, and a trial that compared two different deliveries of intervention [23, 32] were excluded to reduce heterogeneity (the reasons for exclusion in meta-analysis are summarized in Supplementary Table E3). Effect sizes for parental educational programs were pooled using SCORAD as the majority of the studies used this matrix. The final meta-analysis of SCORAD comprised seven studies and a total of 1853 patients [20, 21, 24–28]. Overall, participants in the group that received therapeutic education had a significantly greater reduction in SCORAD at the end of the follow-up (SMD − 8.22, 95% CI = − 11.29, − 5.15; P < 0.001; Fig. 2a). The heterogeneity was high and statistically significant (P for Cochran Q < 0.001, I2 = 78.6%). Sensitivity analysis revealed that the result was robust to the objective measure of SCORAD (SMD − 7.38, 95% CI = − 10.37, − 4.39; P < 0.001) [20, 21, 24, 28]. The subgroup analyses (Table 2) revealed that follow-up duration and session frequency were sources of heterogeneity (P for Cochran Q < 0.01). The strata of studies with shorter follow-up duration and a single session delivery had greater improvement in SCORAD (P for Cochran Q < 0.01). Tailored sessions (P for Cochran Q = 0.10) showed borderline significance when compared with group sessions. Additionally, the improvements did not seem to be significantly modified by the baseline disease severity (P for Cochran Q < 0.01).
Fig. 2.
Standardized mean difference (SMD) for Scoring of Atopic Dermatitis (SCORAD, a), Dermatitis Family Index (DFI, b), Infants’ Dermatitis Quality of Life (IDQOL, c), and Children’s Dermatology Life Quality Index (CDLQI, d) at the end of study. The dots represent point estimates of SMD while the horizontal lines represent the 95% CI for the SMD. The size of the square around each SMD is proportional to the study weight. I2 represents the degree of heterogeneity in the included studies. The pooled SMD (diamond) was calculated using a random effects model
Table 2.
Subgroup analyses of RCTs included in the meta-analyses of SCORAD
| Parameters | Studies, no. | Treatment/control, no. | SMD (95% CI) | P value | I2, % |
|---|---|---|---|---|---|
| Frequency* | < 0.01 | ||||
| One single session [14, 17, 18, 21] | 4 | 174/173 | − 12.11 (− 14.83, − 9.39) | 0.0 | |
| Cumulative sessions [13, 19] | 2 | 739/626 | − 5.88 (− 9.36, − 2.40) | 73.5 | |
| Follow-up duration, months* | < 0.01 | ||||
| < 6 [17, 18, 21] | 3 | 840/725 | − 11.97 (− 15.28, − 8.65) | 0.0 | |
| ≥ 6 [13, 14, 16, 19] | 4 | 145/143 | − 6.45 (− 9.63, − 3.27) | 77.0 | |
| Disease severity | 0.71 | ||||
| Moderate to severe AD [13, 14, 17, 19] | 4 | 832/720 | − 8.19 (− 11.99, − 4.38) | 82.3 | |
| Unlimited [16, 18, 21] | 3 | 153/148 | − 8.74 (− 15.55, − 1.94) | 78.0 | |
| Delivery | 0.1 | ||||
| Tailored individual session [18] | 1 | 49/50 | − 9.93 (− 14.57, − 5.29) | – | |
| Group session [13, 14, 16, 17, 19, 21] | 6 | 936/818 | − 8.03 (− 11.35, − 4.70) | 79.8 | |
Based on total SCORAD
AD atopic dermatitis, SMD standardized mean difference, CI confidence interval, SCORAD Scoring of Atopic Dermatitis
*Between subgroup Q statistics significant (P < 0.1)
Quantitative Assessment of QoL Measures
The instruments for QoL varied among studies. We were able to pool effects of Dermatitis Family Index (DFI) [21, 22, 24, 28, 30], Children’s Dermatology Life Quality Index (CDLQI) [22–24, 27, 28, 30] and Infants’ Dermatology Quality of Life Index (IDQOL) [23, 24, 27, 28, 30]. For other measures including Family Dermatology Life Quality Index (FDLQI), Dermatology Life Quality Index (DLQI), and pruritus questionnaires, the pooling of data was impractical due to the limited number of studies. Four studies that randomized 462 participants were included in the pooling of DFI and the result was not significant (SMD = − 0.65, 95% CI − 1.49 to 0.18, P = 0.126, Fig. 2b). There was low heterogeneity among the trials (I2 = 0.0%, P = 0.838). Similarly, for IDQOL that randomized 558 participants, therapeutic patient education did not appear to result in a statistically significant improvement (SMD = 0.30, 95% CI − 1.04 to 1.63; P = 0.665; I2= 63.1%; Fig. 2c). Additionally, CDLQI did not significantly change with TPE (SMD = − 1.61, 95% CI − 3.76 to 0.55; P = 0.144; I2= 89.0%; Fig. 2d). The heterogeneity was significant for both measures.
Discussion
The present study reviewed recent literature on disease severity improvements from parental educational interventions. Compared to the most recent systematic review done in 2014 [9], our study added 2 and 6 more to the synthesis of quantitative and qualitative analysis, respectively. Additionally, we conducted subgroup analyses to explore sources of heterogeneity and examined the impact on QoL matrices. Sensitivity analysis using the objective measure of SCORAD was conducted to examine the potential impact of performance bias.
The current review found that the scope of educational interventions varied among studies. First, skin care was an area that was universally covered by all programs, highlighting the importance of using moisturizers to address the defective barrier [11, 33, 34]. In the meantime, it’s also important to clear up misunderstandings about “natural skin products” containing food allergens (e.g., goat’s milk, cow’s milk, peanut oil, almond oil, and oatmeal), as skin sensitization could incur serious consequences, including anaphylaxis [35]. There was also coverage of psychological burdens and managements both by studies included in the current review [20, 25, 27] and by two additional adult studies [36, 37]. The psychological consequences of AD include a higher risk of depression and suicidal ideations [38]. While it has been well acknowledged that asthma and allergic rhinitis are important comorbidities arising from atopic march, other consequences including psychological comorbidities and impaired QoL for both the children and care givers should not be overlooked [20, 22, 25, 27, 39]. In realizing the psychological burdens of AD and its management, more could be done to improve the QoL of both. Food allergy and its link with AD have been extensively studied. Although an avoidance diet may lead to an improvement of the AD symptoms, this could also lead to nutritional and possible immune consequences (the development of immediate-type reactions) [40]. Thus, food avoidance should be initiated with discretion, especially when oral immunotherapy is available. Unfortunately, in a study that explored parents’ attitudes toward food elimination in the general population, 23% intentionally avoided giving at least one food to their children [41]. Guidance on this topic should be offered to reduce consequences of unnecessary food avoidance. Lastly, steroid phobia should be addressed adequately in these programs. Topical steroids are regarded as the mainstay in treating AD. Steroid phobia constitutes one of the major reasons for non-adherence and disease flares [42], affecting up to 60–73% of patients, which indicated that clarifications are needed in a standard patient visit. As studies into this area have mostly been cross sectional, educational trials with an extended follow-up could be more informative in illustrating the learning curve of patients’ Knowledge, Attitudes and Practice (KAP) of topical steroids throughout the post-intervention periods. In conclusion, future TPE programs are encouraged to encompass four areas of focus: skin care, diet, psychological issues, and topical steroid usage. Only three studies in the present review addressed all four areas [20, 23, 27].
In the present study, a significant reduction in SCORAD was found, which supports the role of patient education. In the subgroup analyses, a greater reduction in SCORAD in studies with shorter follow-up durations was observed (P for Cochran Q < 0.01). This was probably influenced by the parents’ knowledge-guided practice returning to their pre-interventional states after a longer washout period in studies with extended post-intervention follow-ups. Interestingly, a greater effect was found in the group of participants who were educated “once and for all”, when compared with those receiving a cumulative curriculum regime (P for Cochran Q < 0.01). This was probably confounded by the shorter follow-up duration of single-session programs when compared with participants who received multiple sessions at intervals, i.e., parents that received a single session of education were universally followed for less than 6 months whereas those that were cumulatively educated were followed for longer periods. For long-term benefits to be achieved, it’s probably recommended to assess the learning curve of parents and to determine whether education should be delivered at iterations of small sessions rather than at serial comprehensive sessions. A borderline (P for Cochran Q = 0.1) significant difference was identified between those who received tailored sessions versus those who were educated as a group; however, as there was only one study in the tailored delivery subgroup, this finding should be viewed with discretion and future studies are required to determine the optimal delivery. Initiatives examining education in digital settings (i.e., daily text messages and videotapes) have yielded inconclusive results. Singer et al. [29] reported a null result for EASI with the intervention of daily text messages while Saritha [31] reported a statistically significant reduction in EASI with videotape. As these studies were all pilot studies with a limited sample size (n = 30 and 10, respectively), future studies with larger sample sizes are required to confirm the findings. Furthermore, patients with different disease severities appeared to benefit alike from educational programs (P = 0.71), indicating that TPE may be implicated regardless of disease severity.
The literature was inconsistent about the correlation of disease severity and QoL [42–45]. One explanation was that severity correlated better with QoL when disease activity was less severe [44], and as the disease proceeded into the moderate to severe spectrum, the association may gradually grow out of linearity. Meanwhile, as previous studies have indicated, a drastic change in disease activity (SCORAD change of 10 points) was only associated with a small improvement in QoL (CDLQI and DLQI of 0.12 and 0.13, respectively) [44, 46], indicating that other factors might be predictors of quality of life for AD patients. One such factor might be the affected body surface area [44, 47]. Another factor is the psychological burden, which has been shown to correlate with the Dermatology Life Quality Index but not EASI [48]. Taken together, these QoL instruments and disease severity instruments offer distinct information and should be separately assessed in efficacy trials of AD. In the present study, the pooled SMDs of QoL measures failed to reveal a significant difference, although the pooled SMD of SCORAD did. One reason might be that all but one study [27] included for the synthesis of QoL measures incorporated psychological management as a part of the program intervention, limiting its capacity to deal with QoL impairments. In the three studies with adequate randomization on baseline QoL that did touch on psychological issues, QoLs were significantly improved by the program [20, 25, 27]. Another attributing factor might be treatment compliance, which requires more time and participation in the treatment group [28], resulting in higher expectations and a lower rating of QoL. Although most studies have investigated improvements in the QoL of patients, less is known about disease burden on family members (n = 6 reported [21, 22, 24, 25, 28, 30]). These matrices should be incorporated, because sleep disturbances and time spent in attending patients were problematic not only for the patients themselves, but also for their family members, particularly in pediatric patients [3]. Validated disease-specific family QoL questionnaires have been developed [49, 50], aiding in the scaling of this matter. In the present study, although we failed to identify a statistically significant difference in the comparison of family QoL, a clear trend favoring the treatment group was observed.
Health economics matrices were under-reported. Educational interventions have the benefit of being low-cost to implement; however, to maintain sustainable effects, long-lived changes in behavior should be achieved. Our study also suggested that that people’s behavior tends to approach baseline when they were followed for longer periods, indicating that TPE programs delivered at iterations across various intervals might be the most effective. On the other hand, this means that an increment in the cost is to be expected. Bosteon et al. reported a comparable medical consumption between the control and intervention groups in adult patients [36]. However, if program implementation and moisturizer consumption were to be calculated as program inputs, an increase in cost in the intervention group might be expected. No such study has been conducted in the pediatric population. Future studies comparing the cost-effectiveness of different methods and frequencies of delivery in pediatric AD patients are warranted. Compared with face-to-face training, web-based training has the benefit of being more cost-effective [51, 52]. One study on adult patients that compared two different deliveries of TPE, i.e., web-based video vs written pamphlets, found that there was a significantly greater reduction in disease severity in the former [53]. It is tempting to suggest that online settings could at least play a complementary role in future TPE programs.
One study [27] in the present review and two others in adult patients [53, 54] used knowledge questionnaires as the outcome of interest, although the instruments were not validated. Participants’ KAP could be quantitatively measured using KAP questionnaires, as was the case for asthma [55], cancer [56] and other chronic diseases [57]. To the best of our knowledge, the only validated questionnaire of such for AD was the TOPICOP questionnaire that measured topical corticosteroid phobia [58], and this has been translated into 15 languages. As was reported by Gonzales et al., a high level of TOPICOP score was associated with poor adherence [59], indicating that KAP instruments could serve as a useful surrogate for the evaluation of treatment adherence. More comprehensive KAP instruments should be developed, validated and incorporated in TPE programs of pediatric AD to facilitate the assessment of patient knowledge and for the development of more targeted education.
The present study was limited by a very low to moderate quality of evidence rated by GRADE (Supplementary Table E2) due to risk in performance and detection bias, i.e., blinding of participants and researchers was impractical and blinding of outcome assessors was not carried out by all studies. One approach to minimize performance bias in the absence of blinding is to use objective investigator-led instruments as the outcome of interest [60], which was the main reason for choosing objective SCORAD over pruritus SCORAD in the sensitivity analysis of the present study.
Furthermore, a trial with non-medication intervention is, by nature, susceptible to intra-group contamination, i.e., participant allocation may get contaminated due to the close proximity of patients and the provision of care by the same team [61, 62]. Intra-group contamination reduces the point estimate of an intervention’s effectiveness which may thereby lead to a type II error [63]. This means that the actual effect size is likely to be even larger than the observed. Compared with individual randomization, cluster randomization offers the privilege of minimizing contamination. Future studies might consider using a cluster-randomized design to obtain more valid results.
In terms of the scope of the present systematic review, the reason for including only educational programs and excluding action plans was to reduce heterogeneity, and it was acknowledged that this might affect the generalizability of our study. Future studies and reviews that focus on AD action plans might offer new perspectives on this topic. Meanwhile, since adult participants were not included, the present review is applicable to only pediatric studies with parental educational interventions.
Conclusions
In summary, present structured education programs benefit pediatric AD patients in terms of disease severity but do not appear to significantly improve quality of life. Future TPE programs were recommended to offer guidance on skin care, diet, psychological issues, and topical steroid usage. Efforts are needed to optimize the frequency, delivery, and cost-effectiveness of program implementation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 11949 kb)
Acknowledgements
Funding
This work was supported by the Special Fund of The Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (XTZD20180502), the Daxing District Science Development Program (KT201902314), and the National Natural Science Foundation of China (81602754). The Journal’s Rapid Service Fee was funded by Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, and Daxing Teaching Hospital, Capital Medical University.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Mutong Zhao and Yuan Liang contributed equally to the work and are equal co-first authors.
Disclosures
Mutong Zhao, Yuan Liang, Chunping Shen, Ying Wang, Lin Ma, and Xiuhua Ma have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11913318.
Contributor Information
Lin Ma, Email: bch_maleen@aliyun.com.
Xiuhua Ma, Email: mxhdxqyy@126.com.
References
- 1.Henriksen L, Simonsen J, Haerskjold A, et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol. 2015;136(2):360-6 e2. doi: 10.1016/j.jaci.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159(8):745–750. doi: 10.1001/archpedi.159.8.745. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Regional Office for, E., therapeutic patient education: continuing education programmes for health care providers in the field of prevention of chronic diseases: report of a WHO working group. Copenhagen: WHO Regional Office for Europe; 1998.
- 6.Lovell MR, Luckett T, Boyle FM, Phillips J, Agar M, Davidson PM. Patient education, coaching, and self-management for cancer pain. J Clin Oncol. 2014;32(16):1712–1720. doi: 10.1200/JCO.2013.52.4850. [DOI] [PubMed] [Google Scholar]
- 7.Zaman S, Zaman SS, Scholtes T, et al. The mortality risk of deferring optimal medical therapy in heart failure: a systematic comparison against norms for surgical consent and patient information leaflets. Eur J Heart Fail. 2017;19(11):1401–1409. doi: 10.1002/ejhf.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168(2):110–120. doi: 10.7326/M17-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ersser SJ, Cowdell F, Latter S, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev. 2014;1:004054. doi: 10.1002/14651858.CD004054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnason S, White-Williams C, Rossi LP, et al. Evidence for therapeutic patient education interventions to promote cardiovascular patient self-management: a scientific statement for healthcare professionals from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2017;10(6):000025. doi: 10.1161/HCQ.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 11.Katayama I, Aihara M, Ohya Y, et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66(2):230–247. doi: 10.1016/j.alit.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 13.Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233. doi: 10.1016/j.jaad.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meropol NJ, Wong YN, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469–478. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogul DB, Henderson ML, Bridges JFP. Expanding the Facebook platform to engage and educate online communities. Am J Gastroenterol. 2018;113(4):457–458. doi: 10.1038/ajg.2017.450. [DOI] [PubMed] [Google Scholar]
- 16.Corcimaru A, Morrell DS, Burkhart CN. The Internet for patient education on atopic dermatitis: friend or foe? J Am Acad Dermatol. 2017;76(6):1197–1198. doi: 10.1016/j.jaad.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 17.Ferdinand KC, Senatore FF, Clayton-Jeter H, et al. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69(4):437–451. doi: 10.1016/j.jacc.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chida Y, Steptoe A, Hirakawa N, Sudo N, Kubo C. The effects of psychological intervention on atopic dermatitis. A systematic review and meta-analysis. Int Arch Allergy Immunol. 2007;144(1):1–9. doi: 10.1159/000101940. [DOI] [PubMed] [Google Scholar]
- 19.Stringer T, Gittler J, Schneider A, Curtiss P, Yin S, Oza V. The readability and suitability of eczema action plans in the United States, Pediatric Dermatology. Conference: 13th world congress of pediatric dermatology. United States. 34 (Supplement 1), pp S38–S39; 2017. Date of publication: July 2017.
- 20.Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332(7547):933–938. doi: 10.1136/bmj.332.7547.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Futamura M, Masuko I, Hayashi K, Ohya Y, Ito K. Effects of a short-term parental education program on childhood atopic dermatitis: a randomized controlled trial. Pediatr Dermatol. 2013;30(4):438–443. doi: 10.1111/pde.12105. [DOI] [PubMed] [Google Scholar]
- 22.Weber MB, de Fontes Neto PT, Prati C, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. J Eur Acad Dermatol Venereol. 2008;22(8):992–997. doi: 10.1111/j.1468-3083.2008.02697.x. [DOI] [PubMed] [Google Scholar]
- 23.Shaw M, Morrell DS, Goldsmith LA. A study of targeted enhanced patient care for pediatric atopic dermatitis (STEP PAD) Pediatr Dermatol. 2008;25(1):19–24. doi: 10.1111/j.1525-1470.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 24.Schuttelaar ML, Vermeulen KM, Drukker N, Coenraads PJ. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. Br J Dermatol. 2010;162(1):162–170. doi: 10.1111/j.1365-2133.2009.09502.x. [DOI] [PubMed] [Google Scholar]
- 25.Pustisek N, Situm M, Vurnek Zivkovic M, Ljubojevic Hadzavdic S, Vurnek M, Niseteo T. The significance of structured parental educational intervention on childhood atopic dermatitis: a randomized controlled trial. J Eur Acad Dermatol Venereol. 2016;30(5):806–812. doi: 10.1111/jdv.13519. [DOI] [PubMed] [Google Scholar]
- 26.Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australas J Dermatol. 2009;50(2):100–106. doi: 10.1111/j.1440-0960.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y. Therapeutic patient education in children with moderate to severe atopic dermatitis: a multicenter randomized controlled trial in China. Clin Exp Allergy. 2018;35(1):70–75. doi: 10.1111/pde.13362. [DOI] [PubMed] [Google Scholar]
- 28.Grillo M, Gassner L, Marshman G, Dunn S, Hudson P. Pediatric atopic eczema: the impact of an educational intervention. Pediatr Dermatol. 2006;23(5):428–436. doi: 10.1111/j.1525-1470.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 29.Cresce N, Zlotoff B, Singer HM. Texting atopic dermatitis patients to optimize learning and eczema area and severity index scores: a pilot randomized control trial. Pediatr Dermatol. 2018;35(4):453–457. doi: 10.1111/pde.13510. [DOI] [PubMed] [Google Scholar]
- 30.Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. Br J Dermatol. 2002;146(3):432–439. doi: 10.1046/j.1365-2133.2002.04603.x. [DOI] [PubMed] [Google Scholar]
- 31.Saritha Kartan M, Minsoo Kim MD. Video-based education in pediatric atopic dermatitis. J Am Acad Dermatol. 2018;79:AB312. doi: 10.1016/j.jaad.2018.05.1230. [DOI] [Google Scholar]
- 32.Park GY, Park HS, Cho S, Yoon HS. The effectiveness of tailored education on the usage of moisturizers in atopic dermatitis: a pilot study. Ann Dermatol. 2017;29(3):360–362. doi: 10.5021/ad.2017.29.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 35.Leyva-Castillo JM, Galand C, Kam C, et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity. 2019;50(5):1262-75 e4. doi: 10.1016/j.immuni.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bostoen J, Bracke S, De Keyser S, Lambert J. An educational programme for patients with psoriasis and atopic dermatitis: a prospective randomized controlled trial. Br J Dermatol. 2012;167:1025–1031. doi: 10.1111/j.1365-2133.2012.11113.x. [DOI] [PubMed] [Google Scholar]
- 37.Heratizadeh A, Werfel T, Wollenberg A, et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140(3):845–853. doi: 10.1016/j.jaci.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Patel KR, Immaneni S, Singam V, Rastogi S, Silverberg JI. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):402–410. doi: 10.1016/j.jaad.2018.08.063. [DOI] [PubMed] [Google Scholar]
- 39.Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry. 2002;52(3):193–204. doi: 10.1016/S0006-3223(02)01347-1. [DOI] [PubMed] [Google Scholar]
- 40.Eigenmann PA, Beyer K, Lack G, et al. Are avoidance diets still warranted in children with atopic dermatitis? Pediatr Allergy Immunol. 2019;31:19–26. doi: 10.1111/pai.13104. [DOI] [PubMed] [Google Scholar]
- 41.Yrjana JMS, Bloigu R, Kulmala P. Parental confusion may result when primary health care professionals show heterogeneity in their knowledge, attitudes, and perceptions regarding infant nutrition, food allergy, and atopic dermatitis. Allergol Immunopathol (Madr) 2018;46(4):326–333. doi: 10.1016/j.aller.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165(4):808–814. doi: 10.1111/j.1365-2133.2011.10449.x. [DOI] [PubMed] [Google Scholar]
- 43.Kong TS, Han TY, Lee JH, Son SJ. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28(3):321–326. doi: 10.5021/ad.2016.28.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haeck IM, ten Berge O, van Velsen SG, de Bruin-Weller MS, Bruijnzeel-Koomen CA, Knol MJ. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26(2):236–241. doi: 10.1111/j.1468-3083.2011.04043.x. [DOI] [PubMed] [Google Scholar]
- 45.Hon KL, Kam WY, Lam MC, Leung TF, Ng PC. CDLQI, SCORAD and NESS: are they correlated? Qual Life Res. 2006;15(10):1551–1558. doi: 10.1007/s11136-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150(2):284–290. doi: 10.1111/j.1365-2133.2004.05776.x. [DOI] [PubMed] [Google Scholar]
- 47.Holm JG, Agner T, Clausen ML, Thomsen SF. Quality of life and disease severity in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30(10):1760–1767. doi: 10.1111/jdv.13689. [DOI] [PubMed] [Google Scholar]
- 48.Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90(6):582–588. doi: 10.2340/00015555-0933. [DOI] [PubMed] [Google Scholar]
- 49.Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol. 1998;138(1):107–113. doi: 10.1046/j.1365-2133.1998.02034.x. [DOI] [PubMed] [Google Scholar]
- 50.Meni C, Bodemer C, Toulon A, et al. Atopic dermatitis burden scale: creation of a specific burden questionnaire for families. J Eur Acad Dermatol Venereol. 2013;27(11):1426–1432. doi: 10.1111/jdv.12180. [DOI] [PubMed] [Google Scholar]
- 51.Oppong R, Smith RD, Little P, et al. Cost-effectiveness of internet-based training for primary care clinicians on antibiotic prescribing for acute respiratory tract infections in Europe. J Antimicrob Chemother. 2018;73(11):3189–3198. doi: 10.1093/jac/dky309. [DOI] [PubMed] [Google Scholar]
- 52.De Bruin EJ, van Steensel FJ, Meijer AM. Cost-effectiveness of group and internet cognitive behavioral therapy for insomnia in adolescents: results from a randomized controlled trial. Sleep. 2016;39(8):1571–1581. doi: 10.5665/sleep.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armstrong AW, Kim RH, Idriss NZ, Larsen LN, Lio PA. Online video improves clinical outcomes in adults with atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:502–507. doi: 10.1016/j.jaad.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 54.Gradwell C, Thomas KS, English JS, Williams HC. A randomized controlled trial of nurse follow-up clinics: do they help patients and do they free up consultants’ time? Br J Dermatol. 2002;147(3):513–517. doi: 10.1046/j.1365-2133.2002.04901.x. [DOI] [PubMed] [Google Scholar]
- 55.Holley S, Knibb R, Latter S, et al. Development and validation of the Adolescent Asthma Self-Efficacy Questionnaire (AASEQ) Eur Respir J. 2019;54(1):1. doi: 10.1183/13993003.01375-2018. [DOI] [PubMed] [Google Scholar]
- 56.Landier W, Chen Y, Namdar G, et al. Impact of tailored education on awareness of personal risk for therapy-related complications among childhood cancer survivors. J Clin Oncol. 2015;33(33):3887–3893. doi: 10.1200/JCO.2015.62.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. Am J Respir Crit Care Med. 2016;194(6):672–680. doi: 10.1164/rccm.201512-2503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moret L, Anthoine E, Aubert-Wastiaux H, et al. TOPICOP(c): a new scale evaluating topical corticosteroid phobia among atopic dermatitis outpatients and their parents. PLoS ONE. 2013;8(10):e76493. doi: 10.1371/journal.pone.0076493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzales F, Ramdane N, Delebarre-Sauvage C, Modiano P, Duhamel A, Lasek A. Monitoring of topical corticosteroid phobia in a population of parents with children with atopic dermatitis using the TOPICOP((R)) scale: prevalence, risk factors and the impact of therapeutic patient education. J Eur Acad Dermatol Venereol. 2017;31(3):e172–e174. doi: 10.1111/jdv.13961. [DOI] [PubMed] [Google Scholar]
- 60.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2011. [Google Scholar]
- 61.Goldstein CE, Weijer C, Taljaard M, et al. Ethical issues in pragmatic cluster-randomized trials in dialysis facilities. Am J Kidney Dis. 2019;5:656–666. doi: 10.1053/j.ajkd.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. New York: Wiley; 2010. [Google Scholar]
- 63.Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ. 2001;322(7282):355–357. doi: 10.1136/bmj.322.7282.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 11949 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article as supplementary information files.