Abstract
Background
Nitrate-rich food can increase NO production and may induce positive effects on brain function. This study examined the feasibility of a randomized clinical trial (RCT) testing the effects of prolonged consumption of incremental doses of dietary nitrate (NO3−) in overweight and obese older participants. Secondary aims tested dose-dependent changes in cognitive, vascular and pulmonary functions and cerebral blood flow (CBF).
Methods
This was a single blind, four-arm parallel RCT conducted in 60 overweight and obese older participants. Eligible participants were randomized to:1) high NO3− (140 ml of beetroot juice (BJ) per day, ~800 mg of NO3−/day), 2) moderate NO3− (70 ml of BJ per day, ~400 mg of NO3−/day), 3) low NO3− (70 ml on alternate days, ~400 mg of NO3−) or 4) NO3− depleted (70 ml on alternate days, ~0.001 mg of NO3). Measurements of cognitive, vascular and pulmonary functions and CBF were conducted at baseline and 13-weeks NO3− intake was assessed by six 24-h recalls, and by measuring NO3− intake biomarkers. Feasibility was assessed by obtaining qualitative feedback and evaluating trial recruitment, retention, compliance with study visits and measurement protocols.
Results
Participant recruitment started in July 2018 and ended in April 2019. Of all the recruitment strategies that were used, advertisement of the study via Facebook generated the highest response rate. Sixty-two participants consented and were enrolled. Overall, characteristics of included participants matched our recruitment criteria.
Conclusion
The findings from this study provide evidence of the acceptability and feasibility of an intervention investigating the effects of incremental doses of high-nitrate BJ over a prolonged period.
Trial registration
The intervention study was registered with clinical trial ISRCTN registry (ISRCTN14746723) on 27 December 2018.
Keywords: Inorganic nitrate, Beetroot juice, Cognition, Cerebral blood flow, Blood pressure, Older adults
1. Introduction
Nitric oxide (NO) is a gaseous signalling molecule which is produced primarily via the activity of inter-connected enzymatic and non-enzymatic pathways [1]. Reduced NO synthesis is associated with learning and memory impairment in rats which is improved by L-arginine supplementation [2]. In humans, reduced NO availability is associated with increased risk of neurodegenerative diseases such as Alzheimer Disease [3]. Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of NO synthase (NOS) and higher plasma ADMA concentrations are associated with reduced NO synthesis, endothelial dysfunction and increased risk for cardiovascular diseases [4,5].
NO production decreases with age as a result of decreased efficiency of the enzymatic synthetic pathway, and is reduced further in older people with metabolic and cardiovascular impairment [6,7]. Key characteristics of vascular ageing include decreased NO production, reduced endothelial integrity and increased arterial stiffness; established risk factors for the development of atherosclerosis [8]. Nutritional and lifestyle interventions capable of maintaining, or restoring, normal NO production may reduce the risk of atherosclerosis and cardiovascular diseases. In turn, this might help to reduce the risk of cognitive dysfunction and dementia since, among older people, better cardiovascular health is associated with a lower incidence of cognitive decline and lower risk of dementia [9].
Recent studies have demonstrated that foods rich in inorganic nitrate (NO3−) can increase NO production and induce positive effects on blood pressure, muscular performance and brain function [10,11]. In particular, some studies have reported improvements in cognitive function (memory and executive performance) and motor skills after dietary NO3− supplementation, which appears to be mediated by augmented cerebral blood flow (CBF) and efficiency of cellular metabolism [12,13]. However, our recent systematic review and meta-analysis found no evidence that NO3− supplementation improves cognitive function and CBF [14]. This systematic review identified several limitations in the current evidence base including that the majority of trials were of short duration and conducted in healthy, normal weight participants (only one study supplemented inorganic nitrite had a duration of 10 weeks whereas the remaining trials had a duration of less than 2 weeks). Hence, the current evidence on the sustained effects of dietary NO3− and nitrite (NO2−) on cognition, brain function and CBF is limited, and studies of longer duration and larger sample size are needed in individuals with reduced NO synthesis and at greater risk of endothelial dysfunction and cognitive impairment.
This pilot RCT was designed primarily to determine the feasibility and acceptability of the protocol for a 13 weeks intervention study in which overweight and obese older participants were asked to consume different doses of NO3−-rich beetroot juice (BJ). Secondary aims of the study included testing whether the different doses of dietary NO3− result in different changes in cognitive, vascular and pulmonary functions and CBF.
2. Methods
2.1. Study design and randomization
This feasibility study was designed as a randomized, single-blind, placebo-controlled, four-arm parallel trial with a duration of 13 weeks. After a screening assessment for the evaluation of the inclusion and exclusion criteria, eligible participants were randomized to one of the four intervention groups. The first intervention group (Group 1) was asked to consume two 70 ml shots of concentrated BJ per day (400 mg of NO3− per shot, Beet-it, James White Company), one every morning (~8am) and one each evening (~9pm). Groups 2 and 3 were asked to consume one shot every evening (~9pm) and every other evening (~9pm), respectively. Group 4 (the control group) received the placebo (NO3− depleted BJ, 0.001 mg of NO3−) and were asked to consume a bottle every other evening (~9pm). The randomization pattern was generated using the RAND function in Excel (Excel Microsoft software, Microsoft corp, Redmond, WA, USA). The study adhered to the SPIRIT guidelines (see Table 1).
Table 1.
Standard protocol items: recommendations for interventional trials (SPIRIT).
|
Time point (week) |
Study period |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Baseline |
Post-allocation |
Follow up |
||||||||||||
| W0 | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | W13 | ||
| Enrolment | |||||||||||||||
| Eligibility screen | X | ||||||||||||||
| Informed consent | X | ||||||||||||||
| Randomization | X | ||||||||||||||
| Allocation | X | ||||||||||||||
| Interventions | |||||||||||||||
| High BJ dose | |||||||||||||||
| Medium BJ dose | |||||||||||||||
| Low BJ dose | |||||||||||||||
| Placebo (control) | |||||||||||||||
| Assessments | |||||||||||||||
| Demographic data | X | ||||||||||||||
| Anthropometric measurement | X | X | X | ||||||||||||
| Physical activity questionnaire | X | X | |||||||||||||
| Blood pressure | X | X | |||||||||||||
| Endothelial function test | X | X | |||||||||||||
| Pulmonary function test | X | X | |||||||||||||
| Cognitive function test | X | X | |||||||||||||
| Cerebral blood flow test | X | X | |||||||||||||
| Dietary intake | X | X | X | X | X | X | |||||||||
| Blood samples | X | X | |||||||||||||
| Saliva samples | X | X | X | X | |||||||||||
| Urine samples | X | X | X | X | |||||||||||
| Salivary Strips | X | X | X | X | |||||||||||
| Participant feedback | X | ||||||||||||||
W = week of the study.
2.2. Study setting
The study visits were conducted at the NU-Food research facility at Newcastle University and the CBF measurements were performed at the Brain Performance Nutrition research centre at Northumbria University.
2.3. Blinding
This was a single-blind study as participants were not informed about whether they were allocated to the NO3− -rich BJ or NO3− -depleted BJ (placebo). Blinding of the researchers to the intervention was not possible due to the nature of the dietary interventions and study design as the frequency and volume of the BJ given to each participant revealed the nature of the interventions.
2.4. Recruitment strategies
Participant recruitment occurred between July 2018 and April 2019. Potential participants were identified through several recruitment strategies:
-
•
Flyers and posters distributed in local communities
-
•
Advertisements in local newspapers and newsletters
-
•
Emails circulated to Newcastle University members of staff
-
•
Search of existing databases held by Newcastle University of participants in previous studies who had consented to be contacted to take part in future research studies
-
•
Advertisement of the study via social media (i.e., Facebook)
-
•
Invitation from recruited participants to friends and relatives
2.5. Retention
Several strategies were adopted to minimize the drop out of participants during the study. All participants were provided with contact details of the lead investigator who replied promptly to participants' queries. Regular emails were sent to participants to remind them to complete the scheduled dietary assessments using Intake24 every two weeks and to collect the urine and saliva samples every 4 weeks. All appointments were scheduled at the participants’ convenience but careful planning was required to ensure that time intervals between visits were similar between participants (deviation from scheduled appointments: ±2 days). Participants received a £60 shopping voucher on completion of the study.
3. Participants
3.1. Inclusion criteria
We aimed to recruit 60 healthy, overweight and obese ((BMI) range: 25–40 kg/m2) male and female older participants (60–75 years).
3.2. Exclusion criteria
-
•
Current participation in other clinical research studies.
-
•
Vegetarian.
-
•
Smokers.
-
•
Systolic BP lower than 115 mmHg and greater than 160 mmHg; diastolic BP lower than 70 mmHg and greater than 100 mmHg.
-
•
Active cancer and any diagnosis of malignant cancer in the last 5 years
-
•
Excessive alcohol intake (>21 units per week) (Alcohol unit = Total alcohol volume (ml) * Alcohol by volume (%)/1000).
-
•
Allergy or intolerance to the intervention food (BJ).
-
•
Diagnosis of chronic or acute metabolic and inflammatory conditions that may interfere with the study outcomes.
-
•
Major surgical operations.
-
•
Use of prescribed psychiatric drugs (antidepressants, sedatives, antipsychotics), diuretics, organic nitrates and proton pump inhibitors.
-
•
Use of prescribed hormonal therapies (oestrogens, thyroxin, and progesterone), anti-hypertensive (Ca++ channel blockers, beta-blockers, and angiotensin-converting-enzyme (ACE) inhibitors), statins and any other anti-dyslipidaemic agent, if the prescription had started, or the dose had been started/changed, in the previous three months.
-
•
Non-prescribed dietary supplements were stopped at least for 2 weeks before starting the trial.
-
•
Use of the mouthwash during the study was not allowed as it interferes with the conversion of oral NO3− into NO2−.
3.3. Screening and consent
Individuals who contacted the research team and expressed an interest to participate received detailed information about the study either by email or by post. A telephone screening was then arranged to assess eligibility based on date of birth, medical history and medication use and commitment and availability to participate to the study over a period of three months. If potential participants met the eligibility criteria at the telephone screening, they were invited to an onsite screening visit for further assessments to confirm the eligibility.
During the on-site screening visit, a member of the research team explained the study protocol and measurement procedures and answered any queries from participants. Potential participants were asked to read and sign the consent form. Next, body weight and height were measured to calculate BMI and resting clinic BP was measured in triplicate. Participants were included in the study if BMI and BP were within the range specified in the study protocol. At the end of the screening visit, eligible participants started a familiarization session for the computerized cognition tasks which included the completion of three consecutive cognitive tasks. This aimed to minimize the risk of a learning effect between baseline and end of study assessments.
4. Outcome measures
4.1. Primary
The primary aim of the study was to assess the acceptability and feasibility of the proposed intervention trial in terms of recruitment rate and time taken to complete the recruitment, adherence, adverse effects and process evaluation. This was achieved through documentation of adherence to the intervention and measurement protocol, recording of any adverse events, as well as measurements of changes in health outcomes that may occur during the intervention period.
4.2. Secondary
The study protocol includes the investigation of changes in:
-
•
Cognitive function
-
•
Cerebral blood flow
-
•
Resting clinic and home BP
-
•
Nitric oxide biomarkers (urinary, plasma and salivary NO2− and NO3−, NO exhaled and whole-body NO production using stable isotopic methods)
-
•
Validation of salivary NO strips for the long-term compliance to NO3− -based nutritional interventions
-
•
Metabolic risk factors (glucose, insulin)
-
•
Oxidative stress biomarkers (3-Nitrotyrosine)
-
•
Inflammatory biomarkers (IL-6)
-
•
Endothelial-dependent and independent microvascular blood flow, measured by Laser Doppler Iontophoresis (LDI).
-
•
Pulmonary function measured by portable spirometer
4.3. Compliance with the intervention
All participants were provided with verbal and written instructions on the tasks to be completed at home during the trial. Participant compliance was checked primarily by a daily compliance log which was used to record the time they consumed the BJ. In addition, they were asked to return any unopened bottles to the study team. After six weeks, participants returned to the research centre for a mid-study assessment visit to check their compliance, to collect information on safety and adverse events that may have occurred during the first part of the study and to provide participants with the final batch of BJ bottles to complete the study.
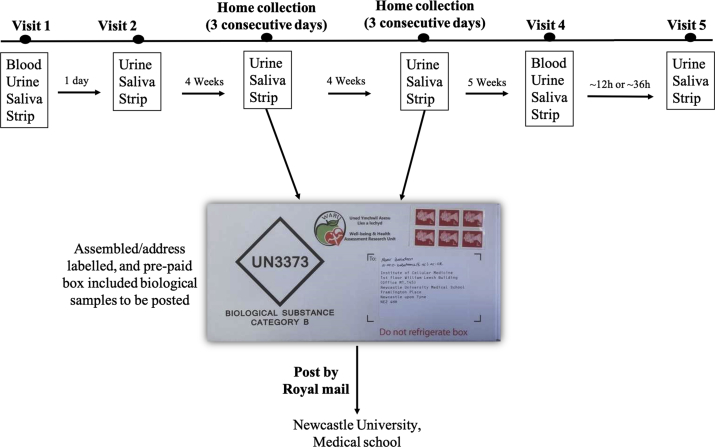
Compliance with the intervention was also assessed objectively by measuring dietary NO3− and NO2− concentrations in plasma, saliva and urine samples and using salivary strips. Plasma samples were collected at baseline and 13 weeks. Salivary strips and urine and saliva samples were collected for 3 consecutive days every 4 weeks. The protocol for the collection of the biological samples is described in Fig. 1. The measurement of NO3−/NO2− concentrations were taken as a measure of the adherence to the intervention and it is expected to find greater concentrations in these biomarkers in the samples collected from high NO3− dose group compared to lower NO3− dose groups and placebo.
Fig. 1.
Overview of the protocol for the collection of the biological samples during the study.
Salivary NO strips were also collected concomitantly with the collection of the saliva samples as part of a validation sub-study to assess the utility of this non-invasive method for the assessment of compliance to prolonged NO3− -based nutritional interventions. The colour of the salivary strips was read immediately after receiving them to determine salivary NO2− concentrations. All participants were instructed not to change their dietary habits or physical activity and to avoid using mouthwash during the study.
4.4. Data collection
Socio-demographic, lifestyle and medical history: A questionnaire was used to collect detailed information on any previous or current illnesses, prescribed and non-prescribed medication use, smoking habits, education and alcohol intake.
Feasibility and acceptability: Feasibility was evaluated by assessing recruitment and retention rates, time taken for recruitment, and number of dropouts, with reasons. In addition, adherence to the intervention and compliance with other components of the protocol to be completed at home were evaluated. Completeness of recording of the outcome measures was reported. Acceptability was evaluated by obtaining feedback from participants on the nutritional interventions and measurement protocols as well as reporting the reasons for discontinuation of the intervention.
Anthropometry and body composition: Height was measured to the nearest 0.5 cm at the screening visit using a stadiometer with an adjustable headpiece. Body weight and body composition parameters (fat mass, fat free mass, body fat % and total body water) were assessed at baseline and at the end of the study by bioelectrical impedance analysis (Tanita BC420 MA, Tanita Corporation, Tokyo, Japan). Weight and height were used to calculate Body Mass Index (BMI). Waist circumference at the midpoint between the lowest margin of the last rib and the top of the iliac crest was measured at baseline and end of the study.
Blood pressure: Resting clinic blood pressure was measured using an automated monitor (model: Omron M3 [HEM-7200-E8(V)]). Participants were in a supine position and measurements were performed after participants had rested for at least 10 min. Three blood pressure measurements were taken and the average of the three readings was calculated. Home resting blood pressure was also measured using the same monitor at baseline and end of the study as part of the protocol for the measurement of whole-body NO production. Each participant was asked to measure blood pressure after they had collected a saliva sample (eight measurements in total). All participants were asked to rest for about 10 min before each measurement (see below for more details).
Peripheral vascular function: Vascular function data were collected at baseline and the end of study using Laser Doppler iontophoresis (LDI, Moor Instruments, Axminster, UK). LDI has been shown to provide a valid measure of skin blood flow [15,16]. One % acetylcholine (Ach, Sigma-Aldrich) and 1% sodium nitroprusside (SNP, Sigma-Aldrich) solution (v/v) were prepared fresh before each visit. One mL of each solution was applied into the iontophoresis chambers placed on the forearm of the participants to quantify changes in endothelial-dependent and independent microvascular blood flow, respectively. Forearm skin erythrocyte flux was measured for 3 min prior to the start of the iontophoresis (baseline), for 5 min during Ach and SNP delivery by iontophoresis (stimulation, current set at 30 μA) and for 10 min after the Ach and SNP delivery (recovery). All assessments were performed with the participant lying supine on a bed.
Cerebral blood flow: Haemodynamic responses were monitored using a frequency domain ‘quantitative’ NIRS system (OxiplexTS Frequency-Domain Near-Infrared Tissue Oximeter, ISS Science) at rest and stimulated conditions (i.e. while participants performed computerized cognitive function tests including serial subtraction 3 and 7, Stroop (congruous & incongruous) and peg and ball tasks, which were mainly executive function tasks). NIRS has been used extensively in neuroscience research as it provides measurements of changes in cerebral blood oxygenation related to brain activity [17]. These measurements were performed at baseline and end of the study. CBF data were collected at a rate of 5 Hz.
Cognitive function: The Computerised Mental Performance Assessment System software (COMPASS, University of Northumbria), which has been shown to be sensitive to a range of nutritional interventions [18,19], and the Trail Making Tasks A and B, were used to assess cognition at baseline and the end of the study. The COMPASS test includes word presentation, immediate word recall, digit vigilance, numeric working memory, choice reaction time, computerized corsi blocks, Stroop, peg and ball, delayed word recalls and words recognition. A description of these cognitive tasks is provided in Supplemental Table 1.
Lung Function: Pulmonary function was assessed using a portable spirometer (Micro Spirometer@, Micro Medical Instruments Ltd, UK), at baseline and the end of the study. The following variables were recorded: forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and the ratio of the two volumes (FEV1/FVC). In addition, the real-time fractional exhaled nitric oxide (FeNO) was assessed using a portable, non-invasive, automated device (NIOX VERO@, Circassia AB, UK).
Dietary Intake: Dietary NO3− intake during the study was assessed using a 24-hr dietary recall every two weeks (6 dietary assessments were performed in total) using an online validated platform (Intake 24) [20]. Dietary NO3− intake was calculated by assigning the specific NO3− content for each reported food (from a comprehensive database including the NO3− concentrations from 3498 individual foods and beverages estimated from sixty different countries [21]) and taking into account the amount consumed. Dietary NO3− intake was reported in mg/day.
5. Collection of biological samples
5.1. Blood
Blood samples were collected after an overnight fast (at least 12 h) at baseline and at 13 weeks post-dose. Blood samples were collected by venepuncture by trained phlebotomists in 3 tubes (4 ml each), containing (LH) lithium heparin, EDTA (Ethylenediaminetetraacetic acid) and sodium fluoride and potassium oxalate, respectively.
The samples were processed within 10 min of collection to minimize nitrite degradation. Samples were spun at 4000 rpm for 10 min at room temperature and plasma aliquots were stored at −80 °C until further analysis. Samples were used to measure the concentrations of NO3−, NO2, cGMP, glucose, insulin, 3-nitrotyrosine (a marker of oxidative stress) and Interleukin-6 (a pro-inflammatory marker).
5.2. Urine and saliva samples and salivary nitrite strips
Salivary nitrite strips, urine and saliva samples were collected at baseline and then again at 4 weeks (3 consecutive days), 8 weeks (3 consecutive days), and 13 weeks post-dose (see Fig. 1).
5.3. Urine
A mid-stream urine sample was collected at the baseline and end of study visits using a sterile, collection kit (Mid-stream urine collection set, UN252, Shermond, UK). After 4 and 8 weeks, urine samples were collected at home by each participant for three consecutive days using a urine collection kit. A pre-paid box with sealed envelopes for the storage of biological samples was provided and each participant was asked to mail the samples to the research team using a fast delivery service. During each three-day collection period, participants were asked to keep urine samples in the fridge. All urine samples were stored at −20 °C until further analyses. Urine samples were used for the measurement of NO3− concentrations using ozone-based chemiluminescence.
5.4. Saliva
Participants were asked to chew a cotton ball for approximately 2 min, which was then placed in the barrel of a 20 mL syringe. The plunger was then inserted back into the syringe and used to squeeze the saliva into a 2 mL Eppendorf tube. Participants were asked to repeat this saliva collection procedure at home as required by the study protocol. Samples collected after 4 and 8 weeks were posted [together with urine samples (see above)], and participants were instructed to keep the saliva samples in the freezer until put in the post. After arrival in the laboratory, saliva samples were stored at −20 °C until required for analysis of NO3− and NO2− concentrations using ozone-based chemiluminescence and sialin concentrations using an ELISA kit (BioAssay™ (Human), Stratech Scientific Ltd, Cambridge, UK).
5.5. Salivary nitrite strips
Berkeley strips (Berkeley Test®, CA, USA) were used as per the manufacturer's guidelines. Specifically, participants were asked to place the test strip with the ‘saliva here’ side on the tongue and swab it over a 10 s period covering different areas including the dorsal surface of the tongue. The two ends of the strip were folded and pressed gently for 10 s. The colour of the NO test pad was then allowed to develop over a 45 s period. The intensity of the colour was compared with a colour chart included in the product package (Depleted, Low, Threshold, Target and High), with darker colours corresponding to higher NO2− concentrations. For the purpose of quantitative analysis, categorical colours were given a categorical number from 1 to 5, where 1 corresponding to “Depleted” and 5 corresponding to “High”. Participants were asked to repeat a similar procedure at home as required by the study protocol. The strips collected at 4 and 8 weeks were posted (with the urine and saliva samples) to the research centre. Participants were asked to keep the strips in a dry place until put in the post. The colour of the strips was recorded as soon as they were received in the laboratory.
5.6. Whole-body NO production
The measurements of whole-body NO production were performed at baseline and at the end of the study. Participant were asked to consume a controlled low NO3− meal at lunch time. Four hours later, a baseline saliva sample was collected followed by ingestion of 4 mg of Na15NO3 dissolved in 100 mL of distilled water. Further saliva samples were collected at 6, 7, 8, 9, 18, 19 and 20 h post-meal. NO3− enrichment in saliva was measured using Gas Chromatography Mass Spectrometry [22] and NO production will be estimated using a validated model proposed by Refs. [6].
5.7. Adverse events reporting
Adverse events are symptoms or signs that may occur during the trial and may or may not be causally related to the intervention. All adverse events potentially related to the BJ product were recorded.
5.8. Sample size calculation
This is a pilot study designed to assess the feasibility and acceptability of the proposed intervention. A sample size of 15 per group was based on the predicted effect size provided by the study and based on the guidelines indicated by Whitehead et al. [23]. This study provides guidance on sample size calculation for pilot studies with the aim of maximising use of resources and to avoid occurrence of a type II error. Specifically, a sample size of 15 individuals per group would provide a 90% power to detect a medium effect size (δ) between 0.3 and 0.7.
5.9. Statistical analysis
Baseline characteristics are presented as mean ± SD. Feasibility was evaluated by the effectiveness of the screening process and recruitment of participants who met the eligibility criteria for the study, together with retention of participants. Retention was evaluated by collection of information on the proportion of participants lost after randomization in each arm of the study. Compliance with the interventions was estimated by calculating the proportion of BJ bottles returned unused relative to the dispensed bottles.
5.10. Confidentiality
Every effort was made to ensure confidentiality. In this study, paper forms are stored in locked filing cabinets and transferred to password secured computer databases accessible only to the researchers. Participant data were anonymised by assigning a unique identification code which was used on all documents and electronic databases used in the study.
6. Results
6.1. Recruitment
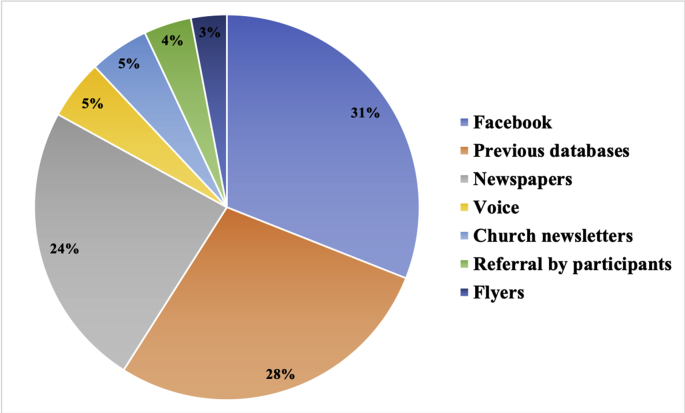
In total, 250 responses were received from use of our various recruitment methods. As shown in Fig. 2, advertisement via Facebook generated the highest response rate with 77 contacts (31%) made within a period of 10 days, followed by 68 contacts (28%) established from recruitment emails that were sent to participants in previous studies who were identified from our databases, 61 responses (24%) from local newspapers, 13 responses (5%) received through the advertisement on the Voice website (https://www.ncl.ac.uk/nica/voice/), 12 responses (5%) from advertisement in the Cathedral church of St Mary newsletter, 11 responses (4%) received after referral by current participants. The least effective strategy was the distribution of flyers, from which only 7 responses (3%) were received.
Fig. 2.
Response rate from each recruitment strategy.
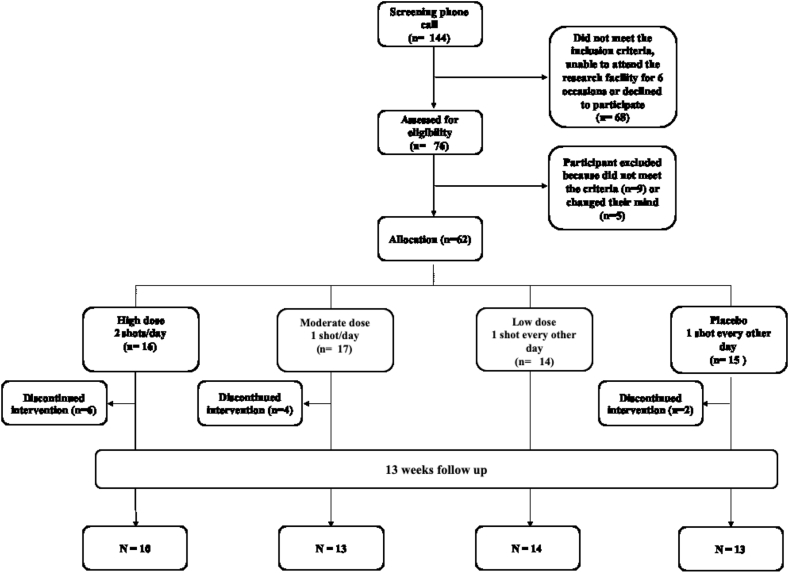
Fig. 3 summarizes the recruitment and retention of participants in the study. A hundred and six participants were either unreachable or declined to participate. The initial telephone screening was conducted with 144 individuals. At this stage, 68 potential participants were not included for the following reasons: 1) taking medications such as antacids, antidepressants or diuretics (54%), 2) co-existent health conditions including cancers, cardiovascular disease, kidney disease, type 1 diabetes or epilepsy (19%), 3) declined to take part after reading the information sheet (12%), 4) unreachable (6%), 5) smokers (4%) and 6) age over 75 years old (4%).
Fig. 3.
Flowchart describing the recruitment of participants into the feasibility study.
A total of 76 participants attended the on-site screening visit to confirm their eligibility. Seventy of these persons were eligible and 62 participants were consented and enrolled. Nine participants (11%) were excluded at the screening visit, and the reasons included high BP (55%), normal BMI < 24.9 kg/m2 (33%) and BMI > 40 mg/m2 (11%). Additional reasons to not participate included: 1) no longer interested to take part (4%), 2) personal bereavement (1%) and 3) being unwell (1%). The recruitment target was 60 participants, but two participants who dropped out the study immediately after the start of the intervention were replaced and, therefore, 62 participants were randomized and had a baseline visit assessment.
The sixty-two participants were randomized to one of the four intervention groups as follows: 16 participants were allocated to Group 1 (2 shots of BJ/day, every morning and evening), 17 participants to Group 2 (1 shots of BJ/day, every evening), 14 participants to Group 3 (1 shot of BJ every other evening) and 15 participants were allocated to Group 4 (Placebo, 1 shot of BJ depleted NO3− every other evening).
6.2. Retention
Of those who were randomly assigned to Group 1 (n = 16), 10 participants completed their 13 weeks assessment. Similarly, 13, 14, and 13 participants from Groups 2, 3 and 4 completed their 13 weeks assessment. The overall attrition rate for the study was 19% with 12 participants dropping from the study.
6.3. Baseline characteristics of participants
Baseline characteristics of the participants are reported in Table 2. The age of participants ranged from 60 to 73 years (mean ± SD, 66 ± 4 years) and 62% were men (n = 38). All included participants were either overweight (n = 35) or obese (n = 27). BMI for all randomized participants ranged from 25 to 39 kg/m2 (30.4 ± 4 kg/m2). The range of SBP was from 110 to 167 mmHg (mean 135 ± 15 mmHg). The range of DBP was from 60 to 100 mmHg (mean 77 ± 10 mmHg).
Table 2.
Baseline characteristics of the study participants including use of medications.
| Total (n = 62) | |
|---|---|
| Characteristics | |
| Gender, M/F | 24/38 |
| Age (years) | 66 ± 4 |
| Education (years) | 15 ± 3 |
| Body weight (kg) | 85 ± 13 |
| BMI (kg/m2) | 30 ± 4 |
| WC (cm) | 103 ± 9 |
| Fat Mass (kg) | 32 ± 9 |
| Fat Mass (%) | 38 ± 8 |
| Fat free mass (kg) | 53 ± 10 |
| SBP (mm Hg) | 135 ± 15 |
| DBP (mm Hg) | 77 ± 10 |
| PA (METs/wk) | 3667 ± 5604 |
| Energy intake (Kcal) | 2440 ± 992 |
| Medication use | |
| Antihypertensive | 6 (9.8%) |
| Hormonal therapy | |
| Thyroxin | 9 (14.5%) |
| Testosterone | 1 (1.6%) |
| Antihistamine | 1 (1.6%) |
| Lipid lowering agents | 10 (16%) |
| Vitamin D | 3 (3%) |
| Aspirin | 1 (1.6%) |
| Corticosteroid inhalers | 2 (3%) |
| No therapy | 35 (56%) |
M/F, male/female; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PA, physical activity; PA, physical activity; Data are expressed as mean ± SD. Medications are presented as n (% of group).
7. Discussion
There has been a growing research interest into the investigation of the potential beneficial effects of NO3− on cardio-metabolic health outcomes including blood pressure, glucose control, dyslipidaemia, cognition, heart failure and peripheral arterial diseases [12,[24], [25], [26], [27]]. However, these studies had limited power due to the small sample sizes and the duration of the studies was short (<8 weeks). To the best our knowledge, this is the longest RCT evaluating the feasibility and potential beneficial effects of different doses of NO3−-rich BJ supplementation on cognitive and vascular functions, CBF and pulmonary functions in overweight and obese older adults. The study involved a mixed-method approach where the primary outcomes on feasibility and adherence were evaluated by asking participants to complete detailed questionnaires with closed and open questions. Questions focussed on process evaluation, adherence to nutritional interventions and measurement protocols, safety of the intervention and procedures and suggestions for future studies. An exit questionnaire was also administered to participants who dropped out of the study asking to explain the reasons for their withdrawal and to provide feedback and suggestions for future studies. Objective measures of adherence were number of dropouts and completion of daily logs to record BJ consumption. In addition, we asked participants to complete online 24-hr dietary recalls every 4 weeks and to collect biological samples (urine, saliva and salivary strips) to measure changes in NO3− concentrations to provide objective evaluation of compliance with the interventions. Additional strengths of the study include a detailed assessment of physiological outcomes using advanced methods for the measurement of cognitive function, peripheral and central vascular function, clinic and home measurements of resting blood pressure and measurement of nitrate and nitrite concentrations using ozone-based chemiluminescence. In addition, this is the first study to employ a non-invasive stable isotopic method for the evaluation of changes in systemic NO production following prolonged NO3− supplementation.
The most challenging aspects of the study included recruiting older participants who met our inclusion criteria and in retaining them in the trial. Some of the difficulties associated with recruiting older people in clinical trials have been documented [28]. We employed a variety of recruitment strategies over a 10-month period and we found that advertising the study on social media (i.e. Facebook) was the most effective strategy. This approach yielded a high response rate within a short period of time and we believe that this recruitment strategy may represent a viable approach to enrol older participants from the community in future nutritional and clinical studies. The use of social media by older people is increasing [29]. In the UK in 2017, 39% of those aged 65–74years used a smartphone and 48% of internet users in this age group have a social media profile [30]. A recent study indicated that the response rate from older people to a social media advertisement for recruiting participants into a clinical trial was higher than for younger adults [31]. Another recent study reported that the use of social media was a successful strategy for recruiting older participants into a clinical trial investigating cardiovascular outcomes [32]. We also recruited participants from a database of participants who had participated previously in other studies at Newcastle University and who had expressed an interest to be involved in future research. This approach was also effective in recruiting participants into the trial, but it was more time-consuming, and it had a lower response rate compared with the social-media approach. The RCT was also promoted in local newspapers, but the response rate was lower, and the costs of the advertisement were considerably higher than the social media approach. Overall, the use of social media (i.e., Facebook, Twitter) appears to offer a cost-effective solution to facilitate the recruitment of older overweight and obese participants from the community into a RCT.
There is limited evidence on the long-term effect of dietary NO3− on physiological functions in older populations. This feasibility study will inform the design and conduct of definitive, larger and longer studies investigating the effects of nitrate-rich BJ on cognitive and vascular outcomes in older people.
Funding
This study was funded by Newcastle University core budget.
Trial status
Data collection in the trial ended in July 2019.
Declaration of competing interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2020.100571.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weitzberg E., Lundberg J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013;33:129–159. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 2.Paul V., Reddy L., Ekambaram P. Prevention of picrotoxin convulsions-induced learning and memory impairment by nitric oxide increasing dose of L-arginine in rats. Pharmacol. Biochem. Behav. 2003;75(2):329–334. doi: 10.1016/s0091-3057(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 3.Venturelli M., Pedrinolla A., Boscolo Galazzo I., Fonte C., Smania N., Tamburin S., Muti E., Crispoltoni L., Stabile A., Pistilli A. Impact of nitric oxide bioavailability on the progressive cerebral and peripheral circulatory impairments during aging and alzheimer's disease. Front. Physiol. 2018;9:169. doi: 10.3389/fphys.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colonna V.D.G., Bianchi M., Pascale V., Ferrario P., Morelli F., Pascale W., Tomasoni L., Turiel M. Asymmetric dimethylarginine (ADMA): an endogenous inhibitor of nitric oxide synthase and a novel cardiovascular risk molecule. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2009;15(4):RA91–RA101. [PubMed] [Google Scholar]
- 5.Liu X., Xu X., Shang R., Chen Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide. 2018;78:113–120. doi: 10.1016/j.niox.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siervo M., Jackson S.J., Bluck L.J.C. In-vivo nitric oxide synthesis is reduced in obese patients with metabolic syndrome: application of a novel stable isotopic method. J. Hypertens. 2011;29(8):1515–1527. doi: 10.1097/HJH.0b013e3283487806. [DOI] [PubMed] [Google Scholar]
- 7.Torregrossa A.C., Aranke M., Bryan N.S. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J. geriatr. cardiol.: JGC. 2011;8(4):230. doi: 10.3724/SP.J.1263.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungvari Z., Kaley G., de Cabo R., Sonntag W.E., Csiszar A. Mechanisms of vascular aging: new perspectives. J. Gerontol. Series A: Biomed. Sci.Med. Sci. 2010;65(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samieri C., Perier M.-C., Gaye B., Proust-Lima C., Helmer C., Dartigues J.-F., Berr C., Tzourio C., Empana J.-P. Association of cardiovascular health level in older age with cognitive decline and incident dementia. Jama. 2018;320(7):657–664. doi: 10.1001/jama.2018.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siervo M., Lara J., Ogbonmwan I., Mathers J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J. Nutr. 2013;143(6):818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 11.Clifford T., Howatson G., West D.J., Stevenson E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7(4):2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Presley T.D., Morgan A.R., Bechtold E., Clodfelter W., Dove R.W., Jennings J.M., Kraft R.A., King S.B., Laurienti P.J., Rejeski W.J., Burdette J.H., Kim-Shapiro D.B., Miller G.D. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24(1):34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wightman E.L., Haskell-Ramsay C.F., Thompson K.G., Blackwell J.R., Winyard P.G., Forster J., Jones A.M., Kennedy D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015;149:149–158. doi: 10.1016/j.physbeh.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Clifford T., Babateen A., Shannon O.M., Capper T., Ashor A., Stephan B., Robinson L., O'Hara J.P., Mathers J.C., Stevenson E. 2018. Effects of Inorganic Nitrate and Nitrite Consumption on Cognitive Function and Cerebral Blood Flow: a Systematic Review and Meta-Analysis of Randomised Clinical Trials', Critical Reviews in Food Science and Nutrition. (just-accepted), pp. 01–31. [DOI] [PubMed] [Google Scholar]
- 15.Kernick D., Shore A. Characteristics of laser Doppler perfusion imaging in vitro and in vivo. Physiol. Meas. 2000;21(2):333. doi: 10.1088/0967-3334/21/2/312. [DOI] [PubMed] [Google Scholar]
- 16.Ferrell W.R., Ramsay J.E., Brooks N., Lockhart J.C., Dickson S., McNeece G.M., Greer I.A., Sattar N. Elimination of electrically induced iontophoretic artefacts: implications for non-invasive assessment of peripheral microvascular function. J. Vasc. Res. 2002;39(5):447–455. doi: 10.1159/000064515. [DOI] [PubMed] [Google Scholar]
- 17.Jackson P.A., Kennedy D.O. The application of near infrared spectroscopy in nutritional intervention studies. Front. Hum. Neurosci. 2013;7:473. doi: 10.3389/fnhum.2013.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haskell C.F., Robertson B., Jones E., Forster J., Jones R., Wilde A., Maggini S., Kennedy D.O. Effects of a multi‐vitamin/mineral supplement on cognitive function and fatigue during extended multi‐tasking. Hum. Psychopharmacol. Clin. Exp. 2010;25(6):448–461. doi: 10.1002/hup.1144. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy D.O., Wightman E.L., Reay J.L., Lietz G., Okello E.J., Wilde A., Haskell C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010;91(6):1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 20.Simpson E., Bradley J., Poliakov I., Jackson D., Olivier P., Adamson A., Foster E. Iterative development of an online dietary recall tool: intake24. Nutrients. 2017;9(2):118. doi: 10.3390/nu9020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon N., Pavey T., Desbrow B., Leveritt M. Developing a nitrate, nitrite, and nitrosamine food and beverage composition database for use with a nitrate food frequency questionnaire: a systematic review. J. Sci. Med. Sport. 2017;20:22. [Google Scholar]
- 22.Jackson S.J., Siervo M., Persson E., McKenna L.M., Bluck L.J. A novel derivative for the assessment of urinary and salivary nitrate using gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom.: An International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass Spectrometry. 2008;22(24):4158–4164. doi: 10.1002/rcm.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead A.L., Julious S.A., Cooper C.L., Campbell M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016;25(3):1057–1073. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen J.D., Giordano T., Kevil C.G. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide. 2012;26(4):217–222. doi: 10.1016/j.niox.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly J., Fulford J., Vanhatalo A., Blackwell J.R., French O., Bailey S.J., Gilchrist M., Winyard P.G., Jones A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(2):R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 26.Khalifi S., Rahimipour A., Jeddi S., Ghanbari M., Kazerouni F., Ghasemi A. Dietary nitrate improves glucose tolerance and lipid profile in an animal model of hyperglycemia. Nitric Oxide. 2015;44:24–30. doi: 10.1016/j.niox.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 27.d’El-Rei J., Cunha A.R., Trindade M., Neves M.F. Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int. J. Hypertens. 2016;2016 doi: 10.1155/2016/6791519. 6791519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridda I., MacIntyre C., Lindley R.I., Tan T. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901–906. doi: 10.1016/j.vaccine.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood S., Perrin A., Duggan M. Social media update 2016. Pew research center. 2016. https://www.pewinternet.org/2016/11/11/social-media-update-2016/ [Online]. Available at:
- 30.Ofcom Rise of the social seniors revealed. 2017. https://www.ofcom.org.uk/about-ofcom/latest/media/media-releases/2017/rise-social-seniors
- 31.Cowie J.M., Gurney M.E. The use of facebook advertising to recruit healthy elderly people for a clinical trial: baseline metrics. JMIR Res. Protoc. 2018;7(1):e20. doi: 10.2196/resprot.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash E.L., Gilroy D., Srikusalanukul W., Abhayaratna W.P., Stanton T., Mitchell G., Stowasser M., Sharman J.E. Facebook advertising for participant recruitment into a blood pressure clinical trial. J. Hypertens. 2017;35(12):2527–2531. doi: 10.1097/HJH.0000000000001477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.