Abstract
Introduction
In canine visceral leishmaniasis (CVL), lymphopenia, and the disorganization of lymphoid organs such as spleen and lymph nodes have been demonstrated. However, the involvement of thymus in CVL has not been evaluated so far. Herein, we investigated whether the thymus can be colonized by Leishmania infantum in naturally infected dogs.
Methods
Thymus were obtained from 16 of 58 dogs and samples of this organ were submitted to immunohistochemistry for laminin and fibronectin detection, histopathology, in situ hybridization and polymerase chain reaction (PCR) targeting the gene ITS‐1 for Leishmania and sequenced. Samples of spleen, skin and popliteal lymph nodes were collected and submitted to immunohistochemistry and parasitological culture followed by multilocus enzyme electrophoresis.
Results
L. infantum was identified in all dogs. DNA and amastigote forms of Leishmania were detected in the thymus from 16 dogs by PCR and in eight by immunohistochemistry. Besides thymus, parasites were detected in spleen, lymph nodes, and skin. A granulomatous or pyogranulomatous thymitis was observed in eight dogs associated to intact amastigotes forms of this parasite. Fibronectin deposition in thymus was higher in dogs with more clinical signs.
Conclusions
These results demonstrate that the thymus of dogs can be parasitized by L. infantum, which may generate inflammatory reactions leading to alterations in thymic microarchitecture.
Keywords: canine visceral leishmaniasis, clinical signs, extracellular matrix, histopathology, Leishmania infantum, parasite load, thymus
The thymus of dogs can be parasitized by L. infantum. Infection generates inflammatory reactions leading to thymic alterations. Thymic extracellular matrix alterations are associated with disease worsening in canine visceral leishmaniasis
1. INTRODUCTION
Visceral leishmaniasis (VL) is a neglected zoonotic disease caused by the protozoan Leishmania infantum in the Americas.1 Dogs play an important role as domestic reservoirs of VL in the urban space since they harbor parasites available for sand fly vectors even in the healthy skin.2 L. infantum infects macrophages and can spread systemically to all canine tissues leading to the appearance of multiple clinical signs.3 Among the most commonly infected organs are the spleen and lymph nodes, which are essential to antigen presentation and the adequate development of a specific immune response.4 The disruption of splenic microarchitecture has been described in canine VL,5 as well as a pyogranulomatous or granulomatous inflammatory infiltrate, high parasite load and hypertrophy.6, 7 These alterations in the spleen are associated with the deposition of collagen, the impairment in cytokine messenger RNA expression and uncontrolled parasite growth in infected dogs.5, 8, 9, 10
In the lymph node, another secondary lymphoid organ, higher parasite load and higher expression of inducible nitric oxide synthases have been reported in symptomatic dogs when compared with asymptomatic animals.11 Histopathological alterations such as pyogranulomatous or granulomatous inflammation, hypertrophy, and hyperplasia of cortical and medullary zones7, 12, 13 and collagen deposition have been reported.8 Both, spleen and lymph nodes are important sites for the development of the specific immune response13 once they receive the majority of mature T lymphocytes from the thymus and form a delicate compartmentalization based on chemotaxis and an intrinsic antigen presentation.9, 14, 15
Although the association between parasitism and histological lesions has been frequently described in the spleen and lymph node of dogs infected by L. infantum, little is known about the parasite occurrence and pathogenesis in the thymus in these cases. The thymus is a primary lymphoid organ that plays a role in T‐cell homeostasis maintenance, and its product can dictate the immunological profile of individuals.16 In the thymus, thymocytes initiate the genic recombination and expression of their TCR receptors and CD4+ and CD8+ surface molecules. Another extremely important function of the thymus is the positive and negative selection of T cells. In this process, macrophages at the corticomedullary junction remove, by phagocytosis, T lymphocytes that recognize both self‐major histocompatibility complex molecules and self‐antigens.17, 18 The extracellular matrix compounds such as laminin and fibronectin are essential to promote thymocyte maturation based on cellular migration from cortical to the medullar region and then to the periphery. After maturation, naïve T cells leave the thymus, reaching the bloodstream and populating the secondary lymphoid organs. However, lymphopenia and failure in cellular immune response have been described in infected dogs.19 In this context, the present study aimed to evaluate the occurrence of L. infantum in the thymus of naturally infected dogs, and to describe the inflammatory infiltrate, the microarchitecture alterations and the deposition of extracellular matrix components (laminin and fibronectin) in this organ associated with parasitism. Herein, we observed infection by L. infantum in the thymus of all studied dogs, as well as an association between thymic microarchitecture alterations and disease‐worsening.
2. MATERIALS AND METHODS
2.1. Ethical statement
The animals included in this study were L. infantum‐naturally infected dogs that were destined for euthanasia as recommended by the Brazilian Ministry of Health polices. All dog owners had provided formal written consent. The samples were collected during necropsies conducted by veterinarians from the Laboratório de Pesquisa Clínica em Dermatozoonoses em Animais Domésticos (LAPCLIN‐DERMZO–INI/FIOCRUZ). This study was approved by the Ethics Committee on Animal Use of the Oswaldo Cruz Foundation (CEUA/FIOCRUZ; Permit Number: LW‐54/13) and performed according to Brazilian Law 11794/08 and the Brazilian Society of Laboratory Animal Science.
2.2. Animals
A descriptive study was conducted using a nonprobabilistic sample of dogs from Barra Mansa (22°32′25.19″S and 44°10′35.33″W), Rio de Janeiro State, Brazil, a VL endemic area.20 These dogs tested seropositive for anti‐L. infantum antibodies by a rapid immunochromatographic test (Dual Path Platform; Bio‐Manguinhos, Rio de Janeiro, Brazil) and by enzyme‐linked immunosorbent assay (Bio‐Manguinhos). The tests were performed by public health services responsible for the VL surveillance and control program of the state of Rio de Janeiro, with the permission of the owners.
The seropositive dogs were sent by the local authorities (Municipal Health Department of Barra Mansa) to be euthanized at Instituto Nacional de Infectologia Evandro Chagas (INI), Fundação Oswaldo Cruz, Brazil. Euthanasia procedure was performed according to recommendations of the Brazilian Ministry of Health for the control of VL. From the 58 animals necropsied, we were able to recover enough thymus samples from only 16 dogs, which were included in the study.
2.3. Clinical evaluation
The animals were submitted to a physical examination that consisted of the inspection of the skin, ocular, and nasal mucosae, as well as palpation of the superficial lymph nodes and organs. The following clinical signs were considered compatible with VL: low body score, cachexia, apathy, pale mucosae, regional or generalized lymphadenomegaly, splenomegaly, hepatomegaly, skin ulcers, furfuraceous desquamation of skin, onychogryphosis, local or generalized alopecia, and keratoconjunctivitis.3, 21 According to the clinical signs compatible with VL, the dogs were classified into three groups: without clinical signs, with few clinical signs (one to three clinical signs) and with many clinical signs (more than three clinical signs).22
2.4. Sample collection
After physical examination, the dogs were euthanized using 1.0 mL/kg of intravenous Thiopental 1.0% (Thiopentax Cristália). After the detection of an absence of corneal reflex induced by deep anesthesia, 10 mL of intravenous potassium chloride 19.1% (Isofarma) were administered. At necropsy, thymus samples were fixed in 10% neutral formalin and processed routinely for embedding in paraffin for immunohistochemistry, in situ hybridization (ISH), histopathology, and Polymerase chain reaction PCR. Additionally, samples of spleen, skin and popliteal lymph nodes were collected aseptically, immersed in sterile saline, and submitted to parasitological culture followed by multilocus enzyme electrophoresis (MLEE) for Leishmania species identification.
2.5. Parasitological culture for isolation and identification of Leishmania species
The samples were seeded onto biphasic NNN/Schneider's Insect Medium (Sigma‐Aldrich Co, St Louis, MO) containing 10% fetal bovine serum and incubated at 26 ± 28°C23 for at least 30 days. The Leishmania promastigotes, isolated from tissues, were further identified at the species level by MLEE using five enzymatic systems according to protocols previously described.24
2.6. Histopathological evaluation
The sections (5‐µm‐thick) were stained with hematoxylin and eosin and examined by light microscopy (Nikon Eclipse E400; Tokyo, Japan). The cortical area (mm2), the medullary area (mm2) of tissue and the cortex:medulla ratio were analyzed using the ImageJ software (NIH).
2.7. Detection and quantification of amastigote forms of Leishmania spp. by immunohistochemistry
The slides were submitted to deparaffinization, rehydration, blocking of endogenous peroxidase, antigen retrieval, blockade of nonspecific protein binding, and incubation with polyclonal rabbit anti‐Leishmania serum diluted 1:500 following a previously described protocol.25 A polymer‐based detection system (HiDef Detection HRP Polymer System; Cell Marque, Rocklin, CA) was used for the detection of amastigote forms of Leishmania spp. according to the manufacturer's recommendations.
For the evaluation of the parasite load in the thymus, macrophages parasitized with amastigote forms of Leishmania spp. were quantified using a 1‐mm2 optical grid and a manual cell counter. The cells were counted in five fields at ×400 magnification in the most parasitized areas of the sections. The mean number of parasitized macrophages was calculated and parasitism was classified as absent, mild to moderate (0.2‐10 parasitized macrophages), and intense (more than 10 parasitized macrophages).
2.8. Detection of laminin and fibronectin by immunohistochemistry
After deparaffinization, rehydration, blocking of endogenous peroxidase, antigen retrieval, blockade of nonspecific protein binding (0.4% bovine serum albumin; Sigma‐Aldrich Co), the specimens were incubated with the primary antibodies directed to laminin and fibronectin (Abcam), followed by incubation with anti‐rabbit secondary antibody (Abcam) and streptavidin‐horseradish peroxidase. Aminoethylcarbazole (AEC kit; Invitrogen) was used as the substrate–chromogen system and the slides were counterstained with Mayer's hematoxylin (Sigma‐Aldrich Co). The slides were examined under a light microscope (Nikon). Laminin and fibronectin deposits were quantified using the ImageJ software (NIH) and we analyzed at least 10 pictures/microscopy field. The results were expressed as area fraction (representative of the percentage of the stained area).
2.9. Leishmania characterization by PCR
The Leishmania present in the thymus were obtained by PCR the internal transcribed spacer‐1 (ITS‐1) gene fragment26 followed by sequencing to determine the species. Only 10 out of 16 samples from infected dogs were successfully sequenced. The six samples left were positive to Leishmania sp but not sequenced. The primer sequences are described in Table 1. PCR was performed following previously described cycling conditions: 94°C for 5 minutes followed by 32 cycles at 94°C for 30 seconds, 52°C for 1 minute and 72°C for 90 seconds and a final extension at 72°C for 10 minutes.
Table 1.
Primers ITS‐1 sequences27
| Sense | Sequence (5′‐3′) | Size, bp |
|---|---|---|
| Forward (LITSR) | CTG GAT CAT TTT CCG ATG | ⋯ |
| Reverse (L5.8S) | TGA TAC CAC TTA TCG CAC TT | 320 |
Abbreviations: ITS‐1, internal transcribed spacer‐1; bp, base pairs.
After amplification, the products were purified with MinElute 96 UF PCR purification kit, Qiagen. The purified products were sequenced using the same primers with BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, EUA) according to the manufacturer's specifications, in 96‐well plates using a concentration of 3.2 μM of each primer (forward and reverse) and 1.6 μL of the purified PCR product. The reactions were performed by Fiocruz sequencing platform (RPT01A; Seqüenciamento de DNA, RJ). The obtained sequences were analyzed using Phred/Phrap/Consed script and aligned with MEGA 7 software (Gordon D, 2003). A search using the BLAST algorithm (http://ncbi.gov) was carried out to confirm the species.
2.10. Detection of amastigote forms of L. infantum by ISH
ISH was used only in thymus samples in which the Leishmania species was not identified by PCR. In these samples, ISH was used to identify amastigote forms of L. infantum by detecting a specific nucleic acid sequence of this parasite. For this purpose, an L. infantum‐specific oligonucleotide probe labeled with digoxigenin that targets the minicircle kinetoplast DNA gene of the parasite28 was used. Five‐micrometer‐thick sections were cut from the paraffin blocks and mounted on silanized slides. These sections were processed as previously described29 using the ZytoFastPlus CISH Implementation Kit AP‐NBT/BCIP1 (Zytovision GmbH, Bremerhaven, Germany). The probe was diluted 1:500 in Hybridization Solution H7782 (Sigma‐Aldrich Co).
2.11. Statistical analysis
In the present study, convenience samples were used, and for analysis purposes, the animals were divided into three groups according to clinical signs: without clinical signs, with few clinical signs and with many clinical signs or parasite load: absent, mild to moderate (0.2 to 10 parasitized macrophages), and intense (more than 10 parasitized macrophages). All variables (laminin and fibronectin deposition, cortical and medullary areas, and parasite load‐amastigotes/mm2) had normal distribution (P > .05) as indicated by Kolmogorov‐Smirnov test in SPSS software. The construction of the Gaussian mixture (Normal distribution) was performed in excel and SPSS 24 software. A table with average, median, standard deviation, and maximum and minimum value of the number of amastigotes/mm2 was constructed. The normal distribution and the division into groups according to the intensity of the parasite load (high and low; Figure 1) were performed based on our previous study.5 The correlations between the variables (clinical score, cortical/medullary areas, laminin/fibronectin depositions, and parasite load) were performed using the Pearson correlation. The comparisons between groups (high/low parasite load and clinical signs) were then analyzed using a t‐independent test.
Figure 1.
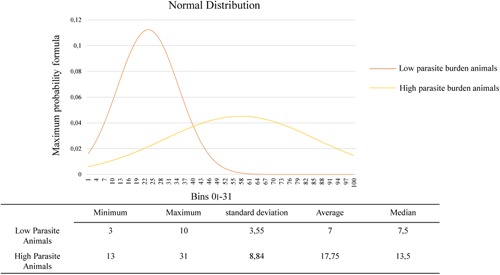
Quantification of parasite load was performed counting the number of amastigotes/mm2 of each animal. A Gaussian mixture (Normal distribution) was performed resulting in two groups: low and high parasite load
3. RESULTS
3.1. Clinical data
Ten of 16 dogs analyzed were males and six were female. The median age was 2.5 (range, 1‐12 years) (Table 2). In the clinical examination, seven dogs had no clinical signs, six had two or more clinical signs and three had one clinical sign (Table 2).
Table 2.
Description of clinical data and detection of amastigotes in the thymus from Leishmania infantum‐naturally infected dogs
| Dog | Breed | Age, y | Sex | Clinical signs | Amastigotes in thymus (IHC) | Parasite load/mm2 | L. infantum in SK (LN and SP) and PC |
|---|---|---|---|---|---|---|---|
| 1 | Mixed‐breed | 3 | M | Alopecia | Positive | 13 | SK(+), LN(+), and SP(+) |
| 2 | Mixed‐breed | 1 | M | Furfuraceous desquamation of skin, thinning, onychogryphosis, and keratoconjunctivitis | Positive | 5 | SK(+), LN(+), and SP(+) |
| 3 | Rottweiler | 6 | F | Absent | Negative | 0 | SK(−), LN(−), and SP(−) |
| 4 | Mixed‐breed | 4 | F | Alopecia, lymphadenomegaly Onychogryphosis | Negative | 0 | SK(+), LN(+), and SP(+) |
| 5 | Mixed‐breed | 2 | F | Absent | Negative | 0 | SK(−), LN(−), and SP(+) |
| 6 | Cane corso | 4 | F | Thinning | Positive | 14 | SK(+), LN(+), and SP(+) |
| 7 | Cane corso | 2 | M | Furfuraceous desquamation of skin, alopecia, cachexia, onychogryphosis, keratoconjunctivitis, lymphadenomegaly, and splenomegaly | Negative | 0 | SK(+), LN(+), and SP(−) |
| 8 | Pinscher | 4 | M | Absent | Negative | 0 | SK(+), LN(+), and SP(+) |
| 9 | Mixed‐breed | 3 | M | Splenomegaly, lymphadenomegaly | Negative | 0 | SK(+), LN(−), and SP(−) |
| 10 | Mixed‐breed | 12 | F | Absent | Negative | 0 | SK(−), LN(−), and SP(+) |
| 11 | Mixed‐breed | 2 | M | Absent | Positive | 13 | SK(+), LN(+), and SP(+) |
| 12 | Mixed‐breed | 2 | M | Splenomegaly | Positive | 03 | SK(+), LN(+), and SP(+) |
| 13 | Mixed‐breed | 5 | M | Absent | Negative | 0 | SK(+), LN(+), and SP(+) |
| 14 | Mixed‐breed | 1 | M | Furfuraceous desquamation of skin, keratoconjunctivitis, lymphadenomegaly, and hepatomegaly | Positive | 10 | SK(+), LN(+), and SP(+) |
| 15 | Mixed‐breed | 2 | F | Hepatomegaly, skin ulcer, lymphadenomegaly, alopecia, and onychogryphosis | Positive | 31 | SK(+), LN(+), and SP(+) |
| 16 | Mixed‐breed | 1 | M | Absent | Positive | 10 | SK(−), LN(+), and SP(+) |
Abbreviations: IHC, immunohistochemistry; LN, lymph node; PC, parasitological culture; SK, skin; SP, spleen.
3.2. L. infantum infecting thymus
All 16 dogs tested positive for Leishmania sp. DNA by PCR in thymus. After ITS‐1 target amplification, the amplified products from the samples of 10 dogs were identified by sequencing and sequence blast revealed that all DNA samples corresponded to L. infantum (Table 3). Amastigote forms within macrophages were detected in the thymic cortex and medulla from eight (50%) dogs by immunohistochemistry (Figure 2A). In the thymus of two dogs that tested positive by PCR, but in which the parasite was not identified at the species level by this technique, ISH was positive for amastigote forms of L. infantum (Figure 2B). The thymus parasite load was assessed by immunohistochemistry, and of 16 dogs evaluated, eight showed no label (50%), four dogs (25%) showed mild to moderate parasitism, and four dogs (25%) intense parasitism (Table 2).
Table 3.
Data description of the sequencing of Leishmania internal transcribed spacer‐1 (ITS‐1) gene fragment
| Identity animal | Max score | Total score | Query cover, % | E value | Degree of similarity sequence, % | Accession | Sequence similar |
|---|---|---|---|---|---|---|---|
| 1 | 361 | 361 | 83 | 6.00E−96 | 98 | KY658231.1 | L. infantum |
| 2 | 424 | 424 | 99 | 7.00E−115 | 98 | KY658235.1 | L. infantum |
| 3 | 315 | 315 | 74 | 5.00E−82 | 96 | KY658235.1 | L. infantum |
| 4 | 438 | 438 | 99 | 3.00E−119 | 99 | KY658231.1 | L. infantum |
| 6 | 442 | 442 | 91 | 2.00E−120 | 99 | KY658231.1 | L. infantum |
| 7 | 438 | 438 | 82 | 3.00E−119 | 99 | KY658231.1 | L. infantum |
| 9 | 235 | 235 | 54 | 3.00E−58 | 98 | KY658231.1 | L. infantum |
| 10 | 353 | 353 | 83 | 9.00E−94 | 99 | FJ555210.1 | L. infantum |
| 12 | 207 | 207 | 40 | 1.00E−48 | 95 | FJ497004.1 | L. infantum |
| 16 | 385 | 385 | 65 | 4.00E−103 | 100 | KY658235.1 | L. infantum |
| IOCL579 (Reference) | 479 | 479 | 37 | 5.00E−131 | 100 | KY658231.1 | L. infantum |
| IOCL566 (Reference) | 468 | 468 | 57 | 6.00E−128 | 100 | KP274863.1 | L. braziliensis |
Figure 2.
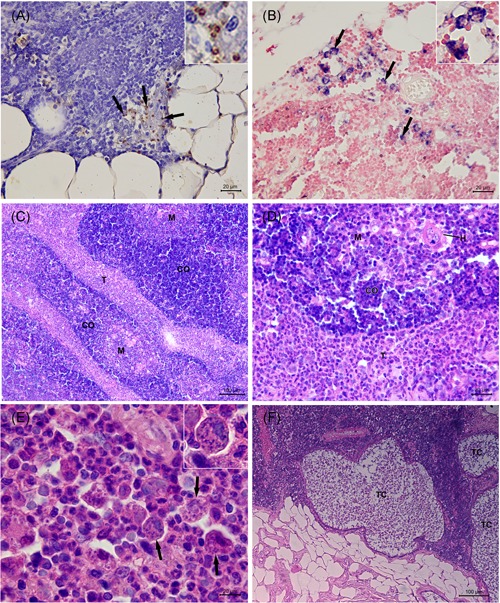
Histopathological evaluation of the thymus from Leishmania infantum‐naturally infected dogs. A, Brown‐stained amastigote forms of Leishmania spp. (arrow and inset) in the cytoplasm of macrophages (immunohistochemistry). B, Blue‐stained amastigote forms of Leishmania spp. (arrow and inset) in the cytoplasm of macrophages (in situ hybridization). C, Thymitis showing thickening of the interlobular trabeculae due to fibrosis and to an intense pyogranulomatous inflammatory infiltrate (HE). D, Detail of (C) showing an intense pyogranulomatous inflammatory infiltrate in the interlobular trabeculae. E, Detail of (D) showing an intense and poorly formed pyogranulomatous inflammatory infiltrate in the interlobular trabeculae composed mainly by macrophages and neutrophils with few lymphocytes and plasma cells. Several amastigote forms of Leishmania spp. (arrow and inset) are observed within the cytoplasm of macrophages (HE). F, Thymic cysts lined by ciliated epithelium in the parenchyma (HE). C, cortex; H , Hassall's corpuscle; HE, hematoxylin and eosin; M, medulla, T, interlobular trabecula; TC, thymic cyst
3.3. L. infantum infecting other organs
Fifteen (94%) of the 16 evaluated dogs were positive for Leishmania spp. in the skin, spleen, or lymph node by parasitological culture (Table 2). The parasite was isolated by culture from spleen, skin and lymph node in 13 (81%), 12 (75%), and 12 (75%) animals, respectively. All these isolates were identified as L. infantum by MLEE.
3.4. Histopathological evaluation
In the thymus of eight dogs positive for Leishmania amastigote forms, a granulomatous or pyogranulomatous thymitis was observed: in six dogs (#1, 2, 11, 12, 14, and 16) and two dogs (#6 and 15), respectively. The granulomatous inflammatory infiltrate was poorly delimited and composed mainly by macrophages with few lymphocytes and plasma cells. The pyogranulomatous inflammatory infiltrate was poorly delimited and composed mainly by macrophages and neutrophils with few lymphocytes and plasma cells. The distribution of the inflammatory infiltrate was multifocal or diffuse and it affected cortical and medullar regions. In one dog (#6), the interlobular trabeculae were thickened due to fibrosis and intense pyogranulomatous inflammatory infiltrate associated with many amastigote forms of Leishmania within macrophages (Figure 2C‐E). In addition, involution with replacement by connective and adipose tissues was observed in all dogs. Thymic cysts, lined by ciliated epithelium, were found in four dogs (#2, 6, 12, 14) (Figure 2F). In the thymus of the eight dogs in which Leishmania amastigote forms were not detected, thymitis was not observed. All of these eight dogs had involution of thymus and thymic cystis was observed in three dogs (#3, 4, and 13).
3.5. Analysis of cortex:medulla ratio
The cortex:medulla ratio was calculated for each dog and varied from 0.13 to 5.18 mm2 (mean ± SEM: 2.5 ± 0.35). We did not observe differences in cortex:medulla ratio when groups based on age, clinical signs or parasite load were compared (P > .05) (Figure 3).
Figure 3.
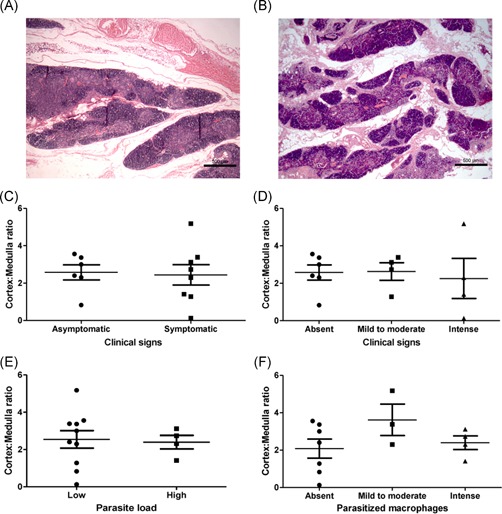
Analysis of the cortex:medulla ratio of the thymus from L. infantum‐naturally infected dogs. A,B, Representative images of the cortex:medulla ratio observed in two animals. C,D, Comparison of cortex:medulla ratio between groups according to clinical signs. E,F, Comparison of cortex:medulla ratio between groups according to parasite load
3.6. Analysis of thymic extracellular matrix compounds (laminin and fibronectin depositions)
As we had observed amastigotes in the thymus associated with in situ inflammatory reaction, we decided to quantify some components of thymic extracellular matrix, such as laminin and fibronectin. These molecules act as a scaffold to thymus and play a role in the events of T‐lymphocyte maturation. Fibronectin and laminin depositions were detected in the thymus from all evaluated dogs and varied from 0.92 to 25.80 area fraction (mean ± SEM: 12.44 ± 1.98) and from 1.18 to 19.20 area fraction (mean ± SEM: 9.06 ± 1.66), respectively (Figures 4 and 5). Although we did not observe differences when comparing the area fraction of fibronectin and laminin deposition with the parasite load, we observed that fibronectin deposition was greater in animals presenting more clinical signs (P = .028) (Figure 5B). There was a positive correlation between fibronectin deposition and clinical signs (Pearson correlation, P = .008; r = .638) (Figure 6A). We also observed a negative correlation between laminin deposition and cortex:medulla ratio (Pearson correlation, P = .016; r = −.628) (Figure 6B).
Figure 4.
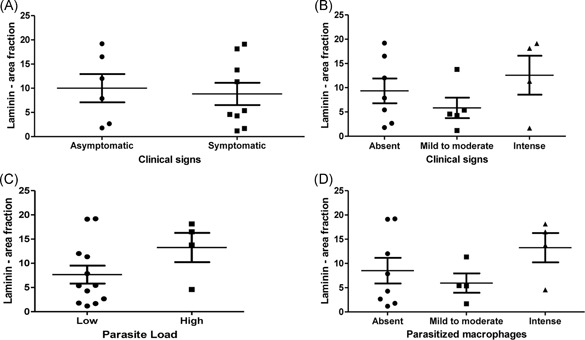
Analysis of laminin deposition in the thymus from L. infantum‐naturally infected dogs. A,B, Comparison of laminin deposition between groups according to clinical signs. C,D, Comparison of laminin deposition between groups according to parasite load
Figure 5.
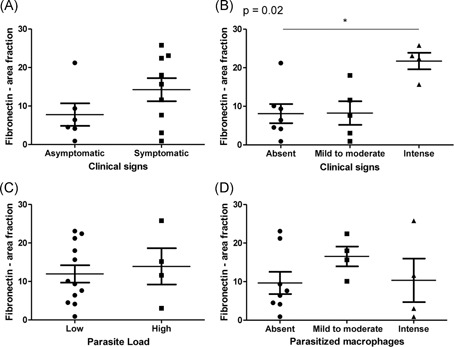
Analysis of fibronectin deposition in the thymus from L. infantum‐naturally infected dogs. A,B, Comparison of fibronectin deposition between groups according to clinical signs. C,D, Comparison of fibronectin deposition between groups according to parasite load
Figure 6.
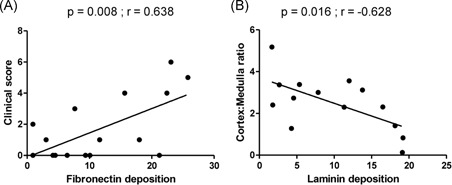
Correlations between (A) fibronectin deposition and clinical score and (B) laminin deposition and cortex:medulla ratio (Pearson correlation)
4. DISCUSSION
In the present study, the thymuses of 16 dogs were evaluated for the presence of the parasite L. infantum. Herein, we detected Leishmania DNA by PCR ITS‐1 in the thymus from all dogs included in the study and intact amastigotes by immunohistochemistry in 50% of them. The animals showing thymus infection also had parasites in other lymphoid organs such as spleen and lymph nodes. Furthermore, skin infection by L. infantum was frequently observed, which suggests that these dogs were potential source of infection for sandy flies.
Histopathological alterations such as granulomatous or pyogranulomatous infiltration were evidenced in the thymuses from eight dogs in which intact amastigotes were visualized in situ. Moreover, dogs presenting more clinical signs showed higher fibronectin depositions in thymus. Altogether, the data suggest the thymus as a site for parasite colonization. Moreover, the alterations in thymic microarchitecture could be associated with inflammatory reactions induced by the parasite in the organ and may influence the antiparasite immune response and disease progression.
The thymic microenvironment is formed mainly by fibronectin and laminin.30 The bone marrow‐derived T‐cell precursors enter in the thymus by the cortical zone and travel through the thymic microenvironment to finish their maturation.30, 31, 32 Cell migration is crucial for intrathymic T‐cell differentiation induced by chemokines and extracellular matrix components such as fibronectin.30 Parasitic infections lead to thymic changes that may affect the immune response of animals.33, 34, 35 In a previous study, amastigotes were evidenced in mice experimentally infected with L. infantum, and the thymuses of those animals exhibited increased weight and significant alterations in chemokine levels.35 Morrot et al33 studying an experimental acute infection by Trypanosoma cruzi, observed massive thymocyte depletion and abnormal release of immature CD4(+)CD8(+) cells to peripheral lymphoid organs, where they acquired an activated phenotype similar to activated effector or memory T cells. They suggested that, in this model, these cells escaped from the negative selection process, and some of them became potentially autoimmune.33 They also observed atrophy of mesenteric lymph nodes, in contrast with lymphocyte expansion in spleen and subcutaneous lymph nodes, illustrating a complex and organ‐specific dynamics of lymphocyte subpopulations.33 Herein, we observed inflammatory reaction and alterations in fibronectin depositions in infected dogs. Since the cortical region is the area where immature lymphocytes migrate while they receive the stimuli for their maturation, the data suggest that Leishmania infection could modify the regular functionality of thymus. In fact, lymphopenia, splenomegaly, and lymphadenopathy have been described in canine visceral leishmaniasis (CVL), as well as microarchitecture disruption of both spleen and lymph nodes.8, 36, 37 Although the presence of amastigotes in spleen and lymph nodes can produce local inflammatory alterations, based on our data, we cannot discard the possible contribution of immature lymphocytes released from the thymus to the described tissue alterations. Therefore, other analyses should be done to verify this hypothesis.
In our study, the animals were in the adult phase and we observed involution of thymus in all of them. In dogs, the complete development of thymus occurs at 2 months of age, entering into a process of involution after 6 months of age, but maintaining its activity during the animal's life and acting as a modulator of other lymphoid organs.18 From 1‐year‐old, the adipose tissue becomes more evident with small islands of remaining thymic tissue,18 which may persist until the old age. In fact, we observed the islands of remaining thymic tissue in one 12‐year‐old dog. These islands had all cell populations as observed in the thymus from young animals but in smaller numbers.18 So, it is reasonable to think that due to infection by Leishmania and consequent tissue damage observed in the thymus, the maturation of lymphocytes could be altered in these thymic islands and might contribute to the immunopathology of secondary lymphoid organs in CVL. Although the reminiscent thymus maintains thymocyte maturation over the whole life of the animal, the effect of thymic infection in the course of leishmaniasis would be more severe in pups, since there is intense thymus activity and immunological maturation in young animals. Thus, studies focused on the thymus of young dogs would better‐clarify this hypothesis.
Regarding the alterations observed in thymic extracellular matrix compounds, fibronectin interconnecting segment domain can be recognized by receptors expressed in macrophages favoring the invasion of parasites and reducing the activation of macrophages.38, 39, 40, 41 In fact, the thymus is an organ that harbors a significant number of TGF‐β (transforming growth factor‐β)‐producing macrophages that act as professional scavengers of apoptotic cells resulting from the regular process of negative selection.42, 43 Due to selection processes, 95% of all the thymocytes formed die and are submitted to clearance by macrophages.44 Although classically activated macrophages (M1‐NOS2 and TNF‐α [tumor necrosis factor‐α] producer cells) play a role in parasite killing,45 the professional scavenger macrophages observed in thymus could act as a site for Leishmania persistence. In fact, we previously observed F4/80+ cells colocalizing with parasites in the thymus of L. infantum‐infected mice.35 In the infected dogs, the amastigotes were observed inside macrophages located at the cortical and medullary regions.
The higher expression of extracellular matrix molecules such as laminin, collagen, and fibronectin in animals with more clinical signs has been demonstrated in lymphoid organs such as spleen and lymph node, and also in the liver.8, 9, 14, 37, 40 In our study, we observed a positive correlation between clinical score and fibronectin deposition. This data suggests a remodeling process of the thymic extracellular matrix probably due to the inflammatory process induced by the presence of the parasite in this organ. The parasite disseminates through various organs, causing inflammatory process, extracellular matrix changes and tissue damage leading to organic dysfunctions, and consequently, the appearance of clinical signs. However, further studies should be performed to elucidate this hypothesis.
The lack of correlation between fibronectin expression and cortex/medulla ratio can be explained by the large variability in measurements of these areas due to the age differences of the evaluated dogs. There are several factors that may influence the thymus histological patterns: diabetes,46 infections,34 malnutrition,35 and age.16, 18 It is well‐described that the thymus suffers involution throughout the life of the animal accompanied by the reduction of the cortex.16, 18 Laminin, in turn, correlated inversely with the cortex/medulla ratio. Laminin is more concentrated in the medullary region, and therefore, the larger the cortical area, the lower the laminin expression. This correlation probably occurred due to age‐related differences but not to the infectious process itself.
Taken together, these results demonstrate that the thymus of dogs can be parasitized by L. infantum, which may generate inflammatory reactions leading to alterations in thymic microarchitecture. These alterations probably compromise the maturation of T lymphocytes in the thymus favoring the failure of the immune response in infected dogs.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
RCM, FNM, and RP conceived and designed the research; AVAS, TLS, FBF, AAVMJ, LCF, CPBF, and PC performed the experiments; AVAS, TLS, RP, RCM, and FNM prepared the figures and wrote the manuscript; AVAS, TLS, RCM, and FNM analyzed the data; AVAS, T.L.S, RP, RCM, and FNM edited the manuscript; and AVAS, TLS, FBF, AAVMJ, LCF, CPBF, PC, RP, RCM, and FNM revised and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful to the administrative staff at Laboratório de Pesquisa em Leishmaniose (LPL‐IOC/FIOCRUZ) and Laboratório de Pesquisa Clínica em Dermatozoonoses em Animais Domésticos (LAPCLIN‐DERMZOO‐INI/FIOCRUZ). This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico‐CNPq (PC Universal grant No. 421223/2018‐9), Fundação de Amparo à Pesquisa do Estado de Rio de Janeiro‐FAPERJ (E‐26/010.001018/2016), and PAEF‐Fiocruz; IOC‐FIOCRUZ, Programa Cientista do Nosso Estado (Faperj E‐26/203.069/2016), Programa Jovem Cientista do Nosso Estado (Faperj E‐26/202.760/2019). RCM and FBF received productivity fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico. We also thank the Programa Print‐Capes for financial support. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
da Silva AVA, de Souza TL, Figueiredo FB, et al. Detection of amastigotes and histopathological alterations in the thymus of Leishmania infantum‐infected dogs. Immun Inflamm Dis. 2020;8:127–139. 10.1002/iid3.285
Rodrigo C. Menezes and Fernanda N. Morgado contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Werneck GL. Visceral leishmaniasis in Brazil: rationale and concerns related to reservoir control. Rev Saude Publica. 2014;48:851‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borja LS, Sousa OMF, Solcà MS, et al. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet Parasitol. 2016;229:110‐117. [DOI] [PubMed] [Google Scholar]
- 3. Koutinas AF, Koutinas CK. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi. Vet Pathol. 2014;51:527‐538. [DOI] [PubMed] [Google Scholar]
- 4. Haley PJ. The lymphoid system: a review of species differences. J Toxicol Pathol. 2017;30:111‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavalcanti AS, Ribeiro‐Alves M, Pereira LOR, et al. Parasite load induces progressive spleen architecture breakage and impairs cytokine mRNA expression in Leishmania infantum‐naturally infected dogs. PLOS One. 2015;10:e0123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lima IS, Silva JS, Almeida VA, et al. Severe clinical presentation of visceral leishmaniasis in naturally infected dogs with disruption of the splenic white pulp. PLOS One. 2014;9:e87742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reis AB, Martins‐Filho OA, Teixeira‐Carvalho A, et al. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet Immunol Immunopathol. 2009;128:87‐95. [DOI] [PubMed] [Google Scholar]
- 8. Silva LC, Castro RS, Figueiredo MM, Michalick MSM, Tafuri WL, Tafuri WL. Canine visceral leishmaniasis as a systemic fibrotic disease. Int J Exp Pathol. 2013;94:133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva JS, Andrade AC, Santana CC, et al. Low CXCL13 expression, splenic lymphoid tissue atrophy and germinal center disruption in severe canine visceral leishmaniasis. PLOS One. 2012;7:e29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silva‐O'Hare J, de Oliveira IS, Klevorn T, et al. Disruption of splenic lymphoid tissue and plasmacytosis in canine visceral leishmaniasis: changes in homing and survival of plasma cells. PLOS One. 2016;11:e0156733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanches FP, Tomokane TY, Da Matta VLR, Marcondes M, Corbett CEP, Laurenti MD. Expression of inducible nitric oxide synthase in macrophages inversely correlates with parasitism of lymphoid tissues in dogs with visceral leishmaniasis. Acta Vet Scand. 2014;56:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keenan CM, Hendricks LD, Lightner L, Webster HK, Johnson AJ. Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease, and clinical pathology. Vet Pathol. 1984;21:74‐79. [DOI] [PubMed] [Google Scholar]
- 13. Sanchez MA, Diaz NL, Zerpa O, Negron E, Convit J, Tapia FJ. Organ‐specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am J Trop Med Hyg. 2004;70:618‐624. [PubMed] [Google Scholar]
- 14. Santana CC, Vassallo J, de Freitas La R, Oliveira GGS, Pontes‐de‐Carvalho LC, dos‐Santos WLC. Inflammation and structural changes of splenic lymphoid tissue in visceral leishmaniasis: a study on naturally infected dogs. Parasite Immunol. 2008;30:515‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch HE, Goldberg GL, Chidgey A, van den Brink MRM, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milićević NM, Milićević Ž. Metallophilic macrophages of the rodent thymus. Prog Histochem Cytochem. 2013;48:1‐46. [DOI] [PubMed] [Google Scholar]
- 18. Agreste FR, Moriconi PR, Barreto‐Vianna ARC, Santos‐Leonardo A, Bombonato PP. Is the vascular growth curve of thymus of dogs (Canis familiaris) time‐dependent? Aust J Basic Appl Sci. 2013;7:334‐342. [Google Scholar]
- 19. Maia C, Campino L. Cytokine and phenotypic cell profiles of leishmania infantum infection in the dog. J Trop Med. 2012;2012:541571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Mello CX, Figueiredo FB, Mendes AAV Júnior, Furtado MC, de Miranda LFC, Fatima Madeira M. Outbreak of canine visceral leishmaniasis in Barra Mansa, State of Rio de Janeiro. Rev Soc Bras Med Trop. 2014;47:788‐790. [DOI] [PubMed] [Google Scholar]
- 21. Marzochi MCA, Coutinho SG, Souza WJS, et al. Canine visceral leishmaniasis in Rio de Janeiro, Brazil. Clinical, parasitological, therapeutical and epidemiological findings (1977‐1983). Mem Inst Oswaldo Cruz. 1985;80:349‐357. [DOI] [PubMed] [Google Scholar]
- 22. de Queiroz NMGP, da Silveira RCV, de Noronha ACF, Oliveira TMFS, Machado RZ, Starke‐Buzetti WA. Detection of Leishmania (L.) chagasi in canine skin. Vet Parasitol. 2011;178:1‐8. [DOI] [PubMed] [Google Scholar]
- 23. Madeira MF, Figueiredo FB, Pinto AGS, et al. Parasitological diagnosis of canine visceral leishmaniasis: Is intact skin a good target? Res Vet Science. 2009;87:260‐262. [DOI] [PubMed] [Google Scholar]
- 24. Cupolillo E, Grimaldi G, Momen H. A general classification of new world leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296‐311. [DOI] [PubMed] [Google Scholar]
- 25. Boechat VC, Mendes Junior AAV, Madeira MF, et al. Occurrence of Leishmania infantum and associated histological alterations in the genital tract and mammary glands of naturally infected dogs. Parasitol Res. 2016;115:2371‐2379. [DOI] [PubMed] [Google Scholar]
- 26. Schönian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349‐358. [DOI] [PubMed] [Google Scholar]
- 27. el Tai NO, Osman OF, el Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single‐strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94:575‐579. [DOI] [PubMed] [Google Scholar]
- 28. Menezes RC, Figueiredo FB, Wise AG, et al. Sensitivity and specificity of in situ hybridization for diagnosis of cutaneous infection by Leishmania infantum in dogs. J Clin Microbiol. 2013;51:206‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliveira VC, Boechat VC, Mendes AAV Junior, et al. Occurrence of Leishmania infantum in the central nervous system of naturally infected dogs: Parasite load, viability, co‐infections and histological alterations. PLOS One. 2017;12:e0175588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Savino W, Mendes‐da‐Cruz DA, Silva JS, Dardenne M, Cotta‐de‐Almeida V. Intrathymic T‐cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002;23:305‐313. [DOI] [PubMed] [Google Scholar]
- 31. Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocr Rev. 2000;21:412‐443. [DOI] [PubMed] [Google Scholar]
- 32. Mendes‐da‐Cruz DA, Smaniotto S, Keller AC, Dardenne M, Savino W. Multivectorial abnormal cell migration in the NOD mouse thymus. J Immunol. 2008;180:4639‐4647. [DOI] [PubMed] [Google Scholar]
- 33. Morrot A, Barreto de Albuquerque J, Berbert LR, de Carvalho Pinto CE, de Meis J, Savino W. Dynamics of lymphocyte populations during trypanosoma cruzi infection: from thymocyte depletion to differential cell expansion/contraction in peripheral lymphoid organs. J Trop Med. 2012;2012:747185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Meis J, Aurélio Farias‐de‐Oliveira D, Nunes Panzenhagen PH, et al. Thymus atrophy and double‐positive escape are common features in infectious diseases. J Parasitol Res. 2012;2012:574020‐574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Losada‐Barragán M, Umaña‐Pérez A, Cuervo‐Escobar S, et al. Protein malnutrition promotes dysregulation of molecules involved in T cell migration in the thymus of mice infected with Leishmania infantum. Sci Rep. 2017;7:45991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Vasconcelos TCB, Doyen N, Cavaillon J‐M, et al. Cytokine and iNOS profiles in lymph nodes of dogs naturally infected with Leishmania infantum and their association with the parasitic DNA load and clinical and histopathological features. Vet Parasitol. 2016;227:8‐14. [DOI] [PubMed] [Google Scholar]
- 37. da Silva AVA, Figueiredo FB, Menezes RC, et al. Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum‐naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens. PLOS Negl Trop Dis. 2018;12:e0006445. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Korom S, Hancock WW, Coito AJ, Kupiec‐Weglinski JW. Blockade of very late antigen‐4 integrin binding to fibronectin in allograft recipients. II. Treatment with connecting segment‐1 peptides prevents chronic rejection by attenuating arteriosclerotic development and suppressing intragraft T cell and macrophage activation. Transplantation. 1998;65:854‐859. [DOI] [PubMed] [Google Scholar]
- 39. Kulkarni MM, Jones EA, McMaster WR, McGwire BS. Fibronectin binding and proteolytic degradation by Leishmania and effects on macrophage activation. Infect Immun. 2008;76:1738‐1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melo FA, Moura EP, Ribeiro RR, et al. Hepatic extracellular matrix alterations in dogs naturally infected with Leishmania (Leishmania) chagasi. Int J Exp Pathol. 2009;90:538‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci. 2014;71:1245‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wakimoto T, Tomisaka R, Nishikawa Y, Sato H, Yoshino T, Takahashi K. Identification and characterization of human thymic cortical dendritic macrophages that may act as professional scavengers of apoptotic thymocytes. Immunobiology. 2008;213:837‐847. [DOI] [PubMed] [Google Scholar]
- 43. Wahl SM, Orenstein JM, Chen W. TGF‐beta influences the life and death decisions of T lymphocytes. Cytokine Growth Factor Rev. 2000;11:71‐79. [DOI] [PubMed] [Google Scholar]
- 44. Sarang Z, Garabuczi É, Joós G, et al. Macrophages engulfing apoptotic thymocytes produce retinoids to promote selection, differentiation, removal and replacement of double positive thymocytes. Immunobiology. 2013;218:1354‐1360. [DOI] [PubMed] [Google Scholar]
- 45. Díaz‐Gandarilla JA, Osorio‐Trujillo C, Hernández‐Ramírez VI, Talamás‐Rohana P. PPAR activation induces M1 macrophage polarization via cPLA2‐COX‐2 inhibition, activating ROS production against Leishmania mexicana. BioMed Res Int. 2013;2013:215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagib PR, Gameiro J, Stivanin‐Silva LG, et al. Thymic microenvironmental alterations in experimentally induced diabetes. Immunobiology. 2010;215(12):971‐979. 10.1016/j.imbio.2010.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


