Abstract
Dairy pastoralism is integral to contemporary and past lifeways on the Eastern Eurasian Steppe, facilitating survival in agriculturally challenging environments. While previous research has indicated that ruminant dairy pastoralism was practiced in the region by c. 1300 BC, the origin, extent and diversity of this custom remains poorly understood. Here we analyze ancient proteins from human dental calculus recovered from geographically diverse locations across Mongolia and spanning 5,000 years in time. We present the earliest evidence for dairy consumption on the Eastern Eurasian Steppe by c. 3000 BC, and the later emergence of horse milking at c. 1200 BC, concurrent with the first evidence for horse riding. We argue that ruminant dairying contributed to the demographic success of Bronze Age Mongolian populations, and that the origins of traditional horse dairy products in Eastern Eurasia are closely tied to the regional emergence of mounted herding societies during the late second millennium BC.
In contemporary central and eastern Eurasia, mobile dairy-based pastoralism is a key subsistence practice for many people1. Much of the Eastern Eurasian Steppe is covered by dryland grasses which, while challenging for grain agriculture, can sustain large meat and dairy-producing herds2,3. Across the steppe, dairy is a staple food and the product of rich culinary traditions. In the Mongolian countryside, fresh, fermented, processed and distilled dairy products provide a major source of hydration and up to 50% of summer caloric intake1,4. Moreover, milk provides a protein- and fat-rich dietary component, while the processing of milk into dairy products enables the creation of a storable and transportable food source. In Mongolia today, dairy livestock, including sheep, goat, horse, cow, yak, reindeer, and camel, are exploited for milk, meat, traction and transport across diverse environmental niches.
The adoption of dairy into adult human diets was a major transition in prehistoric subsistence5, and in Western Eurasian contexts, biomolecular approaches have been extensively applied to investigate its archaeological antiquity. Following their initial domestication in Southwestern Asia, cattle (Bos taurus), sheep (Ovis aries) and goats (Capra hircus) spread eastward across the Eurasian sSeppe into Central Asia6. Biomolecular evidence for dairy lipids has been identified in ceramics from Neolithic Anatolia7 and eastern Europe8, as well as Copper and Bronze Age Kazakhstan9,10, indicating the potential spread of dairying out of Southwestern Asia. As far east as the Tarim Basin of the Xinjiang region in northwest China in the Middle Bronze Age, milk proteins have been identified in a woven basket11 and pieces of well-preserved kefir cheese12 (Figure 1). In Bronze Age Mongolia, a recent study of human dental calculus from individuals across multiple sites in the northern Khövsgöl aimag identified milk proteins from sheep, goat, and cattle1. Ancient DNA analysis of the same population found that almost all individuals were of predominantly local ancestry, and only a single individual had over 10% of Western Steppe Herder (WSH) ancestry. This suggests that by the late second millennium BC, dairy pastoralism had been fully adopted by, or originated with, local northern populations, leaving an open question of when and how dairy subsistence arrived in this region.
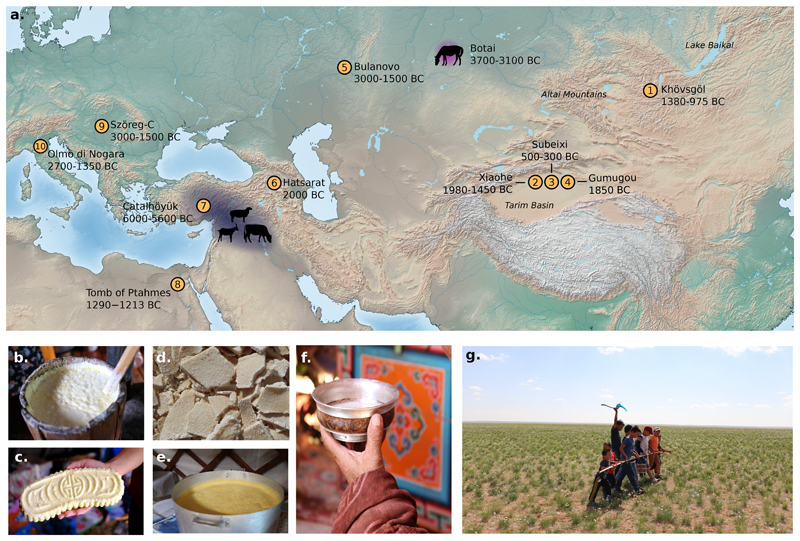
Figure 1. Ruminant and equine dairying in prehistoric Eurasia and contemporary Mongolia.
(a) Map of Eurasia showing major geographical features referred to in the text and sites where evidence of dairying has been previously found using proteomic approaches: (1) Khövsgöl1, (2) Xiaohe11, (3) Gumugou10, (4) Subeixi68, (5) Bulanovo29, (6) Hatsarat29, (7) Çatalhöyük West69 (8), Tomb of Ptahmes70, (9) Szöreg-C (Sziv Utca)29, (10) Olmo di Nogara29. Locations for the earliest evidence of ruminant dairying based on the presence of milk fats in ceramics is shown in blue7 and the earliest evidence of horse dairying9 shown in pink. Details for each site included in this figure are referenced in Supplementary Table S3. (b-f) Mongolian dairy products from Khövsgöl aimag and (g) dairying rituals from Dundgobi aimag, Mongolia. (b) Yogurt starter culture, Khöröngö (ХӨрӨнГӨ); (c) curd from reindeer milk, ‘kurd’; (d) dried curd from mixed yak and cow milk, aaruul (ааруул); (e) clotted cream from mixed yak and cow milk, öröm (ӨрӨм); (f) fermented horse milk, airag (аЙраг); and (g) blessing ritual for the first horse airag production of the season.
Direct evidence into the timing and nature of pastoral economies in Mongolia from other datasets is exceedingly rare. On the Eastern Steppe, the ephemeral nature of pastoral campsites and severe wind deflation in most contexts makes detecting occupational sites with direct information on subsistence economies challenging13,14. As a result, archaeologists have often been forced to form conclusions about local subsistence from materials found in ritual human burials under stone monuments that dominate the Bronze Age archaeological landscape, and which occasionally include satellite animal burials. Specific features of burial mounds (stone type, shape, ringed fences) can be used to identify interred individuals into different culture groups as mound construction styles changed alongside evolving cultural traditions in Bronze Age Mongolia15,16. Prior to the Bronze Age, before the presence of constructed stone burial mounds, there are very few uncovered occupation or ritual sites, and pre-Bronze Age subsistence strategies are not well understood. However, it is assumed that Neolithic subsistence strategies included hunting, gathering, and fishing, although the possibility of pastoralism should not be completely discounted14. Human burials associated with Afanasievo and Chemurchek culture groups (c. 3000-2500 BC) contain faunal remains of ovicaprids, bovines, equids, and dogs15,17–19, yet it is unclear whether the remains of each were of a domesticated variety or their wild relatives, such as Ovis ammon and Capra ibex in the case of ovicaprids20. Later Bronze Age (1500-800 BC) campsites containing ruminant and equine remains suggest the dietary consumption of horses and cattle, as well as sheep and/or goats as part of a fully pastoral economy21. Satellite burials containing multiple animals surrounding ritual human internments attest to pastoral culling and herd management patterns of sheep, goat, cattle, and horses during this period22,23. By 1200 BC, remains of domesticated horses became almost ubiquitous at ritual burials sites in Northern Mongolia, with some of the crania displaying evidence for equine dentistry and horse bridling and riding24,25.
In later time periods, written records from neighboring regions, such as China and the Middle East, as well as within Mongolia, document the importance of ruminant and equine dairy in day-to-day subsistence, particularly the consumption of fermented horse milk as early as the Xiongnu Empire (c. 200 BC - 100 AD)26 along with camel milk by the Mongol period (c. 1206-1398 AD)27,28. Even though many archaeologists and historians assume milk had been included in ancient steppe diets, little direct evidence has been available about where and when specific animal species were first exploited for dairy on the steppe east of the Altai Mountains.
The analysis of ancient proteins extracted from ancient human dental calculus (calcified dental plaque or tooth tartar) has been established as an approach for detecting milk consumption in past individuals1,29–31. Differences in amino acid sequences between taxa enable the detection of the livestock species, enabling ‘zooarchaeology by proxy’ - a method by which to detect past animal use by the analysis of human remains alone. Here we apply the proteomic analysis of ancient dental calculus to 32 individuals spanning from the Early Bronze Age through the Middle Ages to characterize the antiquity and species diversity of ruminant and equine dairying in Mongolia. We report the earliest direct evidence for dairy consumption in East Asia (east of the Altai Mountains), finding that ruminant dairy consumption was a feature of ancient diets in Mongolia from its initial pastoral occupation, c. 3000 BC and occurred in association with archaeological sites linked to Western Steppe cultures. We show that ruminant dairying became widespread by the Middle Bronze Age (1800-1200 BC), and from 1200 BC we observe the onset of horse milk consumption, perhaps the progenitor of the alcoholic drink airag, in tandem with the first evidence for mounted horseback riding and highly mobile economies – a tradition that remained significant through the great nomadic empires and into the modern era.
Results
Milk proteins were identified in 72% of individuals analyzed (23 of 32 individuals, Table 1), indicating the widespread consumption of dairy foods across multiple time periods in prehistoric and historic Mongolia. Specifically, we detected evidence of the milk proteins beta-lactoglobulin (I and II), alpha-S1-casein, kappa-casein, alpha-lactalbumin and beta-casein, lysozyme C, and peptidoglycan recognition protein 1. Beta-lactoglobulin (BLG) was the most frequently detected milk protein, a pattern consistent with previous observations from ancient dental calculus1,32. We observe evidence of milk consumption from diverse taxa across multiple environmental zones within Mongolia (Figure 2). Of the seven dairy livestock species utilized in contemporary Mongolia, we identified milk peptides from goat, sheep, cattle, horse and camel, but did not detect peptides that could be assigned specifically to reindeer or yak milk.
Table 1. Presence of dairy proteins by individual and archaeological site.
Radiocarbon dates are calibrated to a 2-sigma probability (Supplementary Table S6). Bovinae/Ovis* identifications refer to the detection of a polymorphic amino acid position where in the identified peptide TPEVD(D/N/K)EALEK, D is specific to Bovinae, N is specific to Ovis and K is specific to Capra. Because asparagine (N) deamidates to aspartic acid (D), the presence of a D at this position cannot be unambiguously assigned to Bovinae or Ovis. References and details on archaeological site excavations can be found in the Supplementary Table 1. All identified dairy peptides per individual are detailed in Supplementary Data 2.
| Archaeological Culture | Archaeological Site | Individual ID | Calibrated Radiocarbon Date BC | Milk Species/Taxonomic Group Identified |
|---|---|---|---|---|
| Early Bronze | ||||
| Afanasievo | Shatar Chuluu | AT-26 | 3316-2918 | Bovinae/Ovis* |
| Chemurchek | Khundii Gobi | AT-628 | 3310-2919 | Ovis, Bovinae/Ovis |
| Chemurchek/ Afanasievo | Khuurai Gobi | AT-635 | 2618-2487 | None Detected |
| Chemurchek | Yagshiin Khuduu | AT-590b | 2567-2468 | Ovis, Bovinae/Ovis*, Caprinae |
| Middle Bronze | ||||
| Ulaanzuukh | Ulaanzuukh | AT-823 | 1391-1209 | None Detected |
| AT-921 | 1412-1266 | Capra, Ovis, Bovidae | ||
| AT-923 | No Date | Bovinae/Ovis* | ||
| AT-824 | 1402-1279 | None Detected | ||
| AT-769A | 1608-1416 | Bovidae, Bovinae/Ovis* | ||
| AT-769B | 1509-1439 | None Detected | ||
| Late Bronze | ||||
| Baitag | Uliastai River, Central Terrace | AT-676 | 1277-1057 | Equus, Capra, Ovis, Bovinae |
| Deer Stone/ Khirigsuur | Berkh Uul | AT-905 | 1371-1121 | Ovis, Capra, Bovinae/Ovis* |
| Slab Burial | Shunkhlai Uul | AT-233 | 1072-903 | Equus, Capra, Ovis, Bos |
| Undefined | Khoit Tsenkher | AT-398 | 1056-904 | Capra, Bovinae/Ovis* |
| Early Iron | ||||
| Chandmani | Chandman Uul | AT-56 | 971-843 | Equus, Capra, Ovis, Bovinae |
| AT-121 | 358-195 | None Detected | ||
| Slab Grave | Dartsagt | AT-766 | 750-407 | None Detected |
| Late Iron | ||||
| Xiongnu | Tamiryn Ulaan Khoshuu | AT-728 | No Date | Equus |
| Xiongnu | Duulaga Uul | AT-605 | 43 BC - 51 AD | Bos, Bovinae/Ovis*, Caprinae |
| Iron Age | Ulaanzuukh | AT-885 | 96-61 cal AD | Caprinae, Bovinae/Ovis* |
| Early Medieval | ||||
| Turkic | Uliastain dood denj | AT-675 | 650-765 cal AD | None Detected |
| Late Medieval (Mongol Period) | ||||
| Mongol Empire | Sharga Uul | AT-701 | No Date | Equus |
| Mongol Empire | Del Khad | AT-775 | No Date | Equus, Ovis, Camelus |
| Mongol Empire | Zaraa Tolgoi | AT-271 | No Date | Equus, Bovinae/Ovis* |
| Mongol Empire | Banzart Khairkhan | AT-846 | No Date | Equus, Bovinae/Ovis* |
| Mongol Empire | Tahiltyn Khotgot | AT-360 | No Date | Equus |
| Mongol Empire | Burgaldain Khundii | AT-650 | No Date | Equus, Caprinae, |
| Mongol Empire | Ganzagad | AT-835 | No Date | Equus, Capra, Bovinae/Ovis* |
| Mongol Empire | Uguumur Tsuvaraa Uul | AT-549 | No Date | Capra, Ovis, Bovinae |
| Mongol Empire | Khoit Tsenkher, Tarvagatain Am | AT-354 | 1158-1252 AD | Equus, Caprinae, Bovidae |
| Mongol Empire | Kharkhorin | AT-512 | No Date | None Detected |
| Mongol Empire | Mori Baridag | AT-800 | No Date | None Detected |
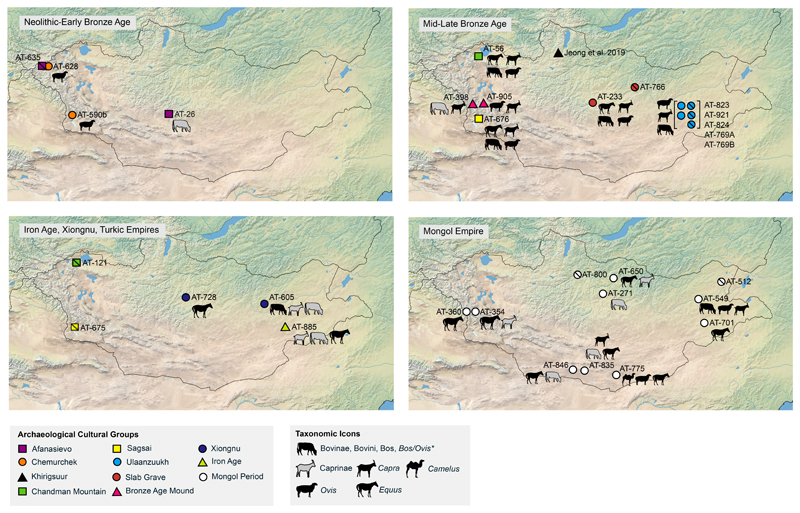
Figure 2. Mongolian dairy consumption by period.
(a) Neolithic to Early Bronze Age; (b) Middle-Late Bronze Age; (c) Iron Age and Early Medieval; (d) Late Medieval. Archaeological site cultural affiliation is indicated by colors and symbols. Solid filled symbols indicate individuals with positive evidence of milk proteins, while symbols bisected with a diagonal line indicate individuals where no milk proteins were identified. Individuals of the same site are contained within brackets. Individual AT-923, associated with Ulaanzuukh, is not directly radiocarbon dated and is not included in this figure. Taxonomic icons only indicate the most specific taxa identified in a phylogenetic branch. The full list of dairy species identified for each individual is listed in Table 1 and Supplementary Data 2. Data used in the creation of this figure is included in Supplementary Table S4.
We find evidence of milk proteins in the earliest directly dated individual in our sample set, AT-26 (3316-2918 cal BC; 2-sigma range) at the Afanasievo burials of Shatar Chuluu, the earliest known mounded burial features associated with pastoral economies in the territory of Mongolia. Specifically, we observe peptides deriving from a taxonomically ambiguous region of the milk whey protein BLG, where the species can be assigned as a bovid in bovinae subfamily (cow, yak, bison, water buffalo) or Ovis genus (sheep), making a more specific taxonomic assignment for this individual’s milk consumption more challenging. In the two individuals associated with Chermurchek culture at the sites of Khundii Gobi (2886-2577 cal BC; 2-sigma range) and Yagshiin Khuduu (2567-2468 cal BC; 2-sigma range), we detect milk peptides matching to the subfamily caprinae (sheep or goat) and the genus Ovis (sheep) from BLG and also alpha-S1-casein. At Khundii Gobi, these identifications are specific to sheep (with 6 BLG peptides identified) among others that are specific only to the subfamily caprinae and the infraorder Pecora (all even-toed ruminant mammals). These results align with recent archaeofaunal data from the early 2nd millennia BC, which suggest a significant role for sheep in the prehistoric economy of pastoral occupants of the Mongolian Altai33.
In the Middle Bronze Age (c. 1800-1200 BC) the consumption of ruminant dairy milk can be seen in four of seven individuals analyzed in the central eastern site of Ulaanzuukh, and in seven of nine individuals in the previously published Khövsgöl sites in northern central Mongolia. Ulaanzuukh individuals date to slightly earlier than the Bronze Age burial sites analyzed in Jeong et al1, where evidence of ruminant milk consumption was found in individuals associated with slope burials and khirigsuur ritual monuments. At Ulaanzuukh, BLG is the most frequently recovered protein across all samples, with peptides from alpha-S1-casein and kappa casein (proteins which are associated more with milk curds than milk whey) identified to a lesser extent. The dental calculus from the three Ulaanzuukh individuals who did not show evidence of dairy consumption exhibited a poor level of protein preservation, with a general absence of typical salivary and bacterial proteins reported previously in dental calculus studies34, which may suggest that an absence of evidence for milk consumption in these individuals could be due to overall poor biomolecular preservation.
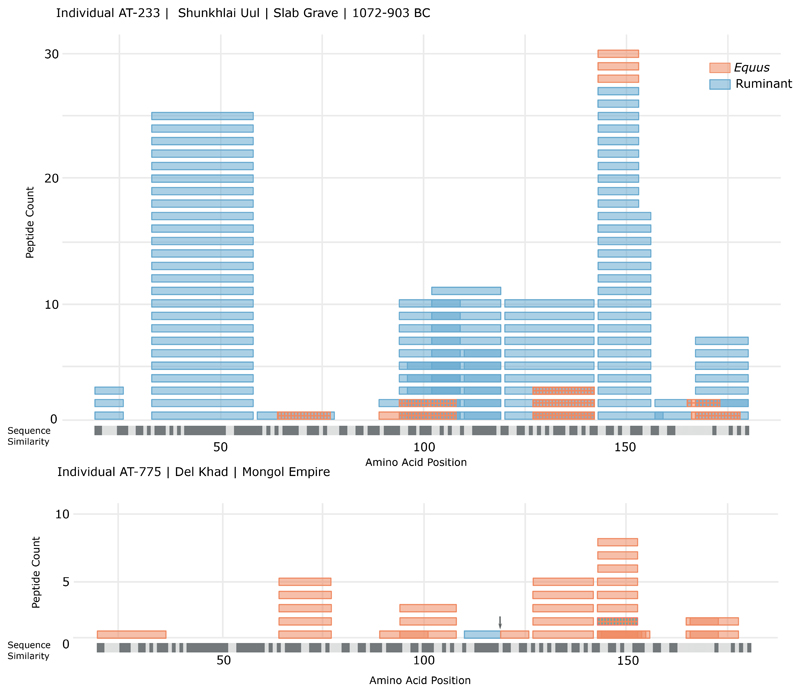
At Bronze Age sites dating to after 1200 BC, we found evidence for dairy consumption in all four individuals tested. We identified ruminant milk peptides from the same two proteins (BLG, alpha-S1-casein) identified in the previous time period, as well as from two additional proteins: alpha lactalbumin and beta-casein. In addition to ruminant milk proteins, we also detected the first palaeoproteomic evidence of horse (Equus) milk, including horse-specific peptides from BLG I and II (horse BLG is derived from two paralogous genes) in 2 of 4 individuals. An individual from the site of Shunklai Uul (c. 1000 BC), in central Mongolia, had 126 peptide spectral matches (PSMs) from ruminant BLG, another 50 from horse BLG I and II (Figure 3), and an additional nine from alpha-S1- and beta-casein and alpha-lactalbumin.
Figure 3. Alignment of observed BLG peptides for two individuals analysed in this study (AT-233, Mid-Late Bronze Age; and AT-775, Mongol Empire), showing the number of Equus (orange) and ruminant (blue) BLG peptides detected.
For Equus, peptides from both BLG1 and BLG2 paralogs are displayed (see Supplementary Table S5 for data associated with figure). Where peptides from these two taxa overlap this has been indicated by a blue/orange cross-hatch pattern. The arrow in Individual AT-775 indicates two contiguous but independent peptides. Beneath each individual is a consensus sequence of Bos taurus BLG (UniProt: P02754) and Equus caballus BLG1 (UniProt: P02758) with dark grey indicating sequence identity, and pale grey indicating sites with sequence differences.
Of the three Early Iron Age individuals (800-400 BC), two from the northwestern Mongolia site of Chandman Mountain and another from Dartsagt (north-central Mongolia), all exhibited moderate to high preservation and contained an abundance of human and oral microbiome proteins. Two of these contained no robustly identified milk peptides, and in contrast one individual from Chandman Mountain contained an abundance of PSMs to milk proteins. During this period, BLG peptides specific to both sheep and goat were detected, along with others that can be assigned to the higher taxonomic orders of caprinae and bovidae. Peptides derived from casein and alpha-lactalbumin specific to caprines, and those from both BLG I and II specific to equine milk.
In the Late Iron Age, during the tenure of the Xiongnu Empire (c. 200 BC - 300 AD), calculus from each of the three individuals studied contained evidence for dairy consumption. One individual had PSMs specific to only horse BLG, and the second had Bos specific beta-lactoglobulin and caprine alpha-S1-casein peptides. The third individual had ruminant casein and whey proteins (BLG, alpha-S1-casein) as well as lysozyme C peptides specific to Equus. In the post-Xiongnu period, a single individual archaeologically classified from the Turkic era did not display any evidence of milk consumption.
Nine of the eleven Mongol Empire individuals displayed evidence for the consumption of dairy, with many individuals showing evidence for the consumption of milk from multiple species. For example, one individual showed evidence for the consumption of ruminant, equine, and camel milk, whilst a further five individuals displayed evidence for the consumption of both ruminant and horse dairy products (Figure 3). During this period, we observed the first evidence for the consumption of camel milk through the detection of peptidoglycan recognition protein 1 (UniProtKB: Q9GK12), an immune protein that has been isolated from modern camel milk.
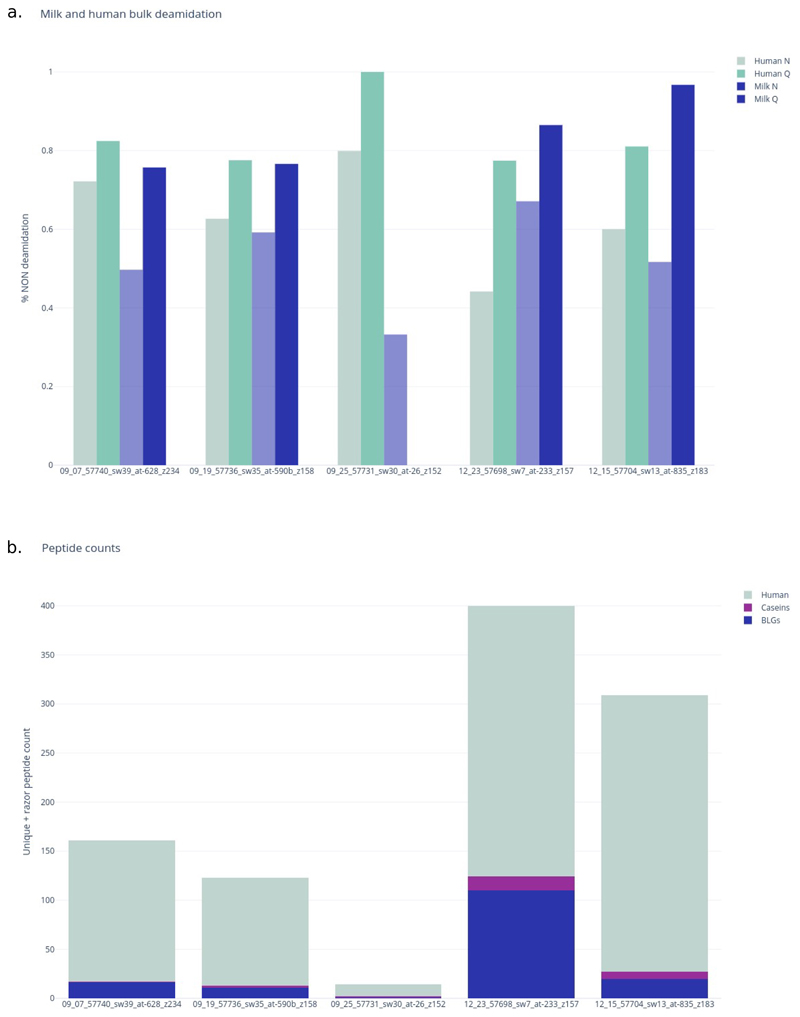
Deamidation of glutamine and asparagine has been proposed as a marker of taphonomic degradation in ancient proteins35. We applied an analysis of bulk deamidation using a previously published approach36 to 5 individuals from this study to examine potential patterns of archaeological degradation in milk, which may suggest that the milk proteins in our samples are ancient, as they appear to be of similar deamidation levels as the human oral proteins in the calculus. All deamidation is reported in Supplementary Table S2. We observed that the older samples generally showed higher levels of deamidation. The milk-origin peptides retrieved from the early Bronze Age individuals displayed an average of 23.9% glutamine deamidation, the Late Bronze Age 13.5%, and the Mongol era 3.3%. The same pattern was observed in the deamidation of asparagine, with an average of 52.6% deamidation of milk-origin peptides in the Early Bronze Age and 32.9% in the Late Bronze Age. However, the milk peptides recovered from the Mongol era individual do not fit this pattern in terms of asparagine deamidation, with 48.3% deamidation (Supplementary Table S2).
Discussion
Earliest evidence for dairying is associated with Western Steppe herder archaeological cultures
Our results demonstrate the oldest known evidence of dairy consumption in Mongolia and the Eastern Eurasian Steppe (c. 3000 BC - 2500 BC), in the form of Early Bronze Age Afanasievo and Chemurchek-associated individuals in both Central and Western Mongolia (Table 1). Previous ancient DNA analysis of one of the individuals at Shatar Chuluu exhibiting ruminant dairy proteins (AT-26) was shown to have a non-local mitochondrial haplogroup consistent with WSH populations37, supporting the interpretation of individuals associated with the Afanasievo culture as migrants from eastern Europe via the Russian Altai38–40 and a likely vector for the initial introduction of domestic animals into Mongolia14. The identification of ruminant milk proteins (Ovis and Bovinae/Ovis) supports the domestic nature of fauna found in these burial features, and indicate that dairy pastoralism formed an important element of the subsistence base of these late 4th millennium BC transcontinental migrants. Most significantly, they suggest that human migrations associated with the expansion of the Afanasievo culture present a viable candidate for the initial introduction of dairy and domestic livestock into Eastern Eurasia. In this study, the earliest individual in this dataset displayed evidence of dairy consumption, and to track the earliest instances of dairy consumption in the Eastern Steppe, it would be necessary to analyze individuals from earlier time periods. This would be particularly informative to untangle the presence or absence of dairying prior to Yamnaya-Afansievo migrations.
The antiquity of eastern steppe horse milk consumption
Temporal patterning in our protein results suggest that horse milk consumption played a key role in the emergence and proliferation of mobile pastoralism in Mongolia (Figure 4). Today, horses play a vital role in traditional Central Asian pastoral lifeways, improving herd management as well as providing a primary source of meat and milk. Horseback herders can manage larger herd sizes, and horses can break through snow and ice to access the sustenance underneath, exposing grass oases for other animals in the herd41–43. We observe direct evidence of horse milk consumption on the Eastern Steppe, in the form of equine (Equus) specific peptides from the milk whey proteins BLG (I and II) and lysozyme C in individuals associated with the Baitag and Slab Grave cultures in Western Mongolia dated to the late second millennium BC (Table 1). In addition to the appearance of horses in dietary assemblages21, this time period is linked with the proliferation of horses in ritual sites, the first direct archaeological evidence for horse bridling and riding25,44, the first evidence for horse breeding and management22,23, innovations in horse healthcare24, an expanded use of dry intermontane grasslands23,24,45 and the emergence of mobile, horse-facilitated pastoralism in Eastern Eurasia. Our findings suggest that the incorporation of horses into dairy herds may have been closely linked to this multi-faceted economic transformation in the use of horses45.
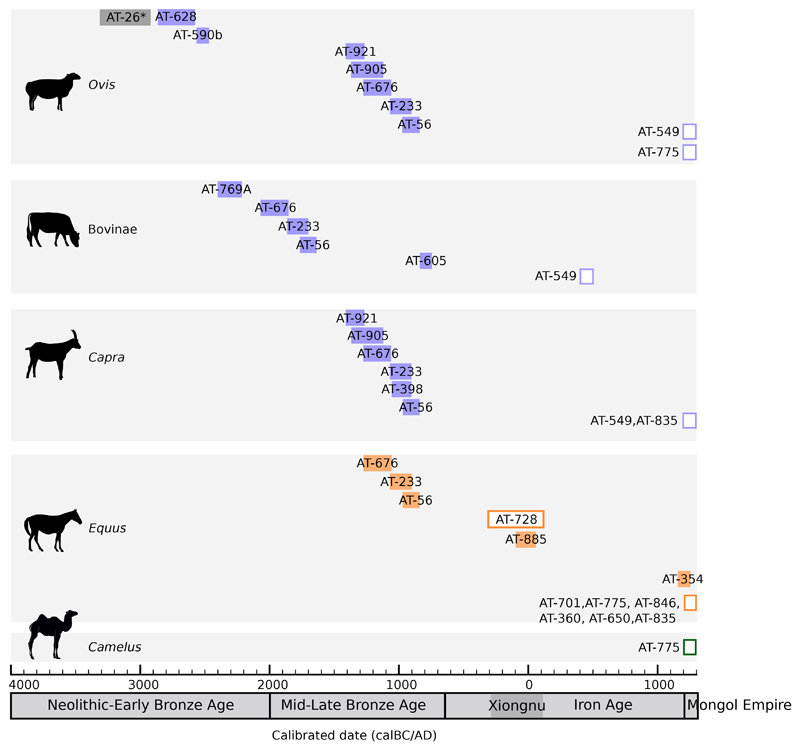
Figure 4. Timeline of evidence for the consumption of different livestock milk in prehistoric and historic Mongolia.
Radiocarbon dates for each individual were calibrated using OxCal (OxCal v4.3.2 Bronk Ramsey66; r:5 IntCal13 atmospheric curve67) and resulting radiocarbon probabilities were grouped by the taxa of dairy proteins identified in that individual (indicated by AT- numbers), with ruminant taxa (Ovis, Capra and Bovinae) indicated in purple, Equus indicated by orange and Camelus indicated by green. Dairy peptides identified in individual AT-26 (indicated with an asterisk) are specific to Bovinae/Ovis. Individuals without direct radiocarbon dates are indicated by unfilled boxes and are placed on the timeline based on the estimated time spans for the Xiongnu and Mongol Empires. For data used in this figure, refer to Supplementary Table S6.
Following the Bronze Age, direct proteomic evidence for horse milk consumption continues through the imperial Xiongnu and Mongol periods (Table 1), in agreement with extensive textual and zooarchaeological evidence underscoring their significance to historic economies. During the Iron Age, we identify horse milk proteins at Tamiryn Ulaan Khoshuu, a site within the heartland of the Xiongnu Empire46,47. Historical Chinese documents record that an assortment of dairy products were consumed during the Xiongnu period (c. 200 BC - 100 AD), including dried curds from ruminant milk (aaruul), but lao (“horse alcohol”) was the most consistently referenced dairy product, and it held a prominent place in the cultural practices and identities of the steppe peoples48–50. By the Mongol period, horse milk consumption appears in among >80% (9 of 11) of tested individuals, a finding that matches historical accounts for widespread consumption of fermented mare’s milk, known as airag (Mongolian) or koumiss/kymyz (Turkic languages)27.
Horse milk differs from ruminant milks in important ways, in particular, it contains less curd protein (caseins) and much more lactose41,51,52. While the lower casein content makes it undesirable for producing most dried curd products, like aaruul, a staple of the traditional Mongolian diet, its high lactose content makes it highly suitable for making alcoholic beverages with ethanol contents as high as 10-12%53. Communal airag drinking was and continues to be an important social activity (Figure 1), and it has been frequently noted in both Mongol and foreign historical texts. The cultural significance of airag continued throughout the Mongol period, with social gatherings at the time referred to as “going to drink airag with people”, and social rankings reinforced by how close one sat in relation to the pitchers of airag. Favored associates were charged with serving the airag, and with choosing the order in which airag was served at feasts27. At the Mongol capital of Kharkorum, a silver fountain was said to flow with fermented mare’s milk, dispensing airag54.
Identification of other milks and fermentation agents
In addition to cattle, sheep, goats, and horses, domesticated Bactrian camels (Camelus bactrianus), yaks (Bos grunniens), and reindeer (Rangifer tarandus) are also milked in contemporary Mongolia; however, very little is known about the dairying history of these three species. Here we report protein evidence of camel milk consumption (together with horse and sheep milk) in a Mongol period individual buried in the Gobi Desert (Table 1), a habitat of Bactrian camels55. Although not unique to milk in other mammal species, the camel protein we identify here, peptidoglycan recognition protein 1 (PRP1), is an important component of camel milk whey56,57. Mongol era historical accounts, such as the twelfth-century Secret History of the Mongols27, and historic accounts from foreign travelers into the empire, such as Marco Polo28, include stories of camels used for subsistence (milk, meat, and blood) and transport of people and gers. While likely not the earliest instance of camel milk drinking, the PRP1 protein data presented here are the first biomolecular evidence for its consumption. Camel dairy use is not well understood, and this finding provides a necessary insight into exploitation of this historic traction animal for milk.
We did not detect any milk peptides specifically identified as yak or reindeer in this study. While yak is a commonly herded species in Mongolia today, their past use as a dairying animal is not well understood. Yak proteins are difficult to specifically identify due to their sequence similarity to other cattle species. Yak beta-lactoglobulin, for example, differs from that of cattle at only a single amino acid across the entire BLG protein. While it is possible that some of the peptides assigned in this study to Bos or the higher taxonomies of Pecora, Bovidae, and Bovinae could have originated from domestic yak, we only observed cattle-specific variants when sufficient protein coverage enabled it. The absence of reindeer-specific milk consumption may be expected based on historical accounts on the use of this animal for dairying in this region. Contemporary reindeer herders in Mongolia do milk their animals, but these families migrated from Siberia into northern Mongolia only in the last century58,59.
In the present study, we did not identify any proteins specific to any bacterial taxa or other fermentation agents used in the processing of milk into other dairy products. Although processing agents were not found in the samples analyzed in this study, it does not mean these were not used in ancient and historic Mongolia, and future studies should continue to look for their presence alongside milk peptides.
These results provide the earliest evidence for dairy pastoralism in Mongolia’s first herding societies, pushing back previous estimates of dairying in the Eastern Steppe by more than 1,700 years, and tracing pastoralist connections between the Western and Eastern Steppes to the Early Bronze Age. These results show that within 5,000 years after the earliest evidence for dairying in the Near East, this practice and its associated animals had spread more than 7,000 thousand kilometers eastward to become a successful mode of subsistence on the Mongolian Steppe. While the routes of this movement remain to be fully understood, our data suggest that dairy pastoralism, and in particular the emergence of horse riding and horse milking c. 1200 BC, provided the economic, political, and social support necessary for the success of subsequent nomadic empires on the vast grasslands of Eurasia.
Methods
Sample collection
Dental calculus samples were collected from the Department of Anthropology and Archaeology at the National University of Mongolia (NUM) from 32 previously excavated individuals (Table 1; listed by NUM accession number, See Supplementary Table S1 for site details). Individuals were selected from archaeological sites assigned to time periods between the Neolithic and the Mongol Period. Dental calculus was removed from the tooth using sterilized dental scalars and stored in Eppendorf tubes until extraction. Nitrile gloves were used during sample collection to avoid contamination from skin proteins. Samples were exported to the Max Planck Institute for the Science of Human History under permission from the Ministry of Culture, Education, Science and Sports (Export number 10/413 (7b/52) was received on 2nd February, 2017 #A0109258, MN DE 7 643). Protein extractions were conducted in a dedicated laboratory for the extraction of ancient proteins at the Max Planck Institute for the Science of Human History, Jena, using a Filter Aided Sample Preparation (FASP) protocol previously published in Jeong et al.1. Following protein extraction, digested peptides were stored at -80°C before being analyzed by LC-MS/MS at the Functional Genomics Center Zürich (FGCZ) at the ETH/University of Zürich, Switzerland.
LC-MS/MS Analysis
Mass spectrometry analysis was performed on a Q Exactive HF mass spectrometer (Thermo Scientific) equipped with a Digital PicoView source (New Objective) and coupled to a M-Class UPLC (Waters). Solvent composition at the two channels was 0.1% formic acid for channel A and 0.1% formic acid, 99.9% acetonitrile for channel B. Column temperature was 50°C. For each sample 4 μL of peptides were loaded on a commercial ACQUITY UPLC M-Class Symmetry C18 Trap Column (100Å, 5 μm, 180 μm x 20 mm, Waters) followed by ACQUITY UPLC M-Class HSS T3 Column (100Å, 1.8 μm, 75 μm X 250 mm, Waters). The peptides were eluted at a flow rate of 300 nL/min by a gradient from 5 to 40% B in 120 min. Column was cleaned after the run by increasing to 98 % B and holding 98 % B for 5 min prior to re-establishing loading condition. Samples were acquired in a randomized order. The mass spectrometer was operated in data-dependent mode (DDA), acquiring a full-scan MS spectra (350−1,500 m/z) at a resolution of 120,000 at 200 m/z after accumulation to a target value of 3,000,000, and a maximum injection time of 50 ms followed by HCD (higher-energy collision dissociation) fragmentation on the twelve most intense signals per cycle. HCD spectra were acquired at a resolution of 30,000 using a normalized collision energy of 28 and a maximum injection time of 50 ms. The automatic gain control (AGC) was set to 100,000 ions. Charge state screening was enabled. Singly, unassigned, and charge states higher than eight were rejected. Only precursors with intensity above 90,000 were selected for MS/MS. Precursor masses previously selected for MS/MS measurement were excluded from further selection for 30 s, and the exclusion window was set at 10 ppm. The samples were acquired using internal lock mass calibration on m/z 371.1012 and 445.1200.
Data Analysis
In order to account for as much variation of milk-associated proteins as possible during MS/MS ion searches, a supplementary database of unreviewed milk protein sequences was curated from UniProtKB. As an additional source of dairy protein sequences, genomic data covering the 2 beta-lactoglobulin genes in ancient horses generated by Gaunitz and colleagues60 were translated into amino acid sequences, aligned with their respective modern sequences, and any putatively divergent proteins were concatenated to the supplementary database. In total, 244 additional accessions from UniProtKB and 4 putatively divergent beta-lactoglobulin sequences from ancient horse genomes were added (Supplementary Data 1).
Peak lists were generated from raw files by selecting the top 100 peaks using MSConvert from the ProteoWizard software package version 3.0.1178161. Each sample was searched using Mascot62 (version 2.6.0) against Swiss-Prot in combination with the curated milk protein database (Supplementary Data 1). Results were exported from Mascot as csv files, and further processed through an internally created tool, MS-MARGE63 (https://bitbucket.org/rwhagan/ms-marge/src/master/), to estimate the validity of peptide identifications and summarize the findings. MS-MARGE is an R script which relies on an Rmarkdown file to generate the following: an HTML report summarizing the search (an example is given in at the end of the Supplementary Information file), a csv file containing confidently identified PSMs, and a FASTA file of confidently identified peptide sequences. As input, MS-MARGE accepts a csv file exported from a Mascot MS/MS ion search against an amino acid database containing decoyed sequences with the Group Protein Families option turned off. Additionally, two parameters can be provided: an expect value (e-value) cutoff, and a minimum number of peptides to support an identification (default 0.01 and 2, respectively). In order to estimate FDR at the PSM and protein level, MS-MARGE counts the number of decoy hits after filtering for e-value and minimum peptide support, and divides this value by the number of target hits minus the number of decoys. The resulting value is multiplied by 100 to provide an estimate of FDR in percentage. We aimed for a protein FDR of under 5%, and a peptide FDR of under 2% (Supplementary Data 2). A minimum of two individual PSMs were required for specific protein identifications, and only peptides with an e-value of below 0.01 were accepted. After filtering criteria was applied, we observed a range of variation in the numbers of proteins identified, with samples ranging from 2 to 209 confidently identified protein families.
Deamidation levels in five samples (AT-628, AT-590b, AT-26, AT-233 and AT-835) were calculated in order to assess authenticity using a previously published approach36 (Extended Data 1 and Supplementary Table S2). These samples were specifically chosen to verify the antiquity of the oldest samples in this study, as well as a more recent sample from the Mongol period for comparison of deamination patterns. The raw MS/MS files for each were run through MaxQuant64 version 1.6.2.6a against the previously described milk database and against the human proteome. Settings included a semi-tryptic search strategy allowing for a maximum of two missed cleavages was utilized, and the score cutoff for modified and unmodified peptides was set to 60 with no correction for FDR. Carbamidomethyl (C) was added as a fixed modification, while variable modifications included: Oxidation (M), Acetyl (Protein N-term), Deamidation (NQ), Gln->pyro-Glu, Glu->pyro-Glu, Phospo (ST) and Hydroxyproline. The deamidation levels of all milk proteins were averaged per sample. All identified peptides, including their post-translational modifications, are reported in Supplementary Data 2.
Radiocarbon dating
A total of 26 bones and teeth from 24 individuals were radiocarbon dated at two research facilities, the Oxford Radiocarbon Accelerator Unit (ORAU, lab code OxA) and the Groningen Radiocarbon Laboratory (lab code GrM). The ORAU followed routine pretreatment and measurement procedures65. In brief, between 200-600 mg of bone or dentine was drilled using a hand-held dentist drill, and collagen was extracted through a series of chemical steps that involved immersion in HCl, removal of humic acids using NaOH and removal of adsorbed CO2 via a final HCl wash. Only 4 of the samples prepared at the ORAU (OxA-36230, -36231, -36232, -36233) underwent ultrafiltration using Vivaspin ultrafilters, due to initial indications of poor collagen perseveration. Extracted collagen was frozen overnight and was lyophilized. Between 2-5 mg of collagen was combusted in an elemental analyser (EA) and its C and N stable isotopes were measured at an IRMS instrument linked to the EA, before excess gas CO2 was collected, graphitized and measured at an HVEE accelerator, alongside blanks and standards. These were used for contamination calculation and final correction of the data. For these samples collagen yields ranged greatly from 0.8% to 17.8%, C:N ratio of the extracted collagen fall within expected ranges (3.2-3.4) with the exception of OxA-36233 (C:N=3.6) and %C in the combusted collagen was between 37-46%. The measurements are reported in radiocarbon years before present (BP), where BP=AD 1950. Samples from Oxford were calibrated using OxCal v4.3.266 and an IntCal13 atmospheric curve67.
For radiocarbon dates processed at the Groningen Radiocarbon Laboratory, samples were decalcified over at least a 24-hour period using mild acid (HCl, 2-4% w/vol; RT) at the Center for Stable Isotope Research at the University of Groningen. For each sample still not fully decalcified, the solution was refreshed, removing and storing soft portions separately in demineralised water until further preparation. Soft and pliable fragments were rinsed thoroughly with demineralised water. Extracts were then exposed to NaOH (1%, ~30 min) to eliminate humic acids, rinsed to neutrality and treated once more with acid (HCl, 4% w/vol, 15 min). The raw collagen fraction was denatured to gelatin in acidified demineralised water (pH 3) at 80 °C for 18 hours. Before drying, the dissolved gelatin was filtered through a 50 μm mesh to eliminate any remaining foreign particulates, and the crystalline collagen scraped from the glass. Approximately 4 mg aliquots of the reduced carbon fraction were then weighed into tin capsules for combustion in an Elemental Analyser (EA, IsotopeCube NCS, Elementar®). The EA was coupled to an Isotope Ratio Mass Spectrometer (IRMS, Isoprime® 100), allowing the ∂13C value of the sample to be measured, as well as a fully automated cryogenic system to trap CO2 liberated on combustion. After run completion, the individual reaction vessels were transferred to a graphitisation manifold, where a stoichiometric excess of H2 gas (1: 2.5) was added, and the CO2 gas reduced to graphite over an Fe(s) catalyst. The graphite samples were then pressed, and the radioisotopic ratio determined on a MICADAS accelerator mass spectrometer. Samples from Groningen were calibrated using OxCal v4.3.266 and an IntCal13 atmospheric curve67.
Extended Data
Extended Data Fig. 1. Milk and bulk deamidation and peptide counts.
Supplementary Material
Acknowledgements
We thank the National University of Mongolia and the Ministry of Education, Culture, Science, and Sports for facilitating this research. We thank the Functional Genomics Center Zürich, Laura Kunz and Claudia Fortes for mass spectrometry analysis and helpful comments and suggestions for this study. We thank Dalaimyagmar, Bayandalai and Sukhbat-Nayamsuren from Khövsgöl aimag for sharing their knowledge and insights into traditional Mongolian dairying practices, traditions, and herding strategies, and we thank Björn Reichhardt and Matthäus Rest for use of the photographs displayed in Figure 1. We thank Katerina Douka from the Oxford Radiocarbon Accelerator Unit, and Mike Dee from the Groningen Radiocarbon Laboratory for radiocarbon analysis. This research was supported by the Max Planck Society (S.W., A.V.M, W.T., R.H., M.B., A.S., N.B., C.W. and J.H.), a Max Planck Society Donation Award (J.H. and C.W.), the U.S. National Science Foundation (BCS-1523264 to C.W.), and the European Research Council under the European Union’s Horizon 2020 research and innovation programme under grant agreement number 804884-DAIRYCULTURES (C.W.). The protein database used for this research was produced within the framework of ‘FoodTransforms: transformations of food in the Eastern Mediterranean Late Bronze Age’ (ERC-2015-StG 678901-FoodTransforms) funded by the European Research Council. Deamidation analysis was funded by the Arts and Humanities Research Council (grant number AH/N005015/1).
Footnotes
Data Availability
Raw and processed MS/MS data from blanks, instrument washes and samples are available to download via the PRIDE partner repository (https://www.ebi.ac.uk/pride/archive/) under accession code PXD014730 and 10.6019/PXD014730.
Code Availability
MS-MARGE, an R script used to estimate the validity of peptide identifications and summarize the findings is available for use via Bitbucket: https://bitbucket.org/rwhagan/ms-marge/src/master/. The custom dairy database used to analyze the data in this study is available as to download via the York Research Database (https://pure.york.ac.uk/portal/en/) with the DOI: 10.15124/589742eb-287a-4576-a00a-30df33d9f52c. The authors declare that all other data supporting the findings of this study are available within the paper and its supplementary information files.
Author contributions
S.W., W.T.T.T., N.B., C.W. and J.H. designed the research plan; S.W., M.B., S.G., M.H., S.U., E.M., and J.H. assessed archaeological collections, performed osteological assessments and subsampling. S.W., R.H., A.R., C.T., P.N., and J.H. performed laboratory work, mass spectrometry work and data analysis; R.W.H., A.S., A.R., C.T., J.G., P.W.S, and C.W. contributed to the development of data analysis tools; S.W., A.V.M., W.T.T.T., B.K.M., S.U., L.O., E.M., C.W., and J.H. and contributed to archaeological data interpretation. J.H. and S.W. generated the figures. S.W. and J.H wrote the paper with input from W.T.T.T., L.O. and C.W, and final approval from all authors.
Competing interests
The authors declare no competing interests.
References
- 1.Jeong C, et al. Bronze Age population dynamics and the rise of dairy pastoralism on the eastern Eurasian steppe. Proc Natl Acad Sci U S A. 2018;115:E11248–E11255. doi: 10.1073/pnas.1813608115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherratt A. The secondary exploitation of animals in the Old World. World Archaeol. 1983;15:90–104. [Google Scholar]
- 3.Arbuckle BS, Hammer EL. The rise of pastoralism in the ancient Near East. J Archaeol Res. 2018:1–59. [Google Scholar]
- 4.Ishii S, Samejima K. Dietary survey on the diet of Mongolian nomads. Journal of Home Economics of Japan. 1999;50:845–853. [Google Scholar]
- 5.Sherratt A. Plough and Pastoralism: Aspects of the Secondary Products Revolution. Cambridge University Press; 1981. [Google Scholar]
- 6.Vigne JD. Early domestication and farming: what should we know or do for a better understanding? Anthropozoologica. 2015;50:123–150. [Google Scholar]
- 7.Evershed RP, et al. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature. 2008;455:528–531. doi: 10.1038/nature07180. [DOI] [PubMed] [Google Scholar]
- 8.Salque M, et al. Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature. 2013;493:522–525. doi: 10.1038/nature11698. [DOI] [PubMed] [Google Scholar]
- 9.Outram AK, et al. The earliest horse harnessing and milking. Science. 2009;323:1332–1335. doi: 10.1126/science.1168594. [DOI] [PubMed] [Google Scholar]
- 10.Outram AK, et al. Patterns of pastoralism in later Bronze Age Kazakhstan: new evidence from faunal and lipid residue analyses. J Archaeol Sci. 2012;39:2424–2435. [Google Scholar]
- 11.Xie M, et al. Identification of a dairy product in the grass woven basket from Gumugou Cemetery (3800 BP, northwestern China) Quat Int. 2016;426:158–165. [Google Scholar]
- 12.Yang Y, et al. Proteomics evidence for kefir dairy in Early Bronze Age China. J Archaeol Sci. 2014;45:178–186. [Google Scholar]
- 13.Wright J. Households without Houses: Mobility and Moorings on the Eurasian Steppe. J Anthropol Res. 2016;72:133–157. [Google Scholar]
- 14.Janz L, Odsuren D, Bukhchuluun D. Transitions in palaeoecology and technology: hunter-gatherers and early herders in the Gobi Desert. Journal of World Prehistory. 2017;30:1–80. [Google Scholar]
- 15.Kovalev AA, Erdenebaatar D, Rukavishnikova IV. A ritual complex with deer stones at Uushigiin Uvur, Mongolia: composition and construction stages. Archaeology, Ethnology and Anthropology of Eurasia. 2016;44:82–92. [Google Scholar]
- 16.Taylor W, et al. Radiocarbon dating and cultural dynamics across the early pastoral transition in eastern Eurasia. PLoS One. 2019 doi: 10.1371/journal.pone.0224241. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovalex AA, Sergey G, Chuluunbat M. Earliest Europeans in the Heart of Asia: The Chemurchek (Qiemuerqieke) Cultural Phenomenon. Vol. 2 Saint Petersburg State Museum: Institute of the Roerichs; 2015. [Google Scholar]
- 18.Kovalev A. Earliest Europeans in the heart of Asia: The Chemurchek (Qiemuerqieke) cultural phenomenon. Vol. 1 LEMA; 2014. [Google Scholar]
- 19.Kovalev AA, Erdenebaatar D. Discovery of new cultures of the Bronze Age in Mongolia according to the data obtained by the International Central Asian Archaeological Expedition. Current archaeological research in Mongolia. 2009:149–170. [Google Scholar]
- 20.Honeychurch W. The development of cultural and social complexity in Mongolia. In: Habu J, Lape PV, Olsen JW, editors. Handbook of East and Southeast Asian Archaeology. Springer; New York: 2017. pp. 513–532. [Google Scholar]
- 21.Houle JL. Emergent Complexity on the Mongolian Steppe: Mobility, Territoriality, and the Development of Early Nomadic Polities. University of Pittsburgh; 2010. [Google Scholar]
- 22.Allard F, Erdenebaatar D, Olsen S, Cavalla A, Maggiore E. Ritual and horses in Bronze Age and present-day Mongolia: Some preliminary observations from Khanuy Valley. Social orders and social landscapes. 2007:151–162. [Google Scholar]
- 23.Taylor W. Horse demography and use in Bronze Age Mongolia. Quat Int. 2017;436:270–282. [Google Scholar]
- 24.Taylor WTT, et al. Origins of equine dentistry. Proc Natl Acad Sci U S A. 2018;115:E6707–E6715. doi: 10.1073/pnas.1721189115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor WTT, Tuvshinjargal T. Horseback riding, asymmetry, and changes to the equine skull: Evidence for mounted riding in Mongolia’s Late Bronze Age. In: Bartosiewicz L, Gál E, editors. Care or Neglect: Evidence of Animal Disease in Archaeology. Oxbow Books; 2018. pp. 134–154. [Google Scholar]
- 26.Sima Q. Records of the grand historian: Han dynasty. Vol. 65 Columbia University Press; 1993. [Google Scholar]
- 27.de Rachewiltz I. The Secret History of the Mongols: A Mongolian Epic Chronicle of the Thirteenth Century. 2004. [Google Scholar]
- 28.Smith JM., Jr Dietary decadence and dynastic decline in the Mongol Empire. J South Asian Nat Hist. 2000;34 [Google Scholar]
- 29.Warinner C, et al. Direct evidence of milk consumption from ancient human dental calculus. Sci Rep. 2014;4 doi: 10.1038/srep07104. 7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mays S, et al. Lives before and after Stonehenge: An osteobiographical study of four prehistoric burials recently excavated from the Stonehenge World Heritage Site. Journal of Archaeological Science: Reports. 2018;20:692–710. [Google Scholar]
- 31.Charlton S, et al. New insights into Neolithic milk consumption through proteomic analysis of dental calculus. Archaeol Anthropol Sci. 2019 doi: 10.1007/s12520-019-00911-7. [DOI] [Google Scholar]
- 32.Hendy J, et al. Proteomic evidence of dietary sources in ancient dental calculus. Proc Biol Sci. 2018;285 doi: 10.1098/rspb.2018.0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houle JL. Bronze Age Mongolia. Oxford University Press; 2016. [Google Scholar]
- 34.Warinner C, et al. Pathogens and host immunity in the ancient human oral cavity. Nat Genet. 2014;46:336–344. doi: 10.1038/ng.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeter ER, Cleland TP. Glutamine deamidation: an indicator of antiquity, or preservational quality? Rapid Commun Mass Spectrom. 2016;30:251–255. doi: 10.1002/rcm.7445. [DOI] [PubMed] [Google Scholar]
- 36.Mackie M, et al. Palaeoproteomic Profiling of Conservation Layers on a 14th Century Italian Wall Painting. Angew Chem Int Ed Engl. 2018 doi: 10.1002/anie.201713020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers LL. Understanding ancient human population genetics of the eastern Eurasian steppe through mitochondrial DNA analysis: Central Mongolian samples from the Neolithic, Bronze Age, Iron Age and Mongol Empire periods. Indiana University; 2016. [Google Scholar]
- 38.Allentoft ME, et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. [DOI] [PubMed] [Google Scholar]
- 39.Damgaard P, de B, et al. 137 ancient human genomes from across the Eurasian steppes. Nature. 2018;557:369–374. doi: 10.1038/s41586-018-0094-2. [DOI] [PubMed] [Google Scholar]
- 40.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malacarne M, Martuzzi F, Summer A, Mariani P. Protein and fat composition of mare’s milk: some nutritional remarks with reference to human and cow's milk. International Dairy Journal. 2002;12:869–877. [Google Scholar]
- 42.Barlowska J, Szwajkowska M, Litwinczuk Z, Król J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr Rev Food Sci Food Saf. 2011;10:291–302. [Google Scholar]
- 43.Buell PD. Steppe Foodways and History. Asian Med. 2006;2:171–203. [Google Scholar]
- 44.Taylor WTT, Bayarsaikhan J, Tuvshinjargal T. Equine cranial morphology and the identification of riding and chariotry in late Bronze Age Mongolia. Antiquity. 2015;89:854–871. [Google Scholar]
- 45.Taylor WTT, et al. A Bayesian chronology for early domestic horse use in the Eastern Steppe. J Archaeol Sci. 2017;81:49–58. [Google Scholar]
- 46.Brosseder U, Miller BK. Xiongnu Archaeology. Multidisciplinary Perspectives of the First Steppe Empire in Inner Asia. Bonn Contributions to Asian Archaeology. 2011;5 [Google Scholar]
- 47.Miller BK. Power Politics in the Xiongnu Empire. University of Pennsylvania; 2009. [Google Scholar]
- 48.Kuan H. Huan Kuan 桓寬 (1st BCE) In: 王利器 WLC, editor. Discourse on Salt and Iron: Commentaries. 1958. [Google Scholar]
- 49.Ban G. Han shu. Vol. 5 Ding wen shu ju; 1962. [Google Scholar]
- 50.Sima Q. In: Shiji (Records of the grand historian) Watson B, translator. Qin Dynasty Columbia University Press; 1961. [Google Scholar]
- 51.Raynal-Ljutovac K, Lagriffoul G, Paccard P, Guillet I, Chilliard Y. Composition of goat and sheep milk products: An update. Small Rumin Res. 2008;79:57–72. [Google Scholar]
- 52.Salimei E, Fantuz FL. Equid milk for human consumption. Int Dairy J. 2012;24:130–142. [Google Scholar]
- 53.Badarch D, Zilinskas RA. Mongolia Today: Science, Culture, Environment and Development. Routledge; 2015. [Google Scholar]
- 54.Rubruck W. The Mission of Friar William of Rubruck: His Journey to the Court of the Great Khan Mongke, 1253-1255. Hakluyt Society; 1990. [Google Scholar]
- 55.Reading RP, Mix H, Lhagvasuren B, Blumer ES. Status of wild Bactrian camels and other large ungulates in south-western Mongolia. Oryx. 1999;33:247–255. [Google Scholar]
- 56.Hailu Y, et al. Functional and technological properties of camel milk proteins: a review. J Dairy Res. 2016;83:422–429. doi: 10.1017/S0022029916000686. [DOI] [PubMed] [Google Scholar]
- 57.Kappeler SR, Heuberger C, Farah Z, Puhan Z. Expression of the peptidoglycan recognition protein, PGRP, in the lactating mammary gland. J Dairy Sci. 2004;87:2660–2668. doi: 10.3168/jds.S0022-0302(04)73392-5. [DOI] [PubMed] [Google Scholar]
- 58.Keay MG. The Tsaatan reindeer herders of Mongolia: Forgotten lessons of human-animal systems. Encyclopedia of Animals and Humans. 2006:1–4. [Google Scholar]
- 59.Inamura T. The transformation of the community of Tsaatan reindeer herders in Mongolia and their relationships with the outside world. Senri Ethnol Stud. 2005;69:123–152. [Google Scholar]
- 60.Gaunitz C, et al. Ancient genomes revisit the ancestry of domestic and Przewalski’s horses. Science. 2018;360:111–114. doi: 10.1126/science.aao3297. [DOI] [PubMed] [Google Scholar]
- 61.Chambers MC, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 63.Hagan R. Mpi-Shh-Mascot Report Generator. MS-MARGE; 2018. [Google Scholar]
- 64.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 65.Brock F, Higham T, Ditchfield P, Ramsey CB. Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (Orau) Radiocarbon. 2010;52:103–112. [Google Scholar]
- 66.Ramsey CB. Methods for summarizing radiocarbon datasets. Radiocarbon. 2017;59:1809–1833. [Google Scholar]
- 67.Reimer PJ, et al. IntCal13 and marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 68.Hong C, et al. Identification of milk component in ancient food residue by proteomics. PLoS One. 2012;7:e37053. doi: 10.1371/journal.pone.0037053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendy J, et al. Ancient proteins from ceramic vessels at Çatalhöyük West reveal the hidden cuisine of early farmers. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06335-6. 4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greco E, et al. Proteomic analyses on an ancient Egyptian cheese and biomolecular evidence of brucellosis. Anal Chem. 2018;90:9673–9676. doi: 10.1021/acs.analchem.8b02535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.