Abstract
Context
Glucagon-like peptide-1 (GLP-1) is an incretin hormone used therapeutically in T2DM and obesity. The interplay between ambient fatty acids (FFA) and GLP-1, remains unclear. Acipimox suppresses adipose tissue lipolysis via activation of the PUMA-G (aka HCA2 and GPR109a) receptor.
Objective
To investigate if lowering of serum FFA level with acipimox affects GLP-1 secretion.
Design
Two randomized crossover studies were performed in human subjects. Rat intestine was perfused intraarterially and -luminally and L-cell were incubated with acipimox.
Participants
The participants were healthy overweight subjects and hypopituitary adult patients.
Interventions
The overweight participants received acipimox 250 mg 60 minutes prior to an oral glucose test. The hypopituitary patients received acipimox 250 mg 12, 9, and 2 h prior to and during the metabolic study day, where they were studied in the basal state and during a hyperinsulinemic euglycemic clamp.
Results
Acipimox suppressed FFA but did not affect insulin in the clinical trials. In overweight subjects, the GLP-1 increase after the OGTT (AUC, pmol/lxmin) was more than doubled [4,119±607 [Acipimox] vs. 1,973±375 [Control], P=0.004]. In hypopituitary patients, acipimox improved insulin sensitivity (mg glucose/kg/min): 4.7±0.8 [Acipimox] vs. 3.1±0.5 [Control], P=0.005, and GLP-1 concentrations increased approximately 40%. An inverse correlation between FFA and GLP-1 concentrations existed in both trials. In rat intestine, acipimox did not affect GLP-1 secretion and L-cells did not consistently express the putative receptor for acipimox.
Conclusions
Acipimox treatment enhances systemic GLP-1 levels in both obese subjects and hypopituitary patients. Our in vitro data indicate that the underlying mechanisms are indirect.
Keywords: GLP-1, Insulin sensitivity, OGTT, glucose tolerance, lipolysis, free fatty acids
Introduction
Glucagon-like peptide-1 (GLP-1) is secreted from entero-endocrine L-cells in the intestinal epithelium in response to various macronutrients including luminal glucose (1), protein hydrolysates (2) as well as luminal and vascular lipids (3,4). Indeed, a variety of short-chain and long-chain fatty acids (SCFA and LCFA, respectively) directly stimulate GLP-1 secretion in several non-human, experimental models (4–8) via mechanisms involving free fatty acid (FFA)-sensitive G-protein coupled receptors (FFAR) (9). GLP-1 in turn stimulates insulin secretion and inhibits gastric emptying, and analogs of GLP-1 are used to treat type 2 diabetes (T2D). Moreover, strategies to enhance the endogenous production and secretion of GLP-1 are currently being investigated (9).
Circulating free fatty acids (FFA) are assumed to play a causal role for the pathogenesis of hepatic and peripheral insulin resistance (10–13), which are hallmarks of T2D (5). Acipimox is a nicotinic acid analogue that binds to and activates the protein up-regulated in macrophages by interferon-γ (PUMA-G) receptor (14) [mammal equivalent HCA2 (15) and also known as GPR109a], resulting in inhibition of the hormone sensitive lipase (HSL) and thereby suppression of lipolysis and reduced circulating levels of FFA and triglycerides (16). Acipimox also improves insulin sensitivity in obese individuals (17) by mechanisms that presumably depend on the lipid lowering actions. A recognized challenge when performing and interpreting acipimox studies is a rebound increase in FFA levels induced by feedback stimulation of GH and ACTH (18), which can be circumvented by studying hypopituitary subjects on stable replacement with GH and hydrocortisone (19).
Since the impact of circulating FFA levels on GLP-1 concentration remains unclear, we tested acute and prolonged effects of acipimox on basal and glucose-stimulated GLP-1 concentration in hypopituitary patients and in obese subjects. This was followed by in vitro studies utilizing an isolated perfused rat small intestine model and a GLP-1 secreting cell line.
Research Design and Methods
Clinical studies
Two in vivo studies in human subjects were conducted in accordance with the Helsinki Declaration, where all subjects gave their oral and written informed consent to participate. The regional Ethics Committee System approved both study protocols, and protocol 2 was also approved by the Danish Medicines Agency. The protocols were registered at Clinicaltrials.gov NCT02796950 and NCT01209416 before the onset of enrolment.
Subjects
Study 1: Eight overweight but otherwise healthy men with a mean ± SE age of 30 ± 3 years and a BMI of 30.0 ± 0.7 kg/m2 participated.
Study 2: The subjects in Study 2 comprised eight hypopituitary men, mean age 53 ± 5 years and with a mean BMI of 30.3 ± 4.6 kg/m2, on stable replacement therapy with hydrocortisone and GH, as previously described (20). Mean circulating HbA1c levels at screening were 5.5 ± 0.1 % (37 ± 1 mmol/mol). None of the patients had diabetes or any other concomitant chronic disease.
Study protocols
All participants were examined on two occasions separated by a minimum of two weeks. After an overnight fast, the subjects were studied in a quiet, thermo-neutral indoor environment in the supine position. The subjects fasted during the trials.
Study 1: At the onset of the study, an i.v. cannula was inserted into a dorsal hand vein for frequent blood sampling. The hand was wrapped in a heat pad in order to obtain arterialized venous blood samples. In a randomized, non-blinded study the participants were studied twice after an overnight fast receiving either one oral acipimox dose of 250 mg at t = 0 [Aci] or no medication (including placebo) [Control]. After 60 minutes (t = 60) an oral glucose tolerance test (OGTT) with 75 g of glucose solution was performed. Blood sampling was conducted every 15 minutes from t = 0 to 240 min.
Study 2: All patients continued replacement therapy with GH and hydrocortisone during the study; GH was administered subcutaneously at 22.00 hr. before the metabolic study day and hydrocortisone was administered at 08.00 hr. on the metabolic study day with the patient’s regular replacement dose. Each subject underwent two interventions receiving either acipimox [Aci] or placebo [Control] in a double-blind crossover design. Four doses of oral acipimox capsules 250 mg or placebo were administered, of which two doses were administered at 20.00 and 23.00 hr. on the evening before, and two doses were administered at 06.00 and 10.00 hr. on the day of the metabolic study.
The metabolic studies were performed between 08.00 and 13.00 hr. (0-300 min). The subjects were studied in the basal postabsorptive state ('basal') for 120 min followed by a hyperinsulinemic/euglycemic clamp ('clamp') for 180 min, during which they received a constant infusion of insulin (0.6 mU/kg/min; Actrapid, Novo Nordisk, Gentofte, Denmark). During the insulin infusion, plasma glucose was clamped at 5.0 mmol/l by adjusting the rate of infusion of 20% glucose according to plasma glucose measurements carried out every 10 min. Insulin sensitivity was estimated by the level of glucose infusion rate (GIR) during the last 30 min of the clamp. Additional blood samples were drawn every 30 min and analyzed for insulin, C-peptide, GLP-1, and FFA. Clamp data from this study not including GLP-1 or GIP measurements have previously been published (20).
Animal and cell studies
The studies were conducted with permission from the Danish Animal Experiments Inspectorate (2013-15-2934-00833) and the local ethics committee in accordance with the guidelines of Danish legislation governing animal experimentation (1987) and the National Institutes of Health (publication number 85-23).
Isolated perfused rat intestine procedure: Male Wistar rats were obtained from Janvier (Saint Berthevin Cedex, France), housed two per cage with ad libitum access to standard chow and water, and kept on a 12:12-hr. light-dark cycle. Animals were allowed at least one week of acclimatization. On the respective day of study, (non-fasted) rats (mean ± SE weight 443 ± 28 g) were anesthetized by a subcutaneous injection with Hypnorm/Midazolam, placed on a heated table (37°C) and the abdomen opened by a midline insertion. The entire large intestine was removed after ligation of the supplying vasculature, and the small intestine (still left in situ) was perfused (7.5 mL/min) with a modified Krebs-Ringer buffer through a catheter inserted in the upper mesenteric artery. Immediately after insertion of the first catheter, a second catheter was inserted into the portal vein to collect perfusion effluent. As soon as proper perfusion flow was established, the rat was euthanized by diaphragm perforation. The Krebs-Ringer buffer contained in addition 0.1 % (w/v) BSA (Fraction V; cat. no. 1.12018.0500, Merck, Ballerup, Denmark), 5 % (w/v) dextran T70 to balance osmolarity (Pharmacosmos, Holbaek, Denmark), and 5 mM pyruvate, fumarate and glutamate and 10 μM 3-isobutyl-1-methylxanthine (IBMX, Cat no. I5879, Sigma Aldrich), 3.5 mM glucose. Prior to infusion, the buffer was pH adjusted to 7.4-7.5 and bubbled with 95 % O2, 5 % CO2 to maximally elevate the O2 concentration and ensure a pH of around 7.3-7.4 (monitored throughout). A UP100 Universal Perfusion System from Hugo Sachs (Harvard Apparatus, March Hugstetten, Germany) was used, which amongst others ensures that the perfusion buffer was 37°C when reaching the organ. The preparation was allowed to stabilize for 25 min before collection of the first sample. Hereafter samples were collected each minute and instantly transferred onto ice before being stored at -20°C until hormone analysis (within two weeks). Vascular test stimulus was administered intra-arterially (into the upper mesenteric artery) and consisted of acipimox (200 μM, Cat. no. A7856, Sigma Aldrich), niacin (Cat. no. 1461003-200MG, Sigma Aldrich), 3-hydroxybuturate (Cat. no. 226491-5G, Sigma Aldrich), or bombesin acetate salt hydrate (positive control, 10 nM, Cat. no. B4272, Sigma Aldrich); all prepared in perfusion buffer and adjusted to pH 7.4-7.5. In separate experiments, acipimox (200 μM) was prepared in isotonic saline and administered intra-luminally at an initial rate of 2.5 ml/min for 5 min followed by a flow rate of 0.5 ml/min for the remaining of the stimulus period. The bolus administration was included to rapidly replace the isotonic saline present in the lumen at start of stimulation. At the end of stimulation, a similar bolus of isotonic saline was administered followed by a flow of 0.5 ml/min for the remaining of the experiment. In all experiments, bombesin was added intraarterially at the end of the protocols to control for responsiveness of the perfused preparation. The methods are described in more detail elsewhere (1). Group sizes were 5-7 as indicated in the result section and figure legends.
GLUTag cell studies: GLUTag cells (21) were kindly provided by Prof. Dan J. Drucker (Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Canada). Cells were grown at 37°C, 5 % CO2 in Nucleon coated T75 flasks (Cat. No. EW-01930-54, Thermo Fisher Scientific, MA) using low glucose (1.0 g/L) DMEM medium (Cat No. 6046, Sigma-Aldrich) supplemented with 10 % (v/v) FBS and 1 % (v/v) penicillin (10,000 U/mL)/streptomycin (10,000 μg/mL) and GlutaMAX (Cat. No. 35050061, Gibco, Life Technologies Corporation, CA). Cells were stripped and re-plated when reaching 70-80 % confluence. Approximately 18-24h before secretion experiments, cells were plated onto 24-wells plates pre-coated with matrigel basement membrane (Cat. No. 354234, BD Biosciences, Kongens Lyngby, Denmark). On the following day, the now attached and approximately 80 % confluent cells were thoroughly washed with isotonic saline (37°C) and incubated for 2h with buffer alone (baseline) or test stimulus prepared in buffer. Supernatants were obtained and centrifuged (1500 x g, 4°C, 5 min) to remove potential floating cells of debris. The resulting supernatants were transferred to fresh Eppendorf tubes and samples were stored at -20°C until analysis. Test stimulus consisted of 0.1, 1, 10 or 100 mM sodium-hydroxybutyrate (Cat no. 54965, Sigma Aldrich) or 10 mM glucose + 10 μM forskolin (Cat. no. F3917, Sigma Aldrich) IBMX. The buffer contained 138 mM NaCl, 4.5 mM KCl, 4.2 mM NaHCO3, 1.2 mM NaH2PO4, 2.5mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES supplemented with 0.1 % (w/v) fatty acid-free BSA (Cat. No. A-603-10G, Sigma-Aldrich). Prior to secretion experiments, the buffer was pH calibrated to 7.4.
Cell sorting and RNA sequencing
Single cell digestion and cell sorting: L-cells from three different regions of the GI tract were isolated as previously described (22). In brief, the top and bottom 10 cm of the small intestine and the colon plus rectum were harvested from Glu-Venus mice euthanized by cervical dislocation (UK Home office license 70/7824), washed with cold PBS and the muscle layer removed. Tissue was sliced and digested into single cells with 1 mg/mL collagenase and single cells, after filtering with a 50 μm filter, were sorted on a JAZZFacs at the Cell Phenotyping Hub, Cambridge. 20,000 negative and 2,000-10,000 positive cells were directly collected into lysing solution (RLT+ buffer with 1 % β-mercaptoethanol) and RNA extracted using a micro plus RNeasy kit (Qiagen). RNA quality (>6) and concentration were checked on Bioanalyzer (Agilent). Library construction and sequencing: two ng of RNA from each sample were used for cDNA amplification using an Ovation RNAseq system V2 kit (Nugen) and after fragmentation to ~200 bp (Diagenode sonicator), adaptors for indexing were added (Ovation rapid DR multiplex1-96, Nugen). Samples were pooled and single-end 50 bp sequenced at the Genomic Core, CRUK Cambridge with an Illumina Hiseq4000 system. Sequence reads were demultiplexed using the Cava pipeline (Illumina) and aligned to the mouse genome (GRCm38) using Top Hat v2.1.0 and raw counts were obtained using Cufflinks v2.2.1. Data were then normalized and differential expression assessed using DESeq2 (23).
Biochemical analyses
Plasma glucose was analyzed bedside using the glucose oxidase method (YSI 2300 STAT Plus; YSI Life Sciences, Yellow Springs, OH) whereas serum and plasma samples for other metabolites and hormones were frozen and stored at -20°C or at -80°C (glucagon, GIP, and GLP-1). Serum FFA was analyzed by a commercial kit (Wako Chemicals, Neuss, Germany). Serum GH was analyzed using chemiluminescence technology (IDS-iSYS Multi-Discipline Automated Analyzer, Immunodiagnostic Systems Nordic a/s Herlev, Denmark). Serum insulin was analyzed using time-resolved fluoroimmunoassay assay (AutoDELFIA Insulin kit, catalog no. B080–101, PerkinElmer, Turku, Finland). Samples for GIP and GLP-1 were drawn into EDTA and aprotinin containing chilled tubes (400 kallikrein inhibitor units/mL blood; Bayer, Leverkusen, Germany) and kept on ice. Plasma total GIP and GLP-1 were analyzed as previously described (24,25). GLP-1 secretion from the perfused intestine and GLUTag cells was measured using the same radioimmunoassay for total GLP-1 (measuring intact peptide + the primary metabolite GLP-1 [9–36amide]) using antiserum code no. 89390, recognizing the amidated C-terminus of the molecule, as described previously (26). Since the gut was perfused at a constant rate, concentrations parallel output (= secretion). The choice of targeting the amidated (x-36amide) rather than the glycine extended (x-37) isoform was based on a recent study showing that GLP-1 is predominantly amidated in rats (27). The experimental detection limit was 1 pM, and the intra-assay coefficient of variation of 6 %.
Statistical analysis
Results are expressed as mean ± standard error of the mean (mean ± SEM). Clinical studies: The statistical analyses were performed using SigmaPlot 11.0 (©Systat Software, CA). Effects of acipimox on plasma glucose, GIP, GLP-1, GH, insulin, C-peptide, and serum FFA were analyzed by comparing the areas under concentration-time curves (AUC). GIR and concentrations at single time points were analyzed by Student’s two-tailed paired t-test. Correlation analyses were performed using Pearson’s Product Moment test for normal distributed data and Spearman’s Rank Order for non-normal distributed data. A P value < 0.05 was considered significant. Animal and cell line studies: For perfusion experiments, outputs at baseline and under stimulus administration (response) are shown. Response outputs were calculated as the sum of secretion over the stimulation period (15 min) divided by the time course. Baseline output was also calculated as the averaged secretion over 15 min duration, but in order to correct for potential drift in secretion over the time course of the experiment, values were taken from 8 min that lead immediately up to the stimulus administration and the following 7 min before administration of the positive control (e.g. after acipimox administration). Statistical significance was assessed using GraphPad Prism software (La Jolla, CA) by a Student’s two-tailed paired t test (perfusion experiments) or Oneway ANOVA for repeated measurements with Tukey posthoc comparison (GLUTag studies).
Results
Study 1
Plasma GLP-1
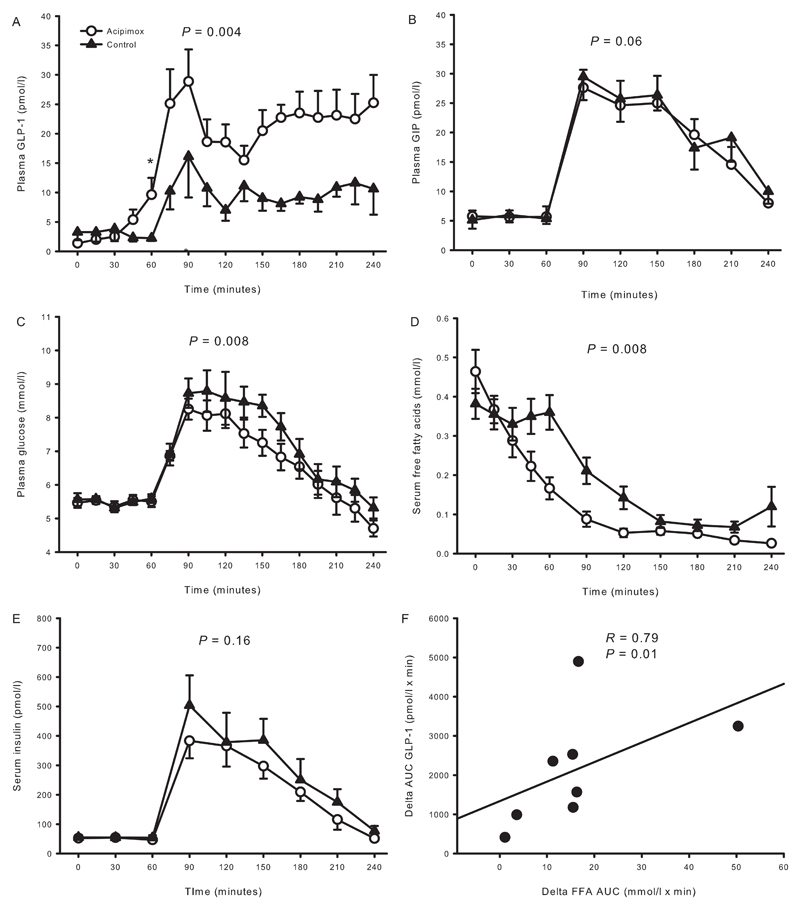
Plasma GLP-1 concentrations were similar at t = 0 min (pmol/l) [1.4 ± 0.2 [Aci] vs. 3.3 ± 1.3 [Control], P = 0.23] (Figure 1A) but increased after 60 min in response to acipimox treatment [9.6 ± 2.9 [Aci] vs. 2.3 ± 0.5 [Control], P = 0.044]. Plasma GLP-1 increased in response to the OGTT but more so in the presence of acipimox pretreatment (AUC, pmol/l x min) [4,119 ± 607 [Aci] vs. 1,973 ± 375 [Control], P = 0.004].
Figure 1.
Hormones and metabolites from study 1 comprising overweight healthy men. A: Plasma GLP-1 increased during acipimox treatment. B: Acipimox did not impact on plasma GIP levels. C: Plasma glucose increased to a lesser extent after the OGTT during acipimox treatment. D: Serum FFA were suppressed more after the OGTT during acipimox treatment. E: Acipimox did not impact on serum insulin levels. F: The change in AUCs (delta FFA: Control minus Acipimox; delta GLP-1: Acipimox minus Control) between acipimox treatment and the control experiment in FFA and GLP-1 were compared and revealed a significant correlation. Acipimox: white circles, control: black triangles. Printed P values refer to comparison of AUCs. Asterisk indicates significant differences in a pairwise comparison. All data are presented as mean ± SEM.
Plasma GIP
Plasma GIP concentrations were similar at t = 0 min (pmol/l) [5.8 ± 1.0 [Aci] vs. 5.1 ± 1.5 [Control], P = 0.65] (Figure 1B). There was no significant effect of acipimox on the OGTT-stimulated GIP concentrations (pmol/l x min) [3,082 ± 357 [Aci] vs. 3,774 ± 516 [Control], P = 0.06].
Plasma glucose
Plasma glucose concentrations were similar at t = 0 min (mmol/l) [5.5 ± 0.1 [Aci] vs. 5.6 ± 0.2 [Control], P = 0.61] (Figure 1C). Plasma glucose was not acutely affected by acipimox treatment [t = 60 min 5.5 ± 0.2 [Aci] vs. 5.9 ± 0.9 [Control], P = 0.51], but the response was blunted in the acipimox experiment during the OGTT (mmol/l x min) [1,222 ± 49 [Aci] vs. 1,320 ± 56 [Control], P = 0.008].
Serum free fatty acids
Serum FFA levels were similar at t = 0 min (mmol/l) [0.46 ± 0.06 [Aci] vs. 0.38 ± 0.04 [Control], P = 0.11] (Figure 1D). FFA concentrations decreased from t = 0 min in response to acipimox administration, and the suppression of FFA in response to the OGTT was accentuated by acipimox (AUC, mmol/l x min) [29.3 ± 3.9 [Aci] vs. 45.5 ± 4.6 [Control], P = 0.008]. The change in FFA and GLP-1 concentrations were compared by a correlation analysis of the change in the area under concentration-time curves of GLP-1 and of FFA, and revealed a significant inverse association as a larger decrease in FFA correlated significantly to a larger increase in GLP-1, R = 0.79, P = 0.01 (Figure 1F).
Serum insulin
Serum insulin concentrations were similar at t = 0 min (pmol/l) [52.4 ± 8.8 [Aci] vs. 55.1 ± 8.5 [Control], P = 0.58] (Figure 1E). Serum insulin increased to approximately the same extent in response to the OGTT [t = 90 min, serum insulin 383.3 ± 59.5 [Aci] vs. 503.9 ± 101.8 [Control], P = 0.23], and the AUCs were similar (pmol/l x min) [42,596 ± 4,684 [Aci] vs. 52,751 ± 9,620 [Control], P = 0.16].
Study 2
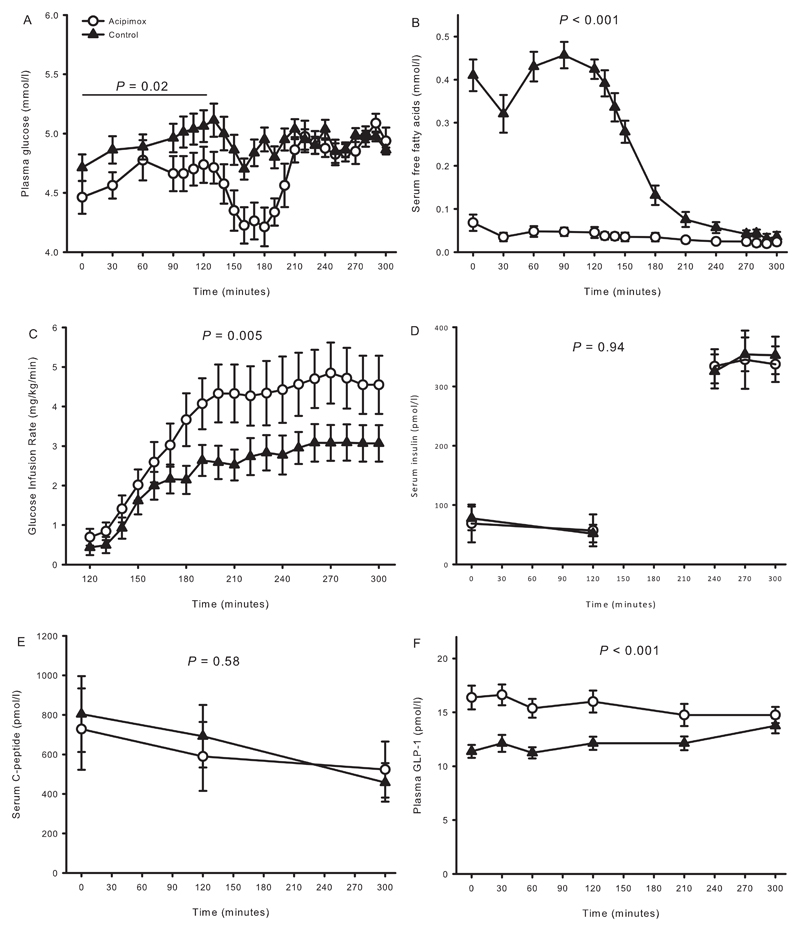
Data on metabolites and insulin sensitivity were reported previously (20). In summary, plasma glucose levels (mmol/l) were similar at baseline (t = 0 min) [4.5 ± 0.1 [Aci] vs. 4.7 ± 0.1 [Control], P = 0.06] (Figure 2A), but became significantly reduced during the ensuing basal period (t = 0-120 min) after acipimox as compared to placebo, P = 0.02. During the terminal 30 minutes of the clamp, plasma glucose levels were similar (mmol/l) [5.0 ± 0.0 [Aci] vs. 5.0 ± 0.1 [Control], P = 0.90]. Acipimox suppressed basal serum FFA levels (mmol/l) [0.07 ± 0.02 [Aci] vs. 0.41 ± 0.04 [Control], P < 0.001] (Figure 2B), and the FFA levels were lower throughout the acipimox study days (P < 0.001). Peripheral insulin sensitivity, as expressed by the GIR (mg/kg/min), improved by acipimox [4.7 ± 0.8 [Aci] vs. 3.1 ± 0.5 [Control], P = 0.005] (Figure 2C). Serum insulin levels (Figure 2D) increased and serum C-peptide (Figure 2E) levels decreased during the clamp independently of treatment.
Figure 2.
Metabolites, glucose infusion rate, and hormones from study 2 comprising hypopituitary men treated with overnight acipimox. A: Plasma glucose was lower during acipimox treatment during basal conditions and later clamped at the same level. B: serum FFA were decreased during acipimox treatment. C: Glucose infusion rates were increased during acipimox treatment. D and E: Acipimox did not impact on serum insulin and C-peptide levels. F: Plasma GLP-1 levels were increased in response to acipimox treatment and were normalized at the very end of the clamp period.
Acipimox: white circles, control: black triangles. Printed P values refer to comparison of AUCs. All data are presented as mean ± SEM.
Plasma GLP-1
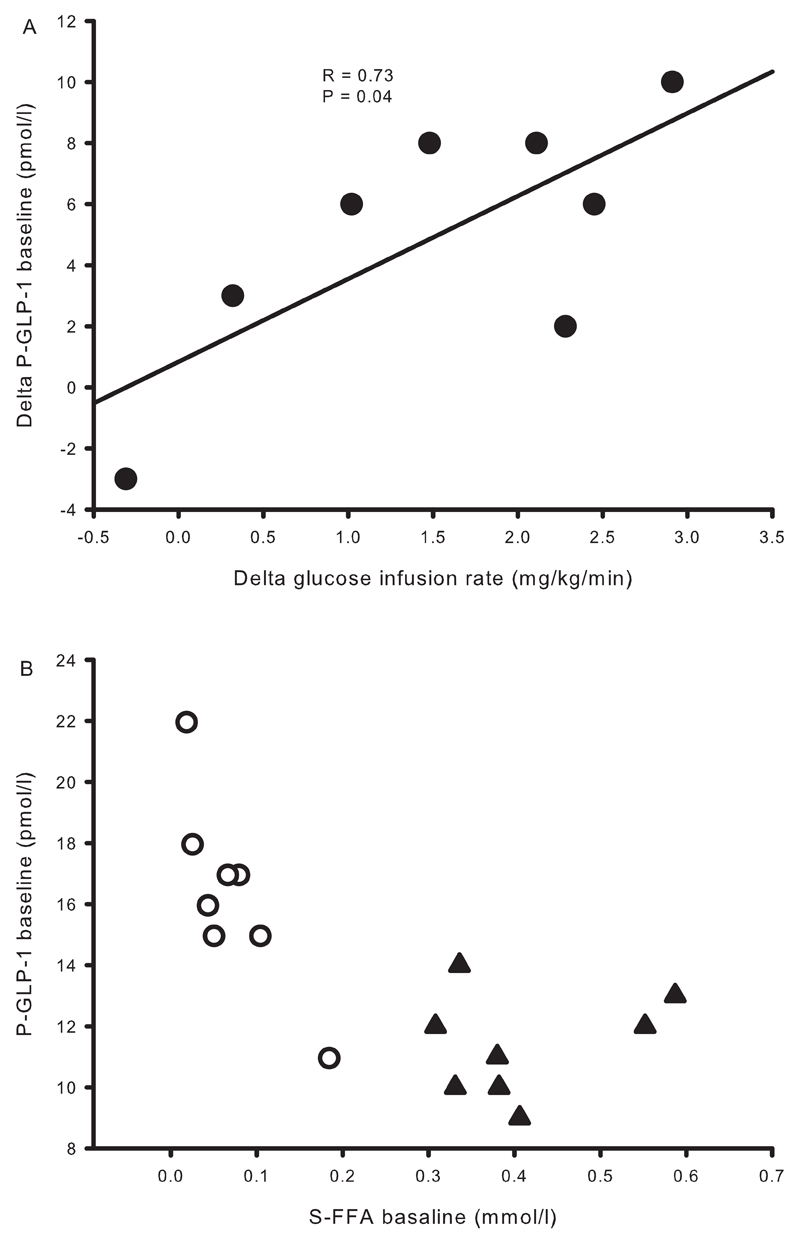
Baseline plasma GLP-1 levels increased with acipimox exposure (pmol/l) [16.4 ± 1.1 [Aci] vs. 11.4 ± 0.6 [Control], P < 0.001] (Figure 2F), and remained elevated during the basal and the clamp periods (P < 0.001, compared to the control situation). The change in basal GLP-1 and the change in insulin sensitivity were compared, and correlation analysis revealed that the increase in basal plasma GLP-1 concentration was positively associated with the increase in insulin sensitivity in response to acipimox treatment, R = 0.73, P = 0.04 (Figure 3A). Baseline serum FFA and plasma GLP-1 levels are shown in Figure 3B, and correlation analyses revealed a significant inverse correlation at baseline, R = -0.73, P = 0.001.
Figure 3.
Correlation analyses from study 2. A: The difference in glucose infusion rates and plasma GLP-1 between acipimox treatment and the control situation shows a positive association. B: Basal concentrations of serum FFA correlated inversely with plasma GLP-1. Acipimox: white circles, control: black triangles.
Isolated perfused rat small intestine
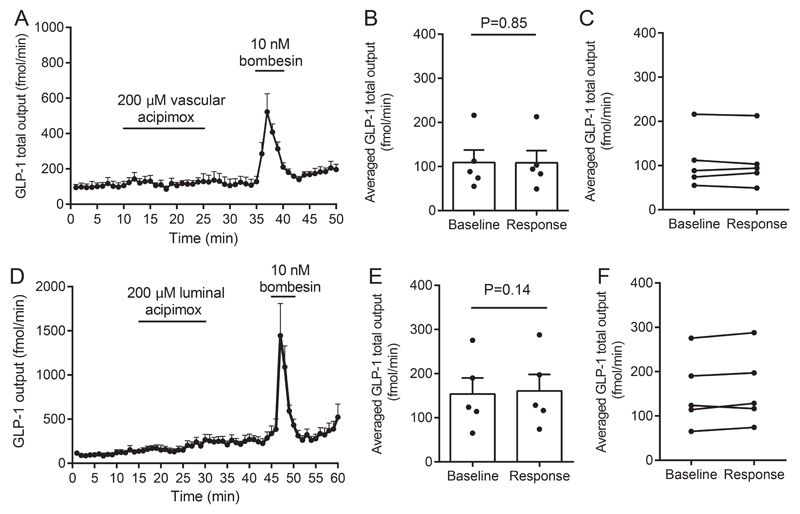
To investigate whether the acipimox-stimulated GLP-1 response observed in humans in vivo could be a direct effect on the intestinal L-cell, we next investigated the effects of acipimox on GLP-1 secretion from the isolated perfused rat small intestine. In one line of experiments, acipimox (200 μM) was administered intra-arterially, which did not affect GLP-1 secretion: Averaged GLP-1 secretion was 109 ± 29 fmol/min within the baseline period and 108 ± 28 fmol/min during intra-arterial acipimox administration (P = 0.85, Figure 4A and 4B, n = 5). In another line of experiments, acipimox was administered intra-luminally (200 μM), which also failed to increase GLP-1 secretion: Averaged baseline secretion = 154 ± 36 fmol/min vs. averaged secretion during acipimox stimulation = 161 ± 37 fmol/min (P = 0.14, Figure 4C and 4D, n = 5). Bombesin was included at the end of all experiments as positive control and robustly elevated GLP-1 secretion in all experiments.
Figure 4.
Effects of intra-arterial and luminal acipimox (200μM) on GLP-1 secretion from isolated perfused rat small intestine. Data are shown as means ± SEM. A: GLP-1 (total) output (fmol/min) at baseline and in response to intra-vascular acipimox (200 μM) or bombesin (BBS, 10 nM, positive control). B and C: Averaged GLP-1 output (fmol/min) during baseline and intra-arterial acipimox administration (response). D: GLP-1 (total) output (fmol/min) at baseline and in response to luminal acipimox (200 μM), E and F: Averaged GLP-1 output (fmol/min) during baseline and intra-luminal acipimox administration (response). Intra-arterial bombesin (BBS, 10 nM) was included in all experiments as positive control. Points indicate individual observations. nA-D = 5.
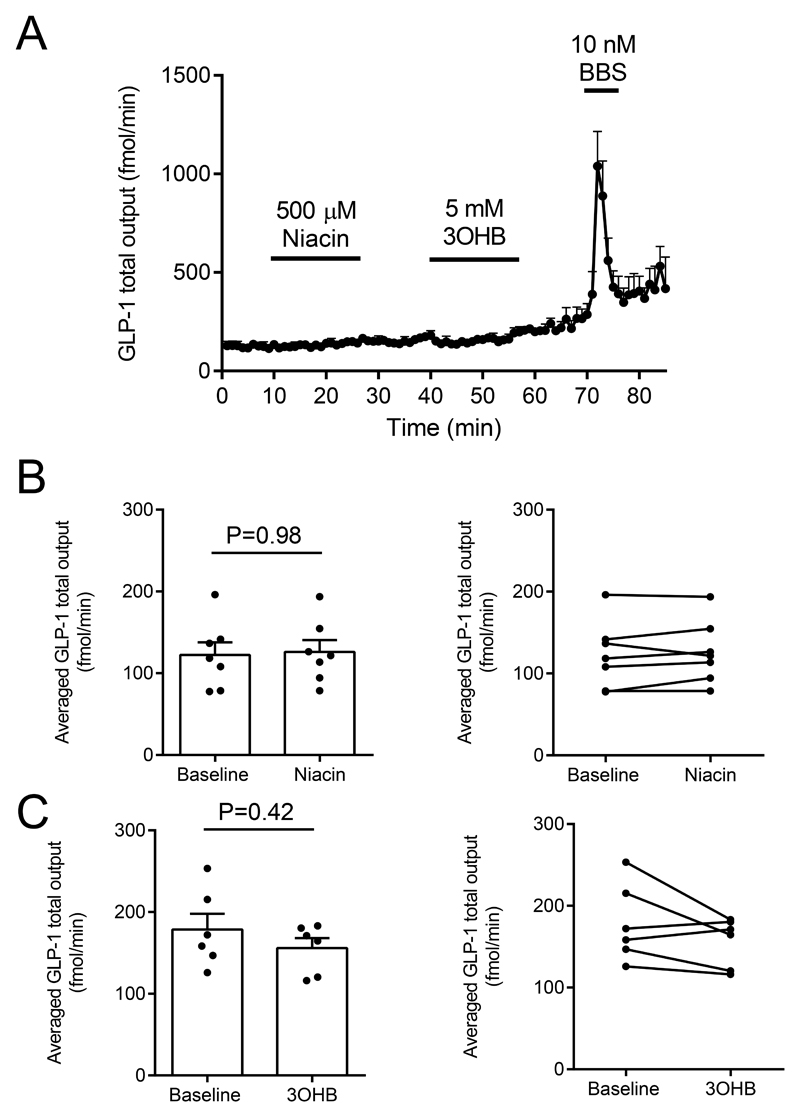
Additional experiments with intra-arterial niacin and 3-hydroxybutyrate administration were subsequently performed because these compounds also are ligands for the HCA2 receptor; these compounds also did not affect GLP-1 secretion (Figure 5, n = 6).
Figure 5.
A-C: Effects of intra-arterial niacin and 3-hydroxybuturate (3OHB) on GLP-1 secretion from isolated perfused rat small intestine. Intra-arterial bombesin (BBS, 10 nM) was included in all experiments as positive control. nA-C = 6.
GLUTag cell studies
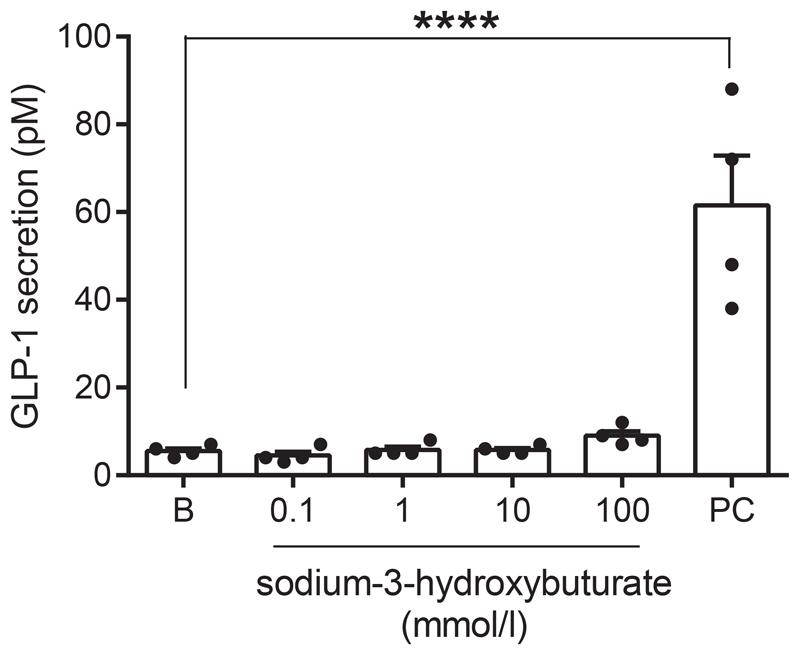
To ensure that the lack of a direct effect of acipimox on the L-cell was not model-dependent, we tested whether 3-hydroxybuturate, which is also a ligand of the PUMA-G receptor, stimulated GLP-1 secretion from the GLUTag cell line. Concentrations from 0.1-100 mM were tested, but in all cases GLP-1 secretion was not different from baseline secretion [Baseline secretion: 5.5 ± 0.6 pM, 0.1 mM: 4.5 ± 0.9 pM, 1 mM: 5.8 ± 0.9 pM, 10 mM: 5.8 ± 0.5 pM, 100 mM: 9.0 ± 1.1 pM, P > 0.99 for all groups compared to baseline] (Figure 6). In same line of experiments, 10 mM glucose + 10 μM FSK/IBMX (positive control) robustly increased GLP-1 secretion to 62 ± 11 pM, n = 4, P < 0.001 compared to baseline.
Figure 6.
GLP-1 secretion from GLUTag cells at baseline (B) and in response to sodium-3-hydroxybuturate in indicated concentrations or the positive control (PC, 10 mM glucose + 10μM FSK/IBMX). nD = 4, ****P < 0.001.
RNA sequencing
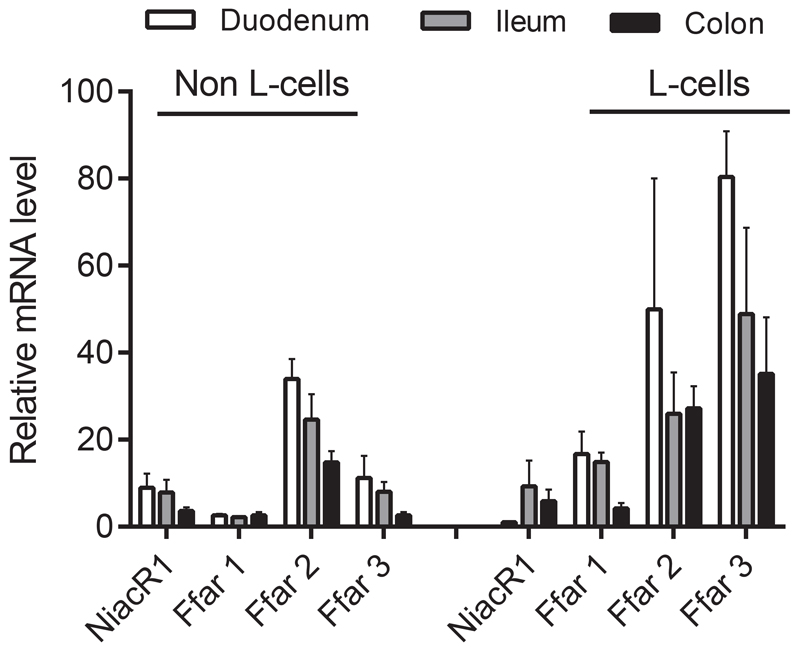
Because of the lack of direct effects of acipimox, niacin and 3-hydroxybuturate on GLP-1 secretion, we next investigated whether the PUMA-G receptor (NiacR1) was expressed by the L-cell. mRNA levels of NiacR1 and FFAR1-3 for comparison were measured in FACS sorted L-cells and non-L-cells (presumably dominated by enterocytes) from the mouse. Cells were sorted from biopsies taken from the upper and lower part of the small intestine and the colon, respectively. Whereas FFAR1-3, which are generally considered as part of the L-cell sensing machinery, were expressed at fairly high levels and to an enriched extent in L-cells, NiacR1 was expressed at low levels down the intestine and not enriched in L-cells (Figure 7).
Figure 7.
Relative expression levels of NiacR1 (also known as HCA2 receptor) in sorted primary L-cells and non-L-cells from mouse mucosa. Expression in biopsies taken from duodenum, ileum and colon is shown. Expression levels of the fatty-acid sensitive receptors FFAR1-3 are shown for comparison. Data are presented as mean ± SEM, n (duodenum) = 2, n (ileum and colon) = 3.
Discussion
This study reveals that acipimox administration in both obese subjects and hypopituitary patients amplify circulating GLP-1 levels, while subsequent experiments in a rat model and in vitro suggested that this is not a direct receptor-mediated effect of acipimox on the L-cell. GLP-1 lowers blood glucose via several mechanisms that include potentiation of glucose-stimulated insulin secretion, inhibition of gastric emptying, inhibition of food-intake, and attenuation of glucagon secretion (29,30). Because of these actions, several types of GLP-1 based drugs are used for the treatment of T2D and, more recently, for treatment of obesity (31). In addition, considerable research is devoted to uncover the molecular mechanisms controlling GLP-1 secretion in order to develop drugs that selectively stimulates GLP-1 secretion (9,32).
The primary aim of the present study was to investigate whether the anti-diabetic effect of acipimox could in part be attributable to a direct stimulatory effect on GLP-1 secretion. We demonstrated that acute and more prolonged acipimox intervention indeed increased circulating GLP-1 concentrations in human subjects, and we went on to investigate whether this was caused by direct interaction with the intestinal L-cells. To this end, we used the isolated perfused rat small intestine model (33), which allows for strict experimental control while maintaining critical physiological factors such as intestinal polarization and removal of secreted and absorbed products (by convective drag).
Acipimox binds to the G-protein coupled receptor named HCA2 encoded by the Hydroxycarboxylic Acid Receptor 2 gene (HCAR2) in adipose tissue and thereby inhibits the hormone sensitive lipase (14,34) leading to acute suppression of circulating FFA levels and improved peripheral insulin sensitivity (17). As mentioned, the FFA lowering effect of acipimox in patients with T2D is transient due to a counter-regulatory lipolytic response mediated by GH and ACTH (18), which can be abrogated by studying hypopituitary patients with GH and ACTH deficiency (19).
It has previously been demonstrated that acipimox acutely increases GLP-1 concentration in obese women (28,35), but it remained to be determined whether this effect was independent of GH and cortisol (18). Moreover, the previous reports on the stimulatory effect of acipimox on GLP-1 concentration measured intact GLP-1 7-36 amide (28,35), which has a very short half-life because of DPP-4 (dipeptidyl peptidase-4) mediated degradation to the inactive GLP-1 9-36amide (36). Thus, plasma levels of intact GLP-1 rarely reflect the secretion rate of GLP-1. In the present study, we measured total GLP-1, which more accurately reflects GLP-1 secretion. In hypopituitary male patients, we observed that GLP-1 concentrations increase by more than 40 % after an overnight fast during acipimox treatment and that the effect of acipimox on insulin sensitivity correlates with the change in GLP-1 levels. To determine whether acipimox stimulates GLP-1 secretion directly, we used the isolated and perfused rat small intestine model and examined the direct effects of intra-arterial as well as intra-luminal acipimox on GLP-1 output. In either case, acipimox failed to affect GLP-1 secretion, and two other ligands of the HCA2 receptor, 3-OHB and niacin also had no effect. Moreover, we performed studies on the GLP-1 secreting GLUTag cells stimulated with 3-OHB and quantified the expression of the PUMA-G receptor in sorted primary mouse L-cells. Consistent with the lack of effects in the isolated perfused rat small intestine model, 3-OHB did not affect GLP-1 secretion, and the expression of the PUMA-G receptor was low. Our studies therefore suggest that the stimulatory effects of acipimox are caused by indirect mechanisms not involving binding to the L-cell or interaction with other enteroendocrine cell types in the gut.
Species-specific differences between humans and rodents should, however, also be taken into account. Fasting induces lipolysis leading to an increase in circulating FFA levels in humans (37). GH secretion plays a significant role for fasting-induced lipolysis in humans (38), which is in contrast to mice (39). Rats do not naturally enter into prolonged fasting periods, and they exhibit a reversed dark-light cycle compared to humans and a different neuroendocrine pattern (40), both of which make extrapolations to human physiology difficult.
From a teleological and physiological point of view, it would make sense that high circulating FFA levels, which mainly occur during fasting conditions, suppress GLP-1 secretion and vice versa in human subjects in vivo. In support of this, an inverse correlation between FFA and GLP-1 has been demonstrated (35). Correlation analyses revealed that the acute decrease in FFA in our study in obese subjects showed a very close inverse correlation with the increase in GLP-1 after acipimox treatment, and FFA also correlated inversely with GLP-1 levels in the hypopituitary patients. In rats and mice, both vascular and luminal perfusion of the gut with LCFA and SCFA increases GLP-1 secretion by mechanisms that may involve FFAR1, -2, and -3 activation (4–8), which indirectly suggest a stimulatory effect of circulating FFA on GLP-1 secretion. Whether the results from our human study reflect a species-specific difference remains to be determined. An alternative interpretation of our data could be that improvement in intestinal insulin sensitivity because of low FFA improves the L-cell response to glucose and thereby amplifies GLP-1 secretion, but this also remains to be investigated.
Acknowledgements
Mrs. E. Hornemann and Mrs. L. Buus are acknowledged for excellent technical assistance. The data in study 2 were presented as an oral presentation at the Endocrine Society Annual Meeting 2015.
Grants and fellowships: The study was supported by a postdoctoral research fellow grant (11-105283) from the Danish Council for Independent Research (Medical Sciences) and grants from the Riisfort Fonden, and the A.P. Moller Foundation. The work in Prof. Holst lab was supported by an unrestricted grant to Prof. Jens Juul Holst from the Novo Nordisk Center for Basic Metabolic Research (Novo Nordisk Foundation, Denmark), a grant to Prof. Holst from the European Research Council (Grant no.695069) as well as a postdoc grant to Rune E. Kuhre from the Lundbeck Foundation (R264-2017-3492).
Footnotes
Author contributions
E.T.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions:
E.T.V: wrote protocol, screened patients, performed the clinical study, collected data, analyzed data, interpreted results, wrote first manuscript draft, edited and revised manuscript, created figures and table, literature research, approved the final manuscript.
N.J: analyzed data, interpreted results, edited and revised manuscript, literature research, approved the final manuscript.
J.J.H: generated and interpreted results, edited and revised manuscript, approved the final manuscript.
N.M: interpreted results, edited and revised manuscript, approved the final manuscript.
J.O.L.J: conceptualized study, study design, wrote protocol, analyzed data, interpreted results, edited and revised manuscript, literature research, approved the final manuscript.
A.J.H: wrote protocol, screened patients, performed the clinical study 1, collected data, analyzed data, interpreted results, approved the final manuscript.
R.E.K: generated and interpreted results, edited and revised manuscript, approved the final manuscript.
P.L: generated and interpreted some results, approved the final manuscript.
F.M.G: designed transcriptomic experiments, edited and approved final manuscript.
F.R: designed transcriptomic experiments, edited and approved final manuscript.
Disclosure statement: The authors of this work declare no potential conflicts of interest relevant to this article.
Clinical trials registration numbers: Clinicaltrials.gov NCT02796950 and NCT01209416.
Précis
This study shows that acipimox treatment enhances GLP-1 concentrations in healthy overweight and hypopituitary otherwise healthy subjects. GLP-1 and free fatty acid levels correlated inversely.
References
- 1.Kuhre RE, Frost CR, Svendsen B, et al. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2014;64:370. doi: 10.2337/db14-0807. [DOI] [PubMed] [Google Scholar]
- 2.Svendsen B, Pedersen J, Albrechtsen NJ, et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156:847–857. doi: 10.1210/en.2014-1710. [DOI] [PubMed] [Google Scholar]
- 3.Moss CE, Glass LL, Diakogiannaki E, et al. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides. 2016;77:16–20. doi: 10.1016/j.peptides.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen LW, Kuhre RE, Janus C, et al. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiological Reports. 2015;3 doi: 10.14814/phy2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolhurst G, Heffron H, Lam YS, et al. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes. 2012;61:364. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen CB, Gabe MBN, Svendsen B, et al. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2018;315:G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 7.Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. International journal of obesity. 2014;39:424. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edfalk S, Steneberg P, Edlund H. Gpr40 Is Expressed in Enteroendocrine Cells and Mediates Free Fatty Acid Stimulation of Incretin Secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gribble FM, Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annual Review of Physiology. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 10.Belfort R, Mandarino L, Kashyap S, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54:1640–1648. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- 11.Gormsen LC, Jessen N, Gjedsted J, et al. Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. Journal of Clinical Endocrinology Metabolism. 2007;92:1834–1842. doi: 10.1210/jc.2006-2659. [DOI] [PubMed] [Google Scholar]
- 12.Randle Pj Fau - Garland PB, Garland Pb Fau - Hales CN, Hales Cn Fau - Newsholme EA, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. The Lancet. 1963;281:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM, Hollenbeck C, Jeng C-Y, et al. Measurement of Plasma Glucose, Free Fatty Acid, Lactate, and Insulin for 24 h in Patients With NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 14.Tunaru S, Kero J, Schaub A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nature medicine. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 15.Offermanns S. Hydroxy-Carboxylic Acid Receptor Actions in Metabolism. Trends Endocrinol Metab. 2017;28:227–236. doi: 10.1016/j.tem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Haemmerle G, Zimmermann R, Hayn M, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 17.Santomauro AT, Boden G, Silva ME, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 18.Saloranta C, Taskinen M-R, Widén E, et al. Metabolic Consequences of Sustained Suppression of Free Fatty Acids by Acipimox in Patients With NIDDM. Diabetes. 1993;42:1559–1566. doi: 10.2337/diab.42.11.1559. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Moller N, Pedersen SB, et al. The effect of long-term pharmacological antilipolysis on substrate metabolism in growth hormone (GH)-substituted GH-deficient adults. Journal of Clinical Endocrinology Metabolism. 2002;87:3274–3278. doi: 10.1210/jcem.87.7.8597. [DOI] [PubMed] [Google Scholar]
- 20.Vestergaard ET, Jessen N, Møller N, et al. Acyl Ghrelin Induces Insulin Resistance Independently of GH, Cortisol, and Free Fatty Acids. Scientific Reports. 2017;7 doi: 10.1038/srep42706. 42706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drucker DJ, Jin T, Asa SL, et al. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Molecular endocrinology. 1994;8:1646–1655. doi: 10.1210/mend.8.12.7535893. [DOI] [PubMed] [Google Scholar]
- 22.Reimann F, Habib AM, Tolhurst G, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deacon CF, Nauck MA, Meier J, et al. Degradation of Endogenous and Exogenous Gastric Inhibitory Polypeptide in Healthy and in Type 2 Diabetic Subjects as Revealed Using a New Assay for the Intact Peptide1. The Journal of Clinical Endocrinology & Metabolism. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 25.Ørskov C, Rabenhøj L, Wettergren A, et al. Tissue and Plasma Concentrations of Amidated and Glycine-Extended Glucagon-Like Peptide I in Humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 26.Ørskov C, Jeppesen J, Madsbad S, et al. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. The Journal of clinical investigation. 1991;87:415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhre RE, Albrechtsen NW, Windelov JA, et al. GLP-1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP-1 secreting cell lines. Peptides. 2014;55:52–57. doi: 10.1016/j.peptides.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Ranganath L, Norris F Fau - Morgan L, Morgan L Fau - Wright J, et al. Inhibition of carbohydrate-mediated glucagon-like peptide-1 (7-36)amide secretion by circulating non-esterified fatty acids. Clin Sci (Lond) 1999;96:335–342. doi: 10.1042/cs0960335. [DOI] [PubMed] [Google Scholar]
- 29.Holst JJ. The Physiology of Glucagon-like Peptide 1. Physiological Reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 30.Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Deacon CF. Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides. 2018;100:150–157. doi: 10.1016/j.peptides.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Albrechtsen NJW, Kuhre RE, Deacon CF, et al. Targeting the intestinal L-cell for obesity and type 2 diabetes treatment. Expert Review of Endocrinology & Metabolism. 2014;9:61–72. doi: 10.1586/17446651.2014.862152. [DOI] [PubMed] [Google Scholar]
- 33.Kuhre RE, Frost CR, Svendsen B, et al. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64:370–382. doi: 10.2337/db14-0807. [DOI] [PubMed] [Google Scholar]
- 34.Carlson LA. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med Scand. 1963;173:719–722. doi: 10.1111/j.0954-6820.1963.tb17457.x. [DOI] [PubMed] [Google Scholar]
- 35.Ranganath L, Norris F Fau - Morgan L, Morgan L Fau - Wright J, et al. The effect of circulating non-esterified fatty acids on the entero-insular axis. Eur J Clin Invest. 1999;29:27–32. doi: 10.1046/j.1365-2362.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, et al. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complications. 2015;29:445–450. doi: 10.1016/j.jdiacomp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Gordon RS, Jr, Cherkes A. Unesterified Fatty Acid in Human Blood Plasma. The Journal of Clinical Investigation. 1956;35:206–212. doi: 10.1172/JCI103265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakharova AA, Horowitz JF, Surya S, et al. Role of Growth Hormone in Regulating Lipolysis, Proteolysis, and Hepatic Glucose Production during Fasting. The Journal of Clinical Endocrinology & Metabolism. 2008;93:2755–2759. doi: 10.1210/jc.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steyn FJ, Leong JW, Huang L, et al. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology. 2012;153:273–282. doi: 10.1210/en.2011-1681. [DOI] [PubMed] [Google Scholar]
- 40.Larue-Achagiotis C, Le Magnen J. Fast-induced changes in plasma glucose, insulin and free fatty acid concentration compared in rats during the night and day. Physiology & behavior. 1983;30:93–96. doi: 10.1016/0031-9384(83)90043-4. [DOI] [PubMed] [Google Scholar]