Abstract
Background
The goal of this work was to characterize the maturation of inhibitory control brain function from childhood to early adulthood using longitudinal data collected in two cohorts.
Methods
Functional MRI during a go/no-go task was conducted in 290 participants, with 88 % undergoing repeated scanning at 1- to 2-year intervals. One group entered the study at age 7–13 years (n = 117); the other entered at age 18–23 years (n = 173). 33.1 % of the sample had two parents with a substance use disorder (SUD), 43.8 % had one parent with an SUD, and 23.1 % had no parents with an SUD. 1162 scans were completed, covering ages 7–28, with longitudinal data from the cohorts overlapping across ages 16–21. A marginal model with sandwich estimator standard errors was used to characterize voxel-wise age-related changes in hemodynamic response associated with successful inhibitory control.
Results
There was significant positive linear activation associated with age in the frontal, temporal, parietal, and occipital cortices. No clusters survived thresholding with negative linear, positive or negative quadratic, or positive or negative cubic contrasts.
Conclusions
These findings extend previous cross-sectional and small-scale longitudinal studies that have observed positive linear developmental trajectories of brain function during inhibitory control.
Keywords: Go/no-go, Functional magnetic resonance imaging, Sandwich estimator, Neuroimaging
1. Introduction
Inhibitory control is the ability to voluntarily suppress goal- or task-irrelevant information. It is key to the control of attention and provides the flexibility needed to guide behavior based on task goals. Inhibitory control is often assessed with tasks that require withholding a prepotent response, such as the go/no-go paradigm, in which individuals respond to frequent “go” stimuli but must inhibit responses to infrequent “no-go” stimuli. The ability to successfully inhibit a response increases from childhood into adulthood as cognitive control abilities mature and goal-directed behaviors develop (Dempster, 1992; Harnishfeger and Bjorklund, 1993; Luna et al., 2004, 2001). During adolescence, a protracted development in top-down self-regulation including inhibitory control is believed to be associated with increased risk-taking behaviors (Casey, 2015; Geier, 2013), such as substance use, which escalates during adolescence and peaks in young adulthood (Schulenberg et al., 2018).
Early functional magnetic resonance imaging (fMRI) studies investigating maturation of inhibitory control circuitry used cross-sectional designs and reported both activation decreases and increases with age. Specifically, significant activation increases with age have been reported in orbitofrontal cortex (Rubia et al., 2006; Tamm et al., 2002), anterior cingulate (Rubia et al., 2006), and inferior frontal gyrus extending into insula (Tamm et al., 2002) during response inhibition in go/no-go tasks. Similarly, during other inhibitory control tasks (i.e., flanker and stop tasks), activation increases with age have been found in inferior frontal cortex (Bunge et al., 2002; Rubia et al., 2007) and anterior cingulate (Bunge et al., 2002), as well as temporal and parietal areas (Bunge et al., 2002; Rubia et al., 2007) that were not reported in the go/no-go studies. Significant activation decreases with maturation have been reported in the superior frontal gyrus (Booth et al., 2003; Tamm et al., 2002), portions of anterior, middle, and posterior cingulate (Booth et al., 2003; Tamm et al., 2002), and dorsolateral prefrontal cortex (Durston et al., 2006), as well as subcortical areas (e.g., caudate and thalamus (Booth et al., 2003)) during go/no-go tasks. A stop-signal task (Cohen et al., 2010) found similar activation decreases with age in the medial prefrontal cortex extending into anterior cingulate.
These studies have not converged on definitive developmental changes in inhibitory control circuitry, likely due to a number of methodological factors. One may be the variable developmental periods during which participants were scanned. For example, some studies have investigated age-related changes cross-sectionally by comparing groups of early or middle adolescents with young adults (Adleman et al., 2002; Booth et al., 2003; Bunge et al., 2002; Casey et al., 1997; Durston et al., 2002b), whereas other studies have used linear regression to identify positive or negative age-related changes (Adleman et al., 2002; Luna et al., 2001; Marsh et al., 2006; Rubia et al., 2000, 2007; Rubia et al., 2006; Schulte et al., 2019; Tamm et al., 2002; Velanova et al., 2008). Gaps between measurement groups (e.g., comparing 12–16 year-olds with 18–21 year-olds) or limiting the age range of participants (e.g., 13–17 years) excludes important developmental time periods and can present an incomplete picture. Additionally, developmental changes are difficult to characterize with cross-sectional studies, as these types of designs are unable to fully capture change over time.
Only a few studies have examined maturation of inhibitory control circuitry using longitudinal designs. Using an accelerated longitudinal design, Ordaz and colleagues demonstrated decreasing activation across ages 9–26 years in the right prefrontal cortex, as well as increasing activation to inhibitory errors in the anterior cingulate during an anti-saccade task (Ordaz et al., 2013). More recently, a similar design was used to investigate age-related change in brain activation during an incentivized anti-saccade task. Particularly relevant here are the findings observed during non-incentivized trials of decreasing activation with age in the prefrontal cortex and a U-shaped developmental trajectory in the posterior parietal cortex (Paulsen et al., 2015). These longitudinal studies converge on a common finding of decreasing prefrontal activation during the inhibition of saccades across adolescence. However, to our knowledge, this has not been investigated in a longitudinal design during the more widely used go/no-go task of motor inhibition. Furthermore, these studies focused on regions of interest (ROI), which restricts the search for maturational changes to a priori regions, thereby potentially missing critical age-related effects outside of these regions.
Here we sought to characterize the development of neural circuitry involved in successful inhibitory control across ages 7–28 years using a go/no-go task during fMRI scanning in a longitudinal design. The development of successful inhibitory control is relevant for elucidating aberrant inhibitory control, which is known to occur in substance use and other disorders. However, most developmental studies have investigated primarily healthy samples that are free from risky behaviors (such as substance use) and disorders (such as depression and ADHD) that are common during childhood, adolescence, and young adulthood. Here, we investigate the development of inhibitory control in a sample that includes youth who display these high risk behaviors and disorders. We used a recently developed toolbox for whole-brain analysis of longitudinal neuroimaging designs—the Sandwich Estimator Toolbox (SwE) (Guillaume, 2015; Guillaume et al., 2014; Guillaume and Nichols, 2015)—to test linear, quadratic, and cubic patterns of activation increases and decreases across age. Based on prior longitudinal work, we expected to see linear decreases in activation with age in the prefrontal cortex.
2. Materials and methods
2.1. Participants
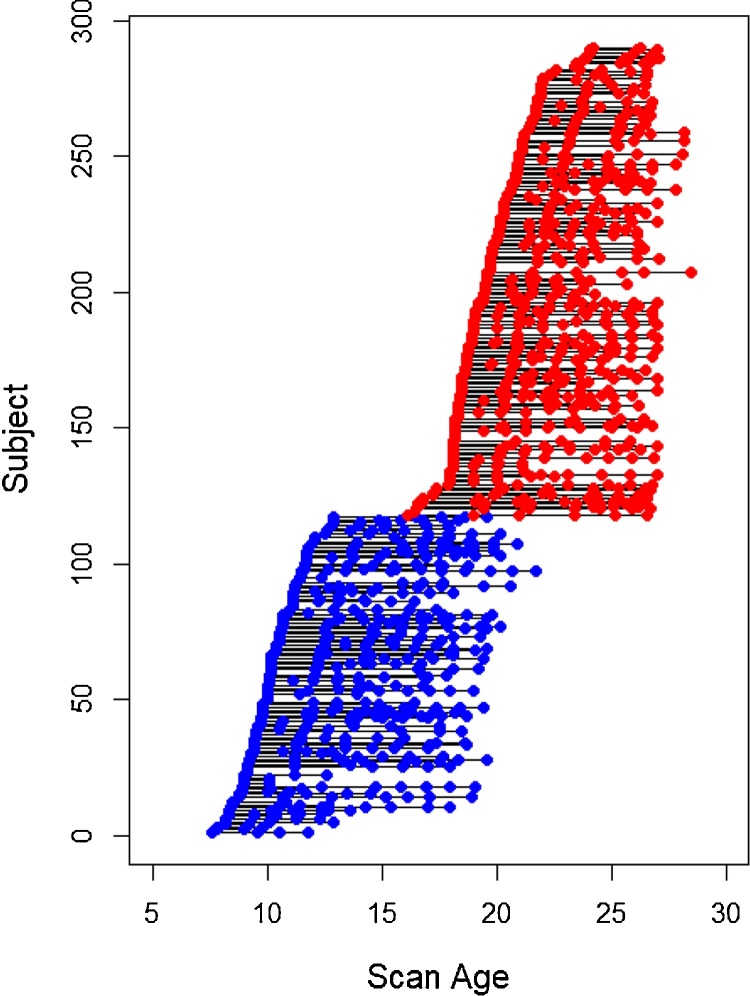
Participants were 290 individuals (111 female) from two cohorts: 117 children and early adolescents aged 7.6–12.9 years at study entry and 173 adolescents and young adults aged 16.1–24.2 years at study entry. Thirty-five participants contributed one scan and 255 participants (87.9 %) contributed between two and eight scans, for a total of 1162 scans (497 scans from the younger cohort and 665 scans from the older cohort) covering the ages of 7.6–28.5 years (with cohort-overlap from 16.1 to 21.7 years of age). Repeat scans took place at approximately 1- to 2-year intervals. See Table 1 for sample size and age by cohort and scan number; see Fig. 1 for a graphical depiction of longitudinal scans by age and cohort.
Table 1.
Sample Size and Age by Scan Number and Cohort.
| Children/Early Adolescents |
Adolescents/Young Adults |
Full Sample | |||||
|---|---|---|---|---|---|---|---|
| n | Mean Age (SD) | Age Range | n | Mean Age (SD) | Age Range | N | |
| Scan 1 | 117 | 10.2 (1.2) | 7.6–12.9 | 173 | 19.7 (1.7) | 16.1–24.2 | 290 |
| Scan 2 | 104 | 12.3 (1.6) | 9.0–16.5 | 151 | 21.8 (1.8) | 19.0–27.0 | 255 |
| Scan 3 | 88 | 14.2 (1.8) | 10.4–18.1 | 129 | 23.3 (1.8) | 20.0–27.1 | 217 |
| Scan 4 | 73 | 15.6 (1.8) | 11.8–19.1 | 98 | 24.3 (1.6) | 21.1–28.1 | 171 |
| Scan 5 | 56 | 16.9 (1.4) | 13.6–19.6 | 60 | 25.2 (1.2) | 22.5–28.2 | 116 |
| Scan 6 | 39 | 18.1 (1.3) | 16.0–20.6 | 37 | 26.0 (0.9) | 24.1–28.5 | 76 |
| Scan 7 | 16 | 19.1 (0.9) | 17.6–20.7 | 16 | 26.5 (0.7) | 25.1–27.8 | 32 |
| Scan 8 | 4 | 20.1 (1.4) | 18.7–21.7 | 1 | 26.3 (—) | — | 5 |
Note. SD, standard deviation.
Fig. 1.
Longitudinal Scans by Age and Cohort.
Circles denote the ages when scans occurred for each participant. Multiple scans from a single participant are connected by lines. Blue circles represent the younger cohort (children and young adolescents), and red circles represent the older cohort (adolescents and young adults).
Participants were offspring from the Michigan Longitudinal Study (MLS; (Zucker et al., 1996, 2000)), a prospective study consisting of a community sample of families with at least one parent with an alcohol use disorder (AUD) and at least one child (approximately two-thirds of the sample) as well as a control sample of families from the same neighborhoods, also with at least one child but where neither parent had a substance use disorder (SUD). Due to initial MLS recruitment strategies, the majority of the present sample was male (61.7 %) and White (80.7 %). Families were excluded if the target child displayed signs of fetal alcohol syndrome or the mother reported drinking during pregnancy. Full details on assessment and data collection in the MLS can be found elsewhere (Zucker et al., 2000).
Participants included in the present analyses are those who participated in the MLS’s neuroimaging sub-study and therefore represent a subset of the larger MLS sample. Participants were excluded if they were left-handed or ambidextrous or had any of the following: neurological, acute, uncorrected, or chronic medical illness; use of centrally active medications either currently or within the past 6 months; a history of psychosis or schizophrenia in first-degree relatives; an IQ less than 70 (as determined by the Wechsler Adult Intelligence Scale (Wechsler, 1981, 1997) or Wechsler Intelligence Scale for Children (Wechsler, 1974, 1991)); or MRI contraindications such as metal implants or claustrophobia. The presence of Axis I psychiatric or developmental disorders other than past or current conduct disorder, attention deficit/hyperactivity disorder (ADHD), and substance use disorder, as well as unmedicated depression or anxiety was exclusionary. Participants who were taking medication for ADHD were asked to abstain for 48 h prior to the scan session. All participants were also instructed to abstain from alcohol and all other substances for at least 48 h prior to the scan session. In participants aged 15 years and older, urine drug screens were conducted immediately before the scan; those who tested positive were rescheduled. Participants under the age of 15 provided verbal confirmation on the day of the scan of drug and alcohol abstinence. Pregnancy was exclusionary: Standard screening for MRI involved asking all female participants if they might be pregnant, and females aged 15 years and older completed a urine pregnancy test immediately before the scan. All participants provided written informed consent (for those 18 and older) or assent (with parental consent, for those 17 and younger) that was approved by the University of Michigan Medical School Institutional Review Board.
2.2. Stimuli and task
An event-related go/no-go task (Durston et al., 2002a; Hardee et al., 2014; Heitzeg et al., 2014, 2010) was used to assess brain activity during successful inhibitory control (i.e., withholding a prepotent response). Participants were instructed to respond via button press to target stimuli (all letters except for “X”; 75 % of stimuli) but withhold their response to nontarget stimuli (the letter “X”; 25 % of stimuli). Stimulus duration was 500 ms, with a 3500 ms inter-stimulus interval consisting of a black screen with a white fixation cross. Participants completed five 3.5-min runs of 49 trials each. Rates of false alarms (pressing the button for nontarget stimuli), hits (pressing the button for target stimuli), misses (not pressing the button for target stimuli), and correct rejections (not pressing the button for a nontarget stimuli) were recorded. Reaction times to false alarms and hits, measured from the beginning of stimulus presentation, were also recorded. All participants practiced the task on a desktop computer prior to scanning.
2.3. Other measures
2.3.1. Psychopathology
Axis I disorders were assessed by a clinical psychologist based on DSM-IV (American Psychiatric Association (1994)) criteria utilizing the Diagnostic Interview Schedule-Version 4 (Robins et al., 2000) for participants aged 18 years and older and the Diagnostic Interview Schedule-Child-Version 4 (Shaffer et al., 2000) for participations under 18 years old.
2.3.2. Family history
Family history of SUD was defined as the number of parents (zero, one, or two) with a lifetime diagnosis of drug or alcohol abuse or dependence, according to DSM-IV criteria.
2.4. fMRI data acquisition
Whole-brain blood oxygen level-dependent (BOLD) functional images were acquired on a 3.0 T GE Signa scanner (Milwaukee, WI, USA) using T2*-weighted single-shot combined spiral in/out sequences (Glover and Law, 2001) with the following parameters: TR = 2000 ms; TE = 30 ms; flip angle = 90 degrees; field-of-view = 200 mm; 64 × 64 matrix; slice thickness = 4 mm, 29 slices. High-resolution anatomical T1 scans were also obtained for spatial normalization. Foam padding around the head and instructions to participants on the importance of keeping still were used to minimize motion.
2.5. Data analysis
2.5.1. fMRI data: preprocessing
An iterative algorithm was used to reconstruct functional images (Noll et al., 2005; Sutton et al., 2003). Motion correction was performed using FSL v5.0.2.2 (FMRIB, Oxford, UK). Runs exceeding 3 mm translation or 3° rotation were excluded. Statistical Parametric Mapping (SPM8; Wellcome Institute of Cognitive Neurology, Oxford, UK) was used to preprocess images. Functional images were first slice-time corrected, spatially normalized to the Montreal Neurological Institute (MNI) template using SPM defaults, and then smoothed with a 6-mm full-width half-maximum smoothing kernel. A high-pass filter (128 s) was used to remove low-frequency noise.
2.5.2. fMRI data: individual level
SPM8 was used to process images at the individual level with a general linear model. Go trials, correct rejections, and false alarms were modeled separately with the standard hemodynamic response function (event duration 4000 ms from stimulus onset); six realignment parameters and white matter signal intensity were also modeled as nuisance variables. Because our primary contrast of interest was activation associated with successful inhibitory control, images that represented the hemodynamic response associated with correct rejections versus implicit baseline were computed for each participant. The implicit baseline was comprised of the 3500 ms fixation only. Because of the high frequency of target trials relative to other event types, an implicit baseline was used as opposed to target stimuli (i.e., “go” stimuli) (DeVito et al., 2013).
2.5.3. fMRI data: group level
Group-level analyses used SPM12 and the Sandwich Estimator Toolbox for Longitudinal and Repeated Measures Data v2.1.0 (SwE; (Guillaume, 2015; Guillaume et al., 2014; Guillaume and Nichols, 2015)) to investigate the development of brain activity associated with successful response inhibition. The SwE toolbox is useful for longitudinal and repeated-measure neuroimaging data, as it fits a marginal model that avoids the need for per-subject dummy variables. Instead of iteratively computing variance components, it uses the non-iterative “sandwich estimator” to find standard errors. The approach used by the SwE toolbox has a number of advantages compared to traditional linear mixed effect models. First, no random effects (e.g., random slopes) need to be specified; only the population model is specified. Second, the use of an unstructured error covariance allows for all possible random effects to be accounted for. Third, a balanced design is not necessary, and even subjects with single data points can be modeled and will contribute to the fitting of cross-sectional effects. Finally, ordinary least squares is used to estimate the population model, which means that the method is non-iterative and avoids convergence failures that can occur in complex mixed-effects models. In accordance with the toolbox’s documentation, the following non-default options were selected: SwE type = classic (no pooling of covariance over subjects, recommended when N is large); degrees of freedom type = naïve; and non-parametric wild bootstrap = yes, with type C2 small-sample adjustments for WB resampling, and 999 bootstraps.
Three different models were used to test the linear, quadratic, and cubic patterns of longitudinal activation change, respectively. In each model, age was decomposed into its between-subject and within-subject components, following Guillaume and colleagues (Guillaume et al., 2014), to avoid an assumption of identical cross-sectional and longitudinal effects. Correct rejection rate was included as a nuisance variable to account for improvements in task performance with age and was similarly decomposed into its between- and within-subject components. We chose to control for task performance so that the interpretation of any differences in brain activation over time would not be confounded by differences in performance. However, for completeness, models that did not include task performance were also run (see Supplemental Material). Because this is a high-risk sample, we also controlled for substance use in supplemental linear mixed models (see Supplemental Material). For each of the three models, both positive and negative associations with age were assessed. Between-subject age was the covariate of interest, and contrast weights were entered accordingly. Correction for multiple comparisons was achieved with a cluster-wise family-wise error (FWE) level of p < .05 (cluster-defining threshold of p < .001).
2.5.4. Task performance
Linear mixed models run in SPSS v24 were used to evaluate the association between age and the following task performance measures: correct rejection rate, false alarm reaction time, hit rate, and hit reaction time. Scan number was entered as a repeated effect, subject as a random factor, and age as a fixed-effect covariate. A first-order autoregressive covariance matrix was chosen due to the presence of repeated measures.
3. Results
3.1. Demographic and psychometric variables
33.1 % of participants (n = 96) had two parents with an SUD, 43.8 % (n = 127) had one parent with an SUD, and 23.1 % (n = 67) had no parents with an SUD. Rates of participants’ lifetime DSM-IV diagnoses including substance use disorders can be found in Table 2. For ADHD, conduct disorder, alcohol abuse or dependence, and cannabis abuse or dependence, there was an increasing rate of disorder across levels of risk, such that participants who had zero parents with an SUD had the lowest rates of disorder (though note that these rates were non-zero), whereas participants who had two parents with an SUD had the highest rates of disorder.
Table 2.
Diagnostic Data by Cohort.
| Children and Early Adolescents (n = 117) |
Adolescents and Young Adults (n = 173) | Full Sample (N = 290)^ | |
|---|---|---|---|
| Parents with an SUD (%: zero/one/two) |
27.4/38.5/34.2 | 20.2/47.4/32.4 | 23.1/43.8/33.1 |
| Lifetime DSM-IV Diagnosis (%)* | |||
| Generalized Anxiety Disorder | 2.2 | 5.2 | 0.0/6.0/4.6 |
| Major Depression | 2.2 | 21.4 | 8.3/17.1/16.1 |
| ADHD | 12.1 | 17.6 | 11.7/15.7/18.6 |
| Conduct Disorder | 2.2 | 9.4 | 5.0/6.1/9.3 |
| Alcohol Abuse or Dep. | 1.1 | 30.0 | 11.7/21.4/24.1 |
| Cannabis Abuse or Dep. | 1.1 | 16.8 | 5.0/12.0/14.9 |
| Nicotine Dep. | 0.0 | 8.7 | 5.0/4.3/8.0 |
| Other Drug Abuse or Dep. | 0.0 | 3.5 | 1.7/3.4/1.1 |
Note. SUD, substance use disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; ADHD, attention deficit/hyperactivity disorder; Dep., Dependence.
^Diagnosis data for the full sample are reported by family history of SUD status: zero/one/two.
Lifetime diagnosis data were missing in 26 participants for all disorders except ADHD and conduct disorder, for which there were missing data in 29 participants.
3.2. Task performance
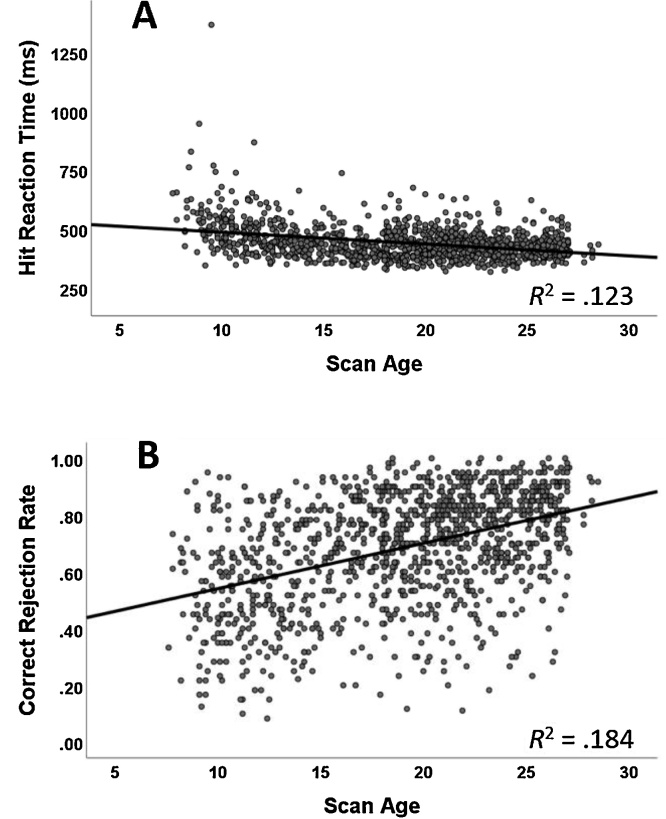
As expected, there was a significant effect of age on all four task performance measures: correct rejection rate (F1,401 = 123.2, p < .001), false alarm reaction time (F1,243 = 96.4, p < .001), hit rate (F1,313 = 29.9, p < .001), and hit reaction time (F1,324 = 127.0, p < .001). Specifically, reaction times decreased and correct inhibitions increased with age. See Fig. 2 for scatterplots of hit reaction time and correct rejection rate by age.
Fig. 2.
Scatterplots of Task Performance by Age.
A) Reaction time for hits (in milliseconds) by scan age in years. B) Correct rejection rate by scan age. In both plots, the linear regression line has been overlaid.
3.3. Longitudinal imaging results
3.3.1. Linear
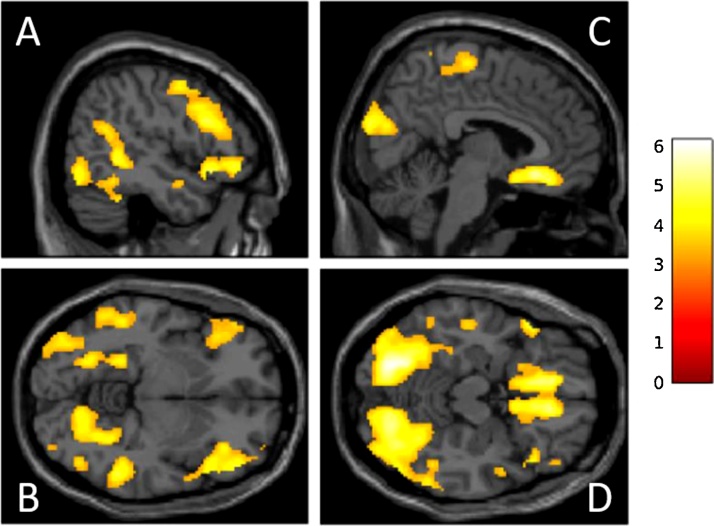
For the model that tested positive linear activation changes associated with age during correct rejections (Table 3, Fig. 3), there were significant increases in areas that included frontal (medial frontal gyrus, inferior frontal gyrus, and middle frontal gyrus), temporal (superior temporal gyrus), parietal (superior parietal lobule), and occipital (fusiform gyrus, lingual gyrus, cuneus) cortex. There were no clusters that survived thresholding with the negative linear contrast.
Table 3.
Correct Rejections vs. Baseline: Increasing Linear Activation Changes Associated with Age.
| Cluster | Cluster Name | Cluster Hemi | Peak Label | BA | k | x | y | z | z-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Occipital | B | Fusiform Gyrus | 19 | 15,522 | −24 | −68 | −14 | 6.16 | |
| Lingual Gyrus | 18 | −22 | −76 | −16 | 5.99 | |||||
| Cuneus | 18 | −24 | −80 | 14 | 5.90 | |||||
| 2 | Orbital Frontal | B | Medial Frontal Gyrus | 11 | 1152 | 6 | 26 | −14 | 5.36 | |
| Subcallosal Gyrus | 11 | −10 | 24 | −14 | 5.25 | |||||
| Medial Frontal Gyrus | 11 | 8 | 34 | −16 | 5.14 | |||||
| 3 | Inferior Frontal/ Temporal Pole | L | Inferior Frontal Gyrus | 45 | 919 | −46 | 26 | 10 | 4.94 | |
| Superior Temporal Gyrus | 38 | −50 | 22 | −16 | 4.60 | |||||
| Inferior Frontal Gyrus | 47 | −42 | 24 | −12 | 4.28 | |||||
| 4 | Middle Frontal | R | Middle Frontal Gyrus | 46 | 1864 | 50 | 20 | 24 | 4.81 | |
| Middle Frontal Gyrus | 46 | 52 | 28 | 22 | 4.54 | |||||
| Postcentral Gyrus | 3 | 28 | −28 | 46 | 4.39 | |||||
| 5 | Inferior Frontal | R | Inferior Frontal Gyrus | 47 | 776 | 38 | 32 | −8 | 4.81 | |
| Inferior Frontal Gyrus | 47 | 50 | 22 | −10 | 4.68 | |||||
| Inferior Frontal Gyrus | 47 | 50 | 40 | −8 | 4.47 | |||||
| 6 | Superior Parietal | L | Superior Parietal Lobule | 7 | 312 | −28 | −62 | 54 | 4.00 | |
| Superior Parietal Lobule | 7 | −30 | −54 | 54 | 3.97 | |||||
| 7 | Medial Frontal | B | Medial Frontal Gyrus | 6 | 449 | 6 | −22 | 64 | 4.00 | |
| Medial Frontal Gyrus | 6 | 10 | −32 | 68 | 3.64 | |||||
| Postcentral Gyrus | 5 | 10 | −48 | 72 | 3.56 | |||||
Note. Hemi, hemisphere; R, right; L, left; B, bilateral; BA, Brodmann area; k, cluster size in voxels.
Coordinates are in Montreal Neurological Institute (MNI) space. Correction for multiple comparisons was achieved with a cluster-wise family-wise error (FWE) level of p < .05 (cluster-defining threshold of p < .001).
Fig. 3.
Correct Rejections vs. Baseline: Increasing Linear Activation Changes Associated with Age.
The color bar represents z-values, and the coordinates (Panel A: x = 50; Panel B: z = -6; Panel C: x = 6; Panel D: z = -16) are in Montreal Neurological Institute (MNI) space. Correction for multiple comparisons was achieved with a cluster-wise family-wise error (FWE) level of p < .05 (cluster-defining threshold of p < .001). Statistics can be found in Table 3.
3.3.2. Quadratic
There were no clusters that survived thresholding with the positive or negative quadratic contrasts.
3.3.3. Cubic
There were no clusters that survived thresholding with the positive or negative cubic contrasts.2
4. Discussion
The goal of this large longitudinal study was to characterize age-related changes in brain function related to successful inhibitory control. We tested linear, quadratic, and cubic effects of age for both positive and negative contrasts. We found that activation in primarily frontal and occipital regions, including bilateral orbital frontal cortex (BA 11), bilateral inferior frontal gyrus (BA 47), and bilateral occipital cortex (BAs 18/19), increased linearly with age. There were no areas with significantly increasing quadratic or cubic activation or decreasing linear, quadratic, or cubic activation.
Functional MRI studies characterizing age-related activation differences in key inhibitory control circuits have primarily reported prefrontal cortex effects (for review, see (Crone and Dahl, 2012)). Prefrontal cortex also undergoes structural changes through early adulthood (e.g., (Gogtay et al., 2004; Shaw et al., 2008; Sowell et al., 2003, 1999)), concomitant with the development of cognitive control abilities (for review, see (Crone and Dahl, 2012)). Developmental effects (both increases and decreases) in the prefrontal cortex—in particular lateral prefrontal cortex—have been reported in other studies across a variety of inhibitory control tasks, such as the go/no-go, stop signal, flanker, anti-saccade, and Stroop tasks. We find a number of these studies are in agreement with the findings reported here, including the right dorsolateral prefrontal cortex (dlPFC; BA 9/46) (Adleman et al., 2002; Bunge et al., 2002; Marsh et al., 2006; Rubia et al., 2006) and bilateral ventrolateral prefrontal cortex (vlPFC; BA 44/45/47) (Adleman et al., 2002; Bunge et al., 2002; Marsh et al., 2006; Rubia et al., 2000, 2007). It has been argued that the vlPFC and dlPFC have distinct, dissociable roles in preparing to inhibit a response; the dlPFC is thought to represent task rules and goal representation in working memory while the vlPFC is thought to implement action control via motor control suppression (Swann et al., 2013). In particular, the inferior frontal gyrus, where the vlPFC is located, plays a crucial role in inhibitory control (for review, see Aron et al., 2014). We also found increasing linear activation in the orbital frontal cortex (OFC), consistent with Rubia and colleagues (Rubia et al., 2006). Neuroimaging studies show that OFC activity is increased in tasks requiring participants to inhibit behavior (Casey et al., 1997; Chikazoe et al., 2009; Horn et al., 2003; Majid et al., 2013; Rubia et al., 2005), demonstrating its involvement in the inhibition of unwanted actions.
Activation in the medial frontal gyrus (BA 6), which includes the supplementary motor area (SMA) and pre-supplementary motor area (pre-SMA), was also found to increase across development—in agreement with another developmental study of inhibitory control (Adleman et al., 2002). The medial frontal gyrus, and particularly the pre-SMA, is consistently activated in inhibitory control tasks (Aron, 2011; Chambers et al., 2009; Meyer and Bucci, 2016). The area of activation reported here covers both SMA and pre-SMA regions (Mayka et al., 2006; Nachev et al., 2008), which are involved in motor sequence processing, planning, and execution (Cona et al., 2017; Hoshi and Tanji, 2004; Hupfeld et al., 2017), as well as the mediation of motor inhibition (Li et al., 2006; Toma et al., 1999). The pre-SMA specifically has been shown to play an important role in switching from an automatic, habitual response to a controlled response (Isoda and Hikosaka, 2007), as well as motor response preparation and selection (Ball et al., 1999; Barber and Carter, 2005). The pre-SMA, along with the inferior frontal gyrus, are key regions for motor inhibition (Aron et al., 2003; Eagle et al., 2008; Floden and Stuss, 2006). The inferior frontal gyrus is thought to first detect stimuli indicating a change in action is needed (i.e., no-go stimuli); it then sends a stop signal to the pre-SMA and subcortical regions (Duann et al., 2009; Sharp et al., 2010; Zandbelt et al., 2013). This is supported by evidence of corticocortical interactions between the pre-SMA and inferior frontal gyrus (Zandbelt et al., 2013), as well as connections between the pre-SMA and subcortical regions such as the caudate (Alexander et al., 1986; Duann et al., 2009).
The largest area showing linear increases in activation with age was comprised of the fusiform gyrus, lingual gyrus, and cuneus (BA 18/19), where activation increases have also been reported in other developmental studies of inhibitory control in adolescents (Adleman et al., 2002; Ordaz et al., 2013). These regions have also been reported in response inhibition studies in adults (Kelly et al., 2004; Liddle et al., 2001; Mathalon et al., 2003; Steele et al., 2013; Tian and Yao, 2008), but are not discussed in detail. However, neuroimaging studies on attentional control indicate that activation in extra-striate regions such as the fusiform and lingual gyri are enhanced when target stimuli fall within the attentional spotlight (Brefczynski and DeYoe, 1999; Heinze et al., 1994; Mangun et al., 1998, 1997; Martinez et al., 1999; Tootell et al., 1998). During tasks like the go/no-go, the no-go stimulus is salient because it appears less frequently, is behaviorally relevant, and demands a change of response. It most likely drives attention in two ways: from a bottom-up involuntary manner, which is stimulus-driven and depends on the physical salience of the object, and from a top-down manner, which depends on the behavioral goals of the individual. It has been suggested that the inferior frontal gyrus, through its connections with inferotemporal visual areas (Barbas and Pandya, 1989; Pandya and Yeterian, 1996), is optimally placed for integrating bottom-up sensory information with top-down, goal-oriented intentions. Additionally, data suggests that the right inferior frontal gyrus may also play a key role in the attentional detection of salient signals (Corbetta et al., 2008; Floden and Stuss, 2006; Hampshire et al., 2010; Sharp et al., 2010). Therefore, activation in the cuneus could reflect modulatory feedback from higher order regions, such as the prefrontal cortex, regarding the behavioral salience of no-go stimuli and the adaptive facilitation of goal-directed behavior.
Together, these results indicate that key components of the response inhibition network, including the prefrontal cortex, pre-SMA, and lingual/fusiform gyrus are increasing linearly in activation across age. This could reflect the view that younger subjects have more diffuse or attenuated patterns of activation across a number of different regions that shifts to focal activation in regions critical to task performance across development (Casey et al., 1997; Durston et al., 2006; Durston et al., 2002b; Schroeter et al., 2004; Velanova et al., 2009). Previous neurodevelopmental studies have reported age-related behavioral improvements that correlated with increases in neural activation magnitude in a number of executive control tasks, including response inhibition tasks (Durston et al., 2006; Marsh et al., 2006; Rubia et al., 2013; Vink et al., 2014).
We did not find maturational effects in several brain regions reported in prior studies of inhibitory control development. For example, cross-sectional developmental studies have identified the anterior cingulate as showing greater activation in adults relative to children or adolescents during incongruent trials of the Stroop task (Adleman et al., 2002), correct no-go trials during a Flanker task (Bunge et al., 2002), and correct no-go trials during a go/no-go task (Rubia et al., 2006). However, longitudinal work using an anti-saccade task has found increased anterior cingulate activation with age only during inhibitory errors but not during correct inhibition (Ordaz et al., 2013). This latter finding is consistent with the current findings and with the role of the anterior cingulate in error processing and performance adjustment (Alexander and Brown, 2010; Botvinick et al., 2001; Carter et al., 1998; Ridderinkhof et al., 2004). We also did not find activation changes in subcortical regions such as the thalamus and striatum, which have been reported previously in developmental studies of inhibitory control, but with mixed directionality. Some studies have reported greater thalamic and/or basal ganglia activation for children/adolescents relative to adults (caudate: (Booth et al., 2003; Rubia et al., 2000, 2007; Rubia et al., 2006); thalamus: (Rubia et al., 2007), while others have reported greater activation for adults compared to children/adolescents (caudate: (Rubia et al., 2007); thalamus: (Luna et al., 2001; Rubia et al., 2006)). The basal ganglia has direct connections to primary motor cortex and is thought to suppress planned or ongoing movements during inhibitory control tasks (Vink et al., 2005). It has been shown to be reliably recruited during motor inhibition tasks (e.g., (Aron and Poldrack, 2006; Boehler et al., 2010; Kelly et al., 2004; Padmala and Pessoa, 2010; Rubia et al., 2005; Vink et al., 2005)). One potential explanation for not finding changes across time in these regions is that activations there are associated with fundamental processes that develop relatively early (Sussman et al., 2016; Velanova et al., 2008), and the use of a wide age-range that included late adolescents and young adults may have “washed out” any such effects.
Contrary to our hypotheses, we did not see significant activation decreases over time in any regions. Although some previous cross-sectional studies did not find any regions that were significantly more active in children relative to adults (e.g., (Adleman et al., 2002; Bunge et al., 2002)), prior longitudinal work found decreasing prefrontal activation during inhibition with age (Ordaz et al., 2013; Paulsen et al., 2015). Differences between the findings from this study and those of previous longitudinal studies could be due to methodological differences, including task type (go/no-go vs. anti-saccade), neural contrast (correct rejections > implicit baseline vs. anti-saccade correct > fixation baseline), and analytic approach (e.g., SwE vs. ROIs). In addition, the majority of participants in the present study were scanned four times, resulting in a more robust longitudinal dataset than in other longitudinal studies. Furthermore, sample characteristics such as age range (e.g., 7–28 vs. 10–22 years-old) and risk status (high-risk for substance use vs. non-high-risk) differed between this and previous studies. Inconsistencies between this study and previous longitudinal studies (Ordaz et al., 2013; Paulsen et al., 2015) are unlikely to be due to our decision to include task performance in the analysis, as we found that results from analyses performed both with and without task performance are similar.
With regard to risk status and substance use, findings may not generalize to populations that are free from substance use and psychiatric disorders due to the sample consisting predominately of youth with either one or two parents with an SUD—with many of these youth having mental health and substance use problems themselves. Moreover, with the exception of generalized anxiety disorder, each SUD and psychiatric disorder was present at all levels of risk (i.e., having zero, one, or two parents with an SUD). In addition to the issue of generalization, we did not control for substance use. However, in supplemental analyses, we found that the association between brain activity and age remained significant even after controlling for alcohol consumed in the year prior to each scan, suggesting that use of the most commonly used substance among this age group was not a driver of the observed effects. We plan on evaluating the effect of substance use more fully after the younger cohort has had time to age into late adolescence and young adulthood. Nonetheless, experimentation with drugs and alcohol during adolescence and young adulthood is normative (Miech et al., 2018), and the results presented here are relevant for understanding how the brain changes with age during successful inhibitory control in individuals with varying levels of substance use and psychiatric disorders (e.g., depression, ADHD). The relevancy of these findings is considerable for those youth at highest risk for the development of these problems as they move into adulthood.
To our knowledge, this study is the largest-scale (i.e., over 1000 observations), widest age-range longitudinal study to assess developmental changes in inhibitory control. As such, it builds on the findings of previous cross-sectional and smaller-scale longitudinal studies and interrogated linear, quadratic, and cubic patterns of age-related changes in inhibitory control activation from age 7 to 28. We also controlled for task performance, which accounts for age-related behavioral improvements in inhibitory control. Finally, use of the Sandwich Estimator is a strength because of its advantages over traditional methods. Specifically, the method enables a whole-brain search for developmental effects by allowing subjects with single data points to be modeled along with subjects with multiple data points. In addition, ordinary least squares is used to estimate the population model, which means the method is non-iterative and avoids convergence failures that can occur in complex mixed-effects models.
In conclusion, the findings presented here are an extension of previous cross-sectional and small-scale longitudinal studies aimed at identifying the developmental correlates of successful inhibitory control. Results indicate linear increases in activation with age in frontal, temporal, parietal, and occipital areas. These results are relevant for understanding how the brain changes functionally over time and matures to support the development of successful inhibitory control.
Declarations of Competing Interest
None.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers K01 DA044270, K01 AA024804, T32 AA007477, R01 AA07065, and R01 AA025790) and the Wellcome Trust (100309/Z/12/Z).
The authors would like to thank Bennet Fauber for his essential help with implementing the Sandwich Estimator Toolbox.
Footnotes
In light of potential differences in the development of inhibitory control circuitry between participants with and without a family history of substance use disorder, we also ran models that explicitly compared them. There were no significant findings in any of the models (i.e., linear, quadratic, or cubic effects for increasing or decreasing activation with age). In addition, we tested for differences between family history groups on the four task performance measures using linear mixed models and did not find any significant differences between groups.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100781.
Contributor Information
Lora M. Cope, Email: lcope@med.umich.edu.
Jillian E. Hardee, Email: jhardee@med.umich.edu.
Meghan E. Martz, Email: mmartz@med.umich.edu.
Robert A. Zucker, Email: zuckerra@med.umich.edu.
Thomas E. Nichols, Email: thomas.nichols@bdi.ox.ac.uk.
Mary M. Heitzeg, Email: mheitzeg@med.umich.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alexander W.H., Brown J.W. Computational models of performance monitoring and cognitive control. Top. Cogn. Sci. 2010;2(4):658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: Author. [Google Scholar]
- Aron A.R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69(12):e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26(9):2424–2433. doi: 10.1523/jneurosci.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Ball T., Schreiber A., Feige B., Wagner M., Lucking C.H., Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage. 1999;10(6):682–694. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- Barbas H., Pandya D.N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barber A.D., Carter C.S. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb. Cortex. 2005;15(7):899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Boehler C.N., Appelbaum L.G., Krebs R.M., Hopf J.M., Woldorff M.G. Pinning down response inhibition in the brain – conjunction analyses of the Stop-signal task. Neuroimage. 2010;52(4):1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brefczynski J.A., DeYoe E.A. A physiological correlate of the’ spotlight’ of visual attention. Nat. Neurosci. 1999;2(4):370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R.J., Orendi J.L., Schubert A.B., Nystrom L.E., Giedd J.N. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J. Cogn. Neurosci. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Garavan H., Bellgrove M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Hirose S., Yamashita K., Miyashita Y., Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009;29(50):15870–15877. doi: 10.1523/jneurosci.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Front. Hum. Neurosci. 2010;4:1–12. doi: 10.3389/fnhum.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona G., Marino G., Semenza C. TMS of supplementary motor area (SMA) facilitates mental rotation performance: evidence for sequence processing in SMA. Neuroimage. 2017;146:770–777. doi: 10.1016/j.neuroimage.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dempster F.N. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev. Rev. 1992;12(1):45–75. doi: 10.1016/0273-2297(92)90003-K. [DOI] [Google Scholar]
- DeVito E.E., Meda S.A., Jiantonio R., Potenza M.N., Krystal J.H., Pearlson G.D. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology. 2013;38(10):1854–1863. doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 2009;29(32):10171–10179. doi: 10.1523/jneurosci.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Worden M.S., Yang Y., Casey B.J. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16(2):449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Uluğ A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002;5(4):F9–F16. [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eagle D.M., Baunez C., Hutcheson D.M., Lehmann O., Shah A.P., Robbins T.W. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex. 2008;18(1):178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Floden D., Stuss D.T. Inhibitory control is slowed in patients with right superior medial frontal damage. J. Cogn. Neurosci. 2006;18(11):1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- Geier C.F. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm. Behav. 2013;64(2):333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Glover G.H., Law C.S. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn. Reson. Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B. Maastricht University/University of Liege; 2015. Accurate Non-iterative Modelling and Inference of Longitudinal Neuroimaging Data. [Google Scholar]
- Guillaume B., Nichols T.E. Paper presented at the Organization for Human Brain Mapping (OHBM); Honolulu, HI: 2015. Non-parametric Inference for Longitudinal and Repeated-measures Neuroimaging Data With the Wild Bootstrap. [Google Scholar]
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee J.E., Weiland B.J., Nichols T.E., Welsh R.C., Soules M.E., Steinberg D.B. Development of impulse control circuitry in children of alcoholics. Biol. Psychiatry. 2014;76(9):708–716. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnishfeger K.K., Bjorklund D.F. Springer; New York: 1993. The Ontogeny of Inhibition Mechanisms: a Renewed Approach to Cognitive Development. [Google Scholar]
- Heinze H.J., Mangun G.R., Burchert W., Hinrichs H., Scholz M., Munte T.F. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372(6506):543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Heitzeg M.M., Nigg J.T., Yau W.Y., Zucker R.A., Zubieta J.K. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry. 2010;68(3):287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M.M., Nigg J.T., Hardee J.E., Soules M., Steinberg D., Zubieta J.K., Zucker R.A. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn N.R., Dolan M., Elliott R., Deakin J.F., Woodruff P.W. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hoshi E., Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. J. Neurophysiol. 2004;92(6):3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Hupfeld K.E., Ketcham C.J., Schneider H.D. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp. Brain Res. 2017;235(3):851–859. doi: 10.1007/s00221-016-4848-5. [DOI] [PubMed] [Google Scholar]
- Isoda M., Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. https://www.nature.com/articles/nn1830#supplementary-information [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Hester R., Murphy K., Javitt D.C., Foxe J.J., Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur. J. Neurosci. 2004;19(11):3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Li C.S., Huang C., Constable R.T., Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32(4):1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Kiehl K.A., Smith A.M. Event-related fMRI study of response inhibition. Hum. Brain Mapp. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Majid D.S., Cai W., Corey-Bloom J., Aron A.R. Proactive selective response suppression is implemented via the basal ganglia. J. Neurosci. 2013;33(33):13259–13269. doi: 10.1523/jneurosci.5651-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun G.R., Hopfinger J.B., Kussmaul C.L., Fletcher E.M., Heinze H.J. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum. Brain Mapp. 1997;5(4):273–279. doi: 10.1002/(sici)1097-0193(1997)5:4<273::aid-hbm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mangun G.R., Buonocore M.H., Girelli M., Jha A.P. ERP and fMRI measures of visual spatial selective attention. Hum. Brain Mapp. 1998;6(5-6):383–389. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R., Zhu H., Schultz R.T., Quackenbush G., Royal J., Skudlarski P., Peterson B.S. A developmental fMRI study of self-regulatory control. Hum. Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Anllo-Vento L., Sereno M.I., Frank L.R., Buxton R.B., Dubowitz D. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat. Neurosci. 1999;2(4):364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Mathalon D.H., Whitfield S.L., Ford J.M. Anatomy of an error: ERP and fMRI. Biol. Psychol. 2003;64(1-2):119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mayka M.A., Corcos D.M., Leurgans S.E., Vaillancourt D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31(4):1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H.C., Bucci D.J. Neural and behavioral mechanisms of proactive and reactive inhibition. Learn. Mem. 2016;23(10):504–514. doi: 10.1101/lm.040501.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R.A., Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E., Patrick M.E. Ann Arbor; 2018. Monitoring the Future National Survey Results on Drug Use, 1975-2017: Volume I, Secondary School Students. [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. https://www.nature.com/articles/nrn2478#supplementary-information [DOI] [PubMed] [Google Scholar]
- Noll D.C., Fessler J.A., Sutton B.P. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans. Med. Imaging. 2005;24(3):325–336. doi: 10.1109/tmi.2004.842452. [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33(46):18109–18124. doi: 10.1523/jneurosci.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48(2):558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D.N., Yeterian E.H. Comparison of prefrontal architecture and connections. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1996;351(1346):1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Paulsen D.J., Hallquist M.N., Geier C.F., Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev. Cogn. Neurosci. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robins L.N., Cottler L.B., Bucholz K.K., Compton W.M., North C.S., Rourke K. Washington University School of Medicine; St. Louis, MO: 2000. The Diagnostic Interview Schedule for DSM‐IV (DIS‐IV) [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C.R., Simmons A. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neurosci. Biobehav. Rev. 2000;24(1):13–19. doi: 10.1016/S0149-7634(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Toone B., Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry. 2005;162(6):1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Woolley J., Nosarti C., Heyman I., Taylor E., Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum. Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum. Brain Mapp. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Lim L., Ecker C., Halari R., Giampietro V., Simmons A. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Zysset S., Wahl M., von Cramon D.Y. Prefrontal activation due to Stroop interference increases during development--an event-related fNIRS study. Neuroimage. 2004;23(4):1317–1325. doi: 10.1016/j.neuroimage.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schulenberg J.E., Johnston L.D., O’Malley P.M., Bachman J.G., Miech R.A., Patrick M.E. Ann Arbor; 2018. Monitoring the Future National Survey Results on Drug Use, 1975-2017: Volume II, College Students and Adults Ages 19-55. [Google Scholar]
- Schulte T., Hong J.-Y., Sullivan E.V., Pfefferbaum A., Baker F.C., Chu W. Effects of age, sex, and puberty on neural efficiency of cognitive and motor control in adolescents. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Bonnelle V., De Boissezon X., Beckmann C.F., James S.G., Patel M.C., Mehta M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U.S.A. 2010;107(13):6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/jneurosci.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Steele V.R., Aharoni E., Munro G.E., Calhoun V.D., Nyalakanti P., Stevens M.C. A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav. Brain Res. 2013;256:529–536. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D., Leung R.C., Chakravarty M.M., Lerch J.P., Taylor M.J. The developing human brain: age-related changes in cortical, subcortical, and cerebellar anatomy. Brain Behav. 2016;6(4) doi: 10.1002/brb3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B.P., Noll D.C., Fessler J.A. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans. Med. Imaging. 2003;22(2):178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- Swann N.C., Tandon N., Pieters T.A., Aron A.R. Intracranial electroencephalography reveals different temporal profiles for dorsal- and ventro-lateral prefrontal cortex in preparing to stop action. Cereb. Cortex. 2013;23(10):2479–2488. doi: 10.1093/cercor/bhs245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tian Y., Yao D. A study on the neural mechanism of inhibition of return by the event-related potential in the Go/NoGo task. Biol. Psychol. 2008;79(2):171–178. doi: 10.1016/j.biopsycho.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Toma K., Honda M., Hanakawa T., Okada T., Fukuyama H., Ikeda A. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: An event-related fMRI study. J. Neurosci. 1999;19(9):3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B., Hadjikhani N., Hall E.K., Marrett S., Vanduffel W., Vaughan J.T., Dale A.M. The retinotopy of visual spatial attention. Neuron. 1998;21(6):1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29(40):12558–12567. doi: 10.1523/jneurosci.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Kahn R.S., Raemaekers M., van den Heuvel M., Boersma M., Ramsey N.F. Function of striatum beyond inhibition and execution of motor responses. Hum. Brain Mapp. 2005;25(3):336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Zandbelt B.B., Gladwin T., Hillegers M., Hoogendam J.M., van den Wildenberg W.P. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum. Brain Mapp. 2014;35(9):4415–4427. doi: 10.1002/hbm.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; New York: 1974. Wechsler Intelligence Scale for Children-Revised. [Google Scholar]
- Wechsler D. The Psychological Corporation; New York: 1981. Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1991. Wechsler Intelligence Scale for Children-3rd Edition. [Google Scholar]
- Wechsler D. Harcourt Assessment; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale-3rd Edition. [Google Scholar]
- Zandbelt B.B., Bloemendaal M., Hoogendam J.M., Kahn R.S., Vink M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci. 2013;25(2):157–174. doi: 10.1162/jocn_a_00309. [DOI] [PubMed] [Google Scholar]
- Zucker R.A., Ellis D.A., Fitzgerald H.E., Bingham C.R., Sanford K. Other evidence for at least two alcoholisms 2. Life course variation in antisociality and heterogeneity of alcoholic outcome. Dev. Psychopathol. 1996;8(4):831–848. [Google Scholar]
- Zucker R.A., Fitzgerald H.E., Refior S.K., Puttler L.I., Pallas D.M., Ellis D.A. The clinical and social ecology of childhood for children of alcoholics: description of a study and implications for a differentiated social policy. In: Fitzgerald H.E., Lester B.M., Zucker R.A., editors. Children of Addiction: Research, Health and Policy Issues. Routledge Falmer; New York: 2000. pp. 109–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.