Abstract
Background
While tenosynovitis in the hands is associated with rheumatoid arthritis (RA) it is unknown whether tenosynovitis of the forefoot is associated with RA.
Purpose
To determine the anatomy of tendon sheaths of the forefoot and the relationship between MRI-detected tenosynovitis at metatarsophalangeal(MTP) joints and rheumatoid arthritis.
Materials and Methods
14 forefeet of donated bodies were examined at flexor-tendons and extensor-tendons for the presence and course of tendon sheaths. In the prospective study between June 2013 and March 2016, newly presenting patients with RA, patients with other early arthritides, and healthy controls all underwent MRI of unilateral MTP (1-5)-joints. MRI studies were scored by two independent readers for tenosynovitis, synovitis and bone marrow edema. The association of the presence of these features with RA were examined using logistic regression.
Results
Macroscopically, all extensor and flexor tendons crossing MTP joints demonstrated sheaths surrounding tendons. Microscopically a synovial sheath was present. 634 participants were evaluated by MRI: 157 newly presenting patients with RA (109 women; mean age 59 years ±11 SD), 284 patients with other early arthritides (158 women; 56 years ±17 SD), and 193 healthy controls (136 women; 50 years ±16 SD). MRI-detected tenosynovitis was associated with RA, both when compared to patients with other arthritides (odds ratio (OR) 2.5 (95%CI 1.7-3.9, P<0.001) and healthy controls (OR 46, 95%CI 14-151, P<0.001). The association was OR 2.4, (95%CI 1.5-3.8, P<0.001) for flexor and OR 3.1, (95%CI 1.9-5.2, P<0.001) for extensor tendons. The sensitivity of tenosynovitis in RA was 65/157 (42%, 95%CI 35-50). The specificity was 63/284 (78%, 95%CI 72-82) compared to other arthritides, and 3/193 (98%, 95%CI 96-99) compared to healthy controls.
Conclusion
Tendons at metatarsophalangeal-joints are surrounded by tenosynovium. MRI-detected tenosynovitis at metatarsophalangeal-joints was specific for rheumatoid arthritis when compared to patients with other arthritides and healthy controls.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is characterized by inflammation of the synovial joints.(1) MRI is recommended for the early detection of inflammation in RA-research, as it is sensitive in measuring synovitis, bone marrow edema (BME) and tenosynovitis.(2) Tenosynovitis is defined as inflammation of the synovial lining of the tendon sheaths. MRI-studies have showed that tenosynovitis at the level of metacarpophalangeal(MCP) and wrist joints is an early phenomenon in patients at risk for RA that is predictive for RA-development,(3–5) and when compared to patients with arthritis with another diagnosis, is highly specific for RA.(6) In addition, tenosynovitis is associated with functional impairment in daily life.(7,8)
Tendon sheaths help prevent tendon injury and help with load bearing.(9) Not all tendons possess a sheath; for example at the MCP-joints, a sheath is present around the flexor tendons but believed to be absent around the extensor tendons.(6) Regarding the metatarsophalangeal(MTP) joints, there is no consensus in the anatomic literature on the presence or absence of a tendon sheath. On the extensor side of the MTP joints, some sources portrayed extensor tendons without sheaths,(10–12) while other sources neglect to provide information about this region.(13) Regarding the flexor side of MTP joints there is controversy between sources: in one example a fibrous and synovial sheath was portrayed around the distal flexor tendons,(10) in another case it was described as solely fibrous,(14) and others did not provide any information on this matter.(11,12)
In addition to the lack of consistent information on the normal human anatomy, the prevalence of inflammation at the level of the tendons of MTP joints and its association with RA are unknown. A few MRI studies reported on the presence of tenosynovitis, but these studies were small (≤30 patients with RA), did not include information at joint level, and/or did not compare findings of patients with RA with reference populations (15–18). The sensitivity and specificity of MRI-detected inflammation of the tendon sheath at the MTP level for RA thus remain unknown.
This study aimed to 1) elucidate the anatomical presence of a tendon sheath at the flexor- and extensor-side of the MTP joints in an anatomical study to clarify if contrast-enhancement around the tendons at MTP level can be interpreted as tenosynovitis, and 2) unravel whether MRI-detected tenosynovitis at MTP joints is associated with RA, in a large cross-sectional MRI study comparing patients newly presenting with early RA with patients diagnosed with other arthritides and healthy controls.
Materials and Methods
Anatomical dissection
Macroscopy
This section of the study was conducted on 11 formalin-phenol embalmed and 3 fresh-frozen human feet at the Anatomy & Embryology lab of the Leiden University Medical Center in Leiden, The Netherlands. All specimens were obtained from bodies that were donated according to the Dutch Burial and Cremation Act to the department of Anatomy and Embryology at the Leiden University Medical Center for use in scientific research and medical education.
The cutis and subcutis at the flexor side of 11 embalmed feet (8 man, 1 woman, 2 unknown sex; age range 70-95yrs) and at the extensor side of three fresh-frozen feet (sex and age unknown) were removed. Tissue surrounding the tendons of interests were injected with epoxy resin at the flexor side and with silicone rubber at the extensor side. Gentle massage was used to promote dissemination of the fluids. Photographs were taken of all specimens. For a more detailed description, see the Supplementary Methods and Figures.
Microscopy
After dissection of the plantar cutis and subcutis of a non-injected foot, a block containing the flexor hallucis longus tendon and the surrounding connective tissue was removed 1-2 cm proximal to the MTP joint. In a separate non-injected foot, a block containing the extensor hallucis longus tendon and surrounding connective tissue was removed. Harvested tissues were embedded in paraffin. The paraffin blocks were transversely sliced in sections of 10 μm. The morphology of all tissues was examined by a hematoxylin and eosin (HE) staining and interpreted by an anatomist (FJ, 5 years experience) and pathologist (AC, 11 years experience) blinded to clinical data.
Imaging studies
Study Participants
In this prospective study, between June 2013 and March 2016, 447 consecutive patients newly presenting with clinically confirmed arthritis and symptom duration <2 years who were naïve to disease-modifying antirheumatic drugs were included in the Leiden Early Arthritic Clinic (EAC).(19) This is an inception cohort were at baseline, swollen joint counts were performed, serum samples were taken and patients underwent MRI. MRIs of 6 patients were excluded because of inhomogeneous fat suppression. RA was defined as a clinical diagnosis plus fulfillment of the American College of Rheumatology/European League Against Rheumatism 2010 classification criteria during the first year of follow-up.(1)
Healthy controls were recruited by advertisements in local newspapers and websites, as previously reported.(20) They had no history of inflammatory rheumatic disease, no joint symptoms during the last month and no arthritis at physical examination.
Studies using data from the EAC and from the healthy controls have been published in the past,(20,21) however MRI-data of tenosynovitis at the MTP joints has thus far not been evaluated.
The early arthritis and the healthy control studies were both approved by the local medical ethics committee (approval numbers P10.108 and P11.210, respectively). Written informed consent was obtained from all participants.
MRI protocol
Patients underwent unilateral MRI of MTP 1-5 joints of the most painful side or the dominant side in the case of equally severe symptoms on both sides ≤2 weeks after the first presentation and before starting disease-modifying antirheumatic drugs. In healthy controls, the dominant side was scanned. A musculoskeletal-extremity 1.5T-MRI scanner (Oni, General Electric, Wisconsin, Madison) was used using a 145mm coil for the foot. After intravenous injection of gadolinium contrast the following sequences were obtained: T1-weighted fast spin-echo (FSE) with fat suppression in the axial plane (TR/TE 700/9.5ms; acquisition matrix 364x224, ETL 2) and: T1-weighted FSE with fat suppression in the coronal plane (perpendicular to the axis of the MTPs) (TR/TE 540/7.5ms; acquisition matrix 320x192, ETL 2). Field-of-view was 140mm. Coronal sequences had 20 slices with a slice thickness of 3mm and a slice gap of 0.3mm. Axial sequences had a slice thickness of 3mm and a slice gap of 0.3mm with 14 slices.
MRI evaluation
Tenosynovitis of the MTP joints was scored according to the method by Haavardsholm et al.(22) This method is developed and validated for scoring tenosynovitis in the wrist. BME and synovitis were scored according to The Outcome Measures in Rheumatology Clinical Trials Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (OMERACT-RAMRIS),(23) with the exception that BME was assessed on a contrast enhanced T1-weighted fat-suppressed sequence, as it allows a shorter scan time and has a higher signal to noise ratio.(24–26) The method by Haavardsholm and the OMERACT-RAMRIS have been designed for scoring inflammation in the hands, and were found reliable when applied to the-MTP joints.(27,28) All tendons, joints and bones were scored on a scale of 0-3. For tenosynovitis the score is based on the thickness of peritendinous effusion or synovial proliferation with enhancement (normal, <2, 2-5, >5mm), for synovitis on the presumed volume of enhancing tissue in the synovial compartment (none, mild, moderate, severe) and for BME based on the affected volume of the bone (no BME, <33%, 33-66%, >66%). Tenosynovitis was scored at the flexor and extensor tendons at the MTP joints, and BME was scored in the proximal and distal part of the MTP joint. Proximal and distal BME-scores were summed per joint.
Each MRI was scored independently by two trained readers, blinded to clinical data. A total of 6 readers scored the images: The first 215 EAC-patients were scored by WN and EN, the remaining 226 EAC-patients by DB and AB and 193 healthy controls by HS and LM. All 6 readers were medical doctors and PhD candidates in the field of Rheumatology and Radiology that had scored >400 MRIs according to the scoring systems during a training period of several months prior to evaluating the MRIs that are part of this study, with 1-2 years of experience with the scoring systems. Intraclass correlation coefficients (ICCs) were calculated. Interreader ICC was ≥0.93, intrareader ICCs ≥0.88 for arthritis patients (Supplementary Table 1). The inter- and intrareader ICCs of the two readers of the healthy controls were ≥0.96.(20)
Statistical Analysis
Non-normally distributed MRI-scores were analyzed using Kruskal-Wallis tests followed by a Bonferroni correction for all pairwise comparisons. Next, data of MRI scores were dichotomized per MTP joint; tenosynovitis, synovitis and BME were considered present if scored ≥1 by both readers. Logistic regression analysis was used to compare MRI-detected tenosynovitis in patients with RA to other arthritides and to healthy controls. Multivariable logistic regression analyses comparing RA to other arthritides corrected for MRI-detected synovitis and BME in the same joint and subsequent analyses additionally corrected for age, swollen joint count, C-reactive protein and anti-citrullinated peptide antibodies. Test characteristics were calculated for MRI-detected tenosynovitis.
SPSS 23.0 was used (IBM, Chicago, Ill). P-values <0.05 were considered significant.
Results
Anatomical studies
Macroscopy
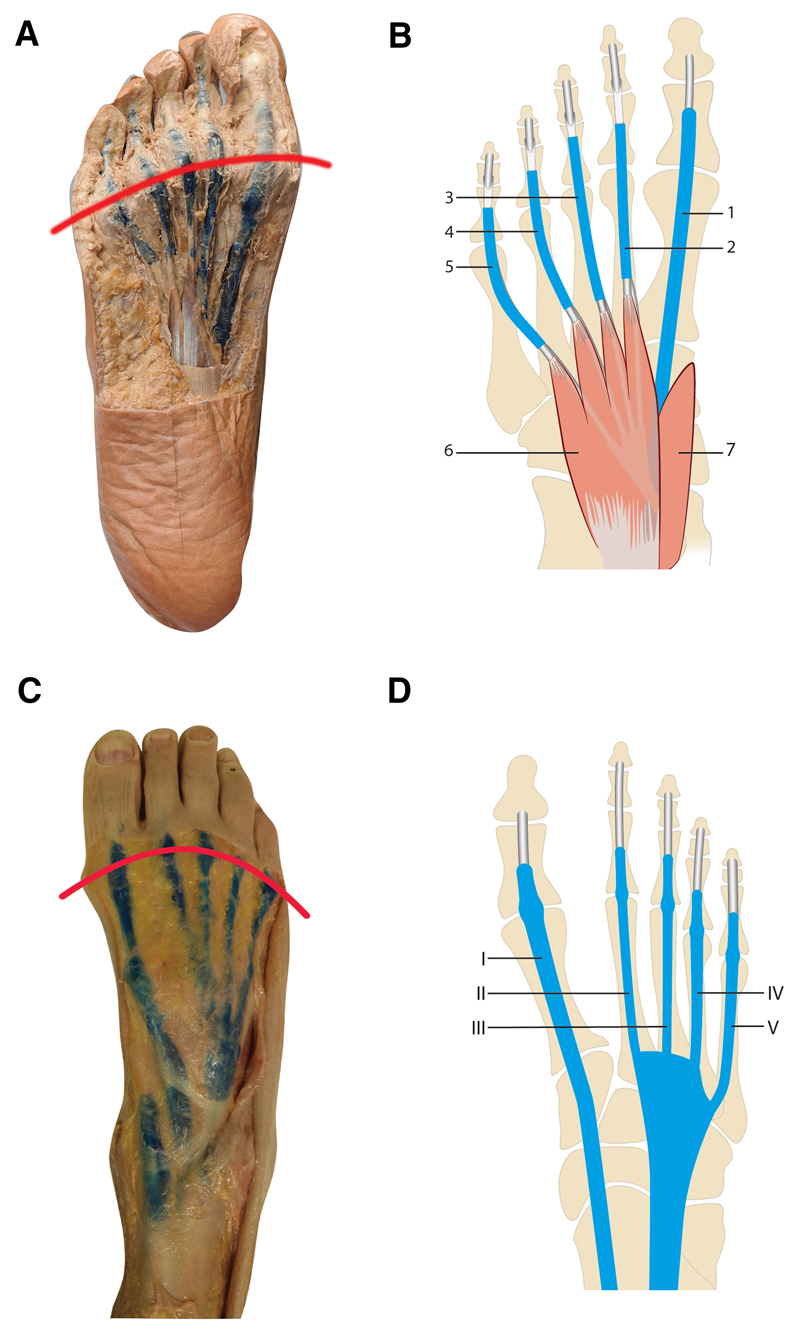
Hardened resin (flexor side) and silicone rubber (extensor side) were found to be confined to a sharply demarcated ‘sheath-like’ structure surrounding the flexor and extensor tendons of all five digits in the foot, as is presented in Figure 1 (additional examples are presented in Supplementary Figure 1). This resembled the expected image of tendon sheaths surrounding their tendons. On both the flexor and the extensor side the distal extensions of all tendon sheaths crossed the MTP joints. There was no exception in this pattern, but variation existed in the distal termination, which reached as far as the distal phalanx. For a more detailed description, see Supplementary Results.
Figure 1. Macroscopic images and schematic drawings of tendon sheaths in the forefeet.
Drawn red lines represent the level of the MTP-joints. Sheaths are in blue. A. Plantar view of a foot with resin in the flexor tendon sheaths, extending proximal and distal of the MTP joints; B: Schematic: tendon sheaths of the m. flexor hallucis longus tendon (FHL) (1) and the common mm. flexor digitorum longus (FDL) and common flexor digitorum brevis (FDB) tendons (2–5). Proximally the four tendons of the FDL run deep of the FDB muscle (6) and extend distally with a common tendon sheath for the FDL and FDB tendons. The tendons of the FDB split to course in a more dorsal position before inserting into the middle phalanx; the tendon of the FDL continue in a straight course and attach to the base of the distal phalanx. The tendon of the FHL (1) proximally runs deep from the musculus abductor hallucis (7) and FDB and inserts at the base of the distal phalanx. C. Dorsal view of a foot with silicone in extensor tendon sheaths, extending from the anterior ankle to distal of the MTP joints; D: Schematic: tendon sheaths of the extensor hallucis longus (EHL) (I) and extensor digitorum longus tendons (EDL) (II-V), forming a common sheath from proximal of the metatarsals to the anterior ankle. The EHL and EDL insert at the dorsal aspect of the distal phalanges. The extensor digitorum brevis (EDB) tendons insert into the EDL II-IV tendons at the MTP-joints and are not portrayed.
Microscopy
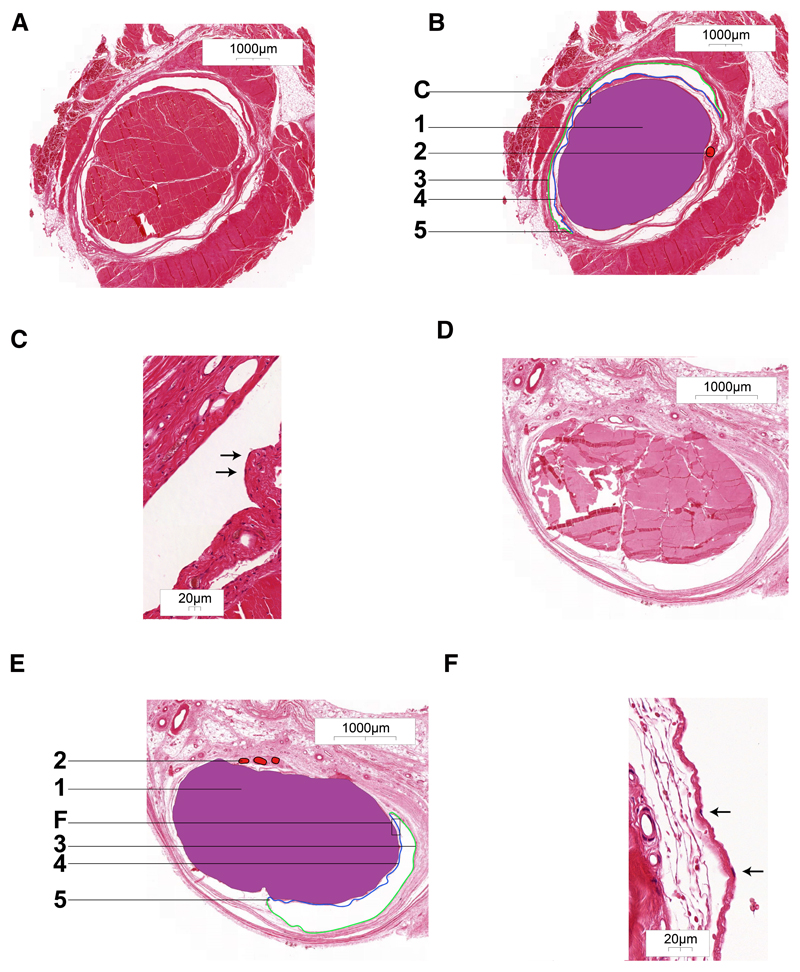
Surrounding the flexor hallucis longus tendon a completely delimited space was noticed (Figure 2A,B). Deep to the tendon, two cul-de-sacs connecting the parietal and visceral part of the sheath were visible and a vinculum between these cul-de-sacs was also visible, allowing vasculature to reach the tendon. 40x magnification showed the delimiting cells as squamous epithelial cells representing synoviocytes (Figure 2C). At the extensor hallucis longus tendon, similar results were observed (Figure 2D,E,F).
Figure 2. Histology of HE-stained, transversely sectioned tendon sheath of the flexor hallucis longus tendon (A, B and C) and of the extensor hallucis longus tendon (D, E and F).
A. Overview of tendon sheath surrounding flexor hallucis longus tendon. B. Overlay with 1. tendon; 2. artery in vinculum; 3. parietal layer of tendon sheath; 4. visceral layer of tendon sheath; 5. cul-de-sac of tendon sheath. C. detail of fig. B. with visible synoviocytes (arrows).
D. Overview of tendon sheath surrounding extensor hallucis longus tendon. E. Overlay with 1. tendon; 2. vessels in vinculum; 3. parietal layer of tendon sheath; 4. visceral layer of tendon sheath; 5. cul-de-sac of tendon sheath. F. detail of fig. E. with visible synoviocytes (arrows).
As a tendon sheath was present at both the flexor and extensor side, we assumed that MRI-detected contrast enhancement around the tendons at both sides of the MTP joints represents tenosynovitis.
Imaging studies
Patient characteristics
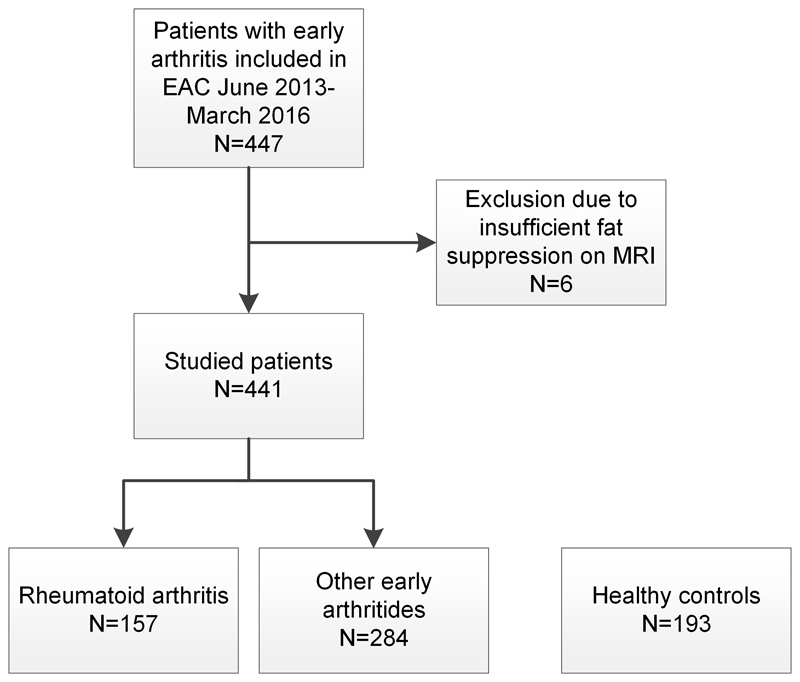
Of the 441 included EAC-patients, 157 were classified as RA (109 women; mean age 59 ± 11 years standard deviation (SD)), the remaining 284 received alternative diagnoses and were grouped together as ‘other arthritides’ (158 women; mean age 56 ± 17 SD) (Figure 3). 193 healthy controls were recruited (136 women; mean age 50 ±16 SD). The baseline characteristics are presented in Table 1. Patients with RA had a median symptom duration of 10 weeks (interquartile range (IQR) 5-28) and 59% were anti-citrullinated peptide antibody-positive. The semiquantitative MRI-tenosynovitis scores were higher in patients with RA than in patients with other arthritides (1.9 and 0.7, respectively, P<0.001, Table 1). Next, the presence of tenosynovitis was dichotomized as described in the methods.
Figure 3. Flow chart of participant selection.
EAC: early arthritis cohort; MRI: magnetic resonance imaging. RA was defined according to clinical diagnosis plus fulfilment of the 2010 classification criteria. The ‘other early arthritides’ included the following diagnoses: unclassified arthritis (n=148), psoriatic arthritis or spondyloarthritis (n=45), inflammatory osteoarthritis (n=23), reactive arthritis (n=7), crystal arthropathy (n=21), remitting seronegative symmetrical synovitis with pitting edema (n=12) and other diagnoses (n=28).
Table 1. Baseline characteristics of patients with RA, patients with other arthritides, and healthy controls.
| Parameter | RA | Other arthritides* | P-value RA vs other arthritides | Healthy controls | P-value RA vs healthy controls |
|---|---|---|---|---|---|
| n=157 | n=284 | n=193 | |||
| Clinical parameters | |||||
| Age, mean (SD) | 59 (14) | 56(17) | 0.07 | 50 (16) | <0.001 |
| Woman, n(%) | 109 (69) | 158 (56) | 0.005 | 136 (71) | 0.83 |
| BMI, mean (SD) | 26 (5) | 27 (4) | 0.52 | 25 (4) | 0.003 |
| Current smoker, n (%) | 31 (21) | 44 (18) | 0.44 | 17 (9) | 0.001 |
| Symptom duration in weeks, median (IQR) | 10 (5-28) | 8 (4-26) | 0.13 | - | - |
| Swollen joint count, median (IQR) | 7 (2-11) | 2 (1-4) | <0.001 | - | - |
| CRP, mg/L, median (IQR) | 9 (4-26) | 6 (3-16) | <0.001 | - | - |
| RF positive, n (%) | 106 (68) | 51 (18) | <0.001 | - | - |
| ACPA positive, n (%) | 87 (59) | 69 (25) | <0.001 | - | - |
| MRI features | |||||
| Tenosynovitis, mean (SD) | 1.9 (2.5) | 0.7 (1.3) | <0.001 | 0.1 (0.3) | <0.001 |
| Synovitis, mean (SD) | 1.9 (2.2) | 0.9 (1.2) | <0.001 | 0.2 (0.4) | <0.001 |
| BME, mean (SD) | 1.9 (2.8) | 1.0 (1.9) | <0.001 | 0.3 (0.8) | <0.001 |
RA: rheumatoid arthritis; SD: standard deviation; BMI: body mass index; IQR: interquartile range; CRP: C-reactive protein; RF: rheumatoid factor; ACPA: anti-citrullinated protein antibody; BME: bone marrow edema; the 66 swollen joint count was assessed.
This included the following diagnoses: unclassified arthritis (n=148), psoriatic arthritis or spondyloarthritis (n=45), inflammatory osteoarthritis (n=23), reactive arthritis (n=7), crystalarthropathy (n=21), remitting seronegative symmetrical synovitis with pitting edema (n=12) and other diagnoses (n=28).
Association of MTP tenosynovitis at patient-level with RA compared to other arthritides
Tenosynovitis was present at ≥1 MTP-location in 65 out of 157 (42%) patients with early RA and 63 out of 284 (22%) of patients with other early arthritides (odds ratio (OR) 2.54 (95% confidence interval (CI) 1.65-3.87), P<0.001) (Table 2).
Table 2. Results of logistic regression analyses for the association of MTP-tenosynovitis with early RA when compared to other early arthritides.
| Patients with MRI-features, n (%) | Univariable analyses | Multivariable analyses: adjusted for local synovitis and BME | Multivariable analyses: adjusted for local synovitis and BME and age, SJC, CRP and ACPA | |||||
|---|---|---|---|---|---|---|---|---|
| RA | Other arthritides | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Presence of Any tenosynovitis | 65 (42) | 63 (22) | 2.5 (1.7-3.9) | <0.001 | 2.3 (1.4-3.5) | <0.001 | 1.7 (1.0-2.9) | 0.048 |
| Any flexor tenosynovitis* | 49 (32) | 46 (16) | 2.4 (1.5-3.8) | <0.001 | 2.1 (1.3-3.4) | 0.002 | 1.9 (1.0-3.4) | 0.040 |
| Any extensor tenosynovitis* | 44 (29) | 32 (11) | 3.1 (1.9-5.2) | <0.001 | 2.7 (1.6-4.6) | <0.001 | 2.1 (1.1-3.9) | 0.017 |
| Flexor tenosynovitis | ||||||||
| MTP 1 | 18 (12) | 13 (5) | 2.7 (1.3-5.7) | 0.009 | 2.2 (1.0-4.9) | 0.049 | 2.7 (1.0-6.9) | 0.04 |
| MTP 2 | 26 (17) | 27 (10) | 1.9 (1.1-3.5) | 0.03 | 1.3 (0.7-2.6) | 0.41 | 1.4 (0.6-3.3) | 0.44 |
| MTP 3 | 27 (18) | 20 (7) | 2.8 (1.5-5.2) | 0.001 | 1.9 (0.9-3.8) | 0.81 | 1.8 (0.7-4.3) | 0.22 |
| MTP 4 | 26 (17) | 19 (7) | 2.8 (1.5-5.3) | 0.001 | 1.8 (0.8-3.7) | 0.14 | 1.6 (0.7-3.8) | 0.31 |
| MTP 5 | 14 (9) | 2 (1) | 14.0 (3.1-62.5) | 0.001 | 5.3 (1.1-25.7) | 0.04 | 9.3 (1.5-57.0) | 0.015 |
| Extensor tenosynovitis | ||||||||
| MTP 1 | 31 (20) | 15 (5) | 4.5 (2.3-8.6) | <0.001 | 3.9 (2.0-7.5) | <0.001 | 3.1 (1.4-6.9) | 0.005 |
| MTP 2 | 4 (3) | 4 (1) | 1.8 (0.5-7.4) | 0.40 | 1.0 (0.2-4.4) | 0.98 | 0.5 (0.1-2.9) | 0.46 |
| MTP 3 | 10 (7) | 14 (5) | 1.3 (0.6-3.0) | 0.53 | 0.7 (0.3-1.9) | 0.51 | 0.9 (0.3-2.5) | 0.89 |
| MTP 4 | 4 (3) | 8 (3) | 0.9 (0.3-3.1) | 0.90 | 0.6 (0.2-2.0) | 0.37 | 0.3 (0.1-1.6) | 0.32 |
| MTP 5 | 14 (10) | 4 (2) | 7.0 (2.3-21.6) | 0.001 | 2.9 (0.8-9.8) | 0.09 | 3.5 (0.9-13.1) | 0.07 |
Tenosynovitis was considered present if scored ≥1 by both readers. BME: bone marrow edema; SJC66: swollen joint count; CRP: C-reactive protein; ACPA: anti-citrullinated peptide antibodies; RA: rheumatoid arthritis; OR: odds ratio: CI: confidence interval; MTP: metatarsophalangeal joint. Other arthritides: patients with early arthritis presenting with arthritis with a diagnosis other than RA.
In some patients, flexor tenosynovitis and extensor tenosynovitis occurred simultaneously, therefore the percentages of patients with any flexor tenosynovitis and any extensor tenosynovitis do not add up to the percentage of patients with any tenosynovitis.
Tenosynovitis regularly co-occurred with synovitis and BME. Therefore, a multivariable analysis including the three types of MRI-detected inflammation was performed that showed tenosynovitis was independently associated with RA (OR 2.25 (95% CI 1.4-3.5), P<0.001) after controlling for the presence of synovitis (OR 1.6, 95% CI 1.0-2.5, P=0.06) and BME (OR 0.9, 95% CI 0.6-1.5, P=0.68) (Table 2 and Supplementary Table 2). In a subsequent multivariable analysis that also adjusted for age, swollen joint count, C-reactive protein, and anti-citrullinated peptide antibodies, tenosynovitis remained associated with RA (OR 1.68, 95% CI 1.0-2.9, P=0.048) (Table 2).
Next, presence of any tenosynovitis at the flexor and extensor side were studied separately. When compared to other arthritides, both presence of tenosynovitis at the flexor and the extensor side was associated with RA (OR 2.4, 95% CI 1.5-3.8, P<0.001 and OR 3.1, 95% CI 1.9-5.2, P<0.001, respectively). Tenosynovitis at flexor and extensor tendons remained statistically associated with RA after controlling for local synovitis and BME and after additional correction for clinical characteristics (Table 2).
Association of MTP tenosynovitis at joint level with RA compared to other arthritides
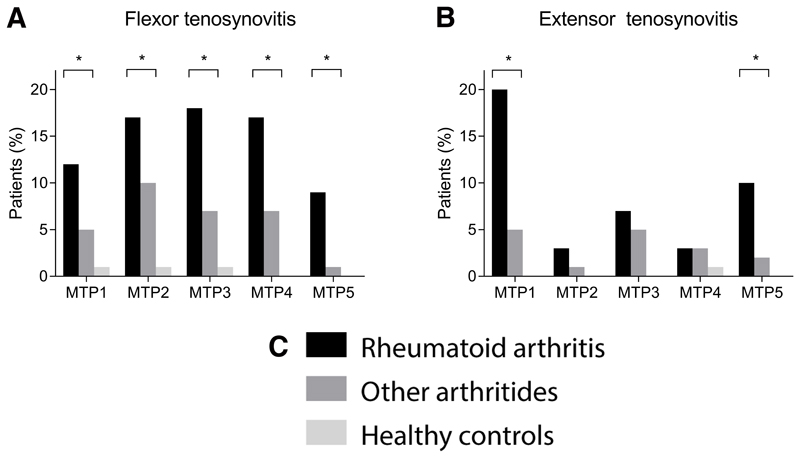
Then, tenosynovitis was studied at the level of individual joints. Frequencies per joint are provided in Figure 4. In univariable analyses, the following features were associated with RA: flexor tenosynovitis at all MTP joints, extensor tenosynovitis at MTP-1 and MTP-5 (Table 2). After correction for BME and synovitis, flexor and extensor tenosynovitis at MTP-1 were associated with RA (OR 2.2, 95% CI 1.0-4.9, P=0.049 and OR 3.9, 95% CI 2.0-7.5, P<0.001, respectively) and flexor tenosynovitis at MTP-5 (OR 5.3, 95% CI 1.1-26, P=0.04). After correction for clinical characteristics similar results were obtained (Table 2). Examples of MRI-detected tenosynovitis are presented in Figure 5.
Figure 4. Frequencies of the presence of (A) flexor and (B) extensor tenosynovitis per MTP-joint of patients newly presenting with rheumatoid arthritis, patients presenting with other early arthritides and of healthy controls.
MTP: metatarsophalangeal joints. *P<0.05 in univariable logistic regression analyses when comparing RA-patients with other arthritides
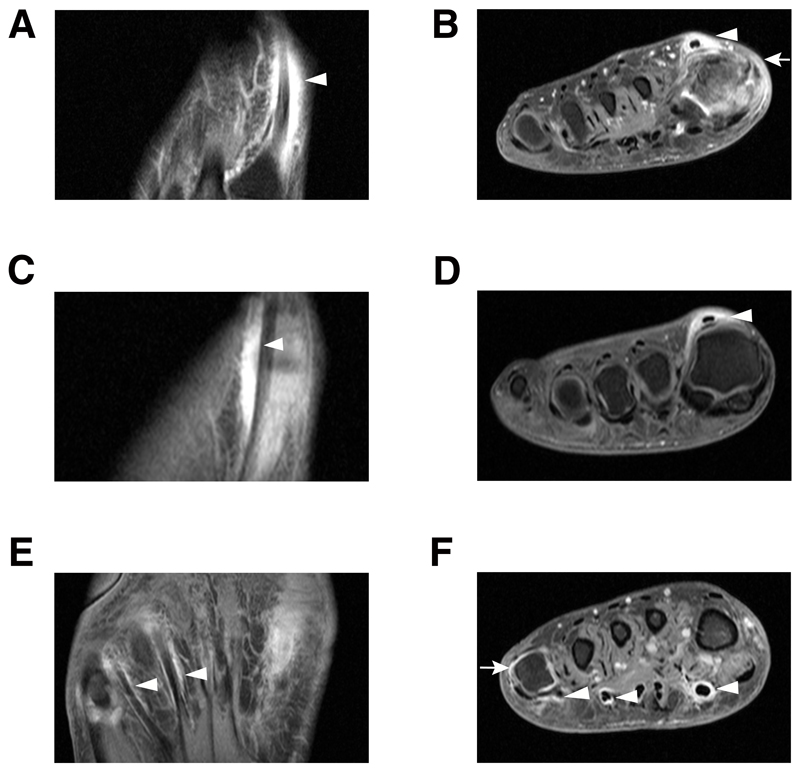
Figure 5. Contrast-enhanced 1.5T MRI of the forefoot in three different patients in corresponding axial (A, C and E) and coronal (B, D and F) planes showing extensor tenosynovitis at MTP 1 (A, B, C, D) and flexor tenosynovitis at MTP 1, 3, 4, 5 (E, F).
All images are T1-weighted fast spin-echo (FSE) with fat suppression after intravenous contrast administration. In A and B circular enhancement is seen of the extensor hallucis longus tendon, shown from the level of MTP 1 to the base of MT1, consistent with tenosynovitis (arrowheads). Synovitis of MTP 1 is visualized and edema of the skin and subcutis medially (arrow). The axial sequences (A) illustrate that the tenosynovitis is ‘sheath-like’ and continues distally to the MTP-joints (arrowheads). In C andD circular enhancement is present at the extensor hallucis longus tendon without concomitant synovitis of MTP 1 (arrowhead). In E and F circular enhancement is present at the tendons of the flexor hallucis and common flexor digitorum of MTP 1, 4 and 5 (arrowheads). There is coexisting synovitis at MTP 5 (arrow).
Association of MTP tenosynovitis with RA compared to healthy controls
To further characterize MRI-detected tenosynovitis, the occurrence of tenosynovitis in patients with RA was compared to healthy controls. In the latter group tenosynovitis at the MTP joints was rare, as it occurred in 3 out of 193 persons (1.6%, compared to 42% of patients with RA). Consequently, a strong association for RA compared to healthy controls was observed for the presence of any tenosynovitis at ≥1 location (OR 46, 95% CI 14-151, P<0.001)(Supplementary Table 3). Also, flexor tenosynovitis and extensor tenosynovitis were both separately highly associated with RA when compared to healthy controls (OR 45, 95% CI 11-187, P<0.001 and OR 78, 95% CI 11-570, P<0.001, respectively).
Test characteristics of MRI-detected tenosynovitis
Finally, the test characteristics for the presence of tenosynovitis were determined. The sensitivity of MRI-detected tenosynovitis at any MTP joint was 42%. The specificity of tenosynovitis at the level of MTPs was 78% compared to other arthritides and 98% with healthy controls as reference (Supplementary Table 4). Analyses for flexor and extensor tenosynovitis separately showed that tenosynovitis at the flexor side had a sensitivity of 32%, and a specificity of 84% when compared to other arthritides, and 99% when compared to healthy controls. For tenosynovitis at the extensor side, these numbers were 29%, 89%, and 99%, respectively.
Discussion
The forefoot is a preferential location for inflammation in rheumatoid arthritis (RA).(29,30) Nonetheless, the feet are less often evaluated than the small joints of the hands. For instance, the Disease Activity Score 28 does not include the feet. Also, the majority of MRI-studies performed thus far did not scan the feet.(31) This prompted us to study tenosynovitis at the level of the forefeet in a large MRI-study of patients with early arthritis. Moreover, since anatomical literature did not provide consistent information of the presence of a tendon sheath at the flexor- and extensor-side of the MTP joints we also performed an anatomical study to determine if tenosynovial sheaths are indeed present at the MTP joints. The aim of this post-mortem and MRI study was to increase the comprehension of the involvement of inflammation surrounding the tendons in RA. In all feet that were examined anatomically, we found a sheath to be present surrounding all extensor and flexor tendons at MTP joints. Histology showed an image fitting a synovial tendon sheath. Because of these anatomical findings, we assumed that MRI-detected contrast-enhancement around the tendons at both sides represented tenosynovitis. This MRI-detected tenosynovitis was associated with RA (P<0.001) and had a high specificity for RA, both when compared to patients with other arthritides (78%) and to healthy controls (98%). Together these data showed that tenosynovitis at MTP level is a characteristic of RA.
Our results are in line with previous studies of the hands that found MRI-detected tenosynovitis to be specific for RA.(6) MRI-detected inflammation is increasingly used as an outcome measure in clinical trials in RA.(2) Our data imply that tenosynovitis at MTP level can be included in this outcome; this would require further validation according to the OMETACT-Filter.(31,32)
In our study imaging was performed with MRI. Ultrasound is an alternate imaging modality that is used, and both methods are recommended in the clinical management of RA.(2) Both modalities have advantages and disadvantages. While ultrasound is easily available, it is operator dependent. MRI is more expensive but is generally considered to yield more reproducible results and also depicts BME that is not depicted by ultrasound. A recent study found ultrasound to be less sensitive than MRI in detecting tenosynovitis.(33) This supports the choice of using MRI in our current study.
The function of a tendon sheath is to provide a smooth gliding surface and thus prevent tendon injury.(9) Although the presence of a tenosynovium at a high-friction location like the MTP joints seems logical, it is surprising that anatomical literature thus far left it undetermined if tendons at these joints possess a synovial sheath, and some even portrayed this sheath to be absent. In this anatomic literature, no source studies were mentioned.(10–14) For the dissection, we started with embalmed bodies for the flexor side and when fresh-frozen bodies became available, they were used for the extensor side. The injection of tendon sheaths was performed in a similar fashion on both sides, therefore, we believe that the different methods of preserving the bodies have not negatively affected our results.
Microscopy indicated an image that was typical for a tendon sheath, with a visceral synovial layer covering the tendon surface, a parietal synovium attached to the surrounding tissues, and a vinculum for the intrinsic blood supply of the tendon.(9).
Our study has several limitations. Microscopically studying these lesions in patients with RA would be the optimal proof that MRI-detected contrast enhancement around the tendons at MTP level is tenosynovitis. This, however, was unfeasible as it would either require biopsies from tenosynovial sheaths at the MTPs from living patients with RA, or the use of donated bodies of patients with RA that had MRI-detected tenosynovitis at the end of their life. In addition, the MRI-scoring systems used are validated for the hand but not for the foot. However, our intra- and inter-reader ICCs were high, indicating a high reliability of scoring. The RAMRIS was recently updated, and now includes tenosynovitis.(34) For tenosynovitis we used the method described by Haavardsholm, as the updated RAMRIS was not yet published at the start of our study.(22) The included patients had an established diagnosis of RA. MRI-studies in other early arthritis populations are relevant to validate the diagnostic value of tenosynovitis at the MTP joints. For the predictive value, we recently observed in a longitudinal study that MTP-tenosynovitis in undifferentiated arthritis is associated with the development of RA,(35) which underlines the possible diagnostic value of tenosynovitis at MTP joints. It is important to note that while tenosynovitis at the MTP joints is specific for RA, the sensitivity of 42% is low. On the other hand, RA is known to be a disease where the hands and feet are involved. A recent study in the hands has revealed a sensitivity 75% for tenosynovitis at the hands.(6) Combining hands and feet will presumably yield a higher sensitivity for tenosynovitis in RA.
In conclusion, this anatomical and imaging study shows that tendons at the MTP joints possess a synovial sheath (both macro- and microscopically, and at flexor and extensor sides), and that MRI-detected tenosynovitis at the MTP joints is associated with, and highly specific for, RA. This study has increased the comprehension of tissues that can be involved in the feet of patients with RA by showing that MRI-detected tenosynovitis at the MTP joints is a feature of RA.
Supplementary Material
KEY Results.
Tendons crossing metatarsophalangeal joints had a synovial sheath (micro- and macroscopically) at the flexor and extensor side.
At the metatarsophalangeal-joints, MRI-detected tenosynovitis was associated with rheumatoid arthritis, both when compared to patients with other arthritides (odds ratio (OR) 2.5, P<0.001) and healthy controls (OR 46, P<0.001).
MRI-detected tenosynovitis of the metatarsophalangeal joints was associated with rheumatoid arthritis for flexor tendons (OR 2.4, P<0.001) and extensor tendons (OR 3.1, P<0.001)
The sensitivity of tenosynovitis of the metatarsophalangeal joints in rheumatoid arthritis was 42%. Specificity was 78% compared to other arthritides and 98% compared to healthy controls
Summary Statement.
Flexor and extensor tendons at metatarsophalangeal joints are surrounded by tenosynovium. MRI-detected tenosynovitis of flexor and extensor tendons at metatarsophalangeal joints was specific for rheumatoid arthritis.
Acknowledgements
The authors thank pathologist A.H.G. Cleven for interpreting the microscopy images, and G. Kracht for the schematic drawings of the tendons at the MTP joints.
Abbreviations
- RA
rheumatoid arthritis
- MTP
metatarsophalangeal
- OR
odds ratio
- CI
confidence interval
- BME
bone marrow edema
- MCP
metacarpophalangeal joint
- RAMRIS
Rheumatoid Arthritis Magnetic Resonance Imaging Score
Footnotes
Author Contributions
YJD, FPJ, MdR, MR and AHMvdHvM contributed to the conception and study design. YJD and FPJ analysed the data. YJD, FPJ, MdR, MR and AHMvdHvM contributed to interpretation of the data. YJD and FPJ contributed to acquisition of the data. YJD, FPJ and AHMvdHvM wrote the first version of the manuscript and MdR and MR revised it critically. All authors read and approved the final version of the document.
Disclosure of Conflics of Interest
The authors YJD, FPJ, MdR, MR and AHMvdHvM disclosed no relevant relationships.
References
- 1.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 2.Colebatch AN, Edwards CJ, Ostergaard M, van der Heijde D, Balint PV, D'Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804–14. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 3.Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology (Oxford) 2009;48(8):887–91. doi: 10.1093/rheumatology/kep136. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwenhuis WP, van Steenbergen HW, Mangnus L, Newsum EC, Bloem JL, Huizinga TWJ, et al. Evaluation of the diagnostic accuracy of hand and foot MRI for early Rheumatoid Arthritis. Rheumatology (Oxford) 2017;56(8):1367–77. doi: 10.1093/rheumatology/kex167. [DOI] [PubMed] [Google Scholar]
- 5.van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2016;75(10):1824–30. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuis WP, Krabben A, Stomp W, Huizinga TW, van der Heijde D, Bloem JL, et al. Evaluation of magnetic resonance imaging-detected tenosynovitis in the hand and wrist in early arthritis. Arthritis Rheumatol. 2015;67(4):869–76. doi: 10.1002/art.39000. [DOI] [PubMed] [Google Scholar]
- 7.Burgers LE, Nieuwenhuis WP, van Steenbergen HW, Newsum EC, Huizinga TW, Reijnierse M, et al. Magnetic resonance imaging-detected inflammation is associated with functional disability in early arthritis-results of a cross-sectional study. Rheumatology (Oxford) 2016;55(12):2167–75. doi: 10.1093/rheumatology/kew334. [DOI] [PubMed] [Google Scholar]
- 8.Glinatsi D, Baker JF, Hetland ML, Horslev-Petersen K, Ejbjerg BJ, Stengaard-Pedersen K, et al. Magnetic resonance imaging assessed inflammation in the wrist is associated with patient-reported physical impairment, global assessment of disease activity and pain in early rheumatoid arthritis: longitudinal results from two randomised controlled trials. Ann Rheum Dis. 2017;76(10):1707–15. doi: 10.1136/annrheumdis-2017-211315. [DOI] [PubMed] [Google Scholar]
- 9.Ettema AM, Amadio PC, Zhao C, Wold LE, O'Byrne MM, Moran SL, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118(6):1413–22. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 10.Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 41th edition. Elsevier; 2015. GH. 1419+42. [Google Scholar]
- 11.Netter FH. Atlas of Human Anatomy. 5th edition. Elsevier; 2014. pp. 557–8. [Google Scholar]
- 12.Paulsen F, Waschke J. Sobotta Atlas of Human Anatomy. 15th edition. Vol. 1. Elsevier; 2013. pp. 318–9. [Google Scholar]
- 13.Agur AMR, Dalley AF. Grant's Atlas of Anatomy. 13th edition. Lippincott Williams And Wilkins; 2013. p. 439. international. [Google Scholar]
- 14.Moore KL, D AF, Agur AMR. Clinically Oriented Anatomy. 7th edition. 2017. p. 769. [Google Scholar]
- 15.Boutry N, Larde A, Lapegue F, Solau-Gervais E, Flipo RM, Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol. 2003;30(4):671–9. [PubMed] [Google Scholar]
- 16.Calisir C, Murat Aynaci AI, Korkmaz C. The accuracy of magnetic resonance imaging of the hands and feet in the diagnosis of early rheumatoid arthritis. Joint Bone Spine. 2007;74(4):362–7. doi: 10.1016/j.jbspin.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Ostendorf B, Scherer A, Modder U, Schneider M. Diagnostic value of magnetic resonance imaging of the forefeet in early rheumatoid arthritis when findings on imaging of the metacarpophalangeal joints of the hands remain normal. Arthritis Rheum. 2004;50(7):2094–102. doi: 10.1002/art.20314. [DOI] [PubMed] [Google Scholar]
- 18.Zubler V, Agten CA, Pfirrmann CW, Weiss BG, Dietrich TJ. Frequency of arthritis-like MRI findings in the forefeet of healthy volunteers versus patients with symptomatic rheumatoid arthritis or psoriatic arthritis. AJR Am J Roentgenol. 2017;208(2):W45–W53. doi: 10.2214/AJR.16.16626. [DOI] [PubMed] [Google Scholar]
- 19.de Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Predicting arthritis outcomes--what can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 2011;50(1):93–100. doi: 10.1093/rheumatology/keq230. [DOI] [PubMed] [Google Scholar]
- 20.Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AH. Magnetic Resonance Imaging-Detected Features of Inflammation and Erosions in Symptom-Free Persons From the General Population. Arthritis Rheumatol. 2016;68(11):2593–602. doi: 10.1002/art.39749. [DOI] [PubMed] [Google Scholar]
- 21.Boeters DM, Nieuwenhuis WP, van Steenbergen HW, Reijnierse M, Landewe RBM, van der Helm-van Mil AHM. Are MRI-detected erosions specific for RA? A large explorative cross-sectional study. Ann Rheum Dis. 2018;77(6):861–8. doi: 10.1136/annrheumdis-2017-212252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66(9):1216–20. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30(6):1385–6. [PubMed] [Google Scholar]
- 24.Mayerhoefer ME, Breitenseher MJ, Kramer J, Aigner N, Norden C, Hofmann S. STIR vs. T1-weighted fat-suppressed gadolinium-enhanced MRI of bone marrow edema of the knee: computer-assisted quantitative comparison and influence of injected contrast media volume and acquisition parameters. J Magn Reson Imaging. 2005;22(6):788–93. doi: 10.1002/jmri.20439. [DOI] [PubMed] [Google Scholar]
- 25.Stomp W, Krabben A, van der Heijde D, Huizinga TW, Bloem JL, van der Helm-van Mil AH, et al. Aiming for a shorter rheumatoid arthritis MRI protocol: can contrast-enhanced MRI replace T2 for the detection of bone marrow oedema? Eur Radiol. 2014;24(10):2614–22. doi: 10.1007/s00330-014-3272-0. [DOI] [PubMed] [Google Scholar]
- 26.Sudol-Szopinska I, Jurik AG, Eshed I, Lennart J, Grainger A, Ostergaard M, et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin Musculoskelet Radiol. 2015;19(4):396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 27.Baan H, Bezooijen R, Avenarius JK, Dubbeldam R, Drossaers-Bakker WK, van de Laar MA. Magnetic resonance imaging of the rheumatic foot according to the RAMRIS system is reliable. J Rheumatol. 2011;38(6):1003–8. doi: 10.3899/jrheum.100906. [DOI] [PubMed] [Google Scholar]
- 28.Dakkak YJ, Matthijssen XME, van der Heijde DM, Reijnierse M, van der Helm-van Mil AHM. Reliability of Magnetic Resonance Imaging (MRI)-scoring of the Metatarsophalangeal-joints of the Foot According to the Rheumatoid Arthritis-MRI Score (RAMRIS) J Rheumatol. 2019 doi: 10.3899/jrheum.190258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borman P, Ayhan F, Tuncay F, Sahin M. Foot problems in a group of patients with rheumatoid arthritis: an unmet need for foot care. Open Rheumatol J. 2012;6:290–5. doi: 10.2174/1874312901206010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otter SJ, Lucas K, Springett K, Moore A, Davies K, Cheek L, et al. Foot pain in rheumatoid arthritis prevalence, risk factors and management: an epidemiological study. Clin Rheumatol. 2010;29(3):255–71. doi: 10.1007/s10067-009-1312-y. [DOI] [PubMed] [Google Scholar]
- 31.Dakkak YJ, van der Heijde DM, Reijnierse M, van der Helm-van Mil AHM. Validity of the rheumatoid arthritis MRI score applied to the forefeet using the OMERACT filter: a systematic literature review. RMD Open. 2018;4(2):e000796. doi: 10.1136/rmdopen-2018-000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d'Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67(7):745–53. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Ohrndorf S, Boer AC, Boeters DM, Ten Brinck RM, Burmester GR, Kortekaas MC, et al. Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthritis Res Ther. 2019;21(1):59. doi: 10.1186/s13075-019-1824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostergaard M, Peterfy CG, Bird P, Gandjbakhch F, Glinatsi D, Eshed I, et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol. 2017 doi: 10.3899/jrheum.161433. [DOI] [PubMed] [Google Scholar]
- 35.Dakkak YJ, Boeters DM, Boer AC, Reijnierse M, van der Helm-van Mil AHM. What is the additional value of MRI of the foot to the hand in undifferentiated arthritis to predict rheumatoid arthritis development? Arthritis Res Ther. 2019;21(1):56. doi: 10.1186/s13075-019-1845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.