Abstract
Background
In this investigation, the in vivo efficacy and safety of biogenic selenium nanoparticles (SeNPs) are assessed against acute toxoplasmosis caused by Toxoplasma gondii (Sarcocystidae) in the mice.
Methods
Male NMRI mice were orally treated with normal saline (control group) and SeNPs at the doses of 5 and 10 mg/kg once a day for 14 days. On the 15th day, the mice were infected with 104 tachyzoites of T. gondii RH strain by the intraperitoneal route. The mortality rate and parasite load were determined in the infected mice. The mRNA levels of IFN-γ, IL10, IL12, and inducible nitric oxide synthase were also examined in the infected mice by quantitative real-time PCR.
Results
The rate of mortality in the infected mice receiving SeNPs at the doses of 5 and 10 mg/kg compared with the mice in the control group was 100% on the 9 and 10 days after the administration. The mean number of tachyzoites in the infected mice receiving SeNPs was significantly lower than that in the control group. No significant difference (p > 0.05) was found in the biochemical parameters between the mice treated with SeNPs and the mice in the control group. The results revealed that mRNA levels significantly improved in the infected mice treated with SeNPs compared with those in the control group.
Conclusion
Findings of the present investigation showed the considerable efficacy of SeNPs with no important toxicity for curing acute toxoplasmosis in the mice model. However, further studies are needed to clarify the accurate anti-Toxoplasma mechanisms of SeNPs.
Keywords: Prophylactic, Selenium, Toxoplasma gondii, Treatment, Toxicity
Highlights
-
•
We evaluated the efficacy of Biogenic Selenium Nanoparticles (SeNPs) against acute toxoplasmosis.
-
•
Mice were orally treated with SeNPs at the doses of 5 and 10 mg/kg once a day for 14 days.
-
•
The average number of tachyzoites and mortality rate was significantly lower in treated mice.
-
•
No significant difference (p > 0.05) observed in the biochemical parameters between the mice treated.
-
•
The mRNA levels of meaningfully improved in infected mice treated with SeNPs.
1. Introduction
Toxoplasma gondii as a universal intracellular parasite which is observed all over the world infects a broad spectrum of animals and about 30% of humans (1(. At present, the three main ways of infection by this parasite, which are almost proven, are as follows: (i) digestion of raw or undercooked meat contaminated with tissue cysts, (ii) eating of sporulated oocysts with food or (iii) congenitally from mother to fetus during pregnancy)2, 3(. In relation to the clinical signs of the T. gondii infections (toxoplasmosis), there are various forms of this disease, from asymptomatic forms to dangerous and, even deadly, ones)4, 5(. Nowadays, the best medication used to treat toxoplasmosis is the concomitant use of pyrimethamine and sulfadiazine; however, studies in recent years have suggested that the side effects of these drugs such as osteoporosis, sepsis, and teratogenic properties, especially in immuno compromised individuals, should not be neglected [[6], [7], [8]]. Considering the explanations given above, it seems that finding a new drug with the same and even higher effectiveness as well as less toxicity would be the intellectual concern for researchers in the world.
Nowadays, it has been proven that selenium (Se) is one of the most important and vital elements for humans and animals, the deficiency of which causes irreparable diseases and injuries such as immune impairment [9]. This element exists in a range of functional proteins that play vital roles including anti-cancer effects and strengthen the immune system against infectious agents [10,11]. Since nanoparticles (NPs) have a high surface-to-volume ratio, they have extensive biological activities [12]. Several investigations have demonstrated that NPs, especially Se NPs, can considerably prevent the growth of some microbial pathogenic strains such as Staphylococcus aureus, Escherichia coli, and Leishmania spp [13,14].
Although the main antimicrobial mechanisms of these nanoparticles are still unclear, a number of investigations have reported that some forms of selenium can show the potent antimicrobial effects through reaction with membrane peroxidases and, subsequently, produce oxygen-free radicals [15]. On the other hand, some studies have exhibited that biogenic Se NPs by activating apoptosis in the Leishmania major promastigotes show their antimicrobial mechanisms [16,17].
Since there is no documentary study on the efficacy of biogenic Se NPs for acute toxoplasmosis, the present study was designed to evaluate the efficacy of biogenic Se NPs against acute toxoplasmosis in the mice model.
1.1. Materials and methods
1.1.1. Biosynthesis and characterization of the Se NPs
In the present investigation, biosynthesis of biogenic Se NPs was carried out according to the method performed by Shakibaei et al. [9]. After the extraction and purification of the Se NPs, the electron microscopy of SeNPs was conducted by carbon-coated copper transmission electron microscope grids and dried under an IR lamp. Moreover, the crystalline structure of the Se NPs was assessed using the X-ray diffraction (XRD) as previously explained [9].
1.2. Animals
Forty-eight male NMRI mice aged 40–45 days and weighting from 20 to 25 g were obtained from Pasteur Institute, Tehran, Iran, to induce the acute toxoplasmosis and safety of Se NPs. Mice were kept in a colony room with 12 h of light and 12 h of darkness at the room temperature.
1.3. Ethical statement
The protocol of this survey was permitted by Ethics Committee of Kerman University of Medical Science (Permit Number: 93/110). The work was also carried out in line with the ARRIVE Guidelines for Reporting Animal Research [10].
1.4. Parasite
The T. gondii virulent RH strain was prepared by Department of Parasitology and Mycology, Kerman University of Medical Sciences, Kerman, Iran. Tachyzoites of T. gondii RH strain (1 × 104) was inoculated intraperitoneally (IP) to the mice in order to establish an animal model of acute toxoplasmosis.
1.5. Experimental design
Fig. 1 shows the experimental design of the present study. After treatment of the mice with SeNPs at the dose of 5 and 10 mg/kg once a day for 14 days, the mice were infected with 1 × 104 tachyzoite of T. gondii RH strain IP. Then, the following parameters were performed to evaluate the efficacy of oral SeNPs against acute toxoplasmosis.
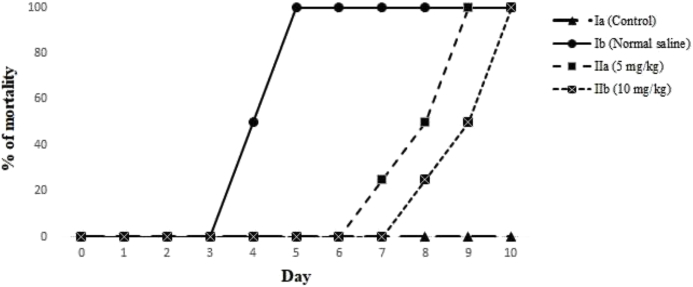
Fig. 1.
Effect of oral administration of SeNPs on mortality rate of mice with acute toxoplasmosis infected with T. gondii RH strain.
1.6. Mortality rate (MR)
The following formula was applied to calculate the MR:
1.7. Parasite load
After the aspiration of the peritoneal fluid of each of the infected mice, the mean number of the T. gondii tachyzoites was recorded under light microscope using the 10 X high power field [11].
1.8. Evaluating safety of SeNPs on biochemical parameters
In order to evaluate the toxicity effects of SeNPs, the mice in the subgroups 1c1 and 1c2 compared with those in the control group Ia were fasted overnight. Animals were anesthetized using ketamine (100 mg/kg)-xylazine (10 mg/kg) and, after opening thoracic cavity, total blood was collected from the heart of the mice. For assessment of the serum biochemical factors, the rest of the collected blood samples was put into tubes without any anticoagulant. Then, the serum was collected using centrifugation at 5000g for 5 min. In the next step, some of the main biochemical factors including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatinine (Cr), blood urea nitrogen (BUN), and bilirubin (direct and total) were measured using the Roche Diagnostics kits (Mannheim, Germany) [12].
1.9. Analysis of mRNA expression by real-time PCR
Since selenium affects all components of the immune system, particularly cellular immunity, the mRNA levels of IFN-γ, IL10, and IL12 as well as inducible nitric oxide synthase (iNOs) as the main effective mechanisms for control and resistance to T. gondii during infection were examined in the infected mice treated with SeNPs by quantitative real-time PCR.
To extract the total RNAs from the blood samples, commercial RNeasy kits (Qiagen, Hilden, Germany) were employed. After the extraction, all the obtained RNAs were reverse transcribed by means of RT premix kit (Qiagen, Hilden, Germany) based on the manufacture's procedure. Then, complementary DNA (cDNA) was used not only for the conventional PCR, but also for real-time PCR.
Real-time PCR was carried out via the iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA), whereas the SYBR green was applied to determine the products [13]. Conditions of reactions were established according to the method described elsewhere. Finally, the obtained data was analyzed by means of iQ™5 Optical System software (Bio-Rad). Table 1 indicates the primer sequences used for IFN-γ TNF-α, IL-12, iNO, and β-actin (house-keeping gene).
Table 1.
Sequences of primers used for real-time PCR.
| Amplicon | Primers | Sequence (5′–3′) |
|---|---|---|
| IL12 | F | ACGACATTCGTCAACTGCAA |
| R | TAAATTGGCACCCTGTAGGC | |
| IFN-γ | F | GATCGTGTCGTCACCAGAAAGG |
| R | TGCCTGGTAACGAGTTGTCC | |
| NO | F | CGTGAAAGGATCCAGAAAGG |
| R | TCCACGATGCCTGGTAGTTG | |
| TNF-α | F | ATTTCTCACGCCAGGATTTG |
| R | GATCGGCAAAGGTTAGGTCA |
1.10. Statistical analysis
Analysis of the data was performed by SPSS statistical software (ver. 22.0). To compare the differences between the experimental groups, one-way ANOVA with Tukey's post-hoc test was applied. p < 0.05 was also measured as statistically significant.
2. Results
2.1. Estimation of the mortality rate
Fig. 1 demonstrates the mortality rate of the infected mice after treatment with SeNPs at doses of 5 and 10 mg/kg for two weeks. The results indicated that the rate of mortality in the infected mice receiving SeNPs at the doses of 5 and 10 mg/kg compared with the mice in control group was 100% on 9 and 10 days after the administration. Obtained findings exhibited that there was a significant difference (P < 0.05) in survival rate among the mice receiving SeNPs with the mice in the control group.
2.2. Parasite load
The findings also demonstrated that the mean number of tachyzoites in the infected mice receiving SeNPs at the doses of 5 and 10 mg/kg was 127 × 104 and 56 × 104 and significantly lower than those in the control group (288 × 104 tachyzoites).
2.3. Safety of SeNPs on biochemical parameters
To examine the safety of SeNPs, some main biochemical parameters were evaluated on the mice in the subgroups 1c1 and 1c2 in comparison with those in the control group 1a after two weeks of SeNPs administration. There was no mortality in the mice in the subgroups 1c1 and 1c2 after two weeks. The findings of the biochemical parameters are presented in Table 2. These results revealed no significant difference (p > 0.05) in the biochemical parameters between the mice in the subgroups 1c1 and 1c2 compared with those in the control group Ia.
Table 2.
Clinical biochemistry parameters in serum of tested mice.
| Clinical biochemistry parameters | BUN(mg/dL) | Cr (mg/dL) | AST (U/L) | ALT (U/L) | ALP (U/L) | TB |
|---|---|---|---|---|---|---|
| Control | 31.4 | 0.24 | 130.6 | 38.7 | 133.3 | 0.15 |
| Se NPs (5 mg/kg) | 33.5 | 0.29 | 141.8 | 44.2 | 137.2 | 0.16 |
| Se NPs (10 mg/kg) | 36.6 | 0.31 | 148.3 | 47.7 | 140.1 | 0.18 |
BUN, Blood urea nitrogen; Cr, creatinine; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; TB, total bilirubin.
2.4. Analysis of cytokine mRNA expression by real-time PCR
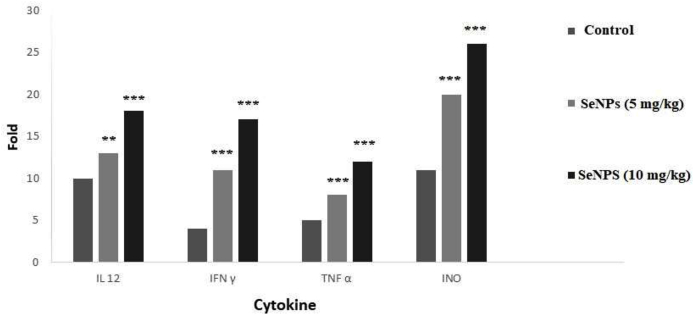
The mRNA levels of IFN-γ, TNF-α, IL-12, as well as iNO were assessed in the infected mice using real-time PCR. As demonstrates in Fig. 2, the results revealed that mRNA levels of IFN-γ (P < 0.001), TNF-α (P < 0.001), IL-12 (P < 0.05), and iNOs (P < 0.05) significantly improved in the infected mice treated with SeNPs compared with those in the control group.
Fig. 2.
The mRNA levels of IFN-γ, TNF-α, IL-10, IL-12, as well as iNO in mice. **p < 0.01; ***p < 0.001 statistically significant.
3. Discussion
Currently, the main treatment for toxoplasmosis is the use of the combination of pyrimethamine and sulfadiazine; however, this combination therapy has some limitations because of possessing dangerous side effects, mainly in the patients with immune system disorders such as AIDS patients [2,3]. Therefore, it seems necessary to search for an alternative agent with higher efficacy as well as less side effects.
The obtained findings revealed that the rate of mortality in the infected mice receiving SeNPs at the doses of 5 and 10 mg/kg compared with the mice in the control group was 100% on days 9 and 10 after the administration. Furthermore, the mean number of tachyzoites was considerably reduced for the infected mice treated with SeNPs at the doses 5 and 10 mg/kg.
Today, selenium compounds have shown a wide range of therapeutic applications such as non-carcinogenic, antioxidant, and antimicrobial [[6], [7], [8]]. In recent years, studies have demonstrated that nanoparticles can be applied for various therapeutic purposes, since these particles have small nanoscale sizes as well as great surface-to-volume percentages, which could produce more active areas for cooperating with biological molecules such as microbes.
In toxoplasmosis, both innate and adaptive immune responses (humoral and cellular immunity), are required to control this infection in humans [14]. Reviews have reported that selenium can motivate the immune responses; moreover, Yazdi et al. (2015) demonstrated that, by sandwich ELISA, levels of cellular immunomodulatory cytokines such as granzyme B, IL-12, IFN-γ, and IL-2 considerably improved (P < 0.05) in the mice treated with both SeNPs [15,16]. Similarly, it was found that the expression levels of IFN-γ TNF-α, IL-12, IL-10, as well as iNO were considerably increased in the infected mice treated with SeNPs compared with the untreated BALB/c mice. These results indicated that the increased survival time in the infected mice after the oral administration of SeNPs might be due to the strengthening of the immune system, especially the cellular immunity of the animal, which caused resistance to the infection.
Several investigations have reported the antimicrobial activity of SeNPs; for example, Beheshti et al. (2013) demonstrated that Se NPs as a new antileishmanial drug might be used to treat the cutaneous leishmaniasis in mice [17]. In another work, Se NPs, particularly in combination with meglumine antimoniate, was reported to considerably inhibit the promastigote and amastigote stages of sensitive and glucantime resistance Leishmania tropica in the in vitro conditions. Mahmoudvand et al. [18] demonstrated that Se NPs could be described as a novel protoscolicidal agent during hydatid cyst surgery. Other studies conducted by Tran et al. [19] and Yang et al. [20] have shown that Se NPs have potent efficacy to prevent the growth of S. aureus and E. coli.
Nowadays, in order to investigate the sub-acute toxicity of some pharmacological agents in animal models, researchers employ the evaluation of the liver and renal enzyme activities such as ALT, AST, ALP, bilirubin (total, direct), Cr, and BUN as well as hematological parameters. These results reveal no significant difference (p > 0.05) in the biochemical and hematological parameters between the mice in the subgroups 1c1 and 1c2 compared with those in the control group Ia. These results consistent with the toxicity classification indicate that SeNPs have no significant toxicity against the male BALB/c mice [21].
4. Conclusion
The findings of the present investigation showed the considerable efficacy of SeNPs with no important toxicity to cure the acute toxoplasmosis in the mice model. However, further studies are needed to clarify the accurate anti-Toxoplasma mechanisms of SeNPs.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
None.
Provenance and peer review.
Not commissioned, externally peer reviewed.
Declaration of competing interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.04.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dubey J.P. Toxoplasmosis-a waterborne zoonosis. Vet Parasitol. 126: 57-72. Hill D, Dubey J. 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2004;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 2.Khryanin A.A., Reshetnikov O.V., Kuvshinova I.N. Toxoplasmosis: epidemiology, diagnosis, treatment. Antibiot. Khimioter. 2015;60:16–21. [PubMed] [Google Scholar]
- 3.Lewis J.M., Clifford S., Nsutebu E. Toxoplasmosis in immunosuppressed patients. Rheumatology. 2015;54:1939–1940. doi: 10.1093/rheumatology/kev115. [DOI] [PubMed] [Google Scholar]
- 4.Halonen S.K, Weiss L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013;114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoudvand H., Ziaali N., Ghazvini H., Shojaee S., Keshavarz H., Esmaeilpour K., Sheibani V. Toxoplasma gondii infection promotes neuroinflammation through cytokine networks and induced hyper algesia in BALB/c mice. Inflammation. 2016;39(1):405–412. doi: 10.1007/s10753-015-0262-6. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudvand H., Sheibani V., Keshavarz H., Shojaee S., Esmaeelpour K., Ziaali N. Acetylcholinesterase inhibitor improves learning and memory impairment induced by Toxoplasma gondii infection. Iran. J. Parasitol. 2016;11:177–185. [PMC free article] [PubMed] [Google Scholar]
- 7.Antczak M., Dzitko K., Długońska H. Human toxoplasmosis-searching for novel chemotherapeutics. Biomed. Pharmacother. 2016;82:677–684. doi: 10.1016/j.biopha.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Montazeri M., Sharif M., Sarvi S., Mehrzadi S., Ahmadpour E., Daryani A. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006-2016) Front. Microbiol. 2017;8:25–32. doi: 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shakibaie M., Khorramizadeh M.R., Faramarzi M.A., Sabzevari O., Shahverdi A.R. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol. Appl. Biochem. 2010;56:7–15. doi: 10.1042/BA20100042. [DOI] [PubMed] [Google Scholar]
- 10.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience Research reporting: the ARRIVE Guidelines for reporting animal Research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavakoli Kareshk A., Keyhani A., Mahmoudvand H., Tavakoli Oliaei R., Asadi A., Andishmand M., Azzizian H., Babaei Z., Zia-Ali N. Efficacy of the bunium persicum (boiss) essential oil against acute toxoplasmosis in mice model. ranian J of Parasitol. 2015;10(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- 12.Keyhani A., Ziaali N., Shakibaie M., Karesh A.T, Shojaee S., Asadi-Shekaari M., Sepahvand M., Mahmoudvand H. Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J. Med. Microbiol. 2020;64(1):104–110. doi: 10.1099/jmm.0.001111. [DOI] [PubMed] [Google Scholar]
- 13.Ha T.Y., Chang K.A., Kim J.A., Kim H.S., Kim S., Chong Y.H., Suh Y.H. S100a9 knockdown decreases the memory impairment and the neuropathology in Tg2576, AD animal model. PloS One. 2010;21(5) doi: 10.1371/journal.pone.0008840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont C.D., Christian D.A., Hunter C.A. Immune response and immunopathology during toxoplasmosis. Semin. Immunopathol. 2012;34(6):793–813. doi: 10.1007/s00281-012-0339-3. 2012 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazdi M.H., Varasteh moradi B., Faghfuri E., Mavandadnejad F., Mahdavi M., Shahverdi A.R. Adjuvant effect of biogenic selenium nanoparticles improves the immune responses and survival of mice receiving 4T1 cell antigens as vaccine in breast cancer murine model. J. Nanosci. Nanotechnol. 2015;15(12):10165–10172. doi: 10.1166/jnn.2015.11692. [DOI] [PubMed] [Google Scholar]
- 16.Yazdi M.H., Mahdavi M., Faghfuri E., Faramarzi M.A., Sepehrizadeh Z., Hassan Z.M., Gholami M., Shahverdi A.R. Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: preliminary vaccine model. Iran. J. Biotechnol. 2015;13(2):1–9. doi: 10.15171/ijb.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beheshti N., Soflaei S., Shakibaie M., Yazdi M.H., Ghaffarifar F., Dalimi A., Shahverdi A.R. Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J. Trace Elem. Med. Biol. 2013;27:203–207. doi: 10.1016/j.jtemb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudvand H., Fasihi Harandi M., Shakibaie M., Aflatoonian M.R., ZiaAli N., Makki M.S., Jahanbakhsh S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 2014;12:399–403. doi: 10.1016/j.ijsu.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Tran A.P., Webster T. Selenium nanoparticles inhibit. Int. J. Nanomed. 2011;6:1553–1558. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Huang K., Qin S., Wu X., Zhao Z., Chen F. Antibacterial action of selenium-enriched probiotics against pathogenic. Dig. Dis. Sci. 2009;54:246–254. doi: 10.1007/s10620-008-0361-4. [DOI] [PubMed] [Google Scholar]
- 21.Loomis T.A. Lea and Febige; Philadelphia ( PA ): 1968. Essential of Toxicology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.