Abstract
GLUTag, NCI-H716 and STC-1 are cell lines that are widely used to study mechanisms underlying secretion of glucagon-like peptide (GLP-1), but the extent to which they resemble native L-cells is unknown. We used validated immunoassays for 14 different hormones to analyze peptide content (lysis samples; n=9 from different passage numbers) or peptide secretion in response to buffer (baseline), and after stimulation with 50 mM KCl or 10 mM glucose + 10 μM forskolin/3-isobutyl-1-methylxanthine (n=6 also different passage numbers). All cell lines produced and processed proglucagon into GLP-1, GLP-2, glicentin and oxyntomodulin in a pattern (prohormone convertase (PC)1/3 dependent) similar to that described for human gut. All three cell lines showed basal secretion of GLP-1 and -2 which increased after stimulation. In contrast to freshly isolated murine L-cells, all lines also expressed PC2 and secreted large amounts of pancreatic glucagon. Neurotensin and somatostatin storage was low and secretion was not consistently increased by stimulation. STC-1 cells released more glucose-dependent insulinotropic polypeptide (GIP) than GLP-1 at baseline (P<0.01) and KCl elevated its secretion (P<0.05). PYY, which normally co-localizes with GLP-1 in distal L-cells, was not detected in any of the cell lines. GLUTag and STC-1 cells also expressed vasoactive intestinal peptide, but none expressed pancreatic polypeptide or insulin. GLUTag contained and secreted large amounts of cholecystokinin while NCI-H716 did not store this peptide and STC-1 contained low amounts. Our results show that hormone production in cell line models of the L-cell have limited similarity to the natural L-cells.
Keywords: GLP-1 producing cell lines, peptide production, L-cells
Introduction
The gut hormone, glucagon-like peptide-1 (GLP-1), is a key hormone in the regulation of blood glucose and satiety in humans. It is produced by enteroendocrine L-cells located in the gut epithelium, and is particularly known for its incretin actions ie. the enhancement of meal-stimulated insulin secretion. This serves to limit postprandial blood glucose levels(1), and therefore, GLP-1-based drugs are used to treat type 2 diabetes. Many studies investigating the secretion of GLP-1 have utilized animal models and while these are useful for studying physiological processes in the broad sense, they are not always ideal for distinguishing direct vs. indirect effects on the L-cell, or for dissecting the underlying molecular mechanisms coupling stimulus to secretion. Therefore, it was not until the development of three GLP-1 producing cell lines, namely GLUTag, STC-1 and NCI-H716, in the 1990s that the specific molecular mechanisms leading to the secretion of GLP-1 began to be unveiled. Naturally, the physiological relevance of studies with these cell lines crucially depends on their degree of resemblance to natural L-cells, but this has not been characterized in any detail. All three cell-lines are derived from carcinomas; GLUTag and STC-1 cells originated from tumours of the large bowel and small intestine of mice, respectively, while NCI-H716 cells derived from ascites fluid from a human with adenocarcinoma of the colon(2–6).
In the L-cell, prohormone convertase (PC) 1/3 processing of the glucagon precursor, proglucagon, results in the formation of GLP-1, together with GLP-2 and glicentin(1). Glicentin may be processed further into oxyntomodulin (OX) and glicentin-related pancreatic peptide (GRPP). The pancreatic α-cells also produce proglucagon, but here the precursor is predominantly processed by PC2 to generate glucagon and the major proglucagon fragment (7,8). Recently, however, several groups have provided evidence that some populations of L-cells exist in which GLP-1 is co-localized with other hormones not derived from proglucagon. Thus proximal L-cells may also contain cholecystokinin (CCK) and neurotensin (NT), whereas distal L-cells typically also produce peptide YY (PYY)(9–12). None, however, seem to contain somatostatin (SST)(11).
As GLUTag, NCI-H716 and STC-1 cells all are derived from carcinomas, we hypothesized that they may, depending on their respective differentiation state, express, process and secrete hormones that normally are not produced by native L-cells. Furthermore, all of these cells lines are immortal, which also distinguishes them from the natural L-cells. We, therefore, undertook the present study to explore to what extent these ‘L-cell models’ actually resemble natural L-cells by examining their expression and secretion of a panel of intestinal and pancreatic hormones.
Methods
Cell work
Cells were cultured following previously described protocols(13,14). In brief, cells were kept at 37°C, 5% CO2 until 80-85% confluent, then split and transferred to fresh nucleon coated T75flasks (Cat. No. EW-01930-54, Thermo Fisher Scientific, MA, USA) for GLUTag and STC-1 cells, or anti-attachment coated T25 flasks (Cat. No. C6731, Sigma Aldrich, Buchs, SG, Germany) for NCI-H716 cells. Cell medium composition was based on publications from several groups(4,15–18), and comprised 1) for GLUTag: Low glucose (1 g/L) Dulbecco’s modified Eagle’s medium (DMEM) (Cat no. 6046, Sigma Aldrich) supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (v/v) penicillin (10,000 U/ml)/streptomycin (10,000 μg/ml) (p/s) and glutamax (200 mM; Cat. No. 35050061, Gibco, Life Technologies Corporation, CA, USA); 2) for NCI-H716: low glucose (2.0 g/L) 1640 RPMI (Roswell Park Memorial Institute medium) (Cat. No. R8750, Sigma Aldrich) supplemented with 10% (v/v) FBS and 1% (v/v) p/s; and 3) for STC-1: high glucose (4.5 g/L) DMEM (Cat No. 6429, Sigma Aldrich) supplemented with 15% (v/v) horse serum (HS), 2.5% (v/v) FBS.
For secretion studies, cells were plated onto 24 well plates pre-coated with matrigel basement membrane (catalog no. 354234; BD Biosciences, Bedford, MA, USA) 18-24 hours prior to the study. On the following day, cells (~80% confluent) were incubated for 2 hours with buffer (baseline secretion) or buffer to which was added 50 mM KCl or 10 mM glucose and 10 μM forskolin/3-Isobutyl-1-methylxanthine (stimulants). The buffer consisted of 138 mM NaCl, 4.5 mM KCl, 4.2 mM NaHCO3, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES supplemented with 0.1% (wt/vol) fatty acid-free BSA (Cat. no. A-603-10G, Sigma Aldrich); pH = 7. Supernatants were obtained and centrifuged (1,500 x g, 4°C, 5 min) to remove any floating cells or debris. The resulting supernatants were transferred to fresh Eppendorf tubes and samples were either directly processed for hormone quantification while kept on ice or stored at -20°C until analysis. In either case, samples were set up non-extracted.
Lysis samples were obtained by incubating the cells with ice-cold lysis buffer for 5 min while kept on ice, followed by thorough mechanical disruption by scraping, as described previously(13). Lysis buffer consisted of: 1% Igepal CA-630 (Cat.No. I8896, Sigma Aldrich,), 0.12 M sodium deoxycholate monohydrate (Cat. No. D5670, Sigma Aldrich), 0.05 M Tris–HCl (Cat. No.A1087, AppliChem GmbH, Darmstadt, Germany), 0.15 M NaCl and one tablet EDTA-free protease inhibitor cocktail/50 ml (Cat. No.05056489001, F. Hoffmann-La Roche, Basel, Switzerland). Samples were stored at -80ºC, after which peptides were purified using Sep-Pak C18 cartridges (Cat. no. WAT036810, Waters, MA, USA), eluted with 70% ethanol + 0.1% Trifluroroacetic acid (TFA) and dried under a gentle stream of compressed air overnight. The purified samples were reconstituted in sodium phosphate buffer supplemented with 0.1% (w/v) human serum albumin (Cat. No. 12666, Merck KGaA, Darmstadt, Germany). Total protein content was quantified by a BCA protein assay kit (Millipore, cat. no. 71285-3).
Gel filtration analysis
Pooled extracted cell lysis samples (n = 9) were centrifuged (4 ºC, 4 min, 4,500 x g) and the supernatants fractionated by gel filtration on a Sephadex G50SF-packed K16-100 column (Pharmacia, Uppsala Sweden), equilibrated and eluted with the same sodium phosphate buffer as above. Gel filtration effluents were collected automatically in fractions corresponding to approximately 1/50 of the fractionation volume of the column. The column was precalibrated using 125I-labelled albumin (V0), 22Na (Vi) and unlabeled proglucagon-derived peptides: glucagon, GLP-1, GLP-2, oxyntomodulin and glicentin. Coefficients of distribution were calculated as: where Ve is the elution volume for the substance in question, V0 is the Vi exclusion volume and Vi is the available inner volume determined as the difference between the elution volumes of the albumin and sodium calibrators (19). Peptide concentrations were analyzed as described above. In all runs, 22Na and 125I-albumin were added as internal controls. Between runs, the column was washed with buffer; no immunoreactive moieties were detected in fractions from any on these control runs (data not shown).
Biochemical measurements
Protein content in lysis samples was measured with BCA protein assay kit (Cat no. 71285-3, Millipore) according to the manufacturer’s instructions. Hormone contents of lysis and secretion samples were measured by well-characterized in-house radioimmunoassays (RIAs), in-house sandwich ELISA or validated commercially available ELISA. Samples were diluted in the respective assay buffers so that all positive measurements were within the sensitive part of the standard curves. Information regarding assay type (RIA or ELISA), antibody codes, epitopes, sensitivity, specificity and linear range for the assays is provided in supplementary table 1.
Proglucagon derived gut peptides
GLP-1(intact) was measured using an in-house two-site sandwich ELISA involving two monoclonal antibodies: GLP-1 F5 as catching antibody (COOH-terminally directed) and Mab26.1 as a detecting antibody (NH2-terminally- directed)(20). Total GLP-1 was measured with an in-house RIA (antibody 89390) utilizing a C-terminal antibody that binds amidated (x-36amide) but not glycine (x-37) extended GLP-1 isoforms(21). GLP-2 (intact) was measured with an in-house RIA (antibody 92160), which employs an antibody that targets GLP-2 N-terminally(22). For measurements of intact GLP-1 and GLP-2, valine pyrrolidide (a generous gift from Novo Nordisk A/S) was added to the samples (final concentration 0.01 mM) to prevent any DPP-4 mediated degradation. Glicentin/oxyntomodulin was measured by a C-terminally directed in-house RIA with equal affinity for oxyntomodulin and glicentin (antibody 645)(23).
Gut peptides not derived from proglucagon
CCK was measured by an in-house RIA (antibody 92128), which measures all bioactive CCK-forms (i.e., amidated and tyrosyl-O-sulfated CCK-58, -33, -22 and -8) while displaying no cross-reactivity with gastrin (24). 125I-labeled sulfated CCK-8 was used as tracer and CCK-8 as standard (24). Chromogranin A (CgA) was assayed by in-house RIA (antibody:95058) (25) targeting the N-terminus of sequence 340-348 in CgA. Gastrin was measured with an in-house RIA (antibody 2604), which is directed against the C-terminus of gastrin-17 and binds all bioactive (i.e., amidated) gastrins (gastrin -71, -34, -17 and -14) in plasma with equimolar potency without cross-reactivity with CCK peptides(26). GIPtotal was measured using a C-terminally directed antiserum (code: 80867-5), which reacts fully with intact GIP and the N-terminally truncated metabolite GIP (3–42)(27). Intact, bioactive GIP was measured with in-house RIA (antibody 98171), which reacts with the N-terminal part of intact GIP (1–42), and cross-reacts less than 0.1% with GIP (3–42) or the structurally related peptides: GLP-1 (7/9-36)amide, GLP-2 (1–33) or glucagon(28). NT was measured by an in-house N-terminal RIA, thus measuring total NT (antibody: 3D97)(29). For GLUTag and STC-1, PYYtotal was measured using a side-viewing antibody (Bachem, Cat. No. T-4093) that binds murine PYY, with murine/porcine PYY standards and 125I labelled porcine PYY (PerkinElmer, Cat. No. NEX 240)(12). For NCI-H716, PYY total was quantified by in-house RIA (code: MAB8500), reacting with the mid part of human PYY (30). Vasoactive intestinal polypeptide (VIP) was measured by in-house RIA previously described (31).
Pancreatic peptides
For content analysis (fig. 2), glucagon was measured by a commercially available sandwich ELISA (Mercodia, cat. No. 10-1271-01), targeting both the N- and C-terminal of glucagon (thus only measuring fully processed pancreatic glucagon and neither C- nor N-terminally elongated or truncated forms(32)). For gel filtration data, glucagon was measured using an in-house RIA employing an antibody directed against the C-terminal of pancreatic glucagon (antibody: 4305)(33). Insulin was measured by in-house RIAs, reacting with either murine (GLUTag and STC-1) or human (NCI-H716) insulin (antibody 2006-3 and 2004-3, respectively) as described previously (33,34). Pancreatic polypeptide (PP) (total) was measured with in-house RIAs employing antibodies that bind human (antibody: 7198) or murine (antibody: HYB 347-07) PP(35). Somatostatin (SST) was measured with in-house RIA employing a side-viewing antibody (code: 1758-5) which reacts with all molecular forms of SST that possess the mid sequence, including gut-derived SST 1-28 (36).
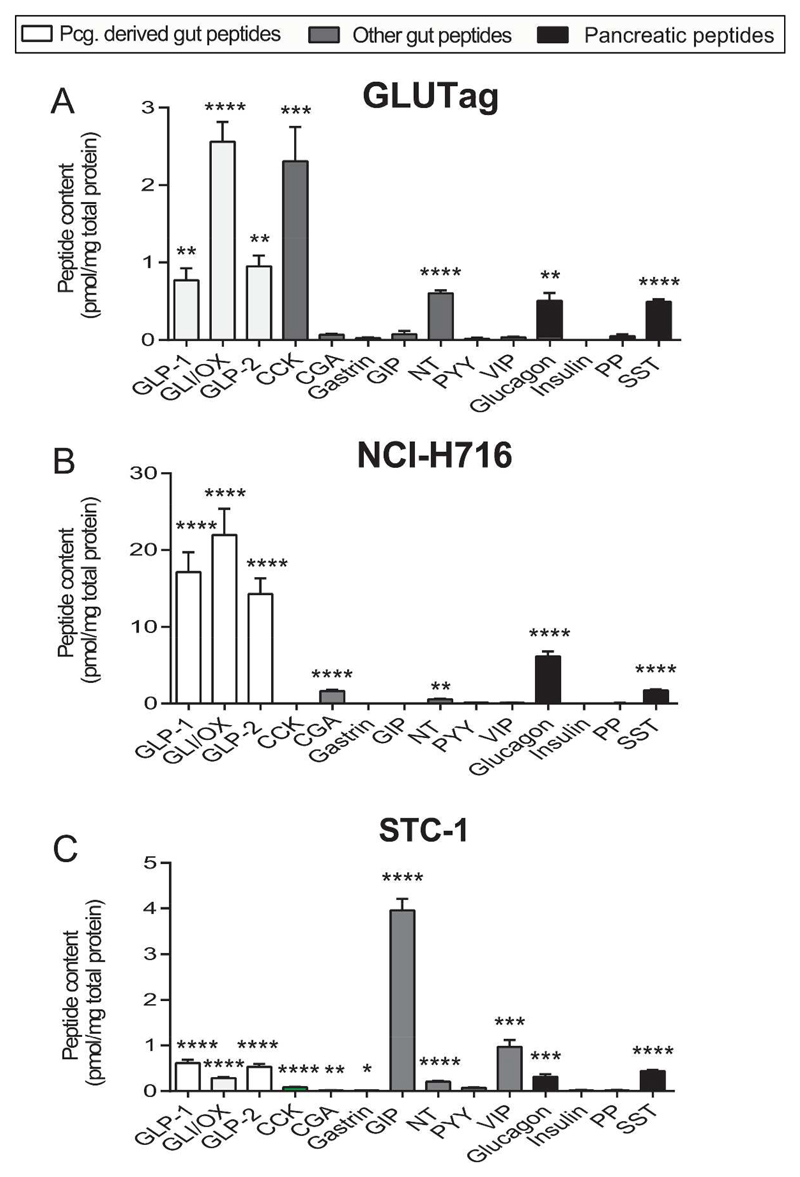
Fig. 2. Gut- and pancreatic hormone contents in GLUTag, NCI-H716 and STC-1 cells.
Data are shown as means ± SEM relative to baseline. Level of significance between baseline and stimulant are indicated with stars; *P < 0.05, **P<0.01, ***P<0.001. N/A: indicates concentrations below lower level of quantification. n = 6. Abbreviations: CCK (active): cholecystokinin, CgA: Chromogranin A, GIP (total): gastric inhibitory peptide, GLI/OX: Glicentin/oxyntomodulin, GLP-1: Glucagon-like peptide-1, GLP-2: Glucagon-like peptide-2, INS: insulin, NT: neurotensin (total), PP: pancreatic polypeptide, PYY: PYY (total), SST: somatostatin (total), VIP: Vasoactive intestinal polypeptide.
Microarray
PC1/3 and -2 expression was determined by microarray. K- and L-cells were isolated by FACS from transgenic mice expressing a fluorescent reporter protein under the control of the preproGIP or the proglucagon promoter, respectively. K- and L-cell negative controls consisted of fluorescence-negative mixed cell populations isolated in parallel from the same preparations and presumably dominated by enterocytes. Further details can be found elsewhere(10,37,38).
Data analysis
Cell content and secretion data (fig. 2 and 3) are presented as means ± 1 SEM, microarray expression data (fig. 1) as means and gel filtration data (fig. 4) as peptide concentrations (pM) in each of the collected fractions (expressed as Kd-values). For cell content data, peptide concentrations (pM) were normalized to protein concentration (mg/l) to adjust for intra-group variation in confluence. Secretion data are expressed as absolute concentration (non-normalized) values (pM) For cell contents, statistical significance was tested by one-sample t-test, for each peptide testing the measured concentrations against the respective assay’s lower limit of detection (2-10 pM, depending on the assay). For secretion, statistical significance was assessed by paired t-test, testing stimuli values against baseline. P < 0.05 was considered significant. Graphs were constructed in Graphpad Prism 5 (La Jolla, CA) and edited in Adobe Illustrator (San Jose, CA).
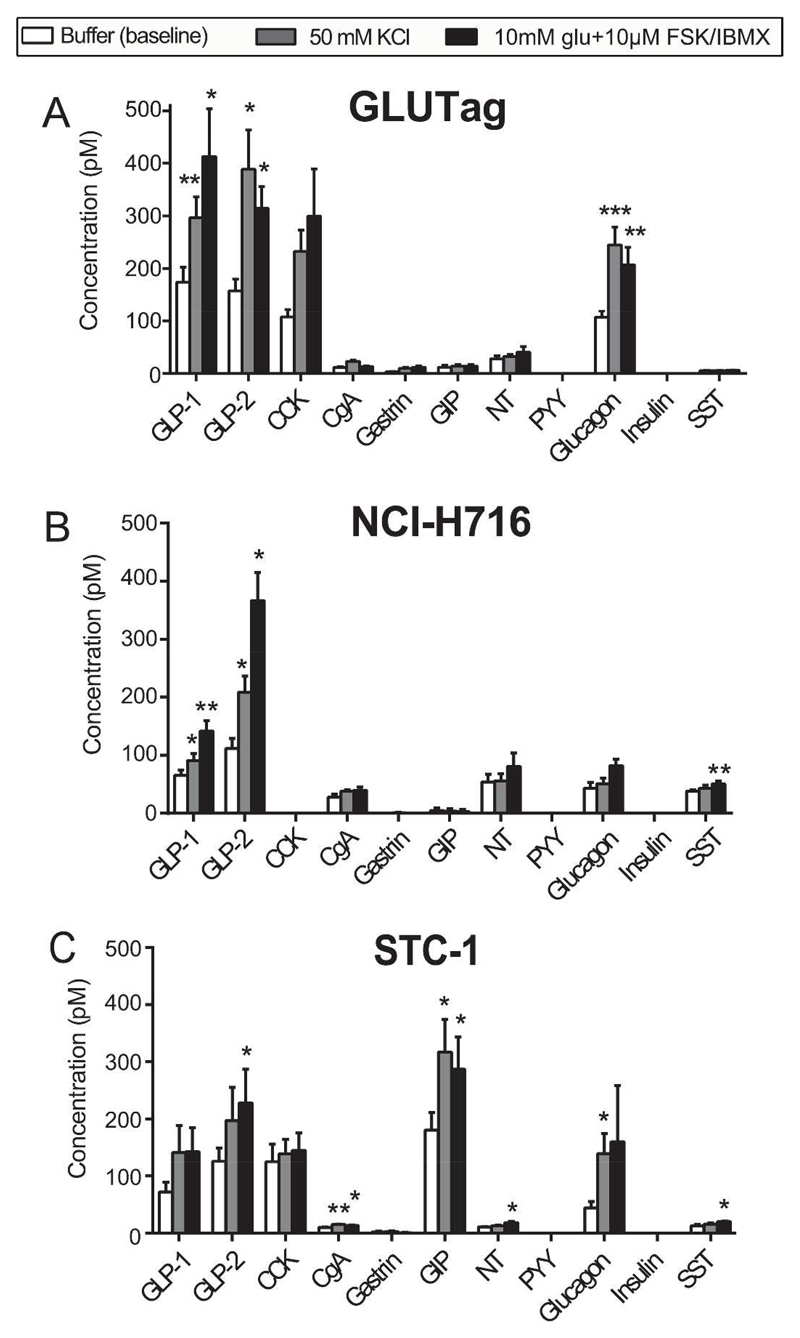
Fig. 3. Secretory repertoire of GLUTag, NCI-H716 and STC-1 cells.
Data are shown as means ± 1SEM. Level of significance between baseline and stimulant are indicated with stars; *P < 0.05, **P<0.01, ***P<0.001. N/A: indicates concentrations below lower level of quantification. n = 6. Abbreviations: CCK: cholecystokinin, CgA: Chromogranin A, GIP: gastric inhibitory peptide, GLI/OX: Glicentin/oxyntomodulin, GLP-1: glucagon-like peptide-1, GLP-2: glucagon-like peptide-2, INS: insulin, NT: neurotensin, PP: pancreatic polypeptide, PYY: peptide YY, SST: somatostatin.
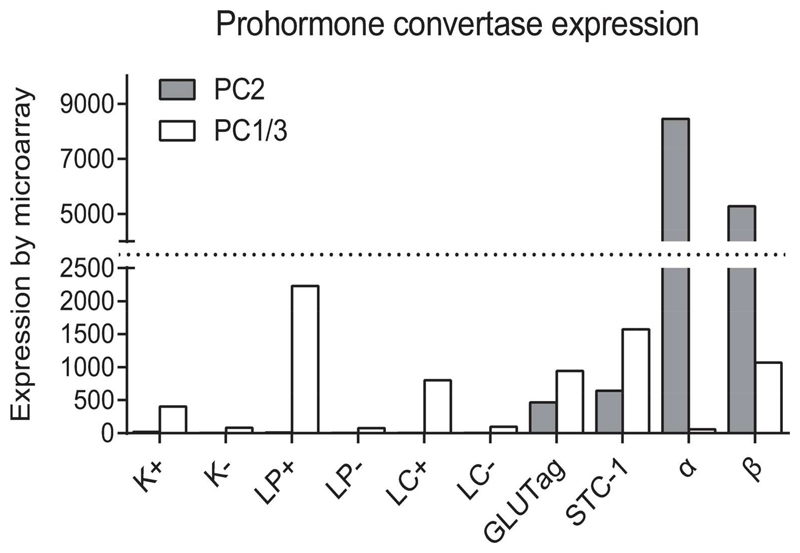
Fig. 1. Microarray expression of Prohormone convertase (PC) 1/3 and 2 in GLUTag and STC-cells, isolated murine K- and L-cells, murine none K- and L-cells, pancreatic α- and β-cells.
Expression levels of PC1/3 and PC2 are shown for FACS sorted mouse K-cells (K+) and proximal L-cells (LP+) collected from the small intestine, and colonic L-cells (LC+), reference tissue (K-, LP- and LC-) collected from identical anatomical sites as K- and L-cells were isolated from, pancreatic mouse α- and β-cells and GLUTag and STC-1 cells.
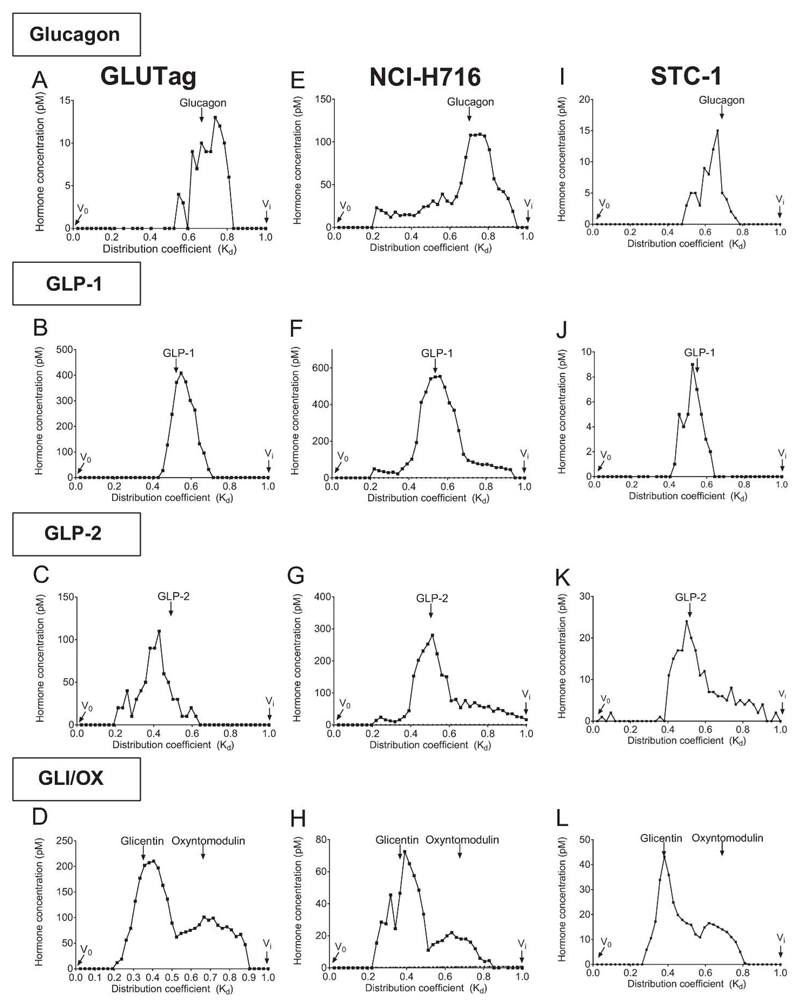
Fig. 4. Processing patterns of proglucagon derived gut peptides.
Gel filtration based characterization of the processing pattern of pro-glucagon. Eluted immunoreactivty (pM) is plotted against coefficient of distribution (Kd). Elution fraction containing the calibrators 22Na and 125I-albumin were defined as V0 and Vi, respectively (indicated on graphs). Representative Kd-values for each of the measured peptides are indicated on top of graphs. For each cell line, 9 extracted cell-lysis samples were pooled.
Results
Expression of PC2 and PC1/3 in primary K- and L-cells, GLUTag and STC-1 cells and α- and β-cells
PC1/3 was expressed by primary murine K-cells and in primary murine L-cells from the small intestine and large intestine, but not in reference tissue collected from same anatomical sites (fig 1). PC2 expression was not detected in K- or L-cells or in reference gut tissue. GLUTag and STC-1 cells expressed PC1/3 but, in addition, both expressed PC2 to about half the level of PC1/3. Primary α-cells from the mouse expressed very high levels of PC2 but no PC1/3, while primary β-cells expressed both PC1/3 and PC2, as expected.
Hormone content of GLUTag, NCI-H716 and STC-1 cells
Overall, the three cell lines varied considerably with respect to the peptides they contained, as well as the relative amounts of each peptide. All three cell lines contained the gut-derived proglucagon products GLP-1, GLP-2 and glicentin/oxyntomodulin. GLP-1 and GLP-2 were present in a 1:1 ratio in all cell lines, but only in NCI-H716 cells were glicentin and oxyntomodulin present in comparable amounts, in the other cells glicentin dominated. All three cell lines also stored glucagon and SST, but neither insulin nor PP. GLUTag cells contained high levels of CCK and NT. NCI-H716 cells also contained low levels of CgA and NT. STC-1 cells contained very high levels of total GIP (fig. 2) and similar levels of intact GIP (data not shown), both significantly higher than the levels of GLP-1 (P < 0.001). In addition STC-1 cells contained comparatively low, but significant concentrations of CCK, gastrin, NT and relatively high concentrations of VIP. All three cell lines were PYY negative.
Secretory repertoire of GLUTag, NCI-H716 and STC-1 cells
GLUTag cells secreted GLP-1, GLP-2 and glucagon in high concentrations and their secretion was enhanced by both stimulants (50 mM KCl and 10 mM glu.+10 μM FSK/IBMX) (fig 3A). CCK was secreted in high concentrations and showed near significant increases after both stimulants compared to baseline (P = 0.054, baseline vs. KCl, P = 0.086, baseline vs. glu. + FSK/IBMX). CgA was secreted in low concentrations but KCl significantly enhanced the secretion compared to baseline. The secretion of NT and SST was not altered by the stimuli. GIP was not secreted in the basal state or in response to the stimuli. NCI-H716 cells secreted GLP-1 and GLP-2 and their secretion was enhanced by both stimulants (fig 3B). NCI-H716 also secreted relatively large amounts of NT, glucagon and SST, but only the secretion of SST was significantly elevated by stimulation and only by glu. + FSK/IBMX. CgA was secreted in low concentrations and its secretion was enhanced by both stimuli. GIP secretion was not detected in any of the treatment groups. STC-1 cells released GLP-1 and GLP-2, but the stimulants only cause a borderline significant elevation of the secretion of GLP-1 compared to basal secretion (P = 0.054 baseline vs. KCl, P = 0.13, baseline vs. glu. + FSK/IBMX), while the GLP-2 response to the positive control reached significance (fig 3C). However, STC-1 cells actually secreted more GIP (measured with both GIP assays) than GLP-1 at baseline (P < 0.01), and its secretion was elevated by both stimulants. CCK was secreted in concentrations similar to GLP-1 and GLP-2, but neither stimulus elevated its secretion compared to baseline. High concentrations of glucagon and low levels of SST and CgA were also secreted by the STC-1 cell at the basal state, and for each, secretion was enhanced by one or both of the stimulants. None of the cell lines secreted PYY.
Processing patterns of proglucagon derived peptides
Gel filtration pattern of extractable glucagon showed that, for all the three cell lines, the majority of immunoreactive material was eluted at the position of unlabeled pancreatic glucagon (Kd ≈ 0.85), although small amounts of larger immunoreactive moieties were also found, particularly in the NCI-H716 cells (19). For GLP-1 and GLP-2, the majority of immunoreactive material was also found at elution positions close to that previously described for synthetic unlabeled GLP-1 and GLP-2 (39)(KdGLP-1 ≈ 0.55 and KdGLP-2 ≈ 0.50 (40)). Single peaks for glicentin and oxyntomodulin were identified for all three cell lines at elution positions close to previously reported Kd values for unlabeled synthetic glicentin and oxyntomodulin (≈ 0.35 and ≈ 0.66, respectively(19)).
Discussion
Enteroendocrine cells are derived from a common pluripotent stem cell that also gives rise to enterocytes, goblet and Paneth cells(41), but there are pronounced differences with respect to the expression pattern of the different gut hormones along the intestine. According to data obtained from immunohistochemical studies of L cells from human, porcine and rat tissue and expression analysis on FACS sorted mouse L-cells, two sub-populations of L-cells seem to exist: a proximal small intestinal population that, in addition to the proglucagon peptides derived from PC1/3 processing, often co-express CCK and/or GIP to some degree, and a more distal L-cell population that co-expresses PYY(9,10,12,42). Supporting this, cell-specific ablation of intestinal L-cells also resulted in dramatic reductions in CCK and PYY expression (mRNA) in proximal and distal small intestine, respectively(43). Similarly, in sorted murine K-cells, PYY was detected by expression analysis in addition to GIP (37). It therefore appears that L-cells are more pluri-hormonal than previously thought.
The GLUTag, NCI-H716 and STC-1 cell lines are frequently used as models to study L-cell physiology, mostly because they are easy to maintain in culture and because no method for culture of pure primary L-cells has been established Such cell lines are useful because they are suitable for cell biological studies for instance live monitoring of the intracellular concentration of free calcium and patch clamp studies(15,44). However, these cell lines also have their limitations, in particular with respect to their lack of the polarization (apical vs. basolateral surfaces) which is characteristic of native L-cells. Furthermore, the translational value of results generated with these cell lines naturally depends on whether the peptide contents and secretory patterns reflect those of natural L-cells. Nevertheless, the peptide profiles of the various cell lines have only sporadically been investigated previously.
We, therefore, characterized the peptide content and secretory repertoire of the three cell lines by assaying for 10 gut- and 4 pancreatic peptides. The major finding is that despite producing GLP-1 and GLP-2 as expected, all three cell lines produce and secrete several other peptides that are not “classical” L-cell products. To the best of our knowledge, this is the first study to show that GLUTag and STC-1 cells express PC2 and that all three cell lines contain and secrete (pancreatic) glucagon, CgA, NT and VIP. Also, this appears to be the first study that has investigated the proglucagon processing in these cell lines.
The detection of SST in both the extracts and incubation medium of all three cell lines was entirely unexpected, since natural L-cells are not thought to express SST(11), as SST producing cells result from an early lineage commitment that solely results in expression of SST. A later lineage commitment then results in cells producing ghrelin/motilin and substance P, while a yet later lineage commitment results in cells expressing CCK, GIP, GLP-1/2, NT and PYY(11).
Unexpectedly, we did not detect any PYY, which often co-localizes with GLP-1 in distal L-cells, in either the lysates or secretion samples from any of the cell lines. A few studies have reported measurable PYY storage in and secretion from STC-1 cells(45) (46), but the study by Geraedts et al. (ref. 45) reports measured concentrations that either are below or at the lower level of detection as reported by the assay manufacturers. However, given that the other peptides measured in our study were present at very high levels, combined with the lower limit of detection sensitivity (< 1 pM) of the applied assays for PYY measurement in the present study, we consider it unlikely that our results are erroneous. Instead, this cell line may show variability with respect to PYY expression.
Surprisingly, the peptide that was stored and released in the highest amounts from the STC-1 cells was GIP, with equivalent concentrations being measured with both N- and C-terminally directed assay (data only shown for intact GIP). Expression of GIP in STC-1 cells has been demonstrated previously although only ~7% of the cells in the tumors that gave rise to the cell line stained for GIP(6). Subsequently a GIP enriched subclone (STC-6-14) was established, exhibiting glucose dependent GIP-secretion, which was further enhanced when co-secreted somatostatin was immune-neutralized (47). Other STC-1 derived cell lines engineered to expressed insulin under control of the GIP-promoter secreted insulin in response to a number of stimuli, although the cell lines differed in their glucose responsiveness (47–49).GIP secretion has also been reported from GLUTag cells in response to the artificial sweetener sucralose (50), although sucralose failed to stimulate GIP secretion from mixed epithelial cultures (37). In this study we did not detect significant amounts of stored or secreted GIP in GLUTag and NCI-H716 cells, in agreement with our previously reported failure to detect GIP mRNA in GLUTag cells (10).
All three cell lines also contained and secreted glucagon in relatively high amounts, consistent with the observation that GLUTag and STC-1 cells, like the pancreatic α-cell (but unlike natural L-cells), express PC2. The finding was not due to cross reactivity of glicentin in the glucagon assay (the entire glucagon sequence is contained in the middle part of glicentin), but the assay employed is highly specific for fully processed pancreatic glucagon, and detects neither glicentin nor oxyntomodulin (nor any elongated or truncated splice variants of glucagon)(32). In accordance with a previous study in GLUTag and STC-1 cells (4), insulin was not detected in any of the cell lines. Our results also confirm data from other studies regarding the presence of GLP-2 in GLUTag and STC-1 cells, and the ability of GLUTag and in particular STC-1 cells, to produce and secrete CCK (46,51–54).
A major strength of our study is that secretion studies were included, since cellular storage of peptide hormones does not necessarily correlate to secretory output, as peptide secretion for instance depends of activation of the exocytotic machinery by molecular sensing mechanisms and intra-cellular sorting of the peptide into secretory vesicles which then have to be recruited to the rapidly releasable vesicle pool. Emphasizing this, the cell lines differed considerably in the amounts of peptides stored (6-20 times higher for NCI-H716 cells), whereas the amounts actually secreted differed only 2-3 fold. Another strength of this study is that we also analyzed the processing pattern of the proglucagon derived peptides. In summary, our analysis indicates that all three cell lines have the same PC1/3 dependent processing pattern of the proglucagon precursor into GLP-1 and -2, GLI and OX as that described for man and pig (19,39,40,55), although unlike native L-cells, all 3 cell lines also stored and secreted glucagon.
Comparing the 3 cell lines, GLUTag and STC-1 cells (derived from mice) contain and secrete more different peptides than the (human) NCI-H716 cells, while NCI-H716 cells contain and secrete larger amounts of the expressed peptides. Of the three cell lines tested, STC-1 cells resemble L-cells the least, since GLP-1 secretion was only weakly elevated by the stimulants, whereas GIP was both expressed and secreted in high concentrations. Therefore, this cell line may be less suitable for GLP-1 secretion studies. GLUTag cells also express and secrete high levels of CCK but not PYY, which suggest that they could be used as a model for proximal rather than distal native L-cells. NCI-H716 showed the most restricted peptide pattern, expressing neither CCK nor PYY but storing and secreting SST. Therefore, NCI-716 cells seem to resemble neither proximal nor distal L-cells. Supporting that NCI-H716 cells represent an aberrant stage of differentiation, another study concluded that the expression of proglucagon in this cell line does not seem to be regulated by the same mechanisms as those activating human and rat proglucagon promoters(56). It would be of immense scientific interest to study the secretory products from isolated native L-cells in response to different stimuli, but unfortunately, attempts to culture isolated (mouse) L-cells have been unsuccessful. In conclusion, results obtained with the cell lines investigated here should be interpreted with some caution and preferably validated in more physiological models including in vivo studies, or studies using isolated perfused organs, where hormone secretion can be assessed directly from the relevant organ while vasculature, paracrine relationships and innervations are preserved.
Supplementary Material
Acknowledgements
This study was supported by a grant to JJH from the Novo Nordisk Centre for Basic Metabolic Research (Novo Nordisk Foundation, Denmark). The funding body had no influence on the design, conduction, interpretation or other aspects of this study. Work in the Reimann/Gribble laboratories was supported by the Wellcome Trust (WT106262/Z/14/Z and WT106263/Z/14/Z) and the MRC (grant MRC_MC_UU_12012
Financial support
This study was supported by a grant to JJH from the Novo Nordisk Centre for Basic Metabolic Research (Novo Nordisk Foundation, Denmark). The funding body had no influence on the design, conduction, interpretation or other aspects of this study.
Footnotes
Author contributions
R.E.K., N.J.W.A and J.J.H. were responsible for idea and design of the study, R.E.K. N.J.W.A, E.B.M, J.F.R, F.R and F.M.G produced and analyzed data. R.E.K. Prepared figures and drafted the manuscript; N.J.W.A, CFD, E.B.M, F.R., F.M.G, J.F.R and J.J.H edited and revised manuscript. R.E.K, CFD, E.B.M., F.R., F.M.G., J.F.R, J.J.H. approved final version of the manuscript.
Disclosures
The authors declare no financial or other conflicts of interests.
Prior Presentation
Some of the findings of this study were presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5-9 June 2015.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Grant SGN, Seidman I, Hanahan D, Bautch VL. Early Invasiveness Characterizes Metastatic Carcinoid Tumors in Transgenic Mice. Cancer Research. 1991;51:4917–4923. [PubMed] [Google Scholar]
- 3.Lee YC, Asa SL, Drucker DJ. Glucagon gene 5'-flanking sequences direct expression of simian virus 40 large T antigen to the intestine, producing carcinoma of the large bowel in transgenic mice. J Biol Chem. 1992;267:10705–10708. [PubMed] [Google Scholar]
- 4.Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8:1646–1655. doi: 10.1210/mend.8.12.7535893. [DOI] [PubMed] [Google Scholar]
- 5.Bruïne A, Dinjens WM, Pijls MJ, Linden EM, Rousch MM, Moerkerk P, Goeij APM, Bosnian F. NCI-H716 cells as a model for endocrine differentiation in colorectal cancer. Virchows Archiv B Cell Pathol. 1992;62:311–320. doi: 10.1007/BF02899698. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Grant GN, Yiangou Y, Ghatei MA, Bloom SR, Bautch VL, Solcia EP, JM Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Am J Pathol. 1990;136:1349–1363. [PMC free article] [PubMed] [Google Scholar]
- 7.Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. Proglucagon processing in porcine and human pancreas. Journal of Biological Chemistry. 1994;269:18827–18833. [PubMed] [Google Scholar]
- 8.Holst JJ. Glucagon and glucagon-like peptides 1 and 2. Results Probl Cell Differ. 2010;50:121–135. doi: 10.1007/400_2009_35. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 10.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM. Overlap of Endocrine Hormone Expression in the Mouse Intestine Revealed by Transcriptional Profiling and Flow Cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer E-M, Olsen J, Sundler F, et al. A Major Lineage of Enteroendocrine Cells Coexpress CCK, Secretin, GIP, GLP-1, PYY, and Neurotensin but Not Somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Torang S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156:847–857. doi: 10.1210/en.2014-1710. [DOI] [PubMed] [Google Scholar]
- 13.Kuhre RE, Albrechtsen NW, Windeløv JA, Svendsen B, Hartmann B, Holst JJ. GLP-1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP-1 secreting cell lines. Peptides. 2014;55:6. doi: 10.1016/j.peptides.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Kuhre RE, Gribble FM, Hartmann B, Reimann F, WJ A, Rehfeld JF, Holst JJ. Fructose stimulates GLP-1 but not GIP secretion in mice, rats and humans. Am J Physiol Gastrointest Liver Physiol. 2014;306:G622–G630. doi: 10.1152/ajpgi.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimann F, Gribble FM. Glucose-Sensing in Glucagon-Like Peptide-1-Secreting Cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 16.Eiki J, Saeki K, Nagano N, Iino T, Yonemoto M, Takayenoki-Iino Y, Ito S, Nishimura T, Sato Y, Bamba M, Watanabe H, et al. A selective small molecule glucagon-like peptide-1 secretagogue acting via depolarization-coupled Ca(2+) influx. J Endocrinol. 2009;201:361–367. doi: 10.1677/JOE-08-0528. [DOI] [PubMed] [Google Scholar]
- 17.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 18.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ. Molecular heterogeneity of glucagon in normal subjects and in patients with glucagon-producing tumours. Diabetologia. 1983;24:359–365. [PubMed] [Google Scholar]
- 20.Wewer Albrechtsen NJ, Bak MJ, Hartmann B, Christensen LW, Kuhre RE, Deacon CF, Holst JJ. Stability of glucagon-like peptide 1 and glucagon in human plasma. Endocr Connect. 2015;4:50–57. doi: 10.1530/EC-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21:73–80. doi: 10.1016/s0196-9781(99)00176-x. [DOI] [PubMed] [Google Scholar]
- 23.Wewer Albrechtsen NJ, Torang S, Hornburg D, Albrechtsen R, Janus C, Jepsen SL, Veedfald S, Meissner F, Mann M, Deacon CF, Holst JJ, et al. A Novel Immune-based Approach for Measurement of the Anorectic Gut Hormone Oxyntomodulin: Changes after Gastric Bypass Surgery. Diabetes. 2015;64:A532. [Google Scholar]
- 24.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem. 1998;44:991–1001. [PubMed] [Google Scholar]
- 25.Børglum Jensen T, Hilsted L, Rehfeld JF. Library of Sequence-specific Radioimmunoassays for Human Chromogranin A. Clinical Chemistry. 1999;45:549–560. [PubMed] [Google Scholar]
- 26.Stadil F, Rehfeld JF. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8:101–112. [PubMed] [Google Scholar]
- 27.Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept. 1984;9:35–46. doi: 10.1016/0167-0115(84)90005-3. [DOI] [PubMed] [Google Scholar]
- 28.Deacon CF, Nauck MA, Meier J, Hucking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 29.Kuhre RE, Bechmann LE, Wewer Albrechtsen NJ, Hartmann B, Holst JJ. Glucose stimulates neurotensin secretion from the rat small intestine by mechanisms involving SGLT1 and GLUT2 leading to cell depolarization and calcium influx. Am J Physiol Endocrinol Metab. 2015 doi: 10.1152/ajpendo.00012.2015. ajpendo 00012 02015. [DOI] [PubMed] [Google Scholar]
- 30.Torang S, Veedfald S, Rosenkilde MM, Hartmann B, Holst JJ. The anorexic hormone Peptide YY3-36 is rapidly metabolized to inactive Peptide YY3-34 in vivo. Physiological reports. 2015;3 doi: 10.14814/phy2.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahrenkrug J, Schaffalitzky de Muckadell OV. Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med. 1977;89:1379–1388. [PubMed] [Google Scholar]
- 32.Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windelov JA, Plamboeck A, Bojsen-Moller KN, Idorn T, Feldt-Rasmussen B, Knop FK, Vilsboll T, Madsbad S, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57:1919–1926. doi: 10.1007/s00125-014-3283-z. [DOI] [PubMed] [Google Scholar]
- 33.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand CL, Jorgensen PN, Knigge U, Warberg J, Svendsen I, Kristensen JS, Holst JJ. Role of glucagon in maintenance of euglycemia in fed and fasted rats. Am J Physiol. 1995;269:E469–477. doi: 10.1152/ajpendo.1995.269.3.E469. [DOI] [PubMed] [Google Scholar]
- 35.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M, Madsen JL, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. International journal of obesity (2005) 2013;37:1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 36.Holst JJ, Bersani M. Assays for Peptide Products of Somatostatin Gene Expression. Metods Neurosciences. 1991;5:3–22. [Google Scholar]
- 37.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ørskov C, Jeppesen J, Madsbad S, Holst J. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buhl T, Thim L, Kofod H, Orskov C, Harling H, Holst JJ. Naturally occurring products of proglucagon 111-160 in the porcine and human small intestine. J Biol Chem. 1988;263:8621–8624. [PubMed] [Google Scholar]
- 41.Roth KA, Kim S, Gordon JI. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol. 1992;263:G174–180. doi: 10.1152/ajpgi.1992.263.2.G174. [DOI] [PubMed] [Google Scholar]
- 42.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen J, Ugleholdt RK, Jørgensen SM, Windeløv JA, Grunddal KV, Schwartz TW, Füchtbauer EM, Poulsen SS, Holst PJ, Holst JJ. Glucose metabolism is altered after loss of L cells and α-cells but not influenced by loss of K cells. American Journal of Physiology - Endocrinology And Metabolism. 2013;304:E60–E73. doi: 10.1152/ajpendo.00547.2011. [DOI] [PubMed] [Google Scholar]
- 44.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 45.Geraedts MC, Troost FJ, Saris WH. Peptide-YY is released by the intestinal cell line STC-1. Journal of food science. 2009;74:H79–82. doi: 10.1111/j.1750-3841.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 46.Hand KV, Bruen CM, O'Halloran F, Panwar H, Calderwood D, Giblin L, Green BD. Examining acute and chronic effects of short- and long-chain fatty acids on peptide YY (PYY) gene expression, cellular storage and secretion in STC-1 cells. Eur J Nutr. 2013;52:1303–1313. doi: 10.1007/s00394-012-0439-9. [DOI] [PubMed] [Google Scholar]
- 47.Kieffer TJ, Huang Z, McIntosh CH, Buchan AM, Brown JC, Pederson RA. Gastric inhibitory polypeptide release from a tumor-derived cell line. Am J Physiol. 1995;269:E316–322. doi: 10.1152/ajpendo.1995.269.2.E316. [DOI] [PubMed] [Google Scholar]
- 48.Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. Journal of cellular physiology. 2002;192:339–350. doi: 10.1002/jcp.10139. [DOI] [PubMed] [Google Scholar]
- 49.Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-Dependent Insulin Release from Genetically Engineered K Cells. Science. 2000;290:1959–1962. doi: 10.1126/science.290.5498.1959. [DOI] [PubMed] [Google Scholar]
- 50.Margolskee R, Dyer J, Kokrashvili Z, Salmon K, Ilegems E, Daly K, Maillet E, Ninomiya Y, Mosinger B, Shirazi-Beechey S. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:6. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordier-Bussat M, Bernard C, Haouche S, Roche C, Abello J, Chayvialle JA, Cuber JC. Peptones stimulate cholecystokinin secretion and gene transcription in the intestinal cell line STC-1. Endocrinology. 1997;138:1137–1144. doi: 10.1210/endo.138.3.5023. [DOI] [PubMed] [Google Scholar]
- 52.Sidhu SS, Thompson DG, Warhurst G, Case RM, Benson RS. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J Physiol. 2000;528(1):165–176. doi: 10.1111/j.1469-7793.2000.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemoz-Gaillard E, Bosshard A, Regazzi R, Bernard C, Cuber JC, Takahashi M, Catsicas S, Chayvialle JA, Abello J. Expression of SNARE proteins in enteroendocrine cell lines and functional role of tetanus toxin-sensitive proteins in cholecystokinin release. FEBS Lett. 1998;425:66–70. doi: 10.1016/s0014-5793(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 54.Hand KV, Bruen CM, O'Halloran F, Giblin L, Green BD. Acute and chronic effects of dietary fatty acids on cholecystokinin expression, storage and secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res. 2010;54(Suppl 1):S93–S103. doi: 10.1002/mnfr.200900343. [DOI] [PubMed] [Google Scholar]
- 55.Orskov C, Holst JJ, Poulsen SS, Kirkegaard P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia. 1987;30:874–881. doi: 10.1007/BF00274797. [DOI] [PubMed] [Google Scholar]
- 56.Cao X, Flock G, Choi C, Irwin DM, Drucker DJ. Aberrant regulation of human intestinal proglucagon gene expression in the NCI-H716 cell line. Endocrinology. 2003;144:2025–2033. doi: 10.1210/en.2002-0049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.