Abstract
Background
Haemostatic activation and hypercoagulability are frequently observed in patients with metastatic colorectal cancer (mCRC), increase risk of venous thromboembolism (VTE) and have been implicated in tumour proliferation and progression. To date, the association of haemostatic biomarkers with oncologic outcomes including overall survival (OS), progression free survival (PFS) and disease control rate (DCR) is incompletely understood.
Methods
Within the framework of the Vienna Cancer and Thrombosis Study, a prospective observational cohort study, we conducted an exploratory analysis to investigate the association of six known biomarkers of haemostasis with oncologic outcomes in 99 patients with mCRC prior to chemotherapy initiation.
Results
Patients with high levels of factor VIII activity (FVIII), D-dimer, prothrombin fragment 1+2 (F1+2) and fibrinogen (defined as levels >75th percentile) had significantly shorter median OS than patients with lower levels. Elevation of four biomarkers was associated with mortality in multivariable analysis, adjusting for age, sex, number of metastatic sites and VTE (hazard ratio [95%CI] for death per doubling of levels: FVIII: 2.06 [1.28-3.30]; sP-selectin: 1.55 [1.07-2.24]; D-dimer: 1.40 [1.18-1.65]; F1+2: 1.64 [1.10-2.46]). Patients with elevated levels had numerically shorter median PFS across all markers and disease control rate (DCR) was significantly smaller in those with high levels of FVIII and F1+2 (adjusted odds ratio [95%CI] for DCR per doubling of levels: 0.23 [0.09-0.62] and 0.36 [0.16-0.82]) compared to patients with lower levels.
Conclusion
Specific elevated haemostatic biomarkers are associated with higher mortality and partially with worse response to chemotherapy in patients with mCRC.
Keywords: Biomarker, haemostasis, metastatic colorectal cancer, survival, mortality
Background
Cancer and the blood coagulation system are tightly connected. Cancer cells are able to activate haemostasis through multiple pathways and thereby induce systemic hypercoagulability.(1, 2) In consequence, patients with cancer are prone to develop venous thromboembolism (VTE), a frequent complication that increases morbidity and mortality.(3–5) On the other hand, procoagulant factors and activated platelets have been suggested to support tumour growth, progression of disease and formation of metastasis by creating a proliferative, pro-invasive and proangiogenic microenvironment.(6–13) In addition to these mechanistic findings, there is some clinical evidence for an association of activation of haemostasis with unfavourable outcomes in patients with cancer. In most of the studies, D-dimer, a fibrin degradation product reflecting systemic blood coagulation activation, has been investigated as haemostatic biomarker.(2, 14–16) In colorectal cancer, D-dimer has been shown to be a predictor for overall survival (OS) and progression free survival (PFS) in inoperable tumours.(17) Changes of D-dimer levels during chemotherapy have been found to be associated with disease progression and OS more accurately than carcinoembryonic antigen, a routine tumour-marker for monitoring of disease.(18, 19) Elevated circulating levels of D-dimer further have been found to be associated with early disease recurrence in the adjuvant setting of colorectal carcinoma(20) and has also been reported to predict response to neoadjuvant treatment in patients with oesophageal cancer(21).
Especially in colorectal cancer, haemostatic biomarkers appear promising in identifying patients with a poor prognosis. Colorectal cancer is the third most common malignancy and the fourth leading cause of cancer-related deaths in the world, with an expected increase in its incidence of 60% until 2030.(22) Despite promising developments in therapeutic approaches, systemic chemotherapy remains the main pillar of treatment for advanced stage colorectal cancer.(23) The clinical scenario of deciding on initiating chemotherapy in patients with metastatic disease is a common oncologic setting. Factors that can predict survival and response to antineoplastic therapy may help personalizing oncologic treatment strategies, identifying patients who are likely to benefit the most from more intense chemotherapy regimens and sparing predicted non-responders from unnecessary side effects. Further, the identification of prognostic and predictive biomarkers contributes to the mechanistic and conceptual understanding of biological processes.
Therefore, the aim of this study was to broadly investigate haemostatic biomarkers as indicators of prognosis in patients with metastatic colorectal cancer (mCRC) and to define the role of haemostatic biomarkers in treatment response prediction. To cover different aspects of the haemostatic system, we evaluated six known haemostatic biomarkers, previously investigated for prediction of cancer-associated VTE, namely D-dimer(16, 24) and prothrombin fragment 1.2 (F1+2)(16) as markers of in vivo activation of the haemostatic system, coagulation factor VIII activity (FVIII)(25) and peak thrombin generation(26) as indicators of potential hypercoagulability, soluble P-selectin (sP-selectin)(27) as a marker of platelet activation and fibrinogen (28, 29) as the substrate for coagulation in vivo. We sought to expand previous knowledge on the association between haemostatic biomarkers, prognosis and therapy response and hypothesized that elevated levels of haemostatic biomarkers prior to initiation of chemotherapy predict shorter overall survival, lower response rates to systemic chemotherapy and shorter time to progression of disease in patients with mCRC.
Methods
Study design
We utilized the dataset of the Vienna Cancer and Thrombosis Study (CATS), an ongoing single-centre prospective observational cohort study, to perform this analysis in the subpopulation of patients with mCRC initiating systemic chemotherapy (Supplementary Figure 1).
Detailed information about the study design and procedures have been described in previous publications.(16) Briefly, adult patients (≥18 years) with a newly diagnosed malignancy or progressive disease after complete or partial remission were eligible for inclusion. The primary endpoint of CATS is VTE.
For the current analysis, mortality, defined as risk of death per time and overall survival (OS), defined as the time from therapy initiation to death from any cause have been defined as main outcomes of interest. For associations of biomarker levels with survival and mortality outcomes, the term “prognostic” is being used.
The secondary outcomes of interest are parameters reflecting response to chemotherapy. First, we chose risk of disease progression per time (confirmed progression of disease by imaging according to RECIST 1.1 [response evaluation criteria in solid tumours, version 1.1] or death from any cause(30)) and progression free survival (PFS), defined as time from start of chemotherapy to radiological progression of disease or death. In other words, PFS depicts the time a patient undergoing oncologic treatment does not experience relevant tumour growth, occurrence of new metastatic lesions or death.
Secondly, disease control rate (DCR), defined as the composite of complete or partial remission and stable disease on imaging according to RECIST 1.1., has been defined as secondary outcome. DCR can be interpreted as the percentage of patients with response to chemotherapy, be it stagnation in tumour growth (stable disease at radiologic restaging) or remission (tumour shrinkage or disappearance on radiological restaging). For associations of biomarker levels with PFS and DCR, the term “predictive” is being used.
Data collection
Data on date and cause of death was gathered by inquiring the official Austrian death-registry. Response to first line of systemic chemotherapy after blood sampling has been identified by screening medical charts for routine follow-up staging procedures, as interpreted by the treating oncologists.
Most parameters have been collected prospectively within CATS, including our main study outcomes (mortality and survival). Data on therapy response has been collected in retrospect from electronic medical charts, utilizing routine clinical parameters as assessed by the treating oncologists.
Blood sampling for biomarker measurement was performed at baseline before the initiation of systemic chemotherapy (pretherapeutic). Detailed information on the design of CATS and technique of biomarker measurement are reported in Supplementary Methods.
Derivation of study cohort
A total number of 2288 patients has been recruited in CATS until March 2019, 197 had histologically confirmed colorectal cancer. Of these, 68 did not have distant metastasis. Of the remaining 129, 11 underwent primary metastasectomy without prior chemotherapy. Eight were treated with alternative treatment approaches for metastasis control (radiotherapy in 7 and thermal-ablation in 1 patient). Of the 110 remaining, 11 were excluded because they either did not receive chemotherapy or had incomplete data on exact therapeutic management. Finally, 99 patients with mCRC (defined as stage IV disease according to the American Joint Committee on Cancer staging system, AJCC)(31) were included in the present analysis, who have received either at least one line of palliative chemotherapy (n=90) or underwent induction chemotherapy with a primary neoadjuvant intent (n=9). (Supplementary Figure 1)
Statistical analysis
Continuous variables were summarized as medians [interquartile-range (IQR)], and count data as absolute frequencies (%). The reverse Kaplan-Meier method was used to estimate specific follow-up time for different endpoints. The association of biomarker levels with outcome variables was evaluated on a continuous scale and by dichotomizing biomarker-measures at the 75th percentile for graphical display, according to previous studies.(16, 25–28)
Due to the probability of collinearity between different markers, as depicted in Supplementary Figures 2 and 3 and Supplementary Table 2, each marker was analysed individually for each outcome variable.
Time-to-event data
OS and PFS was quantified by the method of Kaplan-Meier. The association of biomarker levels on a continuous scale with mortality and risk of disease progression was evaluated in a multivariable cox proportional hazards model, adjusting for age, gender, BMI, number of metastatic organ sites and VTE, with occurrence of VTE being treated as a time-dependent co-variable.
DCR
The association of biomarker levels with DCR was estimated by means of a binary multivariable logistic regression model (adjusting for age, gender, BMI, VTE, number of metastatic organ sites). Further, differences in rates of disease control according to dichotomized biomarker levels have been assessed for statistical significance by Χ2-test. As of the hypothesis-generating nature of this analysis, no post-hoc-test to correct for multiple testing has been conducted.(32)
All statistical analyses have been performed with the commercially-available packages SPSS 24 (SPSS Inc, Chicago IL, USA) and Stata 15.0 (Stata Corp., Houston, TX, USA).
Results
Characteristics of the study population
The median age at the time of study inclusion was 61 years [IQR: 55-67] and 37 (37%) patients were female (Table 1). Three patients (3%) had a positive history of VTE (40, 20 and 5 years prior to study inclusion), one (1%) had a history of arterial thrombotic events (ATE). The median body mass index (BMI) was 24.3 kg/m2 [IQR: 21.7-28.0].
Table 1. Baseline characteristics of the study cohort (n=99).
| Variable | n (% missing) | Median [IQR] or count (%) |
|---|---|---|
| Demographics | ||
| Age (years) | 99 (0%) | 61 [55-67] |
| Female Gender | 99 (0%) | 37 (37.4%) |
| BMI (kg/m2) | 99 (0%) | 24.3 [21.7-27.9] |
| History of smoking | 93 (6.1%) | 37 (39.8%) |
| History of TEE* | 99 (0%) | 4 (4.0%) |
| Tumour specifics | ||
| Location of primum within colorectum | / | / |
| ---Rectum | / | 36 (40.4%) |
| ---Sigmoid colon | / | 24 (27.0%) |
| ---Right sided primary | / | 23 (25.8%) |
| ---Others / not specified | / | 16 (16.2%) |
| Synchronous metastasis | 99 (0%) | 58 (58.6%) |
| Number of metastatic sites | 99 (0%) | 1 [1-2] |
| ---Liver | / | 80 (80.8%) |
| ---Lungs | / | 35 (35.4%) |
| Therapeutic management | ||
| Palliative intent | / | 89 (89.9%) |
| Neoadjuvant intent | / | 10 (10.1%) |
| CTX prior to inclusion* | / | 3 (3.0%) |
| Surgical resection of tumour | 80 (19.2%) | 70 (87.5%) |
| Secondary metastasectomy | 99 (0%) | 28 (28.3%) |
| 1st line CTX data | 98 (1.0%) | / |
| ---FU-oxaliplatin combinations | / | 62 (63.3%) |
| ---FU-irinotecan combination | / | 17 (17.3%) |
| ---FU-monotherapy | / | 8 (8.2%) |
| ---Oxaliplatin-raltitrexed combination | / | 6 (6.1%) |
| ---Bevacizumab | / | 57 (58.2%) |
| ---anti-EGFR-therapy | / | 9 (9.2%) |
| Number of chemotherapy cycles | 94 (5.1%) | 6 [3-6] |
| 2nd line CTX | 99 (0%) | 52 (52.5%) |
| Pretherapeutic haemostatic biomarkers | ||
| Factor VIII (% activity) | 98 (1.0%) | 219 [158-262] |
| sP-Selectin (ng/mL) | 99 (0%) | 41.2 [31.6-53.1] |
| D-dimer (μg/mL) | 93 (6.1%) | 1.05 [0.51-2.24] |
| Prothrombin Fragment 1.2 (pmol/L) | 94 (5.1%) | 265 [217-379] |
| Peak thrombin generation (nmol/L) | 99 (0%) | 430.3 [300.3-573.2] |
| Fibrinogen (mg/dl) | 99 (0%) | 463 [382-555] |
Column 1: Variable name or subsection headline; Column 2: n: number of patients with data present on specific variable; % missing: percentage of missing data in the total study population (99 patients); Column 3: Distribution of continuous variables (median, IQR); absolute and relative frequency of nominal variables;
according to CATS, prior VTE and termination of prior chemotherapy had to be ≥3 months before enrolment;
Abbreviations: IQR: interquartile-range; BMI: body mass index; TEE: thromboembolic events; CTX: chemotherapy; FU: fluoropyrimidine; EGFR: epidermal growth factor receptor;
The most frequent location of the tumour was the rectum (n=36, 36%), followed by the sigmoid colon (n=24, 24%). Twenty-three patients (23%) had a right-sided primary tumour. The most frequent site of metastasis was the liver (n=80, 81%) followed by the lungs (n=35, 35%). The median number of organ sites affected by metastasis was 1 [IQR: 1-2]. Of the 99 patients with mCRC at the time of enrolment, 58 (59%), had metastasis at time of diagnosis (synchronous metastatic disease) the rest had metastatic recurrence of disease after previous curative treatment approaches (metachronous metastatic disease). Seventy-two patients had their primary tumour removed. Twenty-eight patients underwent secondary metastasectomy. Of these patients, 6 did not have disease recurrence over a median time of 97 months (8.1 years). The most frequent chemotherapeutical treatment approaches were fluoropyrimidine-based regimens in 86 patients, either in combination with oxaliplatin (FOLFOX-/CAPOX-regimen, n=61) or irinotecan (FOLFIRI, n=17) or as fluoropyrimidine-monotherapy (n=8). The median number of chemotherapy-cycles was 6 [IQR: 3-6]. The anti-VEGF/A-monoclonal antibody bevacizumab was administered in 57 patients (57.6%) during first line therapy. Nine patients (9.2%) received an EGFR-targeted therapy (cetuximab in 8 and gefitinib in 1 patient). In fifty-two patients (52.5%) a second-line of systemic chemotherapy after disease-progression was initiated. All details are summarized in Table 1, including the distribution of the six haemostatic biomarkers analysed in this study (FVIII, sP-Selectin, D-dimer, F1+2, peak thrombin generation, Fibrinogen).
There were some indications towards collinearity between different markers, as depicted in Supplementary Figure 2. However, only a limited number of patients with levels above the 75th percentile in one markers also had elevations in other markers, indicating some degree of heterogeneity in individual biomarker profiles (Supplementary Table 2, Supplementary Figure 3).
Mortality and therapy response
Eighty-three patients died during the observation period (median follow-up for OS: 88 months [IQR: 59-155]). Tumour progression was listed as cause of death in 81 patients and 2 patients died from a secondary malignancy. Median OS was 21.1 months [95% confidence interval (CI): 15.1-27.2]. The 1, 3 and 5 year OS estimates were 74% [95%CI: 65-81], 37% [95%CI: 28-47%] and 17% [95%CI: 11-26%], respectively. Progression of disease (radiological progression according to RECIST1.1 or death) was observed in 93 patients (either primarily or after initial disease control). Median PFS was 7.8 months (95% CI: 6.3-9.3). Disease control was achieved in 65 patients (DCR: 65.7% (95%CI: 55.4-75.0%)) during systemic chemotherapy (details on therapeutic outcomes are displayed in Supplementary Table 1).
Biomarkers of haemostasis, risk of death and overall survival
In univariable Cox regression analysis, risk of death was significantly elevated with increasing levels of FVIII, sP-selectin, D-dimer, F1+2 and fibrinogen (Table 2). In multivariable Cox regression analysis, adjusted for age, sex, number of metastatic sites and occurrence of VTE (as time-dependent variable), risk of death was significantly elevated in patients with increasing pretherapeutic levels of FVIII (adjusted hazard ratio (HR) [95% CI] per doubling of levels: 2.06 [1.28-3.30]), sP-selectin (1.55 [1.07-2.24]), D-dimer (1.40 [1.18-1.65]) and F1+2 (1.64 [1.10-2.46]). No significant alteration in risk of death was observed for peak thrombin generation (HR: 1.20 [0.95-1.53]) and fibrinogen (HR: 1.94 [0.98-3.83]). (Table 2)
Table 2. Association of haemostatic biomarkers with overall survival.
| Biomarker | HR for death1 | Median OS in months (95% CI)2 | ||
|---|---|---|---|---|
| Univariable HR | Adjusted HR4 | Biomarker ≤Q3 | Biomarker >Q3 | |
| Factor VIII | 2.06 (1.30-3.28) | 2.06 (1.28-3.30) | 24.4 (13.4-35.4) | 13.9 (7.0-20.9) |
| sP-Selectin | 1.55 (1.08-2.21) | 1.55 (1.07-2.24) | 21.1 (9.3-33.0) | 16.3 (4.4-28.1) |
| D-dimer | 1.40 (1.21-1.63) | 1.40 (1.18-1.65) | 26.0 (10.3-41.6) | 13.8 (7.6-19.9) |
| F1+2 | 1.75 (1.25-2.46) | 1.64 (1.10-2.46) | 21.5 (12.9-30.2) | 13.9 (7.0-26.4) |
| Peak TG | 1.17 (0.92-1.48) | 1.20 (0.95-1.53) | 23.7 (14.5-33.0) | 15.3 (10.9-19.6) |
| Fibrinogen | 1.96 (1.05-3.67) | 1.94 (0.98-3.83) | 28.2 (17.8-38.7) | 15.8 (12.9-18.6) |
results indicated in bold represent statistically significant results in Cox-regression analysis of differences in time to death according to doubling of biomarker level on a continuous scale;
results indicated in bold represent statistically significant differences in time to death according to dichotomized biomarkers (method of Kaplan Meier, Log-rank-test);
Adjusted hazard ratio: result of multivariable cox regression analysis to correct for potential confounders (age, gender, BMI, VTE, number of metastatic organ sites);
Abbreviations: OS: overall survival; HR: hazard ratio; Q3: third quartile of biomarker distribution (=75th percentile); sP-selectin: soluble p-selectin; F1+2: prothrombin fragment 1+2; TG: thrombin-generation; BMI: body mass index; VTE: venous thromboembolism;
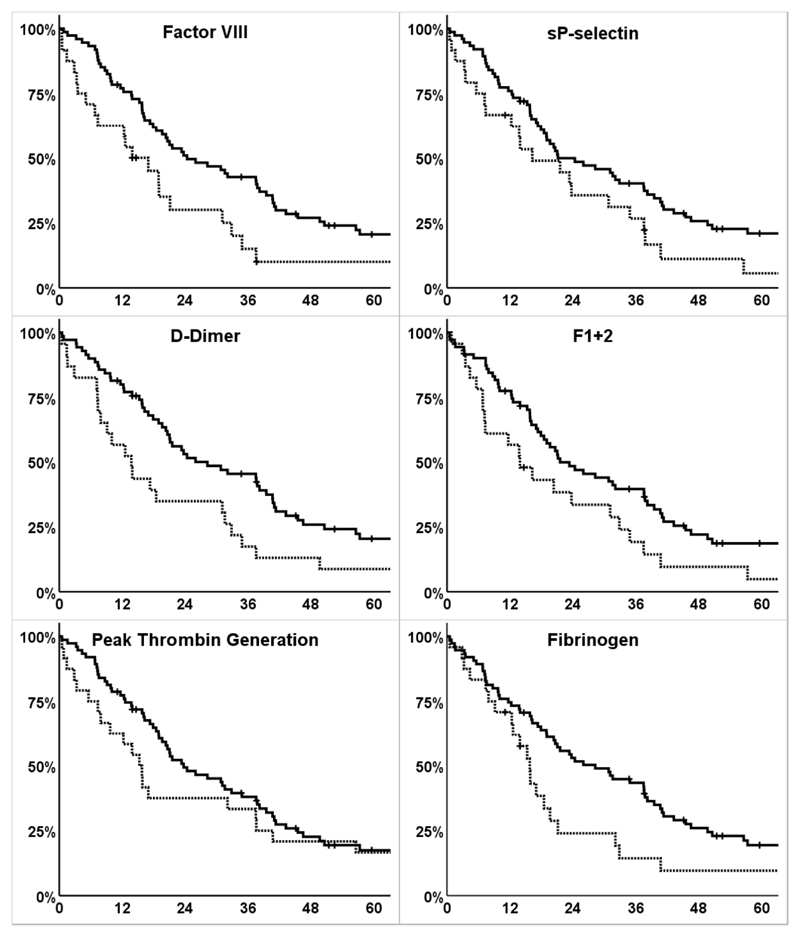
We observed numerically shorter median OS in patients with elevated levels (>75th percentile) compared to those below this cut-off across all biomarkers. In four biomarkers, OS was significantly reduced in patients with high levels compared to those with lower levels (median OS in months [95% CI] of patients ≤75th percentile vs >75th percentile of biomarker distribution, p-value (log-rank-test): FVIII: 24.4 [13.4-35.4] vs. 13.9 [7.0-20.9], p=0.004; D-dimer: 26.0 [10.3-41.6] vs. 13.8 [7.6-19.9], p=0.010; F1+2: 21.5 [12.9-30.2] vs. 13.9 [7.0-26.4], p=0.028; fibrinogen: 28.2 [17.8-38.7] vs 15.8 [12.9-18.6], p=0.019). (Figure 1, Table 2)
Figure 1. Survival functions of overall survival according to dichotomized biomarkers.
y-axis: cumulative survival in percent (%), x-axis: overall survival (time from therapy initiation to death from any cause) in months; continuous line: survival function of patients with biomarker ≤75th percentile; dotted line: survival function of patients with biomarker >75th percentile; crosses indicate data censoring
Biomarkers of haemostasis, risk of disease progression and progression free survival
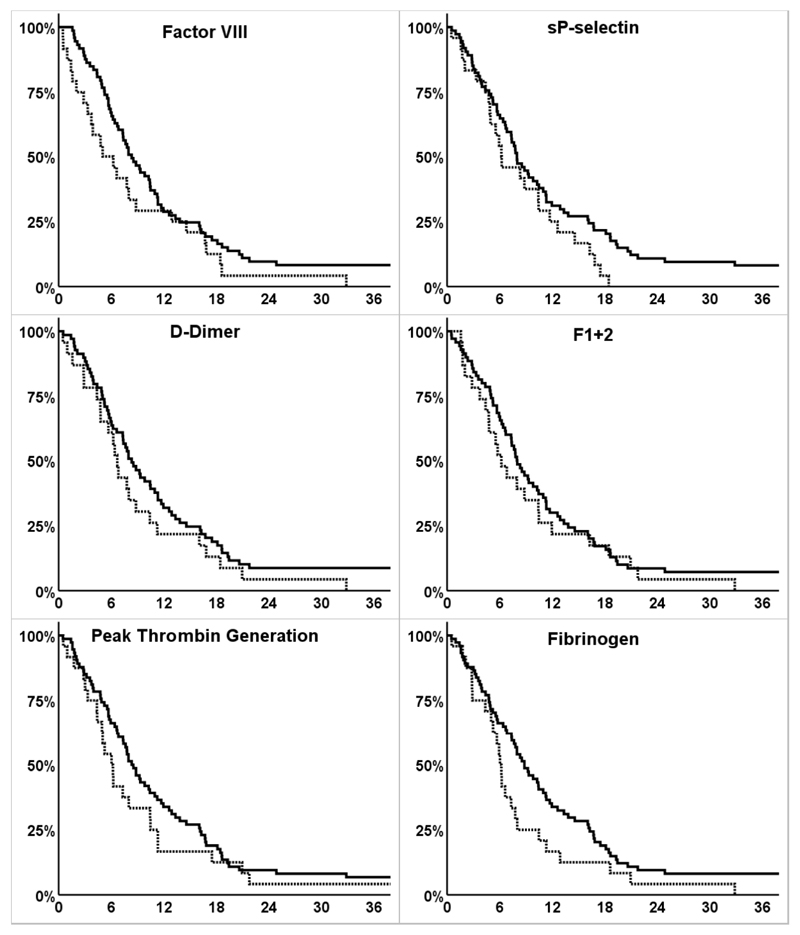
In univariable Cox-regression analysis, levels of two biomarkers were significantly associated with PFS (HR [95%CI] for disease progression per doubling of levels: D-dimer: 1.16 [1.02-1.32]; F1+2: 1.36 [1.02-1.82]). In multivariable Cox-regression-analysis, adjusted for age, sex, number of metastatic site and occurrence of VTE (as time-dependent variable), no statistically significant increase in risk of disease progression has been observed (adjusted HR [95%CI] FVIII: 1.42 [0.92-2.19], sP-selectin: 1.14 [0.80-1.61], D-dimer 1.10 [0.95-1.28], F1+2 1.24 [0.87-1.78], peak-thrombin-generation: 1.16 [0.90-1.49], fibrinogen: 1.01 [0.53-1.94]). (Table 3) We further compared Kaplan-Meier-estimates of PFS for patients with biomarker levels dichotomized at the 75th percentile (above 75th percentile of the distribution vs below or equal to this cut-off). We observed shorter median PFS in patients with elevated levels (>75th percentile) compared to those below this cut-off across all biomarkers, however, the difference did not reach statistical significance. (Figure 2, Table 3)
Table 3. Association of haemostatic biomarkers with progression free survival.
| Biomarker | HR for disease progression1 | Median PFS in months (95%CI)2 | ||
|---|---|---|---|---|
| Univariable HR | Adjusted HR3 | Biomarker ≤Q3 | Biomarker >Q3 | |
| FVIII | 1.39 (0.91-2.13) | 1.42 (0.92-2.19) | 8.3 (6.5-10.1) | 5.0 (1.7-8.2) |
| sP-Selectin | 1.26 (0.90-1.77) | 1.14 (0.80-1.61) | 8.0 (6.5-9.5) | 6.2 (2.9-9.6) |
| D-dimer | 1.16 (1.02-1.32) | 1.10 (0.95-1.28) | 8.3 (6.5-10.2) | 6.7 (5.8-7.6) |
| F1+2 | 1.36 (1.02-1.82) | 1.24 (0.87-1.78) | 8.0 (6.5-9.5) | 6.2 (4.2-8.2) |
| Peak TG | 1.21 (0.94-1.55) | 1.16 (0.90-1.49) | 8.3 (6.8-9.9) | 6.0 (4.6-7.5) |
| Fibrinogen | 1.21 (0.66-2.25) | 1.01 (0.53-1.94) | 8.8 (7.0-10.7) | 6.0 (5.4-6.7) |
results indicated in bold represent statistically significant results in Cox-regression analysis of differences in time to disease progression according to doubling of biomarker level on a continuous scale;
Median PFS and 95% confidence interval according to Kaplan-Meier-estimates;
Adjusted hazard ratio: result of multivariable cox regression analysis to correct for potential confounders (age, gender, BMI, VTE, number of metastatic sites);
Abbreviations: PFS: progression free survival; HR: hazard ratio; Q3: third quartile of biomarker distribution (=75th percentile); sP-selectin: soluble p-selectin; F1+2: prothrombin fragment 1+2; TG: thrombin generation; BMI: body mass index; VTE: venous thromboembolism;
Figure 2. Survival functions of progression free survival according to dichotomized biomarkers.
y-axis: cumulative progression free survival in percent (%), x-axis: progression free survival (time from study inclusion to progression of disease) in months; continuous line: survival function of patients with biomarker ≤75th percentile; dotted line: survival function of patients with biomarker >75th percentile; crosses indicate data censoring
Association of haemostatic biomarkers with disease control rate
To evaluate whether pretherapeutic levels of haemostatic biomarkers were associated with best radiological response during chemotherapy, we analysed the association of haemostatic biomarkers with DCR.
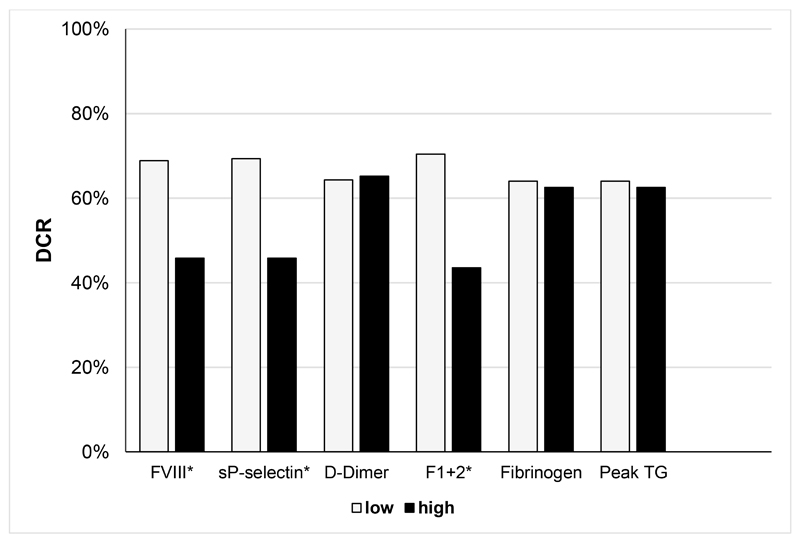
In univariable logistic regression analysis, levels of FVIII and F1+2 very significantly associated with decreased DCR. Also in multivariable analysis (adjusting for age, sex, number of metastatic sites and VTE), FVIII per double increase (HR 0.23 [95%CI: 0.09-0.62]) and F1+2 per double increase (HR 0.36 [0.16-0.82]) were significantly associated with lower DCR during 1st line chemotherapy. Levels of sP-selectin, D-dimer, fibrinogen and peak thrombin generation were not associated with DCR (Table 4). DCR in patients according to dichotomized biomarker levels (above the 75th percentile compared to levels below or equal to this cut-off) was analysed. DCR was significantly reduced in patients with elevated levels compared to those with low levels for FVIII (68.9% vs. 45.8%, p=0.041 (Χ2-test)), sP-selectin (69.3% vs. 45.8%, p=0.037) and F1+2 (70.4% vs. 43.5%, p=0.019). (Figure 3, Table 4)
Table 4. Association of haemostatic biomarkers and disease control rate.
| Biomarker | OR for DCR1 | DCR2 | ||
|---|---|---|---|---|
| Univariable OR | Adjusted OR3 | Biomarker ≤Q3 | Biomarker >Q3 | |
| Factor VIII | 0.28 (0.11-0.70) | 0.23 (0.09-0.62) | 68.9% | 45.8% |
| sP-selectin | 0.75 (0.38-1.48) | 0.77 (0.38-1.54) | 69.3% | 45.8% |
| D-dimer | 0.83 (0.62-1.12) | 0.84 (0.62-1.15) | 64.3% | 65.2% |
| F1+2 | 0.35 (0.16-0.75) | 0.36 (0.16-0.82) | 70.4% | 43.5% |
| Peak TG | 0.94 (0.59-1.50) | 1.03 (0.63-1.68) | 64.0% | 62.5% |
| Fibrinogen | 1.68 (0.55 -5.14) | 1.68 (0.50-5.66) | 64.0% | 62.5% |
results indicated in bold represent statistically significant results in binary logistic regression analysis of the association between DCR and doubling of the levels of biomarkers on a continuous scale;
results indicated in bold represent statistically significant differences in DCR according to dichotomized biomarkers (Χ2-test);
Adjusted hazard ratio: result of multivariable logistic regression analysis to correct for potential confounders (age, gender, BMI, number of metastatic organ sites);
Abbreviations: DCR: disease control rate; OR: odds ratio; Q3: third quartile of biomarker distribution (=75th percentile); sP-selectin: soluble p-selectin; F1+2: prothrombin fragment 1+2; TG: thrombin generation; BMI: body mass index;
Figure 3. Disease control rate (DCR) according to dichotomized biomarkers.
y-axis: percentage of patients who achieved disease control during first line systemic chemotherapy; x-axis: Haemostatic biomarkers, dichotomized at the 75th percentile of level distribution (low: ≤75th percentile, high: >75th percentile). Asterisk (*) indicates statistically significant difference in DCR; Abbreviations: DCR: disease control rate; FVIII: Factor VIII sP-selectin: soluble p-selectin; F1+2: prothrombin fragment F1+2; peak TG: peak thrombin generation
Additive effect of elevated haemostatic biomarkers on outcomes
Levels of all six biomarkers were available in 87 patients. Of those, 27 (31.0%) patients had levels below the 75th percentile across all biomarkers. Eighteen (20.7%) patients had one elevated biomarker (>75th percentile), 20 (23.0%) patients had two and 22 (25.3%) patients three or more elevated biomarkers. Interestingly, we observed less favourable therapy-response-profiles with increasing numbers of elevated biomarkers. Median OS in months for patients with 0, 1, 2 and 3 elevated biomarkers was 41.1, 20.3, 16.3, and 12.6 months (log-rank: p=0.001) and median PFS was 9.3, 8.0, 6.0 and 5.0 months (log-rank: p=0.060). DCR according to number of elevated markers was 77.8%, 66.7%, 60.0% and 50.0% for 0, 1, 2 and ≥3 elevated biomarkers. (Supplementary Figures 3, 4 & 5)
Discussion
Most prominently, we found that that an increase of specific haemostatic biomarkers is associated with shorter overall survival. In addition, we found indications of an association between pretherapeutic measures of some biomarkers with response measures to systemic chemotherapy, namely PFS and DCR. These outcome measures play a major role in clinical practice, with time to first disease progression, as represented by PFS, and stagnation in tumour- and metastatic growth, as indicated by DCR, are crucial factors in evaluating antineoplastic systemic therapy.
In detail, all biomarkers were significantly associated with higher risk of death except for fibrinogen and peak thrombin generation. After dichotomization of biomarker levels (cut-off: 75th percentile of distribution), significantly different median OS was observed for FVIII, D-dimer, F1+2 and fibrinogen. Also for the remaining two biomarkers (sP-selectin and peak thrombin generation), patients with the highest levels had numerically shorter survival than patients with lower levels.
In addition to shorter overall survival times, patients with elevated haemostatic biomarkers seem to respond worse to chemotherapy, as we found that pretherapeutic levels of D-dimer and F1+2 significantly increased risk of disease progression in univariable-, but not multivariable analysis. After dichotomization at the 75th percentile, no significant differences in median PFS were observed. However, patients with elevated biomarkers had numerically shorter median times to disease progression then those with levels below or equal to this cut-off across all markers. The evaluation of radiological response to chemotherapy showed that doubling of measures of FVIII and F1+2 significantly decreased DCR multivariable analysis. After dichotomization, high levels (>75th percentile) were significantly associated with lower DCR compared to low levels for FVIII and F1+2. Additionally, high levels of sP-selectin also significantly predicted lower DCR.
As systemic hypercoagulability in patients with cancer can be mediated by a variety of mechanisms, we evaluated whether haemostatic activation in patients with mCRC tends to be canonical, meaning patients with an elevation in one haemostatic biomarker also have elevated levels in other ones, or if patients have elevated levels in certain haemostatic biomarkers, while others can be low. Interestingly, we found that patients with mCRC are heterogeneous in respect to their profile of haemostatic biomarkers. Moreover, individuals with an increasing number of elevated biomarkers, indicating more profound systemic hypercoagulability, have been found to have worse outcomes (shorter OS, lower DCR and shorter PFS).
Especially D-dimer and FVIII seemed to be associated with clinical outcomes in patients. As of the routine availability of these markers, the threshold of further evaluation and potential future clinical implication of these candidate-biomarkers is lowered.
Systemic hypercoagulability and haemostatic activation seem to be closely related to aggressive biological behaviour in cancer. Especially in colorectal cancer, elevated biomarkers of haemostatic activation have been associated with unfavourable clinical outcomes. However, this has so far only been investigated for single haemostatic biomarkers and mostly in the non-metastatic setting. By evaluating a broad panel of six known indicators of cancer-associated hypercoagulability in patients with mCRC, we aimed at expanding previous knowledge towards the prognostic value of haemostatic biomarkers and explore the role of these markers in predicting response to antineoplastic chemotherapy. The selected biomarkers are reflective of different compartments of the haemostatic system, either of activation of coagulation and fibrinolysis in vivo (D-dimer, fibrinogen), of potential hypercoagulability (factor VIII activity), of thrombin generation (peak thrombin generation, prothrombin fragment F1+2) or of platelet activation (sP-selectin).
Our findings might be explained in two ways. First, the results are in line with results from basic and translational investigations that implicate a role of haemostatic activation in tumour progression and metastasis.(6, 33, 34) Tumour cells that are able to activate the haemostatic system seem to have an evolutionary advantage and tend to be more aggressive in their natural behaviour. Another potential explanation might be a non-causative association. Fast growing, aggressive tumours often exceed natural blood-vessel growth and thereby develop areas of necrosis. This ongoing local trigger might cause systemic hypercoagulability.
Our results are consistent with previous clinical studies in colorectal cancer that suggested elevated haemostatic biomarkers as markers for shorter OS and time to recurrence in patients after tumour resection in the non-metastatic setting and for worse prognosis and therapy response for patients with unresectable or metastatic disease.(17–20, 35, 36) By utilizing a pre-existing dataset of an ongoing prospective, observational study (Vienna Cancer and Thrombosis Study, CATS) and a panel of different biomarkers, we were able to create a cohort that is homogeneous in respect of clinical scenario, as all patients included in the present analysis were initiating systemic chemotherapy due to metastatic disease with high quality of follow up data, due to existing parameters collected within the framework of a prospective observational study and the routine nature of the parameters collected in retrospect.
However, our analysis has several limitations: (a) Despite numerical differences and a clear trend in our outcome variables, for example in PFS, several results in our analysis did not reach statistical significance and some did not show consistent results in separate statistical models. As an example, sP-selectin was associated with significantly higher mortality in uni- and multivariable Cox-regression, however, no significant difference in median OS was seen in Kaplan-Meier-estimates. There could be various explanations for the inconsistent results. The study design might lack statistical power for specific endpoints and thereby increase the rate of false-negative test results, as implied by large quantitative differences in outcomes estimates in combination with broad confidence-intervals. Moreover, the haemostatic system is influenced by a variety of processes (e.g. inflammation, aging, thrombotic events, etc.) and therefore these markers might have some degree of variability due to lack of specificity. The results of the thrombin generation assay did not significantly predict for any oncologic outcomes in this setting. This marker is the only one in our panel that does not directly indicate in-vivo activity of the coagulation system, but rather represents the result of an in-vitro assay, displaying hypothetical activation potential of haemostasis and seem not to be well suited in predicting oncological outcomes in patients with mCRC. (b) Our analysis could further be limited by its procedure of data collection. Some of our endpoints had to be redefined and the variables had to be collected in retrospect, as they have not been originally defined within CATS. This might lead to limitations in data quality. However, the outcome parameters are all easily objectified and routinely assessed and documented in clinical follow-up of an oncological patient as they reflect either radiological confirmed therapy response or death, therefore the likelihood of bias is low. (c) Another limitation of this study is the possibility of an inflation of the type-1-error due to multiple testing. Therefore, we would like to emphasize the hypothesize-generating nature of the present study. Subsequent studies have to be conducted before clinical implication should be drawn. (d) As a limiting factor in regard of biomarker measurement, for certain biomarkers used within the analysis, such as the thrombin generation assay, no standardized methodology is available. Further, residual influences of known and unknown factors interfering with haemostatic biomarker measurements cannot be fully excluded. However, all measurements were conducted in accordance to previous publications and the manufacturer′s instructions of utilized assays. (e) An additional limiting factor is the selection of an appropriate cut-off for dichotomization of biomarkers. To select a uniting threshold rather than individual cut-offs for each marker simplifies interpretation of results. However, the selection of a specific cut-off is limited by its subjectivity. As we were interested in clinical outcomes of patients with especially high haemostatic biomarkers, the comparison of patients above the 75th patients to those below has been chosen, according to previous publications.(16, 27)
Conclusion
In conclusion, we found an association of specific haemostatic biomarkers with decreased survival and increased mortality. Further, we observed an association of selected elevated biomarkers with worse therapy response profiles. Thus, our results provide clinical evidence for an association between elevated haemostatic biomarkers and aggressive biological behaviour in metastatic colorectal cancer.
Supplementary Material
Declarations & Acknowledgments
Funding
This work was supported by the Anniversary Fund of the Austrian National Bank [grant number 17828]; and the Austrian Science Fund (FWF) [Special Research Program (SFB) 54].
Abbreviations
- ATE
Arterial thrombotic event
- BMI
Body mass index
- CATS
(Vienna-) Cancer and Thrombosis Study
- CI
Confidence Intervals
- DCR
Disease control rate
- EGFR
Epidermal growth factor receptor
- F1+2
Prothrombin fragment F1+2
- FVIII
Coagulation Factor VIII
- HR
Hazard ratio
- IQR
Inter-quartiles range
- mCRC
Metastatic colorectal cancer
- OR
Odds ratio
- OS
Overall survival
- PARs
Proteinase activated receptors
- PFS
Progression free survival
- sP-selectin
soluble P-selectin
- TF
Tissue factor
- VEGF
Vascular endothelial growth factor
- VTE
Venous thromboembolism
Footnotes
Ethics approval and consent to participate
The study was approved by the local ethics committee (ethics committee of the Medical University of Vienna; number: 126/2003; ethik-kom@meduniwien.ac.at) and conducted in accordance with the declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Authors: Florian Moik (FM), Florian Posch (FP), Ella Grilz (EG), Werner Scheithauer (WS), Ingrid Pabinger (IP), Gerald Prager (GP), Cihan Ay (CA)
Conceived and designed the study: FM IP CA; Conducted study procedures (patient recruitment, blood sampling, biomarker measurement): FM FP EG IP CA; Collected data: FM FP EG; Analyzed the data: FM FP EG CA; Interpreted the results: FM FP EG WS IP GP CA; Wrote the first draft of the manuscript: FM CA; Contributed to the writing of the manuscript: FM FP EG WS IP GP CA
References
- 1.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(29):4902–11. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falanga A, Marchetti M. Hemostatic biomarkers in cancer progression. Thrombosis research. 2018;164(Suppl 1):S54–s61. doi: 10.1016/j.thromres.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thrombosis and haemostasis. 2017;117(2):219–30. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 4.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. European journal of cancer (Oxford, England : 1990) 2013;49(6):1404–13. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon W, Melton L, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Archives of Internal Medicine. 2000;160(6):809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 6.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Bioscience reports. 2013;33(5):e00064. doi: 10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(10):2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 8.Yin YJ, Salah Z, Grisaru-Granovsky S, Cohen I, Even-Ram SC, Maoz M, et al. Human protease-activated receptor 1 expression in malignant epithelia: a role in invasiveness. Arterioscler Thromb Vasc Biol. 2003;23(6):940–4. doi: 10.1161/01.ATV.0000066878.27340.22. [DOI] [PubMed] [Google Scholar]
- 9.Schaffner F, Ruf W. Tissue factor and protease-activated receptor signaling in cancer. Seminars in thrombosis and hemostasis. 2008;34(2):147–53. doi: 10.1055/s-2008-1079254. [DOI] [PubMed] [Google Scholar]
- 10.Stegner D, Dutting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thrombosis research. 2014;133(Suppl 2):S149–57. doi: 10.1016/S0049-3848(14)50025-4. [DOI] [PubMed] [Google Scholar]
- 11.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell. 2011;20(5):576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 13.Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128(1):24. doi: 10.1182/blood-2016-01-636399. [DOI] [PubMed] [Google Scholar]
- 14.Arpaia G, Carpenedo M, Verga M, Mastrogiacomo O, Fagnani D, Lanfredini M, et al. D-dimer before chemotherapy might predict venous thromboembolism. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2009;20(3):170–5. doi: 10.1097/MBC.0b013e32831bc2de. [DOI] [PubMed] [Google Scholar]
- 15.Stender MT, Frokjaer JB, Larsen TB, Lundbye-Christensen S, Thorlacius-Ussing O. Preoperative plasma D-dimer is a predictor of postoperative deep venous thrombosis in colorectal cancer patients: a clinical, prospective cohort study with one-year follow-up. Diseases of the colon and rectum. 2009;52(3):446–51. doi: 10.1007/DCR.0b013e318197e2b2. [DOI] [PubMed] [Google Scholar]
- 16.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac A-L, Drach J, et al. D-Dimer and Prothrombin Fragment 1 + 2 Predict Venous Thromboembolism in Patients With Cancer: Results From the Vienna Cancer and Thrombosis Study. Journal of Clinical Oncology. 2009;27(25):4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Liu B, Zhao Y, Liu L, Yang C, Yang Y, et al. High levels of D-dimer correlated with disease status and poor prognosis of inoperable metastatic colorectal cancer patients treated with bevacizumab. Journal of Cancer Research and Therapeutics. 2014;10(8):246–51. doi: 10.4103/0973-1482.151451. [DOI] [PubMed] [Google Scholar]
- 18.Inanc M, Er O, Karaca H, Berk V, Ozkan M, Dikilitas M, et al. D-dimer is a marker of response to chemotherapy in patients with metastatic colorectal cancer. Journal of BUON : official journal of the Balkan Union of Oncology. 2013;18(2):391–7. [PubMed] [Google Scholar]
- 19.Blackwell K, Hurwitz H, Lieberman G, Novotny W, Snyder S, Dewhirst M, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. doi: 10.1002/cncr.20336. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe A, Araki K, Harimoto N, Kubo N, Igarashi T, Ishii N, et al. D-dimer predicts postoperative recurrence and prognosis in patients with liver metastasis of colorectal cancer. International Journal of Clinical Oncology. 2018;23(4):689–97. doi: 10.1007/s10147-018-1271-x. [DOI] [PubMed] [Google Scholar]
- 21.Tomimaru Y, Yano M, Takachi K, Kishi K, Miyashiro I, Ohue M, et al. Correlation between pretherapeutic d-dimer levels and response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2008;21(4):281–7. doi: 10.1111/j.1442-2050.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 22.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 23.Tejpar S, Van Cutsem E, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Annals of Oncology. 2016;27(8):1386–422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 24.Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158. doi: 10.3324/haematol.2011.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vormittag R, Simanek R, Ay C, Dunkler D, Quehenberger P, Marosi C, et al. High Factor VIII Levels Independently Predict Venous Thromboembolism in Cancer Patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(12):2176–81. doi: 10.1161/ATVBAHA.109.190827. [DOI] [PubMed] [Google Scholar]
- 26.Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of Venous Thromboembolism in Patients With Cancer by Measuring Thrombin Generation: Results From the Vienna Cancer and Thrombosis Study. Journal of Clinical Oncology. 2011;29(15):2099–103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 27.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008;112(7):2703. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 28.Tiedje V, Dunkler D, Ay C, Horvath B, Quehenberger P, Pabinger M, et al. The role of fibrinogen plasma levels, the -455G>A fibrinogen and the factor XIII A subunit (FXIII-A) Val34Leu polymorphism in cancer-associated venous thrombosis. Thrombosis and haemostasis. 2011;106(5):908–13. doi: 10.1160/TH11-04-0278. [DOI] [PubMed] [Google Scholar]
- 29.Koster T, Rosendaal FR, Reitsma PH, van der Velden PA, Briet E, Vandenbroucke JP. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms--the Leiden Thrombophilia Study (LETS) Thrombosis and haemostasis. 1994;71(6):719–22. [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer (Oxford, England : 1990) 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Weiser MR. AJCC 8th Edition: Colorectal Cancer. Annals of surgical oncology. 2018;25(6):1454–5. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 32.Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 33.Buller HR, Van Doormaal FF, Van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. Journal of Thrombosis and Haemostasis. 2007;5(s1):246–54. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 34.Langer F, Bokemeyer C. Crosstalk between cancer and haemostasis. Hamostaseologie. 2012;32(02):95–104. doi: 10.5482/ha-1160. [DOI] [PubMed] [Google Scholar]
- 35.Kilic M, Yoldas O, Keskek M, Ertan T, Tez M, Gocmen E, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2008;10(3):238–41. doi: 10.1111/j.1463-1318.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 36.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31(8):388–94. doi: 10.1093/jjco/hye075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.