Abstract
Introduction
Exposure to biomass smoke, cigarettes, alcohol, and the impairment of immunoregulation are considered to be risk factors for tuberculosis. Tumour necrosis factor (TNF) –308G/A and –238G/A gene polymorphisms have been associated with tuberculosis. However, the results remain inconsistent. The aim of this study was to determine the association between TNF polymorphisms and tuberculosis in the presence of biomass smoke, cigarettes, and alcohol in a Mexican population.
Material and methods
TNF polymorphisms were determined in 118 tuberculosis patients and 223 controls. We performed a univariate, bivariate, stratified analysis. Odds ratios, confidence intervals, and p-values were calculated.
Results
Occupational biomass smoke exposure was associated with tuberculosis between the patients and controls (OR = 1.70, 95% CI: 1.08–2.70, p = 0.02). We also found an association of the –308A allele carriers between the patients and controls without exposure to occupational (p = 0.04, OR = 0.16, 95% CI: 0.01–0.92) and in-home (p = 0.02, OR = 0.14, 95% CI: 0.01–0.81) biomass smoke, as well as an association with alcohol (p = 0.01, OR = 0.24, 95% CI: 0.05–0.75). The haplotype analysis revealed an association of the –308A/–238G haplotype between patients and nonconsanguineous controls without exposure to occupational (p = 0.02, OR = 0.12, 95% CI: 0.01–0.99) and in-home (p = 0.01, OR = 0.1, 95% CI: 0.01–0.9) biomass smoke, cigarette use (p = 0.04, OR = 0.28, 95% CI: 0.08–0.98), and alcohol (p = 0.02, OR = 0.22, 95% CI: 0.05–0.88) intake.
Conclusions
The TNF –308A allele and the –308A/–238G haplotype are associated with tuberculosis, as are exposure to biomass smoke, cigarettes, and alcohol. No association for the –238G/A polymorphism was found. Our results provide insight into a possible protective role of TNF polymorphisms in tuberculosis in our population.
Keywords: tuberculosis, biomass smoke, cigarette, alcohol, polymorphisms, protection, risk
Introduction
Smoking is considered to be a risk factor for tuberculosis, among other diseases [1, 2], which increases with consumption [3]. An immune response in the lungs is induced by cigarette smoke, recruiting inflammatory cells such as macrophages and T (CD8*) lymphocytes. Macrophages are the main defence cells in nonsmokers; bronchoalveolar aspiration in smokers (when compared with nonsmokers) shows a fivefold increase in the number of inflammatory cells in the lungs, with 85–90% being macrophages [4].
A few studies have evaluated the impact of biomass smoke as a risk factor for tuberculosis. Either occupational or house environment contaminations due to cooking with solid fuels (wood or charcoal) are responsible for respiratory diseases in children and adults [5]. High levels of gaseous compounds and solid particle concentrations within the breathable size range are present in this type of smoke; these compounds are thought to be more harmful to humans [6].
An association between alcohol intake and tuberculosis has also been found; namely, that heavy drinkers have more tuberculosis relapses [7], a worse prognosis, and a higher probability of suffering more destructive forms of tuberculosis [8, 9]. Immunoregulation impairment, involving cytokines, caused by alcohol intake is one of the main risk factors for tuberculosis as well as other pathologies that develop from immunodeficiency, making individuals vulnerable to infectious agents in different tissues [10, 11].
Recently, tumor necrosis factor α (TNF-α) has been associated with heavy alcohol intake [12]. An increased production of cytokines by blood monocytes and Kupffer cells in alcoholic steatosis patients and in animal models with alcohol-induced liver damage has been shown [13–15]. Additionally, excessive TNF-α production depends on the amount of alcohol ingested [16].
In addition to wood smoke, cigarette, and alcohol exposure, genetic factors can alter interindividual cytokine levels [3]. Two polymorphisms in the promoter region of the TNF gene affect its expression: a negative gene regulation that diminishes TNF-α production is caused by the TNF –238G/A polymorphism [17], and an increase in production up to fivefold the basal level is caused by the TNF –308G/A polymorphism [18]. Both polymorphisms have been identified as risk factors for different non-infectious and infectious diseases, including tuberculosis [19–21]. However, the results remain inconsistent and contradictory [22, 23]. The TNF –308AA genotype has been associated with the highest risk for mild airway diseases that could eventually progress to pulmonary obstructive disease [24]. The –308A allele has also been associated with pulmonary disease susceptibility due to cigarette smoke [25]. The aim of this study was to determine the association between TNF polymorphisms and tuberculosis in the presence of biomass smoke, cigarettes, and alcohol.
Material and methods
Study design and subjects
We studied 118 tuberculosis patients and 223 inhome controls. Blood samples from all participants were taken at the National Institute of Respiratory Diseases, Mexico City. The controls were in-home contacts of the patients, all healthy individuals without a previous tuberculosis history, and they were enrolled at the same time as the patients. All cases were microbiologically confirmed according to World Health Organisation guidelines.
DNA was extracted from frozen blood using the salting out procedure without phenol extraction [26]. Briefly, red cells were lysed with Tris-Triton-Sucrose buffer (TTS), then the DNA was extracted from lymphocytes with 5 mM NaCl, 1% sodium dodecyl sulphate (SDS), and saturated NaCl followed by deproteinisation with chloroform : isoamyl alcohol 24 : 1 and precipitation with absolute ethanol. Finally, DNA was dried out and resuspended in sterile water.
Demographic data on the participants were collected through interviews with professional personnel. The information collected included ethnicity, gender, age, biomass combustion exposure (fumes and asbestos for occupational smoke; wood or charcoal for in-home smoke), cigarettes, and alcohol intake. By analysing cooking habits, we classified between modern nonsolid (gas, electricity) and solid fuels (charcoal, wood). Subjects, who had cooked with charcoal or wood and worked with direct exposure to fumes and dust (asbestos) for the year prior to this study were classified as positive for biomass smoke exposure. Subjects with at least a 20-cigarette intake within the last 30 days or one cigarette per month for the last year were classified as smokers. There are questionnaires that consider positive alcohol consumption as the intake of 16 g a day [27] or 25 g per week [11]. However, we defined regular drinkers as those who drank 20 to 40 g per day. Informed consent was signed by all the subjects.
This study was approved by the Institutional Science and Bioethics Committee, code E-05-06.
Genotyping
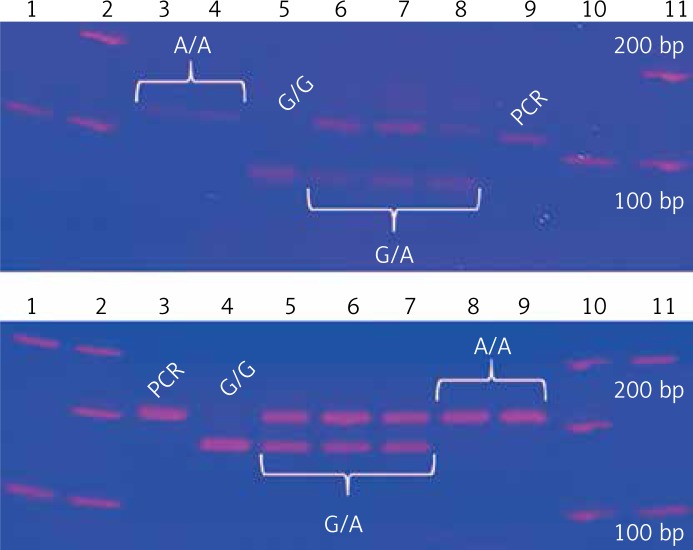
Two TNF gene polymorphisms, previously associated with tuberculosis in European, Mexican American, Hispanic, and Latino populations, were selected [19, 28–31]. TNF –308G/A and –238G/A gene polymorphisms were genotyped by polymerase chain reaction and restriction fragment length polymorphisms (PCR-RFLP) [32]. The –308G/A homozygous AA genotype is identified by a 107 bp fragment, the GG genotype has a combination of 87 and 20 bp bands, and the heterozygous GA has three bands (107, 87, 20 bp). The –238 AA polymorphic genotype is identified by a 152 bp fragment, the GG wild type has two bands (133, 19 bp), and the heterozygous GA has three fragments. Genotypes were confirmed by Sanger sequencing with an ABI Prism 3130 genetic analyser and ABI Prism BigDye Terminator® v3.1 (Applied Biosystems, USA); each genotype was confirmed twice in a bidirectional way by PCR (Figure 1).
Figure 1.
TNF genotypes by polymerase chain reaction and restriction fragment length polymorphisms. Upper image: −308G/A. Lower image: −238G/A
Statistical analysis
Calculated genotypic and allelic frequencies for both TNF polymorphisms in tuberculosis patients and controls were compared and the odds ratio (OR) between polymorphisms was evaluated. We also determined the possible gene-environment interaction between TNF polymorphisms and the exposure factors (biomass smoke, cigarettes, and alcohol). A univariate, bivariate, stratified analysis was performed, and using the χ2 test we calculated p-values in a case-control study design, allowing for the evaluation of the possible association between TNF polymorphisms and pulmonary tuberculosis susceptibility. Haplotype analysis with both TNF polymorphisms was also carried out. All the information gathered from each patient by SPSS version 20.0 (IBM, USA) software was stored in a database.
Results
We studied 118 patients with confirmed pulmonary tuberculosis (PTB) and 223 confirmed healthy controls (TC). One hundred and forty consanguineous contacts (CC) and 83 nonconsanguineous contacts (NCC) comprised the control group. All groups were in Hardy-Weinberg’s equilibrium (p > 0.05). Genotypic and allelic frequencies for both polymorphisms for each group were calculated. Significant differences were found for the TNF –308GA genotype (OR = 0.35, 95% CI: 0.13–0.93, p = 0.03) and the –308A allele (OR = 0.37, 95% CI: 0.14–0.95, p = 0.04) between nonconsanguineous carriers and tuberculosis patients. No association for the TNF –238G/A polymorphism was found. The results are shown in Table I.
Table I.
Genotypic and allelic frequencies for the TNF –308G/A polymorphism (A), TNF –238G/A polymorphism (B)
A
| Genotype/allele | PTB N = 114, n (%) | CC N = 140, n (%) | NCC N = 83, n (%) | TC N = 223, n (%) |
|---|---|---|---|---|
| GG | 107 (93.86) | 128 (91.43) | 70 (84.34) | 198 (88.79) |
| GA | 7 (6.14) | 11 (7.86) | 13 (15.66)* | 24 (10.76) |
| AA | 0 | 1 (0.71) | 0 | 1 (0.45) |
| G | 221 (96.93) | 267 (95.36) | 153 (92.17) | 420 (94.17) |
| A | 7 (3.07) | 13 (4.64) | 13 (7.83)§ | 26 (5.83) |
χ2, p < 0.05.
OR = 0.35, 95% CI: 0.13–0.93, p = 0.03.
OR = 0.37, 95% CI: 0.14–0.95, p = 0.04. PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
B
| Genotype/allele | PTB N = 118, n (%) | CC N = 139, n (%) | NCC N = 83, n (%) | TC N = 222, n (%) |
|---|---|---|---|---|
| GG | 106 (89.83) | 128 (92.09) | 79 (95.18) | 207 (93.24) |
| GA | 11 (9.32) | 11 (7.91) | 4 (4.82) | 15 (6.76) |
| AA | 1 (0.85) | 0 | 0 | 0 |
| G | 223 (94.49) | 267 (96.04) | 162 (97.59) | 429 (96.62) |
| A | 13 (5.51) | 11 (3.96) | 4 (2.41) | 15 (3.38) |
χ2, p < 0.05. PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
Regarding the exposures studied, the median number of years of smoking was 17 (15–19.5), occupational exposure to fumes was 10 years (3–21), stove use in years was 18 (10–30), and alcohol intake time was also 18 years (10–25). Comparisons of PTB with CC, NC, and TC are shown in Table II. Significant p-values with risk OR for occupational biomass smoke exposure between patients, consanguineous controls, and total controls were obtained; in-home biomass smoke exposure had no association.
Table II.
Comparison of exposure factors between the different control groups and tuberculosis patients
| Groups | Occupational biomass smoke | In-home biomass smoke | Cigarette intake | Alcohol intake | ||||
|---|---|---|---|---|---|---|---|---|
| P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | |
| TBP vs. CC | 0.02 | 1.83 (1.11–3.04) | 0.33 | 1.28 (0.78–2.11) | 0.002 | 0.29 (0.12–0.65) | 0.002 | 0.35 (0.18-0.70) |
| TBP vs. NCC | 0.16 | 1.51 (0.85–2.69) | 0.54 | 0.84 (0.47–1.48) | 0.0003 | 0.22 (0.09–0.53) | 0.001 | 0.31 (0.14-0.65) |
| TBP vs. TC | 0.02 | 1.70 (1.08–2.70) | 0.71 | 1.09 (0.69–1.72) | 0.0003 | 0.26 (0.12–0.57) | 0.001 | 0.33 (0.17-0.64) |
TBP – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
No association for the –238G/A polymorphism between patients and controls exposed to biomass smoke (occupational/in-home), cigarettes, and alcohol intake was found. In contrast, the statistical analysis results for the TNF –308G/A variant showed significant differences for the –308A allele carriers (GA and AA genotypes) with protective OR between patients and controls without exposure to biomass smoke (occupational/in-home) and without alcohol intake. In regard to cigarette intake, an association was found only between patients and nonconsanguineous controls (OR = 0.24, 95% CI: 0.06–0.92, p = 0.02) (Table III).
Table III.
A – Genotype frequencies of the TNF –308G/A polymorphism without exposure to the environmental factors. B – Genotype frequencies of the TNF –238G/A polymorphism with exposure to the environmental factors. Comparisons were made between tuberculosis patients and each control group
A
| Exposure factor | Genotype | TBP | CC | NCC | TC |
|---|---|---|---|---|---|
| Occupational biomass smoke | n = 45 | n = 76 | n = 42 | n = 118 | |
| GG, n (%) | 44 (97.8) | 69 (90.8) | 34 (81) | 103 (87.3) | |
| GA, n (%) | 1 (2.2) | 6 (7.9) | 8 (19) | 14 (11.9)§ | |
| AA, n (%) | 0 | 1 (1.3) | 0 | 1 (0.8) | |
| In-home biomass smoke | n = 55 | n = 79 | n = 39 | n = 118 | |
| GG, n (%) | 54 (98.2) | 72 (91.1) | 32 (82.1) | 104 (88.1) | |
| GA, n (%) | 1 (1.8) | 6 (7.6) | 7 (17.9) | 13 (11)¥ | |
| AA, n (%) | 0 | 1 (1.3) | 0 | 1 (0.9) | |
| Cigarette intake | n = 98 | n = 106 | n = 60 | n = 166 | |
| GG, n (%) | 94 (95.9) | 98 (92.5) | 51 (85) | 149 (89.8) | |
| GA, n (%) | 4 (4.1) | 7 (6.6) | 9 (15)* | 16 (9.6) | |
| AA, n (%) | 0 | 1 (0.9) | 0 | 1 (0.6) | |
| Alcohol intake | n = 95 | n = 99 | n = 57 | n = 156 | |
| GG, n (%) | 92 (96.8) | 90 (90.9) | 47 (82.5) | 137 (87.8) | |
| GA, n (%) | 3 (3.2) | 8 (8.1) | 10 (17.5) | 18 (11.6)† | |
| AA, n (%) | 0 | 1 (1) | 0 | 1 (0.6) |
p = 0.04, OR = 0.16, 95% CI: 0.01–0.92;
p = 0.02, OR = 0.14, 95% CI: 0.01–0.81;
p = 0.02, OR = 0.24, 95% CI: 0.06–0.92;
p = 0.01, OR = 0.24, 95% CI: 0.05–0.75. PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
B
| Exposure factor | Genotype | TBP | CC | NCC | TC |
|---|---|---|---|---|---|
| Occupational biomass smoke | n = 61 | n = 55 | n = 39 | n = 94 | |
| GG, n (%) | 56 (91.8) | 52 (94.5) | 34 (87.2) | 86 (91.5) | |
| GA, n (%) | 5 (8.2) | 3 (5.5) | 5 (12.8) | 8 (8.5) | |
| AA, n (%) | 0 | 0 | 0 | 0 | |
| In-home biomass smoke | n = 52 | n = 54 | n = 42 | n = 96 | |
| GG, n (%) | 47 (90.4) | 51 (94.4) | 36 (85.7) | 87 (90.6) | |
| GA, n (%) | 5 (9.6) | 3 (5.6) | 6 (14.3) | 9 (9.4) | |
| AA, n (%) | 0 | 0 | 0 | 0 | |
| Cigarette intake | n = 8 | n = 26 | n = 21 | n = 47 | |
| GG, n (%) | 6 (75) | 24 (92.3) | 17 (81) | 41 (87.2) | |
| GA, n (%) | 2 (25) | 2 (7.7) | 4 (19) | 6 (12.8) | |
| AA, n (%) | 0 | 0 | 0 | 0 | |
| Alcohol intake | n = 12 | n = 34 | n = 24 | n = 58 | |
| GG, n (%) | 9 (75) | 33 (97.1) | 21 (87.5) | 54 (93.1) | |
| GA, n (%) | 3 (25) | 1 (2.9) | 3 (12.5) | 4 (6.9) | |
| AA, n (%) | 0 | 0 | 0 | 0 |
χ2, p < 0.05. PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
Of the four possible haplotypes (GG, AG, GA, AA) originated by the TNF polymorphisms (–308G/A, –238G/A), only the AG polymorphism had a significant association between patients and nonconsanguineous controls without exposure to the environmental factors studied here. The OR values obtained were of protection (Table IV). The analyses with haplotypes GG and GA are shown in the supplementary material (Tables V and VI); no analysis was done with the AA haplotype because it only had an n = 2.
Table IV.
TNF –308A/–238G haplotype associations between groups and exposure factors
| Group | Exposure | P-value | OR | 95% CI | |
|---|---|---|---|---|---|
| PTB vs. TC | Occupational biomass smoke | (+) | 0.91 | 0.93 | 0.29–3.00 |
| (–) | 0.07 | 0.18 | 0.02–1.45 | ||
| In-home biomass smoke | (+) | 0.96 | 1.03 | 0.32–3.24 | |
| (–) | 0.049 | 0.16 | 0.02–1.27 | ||
| Cigarettes | (+) | 0.38 | 2.22 | 0.36–13.66 | |
| (–) | 0.13 | 0.42 | 0.14–1.31 | ||
| Alcohol | (+) | 0.27 | 2.25 | 0.51–9.95 | |
| (–) | 0.056 | 0.30 | 0.08–1.09 | ||
| PTB vs. CC | Occupational biomass smoke | (+) | 0.62 | 1.45 | 0.33–6.41 |
| (–) | 0.19 | 0.26 | 0.03–2.26 | ||
| In-home biomass smoke | (+) | 0.44 | 1.77 | 0.40–7.85 | |
| (–) | 0.13 | 0.22 | 0.02–1.86 | ||
| Cigarettes | (+) | 0.20 | 3.83 | 0.44–33.11 | |
| (–) | 0.40 | 0.58 | 0.16–2.06 | ||
| Alcohol | (+) | 0.08 | 4.75 | 0.71–31.86 | |
| (–) | 0.17 | 0.39 | 0.01–1.58 | ||
| PTB vs. NCC | Occupational biomass smoke | (+) | 0.47 | 0.62 | 0.17–2.29 |
| (–) | 0.02 | 0.12 | 0.01–0.99 | ||
| In-home biomass smoke | (+) | 0.50 | 0.65 | 0.18–2.31 | |
| (–) | 0.01 | 0.10 | 0.01–0.90 | ||
| Cigarettes | (+) | 0.72 | 1.42 | 0.20–9.82 | |
| (–) | 0.04 | 0.28 | 0.08–0.98 | ||
| Alcohol | (+) | 0.78 | 1.25 | 0.25–6.12 | |
| (–) | 0.02 | 0.22 | 0.05–0.88 | ||
PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
Table V.
TNF –308G/–238G haplotype associations between groups and exposure factors
| Group | Exposure | P-value | OR | 95% CI | |
|---|---|---|---|---|---|
| PTB vs. TC | Occupational biomass smoke | (+) | 0.77 | 0.87 | 0.34–2.22 |
| (–) | 0.49 | 1.38 | 0.55–3.47 | ||
| In-home biomass smoke | (+) | 0.66 | 0.82 | 0.33–2.04 | |
| (–) | 0.31 | 1.61 | 0.64–4.03 | ||
| Cigarettes | (+) | 0.38 | 0.45 | 0.07–2.77 | |
| (–) | 0.4 | 1.34 | 0.67–2.68 | ||
| Alcohol | (+) | 0.2 | 0.46 | 0.13–1.56 | |
| (–) | 0.36 | 1.44 | 0.66–3.11 | ||
| PTB vs. CC | Occupational biomass smoke | (+) | 0.41 | 0.62 | 0.19–1.97 |
| (–) | 0.57 | 1.34 | 0.50–3.60 | ||
| In-home biomass smoke | (+) | 0.76 | 0.85 | 0.30–2.40 | |
| (–) | 0.63 | 1.28 | 0.47–3.5 | ||
| Cigarettes | (+) | 0.2 | 0.26 | 0.03–2.25 | |
| (–) | 0.54 | 1.27 | 0.59–2.72 | ||
| Alcohol | (+) | 0.2 | 0.42 | 0.11–1.63 | |
| (–) | 0.6 | 1.26 | 0.53–2.99 | ||
| PTB vs. NCC | Occupational biomass smoke | (+) | 0.69 | 1.24 | 0.42–3.66 |
| (–) | 0.51 | 1.44 | 0.48–4.3 | ||
| In-home biomass smoke | (+) | 0.66 | 0.78 | 0.25–2.39 | |
| (–) | 0.12 | 2.31 | 0.79–6.76 | ||
| Cigarettes | (+) | 0.72 | 0.71 | 0.10–4.90 | |
| (–) | 0.38 | 1.46 | 0.63–3.42 | ||
| Alcohol | (+) | 0.33 | 0.50 | 0.12–2.02 | |
| (–) | 0.24 | 1.75 | 0.69–4.46 | ||
PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls.
Table VI.
TNF –308G/–238A haplotype associations between groups and exposure factors
| Group | Exposure | P-value | OR | 95% CI | |
|---|---|---|---|---|---|
| PTB vs. TC | Occupational biomass smoke | (+) | 0.55 | 1.54 | 0.37–6.39 |
| (–) | 0.25 | 1.88 | 0.63–5.62 | ||
| In-home biomass smoke | (+) | 0.55 | 1.51 | 0.39–5.91 | |
| (–) | 0.37 | 1.66 | 0.54–5.04 | ||
| Cigarettes | (+) | NC | NC | NC | |
| (–) | 0.52 | 1.32 | 0.56–3.15 | ||
| Alcohol | (+) | 0.43 | 2.00 | 0.35–11.45 | |
| (–) | 0.37 | 1.58 | 0.57–4.39 | ||
| PTB vs. CC | Occupational biomass smoke | (+) | 0.52 | 1.75 | 0.31–9.97 |
| (–) | 0.65 | 1.30 | 0.42–4.04 | ||
| In-home biomass smoke | (+) | 0.75 | 0.80 | 0.20–3.17 | |
| (–) | 0.37 | 1.75 | 0.50–6.06 | ||
| Cigarettes | (+) | NC | NC | NC | |
| (–) | 0.9 | 1.06 | 0.42–2.67 | ||
| Alcohol | (+) | 0.72 | 1.38 | 0.23–8.48 | |
| (–) | 0.62 | 1.32 | 0.44–3.96 | ||
| PTB vs. NCC | Occupational biomass smoke | (+) | 0.75 | 1.32 | 0.23–7.58 |
| (–) | 0.06 | 6.47 | 0.74–56.28 | ||
| In-home biomass smoke | (+) | 0.06 | 1.89 | 1.56–2.30 | |
| (–) | 0.58 | 1.50 | 0.35–6.41 | ||
| Cigarettes | (+) | NC | NC | NC | |
| (–) | 0.23 | 2.21 | 0.58–8.38 | ||
| Alcohol | (+) | 0.20 | 4.46 | 0.37–53.7 | |
| (–) | 0.27 | 2.39 | 0.49–11.71 | ||
PTB – tuberculosis patients, CC – consanguineous controls, NCC – nonconsanguineous controls, TC – total controls, NC – no calculable.
Validation
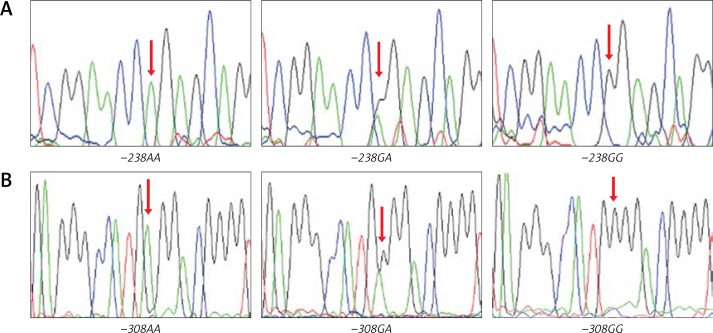
Randomly chosen samples (n = 12) for each genotype were validated by duplicated Sanger sequencing from PCR products, using Big Dye™ terminator v3.1 (Applied Biosystems), sequencing reactions were purified with Sephadex G50® (Sigma-Aldrich) and highly deionised Formamide (Hi-Di)™ (Applied Biosystems). Samples were analysed in an AB3130 Applied Biosystems genetic analyser (Figure 2).
Figure 2.
Electropherograms for TNF −238G/A polymorphism (A), TNF −308G/A polymorphism (B)
Discussion
We found that the presence of the TNF –308A allele is associated with tuberculosis (OR = 0.37, 95% CI: 0.14–0.95, p = 0.04) in our Mexican sample. The same behaviour was observed (protective OR) with the –308GA genotype carriers without exposure to biomass smoke, cigarettes, and alcohol. On the other hand, the –308A/–238G haplotype was also found to be a protective factor against tuberculosis in individuals not exposed to the environmental factors. These results are similar to previous findings that determined that the –308A/–238G haplotype gives protection against tuberculosis in Colombians [29]. Another study of a Greek population found that haplotypes other than GG have a protective effect against the development of sepsis [33]. This could be a result of the fact that every population may have favourable and unfavourable combinations of gene polymorphisms [34] and, in consequence, haplotypes. However, in our population, such protection is lost when exposed to biomass smoke, cigarettes, and alcohol. The explanation for this possibly involves several factors. Biomass smoke, cigarettes, and alcohol impair both the innate and adaptive pulmonary immune response, increasing an individual’s susceptibility to infection by Mycobacterium tuberculosis and subsequent progression to active tuberculosis [4, 35, 36]. Biofuel combustion generates compounds such as cytotoxic polycyclic aromatic hydrocarbons, which increase the secretion of proinflammatory cytokines, such as IL-1β and TNF-α, by alveolar macrophages and pneumocytes. This secretion results in the inability to mount an efficient immune response against infections [37–39], as has been reported in Mexican, Canadian, and Chinese populations [40–42], despite these populations being very different from one other. This may suggest a common mechanism associated with an increased risk of pulmonary tuberculosis. On the other hand, the TNF –308A allele increases expression up to fivefold from its basal levels [43], which favours granuloma formation [44]. The immune response against tuberculosis is mediated by T lymphocytes, whose main function is the activation of macrophages and other cells [45]. Exposure to one or more of these factors (TNF polymorphisms, mycobacteria, alcohol, biomass, cigarette smoke) stimulates TNF-α production by T lymphocytes, which quickly causes macrophage, granulocyte, and cytolytic T lymphocyte activation and maturation, as well as T lymphocyte proliferation. Thus, a positive feedback loop is established, which promotes granuloma formation and maintenance by recruiting macrophages and lymphocytes to the infection site to contain the mycobacteria [46]. This mechanism could explain the possible protective behaviour observed, and we observe that this correlates with our findings because the association is suppressed by exposure to environmental factors.
On the other hand, similar frequencies for the TNF –308G/A polymorphism to the ones we report in this study have been found in Iran [29, 47], Macedonia [48], and Colombia [29]. Merza et al. [47] found an association between this polymorphism and tuberculosis. However, it was with the –308A allele as a risk factor, the other studies did not find any association. This seemingly paradoxical finding needs to be explained in the light of existing literature regarding tuberculosis and TNF polymorphisms.
The genetic structure of the Mexican Mestizo population consists of three primary sources: Amerindians, Caucasians, and Africans [49]. This unique admixture, when compared with other populations, may have some influence on the frequency of the polymorphisms, causing the discrepancy or coincidence observed in our results with respect to other researchers. Supporting this theory, a 2012 meta-analysis involving 18 studies [50] regarding the TNF –308G/A polymorphism and tuberculosis susceptibility revealed that the –308A allele was had a significant risk among Asians, but not in Caucasians, who have an important contribution to the genetic component of the Mexican mestizos.
It has been widely validated that TNF-α inhibitors are drugs that can be used to treat inflammatory conditions successfully, such as rheumatoid arthritis (RA), juvenile arthritis, psoriatic arthritis, inflammatory bowel disease, and psoriasis, among others. These drugs reduce inflammation and stop disease progression by targeting the inflammation-causing TNF-α. However, a major concern is that patients receiving this therapy have an increased risk of bacterial infection, particularly TB reactivation/infection, which interferes with innate immune responses, such as phagosome maturation and apoptosis through complement-mediated cytotoxicity [51]. It is important to clarify that in our study none of the patients or controls had any indications for anti-TNF-α treatment. Our work has the advantage of the available demographic data, which was collected based on a validated questionnaire that is one of the most complete used for respiratory disease epidemiology [52, 53]. Additionally, validation of our technique makes the exposure measurement (polymorphisms) trustworthy. However, biomass smoke exposure levels could not be quantified with adequate equipment, nor were tobacco or alcohol confirmed by lab tests. The only way this information was obtained was through the validated questionnaire, so this could be a limiting factor for our study, which could diminish the precision of these variables due to possible memory bias from interviewed patients. Even though our work is one of the few with a large number of patients analysed, future studies will require even larger sample sizes to conclusively determine the influence of the TNF polymorphisms on tuberculosis in the presence of biomass smoke, cigarettes, and alcohol.
In conclusion, the results indicate that the TNF −308A allele and the –308A/–238G haplotype are associated with tuberculosis and exposure to biomass smoke, cigarettes, and alcohol. However, no association for the –238G/A polymorphism was found. Our results provide insight into a possible protective role of TNF polymorphisms in tuberculosis in our population.
Acknowledgments
We would like to thank all who participated in the making of this study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kałka D, Karpiński L, Gebala J, et al. Sexual health of male cardiac patients – present status and expectations of patients with coronary heart disease. Arch Med Sci. 2017;13:302–10. doi: 10.5114/aoms.2017.65332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankowski P, Czarnecka D, Badacz L, et al. Practice setting and secondary prevention of coronary artery disease. Arch Med Sci. 2018;14:979–87. doi: 10.5114/aoms.2017.65236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Deng J, Su C, et al. Impact of passive smoking, cooking with solid fuel exposure, and MBL/MASP-2 gene polymorphism upon susceptibility to tuberculosis. Int J Infect Dis. 2014;29:1–6. doi: 10.1016/j.ijid.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Demirjian L, Abboud R, Li H, Duronio V. Acute effect of cigarette smoke on TNF-alpha release by macrophages mediated through the erk1/2 pathway. Biochim Biophys Acta. 2006;1762:592–7. doi: 10.1016/j.bbadis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Patra J, Bhatia M, Suraweera W, Morris S, Patra C, Gupta P. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLoS Med. 2015;12:e1001835. doi: 10.1371/journal.pmed.1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swiston J, Davidson W, Attridge S, Li G, Brauer M, Van Eeden S. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–38. doi: 10.1183/09031936.00097707. [DOI] [PubMed] [Google Scholar]
- 7.Selassie A, Wilson D. Quantification of the risk of recurrence in tuberculosis. Ann Epidemiol. 2002;12:524. [Google Scholar]
- 8.Khudzik L, Pankratova D, Riabov B, Vygodchikov I. Social and clinical characteristics of progressive forms of pulmonary tuberculosis in Saratov and Saratov region. Probl Tuberk. 2001;6:24–7. [PubMed] [Google Scholar]
- 9.Breitenfeld D, Trkanjec Z, Thaller V, Breitenfeld T, DeSyo D, Golik V. Tuberculosis and alcoholism in Croatia. Coll Antropol. 1998;22:S217–22. [PubMed] [Google Scholar]
- 10.Erdem B, Peker N, Inci A, et al. Pelvic tuberculosis mimicking ovarian cancer: a series of 13 cases. Arch Med Sci Civil Dis. 2017;2:177–81. [Google Scholar]
- 11.Jelski W, Laniewska-Dunaj M, Orywal K, Kochanowicz J, Rutkowski R, Szmitkowski M. The diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of patients with brain tumor. Arch Med Sci. 2017;13:346–52. doi: 10.5114/aoms.2017.65366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove J, Daly A, Bassendine M, Day C. Association of a tumor necrosis factor promoter polymorphism with susceptibility to alcoholic steatohepatitis. Hepatology. 1997;26:143–6. doi: 10.1002/hep.510260119. [DOI] [PubMed] [Google Scholar]
- 13.McClain C, Cohen D. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–51. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 14.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;21:1304–9. [PubMed] [Google Scholar]
- 15.Pleśniak R, Wawrzynowicz-Syczewska M. Polymorphism of IL28B gene and response to pegylated interferon alpha2a in chronic hepatitis B. Arch Med Sci. 2017;13:70–7. [Google Scholar]
- 16.Stickel F, Sterreicher C. The role of genetic polymorphisms in alcoholic liver disease. Alcohol Alcohol. 2006;41:209–24. doi: 10.1093/alcalc/agl011. [DOI] [PubMed] [Google Scholar]
- 17.Kaluza W, Reuss E, Grossmann S, et al. Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J Invest Dermatol. 2000;114:1180–3. doi: 10.1046/j.1523-1747.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira A, Mendes C, Marano L, et al. Alleles and genotypes of polymorphisms of IL-18, TNF-a and IFN-c are associated with a higher risk and severity of hepatocellular carcinoma (HCC) in Brazil. Hum Immunol. 2013;74:1024–9. doi: 10.1016/j.humimm.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Fan H, Wang Z, Feng F, et al. Association of TNF-alpha -238G/A and -308 G/A gene polymorphisms with pulmonary tuberculosis among patients with coal worker’s pneumoconiosis. Biomed Environ Sci. 2010;23:137–45. doi: 10.1016/S0895-3988(10)60043-8. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K, Zhao Y, Liu L, Wan J. Tumor necrosis factor-alpha 238 G-A polymorphism and risk of hepatocellular carcinoma: evidence from a meta-analysis. Asian Pacific J Cancer Prev. 2013;14:3275–9. doi: 10.7314/apjcp.2013.14.5.3275. [DOI] [PubMed] [Google Scholar]
- 21.Sobjanek M, Zabłotna M, Michajłowski I, et al. -308 G/A TNF-alpha gene polymorphism influences the course of basal cell carcinoma in a Polish population. Arch Med Sci. 2015;11:599–604. doi: 10.5114/aoms.2015.52364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhu H, Pu X, et al. Association between tumor necrosis factor alpha -238G/A polymorphism and tuberculosis susceptibility: a meta-analysis study. BMC Infect Dis. 2012;12:328–35. doi: 10.1186/1471-2334-12-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amirzargar A, Rezaei N, Jabbari H, et al. Cytokine single nucleotide polymorphisms in Iranian patients with pulmonary tuberculosis. Eur Cytokine Netw. 2006;17:84–9. [PubMed] [Google Scholar]
- 24.Hogg J. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 25.Gingo M, Silveira L, Miller Y, et al. Tumour necrosis factor gene polymorphisms are associated with COPD. Eur Respir J. 2008;31:1005–12. doi: 10.1183/09031936.00100307. [DOI] [PubMed] [Google Scholar]
- 26.Buffone GJ, Darlington GJ. Isolation of DNA from biological specimens without extraction with phenol. Clin Chem. 1985;31:164–5. [PubMed] [Google Scholar]
- 27.Lo H, Kuo D, Chen Y. Impact of beverage consumption, age, and site dependency on dual energy X-ray absorptiometry (DEXA) measurements in perimenopausal women: a prospective study. Arch Med Sci. 2017;13:1178–87. doi: 10.5114/aoms.2017.66033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scola L, Crivello A, Marino V, et al. IL-10 and TNF-alpha polymorphisms in a sample of Sicilian patients affected by tuberculosis: implication for ageing and life span expectancy. Mech Ageing Dev. 2003;124:569–72. doi: 10.1016/s0047-6374(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 29.Correa P, Gomez L, Cadena J, Anaya J. Autoimmunity and tuberculosis. Opposite association with TNF polymorphism. J Rheumatol. 2005;32:219–24. [PubMed] [Google Scholar]
- 30.Varahram M, Farnia P, Javad M, Afraei M, Kazempour M, Akbar A. Association of Mycobacterium tuberculosis lineages with IFN-gamma and TNF-alpha gene polymorphisms among pulmonary tuberculosis patient. Mediterr J Hematol Infect Dis. 2014;6:e2014015. doi: 10.4084/MJHID.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas G, Ramirez J, Juarez T, Ramirez S, Carrillo S, Fragoso J. Distribution of the IL-1RN, IL-6, IL- 10, INF-gamma, and TNF-alpha gene polymorphisms in the Mexican population. Genet Test Mol Biomarkers. 2012;16:1246–53. doi: 10.1089/gtmb.2012.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedayati M, Sharifi K, Rostami F, Daneshpour M, Zarif M, Azizi F. Association between TNF-a promoter G-308A and G-238A polymorphisms and obesity. Mol Biol Rep. 2012;39:825–9. doi: 10.1007/s11033-011-0804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retsas T, Huse K, Lazaridis LD, et al. Haplotypes composed of minor frequency single nucleotide polymorphisms of the TNF gene protect from progression into sepsis: a study using the new sepsis classification. Int J Infect Dis. 2018;67:102–6. doi: 10.1016/j.ijid.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Kolovou V, Bilianou H, Giannakopoulou V, et al. Five gene variants in nonagenarians, centenarians and average individuals. Arch Med Sci. 2017;13:1130–41. doi: 10.5114/aoms.2017.68942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stämpfli M, Anderson G. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–84. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 36.Lakshmi P, Virdi N, Thakur J, Smith K, Bates M, Kumar R. Biomass fuel and risk of tuberculosis: a case-control study from Northern India. J Epidemiol Community Health. 2012;66:457–61. doi: 10.1136/jech.2010.115840. [DOI] [PubMed] [Google Scholar]
- 37.Sada I, Ocaña R, Torre L. Humo de biomasa, inmunidad innata y Mycobacterium tuberculosis. Neumol Cir Torax. 2015;74:118–26. [Google Scholar]
- 38.Migliaccio C, Kobos E, King Q, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM (2.5) exposure on macrophage functions. Inhal Toxicol. 2013;25:67–76. doi: 10.3109/08958378.2012.756086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renwick L, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–7. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez E, Brauer M, Perez J, Vedal S. Wood smoke exposure and lung adenocarcinoma in non-smoking Mexican women. Int J Tuberc Lung Dis. 2004;8:377–83. [PubMed] [Google Scholar]
- 41.Ramanakumar A, Parent M, Siemiatycki J. Risk of lung cancer from residential heating and cooking fuels in Montreal, Canada. Am J Epidemiol. 2007;165:634–42. doi: 10.1093/aje/kwk117. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Niu T, Christiani D. Occupational and environmental risk factors for asthma in rural communities in China. Int J Occup Environ Health. 1996;2:172–6. doi: 10.1179/oeh.1996.2.3.172. [DOI] [PubMed] [Google Scholar]
- 43.Ansari A, Hasan Z, Dawood G, Hussain R. Differential combination of cytokine and interferon-gamma +874T/A polymorphisms determines disease severity in pulmonary tuberculosis. PLoS One. 2011;6:e27848. doi: 10.1371/journal.pone.0027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev. 2012;12:352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 45.Jasenosky L, Scriba T, Hanekom W, Goldfeld A. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- 46.Dheda K, Schwander S, Zhu B, Zyl R, Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology. 2010;15:433–50. doi: 10.1111/j.1440-1843.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merza M, Farnia P, Anoosheh S, et al. The NRAMPI, VDR and TNF-alpha gene polymorphisms in Iranian tuberculosis patients: the study on host susceptibility. Braz J Infect Dis. 2009;13:252–6. doi: 10.1590/s1413-86702009000400002. [DOI] [PubMed] [Google Scholar]
- 48.Trajkov D, Trajchevska M, Arsov T, et al. Association of 22 cytokine gene polymorphisms with tuberculosis in Macedonians. Indian J Tuberc. 2009;56:117–31. [PubMed] [Google Scholar]
- 49.Lisker R, Ramirez V, Babinsky V. Genetic structure of autochthonous populations of Meso-America: Mexico. Hum Biol. 1996;68:394–5. [PubMed] [Google Scholar]
- 50.Wang Q, Zhan P, Qiu LX, Qian Q, Yu LK. TNF-308 gene polymorphism and tuberculosis susceptibility: a meta-analysis involving 18 studies. Mol Biol Rep. 2012;39:3393–400. doi: 10.1007/s11033-011-1110-x. [DOI] [PubMed] [Google Scholar]
- 51.Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010;161:1–9. doi: 10.1111/j.1365-2249.2010.04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menezes AM, Victora CG, Perez-Padilla R, PLATINO Team The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15. doi: 10.1186/1471-2288-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Padilla R, Wehrmeister FC, Celli BR, et al. Reliability of FEV1/FEV6 to diagnose airflow obstruction compared with FEV1/FVC: the PLATINO longitudinal study. PLoS One. 2013;8:e67960. doi: 10.1371/journal.pone.0067960. [DOI] [PMC free article] [PubMed] [Google Scholar]