Significance
Vitamin A has diverse biological functions and is transported to target tissues through the specific interaction between its blood transport protein (RBP) and its multitransmembrane domain receptor. However, how this receptor is regulated was completely unknown. Both insufficient or excessive vitamin A uptake has deleterious effects. Through unbiased mass spectrometry analysis and mechanistic studies, this study identified a cellular mechanism that profoundly influences receptor-mediated cellular vitamin A influx and efflux and may point to new directions to treat diseases associated with vitamin A imbalance.
Keywords: vitamin A, transmembrane transport, retinoid cycle
Abstract
Vitamin A has diverse biological functions and is essential for human survival at every point from embryogenesis to adulthood. Vitamin A and its derivatives have been used to treat human diseases including vision diseases, skin diseases, and cancer. Both insufficient and excessive vitamin A uptake are detrimental, but how its transport is regulated is poorly understood. STRA6 is a multitransmembrane domain cell-surface receptor and mediates vitamin A uptake from plasma retinol binding protein (RBP). STRA6 can mediate both cellular vitamin A influx and efflux, but what regulates these opposing activities is unknown. To answer this question, we purified and identified STRA6-associated proteins in a native mammalian cell type that takes up vitamin A through STRA6 using mass spectrometry. We found that the major protein repeatedly identified as STRA6-associated protein is calmodulin, consistent with the cryogenic electron microscopy (cryo-EM) study of zebrafish STRA6 associated with calmodulin. Using radioactivity-based, high-performance liquid chromatography (HPLC)-based and real-time fluorescence techniques, we found that calmodulin profoundly affects STRA6’s vitamin A transport activity. Increased calcium/calmodulin promotes cellular vitamin A efflux and suppresses vitamin A influx through STRA6. Further mechanistic studies revealed that calmodulin enhances the binding of apo-RBP to STRA6, and this enhancement is much more pronounced for apo-RBP than holo-RBP. This study revealed that calmodulin regulates STRA6’s vitamin A influx or efflux activity by modulating its preferential interaction with apo-RBP or holo-RBP. This molecular mechanism of regulating vitamin A transport may point to new directions to treat human diseases associated with insufficient or excessive vitamin A uptake.
Vitamin A is essential for human survival and has many essential biological functions including light sensing in vision (1–4) and the regulation of cell growth and development throughout life (5–7). Vitamin A imbalance is associated with a variety of diseases, including visual diseases, abnormal embryonic development, infectious diseases, skin diseases, neurological diseases, and cancer. A variety of natural and synthetic vitamin A substances have also been used to treat different types of cancer (8–10) and skin diseases (11–13).
Plasma retinol binding protein (RBP) is the principal and specific carrier protein of vitamin A in the blood (14, 15). It was first proposed in the 1970s that there exists a specific RBP receptor that mediates cellular vitamin A uptake (16–18). In 2007, STRA6, a membrane protein originally known as a cancer cell-surface marker, was identified as the cell-surface RBP receptor through unbiased protein purification and mass spectrometry analysis (19). STRA6 couples to either lecithin retinol acyltransferase (LRAT) (19–21) or cellular retinol binding protein-I (CRBP-I) (22) to take up vitamin A from holo-RBP. In humans, STRA6 mutations cause a wide spectrum of pathological phenotypes including anophthalmia, mental retardation, congenital heart defects, lung hyperplasia, intrauterine growth retardation, and embryonic lethality (23, 24). STRA6 fulfills dual roles as both the receptor for RBP and the transporter for retinol (22) and mediates both cellular retinol influx and efflux (20, 22, 25). Studies in animal models demonstrated that STRA6 is crucial for maintaining retinoid homeostasis (20, 26, 27).
Tissue levels of vitamin A are homeostatic under various supply conditions, indicating that vitamin A uptake/efflux is a tightly regulated process. In healthy people, STRA6 constantly faces an “all-you-can-eat” amount of vitamin A in holo-RBP because of the micromolar concentrations of holo-RBP in the blood (28). How STRA6-mediated bidirectional vitamin A transport is regulated was unknown. We started to answer this question by identifying STRA6-interacting proteins in the retinal pigment epithelium (RPE) cells, a cell type that naturally expresses STRA6 and is the major vitamin A uptake cell type in vision. We developed a more efficient method to purify STRA6 from the native mammalian RPE cells and identified calmodulin as the major STRA6 interacting protein. This finding agrees with the report on zebrafish STRA6 cryogenic electron microscopy (cryo-EM) structure and calmodulin as a STRA6-interacting protein (29). To understand the function of calmodulin/STRA6 interaction, we used a variety of techniques to reveal that calmodulin has a profound effect on STRA6’s vitamin A influx and efflux activities and these effects are mediated by the influence of calmodulin on STRA6’s differential interaction with apo-RBP or holo-RBP. These studies reveal a molecular mechanism of regulating STRA6's functions as a receptor for RBP and as a transporter for vitamin A.
Results
Unbiased Identification of STRA6-Interacting Proteins in Native RPE Cells Using Affinity Purification and Mass Spectrometry.
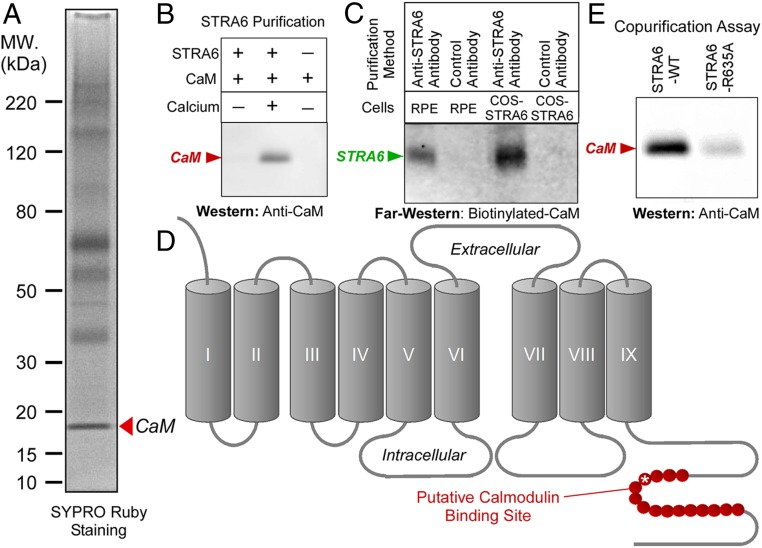
To understand the regulatory mechanism of STRA6, we started to search for STRA6-interacting proteins. We decided to employ a more efficient method of immunoaffinity purification to identify STRA6-interacting proteins in native cells. However, no antibody existed that can efficiently purify STRA6 from native cells given the high hydrophobicity of STRA6 and its relative low abundance in native cells (as compared to an overexpressing system). We decided to target bovine STRA6 for immunoaffinity purification because mouse RPE cells are much more difficult to purify at a sufficient quantity than bovine RPE cells. To generate an antibody that can purify bovine STRA6 using immunoaffinity purification, we generated multiple polyclonal antibodies against the bovine STRA6 protein. Of these antibodies that we produced and characterized, only one is useful in purifying native STRA6 from bovine RPE cells although other antibodies work on Western blot analysis of STRA6. Using this newly produced and purified antibody, we purified STRA6 from freshly dissected RPE cells from bovine eyes. SDS/PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) analysis of the eluted proteins revealed multiple species. However, mass spectrometric analysis identified most bands as STRA6 itself, consistent with the successful purification of STRA6 and the fact that multitransmembrane proteins like STRA6 tend to aggregate in SDS/PAGE. Mass spectrometry identified one protein repeatedly copurified with STRA6 from RPE cells as calmodulin, which is known to regulate diverse cellular processes (Fig. 1A and SI Appendix, Fig. S1). Interestingly, STRA6 and calmodulin represent all of the proteins that are visible on the SDS gel of STRA6 purification from native RPE cells. This finding is consistent with the cryo-EM structural study of zebrafish STRA6, which was copurified with calmodulin when it was produced in cell culture (29).
Fig. 1.
Unbiased identification of STRA6-interacting proteins in the native RPE cells using mass spectrometry. (A) STRA6 was purified from RPE cells freshly dissected from bovine eyes by immunoaffinity purification using an antibody against bovine STRA6. Mass spectrometric analysis revealed that most of the stained bands are STRA6 itself. A distinct band with an apparent molecular mass of about 18 kDa was identified as calmodulin (arrowhead). (B) Calcium-dependent interaction between STRA6 and calmodulin. Calmodulin copurifies with STRA6 only when calcium is present. (C) Calmodulin overlay assay. Biotinylated-calmodulin specifically binds to STRA6 on nitrocellulose membrane. STRA6 was purified from RPE cells or COS-1 cells expressing STRA6 using anti-STRA6 antibody. Negative control is an antibody that does not bind STRA6. (D) The computer-predicted calmodulin binding site on STRA6 is shown in red in the STRA6 topology. (E) The R635A mutation in the putative calmodulin binding site (asterisk in D) significantly suppresses calmodulin binding of STRA6, as shown by copurification of calmodulin with STRA6.
Interaction of Mammalian STRA6 with Calcium/Calmodulin.
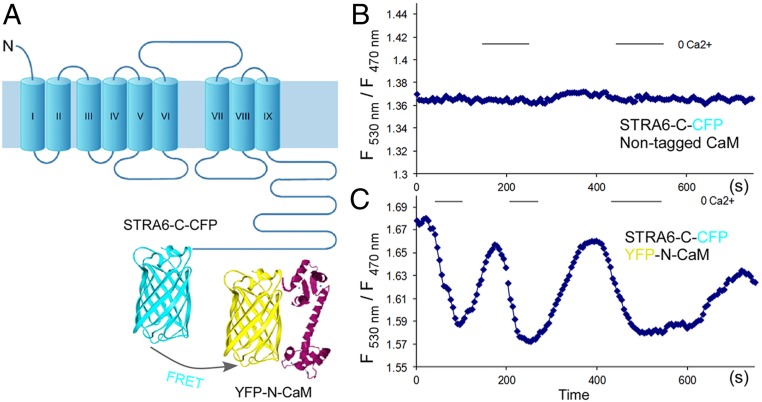
We found that calmodulin interacts with STRA6 in a calcium-dependent manner (Fig. 1B). This association was confirmed by a far-western experiment using biotinylated calmodulin (Fig. 1C). We identified a canonical (basic, amphiphilic alpha-helices) calmodulin binding site near the C terminus of STRA6, as predicted by the calmodulin target database (http://calcium.uhnres.utoronto.ca/ctdb/no_flash.htm) (Fig. 1D). Site-directed mutagenesis of critical residues in this region reduced calmodulin binding (Fig. 1E). To investigate the interaction between STRA6 and calmodulin in real time, we designed constructs to express STRA6-cyan fluorescent protein (CFP) and calmodulin-yellow fluorescent protein (YFP) fusion proteins and performed fluorescence resonance energy transfer (FRET) experiments to study their interaction in real time (Fig. 2A). This real-time monitoring experiment demonstrated that STRA6 and calmodulin interact in a dynamic manner. Lower cellular calcium dissociates their interaction while enhanced intracellular calcium promotes their interaction (Fig. 2 B and C).
Fig. 2.
Calcium-dependent association between STRA6 and calmodulin shown by a real-time FRET assay in live cells. (A) Schematic diagram of the design of the FRET experiment. The FRET signal between STRA6-C-CFP and YFP-N-calmodulin expressed within the same cell is measured by the ratio of fluorescence intensities at 530 nm and 470 nm. Cells on the slide were alternatively perfused with PBS/EDTA (phosphate-buffered saline containing 5 mM ethylenediaminetetraacetic acid) and HBSS (Hank’s Balanced Salt Solution containing 2 mM calcium) every 5 s to induce change in intracellular calcium concentration. (B) Control cells transfected with STRA6-C-CFP and nontagged calmodulin. (C) Cells with coexpression of STRA6-C-CFP and YFP-N-calmodulin.
Intracellular Calcium Regulates STRA6-Mediated Vitamin A Influx and Efflux.
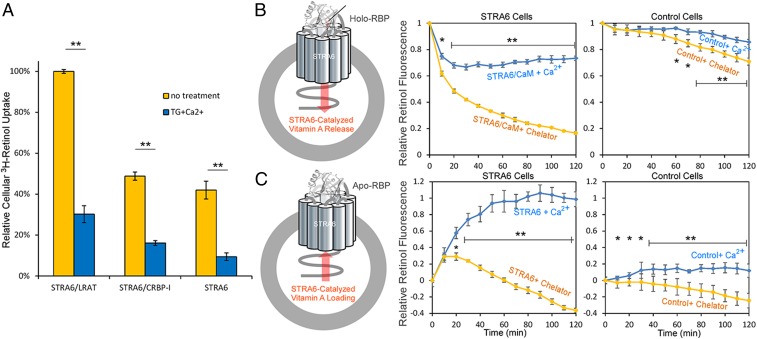
Whether calcium/calmodulin has any effect on STRA6-mediated vitamin A transport was unknown. To determine whether calcium/calmodulin affect STRA6-mediated retinol transport, we performed two types of cell-based STRA6-mediated retinol transport assays: cellular radioactive vitamin A uptake assay from 3H-retinol/RBP complex (19, 22) and real-time retinol transport assay (22, 30). The cellular radioactive vitamin A uptake assay showed that increased intracellular calcium suppresses STRA6-mediated vitamin A uptake (Fig. 3A). The downside of radioactive assay is that it is not real time and each experiment can only generate one data point. We also performed a real-time retinol transport assay using live cells expressing STRA6. These assays showed that decreased intracellular calcium enhanced STRA6-mediated release of retinol from holo-RBP and suppressed STRA6-mediated loading of retinol into apo-RBP (Fig. 3 B and C). These two assays consistently showed that lowered intracellular calcium promotes STRA6-mediated vitamin A influx into the cell and that increased intracellular calcium promotes STRA6-mediated vitamin A efflux.
Fig. 3.
Calcium/calmodulin regulates STRA6-mediated vitamin A transport. (A) Cellular 3H-retinol uptake assay from 3H-retinol/RBP. The same amounts of COS-1 cells were transfected with STRA6/LRAT, STRA6/CRBP-1, or STRA6 alone, and the uptake of 3H-retinol from 3H-retinol/RBP complex was measured by scintillation counting. Thapsigargin (TG), a drug that raises intracellular calcium suppressed cellular retinol uptake. The activity of STRA6/LRAT cells without TG addition is defined as 100%. (B) Real-time retinol fluorescent assay to monitor STRA6-mediated retinol release from holo-RBP. Schematic diagram is shown on the left. 293T cells cotransfected with STRA6 and calmodulin (STRA6 cells) were suspended in HBSS (containing 2 mM calcium) or PBS supplemented with 10 mM EDTA, 20 μM BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid]. Holo-RBP (2 μM) was added at 0 min to start each reaction. Retinol fluorescence was continuously monitored, and its value at 0 min is defined as 1. Lower cellular calcium enhanced STRA6 mediated retinol release from holo-RBP. Control cells are untransfected cells without STRA6. (C) Retinol fluorescent assay to monitor STRA6 mediated retinol loading into apo-RBP. Schematic diagram is shown at Left. Free retinol (2 μM) was added to suspended 293T cells before 2 μM of apo-RBP was added at 0 min to start each reaction. Retinol fluorescence is continuously monitored. The fluorescence value at 0 min is set as 0, and the largest rise of fluorescence signal (STRA6/CaM + Ca2+) is defined as 1. Lower cellular calcium suppressed STRA6-mediated retinol loading into apo-RBP. Control cells are untransfected cells without STRA6. *P < 0.05, **P < 0.05.
Regulation of Vitamin A Uptake into RPE Cells by Calcium/Calmodulin.
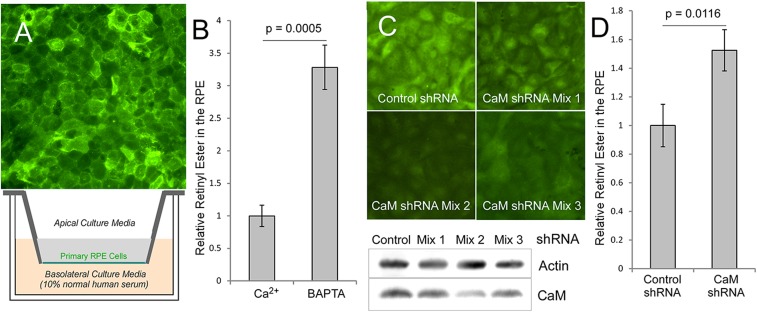
To study whether calcium/calmodulin affects STRA6-mediated vitamin A uptake in a native cell type that depends on STRA6 for vitamin A uptake, we used a primary culture of RPE cells as the model. As the major cell type that takes vitamin A from the blood, RPE both expresses STRA6 and depends on STRA6 for vitamin A uptake from holo-RBP (19, 26, 27). By immunofluorescence staining, we showed that STRA6 is highly expressed in primary RPE culture and is localized to the plasma membrane (Fig. 4A). To study the role of calcium/calmodulin in STRA6’s vitamin A uptake into the RPE, we studied retinyl ester accumulation in the RPE cells using human serum as the source of holo-RBP. Consistent with experiments above, decreased intracellular calcium highly enhanced retinyl ester accumulation (Fig. 4B). To study the role of calmodulin in STRA6-mediated vitamin A uptake, we used RNA interference to knock down calmodulin expression. Calmodulin is one of the most difficult genes to knock down or knock out because this protein is encoded by three independent genes in vertebrates. To knock down its expression in primary RPE cells using short hairpin RNA (shRNA), we screened two shRNA constructs for each of the three calmodulin genes for a combination of nine mixtures of shRNA constructs and identified an shRNA mixture that effectively knocked down calmodulin (Fig. 4C and SI Appendix, Fig. S2). Consistent with the experiments using chemical agents to decrease intracellular calcium, calmodulin knockdown significantly enhanced vitamin A uptake in the RPE (Fig. 4D). This experiment supports the role of calmodulin in suppressing STRA6-mediated vitamin A uptake in the RPE.
Fig. 4.
Primary RPE cell culture model of vitamin A uptake using human serum as the source of RBP. (A) The primary RPE culture. (Upper) Expression of STRA6 in the RPE as shown by immunofluorescence staining of the primary RPE cells derived from the naturally nonpigmented region of tapetum lucidum. (Lower) Schematic diagram of the transwell culture of primary RPE cells. During each vitamin A uptake experiment, normal human serum, which is a source of holo-RBP, is added to the basolateral culture media to initiate each experiment. (B) HPLC analysis of retinyl ester levels in the RPE after vitamin A uptake from human serum. BAPTA (20 μM), a calcium-specific chelator, leads to increased uptake. Control level (without calcium chelation) is defined as 1. (C) Knockdown of calmodulin in the RPE using a mixture of shRNA for each of the three calmodulin genes. Calmodulin shRNA mixture 2 is most effective in knocking down calmodulin expression. (Upper) Immunostaining of calmodulin in the RPE (calmodulin signal in green and DNA in blue). (Lower) Western blot demonstrating the knockdown of calmodulin. (D) Knocking down calmodulin significantly enhances RPE’s vitamin A uptake from holo-RBP, as revealed by retinyl ester analysis using HPLC. Retiny ester level of control shRNA treated cells is defined as 1. *P < 0.05, **P < 0.05.
Effect of Calcium or Calmodulin on STRA6’s Binding to RBP.
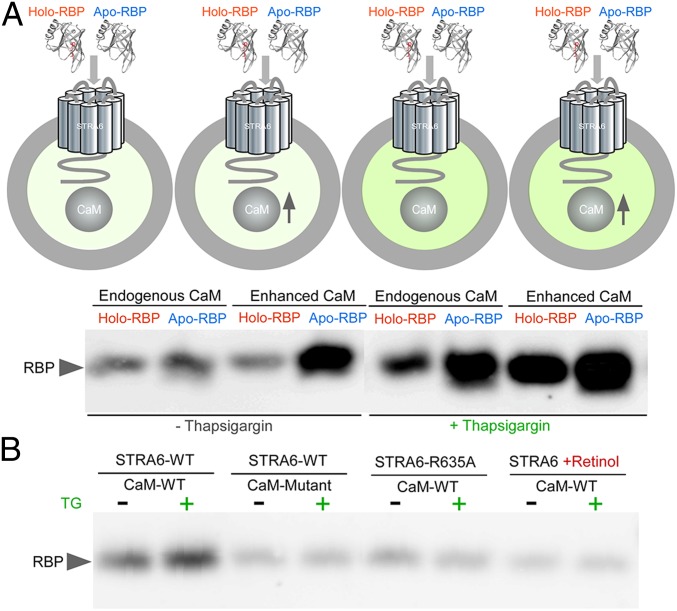
How does the binding of calcium/calmodulin to STRA6 affect its activity in vitamin A transport? Because STRA6 binds to RBP to promote vitamin A release from RBP, we hypothesized that calcium/calmodulin might affect STRA6’s interaction with RBP. We tested this hypothesis by studying the binding of holo-RBP or apo-RBP to STRA6 on live cells. We tested the binding using both the endogenous calmodulin and ectopically higher expression of calmodulin (“enhanced calmodulin”). We found that the enhanced expression of calmodulin causes dramatically enhanced binding of apo-RBP but not holo-RBP to STRA6. Increased intracellular calcium enhances the binding of both holo-RBP and apo-RBP to STRA6, but the enhancement of apo-RBP binding is much more pronounced. Under the condition of increased intracellular calcium, enhanced calmodulin further boosts the binding of both forms of RBP to STRA6, and again the enhancement for apo-RBP binding is much more pronounced (Fig. 5A and SI Appendix, Fig. S3). These experiments suggest that calcium/calmodulin suppress vitamin A influx and promotes vitamin A efflux by promoting the interaction between STRA6 with apo-RBP, which promotes vitamin A efflux and suppress vitamin A influx by competing with holo-RBP.
Fig. 5.
Differential binding of holo-RBP or apo-RBP to STRA6 as regulated by calcium/calmodulin. (A) Schematic diagram of the experimental design of binding holo-RBP or apo-RBP to STRA6 on live cells (darker green color indicates higher intracellular calcium). STRA6 was coexpressed without (“Endogenous CaM”) or with ectopic expression of calmodulin (“Enhanced CaM”) in COS-1 cells. The cells were incubated with holo-RBP or apo-RBP in the absence or presence of 1 μM thapsigargin (TG). Unbound RBP was washed off and the bound RBP was detected by Western blot. (B) How calmodulin mutations that abolish calmodulin binding to calcium (CaM-Mutant), a mutation of the calmodulin binding site on the C terminus of STRA6 (STRA6-R635A) and retinol affect calmodulin’s effect on STRA6/RBP interaction.
To gain additional insights into the role of calmodulin in influencing STRA6’s interaction with RBP, we tested a calmodulin mutant that has all calcium-binding sites abolished (31) and a STRA6 mutant with a point mutation on its putative calmodulin-binding site on its C terminus (Fig. 1E). Both the calmodulin mutations and the STRA6 mutation abolish the effect of calmodulin to enhance STRA6’s binding to apo-RBP (Fig. 5B and SI Appendix, Fig. S4). Because STRA6 catalyzes the loading of retinol into apo-RBP so that apo-RBP becomes holo-RBP (20, 22), we tested the effect of retinol on the STRA6/RBP interaction. We found that retinol highly diminishes the effect of calmodulin on the binding of STRA6 to RBP, consistent with the loading of retinol into RBP to become holo-RBP. This result suggests the preferential effect of calmodulin on promoting STRA6’s interaction with apo-RBP.
Calcium/Calmodulin-Induced Conformational Changes in STRA6’s Transmembrane Domains.
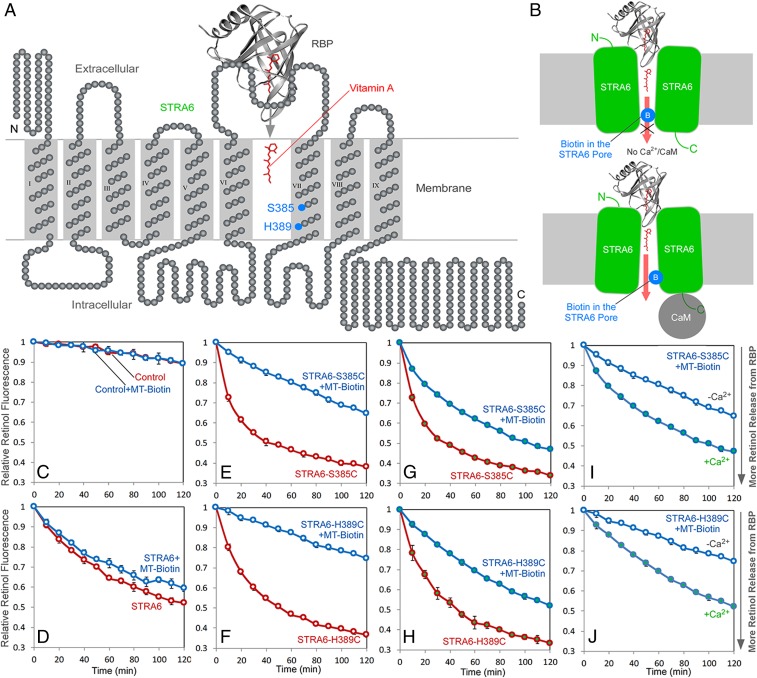
The ability of calcium/calmodulin to affect STRA6’s RBP binding implies that conformational changes are transmitted from the intracellular face to the extracellular face of the transmembrane receptor. In addition to its function as a receptor that binds to RBP, STRA6 also functions as a transporter that facilitates the release of retinol from holo-RBP into the cell. We previously identified positions in or near the transmembrane domains of STRA6 that define the vitamin A transport “pore” through which retinol is transported into the cell using acute chemical modification by MTSEA-biotin [2-((Biotinoyl)amino)ethyl methanethiosulfonate] to block vitamin A transport (30). Acute chemical modification at any one of these positions can effectively block vitamin A transport by STRA6. This is analogous to the blocking of the pore of an ion channel by channel blockers. We investigated the effect of calcium/calmodulin on pore blocking by MTSEA-biotin and found that calcium/calmodulin alleviates the blocking effect by MTSEA-biotin on STRA6’s pore at two key positions: S385 and H389 in the transmembrane domain 7 (Fig. 6). This is consistent with a conformational change in the STRA6’s transmembrane domains that makes the pore blocking less effective.
Fig. 6.
Calcium/calmodulin-induced conformational change in STRA6. MTSEA-biotin blocking of STRA6’s vitamin A transport pore is influenced by calcium calmodulin binding to STRA6. (A) A schematic diagram of STRA6 topology. Resides 385 and 398 are located in the vitamin A transport pore of STRA6 and are labeled in blue. (B) A model on how calcium/calmodulin affects the biotin blocking of the STRA6 pore. In the absence of calcium, MTSEA-biotin can acutely block vitamin A transport through STRA6 by interacting with a cysteine residue on position 385 or 389 in STRA6 vitamin A transport pore. Calcium/calmodulin binding to STRA6 induces its conformational change in the transmembrane domains and relieves the blocking by MTSEA-biotin. (C–J) Real-time analysis of STRA6-catalyzed retinol release from holo-RBP. Holo-RBP was added at time 0. Retinol fluorescence at time 0 is defined as 1. Open and closed symbols represent the absence of presence of calcium, respectively. Blue traces represent MTSEA-biotin (MT-Biotin) treated samples (treatment was done before the addition of holo-RBP). Red traces represent experiments without MTSEA-biotin treatment. Although MTSEA-biotin treatment suppresses STRA6-mediated retinol release for both S358C (E and G) and H389C (F and H) as shown by comparing the blue and red traces, the suppression is much more pronounced in the absence (E and F) as compared in the presence of calcium (G and H). The direct comparison of MTSEA-biotin–inhibited STRA6 in the presence or absence of calcium are shown in I and J.
A Model on How Calmodulin/STRA6 Interaction Affects Vitamin A Transport.
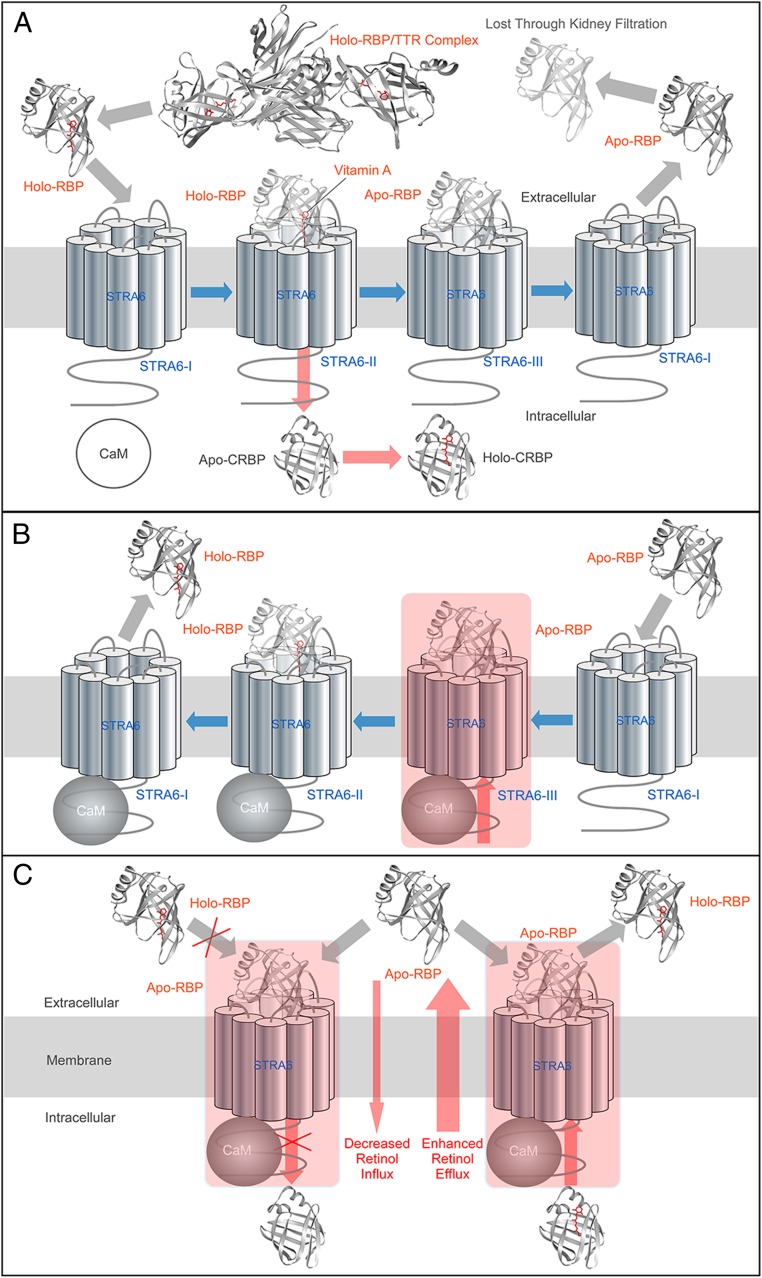
We employed a diverse set of techniques including radioactive vitamin A uptake assay, real-time vitamin A transport assay, HPLC-based vitamin A uptake assay using primary cell culture, shRNA knockdown, and live cell-based RBP/STRA6 binding assay to study the functional consequences of calmodulin/STRA6 interaction. There are three major findings in this study. First, calcium/calmodulin profoundly inhibits STRA6’s vitamin A uptake activity. Second, calcium/calmodulin promotes vitamin A efflux through STRA6. Third, calcium/calmodulin promotes the binding of apo-RBP to STRA6. These findings clearly and consistently point to a unifying model for calcium/calmodulin-mediated regulation of STRA6’s vitamin A transport activity: Calcium/calmodulin suppresses STRA6-mediated vitamin A uptake by promoting the binding of apo-RBP to STRA6. Binding of apo-RBP to STRA6 both prevents the binding of holo-RBP to STRA6, which is required for vitamin A uptake, and promotes vitamin A efflux through STRA6, as has been demonstrated previously (20, 22, 25). The most direct experiments demonstrating the effects of calcium/calmodulin on STRA6’s differential interaction with holo-RBP and apo-RBP and on STRA6’s influx and efflux activities are shown in Figs. 3 and 5. This model is depicted schematically in Fig. 7 in the context of the known molecular mechanism of vitamin A uptake by STRA6 and how calmodulin regulates the bidirectional vitamin A transport mediated by STRA6. Fig. 7A depicts a general model of STRA6-mediated vitamin A uptake from holo-RBP. Fig. 7B is a schematic model on how calcium/calmodulin promotes vitamin A efflux from STRA6. Fig. 7C illustrates the differential effects of calcium/calmodulin on vitamin A influx and efflux through STRA6.
Fig. 7.
Schematic diagrams of regulation of STRA6-mediated vitamin A transport by calcium/calmodulin. In these diagrams, blue arrows indicate the shifting of STRA6 between states (STRA6-I, no RBP bound; STRA6-II, holo-RBP bound; STRA6-III, apo-RBP bound). Red arrows indicate the transport of vitamin A. Gray arrows indicate the movement of RBP. (A) The current overall model of STRA6-mediated vitamin A uptake from holo-RBP. Holo-RBP dissociates from the RBP/TTR complex and binds to STRA6 (STRA6-II state). The latter mediates the uptake of vitamin A, by coupling to CRBP-I or LRAT (not shown for simplicity). After vitamin A is transported into the cell, apo-RBP is bound to STRA6 (STRA6-III state). After apo-RBP dissociates from STRA6, STRA6 returns to STRA6-I state and can mediate the uptake of the next vitamin A molecule. (B) A model on how calcium/calmodulin promotes vitamin A efflux from STRA6. At higher intracellular calcium, calmodulin binds to STRA6 and strongly promotes the binding of STRA6 to apo-RBP (STRA6-III state). Apo-RBP binding to STRA6 promotes vitamin A efflux because once apo-RBP is loaded with vitamin A to become holo-RBP, it is more likely to dissociate as compared to apo-RBP. This process causes vitamin A efflux. (C) Comparing the effects of calcium/calmodulin on vitamin A influx and efflux through STRA6. The preferentially enhanced binding of STRA6 to apo-RBP in the presence of calcium/calmodulin prevents the next holo-RBP from binding to STRA6 and inhibits vitamin A influx (Left). This preferential binding to apo-RBP also promotes STRA6-mediated retinol efflux, as explained in more details in B (Right).
Discussion
Identification of associated proteins is an effective strategy to reveal regulatory mechanisms. For example, identification of cGMP-gated channel in photoreceptor cells as a calmodulin binding protein revealed its regulatory mechanism in phototransduction (32). This study aims to identify STRA6 regulatory mechanisms by first identifying STRA6-associated proteins in an unbiased manner from the RPE cell, the major cell type in the eye responsible for vitamin A uptake and storage. We developed a more-efficient method to purify endogenous STRA6 protein from freshly dissected RPE cells and used unbiased mass spectrometry to identify STRA6-associated proteins. This effort identified calmodulin as the major STRA6-binding protein in the native RPE cells. This finding is consistent with the first cryo-EM structural study of STRA6 that identified zebrafish STRA6 as in a complex with calmodulin (29).
What is the functional consequence of calcium/calmodulin’s interaction with STRA6? Using a variety of techniques including radioactive vitamin A-based assay, HPLC-based assay, and real-time fluorescence-based assay, we found that calcium/calmodulin has a profound inhibitory effect on STRA6’s vitamin A uptake activity. Increased intracellular calcium suppresses STRA6-mediated vitamin A uptake and decreased intracellular calcium enhances STRA6-mediated vitamin A uptake. These effects are demonstrated both in cell lines ectopically expressing STRA6 (Fig. 3) and in primary RPE cultures expressing endogenous STRA6 (Fig. 4). Consistently, shRNA-mediated knockdown of calmodulin in RPE cells also increased cellular vitamin A uptake from holo-RBP (Fig. 4).
How does calcium/calmodulin regulate vitamin A transport mediated by STRA6? We found that calcium/calmodulin strongly promotes STRA6’s binding to apo-RBP (Fig. 5). Enhanced apo-RBP binding to STRA6 can suppress vitamin A uptake in the cell due to two mechanisms. First, apo-RBP’s binding to STRA6 strongly promotes vitamin A efflux from the cell (25). Second, transient RBP/STRA6 interaction is essential for RBP delivery of vitamin A to a cell because each RBP only binds one molecule of vitamin A. Prolonged interaction would prevent the next holo-RBP from delivering the next vitamin A molecule (Fig. 7). Prolonged RBP/STRA6 interaction is pathogenic as revealed by a recent human genetic study of novel RBP mutations (33).
Calcium is a regulator of diverse and seemingly unrelated cellular processes including many membrane transport events and long-term changes in gene expression (34, 35). Previous studies revealed that calcium is a key regulator of phototransduction in photoreceptor cells (36–42). In the RPE, calcium is known to regulate and coordinate many cellular events including membrane transport (43–46) and activating the light peak in the electrooculogram (47). Calcium in RPE is regulated by extracellular signals through purinergic receptors (44, 45, 48, 49), adrenergic receptors (50, 51), and neuropeptide Y receptor (52).
The profound influence of calcium/calmodulin on vitamin A influx and efflux, as revealed in this study, can not only provide mechanistic insights into how vitamin A homeostasis is maintained, but also have potential practical implications in treating human diseases that have benefited from modulating retinoid levels such as vision diseases and cancer. For example, development of novel drugs that specifically block STRA6/calmodulin interaction can promote cellular vitamin A uptake. Assays described in this study can be potentially used in the screening of such small molecule drugs. STRA6 was initially known as a cancer cell-surface marker, and its expression is highly elevated in certain types of cancer cells (up to 100-fold higher) (53). Such drugs can potentially promote excessive uptake of vitamin A and induce differentiation or apoptosis of cancer cells.
Methods
Please refer to SI Appendix for detailed protocols. Briefly, STRA6 was purified from native RPE cells freshly dissected from bovine eyes using immunoaffinity purification to unbiasedly identify STRA6-associated proteins using mass spectrometry. Real-time monitoring STRA6/calmodulin interaction in live cells was done using fluorescence resonance energy transfer between STRA6-C-CFP and YFP-N-calmodulin. The effects of calcium and calmodulin on STRA6-mediated vitamin A influx and efflux were studied using real-time monitoring of STRA6-catalyzed retinol release from holo-RBP, real-time monitoring of STRA6-catalyzed retinol loading into apo-RBP, radioactive vitamin A-based assays, and HPLC-based vitamin A uptake assays. The effects of calcium and calmodulin on STRA6 and RBP interaction were studied using live-cell binding to holo-RBP or apo-RBP. Acute chemical modification was used to study the STRA6’s vitamin A transport pore. The effects of calcium and calmodulin on primary RPE culture were studied by changing intracellular calcium and by shRNA-mediated knockdown of the three calmodulin genes.
Data Availability Statement.
All data discussed in the paper are made available in the paper.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01EY018144 (to H.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.S.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918540117/-/DCSupplemental.
References
- 1.Wald G., Molecular basis of visual excitation. Science 162, 230–239 (1968). [DOI] [PubMed] [Google Scholar]
- 2.Dowling J. E., Night blindness. Sci. Am. 215, 78–84 (1966). [DOI] [PubMed] [Google Scholar]
- 3.von Lintig J., Kiser P. D., Golczak M., Palczewski K., The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong M., Kawaguchi R., Kassai M., Sun H., Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients 4, 2069–2096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans R. M., The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect. 90, 105–117 (1994–1995). [PubMed] [Google Scholar]
- 6.Chambon P., A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 (1996). [PubMed] [Google Scholar]
- 7.Niederreither K., Dollé P., Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 9, 541–553 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Love J. M., Gudas L. J., Vitamin A, differentiation and cancer. Curr. Opin. Cell Biol. 6, 825–831 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Niles R. M., Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res. 555, 81–96 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Verma A. K., Retinoids in chemoprevention of cancer. J. Biol. Regul. Homeost. Agents 17, 92–97 (2003). [PubMed] [Google Scholar]
- 11.Orfanos C. E., Zouboulis C. C., Almond-Roesler B., Geilen C. C., Current use and future potential role of retinoids in dermatology. Drugs 53, 358–388 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Zouboulis C. C., Retinoids–Which dermatological indications will benefit in the near future? Skin Pharmacol. Appl. Skin Physiol. 14, 303–315 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Chivot M., Retinoid therapy for acne. A comparative review. Am. J. Clin. Dermatol. 6, 13–19 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Goodman D. S., “Plasma retinol-binding protein” in The Retinoids, Sporn M. B., Boberts A. B., Goodman D. S., Eds. (Academic Press, Inc., 1984), Vol. 2, pp. 41–88. [Google Scholar]
- 15.Blomhoff R., Green M. H., Berg T., Norum K. R., Transport and storage of vitamin A. Science 250, 399–404 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Heller J., Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J. Biol. Chem. 250, 3613–3619 (1975). [PubMed] [Google Scholar]
- 17.Bok D., Heller J., Transport of retinol from the blood to the retina: An autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp. Eye Res. 22, 395–402 (1976). [DOI] [PubMed] [Google Scholar]
- 18.Rask L., Peterson P. A., In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey’s small intestine. J. Biol. Chem. 251, 6360–6366 (1976). [PubMed] [Google Scholar]
- 19.Kawaguchi R., et al. , A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Isken A., et al. , RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golczak M., et al. , Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 283, 9543–9554 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi R., et al. , Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem. Biol. 6, 1041–1051 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasutto F., et al. , Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 80, 550–560 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golzio C., et al. , Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am. J. Hum. Genet. 80, 1179–1187 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi R., Zhong M., Kassai M., Ter-Stepanian M., Sun H., STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 245, 731–745 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz A., et al. , Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest. Ophthalmol. Vis. Sci. 53, 3027–3039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amengual J., et al. , STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum. Mol. Genet. 23, 5402–5417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills J. P., Furr H. C., Tanumihardjo S. A., Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp. Biol. Med. (Maywood) 233, 1255–1261 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., et al. , Structure of the STRA6 receptor for retinol uptake. Science 353, aad8266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong M., Kawaguchi R., Ter-Stepanian M., Kassai M., Sun H., Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS One 8, e73838 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiser J. R., van Tuinen D., Brockerhoff S. E., Neff M. M., Davis T. N., Can calmodulin function without binding calcium? Cell 65, 949–959 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Hsu Y. T., Molday R. S., Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361, 76–79 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Chou C. M., et al. , Biochemical basis for dominant inheritance, variable penetrance, and maternal effects in RBP4 congenital eye disease. Cell 161, 634–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clapham D. E., Calcium signaling. Cell 131, 1047–1058 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Greer P. L., Greenberg M. E., From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron 59, 846–860 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Koutalos Y., Yau K. W., Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 19, 73–81 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Molday R. S., Calmodulin regulation of cyclic-nucleotide-gated channels. Curr. Opin. Neurobiol. 6, 445–452 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y., Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Palczewski K., Sokal I., Baehr W., Guanylate cyclase-activating proteins: Structure, function, and diversity. Biochem. Biophys. Res. Commun. 322, 1123–1130 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Stephen R., Filipek S., Palczewski K., Sousa M. C., Ca2+ -dependent regulation of phototransduction. Photochem. Photobiol. 84, 903–910 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C. K., Woodruff M. L., Chen F. S., Chen D., Fain G. L., Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J. Neurosci. 30, 1213–1220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai K., Chen J., Kefalov V. J., Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda Y., Steinberg R. H., Chloride currents in freshly isolated rat retinal pigment epithelial cells. Exp. Eye Res. 58, 331–342 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Peterson W. M., Meggyesy C., Yu K., Miller S. S., Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J. Neurosci. 17, 2324–2337 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan J. S., Baldridge W. H., Kelly M. E., Purinergic regulation of cation conductances and intracellular Ca2+ in cultured rat retinal pigment epithelial cells. J. Physiol. 520, 745–759 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wimmers S., Halsband C., Seyler S., Milenkovic V., Strauss O., Voltage-dependent Ca2+ channels, not ryanodine receptors, activate Ca2+-dependent BK potassium channels in human retinal pigment epithelial cells. Mol. Vis. 14, 2340–2348 (2008). [PMC free article] [PubMed] [Google Scholar]
- 47.Strauss O., The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Collison D. J., Tovell V. E., Coombes L. J., Duncan G., Sanderson J., Potentiation of ATP-induced Ca2+ mobilisation in human retinal pigment epithelial cells. Exp. Eye Res. 80, 465–475 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Tovell V. E., Sanderson J., Distinct P2Y receptor subtypes regulate calcium signaling in human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 49, 350–357 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Quinn R. H., Quong J. N., Miller S. S., Adrenergic receptor activated ion transport in human fetal retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 42, 255–264 (2001). [PubMed] [Google Scholar]
- 51.Rymer J., Miller S. S., Edelman J. L., Epinephrine-induced increases in [Ca2+](in) and KCl-coupled fluid absorption in bovine RPE. Invest. Ophthalmol. Vis. Sci. 42, 1921–1929 (2001). [PubMed] [Google Scholar]
- 52.Ammar D. A., Hughes B. A., Thompson D. A., Neuropeptide Y and the retinal pigment epithelium: Receptor subtypes, signaling, and bioelectrical responses. Invest. Ophthalmol. Vis. Sci. 39, 1870–1878 (1998). [PubMed] [Google Scholar]
- 53.Szeto W., et al. , Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 61, 4197–4205 (2001). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are made available in the paper.