Abstract
Impaired glucagon-like peptide (GLP-1) secretion or response may contribute to ineffective insulin release in type 2 diabetes. The conditionally-essential amino acid glutamine stimulates GLP-1 secretion in vitro and in vivo. In a randomized, cross-over study, we evaluated the effect of oral glutamine, with or without sitagliptin, on postprandial glycemia and GLP-1 concentration in 15 type 2 diabetes patients (glycated hemoglobin [HbA1c] 6.5±0.6%). Participants ingested a low-fat meal (5% fat) after receiving either water (control), 30 g L-glutamine (Gln-30), 15 g L-glutamine (Gln-15), 100 mg sitagliptin (SIT), or 100 mg sitagliptin and 15 g L-glutamine (SIT+Gln-15). Studies were conducted 1-2 wk apart. Blood was collected at baseline and postprandially for 180 min for measurement of circulating glucose, insulin, C-peptide, glucagon, and total and active GLP-1. Gln-30 and SIT+Gln-15 reduced the early (t=0-60 min) postprandial glycemic response compared to control. All Gln treatments enhanced the postprandial insulin response from t=60-180 min, but had no effect on the C-peptide response compared to control. The postprandial glucagon concentration was increased by Gln-30 and Gln-15 compared to control, but the insulin:glucagon ratio was not affected by any treatment. In contrast to Gln-30 which tended to increase the total GLP-1 AUC, SIT tended to decrease the total GLP-1 AUC relative to control (both P=0.03). Gln-30 and SIT increased the active GLP-1 AUC compared to control (P=0.008 and P=0.01, respectively). In summary, Gln-30 decreased the early postprandial glucose response, enhanced late postprandial insulinemia, and augmented postprandial active GLP-1 responses compared to control. These findings suggest that glutamine may be a novel agent for stimulating GLP-1 concentration and limiting postprandial glycemia in type 2 diabetes.
Keywords: Glutamine, GLP-1, Postprandial glycemia, Type 2 diabetes
Introduction
Defective insulin secretion is a key abnormality contributing to hyperglycemia and type 2 diabetes (1, 2). Incretin hormones, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), play a major role in mediating physiological insulin release following a meal (3, 4). Although controversial (5, 6), some evidence suggests that GLP-1 secretion is defective in type 2 diabetes (2, 7–9), developing as a consequence, rather than cause, of the hyperglycemic state (2, 6, 7). Insulin release from the beta cell in response to endogenous GLP-1 is preserved in well-controlled type 2 diabetes (10). However, the potency of GLP-1 to enhance insulin secretion may be decreased in more advanced disease (11). In contrast, GIP secretion is intact in diabetes, although the insulinotropic response to this incretin hormone is impaired (12). Interestingly, the blunted insulin response to incretins in poorly-controlled type 2 diabetes may be restored when glycemic control is improved (11).
There has been much recent interest in developing methods by which GLP-1 action can be enhanced in diabetes. An alternative approach to the use of GLP-1 receptor agonists and inhibitors of dipeptidyl peptidase-IV (DPP-IV) is the direct stimulation of GLP-1 secretion from intestinal L-cells. This approach has the additional benefit of stimulating other entero-endocrine peptides, including peptide YY (PYY) and oxyntomodulin, which suppress appetite and reduce food intake (13, 14), and GLP-2, which stimulates regeneration and repair of intestinal epithelium (15). Moreover, stimulation of L-cell secretion will increase GLP-1 9-36 concentration, the cleaved product of DPP-IV, which is a weak insulinotropic agonist that suppresses hepatic glucose production and possibly exerts antioxidant actions in the heart and vasculature (16).
We have previously demonstrated that oral glutamine increases GLP-1 concentration in lean, insulin-resistant obese and diabetic individuals, an effect associated with increased circulating insulin concentration (17). Glutamine is one of the most abundant free amino acid in humans, comprising 20% of the amino acid pool in plasma and 50% in human skeletal muscle (18). Interestingly, circulating glutamine concentration is reduced in well-controlled type 2 diabetes of short duration (19). Oral glutamine doses of 0.35 – 0.65 g·kg-1 result in peak concentrations at 30 – 60 min (17, 20), with similar concentrations attained in individuals with and without diabetes (17). Oral glutamine intake of up to 0.5 g·kg-1 is relatively palatable (20) and has been shown to be safe over 14 d, with no adverse effects on liver and renal function in middle-aged and elderly individuals (18).
Whether oral glutamine reduces postprandial glycemia when consumed with a meal in patients with type 2 diabetes remains unknown. The aims of this study were to determine: (i) whether glutamine attenuates postprandial glycemia in patients with type 2 diabetes when consumed with a meal and (ii) whether glutamine enhances postprandial circulating insulin, C-peptide and GLP-1 concentrations.
Materials and Methods
Type 2 diabetes patients were recruited through advertisements at the St Vincent’s Hospital precinct, Sydney and in local newspapers. Exclusion criteria included treatment with oral hypoglycemic agents other than metformin, ethanol intake of 40 g/d, liver or kidney disease, weight change of >2 kg in the preceding 6-mo, use of weight loss medications, previous bowel surgery and documented malabsorption. The study was of a randomized cross-over design and approved by the Human Research and Ethics Committee at St Vincent’s Hospital. All participants gave written informed consent.
Study Design
Participants attended the Clinical Research Facility at the Garvan Institute of Medical Research on 5 separate occasions, fasting from 22:00 h the previous night and received, in a random order: water (control); 30 g of L-glutamine (Gln-30); 15 g L-glutamine (Gln-15); 100 mg sitagliptin (SIT); and 100 mg sitagliptin plus 15 g L-glutamine (SIT + Gln-15). Following these treatments, participants consumed a meal comprising 33 g Wheat-Bix and 250 mL low fat milk, providing 963 kJ (37 g carbohydrate, 1.3 g fat and 16 g protein). Sitagliptin was administered 25 min prior to the meal (t = -25) with 50 mL of water. L-glutamine powder (Cambridge Commodities, Cambridge UK) was consumed in 300 mL of ice-cold water, to avoid its transformation to pyroglutamic acid (21), over 2 min immediately prior to the meal, which was consumed over 10 min (t = -10 - 0 min). As glutamine at high concentration does not dissolve completely in water, we used the ‘swish and swallow’ technique, as previously described (20). Participants were instructed to complete the meal, which was monitored by the study nurse. t = 0 corresponds to the end of meal ingestion.
Study visits were generally separated by 1-2 wk. Participants taking metformin omitted this medication on study days. A large-bore intravenous indwelling cannula was inserted into a large antecubital vein on each visit for blood sampling. At the first visit, weight and height were measured with the participant wearing light street clothing and BMI was calculated (weight in kg divided by the square of the height in meters, kg·m-2). On each study day, two fasting baseline blood samples were collected at t = -35 and -25 min (prior to sitagliptin administration). After consumption of the meal, blood samples were collected at t = 15, 30, 45, 60, 90, 120, 150 and 180 min for blood glucose, serum insulin and C-peptide and plasma glucagon and GLP-1 (total and active). Satiety was assessed fasting, immediately after meal ingestion (t = 0) and half-hourly for 180 min using a visual analogue scale (VAS).
Analytical Methods
Blood for glucose was collected in a fluoride oxalate tube and assayed immediately after collection, by the glucose oxidase electrode (Yellow Springs Instrument Company, YSI; Life Sciences). HbA1c was analyzed by cation-exchange HPLC using the Variant II analyser (Bio-Rad Laboratories, Gladesville, NSW, Australia). All other assays were performed on plasma and serum samples stored at -80°C. Insulin, C-peptide and glucagon were quantified by radioimmunoassay (Linco Research, St Charles, USA). Blood for total and active GLP-1 was collected into chilled EDTA-coated tubes (with DPP-IV inhibitor and trasylol in the active GLP-1 testing tube to prevent DPP-IV and protease activity, respectively), which were immediately centrifuged (7 min at 4100 x g), snap-frozen and stored at -80°C until analysis. Total GLP-1 concentrations were measured by radioimmunoassay after extraction of plasma with 70% ethanol (v:v, final concentration). Carboxy-terminal GLP-1 immunoreactivity was determined using antiserum 89390, which has an absolute requirement for the intact amidated carboxyl terminus of GLP-1 7-36 amide and cross-reacts < 0.01% with carboxy-terminally truncated fragments and 89% with GLP-1 9-36 amide, the primary metabolite of DPP-IV-mediated degradation. The sum of the 2 components (total GLP-1 concentration) reflects the rate of secretion of the L-cell (22). Active GLP-1 was analyzed at t = -35, -25, 15, 30, 60, 120 and 180 min (limited number to ensure all samples from the same participant were analyzed on the same plate) using an enzyme-linked immunosorbent assay on unextracted plasma, as reported previously (23). For both assays, sensitivity was <1 pmol/L and intra-assay CV <6%.
Statistical Analysis
Baseline characteristics of the cohort are presented as mean ± SD. Fasting baseline glucose, insulin, C-peptide, glucagon and GLP-1 data were calculated as the mean of the t = -35 and -25 results of all 5 visits. Insulin data were not normally distributed and were log10-transformed prior to statistical analysis. As there were no differences in baseline concentrations among treatments for glucose, insulin, C-peptide, glucagon, or total and active GLP-1 by one-way ANOVA, AUC are presented. AUC were calculated using the trapezoidal rule. When calculating the AUC, t = -35 and -25 timepoints were averaged to serve as the baseline value. Consistent with our previously reported biphasic GLP-1 response to glutamine and glucose (17), first (0 – 60 min) and second (60 – 180 min) phase AUC are also reported. The treatments were compared to the control using paired t-tests. Statistical significance was calculated using the Dunn-Bonferroni correction (24) for the 4 control-vs-treatment pairs, at an overall statistical significance threshold of 0.05. Thus, an individual paired t-test of P < 0.0125 (0.05/4) was deemed statistically significant. Data were analyzed using SPSS version 15 (Chicago, IL). Comparisons between treatments were not performed. There was no effect of gender on the data and thus data for the whole cohort is presented. There were 13 different combinations for the order of treatments in the present study, thus the effect of treatment order on the results cannot be tested. In any case, treatments were separated by at least 1 wk, thus the order of the treatments is not expected to affect the results.
Results
Cohort Characteristics
Fifteen participants (9 males and 6 females) were studied. Mean age was 63.6 ± 5.2 y and BMI 29.7 ± 4.4 kg·m-2. Type 2 diabetes was of short duration (2.4 ± 1.2 y). Participants were treated with lifestyle alone (n = 4) and/or metformin therapy (n = 11) and glycemia was well-controlled (HbA1c 6.5 ± 0.6 %). Averaged across the 5 visits, fasting results were as follows: blood glucose 6.2 ± 0.8 mmol/L, serum insulin 146 ± 90 pmol/L, serum C-peptide 3.3 ± 1.4 μg/L, plasma glucagon 77.1 ± 27.7 ng/L, plasma total GLP-1 23.1 ± 7.9 pmol/L, and plasma active GLP-1, 4.4 ± 3.4 pmol/L.
Circulating metabolites
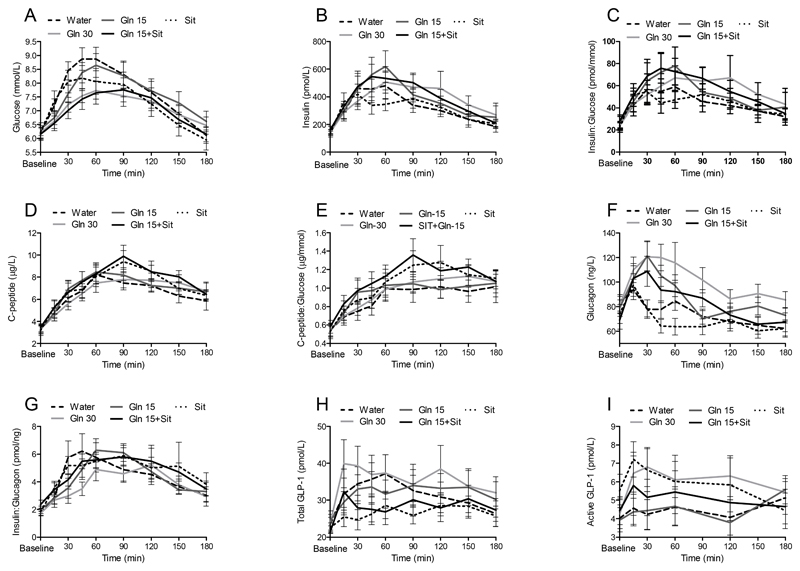
Gln-30 and SIT+Gln-15 reduced the postprandial glucose response compared to control, an effect limited to the first phase (t = 0 - 60 min, Fig. 1, Table 1). Gln-15 decreased the first phase glucose response from t = 0 – 60 min (P=0.016). SIT did not affect postprandial glycemia during either phase (Table 1).
Figure 1.
Circulating glucose (A), insulin (B), the insulin:glucose ratio (C), C-peptide (D), the C-peptide:glucose ratio (E), glucagon (F), the insulin:glucagon ratio (G), total GLP-1 (H) and active GLP-1 (I) concentrations in individuals with type 2 diabetes in response to a high-carbohydrate, low-fat meal following ingestion of water, Gln-30, Gln-15, SIT+Gln-15, or SIT.
Values are means ± SEM, n=15.
Table 1. Circulating glucose, insulin, insulin:glucose ratio, C-peptide, C-peptide:glucose ratio, glucagon, insulin:glucagon ratio, total, and active GLP-1 AUC following ingestion of a high-carbohydrate, low-fat meal with water, Gln-30, Gln-15, SIT+Gln-15, or SIT.
| Water | Gln-30 | Gln-15 | SIT+Gln-15 | SIT | ||
|---|---|---|---|---|---|---|
| 1Glucose | AUCt=0-180 min, mmol/L·180 min | 13.91±0.62 | 12.91±0.48* | 13.80±0.65 | 12.82±0.56** | 13.21±0.72 |
| AUCt=0-60 min, mmol/L·60 min | 4.80±0.18 | 4.25±0.15** | 4.53±0.18 | 4.18±0.18** | 4.61±0.23 | |
| AUCt=60-180 min, mmol/L·120 min | 9.12±0.45 | 8.65±0.37 | 9.27±0.48 | 8.64±0.40 | 8.60±0.50 | |
| 1,3 Insulin | AUCt=0-180 min, Log10pmol/L·180 min | 4.32±0.12 | 4.46±0.12* | 4.47±0.10** | 4.52±0.10 | 4.33±0.10 |
| AUCt=0-60 min, Log10pmol/L·60 min | 1.46±0.05 | 1.46±0.04 | 1.49±0.05 | 1.51±0.04 | 1.45±0.04 | |
| AUCt=60-180 min, Log10pmol/L·120 min | 2.86±0.07 | 2.99±0.08* | 2.98±0.06* | 3.01±0.07* | 2.88±0.07 | |
| 1,3 Insulin:glucose ratio | AUCt=0-180 min, Log10pmol/mmol·180 min | 2.74±0.14 | 2.93±0.13* | 2.89±0.12** | 3.00±0.11* | 2.79±0.12 |
| AUCt=0-60 min, Log10pmol/mmol·60 min | 0.93±0.05 | 0.95±0.04 | 0.97±0.05* | 1.01±0.04* | 0.92±0.05 | |
| AUCt=60-180 min, Log10pmol/mmol·120 min | 1.81±0.09 | 1.98±0.09* | 1.92±0.07* | 1.99±0.07* | 1.87±0.07 | |
| 1C-peptide | AUCt=0-180 min, μg/L·180 min | 11.97±1.15 | 12.56±1.07 | 12.77±0.81 | 13.90±1.12 | 13.32±1.10 |
| AUCt=0-60 min, μg/L·60 min | 3.62±0.40 | 3.39±0.31 | 3.82±0.35 | 3.91±0.45 | 3.80±0.42 | |
| AUCt=60-180 min, μg/L·120 min | 8.35±0.77 | 9.17±0.78 | 8.95±0.53 | 9.99±0.69 | 9.67±0.77** | |
| 1C- peptide:glucose ratio | AUCt=0-180 min, μg/mmol·180 min | 1.64±0.21 | 1.76±0.17 | 1.74±0.17 | 2.02±0.19* | 1.92±0.21* |
| AUCt=0-60 min, μg/mmol·60 min | 0.45±0.06 | 0.47±0.05 | 0.52±0.06* | 0.56±0.06* | 0.50±0.06 | |
| AUCt=60-180 min, μg/mmol·120 min | 1.19±0.16 | 1.30±0.13 | 1.23±0.12 | 1.46±0.13 | 1.42±0.15** | |
| 2Glucagon | AUCt=0-180 min, ng/L·180 min | 13.32±1.22 | 18.11±1.64** | 15.65±1.32** | 14.94±1.34 | 12.32±1.15 |
| AUCt=0-60 min, ng/L·60 min | 4.99±0.43 | 6.90±0.67** | 6.20±0.53** | 5.79±0.62 | 4.64±0.42 | |
| AUCt=60-180 min, ng/L·120 min | 8.33±0.80 | 11.37±1.02** | 9.44±0.82 | 9.16±0.75 | 7.68±0.74 | |
| 1,3 Insulin:glucagon ratio | AUCt=0-180 min, Log10pmol/ng·180 min | 1.01±0.09 | 0.91±0.09 | 1.03±0.07 | 1.13±0.07 | 1.09±0.08 |
| AUCt=0-60 min, Log10pmol/ng·60 min | 0.33±0.04 | 0.24±0.03* | 0.30±0.03 | 0.34±0.03 | 0.34±0.03 | |
| AUCt=60-180 min, Log10pmol/ng·120 min | 0.69±0.06 | 0.67±0.06 | 0.74±0.05 | 0.79±0.05 | 0.75±0.06 | |
| 2Total GLP-1 | AUCt=0-180 min, pmol/L·180 min | 5.72±0.72 | 6.44±0.85 | 5.81±0.68 | 5.14±0.64 | 4.90±0.44 |
| AUCt=0-60 min, pmol/L·60 min | 1.98±0.24 | 2.20±0.32 | 1.86±0.21 | 1.68±0.23* | 1.54±0.15* | |
| AUCt=60-180 min, pmol/L·120 min | 3.73±0.49 | 4.27±0.60 | 3.95±0.54 | 3.47±0.46 | 3.36±0.31 | |
| 2Active GLP-1 | AUCt=0-180 min, pmol/L·180 min | 0.80±0.14 | 1.10±0.14* | 0.80±0.15 | 0.90±0.15 | 1.04±0.17* |
| AUCt=0-60 min, pmol/L·60 min | 0.27±0.04 | 0.37±0.05* | 0.26±0.06 | 0.32±0.05 | 0.38±0.05* | |
| AUCt=60-180 min, pmol/L·120 min | 0.54±0.11 | 0.73±0.10 | 0.54±0.11 | 0.59±0.10 | 0.66±0.12 |
Data are mean ± SEM, n=15
AUC/100
AUC/1000
Data were log10-transformed for statistical analysis
Asterisks indicate different from water (control): *P<0.0125 **P<0.001
Gln-30 and Gln-15 increased, and SIT+Gln-15 tended to increase (P=0.017), the postprandial insulin response compared to control, an effect primarily due to the t = 60 - 180 min period (Table 1). SIT did not increase the insulin concentration in either phase (Table 1). The effect of the treatments on insulin should be viewed relative to the prevailing glucose level; therefore, the insulin:glucose ratio was calculated and the results were similar (Fig. 1, Table 1).
Different from its effect on insulin, glutamine did not enhance the postprandial C-peptide response (Table 1). However, Gln-15 increased the C-peptide:glucose ratio at t = 0 – 60 and sitagliptin increased this ratio when taken alone or in combination with 15 g glutamine (Fig. 1, Table 1).
Postprandial glucagon concentration was increased by Gln-30 and Gln-15 (Table 1) and tended to be increased by SIT+Gln-15 (P=0.018). The ratio of insulin:glucagon was also calculated (Fig. 1). Gln-30 decreased this ratio at t= 0-60 min. Otherwise, due to parallel increases in insulin and glucagon concentrations, the ratio was not different from control for all treatments (Table 1).
Gln-30 tended to increase the total GLP-1 AUC (P=0.03) compared to control. In contrast, SIT+Gln-15 and SIT alone both tended to decrease the t = 0-180 AUC compared to control (P=0.013 and P=0.03) and significantly decreased the total GLP-1 AUC at t = 0-60 min (Table 1) relative to control. Active GLP-1 AUC was enhanced by both Gln-30 and SIT compared to control, an effect driven by the t = 0- 60 min period (Table 1).
Adverse Effects and Satiety
Glutamine was generally well tolerated, with no patient experiencing diarrhea or vomiting. One participant felt nauseated after Gln-15 and Gln-30 and another after Gln-30 only. Headache was reported by one participant after all three glutamine treatments, by another after Gln-30 only and in a third patient after Gln-15 only. None of the treatments affected satiety, as evaluated by VAS (data not shown).
Discussion
In this randomized cross-over study, we demonstrated that a single-dose of 30 g of glutamine, or 15 g glutamine in combination with sitagliptin, reduced postprandial glycemia in patients with type 2 diabetes relative to control. Both treatments also augmented the postprandial insulin response, particularly when considered relative to the reduced glycemia.
We have previously shown that oral glutamine increases the circulating GLP-1 concentration when consumed without a meal in lean, obese non-diabetic, and obese diabetic individuals (17). In the present study, when given with a meal to type 2 diabetes patients, 30 g of glutamine tended to increase total GLP-1 and increased active GLP-1 concentration relative to the control, suggesting increased GLP-1 secretion from intestinal L-cells. Similar to previous findings in humans in response to a meal (9), glucose (17, 25), or glutamine (17), the total GLP-1 response in the present study was biphasic, with an early peak at ~15 min and a second peak from 90 – 120 min. Our current and previous results are consistent with in vitro studies demonstrating that glutamine stimulates the release of GLP-1 from the GLP-1-secreting cell line GLUTag (26). Specifically, at concentrations that mimic the postprandial phase, glutamine stimulated GLP-1 secretion from GLUTag cells shortly after its application (26). Furthermore, glutamine was a more potent GLP-1 secretagogue than glucose or other amino acids (26). In vitro, glutamine triggered membrane depolarisation, initiated action potential and calcium entry to the cells but also had an independent effect on GLP-1 secretion (26). However, it remains unclear whether the mechanisms characterised in cell lines are preserved in vivo (27).
The current study suggests that the glucose-lowering effect of glutamine is due, at least in part, to increased GLP-1 concentrations. A critical question is whether glutamine-induced increases in GLP-1 reduce glycemia by increasing insulin secretion or slowing of gastric emptying, or both. Our data suggest that the latter is likely to be more important. Firstly, we observed that the reduction in postprandial glycemia preceded any increase in insulinemia. Secondly, although we found that glutamine increased the postprandial insulin response, there was no corresponding increase in C-peptide, suggesting that glutamine may affect insulin clearance, rather than secretion. These data indicate that the effect of glutamine on glycemia is predominantly mediated through slowing of gastric emptying. Indeed, in healthy humans, a glutamine and carbohydrate mixed solution prolonged gastric emptying compared to carbohydrate alone (28). Slowed gastric emptying in response to glutamine in the present study may be due to the increase in GLP-1 (29) or to the increased energy with glutamine consumption.
Amino acids have previously been reported to be strong stimulants of glucagon release in dogs (30), as we have recently shown for glutamine in humans (17). Consistently, glutamine increased the postprandial glucagon concentration in the present study. This may be expected to counteract a potential benefit of glutamine on glycemia, via enhanced hepatic glucose production (31). In the fasting state, glucagon maintains normal blood glucose concentration and is maximally active when glucose and insulin concentrations are low. In the present study, the postprandial increase in glucagon following glutamine consumption was paralleled by an increase in insulin concentration and thus would not be expected to affect hepatic glucose production, which is relevant in the fasting state.
Sitagliptin led to relatively lower total GLP-1 concentration, but a higher active GLP-1 concentration compared to control, consistent with the known mechanism of action of DPP-IV inhibitors (32). The lower total GLP-1 concentration is likely to be a response to negative feedback by active GLP-1 (32, 33). When sitagliptin was given in combination with glutamine, total GLP-1 secretion decreased, which was likely due to sitagliptin.
Adverse effects of glutamine were uncommon in the current study. Glutamine was well-tolerated and led to minor gastrointestinal symptoms in two participants only. In a recent study which examined the safety of glutamine given at a dose of 0.5 g·kg-1 body weight·d-1 for 14 d in a similar age and weight group, glutamine was well-tolerated without adverse effects noted on clinical and laboratory meausres, including renal and liver function, and lactate and ammonia concentrations (18).
Our study has some limitations. We limited recruitment to individuals with diabetes of <5 year duration and therefore are unable to comment as to whether glutamine has equally beneficial effects on glycemia in patients with type 2 diabetes of longer duration. The relatively intact beta cell function in individuals with a shorter duration of diabetes may limit the beneficial effects of glutamine to such participants. Moreover, in patients with well-controlled type 2 diabetes, the action of GLP-1 on insulin secretion is preserved (10); thus, glutamine is more likely to be effective in this group of participants. A second limitation is the lack of an amino acid comparator, which would help determine whether the effect on GLP-1 is glutamine-specific or a generalized amino acid effect. However, our recent observations in humans (17) and in vitro (26) suggest that the GLP-1 response is specific to glutamine. Thirdly, we cannot exclude the possibility that the greater energy intake with glutamine supplementation accounted for some of the effects observed in the present study.
In summary, we demonstrate that the consumption of 30 g of glutamine or 15 g of glutamine plus sitagliptin markedly reduced postprandial glycemia in patients with well-controlled type 2 diabetes of short duration. These effects are likely to be mediated through GLP-1-induced slowing of gastric emptying. Our results suggest that glutamine may represent a novel approach to increasing GLP-1 concentration and reducing postprandial glycemia in type 2 diabetes, a state of relative glutamine deficiency (19). With poor adherence to the multiple medications required to treat type 2 diabetes, nutritional supplementation with an amino acid may prove beneficial. The longer-term effects of such treatment require further investigation, particularly in light of recommendations regarding dietary protein intake in patients with type 2 diabetes (34).
Acknowledgements
JRG designed research, conducted research and had primary responsibility for final content. OW, E-LS, NP, AD, JJH conducted research. DS-B analyzed data and wrote paper. FMG and DJC designed research. All authors critically reviewed and approved the final manuscript. We thank Lene Brus Albaek and Signe Jorgensen for performing the GLP-1 assays, Louise Purtell for technical advice and Adelle Coster for statistical advice.
Sources of financial support: The study was supported by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (#535949), Diabetes Australia Research Trust Grant and a research grant from the Investigator-Initiated Studies Program of Merck. JRG was supported by a Neil Hamilton Fairley Fellowship from NHMRC and the Don Chisholm Fellowship (funds from Garvan Research Foundation, including support from GlaxoSmithKline, Australia, Diabetes Australia Research Trust, The Commonwealth Department of Health and Ageing).
Author-defined abbreviations
- DPP-IV
Dipeptidyl peptidase-IV
- GLP
Glucagon-like peptide
- GIP
Glucose-dependent insulinotropic polypeptide
- Gln
Glutamine
- HbA1c
Glycated hemoglobin
- Gln-15
L-glutamine 15 g
- Gln-30
L-glutamine 30 g
- PYY
Peptide YY
- SIT
Sitagliptin
- SIT+Gln-15
Sitagliptin + 15 g L-glutamine
- VAS
Visual analogue scale
Footnotes
Clinical trial registration: www.clinicaltrials.gov (NCT-00673894).
Conflict of interest: This study was funded in part by a research grant from the Investigator-Initiated Studies Program of Merck. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck.
Samocha-Bonet D- no conflict of interest, Wong O- no conflict of interest, Synnott E-L- no conflict of interest, Piyaratna N- no conflict of interest, Douglas A- no conflict of interest, Gribble F- no conflict of interest, Holst J- no conflict of interest, Chisholm D- no conflict of interest, Greenfield J- received travel assistance from Merck Sharp & Dohme (MSD) Australia to travel to a scientific meeting.
Literature Cited
- 1.Bonadonna RC, Stumvoll M, Fritsche A, Muggeo M, Haring H, Bonora E, van Haeften TW. Altered homeostatic adaptation of first- and second-phase beta-cell secretion in the offspring of patients with type 2 diabetes: studies with a minimal model to assess beta-cell function. Diabetes. 2003;52:470–80. doi: 10.2337/diabetes.52.2.470. [DOI] [PubMed] [Google Scholar]
- 2.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vanttinen M, Stancakova A, Jansson PA, Pellme F, Holst JJ, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51:502–11. doi: 10.1007/s00125-007-0899-2. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 5.Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst J, Vilsboll T. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155:485–93. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer K, Holst JJ, Baher B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer K, Gardiwal H, Menge BA, Goetze O, Deacon CF, Schmidt WE, Holst JJ, Meier JJ. Hyperglycemia Acutely Lowers the Postprandial Excursions of Glucagon-Like Peptide-1 and Gastric Inhibitory Polypeptide in Humans. J Clin Endocrinol Metab. 2009;94:1379–85. doi: 10.1210/jc.2008-2197. [DOI] [PubMed] [Google Scholar]
- 8.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 9.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active gluccagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 10.Salehi M, Prigeon RL, Aulinger B, D'Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59:1330–7. doi: 10.2337/db09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hojberg PV, Vilsboll T, Rabol R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 12.Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes. 2004;53(Suppl 3):S190–6. doi: 10.2337/diabetes.53.suppl_3.s190. [DOI] [PubMed] [Google Scholar]
- 13.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 14.Wynne K, Bloom SR. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nat Clin Pract Endocrinol Metab. 2006;2:612–20. doi: 10.1038/ncpendmet0318. [DOI] [PubMed] [Google Scholar]
- 15.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annual Rev Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- 16.Thomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010;21:59–68. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–13. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galera SC, Fechine F, Teixeira MJ, Branco Coelho ZC, de Vasconcelos RC, de Vasconcelos PR. The safety of oral use of l-glutamine in middle-aged and elderly individuals. Nutrition. 2009 doi: 10.1016/j.nut.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Menge BA, Schrader H, Ritter PR, Ellrichmann M, Uhl W, Schmidt WE, Meier JJ. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept. 2010;160:75–80. doi: 10.1016/j.regpep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ward E, Picton S, Reid U, Thomas D, Gardener C, Smith M, Henderson M, Holden V, Kinsey S, et al. Oral glutamine in paediatric oncology patients: a dose finding study. Eur J Clin Nutr. 2003;57:31–6. doi: 10.1038/sj.ejcn.1601517. [DOI] [PubMed] [Google Scholar]
- 21.Gandini C, Delorenzi D, Kitsos M, Massolini G, Caccialanza G. Hplc Determination of Pyroglutamic Acid as a Degradation Product in Parenteral Amino-Acid Formulations. Chromatographia. 1993;36:75–8. [Google Scholar]
- 22.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 23.Lerche S, Soendergaard L, Rungby J, Moeller N, Holst JJ, Schmitz OE, Brock B. No increased risk of hypoglycaemic episodes during 48 h of subcutaneous glucagon-like-peptide-1 administration in fasting healthy subjects. Clin Endocrinol. 2009;71:500–6. doi: 10.1111/j.1365-2265.2008.03510.x. [DOI] [PubMed] [Google Scholar]
- 24.Dunn OJ. Multiple Comparisons among Means. J Am Stat Assoc. 1961;56:52. [Google Scholar]
- 25.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–26. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 26.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 27.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 12:e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 28.Lobo DN, Hendry PO, Rodrigues G, Marciani L, Totman JJ, Wright JW, Preston T, Gowland P, Spiller RC, Fearon KC. Gastric emptying of three liquid oral preoperative metabolic preconditioning regimens measured by magnetic resonance imaging in healthy adult volunteers: a randomised double-blind, crossover study. Clin Nutr. 2009;28:636–41. doi: 10.1016/j.clnu.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, Burgstad C, Jones KL, Chapman MJ, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95:215–21. doi: 10.1210/jc.2009-1503. [DOI] [PubMed] [Google Scholar]
- 30.Barrett EJ, Gusberg R, Ferrannini E, Tepler J, Felig P, Jacob R, Smith D, DeFronzo RA. Amino acid and glucose metabolism in the postabsorptive state and following amino acid ingestion in the dog. Metabolism. 1986;35:709–17. doi: 10.1016/0026-0495(86)90238-6. [DOI] [PubMed] [Google Scholar]
- 31.Henry RR. Protein content of the diabetic diet. Diabetes Care. 1994;17:1502–13. doi: 10.2337/diacare.17.12.1502. [DOI] [PubMed] [Google Scholar]
- 32.Herman GA, Bergman A, Stevens C, Kotey P, Yi BM, Zhao P, Dietrich B, Golor G, Schrodter A, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–9. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 33.Hansen L, Hartmann B, Mineo H, Holst JJ. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Peptides. 2004;118:11–8. doi: 10.1016/j.regpep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Standards of medical care in diabetes-2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]