Abstract
Triglycerides are among the most efficacious stimulators of incretin secretion; however the relative importance of FFA1 (GPR40), FFA4 (GPR120) and GPR119 which all recognize triglyceride metabolites, LCFA and 2-MAG respectively, is still unclear. Here, we find all three receptors to be highly expressed and highly enriched in FACS-purified GLP-1 and GIP cells isolated from transgenic reporter mice. In vivo, the triglyceride-induced increase in plasma GIP was significantly reduced in FFA1 deficient mice (to 34% - mean of four experiments each with 8-10 animals), in GPR119 deficient mice (to 24 %) and in FFA1/FFA4 double deficient mice (to 15%) but not in FFA4 deficient mice. The triglyceride-induced increase in plasma GLP-1 was only significantly reduced in the GPR119 deficient and the FFA1/FFA4 double deficient mice, but not in the FFA1 and FFA4 deficient mice. In mouse colonic crypt cultures the synthetic FFA1 agonists, TAK-875 stimulated GLP-1 secretion to a similar extent as the prototype GLP-1 secretagogue neuromedin C, this however only corresponded to approx. half the maximal efficiency of the GPR119 agonist AR231453 whereas the GPR120 agonist Metabolix-209 had no effect. Importantly, when the FFA1 agonist was administered on top of appropriately low doses of the GPR119 agonist a clear synergistic, i.e. more than additive effect was observed. It is concluded that the 2-MAG receptor GPR119 is at least as important as the LCFA receptor FFA1 in mediating the triglyceride-induced secretion of incretins and that the two receptors act in synergy whereas FFA4 plays a minor if any role.
Keywords: Long chain fatty acids, 2 monoacyl glycerol, olive oil, fat sensing, GLP-1, GIP, incretins
Introduction
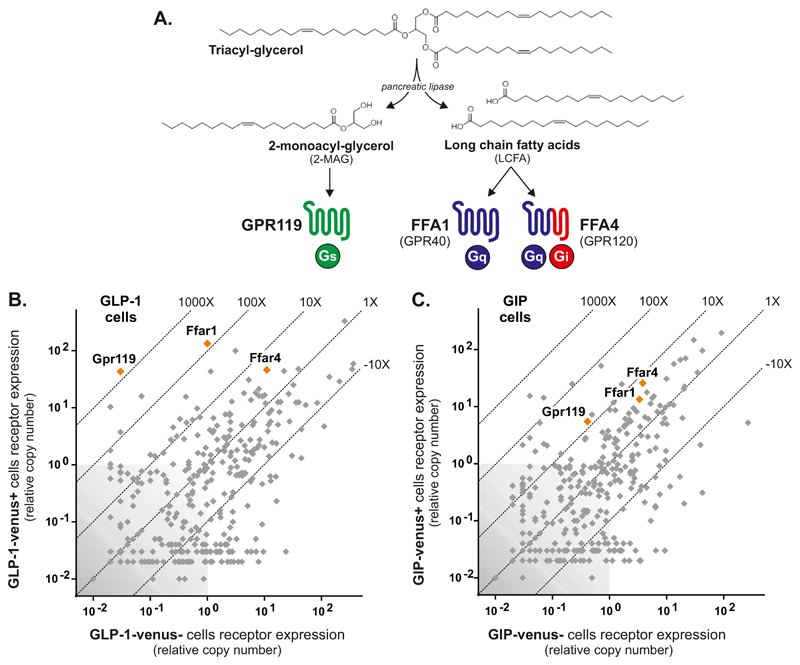
Triglycerides (TG) are known to be highly efficacious secretagogues for gut hormones such as CCK and the incretins, GIP and GLP-1 [1, 2]. It is, however not the intact TGs, which are sensed by the enteroendocrine cells. TGs are digested by pancreatic lipase to generate long chain fatty acids (LCFA) and 2-monoacylglycerol (2-MAG) and it is generally assumed that it is the LCFAs which are stimulating the enteroendocrine cells to release hormones [3–5]. Two G protein-coupled receptors (GPCR), GPR40 (FFA1) and GPR120 (FFA4) are known to be activated by LCFAs [6, 7]. Both receptors are coupled to Gαq and have been reported to be expressed in the gut and specifically in, for example CCK, GIP and GLP-1 cells [8–10]. Both FFA1 and FFA4 have been implicated as important regulators of incretin hormone secretion [7, 11, 12] and it is accordingly assumed that the strong effects of TG on gut hormone secretion in general and incretin hormone secretion in particular is mediated through stimulation of FFA1 and FFA4 by LCFAs liberated from TGs by pancreatic lipase digestion (Fig. 1A).
Fig. 1. Triglyceride metabolite receptors and their expression in GIP and GLP-1 cells.
Panel A – overview of the digestion of dietary TGs by pancreatic lipase generating LCFAs, which potentially could be acting through the two receptors GPR40 and GPR120, and 2-MAG which could be acting through GPR119 to stimulate gut hormone secretion. Panel B – Expression of fat metabolite receptors (orange symbols) in FACS purified murine GIP cells isolated from the GIP-Venus reporter mice versus the expression of these receptors in the neighboring cells. Panel C - Expression of fat metabolite receptors (orange symbols) in FACS purified murine GLP-1 cells isolated from the proglucagon-Venus reporter mice. Grey symbols indicate the relative expression of the main 379 non-olfactory GPCRs.
Recently it was however shown that the other major TG metabolite, 2-monoacylglycerols (2-MAG) and in particular 2-oleoylglycerol (2-OG) can act as an agonist for the Gαs coupled receptor, GPR119, which is also expressed on enteroendocrine cells [10, 13, 14]. Although the potency of 2-OG is only in the micromolar range, the local concentrations of 2-MAG can reach such high levels during ingestion of a lipid-rich meal [14]. Importantly, although GPR119 agonists have not been particularly successful in the clinics as anti-diabetes agents [15, 16], they are nevertheless efficacious incretin secretagogues [17, 18]. Thus, the stimulatory effect of TGs on gut hormone release could potentially be mediated not only by the LCFAs, but also by 2-MAG (Fig. 1A).
The present study was designed to clarify the relative importance of the three receptors which all recognize TG metabolites – FFA1, FFA4 and GPR119 - for the strong stimulatory effect of TG’s on the secretion of the two incretin hormones GIP and GLP-1. In vivo studies in animals deficient in either FFA1, FFA4 or both or deficient in the 2-MAG receptor GPR119 are here combined with ex vivo studies of hormone release in primary cell cultures using highly selective agonists for each of the three receptors either alone or in combinations. Surprisingly, we discovered that the 2-MAG receptor GPR119 is at least as important for the sensing of TGs as the LCFA receptor FFA1, whereas the other LCFA receptor FFA4 apparently plays a rather minor if any role in the direct regulation of hormone secretion.
Results
Receptors for TG metabolites are highly expressed on GLP-1 and GIP cells
The expression of GPCRs were analyzed in FACS-purified Gcg-Venus and Gip-Venus cells isolated from small intestine of the corresponding, previously published reporter mice [9, 10] using a dedicated qPCR array covering 379 receptors [19, 20]. As shown in Fig. 1B, the LCFA receptors FFA1 and FFA4 as well as the 2-MAG receptor GPR119 are all highly expressed in the Gcg-Venus positive cells, i.e. GLP-1 cells. In fact, the transcript for FFA1 was the most highly expressed GPCR in the GLP-1 cells and the transcript for GPR119 was almost equally highly expressed and the most highly enriched (>1000-fold enriched). FFA4 was equally highly expressed but only enriched 4-fold in the GLP-1 cells (Fig. 1B). A similar expression pattern was observed for these receptors in the Gip-Venus positive cells, although none of the receptor transcripts were as enriched as observed in the GLP-1 cells (Fig. 1C). In the GIP cells FFA4 appeared to be the most highly expressed but as in the GLP-1 cells not particularly enriched metabolite receptor.
In vivo dependency of the GLP-1 and GIP responses to TG on FFA1, FFA4 and GPR119
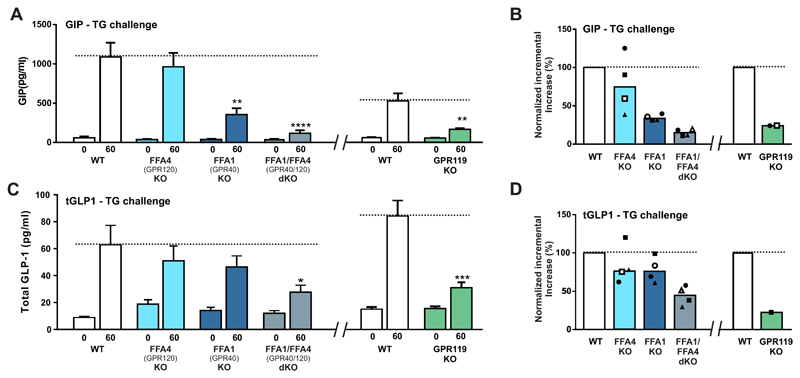
Oral TG is one of the most efficient stimuli for both GIP and GLP-1 secretion. Here we used a standard oral olive oil challenge in mice deficient for FFA1, FFA4 and GPR119, respectively as well as mice deficient in both FFA1 and FFA4 as compared to littermate WT control mice. Plasma GIP and GLP-1 were measured 60 minutes after the TG challenge, i.e. a time point determined to be optimal in prior time-course experiments (Fig. S1). Three experiments with 6-8 animals in each group were performed with littermates versus knock-out animals and one experiment using wild-type C57BL/6 mice as controls. In Fig. 2A and C are shown the GIP and GLP-1 responses from a representative experiment and in Fig. 2B and D are shown normalized results from all four experiments.
Fig. 2. Plasma GIP and GLP-1 responses to oral triglyceride challenge in mice – dependency on the metabolite receptors FFA1 (GPR40), FFA4 (GPR120) and GPR119.
Panel A - Plasma GIP levels before and 60 minutes after an oral gavage challenge with 10ml/kg of olive oil in FFA4 (GPR120), FFA1 (GPR40) and double deficient mice versus littermate wild type control mice (left panel) and in GPR119 deficient mice versus littermate control mice (right panel). A representative experiment with n=8-10 animals in each group is shown. The FFA1 and FFA4 deficient animals were taken from a breeding to obtain the double deficient animals and accordingly the joint littermate controls. Panel B – Mean GIP levels of four experiments similar to the one shown in panel A left panel (indicated in filled square symbols) except that in one experiment wild type C57BL/6 mice were used as controls (open symbols). In the right panel is shown the mean GIP response from two experiments with GPR119 deficient mice. Panel A - Plasma GLP-1 levels before and 60 minutes after an oral gavage challenge with 10 ml/kg of olive oil in FFA4 (GPR120), FFA1 (GPR40) and double deficient mice versus littermate wild type control mice (left panel) and in GPR119 deficient mice versus littermate control mice (right panel). A representative experiment with n=8-10 animals in each group is shown. The FFA1 and FFA4 deficient animals were taken from a breeding to obtain the double deficient animals and accordingly the joint littermate controls. Panel B – Mean GLP-1 levels of four experiments similar to the one shown in panel A (indicated in filled square symbols) except that in one experiment wild type C57BL/6 mice were used as controls (open symbols). In the right panel is shown the mean GLP-1 responses from two experiments with GPR119 deficient mice.
As expected, oral TG induced a robust response in both plasma GIP and GLP-1 in the WT littermates which was also observed in the FFA4(-/-) mice as neither the GIP nor the GLP-1 response to TG were decreased in the knock out animals, although there was some variability from experiment to experiment (light blue columns in Fig. 2B and D). In contrast, a significantly reduced GIP response (p < 0.005) corresponding to only 34% of the response of the WT animals was observed in the FFA1-deficient mice (Fig. 2A and B). However, only a trend towards a reduced GLP-1 response to TG, which did not reach significance, was surprisingly observed in the FFA1 deficient mice (dark blue columns in Fig. 2C and D). In the FFA1/FFA4 double deficient mice, GPR40/120-d(-/-) the GIP response to TGs was even further reduced, i.e. down to 15% of WT (p < 0.0001. In the mice lacking both FFA1 and FFA4 the GLP-1 response was also significantly reduced (p < 0.05) but only down to 34% of the response observed in WT animals (grey columns in Fig 2C and D).
In the GPR119-KO mice the GIP response to TG was reduced significantly (p < 0.005) down to 24% of WT animals (green columns in Fig. 2A and B). In contrast to what was observed in the FFA1 and the FFA4 deficient mice the GLP-1 response to TG was like the GIP response significantly reduced (p < 0.005) in the GPR119(-/-) mice and even down to 22% of the response observed in the WT animals (green columns in Fig. 2C and D).
It is concluded that GPR119 plays a role at least as important as FFA1 in the incretin response to TGs and that the magnitude of the reduction in hormone responses observed in the KO animals indicates that GPR119 and FFA1 may act in synergy. In contrast, FFA4 on its own apparently has very limited if any role in the TG-induced incretin response in vivo.
Stimulation of GLP-1 secretion ex vivo by FFA1, FFA4 and GPR119 selective agonists
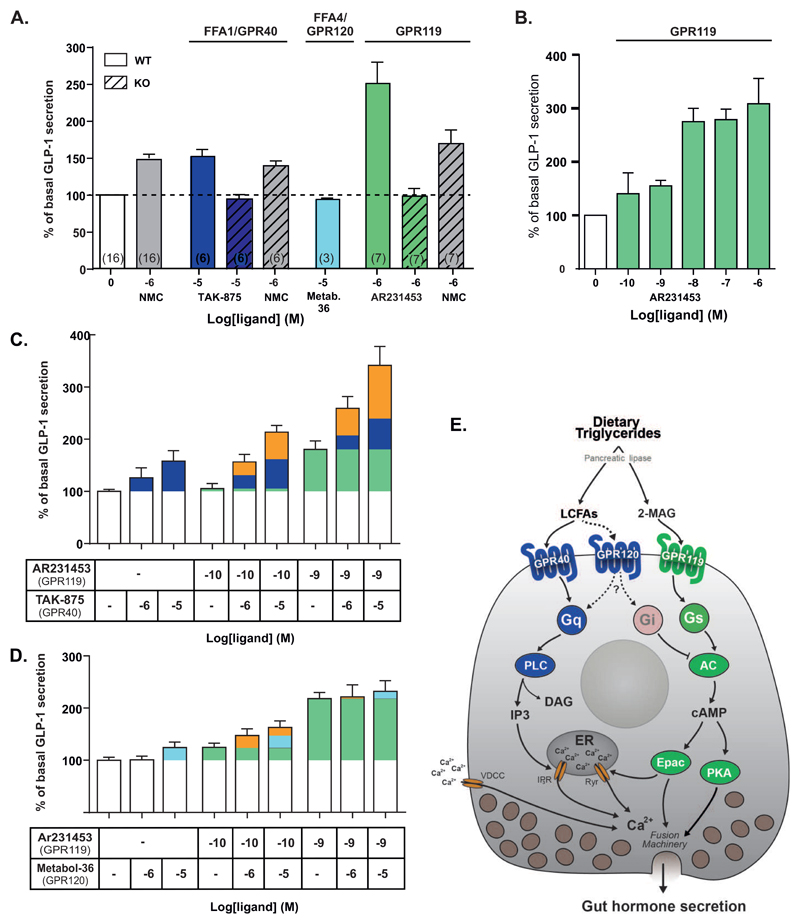
FFA1, FFA4 and GPR119 have all been proposed to stimulate GLP-1 secretion mainly based on experiments with not entirely selective ligands [7, 12, 17, 21]. Today highly selective, synthetic agonists are available for all three receptors [19, 22–24], which we here use to study effects on GLP-1 release from murine colonic crypt cultures. As shown in Fig. 3A, the prototype GLP-1 secretagogue, neuromedin C (NMC), which is a BB2 receptor agonist, increased GLP-1 secretion 1.5 fold. The selective FFA1 agonist, TAK-875 or fasiglifam (10-5 M) stimulated GLP-1 secretion to a similar extent (1.6-fold) as did NMC but in a FFA1-dependent manner as no response was observed in cells isolated from FFA1 deficient mice (Fig. 3A). Surprisingly, no stimulation of GLP-1 secretion was observed in cells stimulated with the FFA4 selective agonist Metabolix-209 (10-5 M), i.e. a compound which we previously have observed efficiently inhibits ghrelin secretion in a FFA4-dependent manner [19]. In contrast, the GPR119 prototype agonist AR231453 (10-6 M) stimulated GLP-1 release with high efficacy, i.e. 2.5 fold and in a GPR119-dependent manner (Fig. 3A). Dose-response experiments demonstrated that the EC50 for AR231453 in respect of stimulation of GLP-1 release from the murine colonic crypt cultures was 2.6 nM (Fig. 3B).
Fig. 3. Secretion of GLP-1 in response to synthetic selective FFA1, FFA4 and GPR119 agonists administered alone or co-administered to probe for potential synergistic effects in mouse colonic crypt cultures.
Panel A – Comparison of the effect on GLP-1 secretion in mouse colonic crypt cultures of maximal doses of the FFA1 agonist TAK875 (dark blue), the GPR120 agonist Metabolex-209 (light blue) and the GPR119 agonist AR231453 (green) with the prototype GLP-1 secretagouge the bombesin 2 receptor (BB2) agonist, neuromedin C (NMC) (grey). For FFA1 and GPR119 the responses in FFA1 and GPR119 deficient animals are indicated in hatched columns. Mean +/- SEM, numbers of experiments are indicated in brackets in the columns. Panel B – dose-response experiment for the highly efficacious GPR119 agonist AR231453 performed to identify appropriately low doses to be used for co-administration studies (panels C and D). Panel C - GLP-1 secretion in response to the GPR40 agonist TAK-875 alone (10-5 and 10-6 M – in dark blue) and in response to the GPR119 agonist AR231453 alone (10-9 and 10-10 M – in green) or the GPR40 agonist together with the GPR119 agonist (orange corresponding to response above the sum of the ‘green’ and the ‘blue’ response). Panel D - GLP-1 secretion in response to the GPR120 agonist Metabolix-209 alone (10-5 and 10-6 M – in light blue) and in response to the GPR119 agonist AR231453 alone (10-9 and 10-10 M – in green) or the GPR120 agonist together with the GPR119 agonist (orange corresponding to response above the sum of the ‘green’ and the ‘light blue’ response). Panel E – Simplified schematic overview of the physiological ligand binding and signal transduction mechanisms for FFA1, FFA4 and GPR120 in a generic enteroendocrine cell. As indicated LCFAs will induce Gαq signaling through FFA1 leading to PLC activation, IP3 accumulation and increase in intracellular Ca++, whereas 2-MAG will induce Gαs signaling through GPR119 leading to adenylate cyclase activation, cAMP accumulation and presumably activation of Epac and PKA which will activate the fusion machinery of the secretory granules. As shown in panel C co-activation of the FFA1/Gαq and the GPR119/Gαs signaling pathway act in synergy to provide a robust GLP-1 secretory response. GPR120 has little or no effect, conceivably due to the fact that the receptor mainly is coupled to Gαi in enteroendocrine cells [19].
Probing for synergy between the metabolite receptors ex vivo
As FFA1 and FFA4 are generally believed to couple mainly through Gαq under physiological circumstances, while GPR119 is a strongly Gαs-coupled receptor, it is likely that these receptors could act in synergy through activation of these different signal transduction pathways in the enteroendocrine cells. In order to be able to observe synergistic effects it is necessary to use highly selective ligands and to use doses which on their own only provide a suitable small response. For the FFA1 agonist, we chose TAK-875 in doses of 10-6 and 10-5 M which gave appropriately small responses (Fig. 3C – blue symbols) and for the GPR119 agonist AR231453 we used doses of 10-10 and 10-9 M based on the dose-response experiments shown in Fig. 1D (Fig. 3C green symbols).
When the GPR119 agonist AR231453 in a dose of 10-10 M, which on its own gave a minimal GLP-1 response (green), was co-administered with the FFA1 agonist TAK875 (blue) to mouse colonic crypt cultures a synergistic, i.e. more than additive effect was observed in respect of stimulation of GLP-1 secretion (Fig. 3C - orange being more than blue plus green). The synergistic effect was also clearly observed with a 10-9 M dose of AR231453 resulting in GLP-1 release corresponding at least to the maximal response normally achieved with a dose of 10-6 M of the GPR119 agonist alone (Fig. 3B and 3C). However, when instead of the FFA1 agonist the co-administration experiments were performed with the FFA4 agonist, which in the 10-5 M dose only gave a small insignificant GLP-1 response, only a minor indication of synergy was observed when using it in combination with the 10-10 dose of the GPR119 agonist and no indication of synergy was observed in combination with 10-9 M of AR231453 (Fig. 3D).
It is concluded that clear synergy between FFA1 and GPR119 is observed in respect of stimulation of GLP-1 release in the ex vivo setting, but only to a very limited degree between FFA4 and GPR119.
Discussion
In the present study we find that GPR119 conceivably through sensing of 2-MAG is at least as important as the LCFA receptor FFA1 for the strong triglyceride-mediated stimulation of incretin hormones. By use of highly selective pharmacological tools we provide evidence that GPR119 and FFA1 act synergistically to stimulate GLP-1 secretion. The other LCFA receptor, FFA4 which also is highly expressed by the GLP-1 and GIP cells appears to play only a minor if any role in the control of incretin secretion in response to triglycerides.
GPR119 is highly important for incretin secretion in response to triglycerides - GPR119 has been known for a while to stimulate GLP-1 secretion as demonstrated by the effect of selective synthetic GPR119 agonists which are among the most efficacious secretagogues for GLP-1 [17, 18, 25, 26]. However, the physiological role of GPR119 has been rather unclear. Since the receptor originally was deorphanized as a sensor of n-acylethanolamines such as OEA a number of lipids have over the years been proposed to function as endogenous ligands for GPR119 [15]. Although it is still rather unclear what the endogenous GPR119 ligand is in the endocrine pancreas, triglyceride derived 2-MAGs such as 2-OG appear to be the most likely physiological stimulator of GPR119 in enteroendocrine cells. 2-OG is rather efficacious but not particularly potent in respect of stimulating cAMP production in cells transfected with human GPR119 [13]. Importantly, intra-jejunal administration of 2-OG in amounts corresponding to what would be liberated from triglycerides during a meal stimulates both GLP-1 and GIP in normal human subjects [13]. Moreover, ingestion of 1,3-dioctanoyl-2-oleoyl glycerol, i.e. an artificial, synthetic triglyceride, which upon digestion by pancreatic lipase generates one molecule of 2-OG plus two molecules of the ‘inactive’ medium-chain fatty acid octanoic acid, stimulated GLP-1 to almost the same degree as olive oil in human subjects [14]. Thus, 2-OG is a powerful stimulus of incretin secretion in vivo in man. In agreement with our results (Fig. 2), Moss and coworkers recently found that the GLP-1 release in response to oral lipid was dramatically reduced in mice lacking GPR119 selectively in proglucagon-expressing cells [27]. These studies combined with the studies with 2-OG administration strongly indicate that sensing of 2-MAG by GPR119 is a key player in the stimulation of incretins by dietary triglycerides.
FFA1 sensing LCFAs acts in synergy with GPR119
For many years it has been generally believed that it was the sensing of LCFA which was responsible for the strong stimulation of incretins by triglycerides [3–5]. Based on similar arguments as presented above for GPR119 and 2-MAG, FFA1 was believed to be ‘the fat sensor’ responsible for this effect [28, 29]. Thus, Edfalk and coworkers demonstrated that FFA1 - at that time known as GPR40 - was expressed in GLP-1 cells and that insulin, GLP-1 and GIP responses to high fat diet were reduced or eliminated in FFA1 KO mice [11] just like Xiong and coworkers found that the GLP-1 response to corn oil was eliminated in FFA1 KO animals [12]. Similarly, FFA1 has been shown to be expressed in CCK cells and the CCK response to olive oil is reduced to 50% in FFA1 KO mice [8]. However, although LCFA apparently stimulate GLP-1 secretion from various cell lines and ex vivo preparations, we found recently that when care is taken to avoid unspecific effects of the LCFAs the actual FFA1 receptor-mediated effect on GLP-1 secretion from colonic crypt cultures was very small compared to the effect of e.g. a prototype GPR119 agonist [26]. Most importantly, in the clinical trials the FFA1 agonist TAK-875 had no effect on GLP-1 and GIP while efficiently lowering basal blood glucose and improving glucose tolerance in the diabetic patients [30]. These ex vivo and in vivo studies indicate that LCFAs and in particularly FFA1 is not a major player in the stimulation of incretin secretion by triglycerides – i.e. on its own.
In agreement with previous reports we observe in the present study that the incretin response to triglyceride is reduced in FFA1 KO mice [11, 12]. However, by use of synthetic selective agonists we find that FFA1 stimulation alone is not very efficacious in respect of stimulating GLP-1 secretion ex vivo, but that FFA1 acts in synergy with GPR119. That is, providing more than additive GLP-1 responses when the GPR40 agonist was administered on top of appropriately low doses of the GPR119 agonists (Fig. 3). We believe these pharmacological experiments mimic the physiological situation where the Gαq-coupled FFA1 and the Gαs-coupled GPR119 receptors on the enteroendocrine cells are stimulated concomitantly by LCFA and 2-MAG (Fig. 3E).
Interestingly, recently we observed that while FFA1 in response to endogenous LCFAs and orthosteric FFA1 agonists such as TAK875 only are able to signal through Gαq as originally described, certain second generation ago-allosteric FFA1 agonists are able to make FFA1 signal not only through Gαq but also through Gαs [26]. Importantly, in contrast to the Gαq-only TAK-875 the Gαq+Gαs FFA1 agonists, as for example AM-5262 stimulated GLP-1 and GIP secretion robustly both ex vivo and in vivo [26]. Thus, it is possible by use of Gαq+Gαs FFA1 agonists to obtain a similarly strong incretin response as obtained with triglycerides presumably acting through their metabolites LCFAs and 2-MAG as Gαq-only FFA1 and Gαs GPR119 agonists, respectively (Fig. 3E).
FFA4 plays a minor if any role in the direct control of incretin secretion
Originally it was reported that FFA4 - at that time known as GPR120 - was highly and exclusively expressed in enteroendocrine GLP-1 cells and through circumstantial evidence it was argued that FFA4 should be involved in the control of GLP-1 secretion [7]. However, over the last years it has become increasingly clear that this is not the case. Xiong and coworker observed that while the GLP-1 response to corn oil was eliminated by FFA1 KO it was not affected by FFA4 KO [12]. Paulsen and coworkers observed FFA4 to be broadly expressed in enterocytes and also concluded that the receptor was not involved in the control of GLP-1 secretion [31]. Here we find that although FFA4 is highly expressed in both GLP-1 and GIP cells it is only slightly enriched, meaning that it is almost as highly expressed in the neighboring cells. Importantly, an otherwise very efficacious FFA4 agonist does not stimulated GLP-1 secretion ex vivo and the GIP and GLP-1 response to olive oil is only marginally if at all reduced in FFA4 KO animals. Thus, there is an increasing consensus that FFA4 in fact is not involved in the direct control of incretin secretion – although it is highly expressed in the enteroendocrine cells.
Nevertheless, there was a trend towards a reduced incretin response to triglycerides in the FFA4 KO animals in some of the experiments (Fig 2B and D) and recently it was reported that the GIP response to lard was reduced in FFA4 animals [32]. Thus it is possible that FFA4 could affect the secretory capacity of the enteroendocrine cells without directly being involved in the secretory process as such. It should also be noted that there was a trend towards a larger effect of the FFA1/FFA4 double KO on both the GIP and GLP-1 response to TG (Fig. 2), indicating that the two LCFA receptors somehow do affect the function of each other.
In other endocrine cells of the gastrointestinal tract and pancreas FFA4 appears to inhibit instead of stimulate hormone secretion. Thus, gastric ghrelin secretion is dose-dependently and efficiently inhibited both ex vivo and in vivo by a selective, synthetic FFA4 agonist and basal ghrelin levels are increased in FFA4 KO animals [19]. Importantly, we found that the inhibitory effect of FFA4 agonists was blocked by pertussis toxin indicating that GPR120 in these cells in fact couples through Gαi [19]. FFA4 agonists also inhibit somatostatin secretion efficiently both in the stomach and in the pancreas [20, 33]. Thus, it is unclear what the role of FFA4 is in the enteroendocrine cells of the intestine, however future work should take into account that the receptor signals not only through Gαq but also through Gαi [19].
Materials and methods
In vivo studies in mice
Fully backcrossed male C57BL/6J GPR40-/-, C57BL/6J GPR120-/-, C57BL/6J GPR119-/- and C57BL/6J GPR40-/-/120-/- were obtained from Taconic (US). Heterozygous mice of GPR40-/-, GPR120-/-, and GPR40-/-/120-/- - strain were bred to give GPR40-/-, GPR120-/-, and GPR40-/-/120-/- KO and littermate wild type controls. GPR119 is X-linked, thus heterozygous female GPR119-/- mice were bred with C57BL/6J male mice to give KO and littermate controls. The mice were housed on a normal 12-h light, 12-h dark cycle and had free access to food and water. The animal studies were conducted in accordance with institutional guidelines and approved by the Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture and Fisheries and the mice handled in a fully AAALAC accredited facility.
Triglyceride challenge
Mice (9-29 weeks) were fasted overnight (16-18 hours) with ad libitum access to water. Olive oil (Sigma Aldrich) was administered p.o. with a dose of 10 ml/kg. A retro orbital blood sample (100 µl) was taken prior to the dosage and was considered 0 min. 60 minutes after the dosage, animals were decapitated and blood was collected. All blood samples were collected in EDTA coated tubes with 0.5 KIU aprotinin/µl blood (Sigma Chemical, St. Louis, MO) and 1 µM DPP4 inhibitor. All blood samples were kept on ice at all times. The blood samples were spun at 9600 G for 10 minutes at 4°C and plasma was stored at -80°C. Total GLP-1 was determined using Total GLP-1 (ver. 2) assay kit (Meso Scale Discovery, Gaithersburg, USA) (model number K150JVC-1). GIP was measured using a Rat/Mouse Total GIP ELISA assay kit (Millipore, St. Charles, MO).
Fluorescence-activated cell sorting (FACS) and quantitative PCR (qPCR)
The upper-half portion of the small intestine was collected from GLP-1-venus [10]) and GIP-venus [9] transgenic mice, washed, minced and treated with collagenase to obtain a single cell suspension as previously described [10]. The cells were sorted based on fluorescence at 530 nm and 580 nm directly into a lysis buffer (Ambion), and the mRNA was purified using "RNAqueous-Micro" micro scale RNA isolation kit (Ambion, Catalogue # 1931). The mRNA was treated with DNase and converted to cDNA using Superscript III (Invitrogen). The GPCR analysis was performed using custom designed 384 well qPCR plates from Lonza (Copenhagen, DK) containing primers for 379 7TM receptors and 3 RAMPs together with primers for Rn18s and genomic DNA. Primer target regions have been published before [19]. A genomic DNA sample was used as calibrator as described previously [19].
GLP-1 secretion from primary colonic crypt cultures
Neuromedin C was purchased from Bachem, AR231453 was a generous gift from Rob Jones, Arena Pharmaceuticals and TAK-875 [23]and Metabolex-209 [24]were synthesized as described. Colonic crypts were prepared from male C57BL/6, GPR119-/- or GPR40-/- mice by collagenase treatment of minced tissue as described previously [10]. Cells were seeded into 24 well plates coated with Matrigel (BD Biosciences). The following day, cells were washed and incubated for 3 hours with ligands dissolved in DMSO (quadruplicates) in standard solution [10] containing 0.1% fatty acid-free BSA (Sigma) and 10 mM glucose. The liquid was aspirated from the wells, centrifuged and the supernatant was kept at -80°C until further use. GLP-1 was measured according to the protocol "Total GLP-1 version 2" from Meso Scale Discovery (#K150JVC-1, MD, USA).
Supplementary Material
Funding
The Novo Nordisk Foundation Center for Basic Metabolic Research (http://www.metabol.ku.dk) is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
References
- 1.Isaacs PE, Ladas S, Forgacs IC, Dowling RH, Ellam SV, Adrian TE, Bloom SR. Comparison of effects of ingested medium- and long-chain triglyceride on gallbladder volume and release of cholecystokinin and other gut peptides. Dig Dis Sci. 1987;32(5):481–6. doi: 10.1007/BF01296030. [DOI] [PubMed] [Google Scholar]
- 2.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahren B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295(4):E779–84. doi: 10.1152/ajpendo.90233.2008. [DOI] [PubMed] [Google Scholar]
- 3.Sundaresan S, Abumrad NA. Dietary Lipids Inform the Gut and Brain about Meal Arrival via CD36-Mediated Signal Transduction. J Nutr. 2015;145(10):2195–200. doi: 10.3945/jn.115.215483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity--oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104(4):613–20. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab. 2013;24(2):92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422(6928):173–6. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 7.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–4. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 8.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140(3):903–12. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52(2):289–98. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–9. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–7. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Swaminath G, Cao Q, Yang L, Guo Q, Salomonis H, Lu J, Houze JB, Dransfield PJ, Wang Y, Liu JJ, et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol Cell Endocrinol. 2013;369(1-2):119–29. doi: 10.1016/j.mce.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96(9):E1409–17. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 14.Mandoe MJ, Hansen KB, Hartmann B, Rehfeld JF, Holst JJ, Hansen HS. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am J Clin Nutr. 2015;102(3):548–55. doi: 10.3945/ajcn.115.106799. [DOI] [PubMed] [Google Scholar]
- 15.Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci. 2012;33(7):374–81. doi: 10.1016/j.tips.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Katz LB, Gambale JJ, Rothenberg PL, Vanapalli SR, Vaccaro N, Xi L, Sarich TC, Stein P. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes Metab. 2012;14(8):709–16. doi: 10.1111/j.1463-1326.2012.01587.x. [DOI] [PubMed] [Google Scholar]
- 17.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149(5):2038–47. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 18.Lan H, Lin HV, Wang CF, Wright MJ, Xu S, Kang L, Juhl K, Hedrick JA, Kowalski TJ. Agonists at GPR119 mediate secretion of GLP-1 from mouse enteroendocrine cells through glucose-independent pathways. Br J Pharmacol. 2012;165(8):2799–807. doi: 10.1111/j.1476-5381.2011.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2(4):376–92. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egerod KL, Engelstoft MS, Lund ML, Grunddal KV, Zhao M, Barir-Jensen D, Nygaard EB, Petersen N, Holst JJ, Schwartz TW. Transcriptional and Functional Characterization of the G Protein-Coupled Receptor Repertoire of Gastric Somatostatin Cells. Endocrinology. 2015;156(11):3909–23. doi: 10.1210/EN.2015-1388. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Swaminath G, Brown SP, Zhang J, Guo Q, Chen M, Nguyen K, Tran T, Miao L, Dransfield PJ, Vimolratana M, et al. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One. 2012;7(10):e46300. doi: 10.1371/journal.pone.0046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semple G, Fioravanti B, Pereira G, Calderon I, Uy J, Choi K, Xiong Y, Ren A, Morgan M, Dave V, Thomsen W, et al. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem. 2008;51(17):5172–5. doi: 10.1021/jm8006867. [DOI] [PubMed] [Google Scholar]
- 23.Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, Ito R, Harada A, Takeuchi K, Suzuki N, Miyazaki J, et al. Discovery of TAK-875: A Potent, Selective, and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2010;1(6):290–4. doi: 10.1021/ml1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi DF, Song J, Ma J, Novack A, Pham P, Nashashibi I, Rabbat CJ, Chen X. GPR120 receptor agonists and uses thereof. WO 2010/080537. Metabolex, Inc; 2010
- 25.Patel S, Mace OJ, Tough IR, White J, Cock TA, Warpman Berglund U, Schindler M, Cox HM. Gastrointestinal hormonal responses on GPR119 activation in lean and diseased rodent models of type 2 diabetes. Int J Obes (Lond) 2014;38(10):1365–73. doi: 10.1038/ijo.2014.10. [DOI] [PubMed] [Google Scholar]
- 26.Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, Weinglass AB, Engelstoft MS, Madsen AN, Luckmann M, Miller MW, et al. GPR40 (FFAR1) - Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab. 2015;4(1):3–14. doi: 10.1016/j.molmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss CE, Glass LL, Diakogiannaki E, Pais R, Lenaghan C, Smith DM, Wedin M, Bohlooly YM, Gribble FM, Reimann F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides. 2015 doi: 10.1016/j.peptides.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol Metab. 2013;24(8):398–407. doi: 10.1016/j.tem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Mancini AD, Poitout V. GPR40 agonists for the treatment of type 2 diabetes: life after 'TAKing' a hit. Diabetes Obes Metab. 2015;17(7):622–9. doi: 10.1111/dom.12442. [DOI] [PubMed] [Google Scholar]
- 30.Kaku K, Araki T, Yoshinaka R. Randomized, double-blind, dose-ranging study of TAK-875, a novel GPR40 agonist, in Japanese patients with inadequately controlled type 2 diabetes. Diabetes Care. 2013;36(2):245–50. doi: 10.2337/dc12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen SJ, Larsen LK, Hansen G, Chelur S, Larsen PJ, Vrang N. Expression of the fatty acid receptor GPR120 in the gut of diet-induced-obese rats and its role in GLP-1 secretion. PLoS One. 2014;9(2):e88227. doi: 10.1371/journal.pone.0088227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki K, Harada N, Sasaki K, Yamane S, Iida K, Suzuki K, Hamasaki A, Nasteska D, Shibue K, Joo E, Harada T, et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology. 2015;156(3):837–46. doi: 10.1210/en.2014-1653. [DOI] [PubMed] [Google Scholar]
- 33.Stone VM, Dhayal S, Brocklehurst KJ, Lenaghan C, Sorhede Winzell M, Hammar M, Xu X, Smith DM, Morgan NG. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia. 2014;57(6):1182–91. doi: 10.1007/s00125-014-3213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.