Abstract
Background
Larger portion sizes (PS) are associated with greater energy intake (EI), but little evidence exists on the appetitive effects of PS reduction.
Objective
To investigate impact of reducing breakfast PS on subsequent EI, postprandial gastrointestinal hormone responses and appetite ratings.
Design and Methods
Randomised crossover design (n=33 adults; mean BMI 29kg/m2) providing a set breakfast based on 25% of gender-specific estimated daily energy requirements; PS reduced by 20% and 40%. EI measured at an ad libitum lunch (240mins) and snack (360mins), and by weighed diet diaries until bed. Blood was sampled until lunch in 20 participants. Appetite ratings were measured using visual analogue scales.
Results
EI at lunch (control:2930±203; 20% reduction:2853±198; 40% reduction:2911±179kJ) and over the whole day except breakfast (control:7374±361; 20% reduction:7566±468; 40% reduction:7413±417kJ) did not differ. Postprandial PYY, GLP-1, GIP, insulin and fullness profiles were lower and hunger, desire to eat and prospective consumption higher following 40% reduction compared to control. Appetite ratings profiles, but not hormone concentrations were associated with subsequent EI.
Conclusions
Smaller portions at breakfast led to reductions in gastrointestinal hormone secretion but did not affect subsequent energy intake, suggesting small reductions in portion size may be a useful strategy to constrain EI.
Keywords: Portion size, appetite, gastrointestinal hormones, energy intake
Introduction
Concurrent with increasing prevalence of obesity has been increased mass of food consumed per eating occasion(1) and the size of commercially available portions(2). Empirical evidence shows larger portion sizes (PS) lead to greater energy intake (EI) at single meals; an effect that continues through 11 days of manipulation(3,4). Reducing PS is a central component in weight management advice, but experimental work to investigate whether PS reduction leads to changes in subsequent EI is limited. Given the asymmetry of appetite control and homeostatic mechanisms to achieve energy balance(5), energy compensation may occur in an environment where food is widely available. Gastrointestinal hormones and the perception of appetite are important biological and psychological appetitive mechanisms. There has been little simultaneous research of these mechanisms and energy intake responses following modest portion size manipulations of mixed meals.
Moreover, most research in relation to the effect of portion size on intake has been conducted among individuals with a healthy weight where appetite control systems may be at their most robust. In contrast, overweight and obese individuals are unstudied yet represent the population most in need of interventions to constrain energy intake. This study investigated whether reducing the PS of a meal is an effective strategy to reduce day-long EI in overweight and obese adults. This study also investigated the impact on gastrointestinal hormones and appetite ratings as measures of biological and psychological appetite control mechanisms.
Methods
Study Design
This was a randomised crossover design involving three PS conditions, presented to each participant at a standardised breakfast time on separate days: a control PS; PS reduced by 20%; and PS reduced by 40%. The control provided 25% of estimated daily energy requirements for the intended average study participant according to gender(6), the energy content of a representative main meal. The test breakfast was consumed in its entirety. Participants were blinded to the specific aims of the study and foods prepared to draw attention away from the intervention. For each individual, study visits were conducted >1week apart, on the same weekday and outside the luteal phase of the menstrual cycle for females.
Participants
Healthy, 18-60y men and women, with a BMI ≥25 and <35kg/m2 were recruited. Participants were excluded for disordered eating (Eating Attitudes Test (EAT-26) score ≥11(7–8)), depressive symptoms (Zung Depression Scale score ≥70(9)), smoking, excessive habitual alcohol intake (women >14units/week, men >21units/week), weight loss/gain within last three months (>4.5kg) or actively trying to lose/gain weight, medical conditions/medications potentially affecting appetite, inflammatory conditions, diabetes or fasting plasma glucose ≥7mmol/l, pregnancy, breastfeeding or planning pregnancy, extremely high levels of exercise (moderate/vigorous activity >420min/week assessed with International Physical Activity Questionnaire (IPAQ)(10)), unable to eat test foods, and not regularly consuming breakfast (breakfast ≤3/week).
A sample size of 33 was recruited to give 83% power to detect a minimum difference of 500kJ lunch EI between any pair of experimental conditions assuming an SD of 950kJ(3,11,12). Biochemical measures were collected in a sub-group of 20 participants.
Recruitment and screening
Participants were recruited from the community, for a study investigating the “relationship between diet and metabolism”. Height, weight, waist circumference, body composition (Tanita body composition analyser BC-418MA), and resting metabolic rate (RMR; IS Gem204 with GEMNutrition2008.4 software) were measured. Participants completed the EAT-26, Zung depression scale, IPAQ and the Three Factor Eating Questionnaire (TFEQ) measuring dietary restraint, disinhibition, and hunger traits(13) and fasting plasma glucose was assessed. Participants were asked to maintain their usual exercise and dietary habits during the study.
Study visits
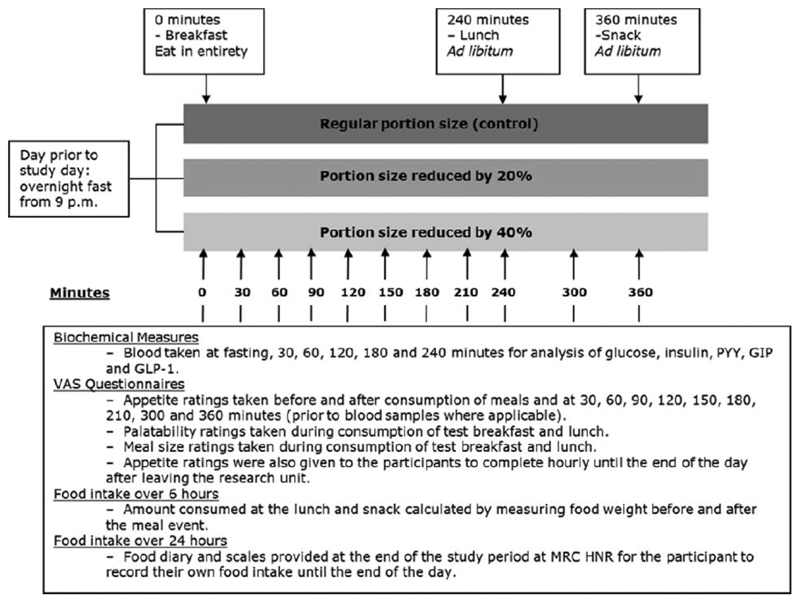
Participants fasted overnight (11h prior to each visit) and were asked to refrain from alcohol and avoid strenuous exercise the 24h before each study day. Provision of the test breakfast marked time zero. Subsequent EI was measured by pre- and post-meal weighing of an ad libitum lunch (240min) and afternoon snack (360min), plus a weighed diet diary to record the remainder of the day’s intake. Visual analogue scale (VAS) questionnaires rating palatability and portion size were given during breakfast and lunch. Appetite ratings were measured using VAS questionnaires at 30min intervals until lunch, then immediately after and at 300 and 360min, then hourly. In a subgroup of 20 participants, blood samples were collected at fasting and 30, 60, 120, 180 and 240mins for the analysis of peptide tyrosine tyrosine 3-36 (PYY3-36), total glucagon-like peptide-1 (GLP-1), total glucose-dependent insulinotropic peptide (GIP), glucose and insulin (Figure 1).
Figure 1.
Overview of the time points for meals and measurements taken during a study day (GIP: glucose-dependent insulinotropic peptide; GLP-1: glucagon-like peptide 1; MRC HNR: Medical Research Council Human Nutrition Research; PYY: peptide tyrosine tyrosine; VAS: visual analogue scales).
At the end of the study participants were fully debriefed on the study aims and asked to verbally report if they had noticed the portion size manipulation between visits. They were reimbursed for travel expenses and given an honorarium. Ethical approval for the study was obtained from Cambridgeshire 2 Research Ethics Committee in November 2010 (Ref: 10/H0308/99) and participants gave informed written consent. The study was conducted at Medical Research Council Human Nutrition Research (MRC HNR) between January 2011 and September 2012.
Study foods
The study breakfast and lunch provided 35% energy from fat, 18% from protein and 47% from carbohydrates(14). The breakfast consisted of a wheat-based breakfast cereal with semi-skimmed milk, scrambled egg, ham, brown toast and butter, and orange juice. For men and women respectively, the control breakfast provided 3310kJ and 2540kJ; the 20% reduction breakfast provided 2650kJ and 2030kJ; the 40% reduction breakfast provided 1990kJ and 1520kJ. The ad libitum lunch consisted of a single course amorphous meal of pasta, minced beef, tomato sauce, mixed vegetables and grated cheese. The lunch provided 8275kJ(men) or 6350kJ(women). The ad libitum snack consisted of ten digestive biscuits (plain cookies) on a plate(3250kJ).
The completed diet diaries and weighed consumption at lunch and snack for each study day were used to calculate energy intake using MRC HNR’s dietary assessment system.
Questionnaires
The appetite VAS questionnaires rated hunger, fullness, desire to eat and prospective consumption, and also included five distractor questions on mood. The palatability questionnaire used VAS to rate palatability parameters, as distractor questions, and portion size. The VAS questionnaires asked participants to mark a horizontal line measuring 100mm with the ends labelled with the extremes of each sensation (e.g. “Not at all” and “Extremely”). The portion size question was “How large is the size of the portion?” anchored with “Extremely small” and “Extremely large”. The distance from the left end to the mark was measured to the nearest millimetre.
Analytic methods
Blood samples were separated on collection and plasma stored at -80°C. Plasma collected on EDTA and treated with dipeptidyl peptidase-IV (DPP-IV) inhibitor immediately on collection (10μl DPP-IV inhibitor/ml of blood) were analysed for PYY3-36 by radioimmunoassay (Millipore®, Massachusetts, USA) (interassay CVs: 15% at 84pg/ml; 7% at 217pg/ml), at University College Hospital, London; total GLP-1 using an electrochemical luminescence immunoassay kit on the MesoScale Discovery® multi-array assay platform (Maryland, USA) (CVs: 16.4%, 11.9% and 11.6% at 5.4pg/ml, 29pg/ml and 83pg/ml respectively), at Core Biochemical Assay Laboratory (CBAL), Cambridge; and total GIP using an enzyme-linked immunosorbent assay (Millipore®, Massachusetts, USA) (CVs: 6.1%, 3.3%, 2.3% and 1.8% at 26pg/ml, 50pg/ml, 134pg/ml and 166pg/ml respectively), at Cambridge Institute for Medical Research. Plasma collected on fluoride oxalate were analysed for glucose using a Dimension® clinical chemistry system (Siemens, Newark, USA) (CVs: 1.69%, 2.23% and 2.56% at 6.23mmol/L, 3.09mmol/L and 18.88mmol/L respectively), at MRC HNR. Plasma collected on lithium heparin were analysed for insulin on a 1235 AutoDELFIA® automatic immunoassay analyzer using a two-step time resolved fluorometric assay (Perkin Elmer Life Sciences, Wallac Oy, Turku, Finland) (CVs: 3.1%, 2.1%, 1.9% and 2.0% at29pmol/L, 79.4pmol/L, 277pmol/L and 705pmol/L respectively) at CBAL, Cambridge.
Statistical analysis
Mixed effects models for continuous responses(15) were used for analysis, which extend standard linear regression to account for within-person variation through random effects. EI and perceived PS at breakfast were modelled with PS condition as the explanatory variable, controlling for gender and BMI. Dietary restraint, disinhibition and hunger, were tested for inclusion as covariates, but were omitted for no effects on the associations of interest.
The effect of PS condition on biochemical measures and appetite ratings was assessed by the interaction between condition and time, which estimated differences at each time-point. Area under the curve (AUC) was calculated using the trapezoidal rule for the time periods of fasting to the pre-lunch time-point for biochemical measures and appetite ratings, and over the whole day for appetite ratings. Models of whole-day appetite ratings AUC included PS condition as the explanatory variable, controlling for time over which appetite ratings were made.
Models predicting EI at lunch included explanatory variables of either the pre-lunch or AUC for each biochemical measure or appetite rating, and controlled for condition, gender and BMI. Similar models assessed the relationship between the whole-day AUC of appetite rating with whole day EI (except breakfast), also controlling for time over which appetite ratings were made.
To examine the relationship between biochemical measures and appetite ratings, appetite ratings were modelled separately with each biochemical measure as the explanatory variable. Time, a quadratic term for time, condition, gender and BMI were covariates.
Potential carry-over and sequence effects, gender, BMI and age, unless specified above as included a priori, were omitted as covariates as there were no effects on the associations of interest. To account for correlation induced by multiple observations/individual (three visits), a random intercept was incorporated into the models. The models for biochemical and appetite ratings profiles as outcomes had two levels of clustering due to repeated sampling time-points and the crossover design. Therefore, a random intercept and slope for time were added to model within-individual variation. Models were fitted using maximum likelihood estimation and likelihood ratio tests were used for model comparison. Plots of residuals were used to check the goodness of fit for each outcome. Insulin and GIP data were transformed (natural logarithm and square root respectively) for analyses, for a symmetrical distribution. All analyses used STATA®12.0 software (StataCorp, Texas, USA). Statistical significance was set at p<0.05. Data are presented as mean±SEM unless indicated otherwise.
Results
Participant characteristics
The characteristics of the study participants are shown in Table 1.
Table 1. Participant characteristics.
| Participant characteristic | All participants (n=33) | Blood sample subgroup (n=20) | Non-blood subgroup (n=13) |
|---|---|---|---|
| Number of men/women | 15/18 | 9/11 | 7/6 |
| Height (m) | 1.69 ± 0.01 | 1.69 ± 0.01 | 1.71 ± 0.03 |
| Weight (kg) | 83.8 ± 1.5 | 82.9 ± 2.1 | 85.3 ± 2.0 |
| BMI (kg/m2) | 29.0 ± 0.4 | 29.0 ± 0.5 | 29.2 ± 0.8 |
| Age (years) | 42.5 ± 2.0 | 40.8 ± 2.5 | 45 ± 3.4 |
| Dietary restraint | 7.2 ± 0.7 | 6.5 ± 0.9 | 8.2 ± 1.1 |
| Disinhibition | 6.7 ± 0.6 | 6.5 ± 0.7 | 6.9 ± 1.1 |
| Hunger trait | 6.3 ± 0.7 | 6.2 ± 0.8 | 6.5 ± 1.1 |
| RMR (kJ/day) | 6594 ± 160 | 6704 ± 224 | 6425 ± 220 |
| Fasting glucose (mmol/L) | 4.8 ± 0.1 | 4.7 ± 0.1 | 4.9 ± 0.1 |
| Body fat (%) | 32.8 ± 1.5 | 31.9 ± 1.8 | 34.2 ± 2.6 |
| Vigorous physical activity (mins per week) | 65 ± 13 | 55 ± 14 | 80 ± 24 |
| Moderate physical activity (mins per week) | 142 ± 21 | 173 ± 29 | 94 ± 26 |
| Walking (mins per week) | 254 ± 30 | 270 ± 37 | 231 ± 53 |
Mean ± SEM.
BMI: Body Mass Index. RMR: Resting metabolic rate.
Energy intake
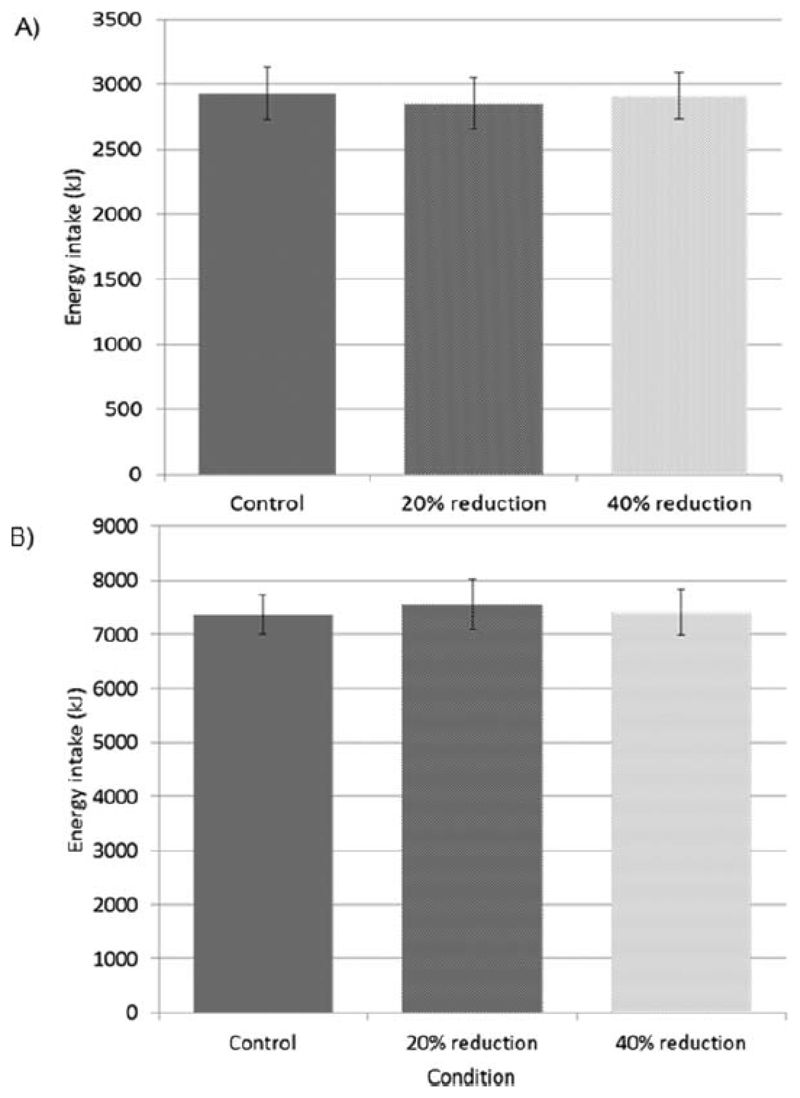
EI was not different between conditions at lunch (control: 2930±203; 20% reduction condition: 2853±198; 40% reduction condition: 2911±179kJ) or over the whole day except breakfast (control: 7374±361; 20% reduction condition: 7566±468; 40% reduction condition: 7413±417kJ) (Figure 2). Daily EI including breakfast was 10287±395kJ, 9897±491kJ and 9161±437kJ in control, 20% reduction and 40% reduction conditions respectively.
Figure 2.
Mean (± SEM) energy intake at A) lunch were not different between conditions (control vs. 20% reduction, β=-76.6, p=0.429; 20% reduction vs. 40% reduction, β=58.2, p=0.547; control vs. 40% reduction, β=-18.3, p=0.850); and B) over the whole day except breakfast (control vs. 20% reduction, β=192.3, p=0.555; 20% reduction vs. 40% reduction, β=-152.8, p=0.639; control vs. 40% reduction, β=39.5, p=0.904).
Biochemical measures
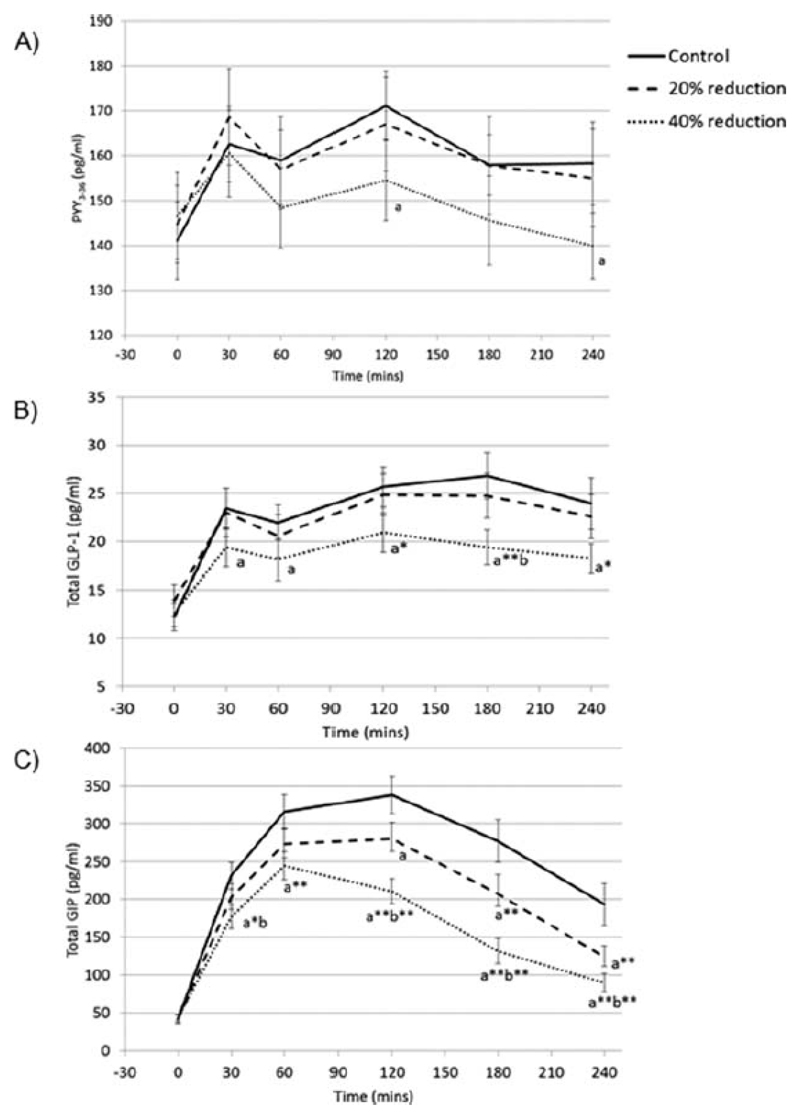
Figure 3 shows the postprandial profiles for each of the gastrointestinal hormones. Compared to control, there was a reduction in PYY in 40% reduction condition at 120 and 240mins(p<0.05), but no condition-time interaction for 40% reduction compared to 20% reduction condition(p>0.076), or 20% reduction compared to control(p>0.42). Compared to control, GLP-1 was lower in 40% reduction condition at all postprandial time-points(p<0.05). GLP-1 was also lower in 40% reduction condition compared to 20% reduction at 180mins(p=0.038), but there was no condition-time interaction for 20% reduction condition compared to control(p>0.056). GIP was lower in 40% reduction condition compared to control at all postprandial time-points(p<0.001), and compared to 20% reduction condition at 30, 120, 180 and 240mins(p<0.05). GIP was lower at 120, 180 and 240mins in 20% reduction condition compared to control(p<0.05).
Figure 3.
Postprandial response (mean ± SEM) of A) plasma PYY3-36, B) plasma total GLP-1, and C) plasma total GIP, according to condition.
‘a’ indicates the mean of the condition is significantly different to the mean of the control condition at that time point. ‘b’ indicates the mean is significantly different to the mean of the 20% reduction condition at that time point (mixed effects models): p<0.05. Addition of * to the letter indicates p<0.01 and ** indicates p<0.001.
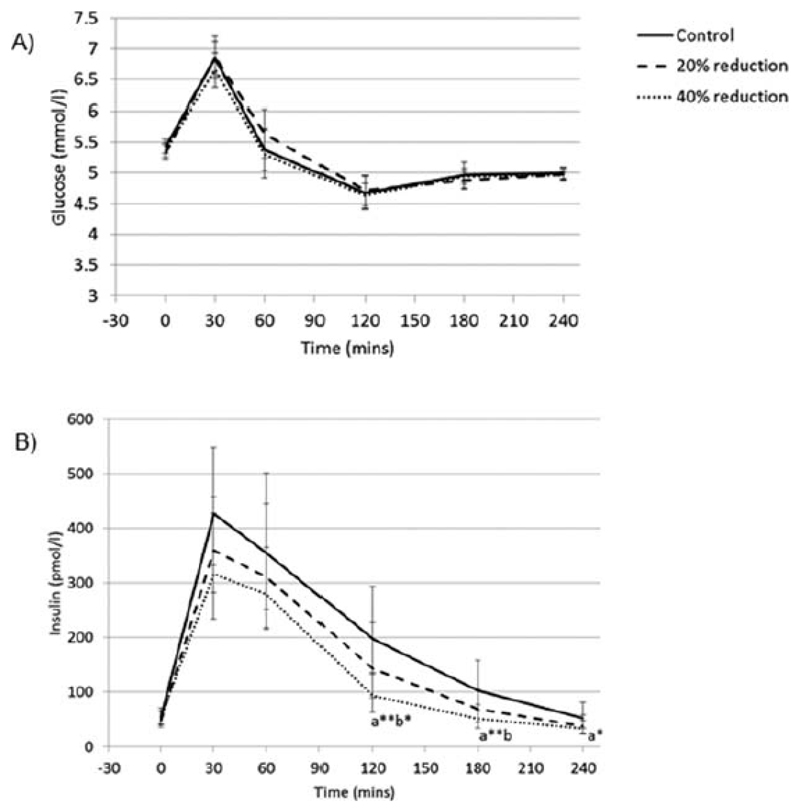
Glucose and insulin profiles are shown in Figure 4. There was no condition-time interaction for glucose(p>0.2 for all comparisons). There was a condition-time interaction such that insulin was less in 40% reduction condition compared to control and 20% reduction condition at 120 and 180(p<0.05), and compared to control at 240mins(p<0.01)There was no condition-time interaction for 20% reduction condition compared to control(p>0.083).
Figure 4.
Postprandial response of A) plasma glucose (mean ± SEM), and B) plasma insulin (geometric mean ± 95% confidence intervals), according to condition.
‘a’ indicates the mean of the condition is significantly different to the mean of the control condition at that time point. ‘b’ indicates the mean is significantly different to the mean of the 20% reduction condition at that time point (mixed effects models): p<0.05. Addition of * to the letter indicates p<0.01 and ** indicates p<0.001.
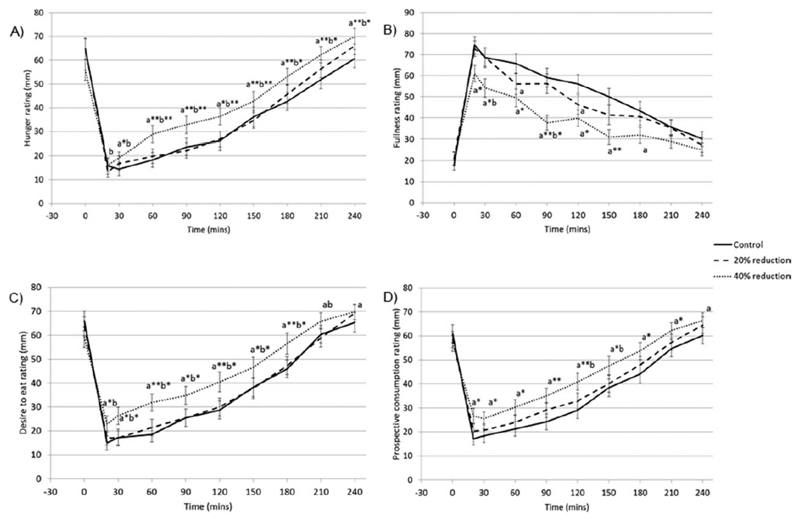
Appetite ratings
Figure 5 shows the appetite ratings. Hunger was greater in 40% reduction condition at all time-points from 30-240min (p<0.006) compared to control, and at all postprandial time-points compared to 20% reduction condition (p<0.021). There was no condition-time interaction for 20% reduction condition compared to control (p>0.291). Fullness was lower in 40% reduction condition at all time-points from 20-180mins(p<0.019) compared to control and at30(p=0.017) and 90min(p=0.003) compared to 20% reduction condition. Also fullness was lower in 20% reduction condition compared to control at 60(p=0.041) and 120mins(p=0.040). Desire to eat ratings were greater in 40% reduction condition at all time-points postprandially(p<0.023) compared to control, and at all time-points from 20-210min (p<0.037) compared to 20% reduction condition. There was no condition-time interaction for 20% reduction condition compared to control(p>0.223). Prospective consumption was greater in 40% reduction condition compared to control at all time-points postprandially(p<0.011) and compared to 20% reduction condition, at 120(p=0.018) and 150min(p=0.027). There was no condition-time interaction for 20% reduction condition compared to control(p>0.068).
Figure 5.
Postprandial ratings (mean ± SEM) for A) perceived hunger, B) perceived fullness, C) perceived desire to eat, and D) perceived prospective consumption, according to condition.
‘a’ indicates the mean of the condition is significantly different to the mean of the control condition at that time point. ‘b’ indicates the mean is significantly different to the mean of the 20% reduction condition at that time point (mixed effects models): p<0.05. Addition of * to the letter indicates p<0.01 and ** indicates p<0.001.
AUCs over the whole day for hunger, desire to eat and prospective consumption were greater in 40% reduction condition compared to control, and smaller for fullness (hunger β=2423.9,p=0.025; fullness β=-4857.9,p=0.001; desire to eat β=3832.5,p=0.001; prospective consumption β=3427.9,p=0.001). AUC for prospective consumption ratings was greater in 20% reduction condition compared to control(β=2284.1,p=0.025), but AUC for hunger(p=0.232), fullness(p=0.136), and desire to eat(p=0.118) did not differ. There were no differences in hunger or fullness when comparing 20% and 40% reduction conditions (data not shown).
Predictors of energy intake
Most of the biochemical measures did not predict EI at lunch (p>0.137) (Table 2). However, AUC (p=0.032) and pre-lunch (p=0.049) measures of PYY were positively associated with EI at lunch. AUCs and pre-lunch measures of hunger, desire to eat and prospective consumption were positively associated with lunch EI (p<0.02). Pre-lunch fullness was negatively associated with lunch EI (p<0.002), but fullness AUC was not (p=0.085). AUCs for hunger, desire to eat and prospective consumption, but not fullness (p=0.469), were positively associated with EI over the day (p<0.026).
Table 2.
Estimated regression coefficients to measure associations between biochemical measures and appetite ratings (predictor variables) with energy intake at lunch and over the whole day apart from breakfast (outcome variables), from mixed effects models.
| AUC as predictor | Pre-lunch measure as predictor | |||
|---|---|---|---|---|
| Predictor of lunch EI | Regression coefficient (SE) | p-value | Regression coefficient (SE) | p-value |
| Biochemical measure | ||||
| PYY | 0.029 (0.014) | 0.032 | 4.442 (2.257) | 0.049 |
| GLP-1 | 0.019 (0.071) | 0.790 | 15.95 (11.17) | 0.154 |
| GIP | 2.666 (4.446) | 0.549 | 39.08 (33.06) | 0.237 |
| Glucose | -0.916 (0.710) | 0.197 | -365.5 (245.8) | 0.137 |
| Insulin | -197.0 (259.8) | 0.448 | -157.0 (168.7) | 0.352 |
| Appetite rating | ||||
| Hunger | 0.091 (0.022) | <0.001 | 11.96 (3.934) | 0.002 |
| Fullness | -0.029 (0.017) | 0.085 | -10.43 (3.389) | 0.002 |
| Desire to eat | 0.087 (0.018) | <0.001 | 8.788 (3.783) | 0.020 |
| Prospective consumption | 0.100 (0.022) | <0.001 | 19.21 (4.384) | <0.001 |
| Predictor of whole day EI | Regression coefficient (SE) | p-value | ||
| AUC appetite rating | ||||
| Hunger | 0.057 (0.025) | 0.026 | ||
| Fullness | -0.016 (0.021) | 0.469 | ||
| Desire to eat | 0.057 (0.023) | 0.013 | ||
| Prospective consumption | 0.068 (0.025) | 0.007 | ||
AUC: area under the curve. EI: energy intake. SE: standard error.
Area under the curve was calculated for between the fasting and pre-lunch time points for predicting energy intake at lunch. Area under the curve for the whole day was calculated for predicting energy intake over the whole day apart from breakfast. Each predictor was analysed in a separate mixed effects model.
Values are given to 4 significant figures. Those in bold are significant.
Biochemical measures and appetite ratings associations
GLP-1, GIP, glucose and insulin were negatively associated with hunger, desire to eat, and prospective consumption, and positively associated with fullness (p<0.012). PYY was not associated with any of the appetite ratings (p>0.068) (Table 3).
Table 3.
Estimated regression coefficients to measure associations between biochemical measures (predictor variables) and appetite ratings (outcome variables) from baseline to the pre-lunch time-point, from mixed effects models.
| Appetite rating | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hunger | Fullness | Desire to eat | Prospective consumption | |||||
| Biochemical measure | Regression coefficient (SE) | p-value | Regression coefficient (SE) | p-value | Regression coefficient (SE) | p-value | Regression coefficient (SE) | p-value |
| PYY | -0.032 (0. 038) | 0.409 | 0.041 (0.041) | 0.315 | -0.018 (0.040) | 0.650 | -0.028 (0.031) | 0.366 |
| GLP-1 | -0.494 (0.172) | 0.004 | 0.631 (0.186) | 0.001 | -0.442 (0.176) | 0.012 | -0.421 (0.138) | 0.002 |
| GIP | -3.271 (0.373) | <0.001 | 3.357 (0.416) | <0.001 | -3.143 (0.379) | <0.001 | -2.629 (0.305) | <0.001 |
| Glucose | -6.650 (1.058) | <0.001 | 6.058 (1.186) | <0.001 | -5.493 (1.087) | <0.001 | -4.396 (0.884) | <0.001 |
| Insulin | -14.07 (1.227) | <0.001 | 14.33 (1.391) | <0.001 | -13.63 (1.250) | <0.001 | -11.86 (0.990) | <0.001 |
SE: standard error.
Each predictor was analysed in a separate model.
Values are given to 4 significant figures. Those in bold are significant.
Perceived portion size
The ratings of portion size at breakfast were different between conditions. Portion size was perceived to be smaller in 40% reduction condition compared to both control (β=-15.6, p<0.001) and 20% reduction (β=-10.8, p<0.001), and portion size was perceived to be smaller in 20% reduction condition compared to control(β=-4.8, p=0.049) (portion size rating: control 53±2mm; 20% reduction 48±3mm; 40% reduction 37±3mm. However at debriefing only two participants specifically reported noticing the change in PS at breakfast. None of the participants were concerned about the blinding of the aims of the study and consented to data inclusion.
Discussion
Reducing PS at a single meal altered psychological and biological markers of appetite, but there was no energy compensation later in the day. EIs at lunch were strikingly consistent in this standardised laboratory setting. These findings indicate reducing PS of a prepared meal could lead to a net reduction in daily EI. However, the effect on gastrointestinal hormones and appetite ratings, particularly after the 40% reduction in PS, questions the sustainability of this strategy to constrain EI.
There were very few differences in the profiles for PYY and GLP-1 between control and 20% reduction conditions. Moreover, there were few differences in the profiles when comparing 20% and 40% reduction conditions suggesting that the responses in these biochemical measures may not be sensitive to the smaller change in PS (660kJ men and 510kJ women). Indeed, all previous studies where a reduction in energy load has led to attenuated PYY(16,17), GLP-1(18,19), or insulin(20,21) profiles, used energy changes between 920-2096kJ. However, the present study showed distinct differences between all conditions in the postprandial profiles for GIP showing that it is sensitive to energy changes in a clear dose response manner, reflecting its important role as an incretin hormone for the regulation of insulin secretion.
Interestingly, the ratings of perceived PS of the breakfast were different between conditions, although at debriefing most participants reported not noticing the meal manipulation. The effect size for the difference between perceived PS ratings was considerably smaller when comparing control versus 20% reduction than 20% versus 40% reduction conditions (β=-4.8; β=-10.8), although the absolute difference in energy was the same. This difference is likely due to either the relative difference between PS being different (20% for control-20% reduction comparison, and 25% for 20%-40% reduction comparison), or due to the Weber-Fechner law, whereby the ability to perceive stimulus change is proportional to the logarithm of the magnitude of the stimulus(22). Thus, as the reference portion size in the first comparison (control versus 20% reduction) was larger than the second (20% versus 40% reduction), the change in PS detectable for the first pairing would have been larger than the second. It is possible that the perception of how much is provided, and thus consumed, could affect appetite ratings. The smaller effect size of perceived PS between control and 20% reduction conditions could in part account for fewer differences in appetite ratings between these conditions.
Postprandial biochemical responses were poor predictors of subsequent EI, consistent with much of the existing evidence(23–25). However, appetite ratings tended to predict EI at lunch and the rest of the day. This is in agreement with some(12,26–28), but not all(29,30), previous studies. The mixed evidence likely reflects the subjective nature of the perception of appetite which leads to measurement variability, but differences are more easily detected in crossover than parallel design studies(31). Although associations between appetite ratings and EI in the present study were highly significant, the effect sizes were small. This, coupled with relatively small differences in postprandial appetite ratings response to the manipulated meal, could in part explain the lack of compensation for the changes in energy. In contrast with the known function of PYY, where exogenous administration reduces EI(16,32,33), there was a small but significant positive effect of AUC and pre-lunch PYY on subsequent EI. However, the effect decreased after adjustment for additional participant characteristics, indicating it may be confounded by other factors. Thus there is uncertainty about these present findings relating to PYY. In contrast to the clear exogenous effect, endogenous postprandial responses in PYY were not associated with subsequent EI(12,24,34), possibly as exogenous PYY tends to be supra-physiological(12).
GLP-1, glucose and insulin were positively related to fullness and negatively related to hunger, desire to eat and prospective consumption consistent with previous research(21,23,35–37), indicating that these biochemical measures are likely to play roles in the perception of appetite sensations. However, some studies have found no relationship, or mixed results, between glucose or insulin and appetite ratings(23,28), possibly because they have reported correlations between the mean AUC or peak values rather than examining within-person relationships. Previous findings with respect to the relationship between postprandial PYY and appetite are mixed, including positive associations between PYY and perceived fullness(35,38), while others, consistent with the present findings, have found no associations(12,37,39), or associations in lean but not obese participants(34). Thus, the robustness of the association of endogenous PYY with appetite ratings is questionable. It is unclear whether GIP plays a role in influencing appetite and EI(40), however the present findings showed GIP was associated with appetite ratings. The distinct similarity between GIP and appetite ratings profiles may have led to these associations, but causality cannot be assumed. The lack of association between GIP and subsequent lunch EI is in agreement with the perspective that GIP does not influence EI.
The present findings support the concept that reducing the PS of pre-prepared meals or unit foods could constrain EI and contribute to prevention of weight gain. However as weight control advice is inherently overt, it is important to establish whether similar effects are seen when participants are aware of the reduction in PS.
There are several limitations to this study. It was conducted in a laboratory setting and, although the specific hypothesis was concealed, participants were aware of their eating behaviour being observed. The frequency and type of food provided at lunch was fixed, thus only the amount could vary potentially limiting compensation by removing some of the environmental cues that are profuse in a free-living environment and can influence EI. This setting also prevented any self-initiated eating episodes between breakfast and lunch. Some of the appetite and hormone profiles suggest effects of PS reduction may have diminished over time and compensation might be seen in a free-living environment during this period. The use of a different control PS (in terms of percentage of estimated daily energy requirements) may alter the relative effects of a percentage reduction. The overweight and obese sample may limit comparison with previous work, however it was most appropriate to study portion size reduction in this group as those with an elevated BMI are most in need of interventions to constrain energy intake, and are to date an understudied population in this field. The study was conducted over a single day and it is possible that a longer period of consuming portions set to provide energy below requirements could lead to adaptation and energy compensation. Future studies should attempt to examine PS reduction in a more realistic setting and with prolonged exposure to smaller portions.
Conclusions
Reductions in PS led to lower EI, despite changes in biological and behavioural measures that tend to favour energy compensation. Although the effect size was small, if sustained this will be of public health benefit in constraining energy intake.
What is already known about this subject.
Larger portion sizes are linked with increased energy intake.
There are strong homeostatic mechanisms to guard against low energy intakes. There is a paucity of data of the effects of smaller portions on appetite control systems and energy intake.
What this study adds
New evidence of the effects of smaller portions on gastrointestinal hormone responses to food.
Data on the effect of portion size reductions on appetite ratings and subsequent energy intake in overweight and obese adults.
Support for portion size reduction as a potential strategy to constrain energy intake.
Acknowledgements
HBL and SAJ were responsible for project conception. HBL, ALA and SAJ developed the protocol. IS-T advised on statistical analysis. HBL conducted research, analysed data, interpreted results, and drafted the manuscript. ALA, IS-T, CGW, FR, FMG and SAJ contributed to the data interpretation and critical revision of the manuscript. All authors read and approved the final manuscript.
We thank the volunteers who participated and the research assistants/students who assisted with data collection. Thank you to Johannes Grosse for discussion and enabling GLP-1 analysis. Thank you to JJ Laboratory Services, London for analysis of PYY; Core Biochemical Assay Laboratory, Cambridge for analysis of insulin and GIP; MRC HNR for analysis of glucose and diet diary coding; and Biochemistry and Immunology Department at Addenbrooke’s Hosptial, Cambridge for screening fasting plasma glucose analysis. This study was supported by a programme grant from the UK Medical Research Council (U105960389). GLP-1 analysis was funded by Takeda Cambridge Ltd., UK.
Footnotes
Conflict of interest
SAJ is the independent Chair of the Department of Health Responsibility Deal Food Network in England, which includes voluntary agreements with industry to reduce the portion size of some food and drinks. No other authors declare a conflict of interest.
References
- 1.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977-1998. JAMA. 2003;289(4):450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 2.Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92(2):246–249. doi: 10.2105/ajph.92.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. Am J Clin Nutr. 2002;76(6):1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- 4.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity. 2007;15(6):1535–1543. doi: 10.1038/oby.2007.182. [DOI] [PubMed] [Google Scholar]
- 5.Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004;62(7):S98–S104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 6.Scientific Advisory Committee on Nutrition. Dietary Reference Values for Energy. London: TSO: 2011. [www document] URL: http://www.sacn.gov.uk/reports_position_statements/reports/sacn_dietary_reference_values_for_energy.html. [Google Scholar]
- 7.Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9(2):273–279. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 8.Orbitello B, Ciano R, Corsaro M, Rocco PL, Taboga C, Tonutti L, et al. The EAT-26 as screening instrument for clinical nutrition unit attenders. Int J Obes. 2006;30(6):977–981. doi: 10.1038/sj.ijo.0803238. [DOI] [PubMed] [Google Scholar]
- 9.Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 10.The IPAQ Group. International Physical Activity Questionnaire. [www document] URL: https://sites.google.com/site/theipaq/
- 11.Kral TVE, Roe LS, Rolls BJ. Combined effects of energy density and portion size on energy intake in women. Am J Clin Nutr. 2004;79(6):962–968. doi: 10.1093/ajcn/79.6.962. [DOI] [PubMed] [Google Scholar]
- 12.Doucet E, Laviolette M, Imbeault P, Strychar I, Rabasa-Lhoret R, Prud'homme D. Total peptide YY is a correlate of postprandial energy expenditure but not of appetite or energy intake in healthy women. Metabolism. 2008;57(10):1458–1464. doi: 10.1016/j.metabol.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 14.Food Standards Agency and Department of Health. National Diet and Nutrition Survey: Headline results from Year 1 of the Rolling Programme (2008/2009) 2010 [www document] URL: http://www.food.gov.uk/multimedia/pdfs/publication/ndnsreport0809year1results.pdf. [Google Scholar]
- 15.McCulloch C, Searle S. Generalized Linear and Mixed Models. Wiley; New York: 2000. [Google Scholar]
- 16.le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 17.Martins C, Robertson MD, Morgan LM. Impact of restraint and disinhibition on PYY plasma levels and subjective feelings of appetite. Appetite. 2010;55(2):208–213. doi: 10.1016/j.appet.2010.05.091. [DOI] [PubMed] [Google Scholar]
- 18.Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 19.Rijkelijkhuizen JM, McQuarrie K, Girman CJ, Stein PP, Mari A, Holst JJ, et al. Effects of meal size and composition on incretin, α-cell, and β-cell responses. Metabolism. 2010;59(4):502–511. doi: 10.1016/j.metabol.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Borer KT, Wuorinen E, Ku K, Burant C. Appetite Responds to Changes in Meal Content, Whereas Ghrelin, Leptin, and Insulin Track Changes in Energy Availability. J Clin Endocrinol Metab. 2009;94(7):2290–2298. doi: 10.1210/jc.2008-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blom WAM, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HFJ. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81(2):367–375. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 22.Colman AM. Oxford Dictionary of Psychology. 3rd edn. Oxford University Press: Oxford; 2009. [Google Scholar]
- 23.Flint A, Moller BK, Raben A, Sloth B, Pedersen D, Tetens I, et al. Glycemic and insulinemic responses as determinants of appetite in humans. Am J Clin Nutr. 2006;84(6):1365–1373. doi: 10.1093/ajcn/84.6.1365. [DOI] [PubMed] [Google Scholar]
- 24.Willbond SM, Doucet E. Individually timing high-protein preloads has no effect on daily energy intake, peptide YY and glucagon-like peptide-1. Eur J Clin Nutr. 2011;65(1):55–62. doi: 10.1038/ejcn.2010.188. [DOI] [PubMed] [Google Scholar]
- 25.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety - Effect of obesity and weight reduction. Int J Obes. 2001;25(8):1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 26.Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: Predictors of energy intake and weight loss. Appetite. 2007;48(2):159–166. doi: 10.1016/j.appet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Lemmens SG, Martens EA, Born JM, Martens MJ, Westerterp-Plantenga MS. Staggered meal consumption facilitates appetite control without affecting postprandial energy intake. J Nutr. 2011;141(3):482–488. doi: 10.3945/jn.110.133264. [DOI] [PubMed] [Google Scholar]
- 28.Holt SHA, Miller JCB, Petocz P. Interrelationships among postprandial satiety, glucose and insulin responses and changes in subsequent food intake. EurJ Clin Nutr. 1996;50(12):788–797. [PubMed] [Google Scholar]
- 29.Gray RW, French SJ, Robinson TM, Yeomans MR. Dissociation of the effects of preload volume and energy content on subjective appetite and food intake. Physiol Behav. 2002;76(1):57–64. doi: 10.1016/s0031-9384(02)00675-3. [DOI] [PubMed] [Google Scholar]
- 30.De Graaf C, Hulshof T. Effects of weight and energy content of preloads on subsequent appetite and food intake. Appetite. 1996;26(2):139–151. doi: 10.1006/appe.1996.0012. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. B J Nutr. 2000;84(4):405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 32.Batterham RL, Cohen MA, Ellis SM, le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 33.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol- Endoc M. 2007;292(4):E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 34.Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, et al. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol- Gastr L. 2012;303(1):G129–G140. doi: 10.1152/ajpgi.00478.2011. [DOI] [PubMed] [Google Scholar]
- 35.Lemmens SG, Martens EA, Kester AD, Westerterp-Plantenga MS. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am J Clin Nutr. 2011;94(3):717–725. doi: 10.3945/ajcn.110.008631. [DOI] [PubMed] [Google Scholar]
- 36.Blundell JE, Levin F, King NA, Barkeling B, Gustafson T, Hellstrom PM, et al. Overconsumption and obesity: Peptides and susceptibility to weight gain. Regul Pept. 2008;149(1-3):32–38. doi: 10.1016/j.regpep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellström PM, Näslund E, et al. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98(5):E847–E855. doi: 10.1210/jc.2012-3835. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y, Ma LJ, Enriori PJ, Koska J, Franks PW, Brookshire T, et al. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity. 2006;14(9):1562–1570. doi: 10.1038/oby.2006.180. [DOI] [PubMed] [Google Scholar]
- 39.Stock S, Leichner P, Wong ACK, Ghatei MA, Kieffer TJ, Bloom SR, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 40.Paschetta E, Hvalryg M, Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obes Rev. 2011;12(10):813–828. doi: 10.1111/j.1467-789X.2011.00897.x. [DOI] [PubMed] [Google Scholar]