Significance
The world is facing a biodiversity crisis, with up to half a million species under imminent threat of extinction over coming decades. Retaining remaining natural habitat for biodiversity is crucial in limiting extinctions; however, there is ongoing debate as to which areas are the most important to retain and protect. One strategy suggests proactively retaining large contiguous areas with the least human impacts, such as wilderness, while another strategy argues we should focus on areas of remnant habitat in highly modified regions. Our analysis integrates these two perspectives, identifying high-value biodiversity habitat globally, in both intact and highly modified regions. Most of this high-value habitat remains unprotected, requiring focused conservation commitments and actions in order to halt ongoing extinctions.
Keywords: community, condition, conservation, contextual intactness, ecosystem
Abstract
Degradation and loss of natural habitat is the major driver of the current global biodiversity crisis. Most habitat conservation efforts to date have targeted small areas of highly threatened habitat, but emerging debate suggests that retaining large intact natural systems may be just as important. We reconcile these perspectives by integrating fine-resolution global data on habitat condition and species assemblage turnover to identify Earth’s high-value biodiversity habitat. These are areas in better condition than most other locations predicted to have once supported a similar assemblage of species and are found within both intact regions and human-dominated landscapes. However, only 18.6% of this high-value habitat is currently protected globally. Averting permanent biodiversity loss requires clear, spatially explicit targets for retaining these unprotected high-value habitats.
The world is facing a biodiversity crisis, with up to half a million species under imminent threat of extinction over the coming decades (1–4). The most important threat to biodiversity is ongoing degradation and loss of natural habitat (4–6), with more than half of the world’s land surface now under human-dominated land uses (7). Retaining remaining natural habitat is a crucial response to limiting further extinctions, with recent proposals for post-2020 protected area targets of 30% of the planet by 2030 (8) and more ambitious calls for protection of half the terrestrial biosphere by 2050 (9–11).
While there is agreement on the need to retain natural habitat to prevent species extinctions, division remains over which areas are most important for focusing the limited resources available for conservation. Brooks et al. (12) first characterized two contrasting general strategies to prioritizing habitat retention: “Proactive” approaches prioritize retention of the most intact natural systems, such as large areas of wilderness (13–15), whereas “reactive” approaches highlight the benefits of protecting and managing hotspot areas where many threatened species occur within more degraded regions (12, 16, 17). Reconciling these differing perspectives is crucial in identifying which areas we need to most urgently protect and manage in order to best promote the persistence of biodiversity globally (18). This is particularly important in light of discussions around a post-2020 biodiversity framework under the Convention on Biological Diversity (CBD) (19, 20).
Proactive and reactive habitat conservation strategies diverge in how they consider habitat condition—the degree to which a location has been impacted by human activities, such as land-use change, vegetation disturbance, hunting, or invasive alien species. Proactive strategies focus primarily on locations with the highest habitat condition that are surrounded by large areas also in the best condition (13–15). In contrast, reactive strategies focus on those locations supporting species, communities, or ecosystem types that have suffered the greatest reductions in habitat condition across their range, providing an indication of their vulnerability to future degradation (12, 16, 17).
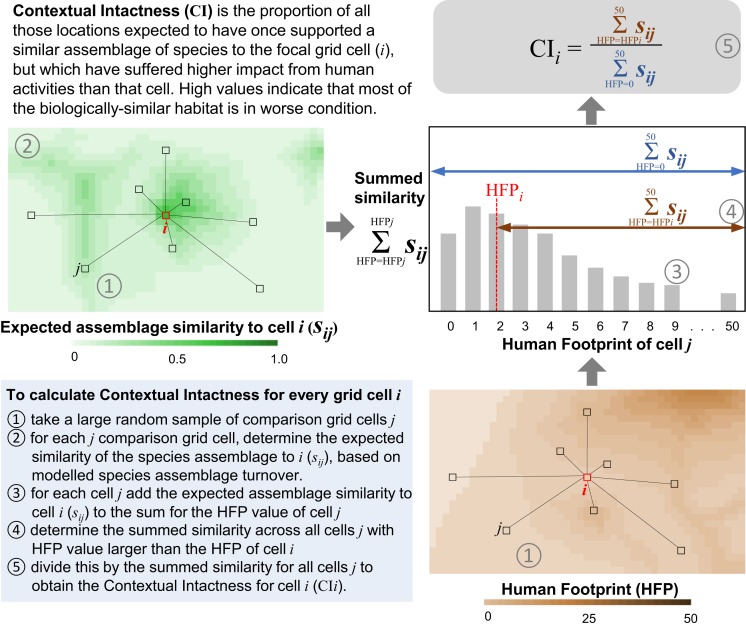
Here, we present an analytical framework that reconciles these differences to help identify priority areas around the world where protection and management will best promote biodiversity persistence. Our approach effectively integrates both the condition of a location itself and the condition of all other locations expected to have supported shared species prior to any habitat degradation. To reconcile proactive and reactive conservation strategies, we analyze where the habitat condition of a given location sits relative to the frequency distribution of condition levels for all biologically similar locations—a property we call “contextual intactness.”
Our analysis combines fine-resolution (1 km) global datasets on habitat condition and spatial biodiversity patterns (SI Appendix). We apply an updated map of the terrestrial human footprint (HFP) on natural systems to describe current habitat condition (SI Appendix, Fig. S1) (21). To inform the biodiversity value of each location, we harness generalized dissimilarity models of species-assemblage turnover for terrestrial vertebrates, invertebrates, and plants, derived by using more than 100 million occurrence records from more than 400,000 species (22, 23). These models enable the estimation of the similarity in species assemblages between any pair of locations and, subsequently, the expected uniqueness of the biodiversity within any terrestrial location globally.
Results and Discussion
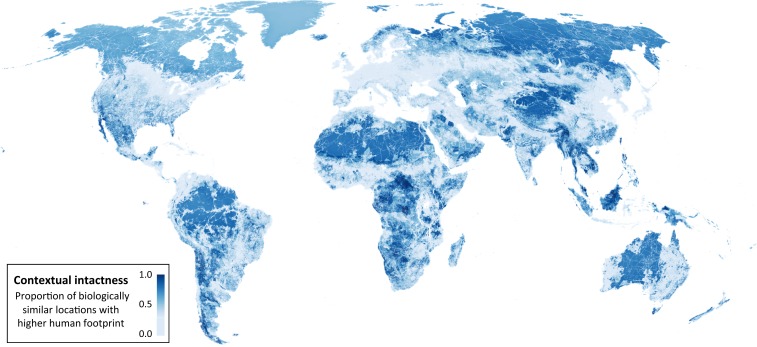
By coupling information on species composition and habitat condition, we assessed the contextual intactness of each location (1-km grid cell) by determining the proportion of habitat predicted to have once supported a similar assemblage of species, but is now in worse condition than the focal location (Fig. 1). Locations with the highest contextual intactness are where all other biologically similar locations are in a worse condition. We found areas of high contextual intactness in every biogeographic realm (Fig. 2), spanning regions with large coverage of wilderness, as well as regions with largely degraded landscapes.
Fig. 1.
Conceptual depiction of the analytical approach to quantifying the contextual intactness for each terrestrial 1-km grid cell globally.
Fig. 2.
Contextual intactness of habitat for biodiversity. For each location, the proportion of habitat expected to have once supported a similar assemblage of species but is now in worse condition than the focal location (higher HFP). The result is averaged across three broad terrestrial taxonomic groups: vertebrates, invertebrates, and vascular plants.
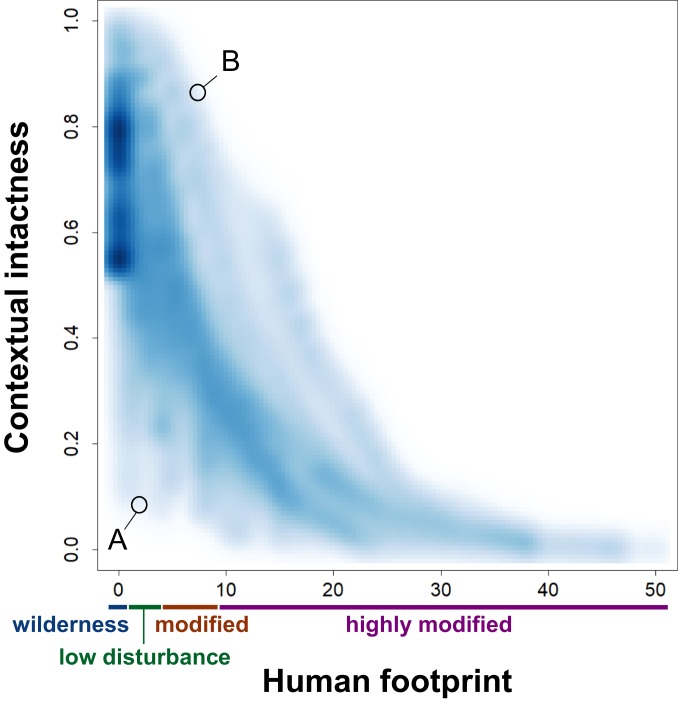
Because contextual intactness integrates both habitat condition and patterns in species-assemblage turnover, it varies markedly within a given level of HFP (Fig. 3). For example, habitat at the edge of wilderness areas often has a higher contextual intactness than core areas, as these edge locations are more likely to share species with locations outside of wilderness and subject to higher levels of human impact (Fig. 2). Indeed, our analysis shows that a location with a higher HFP can still have a higher contextual intactness value, if it is the “best-on-offer” in terms of the condition of all other locations with similar expected species assemblages (Fig. 3). Such areas include remnant habitats within heavily modified regions, such as temperate biomes with extensive agricultural land use. Similarly, while each of the world’s terrestrial biomes contains some habitat with high contextual intactness (SI Appendix, Fig. S2), the distribution of contextual intactness across biomes does not directly align with the distribution of the HFP. This is due to a number of factors, including the degree to which human impacts are more prevalent in particular environments within a biome and the fact that neighboring biomes share some species in common. For example, temperate coniferous forests have a relatively low HFP, but they share some species with even less impacted boreal forests (SI Appendix, Fig. S2) (24).
Fig. 3.
Within a given level of HFP, there is wide variation in contextual intactness values for terrestrial biodiversity. For example, location A has low levels of disturbance but has a high proportion of similar habitat in wilderness areas. In contrast, location B has a higher level of HFP, but most of the areas with similar expected species assemblages are in even more highly modified areas. Data are a sample of 430,000 locations (1-km grid cells) shown as color density with kernel bandwidth of 0.75.
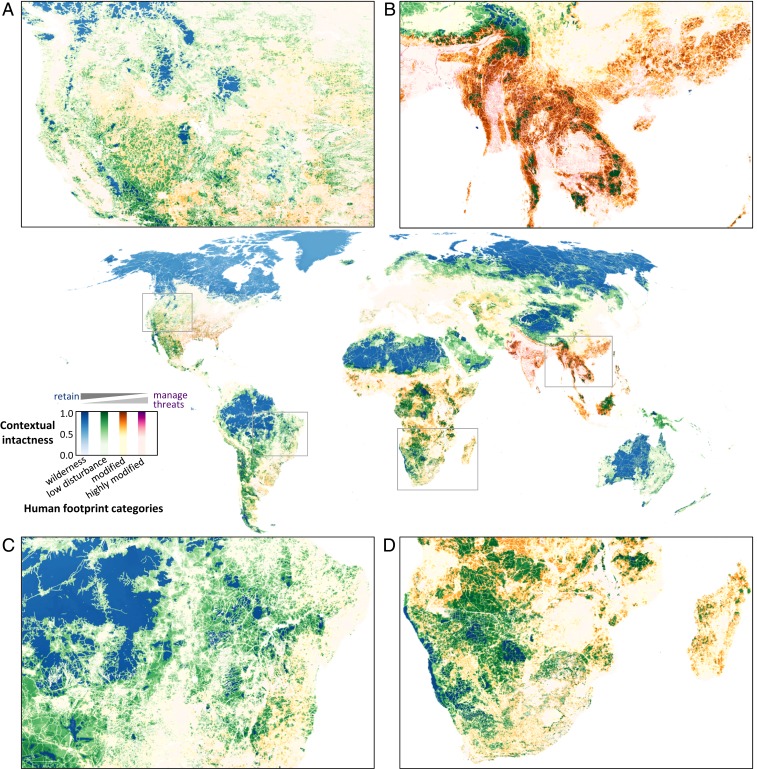
Our analysis integrates both proactive and reactive prioritization strategies into one cohesive conservation framework, which we demonstrate by mapping contextual intactness against four categories of the HFP—“wilderness,” “low disturbance,” “modified,” and “highly modified” (Fig. 4). This shows that while large intact areas are important, partially degraded fragmented habitat can also play a vital role in conserving biodiversity, given that these areas may support species that have lost a large proportion of their habitat elsewhere through human land use (Fig. 4). For example, the tropical forests of southeast Asia have experienced widespread degradation of natural habitats (25, 26), so even if remaining natural habitats are slightly modified, they are still of high value in sustaining the unique diversity of the region (Fig. 4B) (27).
Fig. 4.
High-value biodiversity habitat across levels of HFP. Contextual intactness is represented across four HFP categories: wilderness (blue; HFP = 0), low disturbance (green; HFP = 1 to 3), modified (orange; HFP = 4 to 9), and highly modified (pink; HFP ≥ 10). Darker shades represent higher contextual intactness. Panels show greater detail for northwest North America (A), southeast Asia (B), southeast Amazonia (C), and southern Africa (D).
In addition to the very broad taxonomic scope of our global assessment, a key feature is the spatial resolution at which our analyses are performed (Fig. 4), which is fine compared to many previous global conservation assessments typically implemented at the ecoregion or biome level (10, 12, 28). A fine spatial resolution helps account for local impacts of human activities on nature and the close affinities many species have with environmental and habitat features that can change over very short distances (29). While not obvious at the global scale, most heavily impacted regions contain very small areas of high-value habitat that is important for biodiversity conservation (Fig. 4). Our finely resolved assessment thus provides not only a measure of contextual intactness across the land surface of the planet, but does so at a spatial resolution of relevance to conservation policy, planning, and management at the regional or national level. Contextual intactness could be assessed at even finer resolutions, for regions with customized fine-scale models of assemblage similarity (30) and fine-scale spatial data on habitat condition. Priority actions for high-value habitat will vary from maintaining current low disturbance of wilderness areas to actively managing ongoing threatening processes in highly modified areas (Fig. 4).
To help inform progress toward renewed global protected area targets (8, 10, 11), we identified high-value biodiversity habitat as locations with contextual intactness greater than 0.5. These are locations which are in better condition than more than half of the areas expected to have supported a similar assemblage of species, and, hence, they cover approximately half the land surface of the planet. We then assessed the degree to which these high-value habitats occur within protected areas, finding that only 18.6% of the world’s high-value habitat for biodiversity is currently protected (SI Appendix, Fig. S3). Therefore, protected areas are only slightly better than random at safeguarding the highest-value habitat for biodiversity around the world, given that 14.9% of Earth’s land surface is protected (31). The largest areas of unprotected high-value biodiversity habitat are in wilderness and areas experiencing low human disturbance (SI Appendix, Fig. S3) (15). However, high-value habitat in regions that have been more heavily modified has an even lower proportional coverage in protected areas, reducing to 6% protected (SI Appendix, Fig. S3).
There have been decades of debate on whether it is more urgent to preserve intact landscapes or to protect areas facing high human pressure (12–17). Our findings demonstrate that this conservation dichotomy is unnecessarily artificial and that the situation is actually more nuanced, with high-value biodiversity habitat spanning all levels of human pressure. These results show that the contribution of each location to the persistence of biodiversity is entirely dependent on the regional and environmental context (32), through the fine-scale spatial patterns in both biodiversity and habitat condition. Our analysis effectively considers the representation of a very wide range of species in remaining habitat of varying condition. Preserving the high-value areas identified should be integral to the international commitments to halt biodiversity loss (33), as habitat degradation there would make it much harder to achieve international biodiversity targets.
Retaining high-value habitat for biodiversity also requires supportive measures beyond establishing protected areas, including strong legislation and programs to limit threats to intact habitat (4). This includes establishing and policing laws that limit land clearing and forest degradation, in addition to programs that support sustainable socioeconomic development outside of high-value habitat areas. Jurisdictions where habitat-protection laws and policies have been weakening, rather than strengthening (34, 35), need to be encouraged and supported by the global community to identify ways to improve human wellbeing while retaining the habitat required to support the Earth’s biodiversity (4).
Given the irreversibility of species extinctions, society must act now to retain the Earth’s unique evolutionary heritage. The high-value habitat we have identified will be crucial for the persistence of biodiversity into the future, requiring strong commitments by governments, businesses, and society to stop their loss and degradation.
Methods
Broad Approach.
The broad approach of our analysis was to assess the contextual intactness of every terrestrial location across the globe, by combining data on two features: 1) the current habitat condition of every location, and 2) the expected similarity of the species assemblage occurring in each location relative to every other location. To quantify the current habitat condition, we applied a map of the HFP (21), updated to the year 2013, using some revised spatial datasets, as described below. To quantify the expected similarity in species assemblages between any pair of locations, we utilized generalized dissimilarity models of species-assemblage turnover for terrestrial vertebrates, invertebrates, and plants across the terrestrial surface of the globe (22). These models are also described in more detail below. Using these two data sources, we developed and applied an analysis to derive the contextual intactness across the globe, which for each location is the proportion of habitat that would be expected to host a similar assemblage of species, but is in a worse condition (Fig. 1).
Human Footprint.
The HFP is a standardized measure of the cumulative HFP on the terrestrial environment at 1-km resolution (21). The original global HFP layer was developed by Sanderson et al. (36) based on data from the early 1990s, considering four types of data as proxies for human influence: 1) human population density, 2) land transformation, 3) human access, and 4) electrical power infrastructure. Datasets representing these features were weighted and summed to derive an HFP score ranging from 0 to 72 (36).
To provide an updated assessment of human pressures on the environment, Venter et al. (21) utilized data centered around two timepoints: 1993 and 2009. This analysis harnessed data on eight human pressures: 1) extent of built environments, 2) crop land, 3) pasture land, 4) human population density, 5) night-time lights, 6) railways, 7) roads, and 8) navigable waterways. These pressures were weighted following the approach of Sanderson et al. (36) and then summed to create the standardized HFP for all non-Antarctic land areas, ranging from 0 to 50 (21, 37).
Here, we apply a revised version of the HFP assessment undertaken by Venter et al. (21). The same approach and weighting scheme was used, but harnessing updated datasets centered on the year 2013. This resulted in the same 0 to 50 range of HFP scores, but with each location having a value that reflects the more up-to-date impacts of human activities on the natural environment (SI Appendix, Fig. S1).
In communicating the results of our analysis, we applied four categories of the HFP, based on extensive experience of the coauthors with the HFP index and other studies (7, 38, 39): “wilderness” (HFP = 0), “low disturbance” (HFP = 1 to 3), “modified” (HFP = 4 to 9), and “highly modified” (HFP ≥ 10).
Similarity of Species Assemblages.
To obtain estimates of the similarity in species assemblages between any pair of terrestrial locations, we utilized generalized dissimilarity models derived by Hoskins et al. (22) in the Biogeographic Infrastructure for Large-scaled Biodiversity Indicators (BILBI) modeling framework. Rather than modeling each species individually, this approach models the similarity in the species assemblages between pairs of locations (30-arcsecond grid cells – ∼1 km) based on the environmental conditions at each location and the geographic distance between them. These models then enable prediction of the expected similarity in species assemblage based on spatially complete environmental surfaces. This approach overcomes biases and deficiencies of raw biological data across different regions of the planet, enabling comprehensive biodiversity assessments from regional to global scales (22). While we present an overview of the modeling of pairwise assemblage similarity here, a full description is provided by Hoskins et al. (22).
The BILBI framework includes models of similarity in species assemblage for three broad taxonomic groups: vertebrates, invertebrates, and vascular plants. For each of these three broad taxonomic groups, species occurrence records were obtained from the Global Biodiversity Information Facility and then filtered for accuracy. The subsequent modeling utilized 41,082,043 records for vertebrates (across 24,442 species), 13,244,784 records for invertebrates (across 132,761 species), and 52,489,096 records for vascular plants (across 254,145 species).
As predictors of site–pair species-assemblage similarity, the BILBI framework utilizes a standard set of 15 environmental variables, including five soil variables (bare ground, bulk density, clay, pH, and silt) (40), two terrain variables (topographic roughness index and topographic wetness index) (41, 42), and eight topographically adjusted climate variables (annual precipitation, annual minimum temperature, annual maximum temperature, maximum monthly diurnal temperature range, annual actual evaporation, potential evaporation of driest month, and maximum and minimum monthly water deficit) (43, 44).
Models of species assemblage similarity were fit by using an extension of generalized dissimilarity modeling (GDM) (30), where the response variable is the probability that a pair of species records drawn randomly from two locations represent two different species rather than the same species. This modeled probability was then back‐transformed to the common zero to one measure of pairwise similarity in ecological communities (similar to the Sørensen index) for prediction and analysis (22). For each of the three broad biological groups (vertebrates, invertebrates, and vascular plants), individual models were developed for each of the 61 unique biorealms defined in the World Wildlife Fund’s nested biogeographic realm, biome, and ecoregion framework (45), with species records in neighboring biorealms also used for model fitting. Model performance varied across taxonomic groups and biorealms, with an average Mann–Whitney U statistic of 0.73 for vertebrates, 0.70 for invertebrates, and 0.79 for plants.
The models of site-pair similarity in species assemblages were projected spatially as GDM-transformed grids, using the environmental predictor layers. The GDM-transformed grids enable subsequent prediction of the similarity in species assemblage between any pair of grid cells, hence providing the capacity for a broad range of analyses, rather than providing just a single spatial layer. For example, the BILBI framework has been applied to assess the impacts of global climate and land-use scenarios on plant biodiversity (23), the importance of wilderness areas for the global persistence of biodiversity (15), and forms the basis for two indicators endorsed by the CBD for reporting against Aichi Targets: 1) the Protected Area Representativeness and Connectedness index, and 2) the Biodiversity Habitat Index (https://bipdashboard.natureserve.org).
Quantifying contextual intactness.
To determine the contextual intactness for each location across the globe, we combined the revised HFP spatial layer and the predicted similarity in species assemblages between pairs of locations using the BILBI framework (Fig. 1). For each grid cell i, we selected a spatially regular randomly positioned selection of n other grid cells j to compare to cell i. A sample of comparison cells is required because there are >200 million cells on the 1-km terrestrial grid of the planet, and comparing each grid cell with every other grid cell is computationally prohibitive. For this assessment, the number of other grid cells j was a minimum of 1% of the total grid cells within each of the world’s seven biogeographic realms (Antarctica being excluded) (45).
We then determined the expected similarity (sij) in species assemblages between cell i and each comparison cell j using the BILBI framework (22). The HFP value for cell i (HFPi) and all comparison cells j (HFPj) was also extracted. We then derived a histogram of the summed species assemblage similarity to grid cell i, within integer bands of the HFP value for all of the comparison cells j . From this histogram, we then calculated 1) the sum of the assemblage similarities to i where the comparison cell j had a higher HFP to i , and 2) the total sum of the all of the assemblage similarities between i and j across all HFP scores . The contextual intactness for grid cell i (CIi) was then calculated as the sum of assemblage similarities to i with a higher HFP divided by the total sum of assemblage similarities to i (Fig. 1):
This calculation was repeated for every terrestrial grid cell globally to derive a spatial map of contextual intactness for each taxonomic group (vertebrates, invertebrates, and plants). The spatial layers for these three taxonomic groups were then averaged to derive a single contextual intactness layer for biodiversity (Fig. 2), though future analyses could consider each taxonomic group separately.
While a spatially explicit assessment of confidence for our derived contextual intactness index would be useful, such an assessment currently poses substantial challenges. First, the HFP layer is a composite of a variety of spatial data conceptually linked to human impacts on natural systems (21), and while it has been visually validated (37), there is no direct on-ground measure to use in assessing confidence for this product. Second, the estimates of assemblage similarity are for pairs of locations, not individual points, making it difficult to simplify confidence to locations. The nonlinear spline functions applied in modeling assemblage similarity (22) further complicate derivation of a simple and meaningful confidence metric for the contextual intactness metric presented.
It is also worth noting that there may be a signal of habitat modification in the models of species assemblage similarity we applied. This would be due to the biological records used in model fitting being influenced by both loss of native species and addition of alien species in regions with substantial human disturbance (46). However, as the contextual intactness index focuses on the proportion of biologically similar locations in worse condition (Fig. 1), rather than the predicted amount of similarity, we believe this is not a major issue for the present analysis.
Protected Area Assessment.
To determine the coverage of high-value habitat within protected areas, we first identified high-value intact habitat as those grid cells with contextual intactness value greater than 0.5. These are locations which are in a better condition (lower HFP) than more than half of the similar habitat. We rasterized data from the World Database on Protected Areas (http://www.protectedplanet.net) extracted in August 2018 to 1-km resolution and converted it to a binary raster (protected or not protected). Following Butchart et al. (47) we excluded those internationally designated sites not considered as protected areas, excluded “proposed” sites and those with an unknown status, excluded marine-only sites as well as the marine portion of coastal sites, and finally represented sites without a defined shape as geodetic buffers of the appropriate area. Based on this rasterized protected area layer, we calculated the proportion of high-value habitat within protected areas (SI Appendix, Fig. S3).
Supplementary Material
Acknowledgments
We thank Sam Nicol and Sam Andrew for providing comments on an earlier version of this work. This research was supported collaboratively by Wildlife Conservation Society, University of Queensland, and Commonwealth Scientific and Industrial Research Organisation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The contextual intactness layer derived in this paper is available for download from the Commonwealth Scientific and Industrial Research Organisation Data Access Portal: https://doi.org/10.25919/5e7854cfcb97e.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918373117/-/DCSupplemental.
References
- 1.De Vos J. M., Joppa L. N., Gittleman J. L., Stephens P. R., Pimm S. L., Estimating the normal background rate of species extinction. Conserv. Biol. 29, 452–462 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Johnson C. N., et al. , Biodiversity losses and conservation responses in the Anthropocene. Science 356, 270–275 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Ceballos G., et al. , Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services , “Status and trends—Nature,” in IPBES Global Assessment on Biodiversity and Ecosystem Services, Ichii K., Molnar Z., Obura D., Purvis A., Willis K. J., Eds. (IPBES Secretariat, Bonn, Germany, 2019), chap. 2.2, pp. 1–170. [Google Scholar]
- 5.Hoffmann M., et al. , The impact of conservation on the status of the world’s vertebrates. Science 330, 1503–1509 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Newbold T., et al. , Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Watson J. E. M., et al. , Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conserv. Lett. 9, 413–421 (2016). [Google Scholar]
- 8.Dinerstein E., et al. , A global deal for nature: Guiding principles, milestones, and targets. Sci. Adv. 5, eaaw2869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke H., Nature needs half: A necessary and hopeful new agenda for protected areas. Parks 19, 13–22 (2013). [Google Scholar]
- 10.Dinerstein E., et al. , An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson J. E. M., Venter O., Ecology: A global plan for nature conservation. Nature 550, 48–49 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Brooks T. M., et al. , Global biodiversity conservation priorities. Science 313, 58–61 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Watson J. E. M., et al. , Catastrophic declines in wilderness areas undermine global environment targets. Curr. Biol. 26, 2929–2934 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Watson J. E. M., et al. , The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Di Marco M., Ferrier S., Harwood T. D., Hoskins A. J., Watson J. E. M., Wilderness areas halve the extinction risk of terrestrial biodiversity. Nature 573, 582–585 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Veach V., Moilanen A., Di Minin E., Threats from urban expansion, agricultural transformation and forest loss on global conservation priority areas. PLoS One 12, e0188397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wintle B. A., et al. , Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. U.S.A. 116, 909–914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marco M., Watson J. E. M., Venter O., Possingham H. P., Global biodiversity targets require both sufficiency and efficiency. Conserv. Lett. 9, 395–397 (2016). [Google Scholar]
- 19.Visconti P., et al. , Protected area targets post-2020. Science 364, 239–241 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Convention on Biological Diversity, “Zero draft of the post 2020 global biodiversity framework” (Convention on Biological Diversity, Montreal, Canada, 2020).
- 21.Venter O., et al. , Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoskins A. J., et al. , Supporting global biodiversity assessment through high-resolution macroecological modelling: Methodological underpinnings of the BILBI framework. bioRxiv:10.1101/309377 (4 June 2019).
- 23.Di Marco M., et al. , Projecting impacts of global climate and land-use scenarios on plant biodiversity using compositional-turnover modelling. Glob. Change Biol. 25, 2763–2778 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Nilsson S. G., “Forests in the temperate–boreal transition—natural and man-made features,” in Ecological Principles of Nature Conservation: Application in Temperate and Boreal Environments, Hansson L., Ed. (Springer US, Boston, MA, 1992), pp. 373–393. [Google Scholar]
- 25.De Palma A., et al. , Annual changes in the Biodiversity Intactness Index in tropical and subtropical forest biomes, 2001-2012. bioRxiv:10.1101/311688 (21 March 2019). [DOI] [PMC free article] [PubMed]
- 26.Hansen M. C., et al. , High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Wilcove D. S., Giam X., Edwards D. P., Fisher B., Koh L. P., Navjot’s nightmare revisited: Logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol. Evol. 28, 531–540 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Brauneder K. M., et al. , Global screening for critical habitat in the terrestrial realm. PLoS One 13, e0193102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschalk T., Aue B., Hotes S., Ekschmitt K., Influence of grain size on species-habitat models. Ecol. Modell. 222, 3403–3412 (2011). [Google Scholar]
- 30.Ferrier S., Manion G., Elith J., Richardson K., Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 13, 252–264 (2007). [Google Scholar]
- 31.United Nations Environment World Conservation Monitoring Centre, International Union for Conservation of Nature, National Geographic Society , “Protected planet report 2018” (Job No. DEP/2203/CA, United Nations Environment Programme, Cambridge, UK, 2018).
- 32.Sacre E., Bode M., Weeks R., Pressey R. L., The context dependence of frontier versus wilderness conservation priorities. Conserv. Lett. 12, e12632 (2019). [Google Scholar]
- 33.Convention on Biological Diversity , “Post-2020 global biodiversity framework: Discussion paper” (CBD/POST2020/PREP/1/1, Convention on Biological Diversity, Montreal, Canada, 2019).
- 34.Evans M. C., Deforestation in Australia: Drivers, trends and policy responses. Pac. Conserv. Biol. 22, 130–150 (2016). [Google Scholar]
- 35.Freitas F. L. M., et al. , Potential increase of legal deforestation in Brazilian Amazon after forest act revision. Nat. Sustain. 1, 665–670 (2018). [Google Scholar]
- 36.Sanderson E. W., et al. , The human footprint and the last of the wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. Bioscience 52, 891–904 (2002). [Google Scholar]
- 37.Venter O., et al. , Global terrestrial human footprint maps for 1993 and 2009. Sci. Data 3, 160067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Bryan C. J., et al. , Intense human pressure is widespread across terrestrial vertebrate ranges. Glob. Ecol. Conserv. 21, e00882 (2020). [Google Scholar]
- 39.Jones K. R., et al. , One-third of global protected land is under intense human pressure. Science 360, 788–791 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Hengl T., et al. , SoilGrids1km—Global soil information based on automated mapping. PLoS One 9, e105992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannes R., Hengl T. K., “Worldgrids—A public repository of global soil covariates” in Digital Soil Assessments and Beyond: Proceedings of the 5th Global Workshop on Digital Soil Mapping, Minasny B., Malone B. P., McBratney A. B., Eds. (CRC Press, 2012), p. 287. [Google Scholar]
- 42.Amatulli G., et al. , A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Sci. Data 5, 180040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 44.Reside A. E., et al. , “Climate change refugia for terrestrial biodiversity: Defining areas that promote species persistence and ecosystem resilience in the face of global climate change” (NCCARF Publication 73/13, National Climate Change Adaptation Research Facility, Gold Coast, Australia, 2013).
- 45.Olson D. M., et al. , Terrestrial ecoregions of the world: A new map of life on earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51, 933–938 (2001). [Google Scholar]
- 46.McKinney M. L., Lockwood J. L., Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Butchart S. H. M., et al. , Shortfalls and solutions for meeting national and global conservation area targets. Conserv. Lett. 8, 329–337 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.