Abstract
Purpose:
The use of the opioid antagonist naltrexone (NTX) for pregnant women with opioid use disorder (OUD) remains understudied. The purpose of this pilot study was to examine pregnancy and neonatal outcomes in a cohort of NTX-treated women.
Methods:
This single-center, retrospective cohort study included 6 mother–infant dyads taking NTX compared with 13 taking buprenorphine (BUP) between 2017 and 2019. Maternal demographic characteristics, any unprescribed or illicit opioid use per urine toxicology or provider report during the pregnancy or 6 months’ postdelivery, delivery outcomes, gestational age, birth weight, Apgar scores, neonatal intensive care unit admission, and neonatal abstinence syndrome (NAS) outcomes (NAS diagnosis, pharmacologic treatment, and total hospital length of stay) were compared.
Findings:
Maternal and infant demographic characteristics were similar between the 2 groups, with the exception of cigarette smoking in the BUP group being more common (92% vs 33%; P = 0.02). None of the women on NTX versus 23% of the women on BUP had documented opioid misuse (P = 0.52). No infants in the NTX group had a NAS diagnosis versus 92% in the BUP group (P < 0.001). Forty-six percent of the BUP-exposed infants were treated for NAS versus 0% in the NTX group (P < 0.001). NTX-exposed infants had a shorter length of stay (mean [SD], 3.2 [1.6] vs 10.9 [8.2] days; P = 0.008).
Implications:
Maintaining women on NTX during pregnancy was associated with favorable outcomes. These results support the need for larger multicenter studies sufficiently powered to detect possible differences between the medications on long-term maternal and child safety and efficacy outcomes.
Keywords: buprenorphine, naltrexone, neonatal abstinence syndrome, neonatal opioid withdrawal syndrome, opioid use disorder, pregnancy, Vivitrol
INTRODUCTION
The United States is experiencing an opioid epidemic that has not spared women of childbearing age, with an estimated 5.6 per 1000 women in the United States with opioid use disorder (OUD) during pregnancy.1 The standard of care for pregnant women with OUD is medication treatment with a long-acting opioid such as methadone (MTD) or buprenorphine (BUP), proven to decrease the risk for overdose, relapse, mortality, pregnancy loss, and preterm birth.2,3 As rates of OUD in pregnancy continue to rise, so does the incidence of neonatal abstinence syndrome (NAS), now affecting between 5 and 20 per 1000 live births in the United States.1,4 Infants with NAS are frequently pharmacologically treated with opioids for withdrawal, with an average hospitalization of 22 days.1
Numerous studies have compared the 2 standard-of-care treatments for pregnant women with OUD: MTD and BUP. A systematic review and meta-analysis concluded that BUP was associated with lower risk of preterm birth, greater birth weight, larger head circumference, and less severe NAS compared with MTD.5 Women treated with BUP also have improved compliance with prenatal care and less unprescribed substance misuse during the pregnancy compared with MTD-treated women.6
In nonpregnant patients, naltrexone (NTX) is one of the three standard treatments for OUD.7 NTX is a pure opioid antagonist with high affinity for the mu-opioid receptor. NTX is available in an oral formulation as well as an extended-release injectable formulation,* which was approved to treat OUD in the United States in 2010. Currently, NTX is used by 11% of young adults with OUD, with a large increase in the past 5 years.8 NTX blocks the euphoric effects of opioid agonists, is devoid of abuse potential and tolerance, and has a modest adverse effect profile.9 It has been tested in randomized controlled trials outside of the United States, showing opioid abstinence efficacy with no reported significant adverse events.10 In addition, it is reportedly particularly helpful in select populations who are highly motivated to end their dependencies (eg, inmates).11 The mortality rate associated with NTX is similar to that of MTD and BUP.10,12 In recent years, the idea of using NTX to treat pregnant women with OUD has been proposed.13 As NTX is becoming an increasingly common treatment for OUD, this will likely lead to more women becoming pregnant while taking NTX. Thus, there is a critical need for maternal, fetal, and infant safety and efficacy data to inform clinicians on how to best manage these pregnancies.
Animal research with exposures 50 to 200 times the human therapeutic doses of NTX have been shown to not alter pregnancy outcomes, maternal health, or increase the rate of congenital malformations.14,15 At doses 100 times the human dose, animal studies have shown a relation between NTX exposure and increased prenatal growth, altered pain responses, and neurobehavior.16,17 There are limited human data on the use of NTX in pregnancy. For the oral formulations, no adverse fetal or neonatal effects have been reported.18 Previous case series with use of an NTX implant in pregnancy showed normal birth outcomes.19,20 These preliminary reports have shown similar gestational ages and birth weights in these NTX pregnancies compared with BUP, as well as rates of NAS that are <10% with shorter lengths of hospital stay (LOS).20,21 Birth growth parameters in a cohort of infants on NTX were larger than those who were MTD exposed.21
Current gaps in research include knowledge regarding the relative safety and efficacy of NTX in pregnant women, impact on maternal abstinence from unprescribed opioids throughout the pregnancy and postpartum period, and impact on the range of neonatal and childhood outcomes.
The purpose of the current study was to examine pregnancy and neonatal outcomes in a cohort of NTX-treated women. Maternal demographic characteristics and OUD history, misuse of opioids, delivery outcomes, gestational age, birth weight, Apgar scores, neonatal intensive care unit admission, and NAS outcomes were compared with a cohort treated with BUP.
Subjects and Methods
This was a retrospective cohort study comparing pregnant women with OUD treated at a single academic center with NTX versus women treated with BUP. BUP was chosen for the control group due to lower addiction severity compared with MTD and similar demographic attributes as women on NTX presenting for care at our institution.5,22 NTX pregnancies between January 1, 2017, and December 31, 2018, were identified with the use of our clinic patient lists and the Boston University Medical Campus Clinical Data Warehouse. Women who were initiated on NTX prepregnancy or in the first trimester were considered for inclusion. Pregnant women on BUP were identified based on our patient lists, matched according to month of delivery in the NTX cohort. If multiple infants were born exposed to BUP in a given month, the infant with the first birth date in that month was selected. We elected to match according to month of delivery to eliminate any potential confounding that might arise from temporal trends in treatment and outcomes. Both groups had to have care at our center for the pregnancy, delivery, and immediate postpartum period. Exclusion criteria included women who were on NTX for the treatment of alcohol use disorder.
Clinical Care Protocols
Boston Medical Center is a tertiary care urban safety net medical center with an integrated addiction and prenatal care program called Project RESPECT (Recovery, Empowerment, Social Services, Prenatal care, Education, Community and Treatment). For pregnant women receiving BUP, women were instructed to take the sublingual medication every 12–24 hours. The mean dose at delivery of BUP was 12.4 mg (95% CI, 10.9–13.9) per day. From January 2017 to June 2018, the BUP mono-product† was used, then starting in July 2018, all women transitioned to the combination BUP/naloxone‡ product due to a change in clinic protocol. During labor, all women are offered regional anesthesia with an epidural for labor pain. Postpartum pain control regimens for vaginal deliveries include nonsteroidal anti-inflammatory agents (NSAIDs) and nonpharmacologic interventions. After a cesarean section (C-section), pain regimens include the scheduled IV NSAID ketorolac for 72 hours followed by the scheduled oral NSAID ibuprofen, short-acting oral opioid hydromorphone as needed, and nonpharmacologic interventions.
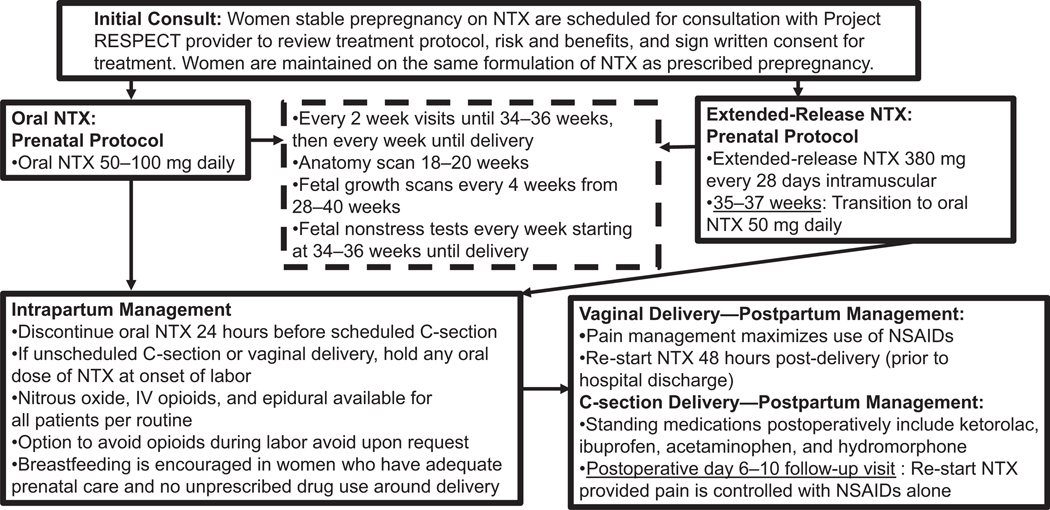
The Boston Medical Center NTX treatment algorithm is provided in the Figure 1. Women who become pregnant while receiving NTX are referred to Project RESPECT for consultation. After consent for treatment by the Project RESPECT team, the patient continues on extended-release NTX 380 mg every 28 days by intramuscular gluteal injection or oral NTX 50–100 mg daily. Women are transitioned from extended-release NTX to oral NTX starting at 35–37 weeks’ gestational age and continued on this regimen until admission for labor or discontinued 24 hours before the scheduled C-section. Intrapartum, there is a patient-directed pain management plan with epidurals (offered per routine), IV opioids, and nitrous oxide. Women may request avoidance of opioids if desired. For vaginal deliveries, women re-start NTX at 48 hours’ postdelivery. For C-sections, re-starting of NTX is delayed until postoperative pain is controlled on NSAID medications alone.
Figure 1.
Naltrexone (NTX) in pregnancy treatment protocol, Boston Medical Center. C-section = cesarean section; NSAIDs = nonsteroidal anti-inflammatory agents; RESPECT = Recovery, Empowerment, Social Services, Prenatal care, Education, Community and Treatment.
Boston Medical Center practices a rooming-in model of care in which infants remain with their mothers on the postpartum unit until maternal discharge. The infant is then transferred to the inpatient pediatric unit for continued monitoring and NAS treatment where rooming-in is encouraged. Infants are assessed with the Eat-Sleep-Console NAS Assessment tool using a standardized NAS treatment algorithm with MTD as first-line pharmacologic treatment.23 A NAS diagnosis was made via chart abstraction with documented signs and symptoms of opioid withdrawal by clinicians, with correlating positive opioid urine or meconium toxicology screen in the infant, and/or urine toxicology screen in the mother. Mothers were encouraged to breastfeed if they were enrolled in Project RESPECT, had attended adequate prenatal care visits (defined as attending at least 50% of recommended visits, and being seen at least twice in the 2 months before delivery), and had no illicit or unprescribed drug use close to the time of delivery assessed by urine toxicology testing.
The Project RESPECT policy is for all women to receive urine toxicology testing at each prenatal encounter and upon admission to the labor and delivery unit. The urine screening panel includes amphetamines, barbiturates, benzodiazepines, cocaine, and opiates. Confirmatory toxicology testing is sent for any positive screen, in addition to an expanded opioid panel (MTD, BUP, oxycodone, and fentanyl). Urine and meconium toxicology screening is sent from the infant after delivery. The meconium toxicology panel includes phencyclidine, cocaine, benzodiazepines, barbiturates, amphetamines, MTD, BUP, and opiates.
Data Collection
This study was approved by the Boston University Medical Campus Institutional Review Board. Prenatal characteristics were collected on all mothers, including demographic characteristics (maternal age, race, health insurance, and prenatal care visits), co-exposures (psychiatric medications and hepatitis C), nicotine use (defined as any amount of nicotine smoking documented in the medical record in the third trimester), OUD history (type of opioid and years of dependence), psychiatric comorbidities, any unprescribed or illicit drug use during the pregnancy or 6 months’ postdelivery (via urine toxicology or documented provider report), delivery type (vaginal/C-section), maternal LOS, congenital anomalies (by chart review), and gestational age at delivery. For the infants, Apgar scores, birth growth parameters (birth weight and head circumference), any medical diagnoses, neonatal intensive care unit admission, any breastfeeding (defined as any amount of breast milk consumed by the infant during the hospitalization), NAS outcomes (diagnosis of NAS [yes/no] defined as documented signs of opioid withdrawal requiring inpatient monitoring for 5–7 days with correlating opioid-positive toxicology screening, receipt of any pharmacologic treatment for NAS [yes/no], and total infant LOS in days), and infant discharge destination (parental custody vs nonparental custody). Data were hand-abstracted from the electronic medical record and entered into an electronic Excel database (Microsoft Corporation, Redmond, Washington).
Statistical Methods
Maternal and infant outcomes were compared between the NTX and BUP cohorts by using bivariate analyses. Two-sample t tests were used to determine differences in means of continuous measures between the NTX and BUP groups. Due to the small sample size, comparisons of categorical measures between the 2 groups were assessed by using Fisher’s exact test. Mean differences and odds ratios (ORs) were calculated by using BUP as the reference group. The sample size was insufficient for any regression modeling. All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
A total of 217 charts were considered for inclusion. There were 113 dyads excluded due to MTD treatment. A total of 12 NTX-maintained pregnancies were identified, of which 6 were excluded due to a primary indication of alcohol use disorder. For the remaining 6 NTX-treated patients, 3 of the women were on oral NTX and 3 were on extended-release NTX. The 92 BUP dyads were matched by infant date of delivery to yield a total of 13 control BUP dyads.
Maternal characteristics are shown in Table I. The average duration of NTX use before the pregnancy ranged from a few months to 3 years. Most maternal demographic characteristics and health outcomes did not differ between groups. However, more women in the BUP group smoked cigarettes during the pregnancy (92% vs 33%; P = 0.02).
Table I.
Maternal demographic characteristics and outcomes. Values are given as mean (SD) unless otherwise indicated.
| Variable | Buprenorphine (n = 13) | Naltrexone (n = 6) | MD (95% CI) or OR(95% CI)* | P |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Maternal age at delivery, y | 30.5 (4.7) | 32.4 (4.0) | 2.1 (−2.5 to 6.8) | 0.78 |
| Race | 6 (100%) | Not calculated | 0.60 | |
| White | 11 (85%) | |||
| Hispanic | 1 (8%) | |||
| Other | 1 (8%) | |||
| Public insurance | 12 (92%) | 4 (67%) | 0.2 (0.003–4.5) | 0.44 |
| Opioid use disorder history | ||||
| Total no. of years of OUD | 8.3 (6.0) | 5.7 (7.5) | −2.6 (−9.4 to 4.1) | 0.43 |
| Type of opioid dependency | ||||
| Prescribed pills | 7 (54%) | 0 | Not calculated | 0.05 |
| Heroin | 6 (46%) | 6 (100%) | ||
| Unprescribed opioid misuse | 3 (23%) | 0 | Not calculated | 0.52 |
| Co-exposures and diagnoses | ||||
| Any psychiatric diagnosis | 10 (77%) | 4 (67%) | 0.6 (0.1–10.1) | 0.99 |
| Depression | 8 (61%) | 2 (33%) | ||
| Anxiety | 7 (54%) | 3 (50%) | ||
| Bipolar disorder | 1 (8%) | 2 (33%) | ||
| PTSD | 4 (31%) | 3 (50%) | ||
| OCD | 2 (15%) | 0 | ||
| None | 3 (23%) | 2 (33%) | Reference | |
| Prescribed psychiatric medications† | 4 (31%) | 3 (50%) | 2.3 (0.2–24.7) | 0.76 |
| Nicotine smoking in third-trimester | 12 (92%) | 2 (33%) | 0.04 (0.001–0.9) | 0.03 |
| Hepatitis C carrier | 6 (46%) | 3 (50%) | 1.7 (0.1–12.3) | 0.99 |
| Pregnancy and delivery | ||||
| GA at the first prenatal visit, wk | 13.2 (7.8) | 18.5 (6.5) | 5.3 (−2.5 to 13.0) | 0.17 |
| No. of prenatal visits | 23.5 (7.1) | 22.5 (6.6) | −1.0 (−8.2 to 6.3) | 0.78 |
| Delivery type | ||||
| Vaginal | 7 (54%) | 5 (83%) | 4.3 (0.3–235.9) | 0.33 |
| Cesarean section | 6 (46%) | 1 (17%) | Reference | |
| Anesthesia during labor | ||||
| Spinal | 4 (31%) | 1 (17%) | 0.5 (0.008–6.8) | 0.96 |
| None | 2 (15%) | 3 (50%) | ||
| Epidural | 7 (54%) | 1 (17%) | Reference | |
| Hospital length of stay after delivery, d | 3.8 (1.3) | 3.2 (1.0) | −0.6 (−1.9 to 0.7) | 0.33 |
GA = gestational age; MD = mean difference; OCD = obsessive-compulsive disorder; OUD = opioid use disorder; PTSD = posttraumatic stress disorder.
Odds ratio (OR) for naltrexone versus buprenorphine.
Methylphenidate, Citalopram, Fluoxetine, Clonidine, Quetiapine, Lamotrigine, Sertraline.
Overall, 4 (31%) of the 13 women taking BUP and 2 (33%) of the 6 NTX-treated women had a urine toxicology screen or provider report of misuse of an unprescribed substance (P = NS). Opioid misuse did not differ between the 2 groups (Table I). In the NTX group, 2 women on the extended-release formulation had positive screens for cocaine. In the BUP group, 3 women had positive screens for cocaine, 3 for opiates, and 2 for unprescribed benzodiazepines.
Infant outcomes are reported in Table II. Mean gestational age at birth and birth weights were not significantly different between the groups. One NTX-exposed infant had a reported congenital anomaly (moderate unilateral renal pyelectasis). None of the infants in the NTX group met criteria for a NAS diagnosis compared with 12 (92%) in the BUP group (P < 0.001), with significantly shorter LOS for infants in the NTX group. One infant in the BUP group had respiratory distress syndrome; there were no other documented medical problems in the immediate perinatal period.
Table II.
Infant outcomes. Values are given as mean (SD) unless otherwise indicated.
| Variable | Buprenorphine (n = 13) | Naltrexone (n = 6) | MD (95% CI) or OR(95% CI)* | P |
|---|---|---|---|---|
| Birth outcomes | ||||
| GA at birth, wk | 39.3 (1.4) | 39.1 (1.3) | −0.2 (−1.7 to 1.3) | 0.78 |
| Infant birth weight, g | 3047 (455) | 3263 (360) | 216.9 (−230.4 to 664.0) | 0.32 |
| Head circumference at birth, cm | 33.1 (1.7) | 33.5 (1.7) | 0.4 (−1.4 to 2.2) | 0.62 |
| Apgar score at 1 min | 8.1 (0.8) | 8.5 (0.6) | 0.4 (−0.3 to 1.2) | 0.24 |
| Apgar score at 5 min | 8.8 (0.4) | 9.2 (0.4) | 0.4 (0–0.9) | 0.08 |
| Congenital anomaly | 0 | 1 (17%)† | Not calculated | 0.32 |
| Breastfeeding initiated | 8 (62%) | 5 (83%) | 3.1 (0.2–176.8) | 0.70 |
| NICU admission | 1 (8%) | 0 | Not calculated | 0.99 |
| Discharged in nonparental custody | 0 | 1 (17%) | Not calculated | 0.32 |
| NAS outcomes | ||||
| NAS diagnosis | 12 (92%) | 0 | Not calculated | <0.001 |
| Pharmacologic treatment for NAS | 6 (46%) | 0 | Not calculated | 0.11 |
| Length of hospital stay, d | 10.9 (8.2) | 3.2 (1.6) | −7.8 (−13.0 to −2.5) | 0.008 |
GA = gestational age; MD = mean difference; NAS = neonatal abstinence syndrome; NICU = neonatal intensive care unit.
Odds ratio (OR) for naltrexone versus buprenorphine.
Mild unilateral renal pyelectasis.
DISCUSSION
We identified preliminary evidence that pregnancy and neonatal outcomes were comparable between BUP-and NTX-maintained pregnancies for women with OUD. None of the women treated with NTX had any documented misuse of opioids, and none of the infants developed signs of opioid withdrawal, resulting in significantly shorter infant LOS compared with BUP. If proven to be safe and efficacious across an array of maternal and infant outcomes, NTX continuation during pregnancy represents a novel treatment approach that could have a significant impact on clinical care. Being able to seamlessly continue a medication during pregnancy that has provided the woman with therapeutic benefit may help improve care by reducing the need to discontinue the patient from NTX or transition her to another medication, which can create vulnerability and risk for relapse to opioids.
Our results are consistent with previous studies of NTX-maintained pregnancies that reported favourable pregnancy and neonatal outcomes without evidence of any immediate complications.18,21,24 We found no risk for NAS, similar to previous published studies. We also found that NTX-exposed infants had healthy birth weights and were born at term, consistent with earlier findings. Strengths of our study include the expansion on these previous Australian studies in the United States through an in-depth comparison of maternal and infant characteristics, as well as additional outcomes not previously explored. This approach includes looking at OUD history, prenatal care utilization, psychiatric diagnoses, and misuse of illicit and unprescribed substances during the pregnancy and postpartum period, a key driver of long-term health for the dyad.
Although not statistically significant, an interesting finding in our study is that women in the NTX group presented later for care than those on BUP; this finding may be because it was unclear to the patients or the providers if NTX could safely be continued during the pregnancy. In addition to the 6 NTX dyads presented in this study, there were several additional pregnancies at our institution over the past 2 years in which women were taken off their NTX once finding out they were pregnant before reaching Project RESPECT. Anecdotally, these women all relapsed with opioids, the highest risk scenario for fatal overdose. They experienced high-risk gaps in care and were then all re-engaged in treatment with stabilization on MTD or BUP for the remainder of their pregnancies. This is the clinical scenario that is best to avoid: a woman who is stable in her recovery whose course is derailed due to insufficient data and guidelines on how to manage these pregnancies.
Another finding to highlight is that 83% of the women in our NTX cohort breastfed, without any immediate issues in the perinatal period. NTX has been reported in breast milk in one case study in a single subject, with the overall dose received by the infant estimated to be <1% of the maternal dose.25 Further studies of NTX concentrations in breast milk are warranted to make definitive recommendations about breast milk safety.
Although this study did not include patients initiated on NTX during pregnancy, it is feasible that a subset of women with OUD could be initiated on NTX in pregnancy. However, it is pertinent to note that NTX is likely not the ideal choice for all patients and should not be considered first-line treatment for active OUD.12 Although preliminary human studies are reassuring, there are a number of concerns about the use of NTX in pregnancy that must be carefully considered. First, initiation onto NTX is associated with a risk for precipitating withdrawal in an opioid-dependent mother, thus requiring detoxification and an opioid-free period of ~2–3 weeks before initiation.9,13 Detoxification during the pregnancy may negatively affect the fetus and pregnancy, and it could increase the risk of spontaneous abortion or premature delivery. This opioid-free period will leave the pregnant woman vulnerable to relapse.13,26 In the absence of rigorous safety and efficacy data looking at a range of maternal and neonatal outcomes, initiation during pregnancy may not be advisable. The other risk is that of potential overdose due to reduced tolerance and increased receptor sensitivity to opioids among women who have been chronically maintained on NTX.27 Therefore, the best case would be a woman who becomes pregnant while already stabilized on NTX who is then continued on NTX to avoid these scenarios. The next concern is to determine the characteristics and addiction severity profile of women who can safely be initiated on NTX in pregnancy.
The pharmacokinetic profile of NTX in pregnancy is also unknown. NTX dosing regimens for pregnancy are extrapolated from standards for nonpregnant patients. It is unclear what formulation of NTX is best during pregnancy, oral versus extended release, for which there are multiple options.7,28–30 Furthermore, another question that warrants exploration is the impact of NTX treatment on pain management during labor, particularly for women who require a C-section. There was only 1 woman who had a C-section in our NTX cohort, and thus this factor could not be explored in the current study.
Limitations of this study include the small sample size with only 6 NTX-exposed pregnancies, and the retrospective design with limitations of data available through the medical record. Any loss to follow-up in the postnatal period limited the ability to fully capture postpartum outcomes. It is possible that opioid and other substance misuse went undocumented. Also, given that some differences were seen between the 2 groups (eg, less smoking in the NTX group), there is likely unmeasured confounding and differences in addiction severity between the groups, which can be explored in future prospective studies. In addition, the NTX group was heterogeneous with some women on the oral formulation and some on the extended-release formulation. Also, given that the infants in the NTX group were not monitored as inpatients for a mandatory 5–7 days for NAS, it is possible that there were infants who developed signs of opioid withdrawal as outpatients. Given that toxicology screening was sent on all NTX-exposed dyads at the time of delivery with no documented positive opioid screens, we find this scenario unlikely.
CONCLUSIONS
NTX pregnancy and neonatal outcomes do not seem worse than BUP treatment. Thus, it is a promising treatment option for pregnant women with OUD who are already receiving it prepregnancy. Further studies are necessary to evaluate the full range of outcomes for both the mother and infant, including relative safety, efficacy, and long-term outcomes.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development) grant 1R01HD096798-01.
Drs. Wachman and Miller drafted the first draft of the manuscript; Dr. Wachman, Dr. Saia, Dr. Carter, Ms. Shrestha, and Dr. Jones helped to conceptualize the idea for the manuscript and plan the data collection; Dr. Valle and Ms. Shrestha helped to design and perform the data collection; and Drs. Miller and Werler planned and performed the data analysis. All authors critically reviewed the manuscript and made edits, and all approved the final submitted version.
Footnotes
CONFLICTS OF INTEREST
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Trademark: Vivitrol® (Alkermes PLC, Dublin, Ireland).
Trademark: Subutex® (Indivior UK Limited, Hull, United Kingdom).
Trademark: Suboxone® (Indivior UK Limited).
REFERENCES
- 1.Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:667. [DOI] [PubMed] [Google Scholar]
- 2.Klaman SL, Isaacs K, Leopold A, et al. Treating women who are pregnant and parenting for opioid use disorder and the concurrent care of their infants and children: literature review to support national guidance. J Addict Med. 2017;11: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saia KA, Schiff D, Wachman EM, et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep. 2016;5: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A. Hospital variation in neonatal abstinence syndrome incidence, treatment modalities, resource use, and costs across pediatric hospitals in the United States, 2013 to 2016. Hosp Pediatr. 2018;8:15–20. [DOI] [PubMed] [Google Scholar]
- 5.Brogly SB, Saia KA, Walley AY, Du HM, Sebastiani P. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. Am J Epidemiol. 2014;180:673–686. [DOI] [PubMed] [Google Scholar]
- 6.Saia K, Bagley SM, Wachman EM, Patel PP, Nadas MD, Brogly SB. Prenatal treatment for opioid dependency: observations from a large inner-city clinic. Addict Sci Clin Pract. 2017;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri A, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction. 2002;97:1241–1249. [DOI] [PubMed] [Google Scholar]
- 8.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001–2014. JAMA Pediatr. 2017;171: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan MA, Garawi F, Bisaga A, et al. Management of relapse in naltrexone maintenance for heroin dependence. Drug Alcohol Depend. 2007;91:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien C, Cornish JW. Naltrexone for probationers and parolees. J Subst Abuse Treat. 2006;31:107–111. [DOI] [PubMed] [Google Scholar]
- 12.Nunes EV, Krupitsky E, Ling W, et al. Treating opioid dependence with injectable extended-release naltrexone (XR-NTX): who will respond? J Addict Med. 2015;9:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HE, Chisolm MS, Jansson LM, Terplan M. Naltrexone in the treatment of opioid-dependent pregnant women: the case for a considered and measured approach to research. Addiction. 2013;108:233–247. [DOI] [PubMed] [Google Scholar]
- 14.Christian MS. Reproductive toxicity and teratology evaluations of naltrexone. J Clin Psychiatry. 1984;45(9 pt 2): 7–10. [PubMed] [Google Scholar]
- 15.Zagon IS, McLaughlin PJ. Naltrexone modulates body and brain development in rats: a role for endogenous opioid systems in growth. Life Sci. 1984;35:2057–2064. [DOI] [PubMed] [Google Scholar]
- 16.Vorhees CV. Effects of prenatal naloxone exposure on postnatal behavioral development of rats. Neurobehav Toxicol Teratol. 1981;3:295–301. [PubMed] [Google Scholar]
- 17.Harry GJ, Rosecrans JA. Behavioral effects of perinatal naltrexone exposure: a preliminary investigation. Pharmacol Biochem Behav. 1979;11(suppl):19–22. [PubMed] [Google Scholar]
- 18.Hulse GK, O’Neill G, Pereira C, Brewer C. Obstetric and neonatal outcomes associated with maternal naltrexone exposure. Aust N Z J Obstet Gynaecol. 2001;41:424–428. [DOI] [PubMed] [Google Scholar]
- 19.Hulse G, O’Neil G. Using naltrexone implants in the management of the pregnant heroin user. Aust N Z J Obstet Gynaecol. 2002;42:569–573. [DOI] [PubMed] [Google Scholar]
- 20.Hulse GK, O’Neil G, Arnold-Reed DE. Methadone maintenance vs. implantable naltrexone treatment in the pregnant heroin user. Int J Gynaecol Obstet. 2004;85:170–171. [DOI] [PubMed] [Google Scholar]
- 21.Kelty E, Hulse G. A retrospective cohort study of birth outcomes in neonates exposed to naltrexone in utero: a comparison with methadone-, buprenorphine- and non-opioid-exposed neonates. Drugs. 2017;77:1211–1219. [DOI] [PubMed] [Google Scholar]
- 22.Brogly SB, Saia K, Hernández-Diaz S, Werler M, Sebastiani P. The comparative safety of buprenorphine versus methadone in pregnancydwhat about confounding? Addiction. 2016;111: 2130–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for neonatal abstinence syndrome. J Perinatol. 2018;38:1114–1122. [DOI] [PubMed] [Google Scholar]
- 24.Kelty E, Hulse G. A retrospective cohort study of obstetric outcomes in opioid-dependent women treated with implant naltrexone, oral methadone or sublingual buprenorphine, and non-dependent controls. Drugs. 2017;77:1199 e1210. [DOI] [PubMed] [Google Scholar]
- 25.Chan CF, Page-Sharp M, Kristensen JH, O’Neil G, Ilett KF. Transfer of naltrexone and its metabolite 6,beta-naltrexol into human milk. J Hum Lact. 2004;20:322–326. [DOI] [PubMed] [Google Scholar]
- 26.Tran TH, Griffin BL, Stone RH, Vest KM, Todd TJ. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824–839. [DOI] [PubMed] [Google Scholar]
- 27.Digiusto E, Shakeshaft A, Ritter A, O’Brien S, Mattick RP, NEPOD Research Group. Serious adverse events in the Australian National evaluation of pharmacotherapies for opioid dependence (NEPOD). Addiction. 2004;99:450–460. [DOI] [PubMed] [Google Scholar]
- 28.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4: CD001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez JP, Brogden RN. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. [DOI] [PubMed] [Google Scholar]
- 30.Rižner TL, Penning TM. Role of aldoketo reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids. 2014;79:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]