The most fundamental genetic program of an annual plant defines when to grow and reproduce and when to remain dormant in the soil as a seed. With the right timing, plants can even live in hostile regions with only a few months of growth-favorable abundant rains and mild temperatures. To achieve this, plants have evolved molecular pathways that sense environmental variables and trigger their two main lifecycle transitions: germination and flowering. Growing season length—which depends on photoperiod, temperature, and rainfall patterns—drastically changes even over short geographic distances, and therefore, it is common that populations of the same species vary genetically to adjust to the local seasons. The widespread annual plant Arabidopsis thaliana has been a key model to study these seasonal timing adaptations and dissect their genetic components. Previous work on seasonal adaptation heavily focused on temperature-dependent flowering time pathways, including the cold-sensing genes FRIGIDA (FRI) and FLOWERING LOCUS C (FLC). This knowledge pointed at two main strategies: winter and spring annual plants. A new study in PNAS from Martínez-Berdeja et al. (1) comprehensively documents germination timing across the geographic distribution of A. thaliana and supports the gene DELAY OF GERMINATION 1 (DOG1) as one key genetic determinant of seasonal timing adaptation. Using their data, here I visualize the complex interactions of flowering and germination time and local climates across A. thaliana’s native geographic range and synthesize its seasonal timing adaptation into four categories: Mediterranean rapid cyclers, facultative winter cyclers, weedy summer/spring cyclers, and strict Scandinavian winter cyclers.
The extensive genetic knowledge of flowering time control in A. thaliana suggests two main seasonal timing strategies (2). Some genotypes have flowering repressors that are only released with experiencing winter (vernalization). Therefore, these plants germinate in fall, experience the winter cold, and flower in spring. These are called winter cyclers. Other A. thaliana genotypes lack a vernalization requirement, and thus, they can germinate in spring and directly flower without ever experiencing cold. These are called spring or rapid cyclers. Although very insightful, this model did not seem to fully account for the other genetically controlled key lifecycle transition: germination timing. Seeds are a living group of cells that read environmental variables and change their internal phytohormone levels and gene expression levels throughout the year and until germination (3). Many studies have characterized A. thaliana seed dormancy (4), the molecular repression of germination to wait until the favorable growing season. The relationship between dormancy and flowering times is complex (5), seemingly challenging the binary winter vs. spring cycle model. One reason for this complexity might be that dormancy can be acquired in two ways: at the seed’s inception and maturation in the mother plant (primary dormancy) or after the seed is in the soil (secondary dormancy); either can be released in response to climates.
In their PNAS study, Martínez-Berdeja et al. (1) characterize germination in a large-scale time series experiment at different cold temperatures with 525 worldwide A. thaliana ecotypes that are part of the 1001 Genomes Project collection (2). Using principal component analyses, they decompose the time series trends into the overall environment-independent germination rate and the cold-dependent release or induction of dormancy. To uncover genes controlling these behaviors, Martínez-Berdeja et al. (1) conduct a genome-wide association to map the variation in germination onto the A. thaliana genome. Both traits were best explained by different alleles in the same gene DOG1, one of the seven DOG loci involved in control dormancy (6). DOG1 encodes a protein of unknown function that, when accumulated in the seed, blocks germination. A balance between expression, degradation, and ability to accumulate finely tunes germination time (7).
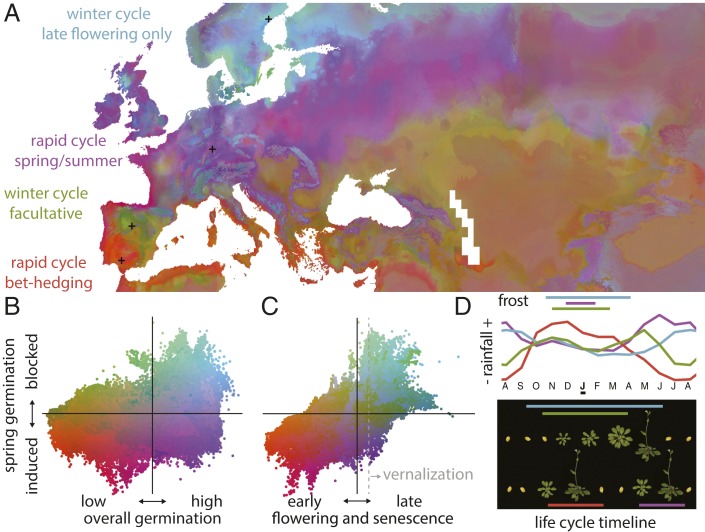
While spring germination induction or block was correlated with flowering time (Spearman’s rank correlation ⍴ = 0.544, P < 10−16), the two germination behaviors did not correlate with each other, creating a complex continuum of seasonal timing behaviors, which can be synthesized into four adaptation strategies: Mediterranean rapid cyclers, facultative winter cyclers, weedy summer/spring cyclers, and strict Scandinavian winter cyclers. To geographically visualize these complex seasonal timing behaviors, I use random forest to model and interpolate the total time from germination to flowering and senescence, temperature-independent germination rate (= primary dormancy; PC1 in ref. 1), and cold-induced/blocked spring germination (= temperature-dependent dormancy induction or release; PC2 in ref. 1). These three ecologically relevant traits had high predictability from 55 climate variables (19 bioclim variables, 12 monthly precipitation, 12 maximum and 12 minimum temperature; http://worldclim.org; trained on n = 520 ecotypes; cross-validation accuracy Pearson’s r = 0.65, 0.63, 0.51).
Following climate patterns, seasonal timing strategies vary dramatically across Europe (Fig. 1A). In the very south along the coasts of the Mediterranean Sea, populations have red to orange hues (Fig. 1 A–C), indicating predictions of early flowering, overall low germination (high primary dormancy), and a preference for spring germination (cold-released dormancy). Hot and dry summers with variable rainfall peaking in winter define Mediterranean regions such as Sevilla (Spain), where the Tri-0 ecotype was collected. These genotypes avoid summer droughts by beginning the cycle in fall or winter and flowering fast before spring droughts arrive again (Fig. 1D). Martínez-Berdeja et al. (1) find that the DOG1 alleles of Spanish lowland ecotypes, such as Andalucia or on the Mediterranean coast of Catalonia, have a DNA sequence deletion that produces an amino acid loss (13th to 16th amino acids: D-RY or D-SY, with “-” indicating a loss). This may reduce DOG1 function, enabling germination in spring. Such premature germination could be deadly in harsh winters but is likely advantageous with mild and wet winters (Fig. 1D), such as is shown in outdoor field experiments in Andalucia conducted in December (9). In contrast, other experiments with Iberian ecotypes indicate that they induce dormancy when exposed to heat (10). These strategies reminds us of annual desert flowers, which practice “bet hedging.” They produce seeds that rarely germinate all at once in the following season, creating multiyear seed banks to minimize the risk of complete offspring loss in a bad rainfall season (11). In agreement, those ecotypes with low overall germination have high survival in drought experiments (⍴ = −0.227, P = 0.004) (12).
Fig. 1.
Distribution of seasonal timing adaptation of A. thaliana. (A) Map of predictions of plant’s time to flowering and senescence, temperature-independent germination rate, and cold-induced/blocked spring germination projected in a red–green–blue space. Black crosses indicate the locations of four ecotypes. (B and C) Relationships between the three variables centered on their averages. The gray dotted line indicates 290 d to senescence, which approximately coincides with the strict requirement of vernalization to flower (8). (D) Months with minimum temperature below 0 °C and monthly precipitation for the four locations in A of representative natural ecotypes: Tri-0 from south Spain (1001 Genomes Project identification code: 9900), Fun-0 from central Spain (1001 Genomes Project identification code: 9542), Hi-4 from Germany (1001 Genomes Project identification code: 9782), and Dör-10 from Sweden (1001 Genomes Project identification code: 5856). Grayscale maps are at https://doi.org/10.6084/m9.figshare.11724039.
Moving up to Mediterranean mountains, such as the Iberian plateau or the Moroccan Atlas, the map is colored with green to brown hues (Fig. 1 A–C), indicating mid- to late flowering time, low overall germination rate, and spring germination block (cold-induced dormancy). In these areas, such as the Guadarrama mountains near Madrid, where the Fun-0 was collected, temperatures fall below 0 °C in winter, and rainfall is bimodal, with peaks in fall and spring (Fig. 1D). This favors ecotypes that germinate in fall, hibernate as small plant rosettes in winter, and flower and reproduce in spring. These genotypes have a functional allele of DOG1 (amino acid sequence ECCY/ECSY) that reduces germination after cold periods, thus avoiding spring germination. The functional DOG1 alleles coexist with less functional ones in the Iberian Peninsula dated to have arisen 281,000–450,000 y ago (1), thus suggesting that both rapid and winter cycle populations survived multiple glacial and interglacial periods together. In fact, in montane Mediterranean regions, populations seem to be facultative cyclers. Natural populations have both winter and spring cohorts (13), in which fitness advantage or disadvantage varies with interannual fluctuations in precipitation (9). With altitude, winters become longer, and spring shortens, increasing the relative frequency of winter vs. spring cyclers (14). Over 800 m above sea level, populations display strict vernalization requirements (i.e., plants need to have passed a winter to flower) (9, 15). A key genetic player of vernalization requirement is the gene FRI, which up-regulates the winter-sensing flowering repressor FLC. Functional alleles of FRI exist at high elevations, whereas loss of function (LoF) alleles are found in the lower montane regions (15). The fact that multiple independent FRI LoF alleles are found in nature suggests that there are recurrent environmental forces that, every once in a while, favor major shifts to early flowering strategy (2).
One such force may be the disturbance of habitats by humans prevalent in the populated and agricultural flatlands of central Europe, where FRI LoFs are common. The map shows purple hues (Fig. 1 A–C), indicating early flowering and high overall germination promoted in spring. Abundant rainfall in central Europe and the United Kingdom (Fig. 1D) enables ecotypes, such as Hi-4 collected near Tübingen (Germany), to grow in highly proliferating and fast-flowering populations with up to two generations per year (one in spring–summer and another in summer–fall) (16), potentially skipping winter frosts altogether. The high overall germination and winter-released germination block are provided by the low-functional DOG1 haplotype (D-RY/D-SY). Most likely, Mediterranean D-RY/D-SY haplotypes were the migration source for central Europe after the last glaciation, with the most weed-like genotypes being favored with the expansion of agriculture some thousands of years ago (17).
In the north range edge of A. thaliana, we find the cyan to green hues (Fig. 1 A–C). These indicate late flowering, intermediate germinability strongly blocked in spring. In areas like the Scandinavian forests near Bollstabruk (Sweden), where Dör-10 was collected, precipitation is high in summer and fall, and it remains inaccessible for plants in the snow cover for many months until late spring. Swedish ecotypes thus germinate in fall but hibernate for months at 0 °C below the snow to flower in spring as snow melts (Fig. 1D). They achieve their long flowering time by strict functional FRI + FLC-mediated vernalization (18). Swedish ecotypes also have an ancestral functional DOG1 haplotype, such as the one present in high-altitude Iberian locations (ECCY), which blocks spring germination (1). Not only do they resemble high-altitude Iberians in their DOG1 haplotype, but multiple pieces of evidence indicate that the Swedish lineage may have migrated from the Mediterranean independently from the central European lineage as the northern ecotypes show unexpectedly high similarities in genome-wide variation and stress tolerance traits (12, 19).
With climates across the world changing in annual averages as well as seasonal patterns (20), a deep understanding on seasonal timing adaptation becomes of crucial importance to foresee impacts of climate change on species. Large-scale datasets that study seasonal timing traits and their genetic basis in a species, such as the one produced by Martínez-Berdeja et al. (1), will be instrumental to develop realistic models that integrate ecological, demographic, and evolutionary processes to anticipate species’ (mal-)adaptive responses to climate change.
Acknowledgments
I thank Martínez-Berdeja et al. for sharing the germination data for Fig. 1 and members of the Moi Laboratory for feedback on the manuscript. This work was funded by Carnegie Endowment Funds (to M.E.-A.)
Footnotes
The author declares no competing interest.
Data deposition: Grayscale maps (Fig. 1) are available on Figshare (DOI: 10.6084/m9.figshare.11724039).
See companion article on page 2526 in issue 5 of volume 117.
References
- 1.Martínez-Berdeja A., et al. , Functional variants of DOG1 control seed chilling responses and variation in seasonal life-history strategies in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 117, 2526–2534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson G. G., Dean C., Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Footitt S., Douterelo-Soler I., Clay H., Finch-Savage W. E., Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 108, 20236–20241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohue K., et al. , The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution 59, 758–770 (2005). [PubMed] [Google Scholar]
- 5.Debieu M., et al. , Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS One 8, e61075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Blanco C., Bentsink L., Hanhart C. J., Blankestijn-de Vries H., Koornneef M., Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164, 711–729 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakabayashi K., Bartsch M., Ding J., Soppe W. J. J., Seed dormancy in Arabidopsis requires self-binding ability of DOG1 Protein and the presence of multiple isoforms generated by alternative splicing. PLoS Genet. 11, e1005737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atwell S., et al. , Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exposito-Alonso M., Brennan A. C., Alonso-Blanco C., Picó F. X., Spatio-temporal variation in fitness responses to contrasting environments in Arabidopsis thaliana. Evolution, 10.1111/evo.13508 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Montesinos-Navarro A., Picó F. X., Tonsor S. J., Clinal variation in seed traits influencing life cycle timing in Arabidopsis thaliana. Evolution 66, 3417–3431 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Gremer J. R., Venable D. L., Bet hedging in desert winter annual plants: Optimal germination strategies in a variable environment. Ecol. Lett. 17, 380–387 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Exposito-Alonso M., et al. , Genomic basis and evolutionary potential for extreme drought adaptation in Arabidopsis thaliana. Nat. Ecol. Evol. 2, 352–358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picó F. X., Demographic fate of Arabidopsis thaliana cohorts of autumn- and spring-germinated plants along an altitudinal gradient. J. Ecol. 100, 1009–1018 (2012). [Google Scholar]
- 14.Vidigal D. S., et al. , Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant Cell Environ. 39, 1737–1748 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Méndez-Vigo B., Picó F. X., Ramiro M., Martínez-Zapater J. M., Alonso-Blanco C., Altitudinal and climatic adaptation is mediated by flowering traits and FRI, FLC, and PHYC genes in Arabidopsis. Plant Physiol. 157, 1942–1955 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burghardt L. T., Metcalf C. J. E., Donohue K., A cline in seed dormancy helps conserve the environment experienced during reproduction across the range of Arabidopsis thaliana. Am. J. Bot. 103, 47–59 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Lee C.-R., et al. , On the post-glacial spread of human commensal Arabidopsis thaliana. Nat. Commun. 8, 14458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stinchcombe J. R., et al. , A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc. Natl. Acad. Sci. U.S.A. 101, 4712–4717 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exposito-Alonso M., Burbano H. A., Bossdorf O., Nielsen R., Weigel D.; 500 Genomes Field Experiment Team , Natural selection on the Arabidopsis thaliana genome in present and future climates. Nature 573, 126–129 (2019). [DOI] [PubMed] [Google Scholar]
- 20.IPCC , “Summary for policymakers” in Global Warming of 1.5°C: An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Masson-Delmotte V., et al., Eds. (World Meteorological Organization, Geneva, Switzerland, 2018). [Google Scholar]