Abstract
Objectives
The objective of this systematic review and meta-analysis study was to identify the different disinfection methods and materials and the existing evidence on their effect on properties of the different impression materials.
Material and methods
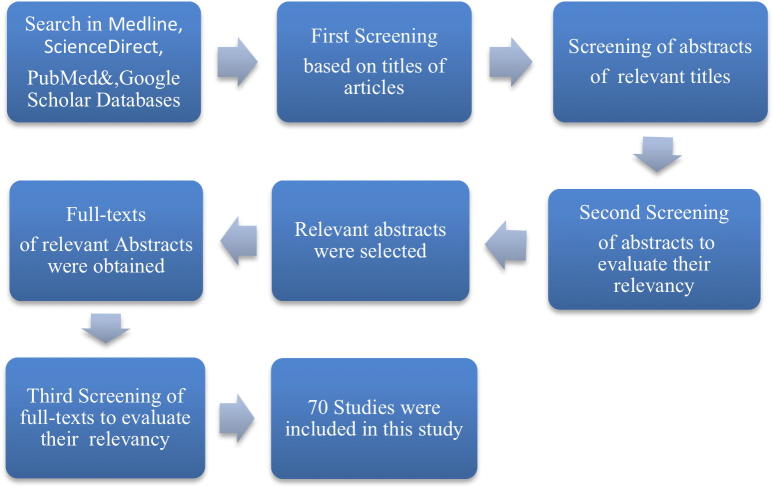
An electronic search of MEDLINE (PubMed), Science Direct, and Google Scholar databases was performed to retrieve related English-language articles published between January 2000 and July 2019. Available studies with search terms such as: Impression disinfection, disinfection method, impression dimensional stability and impression wettability were used. The selected articles were reviewed by screening their titles and abstracts and full text. Finally, a total of 70 articles were considered relevant and were included in this study.
Results
Extensive studies were conducted to determine the effect of the different disinfection methods and materials on the properties of the different impression materials such as dimensional stability, wettability and surface roughness. While some studies reported significant changes in the properties of the impression materials, others reported either no changes or minor insignificant effects.
Conclusions
Some studies reported significant changes in the properties of the impression materials as a result of using different disinfection methods, whereas others reported either minor insignificant or no changes. Although the findings of the studies were controversial, care should be taken to avoid distortion of impressions and loss of their surface details that can adversely affect the fitting accuracy of the restorations. Therefore, better designed and standardized studies are needed to evaluate the effect of different commonly used disinfectants on properties of impression materials. Moreover, manufacturers should be encouraged to recommend specific disinfection methods and materials for disinfecting the impression materials to ensure their optimal accuracy.
Keywords: Chemical disinfection, Microwave irradiation, Steam autoclave, Ultraviolet light radiation, Ozone disinfection, Electrolyzed oxidizing water disinfection
1. Introduction
Micro-organisms present in the patient's saliva and blood may result in contamination of dental impressions. Therefore, immediately after their removal from the patient’s mouth, the impressions are thoroughly washed under tap water to remove contaminants. Although tap water rinsing was found to reduce microbes, it does not eliminate the infection potential of the impressions (Al-Jabrah et al., 2007, Pal et al., 2014, Sousa et al., 2013). Hence, disinfection of impressions is considered mandatory (Al-Jabrah et al., 2007, Matalon et al., 2011) and the American Dental Association (ADA) recommends immediate disinfection of dental impressions immediately after their removal from the patient’s mouth to prevent cross infection between the patients and dental staff in dental offices and laboratories (ADA Council on Scientific Affairs and ADA Council on Dental Practice, 1996).
Several methods of disinfection are used to disinfect the different impression materials. Among these, the most frequently used method is the chemical method where a chemical disinfectant is applied to the surface of the impression materials either by spraying or immersion. Disinfectants with different concentrations may be used such as glutaraldehyde (0.5%, 2%, 2.2%, and 2.45%), sodium hypochlorite (NaOCl) (0.5%, 0.525%, 1%, 4% and 5.25%), iodophors (5% and 10%), phenols (7%), chlorine compounds (0.2% chlorhexidine) and hydrogen peroxide (0.5%); among these, glutaraldehyde and NaOCl disinfectants are widely used. Disinfection by glutaraldehyde was found to be effective in eliminating microbes from the surface of alginate and silicone impression materials without causing changes in their dimensional stability (Demajo et al., 2016).
Other disinfection methods may also be used such as microwave irradiation, ultraviolet (UV) light radiation, steam autoclaving, ozone and electrolyzed oxidizing water (EOW) disinfection. Many studies were conducted to evaluate the effect of the different disinfection methods and materials on different impression materials. The results of the studies varied according to the method of disinfection, application time, and type of impression material to be disinfected. They also depended on the type and concentration of the disinfectant in case of a chemical disinfection. However, the effect of disinfection on different impression materials remains controversial.
The aim of this systematic review and meta-analysis study was to identify the different disinfection methods and materials and the existing evidence on their effect on the properties of the different impression materials.
2. Material and methods
2.1. Data search
An electronic search of MEDLINE (PubMed), Science Direct, and Google Scholar databases was conducted to identify and retrieve articles published between January 2000 and July 2019 that are relevant to the research topic using the following keywords search terms: impression disinfection, disinfection method, impression dimensional stability, and impression wettability.
2.2. Studies selection
The first screening was done based on the title of the articles, followed by a screening of the abstracts of the relevant titles. The second screening was made to evaluate the relevance of the abstracts (Fig. 1). A third screening was conducted to evaluate the relevance of the full texts of the articles with relevant abstracts to the research topic.
Fig. 1.
Flow diagram of the search process of relevant articles to be included in this systemic review and meta-analysis study.
3. Results
The electronic search revealed 70 articles that were related to the topic of this systematic review and meta-analysis study. Accordingly, they were thoroughly reviewed.
Among the several methods used for disinfecting impression materials, the chemical method was most frequently used over the other methods including microwave irradiation, steam autoclave, UV light radiation, ozone, and EOW disinfection.
3.1. Chemical disinfection
Disinfection of impression materials can be achieved by immersing or spraying of the materials with different disinfectants at different concentrations and application times. However, immersion of impression materials in different disinfectants was reported to be more effective than spraying their surfaces. This may be because of a guaranteed disinfection of all surfaces of the impression and the relatively longer exposure time of the impression materials to the disinfectant by immersion than by spraying their surfaces (Kaul et al., 2012). Nevertheless, spray disinfection is the most popular method of disinfection method of impression materials, especially for hydrophilic impression materials such as hydrocolloids (Kaul et al., 2012, Rad et al., 2010). Babiker et al. reported insignificant dimensional changes in gypsum casts obtained from irreversible hydrocolloid impression material that were sprayed with 1% and 5.25% NaOCl, whereas significant dimensional changes were reported when the casts were immersed in NaOCl. Immersion in disinfecting solution promotes water absorption phenomena of hydrophilic impression materials, especially when they are immersed in the disinfectant for a long time allowing chemical interactions to occur between impressions and disinfectants (Kotsiomiti et al., 2008). Therefore, disinfection by spraying with NaOCl rather than immersion has been recommended to disinfect irreversible hydrocolloid impression materials (Babiker et al., 2018). However, Sousa et al. reported a 99.99% reduction in the microbial count when an alginate impression was disinfected by immersion in 0.5% NaOCl for 10 min. Similarly, immersion is preferred for disinfecting hydrophobic impression materials such as vinyl polysiloxane and polysulfide. Moreover, hydrophobic elastomeric materials can be safely immersed for a long time in disinfectants (Kotsiomiti et al., 2008). For instance, the effect of immersion disinfection on the dimensions of polyether and addition silicone in 0.5% glutaraldehyde or ammonium compounds were not clinically significant (Melilli et al., 2008). Furthermore, Pal et al. reported 100% reduction in the microbial count and complete disinfection of the elastomeric impression materials by immersion in 1% and 5% NaOCl and 2% glutaraldehyde. However, insignificant dimensional changes were reported when spray or immersion disinfection of condensation and addition silicone impression materials was conducted for 10 min (Ahila and Thulasingam, 2014). Immersion disinfection of polyether in chlorhexidine was reported to be inappropriate (Ivanis et al., 2000).
Several studies recommended the monitoring of the immersion time of the different impression materials in disinfectants (Blalock et al., 2010, Shetty et al., 2013). They recommended a 10-min disinfection. For instance, 10 and 20 min immersion of condensation silicone material in 1% NaOCl and 2% glutaraldehyde did not result in dimensional changes of the material (Silva and Salvador, 2004). Similarly, addition silicone did not exhibit a significant expansion when disinfected by immersion for 10 min or 1 h in different chemicals (Duseja et al., 2014). While a 10 min immersion of polyether in disinfectants produced significant dimensional changes (Duseja et al., 2014, Silva and Salvador, 2004), Guler et al. recommended a 30-min disinfection of polyether. Bustos et al. reported an effective disinfection with minimal changes in dimensional stability and surface integrity when alginate and silicone impression materials were immersed in 0.5% NaOCl or 2% glutaraldehyde for 5 min rather than for 10 min. Ahila and Thulasingam reported insignificant dimensional changes of silicone impression materials after a 10 min spray or immersion disinfection. A 10 min immersion disinfection in 0.5% NaOCl was recommended for the disinfection of alginate, addition and condensation silicone, and zinc oxide eugenol impression materials (Amin et al., 2009). A 24 h immersion of silicone materials in chlorhexidine resulted in clinically acceptable dimensional changes (Silva and Salvador, 2004). Walker et al. reported dimensional changes of vinyl polysiloxane and polyether due to immersion in 0.5% NaOCl or phenol disinfectants for 10 min and 1 h. These changes conform to the ADA standards. A significant adverse effect was also reported on the surface of polyether as the exposure time to 0.5% NaOCl was increased (Walker et al., 2007). The authors stated that neither 0.5% NaOCl nor phenol disinfectants used for 10 min and 1 h adversely affected the dimensional stability of the more recent formulations of vinyl polysiloxane and polyether (Walker et al., 2007).
In summary, the effect of chemical disinfection on the impression materials varies with the method and duration of disinfection, type and concentration of the disinfectant, and the type of impression material. Generally, disinfection affect not only the dimensional stability and wettability of the impression materials but also the surface quality of a reproduced stone cast. Table 1 presents a summary of the selected studies, indicating the effect of chemical disinfection on the properties of different impression materials.
Table 1.
Summary of selected studies indicating the effect of chemical disinfection on properties of different impression materials.
| Author/Year | Impression Material | Material/Conc. | Method | Time | Property Investigated | Changes |
|---|---|---|---|---|---|---|
| Silva and Salvador (2004) | Condensation Silicone | 1% NaOCl 2% Glutaraldehyde |
Immersion | 10 min 20 min |
Dimensional stability | X |
| Saber et al. (2010) | Condensation Silicone | 5.25% NaOCl 10% Iodophor |
Spray | 10 min |
Dimensional stability | √* |
| Ahila and Thulasingam (2014) | Addition & Condensation Silicone | 4% NaOCl 2.45% Glutaraldehyde 5% Povidone Iodine |
Spray Immersion |
10 min | Dimensional stability & surface roughness |
√ |
| Ivanis et al (2000) | Polyether |
0.5% Chlorhexidine |
Immersion | 10 min 30 min 60 min 24hr |
Dimensional stability | √* |
| Addition & Condensation Silicone | √ | |||||
| Duseja et al. (2014) | Polyether | 0.5% NaOCl 2% Glutaraldehyde Phenol |
Immersion | 10 min | Dimensional stability | √* |
| Addition Silicone | 60 min | √ | ||||
| Kalantari et al. (2014) | Condensation Silicone | 0.5% NaOCl | Immersion | 20 min | Dimensional stability | √* |
| Sinobad et al. (2014) | Silicone | 5.25% NaOCl 0.5% Glutaraldehyde |
Immersion | 10 min | Dimensional stability | √* |
| Ahila and Subramaniam (2012) | Silicone | 4% NaOCl 2.45% Glutaraldehyde 5% Povidone |
Spray Immersion |
10 min 30 min 60 min |
Dimensional stability | √ |
| Rentzia et al. (2011) | Irreversible Hydrocolloid | 0.5% NaOCl 0.55% ortho-phthalaldehyde |
Immersion | 30 sec 60 sec 90 sec 120 sec 180 sec 240 sec 300 sec |
Dimensional stability | X |
| Soganci et al. (2018) | Silicone Polyether |
5.25% NaOCl 2% Glutaraldehyde |
Immersion | 10 min | Dimensional stability | X |
| Carvalhal et al. (2011) | Silicone Polysulfide Polyether |
0.5% NaOCl 2% Glutaraldehyde |
Immersion | 5 min 10 min 20 min |
Dimensional stability | √ |
| 30 min 60 min |
√* | |||||
| 60 min | ||||||
| Bustos et al. (2010) | Alginate Silicone |
0.5% NaOCl 2% Glutaraldehyde |
Immersion | 5 min 10 min |
Dimensional stability | √ |
| Nimonkar et al. (2019) | Silicone | 1% NaOCl 2% Glutaraldehyde |
Immersion | 20 min | Dimensional stability | √* |
| Babiker et al. (2018) | Alginate |
1% NaOCl 5.25% NaOCl |
Spray | 5 min |
Dimensional stability | √ |
| Immersion | √* | |||||
| Shetty et al. (2013) | Polyether | 0.5% NaOCl 5.25% Phenol |
Immersion |
10 min |
Wettability | √* |
| 2% Glutaraldehyde 0.05% Iodophor |
30 min | √ | ||||
| Rad et al. (2010) | Alginate | 5.25% NaOCl 2% Glutaraldehyde |
Spray Immersion |
8 min | Dimensional stability | √* |
| Demajo et al. (2016) | Alginate Addition Silicone |
0.5% Glutaraldehyde | Spray |
10 sec | Dimensional stability | X |
| Yilmaz et al. (2007) | Polyether | 0.525% NaOCl 2% Glutaraldehyde |
Spray Immersion |
10 min | Dimensional stability | √ |
| Dorner et al. (2014) | Irreversible Hydrocolloid | 1% NaOCl | Spray |
10 min | Dimensional stability & surface roughness | √* |
| Guiraldo et al. (2018) | Silicone Polysulfide Polyether |
0.2% Chloramine | Immersion |
15 min | Dimensional stability | X |
| Jagger et al. (2007) | Polyether Silicone |
2% Glutaraldehyde | Immersion |
10 min | Dimensional stability | √ |
| Nassar et al. (2017) | Silicone |
2.5% Glutaraldehyde | Immersion |
30 min | Dimensional stability | X |
| Pal et al. (2014) | Silicone |
1% NaOCl 4% NaOCl 2% Glutaraldehyde |
Immersion |
10 min | Surface quality | X |
| Melilli et al. (2008) | Addition Silicone Polyether |
0.5% Glutaraldehyde | Immersion |
5 min | Dimensional stability | √ |
| Blalock et al. (2010) | Silicone | Hypochlorite-based | Spray | 1 min 20 min 60 min 24hr |
Wettability | √ |
| Lad et al. (2015) | Addition & Condensation Silicone Polyether |
4% NaOCl 2% Glutaraldehyde |
Immersion |
10 min | Wettability | X |
| AlZain (2019) | Addition Silicone Polyether |
0.5% Glutaraldehyde | Spray | 10 min | Wettability | X |
| Panza et al. (2005) | Alginate |
1% NaOCl | Immersion |
10 min | Dimensional stability | √ |
| 15 min | √* | |||||
| Polysulfide Polyether |
2% Glutaraldehyde |
10 min 15 min | √ | |||
| Amin et al. (2009) | Alginate Addition & Condensation Silicone Zinc Oxide-Eugenol |
0.5% NaOCl |
Immersion |
10 min |
Dimensional stability |
√ |
| 1% NaOCl 0.2% Chlorhexidine 2.2% Glutaraldehyde |
5 min |
X: No changes, √: Insignificant changes, √*: Significant changes.
3.2. Microwave irradiation
There are two modes of action of microwaves: thermal and non-thermal. In the thermal mode, the microwave energy is converted into heat by the prolonged kinetic motion of polar molecules. On the Contrary, the non-thermal mode involves direct interaction of the electromagnetic field with the biologic molecule (Bhasin et al., 2013). Most of the microwaves are designed with a rotating platform to avoid “cold spots” (Bhasin et al., 2013). The impression specimens are immersed in distilled water in sterile glass bakers, which allow a uniform heating of the specimens during microwave exposure (Bhasin et al., 2013).
Microwave irradiation method is considered as an effective disinfecting method for reducing microbial count (Vatsal et al., 2015). Goel et al. reported better disinfection by microwave irradiation than by the use of 0.7% NaOCl chemical disinfection. Disinfection through exposure of elastomeric impressions to microwaves at 650 W for 5, 6, and 7 min was found to be effective and convenient (Bhasin et al., 2013). Furthermore, microwave disinfection at 900 W for 5 min was observed to be as effective as chemical immersion disinfection using 0.5% NaOCl for 10 min (Meghashri et al., 2014). Microwave irradiation was also considered as a useful method to disinfect vinyl polysiloxane impression material especially when combined with H2O2, without adversely affecting the physical properties of the impression material (Choi et al., 2014). However, Al Kheraif reported a significant increase in the surface roughness of vinyl polysiloxane after microwave disinfection. Similarly, Ramakrishnaiah et al. observed significant dimensional changes in vinyl polysiloxane impression materials that were considered acceptable by the ADA. They concluded that vinyl polysiloxane impression can be safely disinfected by microwave irradiation because chemical disinfection does not eliminate bacteria (Ramakrishnaiah et al., 2012). Therefore, microwave irradiation was considered as an alternative method to chemical disinfection (Kamble et al., 2015, Kotha et al., 2017).
3.3. Steam autoclaving
Steam autoclaving of impressions is performed at high temperatures which may result in their distortion. However, Millar and Deb reported no statistically significant changes in autoclaved addition and condensation silicone impression materials. Similarly, chemical disinfection and autoclave sterilization produced no statistically significant effect on the surface roughness and wettability of vinyle polysiloxane impression materials (Kotha et al., 2017). The tear strength of the silicone impression materials was not adversely affected by autoclave sterilization at 134 °C for 30 min (Millar and Deb, 2014). Furthermore, autoclaving was found to be suitable for disinfecting vinyl polysiloxane impressions (Al Kheraif, 2013, Ramakrishnaiah et al., 2012). Thus, autoclave sterilization was considered as an effective alternative effective method for disinfecting silicone impression materials (Kotha et al., 2017). Kamble et al. also stated that steam autoclaving is effective and can be used as an alternative to chemical disinfection of elastomeric impression materials (Thota et al., 2014). They considered autoclaving as one of the most effective disinfection methods for condensation and addition silicone but not for polyether impression materials because of their hydrophilic nature.
3.4. Ultraviolet light radiation
Ultraviolet light radiation, which has been introduced in recent decades, effectively inactivates micro-organisms. This, impressions can be disinfected in the chamber of the UV disinfection unit where UV light is emitted so that the impression is simultaneously exposed to UV radiation from different directions; a timer that can be set from 1 to 60 min (Godbole et al., 2014, Nimonkar et al., 2019). The UV light has a powerful bactericidal effect as it acts on the DNA of cells resulting in their destruction (Godbole et al., 2014). The effectiveness of the UV disinfection method of impressions depends on the exposure time, intensity of the radiation, and the accessibility to micro-organisms. Godbole et al. used a radiation of 254 nm wavelength to disinfect vinyl polysiloxane for 10 min whereas Aeran et al. used a similar wavelength (254 nm) to disinfect alginate, addition silicone and polyether for 3, 6, 10 and 15 min. Nimonkar et al. also used the same wavelength to disinfect the vinyl polysiloxane for 20 min. The results showed that disinfection could be achieved for the alginate and addition silicone impression material after a 10 min exposure to UV rays; however, a 10 min UV disinfection resulted in insignificant dimensional changes of vinyl polysiloxane impressions (Godbole et al., 2014, Nimonkar et al., 2019). Similarly, a 3 min exposure to UV rays was considered sufficient to be sufficient for a complete disinfection of polyether (Aeran et al., 2015). Therefore, UV light was recommended for the disinfection of impression materials (Godbole et al., 2014).
In the UV light radiation method, impression materials such as silicones are individually stored in sealed bags and exposed to a standard autoclave cycle at 134 °C for a 30 min (Millar and Deb, 2014), which includes steam autoclaving and drying (Millar and Deb, 2014). A combination of UV radiation and 2% glutaraldehyde immersion was observed to effectively disinfect dental impressions that were infected with HBV and HIV (Zhang et al., 2017).
3.5. Ozone disinfection
Ozone is a gas made up of three oxygen atoms, whereas the oxygen we breathe is composed of two oxygen atoms. Ozone is highly reactive because it is extremely unstable, which makes it a powerful sterilizer. Moreover, ozone is a potent oxidizing agent and has the ability to attack not only the cell membrane and intracellular enzymes but also the DNA of microorganisms (Poulis et al., 2014). Ozone is produced from ozone generators because it is an unstable compound (Poulis et al., 2016). The impression materials are placed inside the disinfection chamber of the ozone disinfection device. Its flow is controlled by a high-precision 0–20 L/min flow meter with an adjustable flow valve, and disinfection is performed by releasing ozone at a constant flow rate (Abinaya et al., 2018, Poulis et al., 2014). A low-flow high-ozone disinfection of dental impressions was recommended by Poulis et al. When a low-flow high-ozone concentration disinfection of light-body addition silicone was performed for 3, 5, 10 and 15 min, a significant elimination of bacteria was reported after 3 min of ozone exposure (Poulis et al., 2014). Albinaya et al. used an ozone concentration of 10 ppm for 20 min whereas Celebi et al. used the same concentration for 30 min. Ozone disinfection was considered as aneffective method to efficiently eliminate bacteria from the surface of the impression materials (Abinaya et al., 2018, Celebi et al., 2018, Poulis et al., 2014). A 10 min immersion of irreversible hydrocolloid impression material in ozonated water can reduce the number of microorganisms (Savabi et al., 2018). Clinically, it has been advised to use ozone water as an alternative to 5.25% NaOCl and 2% glutaraldehyde for the disinfection of silicone impression materials (Abinaya et al., 2018) as well as an alternative to 0.525% NaOCl (Al Kheraif, 2013). Ozone disinfection produced similar surface alterations in polyvinyle siloxane and polyether impression materials as immersion disinfection of them in 0.525% NaOCl (Poulis et al., 2016). Clinically, ozone disinfection is a new method, which needs no consumables, is time saving, and requires limited space in the dental office. This minimizes liquid waste generation resulting in superior environmental protection (Poulis et al., 2014, Poulis et al., 2016). Therefore, ozone is considered as an environment-friendly dental impression disinfection method (Poulis et al., 2014, Poulis et al., 2016). Although laboratory studies suggest a promising antimicrobial potential of ozone, well- designed clinical trials are needed to evaluate the effective and routine use of ozone in dentistry (Azarpazhooh and Limeback, 2008, German et al., 2013, Gupta and Deepa, 2016, Munaz et al., 2011).
3.6. Electrolyzed oxidizing water
Electrolyzed oxidizing water (EOW) has been certified for use in Japan as an effective and safe disinfectant agent. It is environmental-friendly as it reverts to NaCl solution (salt) and water (Mahalakshmi et al., 2019). It is classified according to its pH value as acidic, alkaline, and neutral (Mahalakshmi et al., 2019). Alkaline EOW and 2% glutaraldehyde produced statistically insignificant dimensional changes whereas 1% NaOCl, and acidic and neutral EOW produced statistically significant dimensional changes in polyvinyle siloxane impression materials (Mahalakshmi et al., 2019). However, Nagamatsu et al. considered neutral EOW as the most appropriate disinfection method of alginate impression materials. When EOW was used as an immersion disinfectant of polyvinyle siloxane impression materials, a higher antimicrobial effect was reported as compared with that of 2.4% glutaraldehyde and 1% NaOCl (Jeyapalan et al., 2018). Furthermore, while Mahalakshmi et al. immersed polyvinyle siloxane impression materials in EOW for 10 min, Nagamatsu et al. stated that only a 1 min immersion in neutral EOW could sufficiently disinfect alginate impression materials.
3.7. Self-disinfecting impression materials
The effect of the addition of disinfectants to a powder of alginate impressions on its dimensional stability was studied. Chlorhexidine self-disinfecting irreversible hydrocolloid impression materials have the disinfectant impregnated into their powders and exhibits antibacterial activity without producing adverse effects on the mechanical characteristics of the material (Wang et al., 2007). A modified alginate impression material with 15 wt% povidone PVP-iodine was recommended to produce the self-disinfecting impression material with a less deteriorating effect (Ismail et al., 2016). The surface hardness of gypsum casts poured using impressions made from self-disinfecting alginate and conventional alginates were comparable (Madhavan et al., 2017). Irreversible hydrocolloid impression material mixed with chlorhexidine exhibited varying degrees of antibacterial activity without affecting the dimensional stability of the set material (Benakatti et al., 2017).
A vinyl polysiloxane impression material has been introduced, which is capable of undergoing autoclaving at 134 °C for 18 min at 2.0 psi and a 12 min drying time without undergoing clinically significant dimensional changes (Reddy et al., 2013).
When a topical surface wetting agent was applied on addition and condensation silicones and polyether impression materials, their wettability was improved (Lad et al., 2015). Kang et al. also observed that the application of a wetting agent made the disinfected vinyl polysiloxane impression material specimens less hydrophobic, if the duration of disinfection was less than 6 h. Lad et al. recommended the application of a surface wetting agent after disinfection of silicone and polyether impression materials to obtain accurate casts.
4. Discussion
The response of different impression materials was observed to vary with the disinfection method used, duration of the disinfection, type of disinfectant, and its concentration in case of chemical disinfection. Although the dimensional changes of some of the disinfected materials were found to be in microns, these changes may be of clinical significance in fixed prosthetic cases, which require a high degree of precision (Jagger et al., 2007). In 2008, Kotsiomiti et al. conducted a literature review on the effect of chemical disinfection on the accuracy of impression materials. According to them, it was difficult to compare the results of the different studies because of the variations in the experimental designs. Therefore, they suggested to encourage manufacturers to recommend a specific disinfectant for a particular impression material product to ensure optimal dimensional accuracy (Jagger et al., 2007). Furthermore, Taylor et al. recommended individual analysis of impression materials to determine their suitability to a given disinfection protocol.
Autoclave and microwave and chemical disinfection methods were produced minor dimensional changes of polyether and addition and condensation vinyle polysiloxane impression materials that were within ADA specification. Therefore, autoclave and microwave disinfection methods were recommended as alternatives to chemical disinfection (Kamble et al., 2015).
Furthermore, ozone and EOW disinfection methods have a strong and effective antimicrobial action and are considered as an environment friendly dental impression disinfection method.
5. Conclusions
The conclusions drawn from the different studies on the effect of different disinfecting methods on dimensional stability, wettability and surface quality of the disinfected impressions are controversial. While some studies reported significant changes in the properties of the impression materials, others reported either no changes or minor insignificant effects. This is due to the differences and variations of specimen dimensions, baseline measurements, and the measurement and reporting methods. Hence, better designed and standardized studies are needed to evaluate the effect of different commonly used disinfectants on the properties of impression materials. Therefore, manufacturers should be encouraged to recommend specific disinfection methods and materials for disinfecting the impression materials to ensure their optimal accuracy.
Ethical statement
This research does not require ethical approval. I followed the Helsinki declaration.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abinaya K., Kumar B.M., Ahila S.C. Evaluation of surface quality of silicone impression materials after disinfection with ozone water: an in vitro study. Contemp. Clin. Dent. 2018;9:60–64. doi: 10.4103/ccd.ccd_747_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeran H., Sharma S., Kumar V., Gupta N. Use of clinical UV chamber to disinfect dental impressions: a comparative study. J. Clin. Diagn. Res. 2015;9:67–70. doi: 10.7860/JCDR/2015/14025.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahila S.C., Subramaniam E. Comparative evaluation of dimensional stability and surface quality of gypsum casts retrieved from disinfected addition silicone impressions at various time intervals: an in vitro study. J. Dent. Oral. Hyg. 2012;4:34–43. [Google Scholar]
- Ahila S.C., Thulasingam C. Effect of disinfection on gypsum casts retrieved from addition and condensation silicone impression disinfected by immersion and spray methods. SRM. J. Res. Dent. Sci. 2014;5:163–169. [Google Scholar]
- Al-Jabrah O., Al-Shumailan Y., Al-Rashdan M. Antimicrobial effect of 4 disinfectants on alginate, polyether and polyvinyl siloxane impression materials. Int. J. Prosthodont. 2007;20:299–307. [PubMed] [Google Scholar]
- Al Kheraif A.A. Surface roughness of polyvinyl siloxane impression materials following chemical disinfection, autoclave and microwave sterilization. J. Contemp. Dent. Pract. 2013;14:483–487. doi: 10.5005/jp-journals-10024-1349. [DOI] [PubMed] [Google Scholar]
- AlZain S. Effect of 0.5% glutaraldehyde disinfection on surface wettability of elastomeric impression materials. Saudi. Dent. J. 2019;31:122–128. doi: 10.1016/j.sdentj.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Dental Association (ADA) Council on Scientific Affairs and ADA Council on Dental Practice, 1996. Infection control recommendations for the dental office and the dental laboratory. J.A.D.A. 127, 672–680. [DOI] [PubMed]
- Amin W.M., Al-Ali M.H., Al Tarawneh S.K., Taha S.T., Saleh M.W., Ereifij N. The effects of disinfectants on dimensional accuracy and surface quality of impression materials and gypsum casts. J. Clin. Med. Res. 2009;1:81–89. doi: 10.4021/jocmr2009.04.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarpazhooh A., Limeback H. The application of ozone in dentistry: a systematic review of literature. J. Dent. 2008;36:104–116. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Babiker G.H., Khalifa N., Alhajj M.N. Dimensional accuracy of alginate impressions using different methods of disinfection with varying concentrations. Compend. Contin. Educ. Dent. 2018;39:e17–e20. [PubMed] [Google Scholar]
- Benakatti V.B., Patil A.P., Sajjanar J., Shetye S.S., Amasi U.N., Patil R. Evaluation of antibacterial effect and dimensional stability of self-disinfecting irreversible hydrocolloid: an in vitro study. J. Contemp. Dent. Pract. 2017;18:887–892. doi: 10.5005/jp-journals-10024-2144. [DOI] [PubMed] [Google Scholar]
- Bhasin A., Vinod V., Bhasin V., Mathew X., Sajjan S., Ahmed S.T. Evaluation of effectiveness of microwave irradiation for disinfection of silicone elastomeric impression material. J. Ind. Prosthodont. Soc. 2013;13:89–94. doi: 10.1007/s13191-012-0230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J.S., Cooper J.R., Rueggeberg F.A. The effect of chlorine-based disinfectant on wettability of a vinyl polysiloxane impression material. J. Prosthet. Dent. 2010;104:333–341. doi: 10.1016/S0022-3913(10)60151-5. [DOI] [PubMed] [Google Scholar]
- Bustos J., Herrera R., González U., Martínez A., Catalán A. Effect of immersion disinfection with 0.5% sodium hypochlorite and 2% glutaraldehyde on alginate and silicone: microbiology and SEM Study. Int. J. Odontostomat. 2010;4:169–177. [Google Scholar]
- Carvalhal C.I., Mello J.A., Correr A.B., Sinhoreti M.A. Dimensional change of elastomeric materials after immersion in disinfectant solutions for different times. J. Contemp. Dent. Pract. 2011;12:252–258. doi: 10.5005/jp-journals-10024-1043. [DOI] [PubMed] [Google Scholar]
- Celebi H., Buyukerkmen E.B., Torlak E. Disinfection of polyvinyl siloxane impression material by gaseous ozone. J. Prosthet. Dent. 2018;120:138–143. doi: 10.1016/j.prosdent.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Choi Y.R., Kim K.N., Kim K.M. The disinfection of impression materials by using microwave irradiation and hydrogen peroxide. J. Prosthet. Dent. 2014;112:981–987. doi: 10.1016/j.prosdent.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Demajo J.K., Cassar V., Farrugia C., Sango D.M., Sammut C., Valdramidis V., Camilleri J. Effectiveness of disinfectants on antimicrobial and physical Properties of Dental Impression Materials. Int. J. Prosthodont. 2016;29:63–67. doi: 10.11607/ijp.4358. [DOI] [PubMed] [Google Scholar]
- Dorner A.R., Ferraz S.J.M., Uemura E.S., Borges A.L., Fernandes Junior V.V.B., Yamamoto E.T.C. Effect of disinfection of irreversible hydrocolloid impression materials with 1% sodium hypochlorite on surface roughness and dimensional accuracy of dental stone casts. Eur. J. Gen. Dent. 2014;3:113–119. [Google Scholar]
- Duseja S., Shah R.J., Shah D.S., Duseja S. Dimensional measurement accuracy of recent polyether and addition silicone monophase impression materials after immersion in various disinfectants: An in vitro study. Int. J. Healthc. Biomed. Res. (IJHBR) 2014;2:87–97. [Google Scholar]
- German I.J.S., Rodrigues A.C., Andreo J.C., Pomini K.T., Ahmed F.J., Buchaim D.V., Junior G.M.R., Goncalves J.B.O., Buchaim R.L. Ozone therapy in dentistry: a systematic review. Int. J. Odontostomat. 2013;7:267–278. [Google Scholar]
- Godbole S.R., Dahane T.M., Patidar N.A., Nimonkar S.V. Evaluation of the effect of ultraviolet disinfection on dimensional stability of the polyvinyl silioxane impressions-an in vitro study. J. Clin. Diagn. Res. 2014;8:73–77. doi: 10.7860/JCDR/2014/8461.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraldo R.D., Berger S.B., Punhagui M.F., Moretto T.S., Lopes M.B., Gonini-Júnior A., Sinhoreti M.A.C. Influence of chloramine-T disinfection on elastomeric impression stability. Eur. J. Dent. 2018;12:232–236. doi: 10.4103/ejd.ejd_195_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Deepa D. Applications of ozone therapy in dentistry. J. Oral. Res. Rev. 2016;8:86–91. [Google Scholar]
- Ismail H.A., Asfour H., Shikho S.A. A self-disinfecting irreversible hydrocolloid impression material mixed with povidone iodine powder. Eur. J. Dent. 2016;10:507–511. doi: 10.4103/1305-7456.195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanis T., Babic J.Z., Lazic B., Panduric J. Dimensional stability of elastomeric impression materials disinfected in a solution of 0.5% chlorhexidine gluconate and alcohol. Acta Stomatol. Croat. 2000;34:11–14. [Google Scholar]
- Jagger D.C., Vowles R.W., McNally L., Davis F., O’Sullivan D.J. The effect of a range of disinfectants on the dimensional accuracy and stability of some impression materials. Eur. J. Prosthodont. Restor. Dent. 2007;15:23–28. [PubMed] [Google Scholar]
- Jeyapalan V., Krishnan C.S., Ramasubramanian H., Sampathkumar J., Azhagarasan N.S., Krishnan M. Comparative evaluation of the antimicrobial efficacy of three immersion chemical disinfectants on clinically derived polyvinyl siloxane impressions. J. Prosthodont. 2018;27:469–475. doi: 10.1111/jopr.12518. [DOI] [PubMed] [Google Scholar]
- Kalantari M.H., Malekzadeh A., Emami A. The effect of disinfection with sodium hypochlorite 0.5% on dimensional stability of condensation silicone impression materials of Speedex and Irasil. J. Dent. Shiraz. Univ. Med. Sci. 2014;15:98–103. [PMC free article] [PubMed] [Google Scholar]
- Kamble S.S., Khandeparker R.V., Somasundaram P., Raghav S., Babaji R.P., Varghese T.J. Comparative evaluation of dimensional accuracy of elastomeric impression materials when treated with autoclave, microwave, and chemical disinfection. J. Int. Oral Health. 2015;7:1–3. [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Purra A.R., Farooq R., Khatteb S.U., Ahmad F., Parvez P.A. Infection control in dental laboratories - a review. Int. J. Clin. Cases Investig. 2012;4:19–32. [Google Scholar]
- Kotsiomiti E., Tzialla A., Hatjivasiliou K. Accuracy and stability of impression materials subjected to chemical disinfection – a literature review. J. Oral. Rehab. 2008;35:291–299. doi: 10.1111/j.1365-2842.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Kotha S.B., Ramakrishnaiah R., Divakar D.D., Celur S.L., Qasim S., Matinlinna J.P. Effect of disinfection and sterilization on the tensile strength, surface roughness, and wettability of elastomers. J. Investig. Clin. Dent. 2017;8:e12244. doi: 10.1111/jicd.12244. [DOI] [PubMed] [Google Scholar]
- Lad P.P., Gurjar M., Gunda S., Gurjar V., Rao N.K. The effect of disinfectants and a surface wetting agent on the wettability of elastomeric impression materials: an in vitro study. J. Int. Oral Health. 2015;7:80–83. [PMC free article] [PubMed] [Google Scholar]
- Madhavan R., George N., Thummala N.R., Ravi S.V., Nagpal A. Self-disinfecting alginate vs conventional alginate: effect on surface hardness of gypsum cast-an in vitro study. J. Contemp. Dent. Pract. 2017;18:1061–1064. doi: 10.5005/jp-journals-10024-2176. [DOI] [PubMed] [Google Scholar]
- Mahalakshmi A.S., Jeyapalan V., Mahadevan V., Krishnan C.S., Azhagarasan N.S., Ramakrishnan H. Comparative evaluation of the effect of electrolyzed oxidizing water on surface detail reproduction, dimensional stability and surface texture of poly vinyl siloxane impressions. J. Ind. Prosthodont. Soc. 2019;19:33–41. doi: 10.4103/jips.jips_72_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S., Eini A., Gorfil C., Ben-Amar A., Slutzky H. Do dental impression materials play a role in cross contamination? Quintess. Int. 2011;42:e124–e130. [PubMed] [Google Scholar]
- Meghashri K., Kumar P., Prasad D.K., Hegde R. Evaluation and comparison of high-level microwave oven disinfection with chemical disinfection of dental gypsum casts. J. Int. Oral Health. 2014;6:56–60. [PMC free article] [PubMed] [Google Scholar]
- Melilli D., Rallo A., Cassaro A., Pizzo G. The effect of immersion disinfection procedures on dimensional stability of two elastomeric impression materials. J. Oral Sci. 2008;50:441–446. doi: 10.2334/josnusd.50.441. [DOI] [PubMed] [Google Scholar]
- Millar B.J., Deb S. Effect of autoclave sterilization on the dimensional stability and tear strength of three silicone impression materials. Open J. Stomatol. 2014;4:518–526. [Google Scholar]
- Munaz G., Fernandez A.J.F., Ali J.A., Moscardo A.P., Diago M.A.P. Effect of ozone therapy upon clinical and bacteriological parameters of the oral cavity: an update. J. Clin. Exp. Dent. 2011;3:e325–e327. [Google Scholar]
- Nassar U., Flores-Mir C., Heo G., Torrealba Y. The effect of prolonged storage and disinfection on the dimensional stability of 5 vinyl polyether silicone impression materials. J. Adv. Prosthodont. 2017;9:182–187. doi: 10.4047/jap.2017.9.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar S.V., Belkhode V.M., Godbole S.R., Nimonkar P.V., Dahane T., Sathe S. Comparative evaluation of the effect of chemical disinfectants and ultraviolet disinfection on dimensional stability of the polyvinyl siloxane impressions. J. Int. Soc. Prevent. Communit. Dent. 2019;9:152–158. doi: 10.4103/jispcd.JISPCD_406_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P.K., Kamble S.S., Chaurasia R.R., Chaurasia V.R., Tiwari S., Bansal D. Evaluation of different disinfectants on dimensional accuracy and surface quality of Type IV gypsum casts retrieved from elastomeric impression materials. J. Int. Oral Health. 2014;6:77–81. [PMC free article] [PubMed] [Google Scholar]
- Panza L.H.V., Porto V.C., Salvador M.C.G., Silva Rosa O.P. Evaluation of dimensional stability of impression materials immersed in disinfectant solutions using a metal tray. Revista. Odonto. Ciência. 2005;20:319–323. [Google Scholar]
- Poulis N., Kyriacou A., Kotsou M., Bezirtzogou E., Prombonas A., Drakoulis N. Effectiveness of low-flow high-ozone concentration disinfection of dental impressions: a comparative study to immersion disinfection. Br. J. Appl. Sci. Technol. 2014;4:2528–2537. [Google Scholar]
- Poulis N., PromBonas A., Yannikakis S., Karampotsos T., Katsarou M.S., Drakoulis N. Preliminary SEM observations on the surface of elastomeric impression materials after immersion or ozone disinfection. J. Clin. Diagn. Res. 2016;10:1–5. doi: 10.7860/JCDR/2016/20330.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad F.H., Ghaffari T., Safavi S.H. In vitro evaluation of dimensional stability of alginate impressions after disinfection by spray and immersion methods. Den. Res. Den. Clin. Den. Prosp. (JODDD) 2010;4:130–135. doi: 10.5681/joddd.2010.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnaiah R., Al Kheraif A.A., Bin Qasim S.S. The effect of chemical disinfection, autoclave and microwave sterilization on the dimensional accuracy of polyvinylsiloxane elastomeric impression materials. World. Appl. Sci. J. 2012;17:127–132. [Google Scholar]
- Reddy S.M., Vijitha D., Karthikeyan S., Balasubramanian R., Satish A. Evaluation of dimensional stability and accuracy of autoclavable polyvinyl siloxane impression material. J. Indian. Prosthodont. Soc. 2013;13:546–550. doi: 10.1007/s13191-012-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzia A., Coleman D.C., O’Donnell M.J., Dowling A.H., O’Sullivan M. Disinfection procedures: Their efficacy and effect on dimensional accuracy and surface quality of an irreversible hydrocolloid impression material. J. Dent. 2011;39:133–140. doi: 10.1016/j.jdent.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Saber F.S., Abolfazli N., Kohsoltani M. The Effect of disinfection by spray atomization on dimensional accuracy of condensation silicone impressions. Den. Res. Den. Clin. Den. Prosp. (JODDD) 2010;4:124–129. doi: 10.5681/joddd.2010.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabi O., Nejatidanesh F., Bagheri K.P., Karimi L., Savabi G. Prevention of cross-contamination risk by disinfection of irreversible hydrocolloid impression materials with ozonated water. Int. J. Prev. Med. 2018;9:37. doi: 10.4103/ijpvm.IJPVM_143_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S., Kamat G., Shetty R. Wettability changes in polyether impression materials subjected to immersion disinfection. Den. Res. J. 2013;10:539–544. [PMC free article] [PubMed] [Google Scholar]
- Silva S.M., Salvador M.C. Effect of the disinfection technique on the linear dimensional stability of dental impression materials. J. Appl. Oral. Sci. 2004;12:244–249. doi: 10.1590/s1678-77572004000300016. [DOI] [PubMed] [Google Scholar]
- Sinobad T., Djuricic K.O., Nikolic Z., Dodic S., Lazic V., Sinobad V., Rokvic A.J. The effect of disinfectants on dimensional stability of addition and condensation silicone impressions. Vojnosanit. Pregl. 2014;71:251–258. doi: 10.2298/vsp120709037s. [DOI] [PubMed] [Google Scholar]
- Soganci G., Cinar D., Caglar A., Yagiz A. 3D evaluation of the effect of disinfectants on dimensional accuracy and stability of two elastomeric impression materials. Dent. Mater. 2018;37:675–684. doi: 10.4012/dmj.2017-097. [DOI] [PubMed] [Google Scholar]
- Sousa J.C., Tabio A.M., Silva A., Pereira T., Maia B.S., Vasconcelos M. The effect of water and sodium hypochlorite disinfection on alginate impressions. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2013;54:8–12. [Google Scholar]
- Thota K.K., Jasthi S., Ravuri R., Tella S. A comparative evaluation of the dimensional stability of three different elastomeric impression materials after autoclaving – an in vitro study. J. Clin. Diagn. Res. 2014;8:48–50. doi: 10.7860/JCDR/2014/9768.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsal A., Prasadh S., Deepamala S., Patil A., Sulochana K., Shruthi D.P. Comparative evaluation of dimensional changes of elastomeric impression materials after disinfection with glutaraldehyde and microwave irradiation. J. Int. Oral Health. 2015;7:44–46. [Google Scholar]
- Walker M.P., Rondeau M., Petrie C., Tasca A., Williams K. Surface quality and long-term dimensional stability of current elastomeric impression materials after disinfection. J. Prosthodont. 2007;16:343–351. doi: 10.1111/j.1532-849X.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Wan Q., Chao Y., Chen Y. A self-disinfecting irreversible hydrocolloid impression material mixed with chlorhexidine solution. Angle Orthodont. 2007;77:894–900. doi: 10.2319/070606-277. [DOI] [PubMed] [Google Scholar]
- Yilmaz H., Aydin C., Gul B., Yilmaz C., Semiz M. Effect of disinfection on the dimensional stability of polyether impression materials. J. Prosthodont. 2007;16:473–479. doi: 10.1111/j.1532-849X.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- Zhang W., Mao H., Zhou G. Effect of ultraviolet radiation combined with immersion disinfection of silicone impressions infected with hepatitis B virus and HIV. Biomed. Res. 2017;28:6377–6380. [Google Scholar]
References
Further reading
- Goel K., Gupta R., Solanki J., Nayak M. A comparative study between microwave irradiation and sodium hypochlorite chemical disinfection: a prosthodontic view. J. Clin. Diagn. Res. 2014;8:42–46. doi: 10.7860/JCDR/2014/8578.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler U., Budak Y., Ruh E., Ocal Y., Canay S., Akyon Y. Effect of mixing techniques on bacterial attachment and disinfection time of polyether impression material. Eur. J. Dent. 2013;7:54–59. doi: 10.4103/1305-7456.119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.S., Rueggeberg F., Ramos V., Jr. Effects of chlorine-based and quaternary ammonium-based disinfectants on the wettability of a polyvinyl siloxane impression material. J. Prosthet. Dent. 2017;117:266–270. doi: 10.1016/j.prosdent.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Nagamatsu Y., Chen K.K., Nagamatsu H., Kozono Y., Shimizu H. Application of neutral electrolyzed water to disinfection of alginate impression. Dent. Mater. J. 2016;35:270–277. doi: 10.4012/dmj.2015-132. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Wright P.S., Maryan C. Disinfection procedures: their effect on the dimensional accuracy and surface quality of irreversible hydrocolloid impression materials and gypsum casts. Dent. Mater. 2002;18:103–110. doi: 10.1016/s0109-5641(01)00027-6. [DOI] [PubMed] [Google Scholar]