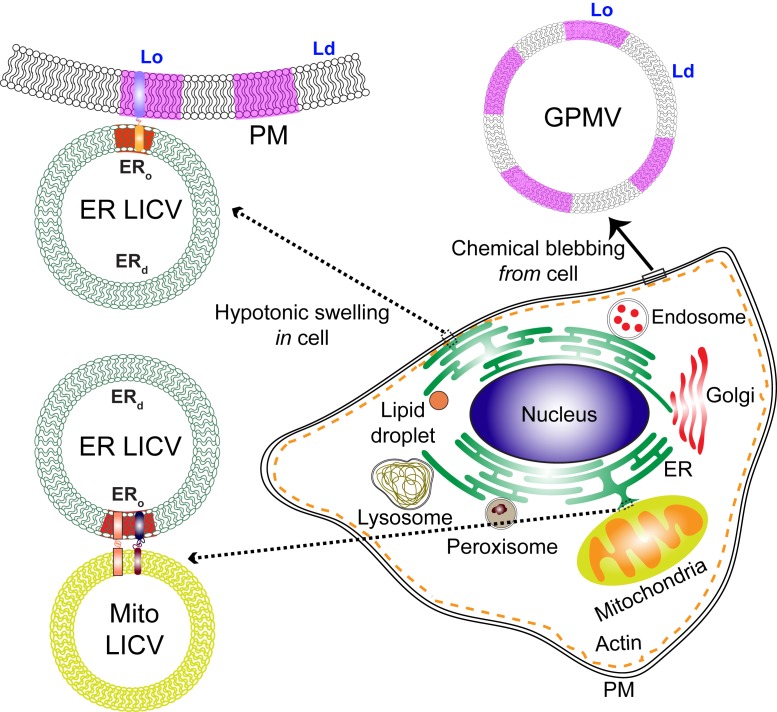
Functioning cells depend on the outward-facing plasma membrane (PM) effectively contacting the endoplasmic reticulum (ER), which serves as a central hub for contacts with mitochondria and other intracellular organelles. The contact sites are critical to intracellular communication because they mediate intermembrane exchange of lipids, ions, and other small molecules that both maintain competent organelles and modulate their activities. The targeting of molecular interactions within and between these cellular membranes underlies a cell’s vitality in its homeostatic environment, and, using the same dynamic infrastructure, the cell is also poised to respond to environmental stimuli. A major question driving current research efforts is how interorganelle contact sites are organized and regulated such that the membranes connect and exchange molecules appropriately. Protein partners serving as tethers have been demonstrated for some intermembrane contact sites, and lipid-based phase separation has been suggested as another possible means for selective targeting of contact-site proteins. However, detailed characterization of contact sites has been hindered by the complexity of the membrane interfaces, which interact dynamically within an intricately networked system (1). In a step to surmount this limitation, King et al. (2) describe the use of hypotonic swelling to create large intracellular vesicles (LICVs) from the ER and other organelles. LICVs both separate the organelle membranes and magnify organelle contacts, providing a relatively simple approach for examining the underlying organization of proteins and lipids. As part of their initial characterization, the authors identify tether proteins in LICV-retained contacts that are known to participate in molecular exchanges across these membranes. Especially exciting is their evidence that ER membranes have the capacity for order/disorder phase separation, which was previously characterized in giant PM vesicles (GPMVs) (3) and may play a similar role sorting proteins in contact sites (Fig. 1).
Fig. 1.
ER-LICVs are created within cells by hypotonic swelling and retain contacts with the PM and LICVs derived from other organelles. ER-LICVs show gel-like (ERo) and fluid (ERd) phase separation when cooled, resembling GPMVs, which bleb from chemically treated cells and separate into Lo-like and Ld-like phases. Coexisting phases provide a mechanism for sorting proteins, which may be important for communication in membrane–membrane contacts. Possible alignment of ordered domains and recruitment of tethering proteins in contact sites is described in the text.
Purification of contact sites in the form of conjoining organelle membranes, together with genetic, proteomic, and lipidomic characterization, previously provided information about protein and lipid composition that can be related to their function. Direct imaging of these connected structures in living cells has been limited by the crowded intracellular space and by the resolution of conventional microscopies. Recent advances have capitalized on sophisticated fluorescence or electron microscopies and computational analysis of complex images (1). However, many of these approaches are low-throughput, and the necessary expertise and equipment are not easily accessible to many researchers working on this problem. The authors found that incubation of cultured mammalian cells in hypotonic medium (5% Dulbecco’s modified Eagle’s medium) causes the network of ER tubules to swell and separate as spherical vesicles of roughly micrometer size, reminiscent of GPMVs. As detected and identified with fluorescent protein constructs, LICVs form from ER in 15 to 40% of the three cell lines tested, indicating the generality of their approach. LICVs form within a few minutes of treatment and are stable for up to an hour. The authors provide confocal microscopy images and analyses of individual cells to further characterize LICVs prepared in fibroblast-like COS7 cells. Fluorescence recovery after photobleaching of probes in ER membrane and lumen confirmed the ER-LICVs to be independent vesicles. They found that LICVs also form from endosomes, lysosomes, and mitochondria. ER-LICVs form visible contact regions with LICVs derived from these other organelles, as well as with peroxisomes, and lipid droplets, which do not form detectable LICVs. They identified several sets of tethering proteins previously shown to pair up in contact sites to facilitate exchange ions and lipids. For example, they found in the apposing membranes visible colocalization of protein pairs vesicle-associated membrane protein-associated protein-B (VAPB) (ER)/protein tyrosine phosphatase-interacting protein 51 (PTPIP51) (mitochondria) and mitofusins MFN1(mitochondria)/MFN2(mitochondria and ER).
In a step to surmount this limitation, King et al. describe the use of hypotonic swelling to create large intracellular vesicles (LICVs) from the ER and other organelles. LICVs both separate the organelle membranes and magnify organelle contacts, providing a relatively simple approach for examining the underlying organization of proteins and lipids.
LICVs appear to be especially valuable for examining lipid phase-like properties that have been suggested for organelle membranes but not previously detected by fluorescence microscopy. They appear similar in many ways to the GPMVs, which opened doors for examining phase separation in the PM and its functional relevance, as described below. Similar to GPMVs, ER-LICVs show microscopic phase separation when the temperature of the preparation medium is reduced below room temperature, as represented by fluorescent probes that prefer an ordered or a disordered membrane lipid environment. Previously, images of deuterated palmitate in intact cells, obtained with stimulated Raman scattering microscopy, showed that the ER lipids coalesce into ordered, gel-like domains upon up-shifts in palmitate metabolism (4). This was surprising because ER membranes are generally expected to be uniformly fluid because of lipid composition, including low levels of cholesterol. King et al. (2) found that more than half of the ER-LICVs in COS7 cells phase-separate when cooled, and this behavior reverses when the temperature is raised. Lateral diffusion of order-sensitive membrane probes in cooled ER-LICVs indicated one phase to be fluid (ERd) and the other phase to be gel-like (ERo). They further discovered that ER-LICV phase separation localizes in contacts with the PM or other organelles, pointing to a mechanism for preferential protein sorting in these regions. They found that the ERo-preferring probe aligns with apposing membranes in contacts with PM, endosomes, mitochondria, and lipid droplets, but not in contacts with lysosomes or peroxisomes. The authors make the interesting correlation that the PM or organelles contacting with the gel-like ERo domains of ER-LICVs are those with established roles in lipid transfer between these membranes. These findings suggest that proteins partnering to facilitate this process in the contacting membranes have a preference for an ordered phase and are thereby recruited to these regions. This points to another possibility that ordered phases align across the apposing membranes.
Participation of lipid-based phase separation to sort proteins in cellular membranes is an attractive concept but difficult to demonstrate experimentally because the heterogenous domains are dynamic and subdiffraction in size under most physiological conditions. So far, many studies have focused on the PM, where the “raft hypothesis” has been evaluated extensively (5). Order/disorder phase coexistence in the PM and organellar membranes may be quite subtle but can be harnessed to sort proteins and target their interactions. To illustrate, we draw on our own investigations of the transmembrane immunoreceptor (FcεRI) in the PM of mast cells, which binds antigen-specific immunoglobulin E (IgE) (6). In its homeostatic steady state, the PM is a heterogeneous distribution of lipids (and accompanying proteins) into nanoscale domains that resemble the liquid-ordered (Lo) and liquid-disordered (Ld) phases defined in model membranes (7). The term “raft” is associated with the ordered, Lo-like domains. Lo and Ld phases as they coexist during homeostasis are small and transient but poised for modulation. How does this manifest in mast cells? Prior to stimulation by antigen, FcεRI’s cytoplasmic tyrosines are not appreciably phosphorylated because of a balanced competition between Lyn tyrosine kinase and a transmembrane tyrosine phosphatase. The balance is disrupted by antigen-mediated cross-linking of IgE–FcεRI, which serves to stabilize an expanded Lo-like region around the clustered FcεRI. This stimulated modulation of membrane heterogeneity significantly increases the probability that the Lo-preferring kinase becomes more proximal and the Ld-preferring phosphatase becomes more distal to the clustered FcεRI, resulting in its net phosphorylation. These initiating events are followed by assembly of intracellular signaling proteins, leading to changes in ER contacts with the PM and with the mitochondria to induce Ca2+ flux, lipid exchange, and consequent cellular responses.
GPMVs have been key for testing the role of phase separation in sorting lipids and proteins in the PM of mast cells and multiple other cell systems. Like LICVs, GPMVs are micrometer-sized spherical vesicles and rather simply prepared. In contrast to LICVs, GPMVs bleb from living cells after a chemical treatment causes PM detachment from the actin cytoskeleton (3). The observation that cooled GPMVs separate reversibly into Lo-like and Ld-like phases cleared the way to evaluate phase preferences of Lyn kinase and other membrane lipids and proteins (8). GPMVs have also been valuable for developing other important hypotheses, such as suppression of PM phase separation by the cytoskeleton (9), which thereby acts as a brake to prevent co-compartmentalization of signaling proteins in the absence of an external stimulus (6).
Based on the success of GPMVs in uncovering membrane biophysics in the PM, we envisage that LICVs will offer exciting new opportunities for examining phase separation and its relevance in interorganelle communications. The initial development and characterization of LICVs in COS7 cells (2) should be readily translatable to other cell types. Some issues remain to be worked out, due in part to LICV creation within the cytoplasmic space where they are stable for a relatively short time. Many LICVs tend to jiggle in that dynamic environment, such that quantified imaging of individual LICVs and contacts is possible only for those vesicles that are relatively immobile. It may be possible to mitigate this limitation on sampling with a fixation technique after the LICVs are formed. With sufficient stabilization LICVs should be readily amenable to superresolution and light-sheet microscopies. Use of optical tweezers to examine the strength of contact junctures could also be very informative but may require that the LICVs be released from entrapping cells.
The relationship between LICVs and the physiological organelle membranes from which they originate is a question that will need further evaluation. Detachment of GPMVs from the cytoskeleton as well as their apparent swelling may contribute to the manifestation of their phase-like behavior. Are LICVs similarly released from some physical constraint when they detach as hypotonically swollen spheres from the ER? Other factors may affect phase-like properties. For example, what changes in bilayer composition or asymmetry result from the treatment? It is known that phosphatidylserine becomes exposed in mostly right-side-out GPMVs, and other lipid changes have also been detected. To what extent are lipids scrambled or altered in LICVs? Are some contacts created or destroyed during the swelling process? We expect that these and other questions will be addressed as LICVs are increasingly used as a tool in multiple laboratories to investigate organelles and intermembrane contacts. In comparison, GPMVs continue to be a valuable model for investigating phase properties and protein sorting in the PM, even as the limitations of GPMVs for this purpose continue to be assessed. An unavoidable limitation for both LICVs and GPMVs is that their energetic states differ from the nonequilibrium states of organelle or PMs in living cells.
What can LICVs tell us about the potential role of phase separation in membrane–membrane communication? As described above for the homeostatic PM in mast cells, Lo-like nanodomains are small and dynamic, and these are poised to be coalesced by antigen-mediated clustering of IgE–FcεRI receptors to facilitate coupling with kinase and consequent transmembrane signaling. A fascinating question is how subdiffraction phase separation in membranes of proximal organelles may be similarly coalesced to create contacts and to target functional proteins. We might speculate, for example, that contacts between ER and mitochondria are initiated by MFN1/MFN2 pairing, and this coalesces nanoscale ERo domains to recruit VAPB in the ER membrane, which then pairs with PIPTP51 in the mitochondrial membrane to facilitate lipid exchange (Fig. 1). Whether MFN2 and VAPB prefer an ERo environment can be tested directly with LICVs. As another possibility, ER contacts with the PM to facilitate extracellular entry of Ca2+ may be stabilized by alignment of ordered membrane domains across the connecting bilayers. Previous studies on mast cells indicated that engagement of the Ca2+ channel (Orai1) occurs in Lo-like domains of the PM (10), and coupling with its protein partner in the ER (STIM1) may be facilitated by an Lo domain aligning with an ERo domain in which STIM1 preferentially resides. Such hypotheses can now be evaluated with LICVs (Fig. 1).
Beyond elucidating the organization of lipids and proteins in contact sites is the overriding question of how this interorganelle communication is regulated under homeostatic conditions and how it is modulated by an external stimulus. There are many examples of metabolic, ion fluxes, autophagic, or other shifts that are mediated by changes in contacts after cell stimulation or by pathological events (1). In the mast cell example described above, antigen engagement with IgE–FcεRI stimulates changes in the contacts between ER and PMs to cause Ca2+ influx. Fluorescence imaging of LICVs may prove to be a valuable tool for elucidating molecular interactions before and after such induced modulation of contacts in cells. Overcoming the technical challenge of sufficient sampling of LICVs and robust statistics over multiple cells is particularly important in these applications because the changes may be subtle.
Acknowledgments
Research in our laboratory is supported by National Institute of General Medical Sciences grant R01 GM117552.
Footnotes
The authors declare no competing interest.
See companion article, “ER membranes exhibit phase behavior at sites of organelle contact,” 10.1073/pnas.1910854117.
References
- 1.Scorrano L., et al. , Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King C., Sengupta P., Seo A. Y., Lippincott-Schwartz J., ER membranes exhibit phase behavior at sites of organelle contact. Proc. Natl. Acad. Sci. U.S.A. 117, 7225–7235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart T., et al. , Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 104, 3165–3170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Y., et al. , Metabolic activity induces membrane phase separation in endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 114, 13394–13399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sezgin E., Levental I., Mayor S., Eggeling C., The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelby S. A., Veatch S. L., Holowka D. A., Baird B. A., Functional nanoscale coupling of Lyn kinase with IgE-FcεRI is restricted by the actin cytoskeleton in early antigen-stimulated signaling. Mol. Biol. Cell 27, 3645–3658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bag N., Holowka D. A., Baird B. A., Imaging FCS delineates subtle heterogeneity in plasma membranes of resting mast cells. Mol. Biol. Cell 31, 709–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorent J. H., et al. , Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 8, 1219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machta B. B., Papanikolaou S., Sethna J. P., Veatch S. L., Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophys. J. 100, 1668–1677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calloway N., et al. , Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P₂ between distinct membrane pools. J. Cell Sci. 124, 2602–2610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]