Abstract
Thrombomodulin (TM) is a 557-amino acid protein with a broad cell and tissue distribution consistent with its wide-ranging physiological roles. When expressed on the lumenal surface of vascular endothelial cells in both large vessels and capillaries, its primary function is to mediate endothelial thromboresistance. The complete integral membrane-bound protein form displays five distinct functional domains, although shorter soluble (functional) variants comprising the extracellular domains have also been reported in fluids such as serum and urine. TM-mediated binding of thrombin is known to enhance the specificity of the latter serine protease toward both protein C and thrombin activatable fibrinolysis inhibitor (TAFI), increasing their proteolytic activation rate by almost three orders of magnitude with concomitant anticoagulant, antifibrinolytic, and anti-inflammatory benefits to the vascular wall. Recent years have seen an abundance of research into the cellular mechanisms governing endothelial TM production, processing, and regulation (including flow-mediated mechanoregulation)—from transcriptional and posttranscriptional (miRNA) regulation of TM gene expression, to posttranslational processing and release of the expressed protein—facilitating greater exploitation of its therapeutic potential. The goal of the present paper is to comprehensively review the endothelial/TM system from these regulatory perspectives and draw some fresh conclusions. This paper will conclude with a timely examination of the current status of TM's growing therapeutic appeal, from novel strategies to improve the clinical efficacy of recombinant TM analogs for resolution of vascular disorders such as disseminated intravascular coagulation (DIC), to an examination of the complex pleiotropic relationship between statin treatment and TM expression.
Keywords: cyclic strain, endothelium, miRNA, therapeutic, thrombomodulin
one of the many functions of a healthy vascular endothelium is to avoid unwanted intralumenal blood clotting and potentially lethal thrombus formation (27, 120). Key to this anticoagulant property is thrombomodulin (TM), an integral membrane type-1 glycoprotein expressed on the lumenal surface of vascular endothelial cells at a density of ∼50,000–100,000 molecules per cell (153). As a pivotal determinant of endothelial thromboresistance, TM-mediated binding of thrombin leading to protein C activation has beneficial anticoagulant, antifibrinolytic, and anti-inflammatory outcomes for the vessel wall (28). Unsurprisingly therefore, endothelial injury and dysfunction, a feature of vascular diseases, manifests reduced TM expression and elevated soluble TM release (15, 58, 101, 114, 124). Recent years have seen an abundance of TM publications, shedding much light on the physiological and pathological mechanisms regulating endothelial TM, from transcriptional and posttranscriptional (miRNA) regulation of TM gene expression to posttranslational processing and shedding of the expressed protein. The specific goal of this review is to comprehensively review the endothelial/TM system from these regulatory perspectives and draw some fresh conclusions. We will also examine the current status of TM's growing therapeutic appeal, from the direct application of recombinant TM analogs to resolve vascular disorders and modify the surface thromboresistance of biomaterials, to the indirect pleiotropic induction of TM expression during statin treatment.
THE VASCULAR ENDOTHELIUM
The vascular endothelium, a continuous monolayer of flattened endothelial cells that forms the lumenal lining of all blood vessels, is vital to the health and homeostatic responsiveness of the mammalian circulatory system (for review see 103). As the central theme of several thousand publications over the last few decades, the highly dynamic nature and functional complexity of the endothelium have been well documented. It exhibits considerable phenotypic heterogeneity across different vascular beds (4), its prime function being to regulate systemic blood flow and tissue perfusion rates through adjustment of vessel diameter and vascular tone (closely interacting with the underlying smooth muscle cells and pericytes), with a vasodilatory phenotype predominating in healthy vessels (52). It also acts as a highly selective barrier controlling the paracellular (and transcellular) exchange of fluids, ions, and macromolecules between circulating blood and tissues (11), a homeostatic function maintained by the coordinated interaction of interendothelial adherens and tight junction complexes (172). In a further dimension to this barrier role, endothelial cells (which normally maintain an anti-inflammatory phenotype) can mediate the recruitment and extravasation (diapedesis) of proinflammatory leukocytes to areas of tissue damage and infection via the lumenal expression/release of cell adhesion molecules (selectins, integrins, PECAM-1) and cytokines (TNF-α, IL-1, IL-6, MCP-1) (33, 41). Finally, the lumenal surface of the healthy endothelium expresses a range of molecules that serve to downregulate platelet and thrombotic mechanisms, thereby creating an anticoagulant surface for blood flow and preventing thrombus formation (27, 120). One of these anticoagulant molecular targets, thrombomodulin (TM), will serve as the focus of the present review.
The endothelium exists within a pressurized fluid environment. Blood flow-associated hemodynamic forces, namely shear stress and cyclic strain, exert a profound influence on endothelial gene expression and signaling (7). Shear stress is the tangential frictional force exerted by blood flow as it drags across the endothelial surface, causing cells to “flatten” and morphologically align in the direction of flow, while cyclic strain, impacting both the endothelial and underlying smooth muscle cells in macrovessels, describes the repetitive outward stretching of the vessel wall in synchronization with the cardiac cycle (36). Endothelial cells possess diverse mechanoreceptor mechanisms that allow them to sense their hemodynamic environment and transduce mechanical signals into appropriate cellular responses (e.g., NO production, gene expression), thus enabling blood vessels to structurally adapt to suit circulatory conditions (174). Within this context, physiological ranges of laminar shear stress and cyclic strain of endothelial cells promote both antiproliferative and anticoagulant characteristics consistent with an atheroprotected phenotype (i.e., manifesting healthy endothelial function and atherosclerotic resistance) (5, 163, 186). However, chronic dysregulation of hemodynamic stimuli (e.g., arterial bifurcations and curvatures, autologous vein grafts) has serious clinical implications ranging from endothelial dysfunction to atherosclerosis, stroke, and vein graft thrombosis (61, 142, 161). The impact of the hemodynamic environment on TM regulation and endothelial thromboresistance will therefore be examined in this review.
THROMBOMODULIN STRUCTURE
Thrombomodulin (THBD, CD141, BDCA3, fetomodulin), discovered and first named in 1982 by Esmon et al. (44), is a multidomain integral membrane protein constitutively expressed on the lumenal surface of vascular endothelial cells. In addition to coating the endothelium throughout all vascular beds, TM expression has been widely detected in several other tissues suggesting a multifunctionality beyond its widely established anticoagulation and anti-inflammatory roles (167). It has been identified in human gestational tissues such as placenta and myometrium (166), the gingival epithelium (100), keratinocytes (138), polymorphonuclear neutrophils (30), monocytes (164), dendritic cells (190), osteoblasts (94), and even platelets (153). TM has also been detected in a variety of cultured cells including smooth muscle cells (142), A549 small cell lung cancer cells (51) and NIH 3T3 cells (39), while soluble variants have been detected in human urine (108) and serum (194).
The human TM gene, separately cloned by Wen et al. (179) and Suzuki et al. (152) from a human cDNA library, is an intronless gene localized to chromosome 20p12-cen (45). It codes for a protein of 575 amino acids (processed to 557 amino acids following removal of the NH2-terminal signal peptide) and has been widely reported as having a molecular weight ranging from 70 to 100 kDa. TM is structurally organized into five domains: D1, an NH2-terminal C-type lectin domain (CTLD); D2, a chain of six extracellular EGF-like repeats; D3, an extracellular serine/threonine (Ser/Thr)-rich region; D4, a transmembrane spanning region; and D5, a short cytoplasmic tail. Over the last two decades, a multitude of studies have uncovered the complex functional heterogeneity of these domains, which range from mediating the anticoagulant and antifibrinolytic properties of TM (D2/D3) (69, 78), to TM internalization and regulation of inflammatory responses (D1) (31, 69). For a highly comprehensive overview of the structural properties of TM, the reader is further directed to a recent review by Conway (28).
THROMBOMODULIN FUNCTIONS
The ability to bind the serine protease thrombin and potentiate its role within the protein C (PC) activation cascade is often viewed as the archetypal function of TM. Indeed, mouse models of TM gene mutation (TMpro/pro mouse) and endothelial cell-specific gene deletion (TMLox− mouse) both exhibit greatly reduced ability to generate activated protein C (APC) within the circulation, leading to thrombosis and hypercoagulable state (66, 127). Thrombin binds to the TM EGF5–6 repeat domain and also, with lower affinity, to the TM CS moiety within the Ser/Thr-rich domain via the thrombin anion binding exosite I and II regions, respectively (86, 189). This has the effect of blocking the interaction of thrombin with circulating procoagulant substrates (e.g., fibrinogen) and enhancing its specificity for PC, leading to a substantially elevated rate of PC activation (over 1,000-fold relative to unbound thrombin) (3). Thrombin cleaves PC to cause the release of a dodecapeptide activation sequence and yield activated PC (APC), a disulfide-linked heterodimer, an event that is significantly enhanced if PC is bound to endothelial protein C receptor (EPCR) (147). Once dissociated from EPCR, APC elicits potent anticoagulant effects primarily through the proteolytic inactivation of factor Va and factor VIIIa (34). Thrombin binding to TM also enhances the substrate specificity of the latter toward thrombin-activatable fibrinolysis inhibitor (TAFI), a plasminogen-bound zymogen, to yield TAFIa (3, 112). TAFIa—also known as procarboxypeptidase B2 (CPB2), carboxypeptidase U (CPU), and plasma carboxypeptidase (pCPB)—exhibits carboxypeptidase-like activity toward COOH-terminal lysine residues from partially degraded fibrin (82, 193), thereby serving as a negative regulator of fibrinolysis. This enhances the stabilization of fibrin clots ensuring greater injury localization during vascular inflammatory events. Interestingly, selective blockade of the TM/thrombin-mediated generation of TAFIa (e.g., using monoclonal antibodies) represents a valid approach to the development of profibrinolytic therapies to reduce clot lysis time (104).
In addition to anticoagulant and antifibrinolytic consequences, the anti-inflammatory properties of TM have also been documented. A relatively recent study by Nara et al. (111) for example describes how treatment of human endothelial cells with an anti-TM monoclonal antibody elicits inflammatory signaling mechanisms (e.g., NF-κB stimulation, IL-6 secretion) leading to endothelial activation, while Takagi and co-workers (155) report that the C-type lectin domain of TM is a modulator of dendritic cell-mediated immunostimulatory events. The anti-inflammatory effects of TM/thrombin binding have also been well documented. While bound to EPCR for example, thrombin-generated APC can suppress monocyte-dependent cytokine production (59) and induce a cytoprotective endothelial gene expression profile (72). EPCR-bound APC can also switch the signaling specificity of protease-activated receptor 1 (PAR1), which serves to orchestrate cellular responses to coagulation proteases such as thrombin, from a proinflammatory to an anti-inflammatory response (24, 125, 126). Similarly, both in vitro (82) and in vivo (12, 96) studies have shown that thrombin-generated TAFIa can proteolytically inactivate a number of endogenous pro-inflammatory mediators such as bradykinin, osteopontin, and the complement-derived anaphylotoxins C3a and C5a via cleavage of their COOH-terminal arginine residues. TM can also negatively regulate the complement system by accelerating factor I-mediated inactivation of C3b. In this respect, missense mutations in TM can lead to a diminished ability to protect against activated complement, a feature of ∼5% of potentially fatal atypical hemolytic-uremic syndrome (HUS) cases (37). It should also be noted that circulating thrombin itself has several potent proinflammatory properties within the vascular endothelium, from induction of proinflammatory molecule expression (e.g., IL-6, IL-8, E-selectin, and MCP-1) (9, 145) and nitric oxide production (107), to activation of NF-κB signaling and monocyte/endothelial adhesion (36). Thrombin is also known to potentiate endothelial activation by TNF-α (9). Thus, by reducing circulating levels of thrombin as well as switching its substrate specificity toward PC and TAFI (to yield anti-inflammatory APC and TAFIa), TM functions as an anti-inflammatory molecule, rendering it both a pivotal player in the progression of inflammatory diseases and a viable target for therapeutic intervention.
TRANSCRIPTIONAL REGULATION
The 5′-untranslated promoter region of the TM gene displays numerous response elements conferring transcriptional sensitivity to various stimuli (159, 188). Oxidative stress (81), hypoxia (114), oxidized LDL (68), C-reactive protein (110), phorbolesters (PMA) (58), cyclic adenosine monophosphate (cAMP) (58), and TNF-α (58) tend to downregulate endothelial TM expression, while an upregulatory effect has been attributed to thrombin (136), VEGF (20), retinoic acid (97), and heat shock (50). In view of so many competing influences, basal endothelial TM levels typically reflect a balance between up- and downregulatory forces. For example, p66Shc-mediated cellular oxidative stress leading to transcriptional repression of Kruppel-like factor 2 (KLF2), a transcription factor positively regulating the TM promoter (Fig. 1), can lead to downregulation of TM expression (81), while upregulation of KLF2 by antioxidant laminar shear stress has the opposite effect (35, 67), a particular aspect of TM regulation discussed in greater detail in the next section (posttranscriptional regulation). Similarly, TNF-α-dependent suppression of endothelial TM levels via NF-κB activation has been well documented (88, 143), an effect that can be counterbalanced by all-trans retinoic acid treatment (97), laminar shear stress (73), and statins (13). Interestingly, the TM promoter does not actually contain a classic NF-κB consensus motif, but NF-κB can mediate cytokine-induced repression of TM expression by competing for binding to p300, a transcriptional coactivator essential for TM expression (143). An intriguing study by Takeda et al. (156) also reports that endothelial TM levels are subject to circadian variation (an important variable in CVD event occurrence) via TM promoter transactivation involving CLOCK/BMAL-2 heterodimer binding to the promoter E-box region (-CANNTG-).
Fig. 1.
Predicted response elements within the 5′-flanking promoter region of the thrombomodulin gene. Consensus motifs and corresponding response element are highlighted. Key: KLF-2, Kruppel-like factor-2; HIF-1, hypoxia inducible factor-1; ETS-1, v-Ets E26 erythoblastosis; AP-1, activating protein-1; CRE, cAMP response element; SP-1, specificity protein-1; GRE, glucocorticoid response element; Sequence shown is for the rat thrombomodulin promoter (GenBank AF022742.1) Promoter, 1–708; ATG start site, 900–902.
Endothelial TM expression also displays sensitivity to blood flow-associated laminar shear stress, a hemodynamic force known to impart an atheroprotective phenotype to the endothelium (163). In view of the anticoagulant and anti-inflammatory characteristics of this phenotype, the observation that shear stress is a positive regulator of TM expression in most studies is therefore unsurprising. Shear-dependent upregulation of TM expression under both acute and chronic treatment paradigms has been reported in human retinal microvascular endothelial cells (67), primate peripheral blood-derived endothelial outgrowth cells (43), HUVECs (14, 154, 187), human abdominal aortic endothelial cells (HAAECs) (129), and even in a mouse transverse aortic constriction model of flow-dependent remodeling (85). The ability of shear stress to offset the downregulatory impact of TNF-α treatment on TM expression in HUVECs has also been reported (73). In stark contrast to these studies, an early publication by Malek et al. (95) has reported that endothelial TM expression exhibits a mild transient increase followed by a (reversible) decrease to just 16% of baseline levels within 9 h of flow onset in bovine aortic endothelial cells (BAECs). One should probably regard this early observation as somewhat atypical, however, in light of the volume of recent studies reporting the opposite effect. The use of BAECs for this study (as opposed to human/primate/mouse models) may serve as a possible explanation. Interestingly, the same authors also report that TM is similarly regulated in BAECs by both laminar and turbulent shear, although they suggest that this unusual observation should be interpreted with caution due to the arbitrary nature of the chosen shearing conditions (i.e., may not reflect the true in vivo situation).
While studied to a lesser extent, contrasting observations also accompany reports on the regulation of TM expression by cyclic strain, the repetitive mechanical deformation of the vessel wall as it rhythmically distends and relaxes with the cardiac cycle. Using a rabbit autologous vein graft model, Sperry et al. (144) have demonstrated that the substantially reduced TM expression in vein grafts (observed both acutely and chronically) occurs as a direct consequence of outward vessel wall distension and, interestingly, not elevated vessel shear rates or local inflammatory response, leading them to conclude that strain-mediated elevation of endothelial thrombogenicity is a principal cause of occlusive thrombosis preceding autologous vein graft failure. In an apparent contrast to this observation, slightly earlier paired studies by Golledge et al. (55) and Gosling et al. (56) employing ex vivo human saphenous vein segments within a validated in vitro flow circuit, report that exposure of vein grafts to arterial flow significantly reduces endothelial TM expression in a cyclic strain-independent manner (although stretch-activated calcium ion channels appear to be involved). One explanation for this difference may possibly be attributed to ineffective external stenting used in the earlier studies to block out cyclic strain influences, with up to 7 ± 2% pulsatile expansion still possible with external polytetrafluoroethylene (PTFE) stents. Another explanation may stem from the fact that the later (144) and earlier (55, 56) studies reflect chronic (weeks) vs. acute (45–90 min) observations, respectively.
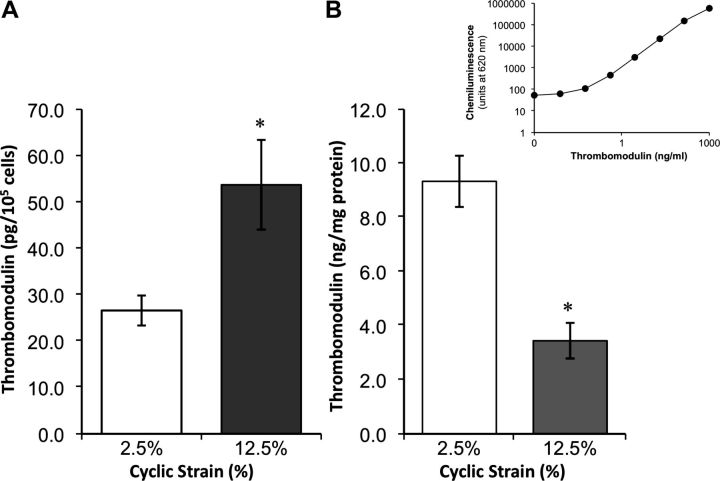
Using both vein graft and in vitro vascular cell culture models, recent studies by Kapur and co-workers (74) into the mechanism of endothelial thromboresistance in vein grafts convincingly demonstrate that cyclic strain-dependent induction and release of transforming growth factor-β1 (TGF-β1) within the medial smooth muscle cell layer could decrease endothelial TM expression in a paracrine manner, albeit via an unknown signaling mechanism. Using a pan-neutralizing TGF-β1 antibody (1D11), these authors were able to block TM downregulation, preserve levels of activated protein C, and reduce thrombus formation in a rabbit vein graft model. Moreover, preliminary work in our own laboratory strongly suggests that chronic elevated cyclic circumferential strain of cultured human aortic endothelial cells (HAECs) could directly reduce cellular expression of TM protein (Fig. 2B) and mRNA, while simultaneously increasing TM release into the media (Fig. 2A) (F. A. Martin and P. M. Cummins, unpublished observations). In stark contrast to these observations, however, (and indeed to those above), a recent paper by Chen et al. (22) demonstrates a sustained increase in TM protein expression in HUVEC cultures following 21% (but not 15%) cyclic strain, with a nitric oxide (NO) signaling mechanism strongly implicated in these events. The authors of this study suggest that as TM promoter activity was not induced by cyclic strain of HUVECs (data not shown), the observed increase in protein expression may putatively be attributed to NO-mediated stabilization of TM protein via S-nitrosylation. In the absence of further data, however, and considering the supraphysiological levels of strain applied, the relevance of this study remains questionable and possibly highlights a limitation of cell culture models in addressing the regulatory impact of cyclic strain on TM expression. Finally, a study by Feng and co-workers report a 2.6-fold downregulation of TM expression in human aortic smooth muscle cells (HASMCs) following 4% equibiaxial cyclic strain for 24 h (48); however, the physiological relevance of this result is questionable in view of the fact that vascular SMCs do not appear to express TM protein or mRNA in vessel walls (142) (again highlighting a limitation of cell culture models).
Fig. 2.
Impact of equibiaxial cyclic strain on thrombomodulin (TM) expression and release in human aortic endothelial cells (HAECs). Cyclic strain was applied to HAECs for 24 h at 60 cycles/min (cardiac waveform) by Flexercell TensionPlus FX-4000T system (Flexercell International). Cells and media were harvested and monitored for TM levels by multiplex ELISA using a 4-plex human vascular injury detection panel (MesoScale Discovery). Histograms display effect of cyclic strain on TM levels in media (A) and cells (B). *P ≤ 0.05 vs. low-strain (2.5%) control. Inset above B shows typical ELISA standard curve for TM (electrochemiluminescent signal at 620 nm vs. TM concentration). In response to elevated chronic cyclic strain (12.5%), TM levels are observed to decrease in cells, while elevated levels of TM shedding into media are observed at high strain (F. A. Martin and P. M. Cummins, unpublished observations).
POSTTRANSCRIPTIONAL REGULATION
MicroRNAs (miRNAs) are endogenously expressed noncoding RNA molecules (18–24 bases) that posttranscriptionally regulate gene expression within eukaryotic genomes, a mechanism that involves miRNA hybridization with the 3′-untranslated mRNA region of target genes leading to gene silencing and translational repression of protein synthesis (16, 62). The vascular endothelium is now a confirmed source of several dozen miRNAs that collectively facilitate regulation of endothelial gene expression and cell fate (146, 150).
Recent findings on the flow-dependent regulation of Krüppel-like factor 2 (KLF2), a vasoprotective transcription factor known to positively regulate endothelial genes including TM (57), provide the most compelling evidence thus far that endothelial TM expression is influenced albeit indirectly by miRNA. Inhibition of miR-92a, a member of the miR-17–92 cluster predicted to bind the 3′-UTR of the KLF2 transcript (17), was found to increase protein and mRNA levels for both KLF2 and TM in HUVECs (65, 187). The same study also demonstrates that miR-92a is downregulated by atheroprotective laminar shear to induce KLF2 and TM levels, consistent with earlier shear studies (35, 67). Thus shear-dependent suppression of miR-92a in endothelial cells likely enhances expression of KLF2 leading to transactivation of the TM promoter. Consistent with these observations, atheroprone endothelial sites (e.g., aortic arch) that exhibit nonlaminar shear flow pattern manifest elevated miR-92a leading to decreased levels of KLF2 (and, one would assume, TM) (46).
MicroRNAs are now established therapeutically viable targets in the regulation of vascular inflammation and senescence (e.g., miR-146a, miR-217, miR-34a, miR-126, miR-21, miR-210, miR-181b) (121, 151, 169, 170), tumor angiogenesis (miR-19b-1) (191), and in vascular diseases such as hypertension (miR-125a/b-5p) (83) and atherosclerosis (miR-92a, miR-27, miR-10a) (23, 46, 47). In view of the established roles for TM in the regulation of inflammatory and thrombotic processes within the vascular wall, in conjunction with its indirect regulation by miR-92a via KLF2 (46, 187), one can anticipate future studies detailing approaches to modulating TM levels in vivo using miRNA-targeting strategies.
POSTTRANSLATIONAL REGULATION
As with many regulatory proteins, TM can undergo an assortment of post-translational modifications that can lead to alterations in its size, structural orientation, amino acid side chain chemistry, and localization, ultimately modulating its vascular homeostatic properties to reflect the tissue environment.
Oxidation.
Early work by Glaser et al. (54) demonstrated that endothelial TM could be almost completely inactivated by oxidation of methionine-388 located within the fifth EGF-like repeat domain (which mediates thrombin binding and anticoagulant function). These authors present evidence pointing to polymorphonucleur neutrophil-derived NADPH oxidase as a probable source of the biological oxidants that cause the TM inactivation typically observed in inflamed tissues. Oxidation of Met-388 leading to lessening of TM functions has also been the subject of studies by Wood et al. (181, 182) and Wang et al. (175), while ROS-dependent inactivation of TM has been reported in a mouse model of vascular dysfunction and thrombosis (2). Furthermore, a relatively recent article by Stites and Froude (148) proposes that the elevated oxidative stress associated with smoking and diabetes is responsible for the prothrombotic state of these conditions by virtue of increased TM Met-388 oxidation and the subsequent decrease in circulating activated protein C levels. Also noteworthy, cellular reducing agents have been shown to modify TM disulfide bonds, rendering the molecule more susceptible to serine protease-mediated cleavage and its subsequent shedding (102).
Glycosylation.
As with many transmembrane proteins boasting an ectodomain, TM is a glycoprotein likely displaying tissue-, organ-, and species-specific glycosylation phenotypes, thus enabling regulatory flexibility with respect to TM:thrombin function in different vascular beds. N-linked glycosylation at asparagine residues has been reported for urinary TM (42, 171). Early studies also report the presence of a TM-associated O-linked glycosaminoglycan (GAG) chain, showing cell-dependent modification of TM at Ser-474 (87). Interestingly, both Edano et al. and Lin et al. also report GAG modification at Ser-472 in C127 mammary tumor cells and CHL-1 melanoma cells, respectively (42, 87). This O-linked GAG modification mediates TM interactions with exogenous GAGs and modulates its thrombin-binding and anti-coagulant activities (79). Indeed, treatment of rabbit TM with chondroitin ABC lyase to remove O-linked GAGs substantially reduced its thrombin-binding capacity (79), while Ye et al. (189) report that the chondroitin sulfate (CS) moiety of TM allows it to bind a second molecule of thrombin. The relevance of the O-linked GAG chain in TM is further clarified in a relatively recent study by Bouton and co-workers (19), who demonstrate in HAECs that the TM CS moiety facilitates complexation with protease nexin-1 (PN-1), a thrombin-inhibiting serpin secreted by endothelial cells. The resultant TM/PN-1 complex has markedly increased inhibitory capacity toward thrombin activity and thrombin-induced fibrinogen clotting compared with either unbound TM or PN-1. Importantly, this study is consistent with an earlier paper by Koyama et al. (79) demonstrating how TM can modulate thrombin inactivation by other heparin-activated serpins (i.e., heparin cofactor II and antithrombin III) as a function of its O-linked CS phenotype in conjunction with the presence or absence of exogenous GAGs (e.g., heparin or dermatan sulfate).
Proteolysis.
Much attention has been devoted to the subject of endothelial TM as a cellular substrate for proteolytic cleavage, frequently leading to its shedding as a soluble variant (sTM). In this respect, sTM has been observed in biological fluids ranging from serum (117) and urine (70) to synovial fluid (29). A study by Abe et al. (1) demonstrated that following incubation of HUVECs with either granulocyte elastase or cathepsin G, both leukocyte-derived lysosomal proteases, cellular TM activity was, respectively, decreased by 90% and 80%, with a concomitant increase in soluble TM variants within the media fraction. Other early studies further confirm likely roles for neutrophil-derived elastase and cathepsin G in the reduction of endothelial cell surface TM activity and elevated TM shedding as causative elements of vascular injury in models of E. coli-induced sepsis, TNF-α-induced endothelial activation, and adult respiratory distress syndrome (ARDS) (15, 77, 93, 124). Moreover, a recent study by Matsuyama et al. (101) has implicated neutrophil-derived enzymes in the proteolytic release of TM from gingival epithelial cells during periodontitis. Other enzymes have also been implicated in TM proteolysis and subsequent shedding. Wu et al. (184) report that LPA-induced shedding of the TM lectin-like domain in HUVECs is MMP-dependent. Moreover, P. gingivalis-derived cysteine proteases (arginine- and lysine-specific gingipains) have been shown to elicit TM degradation and release from the microvascular endothelium in patients with periodontitis, leading to vascular coagulation and inflammation (64). Studies have also confirmed TM cleavage by rhomboids, a family of evolutionarily conserved intramembrane serine proteases originally characterized based on their proteolytic specificity toward Drosophila “Spitz-like” substrates. The TM transmembrane domain can be cleaved by RHBDL2-like rhomboids to release soluble TM, a process mediated through the TM cytoplasmic domain (92). While intriguing, however, the relationship between rhomboids and TM has thus far only been studied in nonvascular cells and dermal wound models (25, 92).
Nonproteolytic mechanisms causing loss of TM from the cell surface are also worth mentioning. Early studies by Maruyama and Majerus (98) and Dittman et al. (39) using A549 and hemangioma cells, respectively, demonstrated how TM/thrombin binding could lead to internalization of surface TM with evidence for partial TM degradation and recycling. Later studies go on to demonstrate that TM cell surface levels can be regulated by endocytosis via non-clathrin-coated pits, an internalization process directed by TM's extracellular lectin-like domain (31, 32). TM shedding from activated endothelium via endothelial microparticles (and exosomes) has also been suggested. These heterogeneous microvesicles facilitate the intercellular exchange of signaling components such as miRNAs, coagulant molecules, and receptors (8). Early work by Satta and co-workers for example has demonstrated that LPS treatment increases TM activity on monocyte-derived MPs by up to 80% (135). Coelevated TM and MP levels in serum have also been observed during systemic inflammatory response syndrome (SIRS) in humans and during heat stroke in baboons (18, 116), while recent work by Duchemin et al. (40) points to an influence of circulating MPs on the “TM resistance” of patients suffering from myeloproliferative neoplasm.
Circulating levels of TM are typically in the low nanogram per milliliter range in healthy human subjects, with pathology frequently causing a moderate, but significant, 1.5- to 2.0-fold increase in patient plasma TM levels (38, 90, 105, 130, 178). While these TM levels are probably too low to have a significant impact on coagulation processes, the consistent elevation in circulating TM levels during pathologies is now widely regarded as an important circulatory biomarker for endothelial dysfunction and vascular risk assessment (15). Consistent with this notion, elevated plasma TM levels have been found to correlate with atherosclerosis (119, 158), cardioembolic stroke (38), obesity (165), Lupus erythomatosus-associated metabolic syndrome (105), preeclampsia (130), sepsis-associated disseminated intravascular coagulation (DIC) (90), and severe acute respiratory syndrome (SARS) (91). Significantly elevated plasma TM levels have also been observed in patients following coronary artery bypass graft (CABG) surgery (10, 78). By contrast, several clinical studies report on vascular pathologies manifesting reduced sTM levels. For example, an inverse relationship between sTM and disease risk has been reported for type-2 diabetes (160) and coronary heart disease (134, 185), while lower levels of sTM have been reported in the serum of patients suffering from acute cerebral infarction (113). Indeed, the more recent MONICA/KORA study demonstrates a lack of any association between sTM levels and CHD risk (75). The incongruity within these collective observations therefore illustrates the need for greater understanding of the precise functional relevance and action of sTM in serum and reinforces the importance of coanalyzing sTM levels with other thrombotic/coagulation markers (e.g., factor VIII, PAI-1) during vascular risk assessment and cohort stratification (6).
THERAPEUTIC CONSIDERATIONS
Soluble recombinant TM.
An improved understanding of the regulation and functions of TM within the vascular endothelium has enabled researchers to better exploit its therapeutic potential. The vasculoprotective properties of various soluble TM preparations (which have been shown to effectively bind circulating thrombin and generate activated protein C) have received particular attention, with studies ranging from animal models to human clinical trials (for review see 106). An early study by Li et al. (84) for example demonstrates how recombinant sTM infusion could reduce neointimal hyperplasia following balloon injury in a rabbit femoral artery model, a therapeutic mechanism that likely encapsulates the anticoagulant and anti-inflammatory properties of the recombinant sTM domains. The ability of sTM to effectively reverse the inflammatory phenotype of the renal microvascular endothelium in rat and murine models of acute ischemic kidney injury and chronic kidney disease, respectively, has also been reported (122, 139). As further testament to its vasculoprotective properties, the therapeutic benefits of recombinant sTM administration toward recovery from severe inflammatory disorders such as heat stroke and radiation toxicity have again been demonstrated in rat and murine models, respectively (53, 60), the former study highlighting an sTM mechanism involving a decrease in high-mobility group box 1 (HMGB1) serum levels in conjunction with blockade of NO overproduction. A human recombinant TM comprising the six EGF-like repeats (domain 2) and Ser/Thr-rich section (domain 3), subsequently referred to as TMD23, has also been reported to have vasculoprotective properties in animal models. Shi et al. (140) illustrate the proangiogenic potential of TMD23 in rat corneal implants, while Wei et al. (177) demonstrate the ability of TMD23 to reduce both neointimal formation (C57BL/6 mouse carotid ligation model) and atherosclerotic lesion formation (ApoE−/− mouse model).
The beneficial effects of soluble TM have also been reported in human clinical trials (132). Approved in 2008 for human therapy in Japan, ART-123 (thrombomodulin alpha, Recomodulin) is a recombinant human soluble TM comprising extracellular domains 1–3, which are essential for the anti-inflammatory and anticoagulant actions of the molecule. ART-123 has been reported to be extremely effective in the rapid resolution of DIC (69, 76, 115), proving safer and more effective as an inhibitor of the propagation of coagulation than traditional low-dose heparin therapy (109). ART-123 has also been used to successfully treat microangiopathies stemming from transplantation-associated sepsis (133) and Lupus-induced thrombosis (150), as well reversing the capillary leakage that accompanies “inflammatory engraftment syndrome” in individuals undergoing hematopoietic stem cell therapy (63). Solulin (sothrombomodulin alpha) is another human recombinant sTM analog that has recently undergone a phase 1 human clinical trial (168). Solulin comprises the same soluble extracellular structure as ART-123, but with specific modifications to further enhance the pharmacokinetic and pharmacodynamic properties of the molecule. These include a series of NH2-/COOH-terminal amino acid deletions and up to four single amino acid exchanges to enhance resistance to oxidation, improve cellular export, and prevent attachment of chondroitin sulfate, collectively improving the stability, homogeneity, and plasma elimination half-life of sTM (168). The efficacy of Solulin in reducing infarct volume during acute ischemic stroke in rats has been attributed to its thrombin binding anticoagulant properties (149), as well as its ability to downregulate inflammatory cytokine gene expression (131), while its anti-fibrinolytic properties are evident in clot stability assays performed on whole blood drawn from hemophilic human and canine subjects (49).
Finally, strategies to improve sTM targeting and therapeutic efficacy offer a means of enhancing the clinical value of recombinant soluble TM. Early work by Wang and co-workers for example demonstrated that fusing a tissue factor (TF) single-chain antibody to an active TM fragment generated a novel fusion protein, Ab(TF)-TM, with a dual mechanism of action, namely, anticoagulant TM-mediated enhancement of protein C activation in conjunction with blockade of prothrombotic TF/factor VIIa-mediated activation of factor IX/X. The fusion protein subsequently demonstrated significantly higher fold efficacy in the resolution of DIC in rats than either sTM or Ab(TF) alone (176). Similarly, linking a PECAM-1-directed antibody single-chain fragment (scFv) to TM recently yielded a fusion protein, scFv(PECAM-1)-TM, for enhanced vascular immunotargeting of TM in mice (21). Moreover, fusion of TM to a red blood cell-directed scFv was recently shown to prolong the circulation time and bioavailability of soluble TM (192).
Biomaterial coating.
The use of recombinant human TM to modulate the surface thromboresistance of blood-contacting biomaterials represents another important therapeutic application. Workers have recently demonstrated for example that coating of ART-123 onto dialyzer membranes can effectively prevent clot formation during dialysis, thereby providing a safe alternative to the drawbacks of heparin administration (99, 118). Moreover, recent evidence demonstrating incomplete endothelialization and low TM expression levels among existing FDA-approved drug eluting stents (DES) has prompted scientists to reconsider the influence of DES agents on endothelial thromboresistance leading to stent thrombosis (71). Paclitaxel, an anti-proliferative DES agent used to prevent neointimal hyperplasia, has been shown to cause TNF-α-induced release of tissue factor leading to endothelial TM downregulation and thereby contributing to a prothrombotic intimal stent surface (173, 183). Reengineering of stents to avoid such thrombogenic complications is now being undertaken. Wong et al. (180) have demonstrated for example that stenting with recombinant human TM-coated PTFE stents could significantly reduce balloon angioplasty-induced neointimal hyperplasia in a pig carotid artery model. Long-term studies, however, need to be conducted to ascertain the resilience of this and other related stent coating improvements for the prevention of arterial thrombosis and graft stenosis following balloon angioplasty and CABG, respectively.
Statins: pleiotropic effects.
Statins reduce endogenous cholesterol biosynthesis through selective inhibition of 3-hydroxy-3-methylglutaryl co-enzyme A reductase (80) and so are widely prescribed for the treatment of dyslipidemias associated with cardiovascular disease and diabetes. Statins also exhibit a multitude of beneficial pleiotropic (nonlipid) effects, of which the vascular endothelium is a key target. Statin-mediated upregulation of endothelial nitric oxide synthase (eNOS), for example, has well-known anti-inflammatory and anticoagulant effects (157). Induction of endogenous TM production leading to anti-inflammatory effects constitutes a further pleiotropic benefit of statins, albeit originally unforeseen for this class of drug (and quite distinct from the predesigned therapeutic modality of recombinant soluble TM). Various studies have documented the upregulation of TM expression in endothelial cells in response to either atorvastatin or simvastatin treatment and demonstrate the anti-inflammatory ability of statins to counteract the suppressive effects of TNF-α (and thus, NF-κB) on TM expression (13, 89, 141). The induction of TM expression and protein C activation in irradiated HUVECs has also recently been reported to be a protective effect of atorvastatin (123). The statin-mediated induction of TM expression has been attributed to transcriptional mechanisms involving both the upregulation of KLF2 expression and the NO-dependent dissociation of HSF1 from heat shock protein 90, with subsequent nuclear translocation of both factors to Kruppel-like factor and heat shock elements within the TM promoter, respectively (50, 137). With respect to KLF2, researchers have also reported that atheroprotective laminar shear stress, through suppression of miR-92a, can upregulate endothelial TM via KLF2-dependent transactivation of the TM promoter (35, 187). Interestingly, the pleiotropic upregulation of endothelial TM by statins was only observed under atheroprotective shearing conditions (consistent with miR-92a suppression and KLF2 induction), and not under conditions of atherogenic oscillatory shear (128). These collective observations suggest that statin-mediated pleiotropic effects on TM are restricted to shear-protected regions of the endothelium and putatively involve suppression of miR-92a. They also lead one to speculate that anti-miR-92a-driven TM upregulation, used in conjunction with statins, may be a potentially viable future therapeutic approach for the improved treatment of vascular pathologies manifesting elevated endothelial thrombogenicity.
CONCLUDING REMARKS
TM is a vasculoprotective integral membrane glycoprotein displaying anticoagulant, antifibrinolytic, and anti-inflammatory properties and, as such, is a pivotal determinant of endothelial thromboresistance. TM is also released/shed from the endothelium as an extracellular soluble form (sTM), this event frequently indicative of inflammatory cellular damage. Consistent with its multifaceted homeostatic roles, TM is subject to physiological (and pathological) regulation at multiple levels, from transcriptional to posttranslational, thus enabling its expression and processing to coordinate with intravascular conditions. Particularly noteworthy, the susceptibility of endothelial TM to mechanoregulation by shear stress and cyclic strain reinforces the homeostatic role of TM under laminar blood flow conditions and strongly implicates a role for TM downregulation (e.g., through KLF-2 and miR-92a mechanisms) in the pathogenesis of chronic flow-associated vascular disorders such as atherosclerosis and vein graft thrombosis. Steady advances in our mechanistic understanding of TM regulation and function within the vasculature will undoubtedly improve and broaden its therapeutic and biomedical potential.
GRANTS
We acknowledge financial support provided through the National Development Plan/Irish Higher Education Authority-Programme for Research in Third Level Institutes Cycle 4 (NDP/HEA-PRTLI Cycle 4: T3 Targeted Therapeutics and Theranostics).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.A.M. and P.M.C. conception and design of research; F.A.M. performed experiments; F.A.M. and P.M.C. analyzed data; F.A.M. and P.M.C. interpreted results of experiments; F.A.M. and P.M.C. drafted manuscript; F.A.M., R.P.M., and P.M.C. edited and revised manuscript; P.M.C. prepared figures; P.M.C. approved final version of manuscript.
REFERENCES
- 1. Abe H , Okajima K , Okabe H , Takatsuki K , Binder BR. Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med : 874–881, 1994. [PubMed] [Google Scholar]
- 2. Adams GN , LaRusch GA , Stavrou E , Zhou Y , Nieman MT , Jacobs GH , Cui Y , Lu Y , Jain MK , Mahdi F , Shariat-Madar Z , Okada Y , D'Alecy LG , Schmaier AH. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood : 3929–3937, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams TE , Huntington JA. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol : 1738–1745, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res : 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Akimoto S , Mitsumata M , Sasaguri T , Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ Res : 185–190, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Aleksic N , Wang YW , Ahn C , Juneja HS , Folsom AR , Wu KK. Assessment of coronary heart disease risk by combined analysis of coagulation factors. Atherosclerosis : 294–300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ando J , Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal : 1389–1403, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Andriantsitohaina R , Gaceb A , Vergori L , Martínez MC. Microparticles as regulators of cardiovascular inflammation. Trends Cardiovasc Med : 88–92, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Anrather D , Millan MT , Palmetshofer A , Robson SC , Geczy C , Ritchie AJ , Bach FH , Ewenstein BM. Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J Immunol : 5620–5628, 1997. [PubMed] [Google Scholar]
- 10. Arazi HC , Doiny DG , Torcivia RS , Grancelli H , Waldman SV , Nojek C , Fornari MC , Badimon JJ. Impaired anti-platelet effect of aspirin, inflammation and platelet turnover in cardiac surgery. Interact Cardiovasc Thorac Surg : 863–867, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Balda MS , Matter K. Tight junctions. J Cell Sci : 541–547, 1998. [DOI] [PubMed] [Google Scholar]
- 12. Beppu T , Gil-Bernabe P , Boveda-Ruiz D , D'Alessandro-Gabazza C , Matsuda Y , Toda M , Miyake Y , Shiraki K , Murata M , Murata T , Yano Y , Morser J , Gabazza EC , Takei Y. High incidence of tumors in diabetic thrombin activatable fibrinolysis inhibitor and apolipoprotein E double-deficient mice. J Thromb Haemost : 2514–2522, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Bergh N , Larsson P , Ulfhammer E , Jern S. Effect of shear stress, statins and TNF-α on hemostatic genes in human endothelial cells. Biochem Biophys Res Commun : 166–171, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Bergh N , Ulfhammer E , Glise K , Jern S , Karlsson L. Influence of TNF-alpha and biomechanical stress on endothelial anti- and prothrombotic genes. Biochem Biophys Res Commun : 314–318, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Boehme MW , Deng Y , Raeth U , Bierhaus A , Ziegler R , Stremmel W , Nawroth PP. Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology : 134–140, 1996. [PMC free article] [PubMed] [Google Scholar]
- 16. Bonauer A , Boon RA , Dimmeler S. Vascular microRNAs. Curr Drug Target : 943–949, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Bonauer A , Dimmeler S. The microRNA-17–92 cluster: still a miRacle? Cell Cycle : 3866–3873, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Bouchama A , Kunzelmann C , Dehbi M , Kwaasi A , Eldali A , Zobairi F , Freyssinet JM , de Prost D. Recombinant activated protein C attenuates endothelial injury and inhibits procoagulant microparticles release in baboon heatstroke. Arterioscler Thromb Vasc Biol : 1318–1325, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Bouton MC , Venisse L , Richard B , Pouzet C , Arocas V , Jandrot-Perrus M. Protease nexin-1 interacts with thrombomodulin and modulates its anticoagulant effect. Circ Res : 1174–1181, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Calnek DS , Grinnell BW. Thrombomodulin-dependent anticoagulant activity is regulated by vascular endothelial growth factor. Exp Cell Res : 294–298, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Chacko AM , Nayak M , Greineder CF , Delisser HM , Muzykantov VR. Collaborative enhancement of antibody binding to distinct PECAM-1 epitopes modulates endothelial targeting. PLoS One : e34958, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen SC , Cheng JJ , Wu SE , Shen HC , Shyu KG , Wang DL. Cyclic strain-induced thrombomodulin expression in endothelial cells is mediated by nitric oxide, but not hydrogen peroxide. Acta Cardiol Sin : 144–150, 2008. [Google Scholar]
- 23. Chen WJ , Yin K , Zhao GJ , Fu YC , Tang CK. The magic and mystery of MicroRNA-27 in atherosclerosis. Atherosclerosis : 314–323, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Cheng T , Liu D , Griffin JH , Fernández JA , Castellino F , Rosen ED , Fukudome K , Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med : 338–342, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Cheng TL , Wu YT , Lin HY , Hsu FC , Liu SK , Chang BI , Chen WS , Lai CH , Shi GY , Wu HL. Functions of rhomboid family protease RHBDL2 and thrombomodulin in wound healing. J Invest Dermatol : 2486–2494, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol : H1209–H1224, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Cines DB , Pollak ES , Buck CA , Loscalzo J , Zimmerman GA , McEver RP , Pober JS , Wick TM , Konkle BA , Schwartz BS , Barnathan ES , McCrae KR , Hug BA , Schmidt AM , Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood : 3527–3561, 1998. [PubMed] [Google Scholar]
- 28. Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol : 107–125, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Conway EM , Nowakowski B. Biologically active thrombomodulin is synthesized by adherent synovial fluid cells and is elevated in synovial fluid of patients with rheumatoid arthritis. Blood : 726–733, 1993. [PubMed] [Google Scholar]
- 30. Conway EM , Nowakowski B , Steiner-Mosonyi M. Human neutrophils synthesize thrombomodulin that does not promote thrombin-dependent protein C activation. Blood : 1254–1263, 1992. [PubMed] [Google Scholar]
- 31. Conway EM , Nowakowski B , Steiner-Mosonyi M. Thrombomodulin lacking the cytoplasmic domain efficiently internalizes thrombin via nonclathrin-coated, pit-mediated endocytosis. J Cell Physiol : 285–298, 1994. [DOI] [PubMed] [Google Scholar]
- 32. Conway EM , Pollefeyt S , Collen D , Steiner-Mosonyi M. The amino terminal lectin-like domain of thrombomodulin is required for constitutive endocytosis. Blood : 652–661, 1997. [PubMed] [Google Scholar]
- 33. Cotran RS , Pober JS. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J Am Soc Nephrol : 225–235, 1990. [DOI] [PubMed] [Google Scholar]
- 34. Dahlbäck B , Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol : 1311–1320, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Dekker RJ , van Soest S , Fontijn RD , Salamanca S , de Groot PG , VanBavel E , Pannekoek H , Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood : 1689–1698, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Delekta PC , Apel IJ , Gu S , Siu K , Hattori Y , McAllister-Lucas LM , Lucas PC. Thrombin-dependent NF-κB activation and monocyte/endothelial adhesion are mediated by the CARMA3·Bcl10·MALT1 signalosome. J Biol Chem : 41432–41442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delvaeye M , Noris M , De Vriese A , Esmon CT , Esmon NL , Ferrell G , Del-Favero J , Plaisance S , Claes B , Lambrechts D , Zoja C , Remuzzi G , Conway EM. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med : 345–357, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dharmasaroja P , Dharmasaroja PA , Sobhon P. Increased plasma soluble thrombomodulin levels in cardioembolic stroke. Clin Appl Thromb Hemost : 289–293, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Dittman WA , Kumada T , Sadler JE , Majerus PW. The structure and function of mouse thrombomodulin: phorbol myristate acetate stimulates degradation and synthesis of thrombomodulin without affecting mRNA levels in hemanigioma cells. J Biol Chem : 15815–15822, 1988. [PubMed] [Google Scholar]
- 40. Duchemin J , Ugo V , Ianotto JC , Lecucq L , Mercier B , Abgrall JF. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res : 238–242, 2010. [DOI] [PubMed] [Google Scholar]
- 41. Ebnet K , Vestweber D. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem Cell Biol : 1–23, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Edano T , Kumai N , Mizoguchi T , Ohkuchi M. The glycosylation sites and structural characteristics of oligosaccharides on recombinant human thrombomodulin. Int J Biochem Cell Biol : 77–88, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Ensley AE , Nerem RM , Anderson DE , Hanson SR , Hinds MT. Fluid shear stress alters the hemostatic properties of endothelial outgrowth cells. Tissue Eng Part A : 127–136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Esmon NL , Owen WG , Esmon CT. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem : 859–864, 1982. [PubMed] [Google Scholar]
- 45. Espinosa R , Sadler JE , Le Beau MM. Regional localization of the human thrombomodulin gene to 20p12-cen. Genomics : 649–650, 1989. [DOI] [PubMed] [Google Scholar]
- 46. Fang Y , Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol : 979–987, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang Y , Shi C , Manduchi E , Civelek M , Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA : 13450–13455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng Y , Yang JH , Huang H , Kennedy SP , Turi TG , Thompson JF , Libby P , Lee RT. Transcriptional profile of mechanically induced genes in human vascular smooth muscle cells. Circ Res : 1118–1123, 1999. [DOI] [PubMed] [Google Scholar]
- 49. Foley JH , Petersen KU , Rea CJ , Harpell L , Powell S , Lillicrap D , Nesheim ME , Sørensen B. Solulin increases clot stability in whole blood from humans and dogs with hemophilia. Blood : 3622–3628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu Q , Wang J , Boerma M , Berbée M , Qiu X , Fink LM , Hauer-Jensen M. Involvement of heat shock factor 1 in statin-induced transcriptional upregulation of endothelial thrombomodulin. Circ Res : 369–377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fujiwara M , Jin E , Ghazizadeh M , Kawanami O. Antisense oligodeoxynucleotides against thrombomodulin suppress the cell growth of lung adenocarcinoma cell line A549. Pathol Int : 204–213, 2002. [DOI] [PubMed] [Google Scholar]
- 52. Furchgott RF , Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature : 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 53. Geiger H , Pawar SA , Kerschen EJ , Nattamai KJ , Hernandez I , Liang HP , Fernández JA , Cancelas JA , Ryan MA , Kustikova O , Schambach A , Fu Q , Wang J , Fink LM , Petersen KU , Zhou D , Griffin JH , Baum C , Weiler H , Hauer-Jensen M. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med : 1123–1129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glaser CB , Morser J , Clarke JH , Blasko E , McLean K , Kuhn I , Chang RJ , Lin JH , Vilander L , Andrews WH. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J Clin Invest : 2565–2573, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Golledge J , Turner RJ , Gosling M , Powell JT. Rapid changes in the coagulant proteins on saphenous vein endothelium in response to arterial flow. Angiology : 693–701, 1999. [DOI] [PubMed] [Google Scholar]
- 56. Gosling M , Golledge J , Turner RJ , Powell JT. Arterial flow conditions downregulate thrombomodulin on saphenous vein endothelium. Circulation : 1047–1053, 1999. [DOI] [PubMed] [Google Scholar]
- 57. Gracia-Sancho J , Russo L , García-Calderó H , García-Pagán JC , García-Cardeña G , Bosch J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut : 517–524, 2011. [DOI] [PubMed] [Google Scholar]
- 58. Grey ST , Csizmadia V , Hancock WW. Differential effect of tumor necrosis factor-alpha on thrombomodulin gene expression by human monocytoid (THP-1) cell vs. endothelial cells. Int J Hematol : 53–62, 1998. [DOI] [PubMed] [Google Scholar]
- 59. Grey ST , Tsuchida A , Hau H , Orthner CL , Salem HH , Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol : 3664–3672, 1994. [PubMed] [Google Scholar]
- 60. Hagiwara S , Iwasaka H , Goto K , Ochi Y , Mizunaga S , Saikawa T , Noguchi T. Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock : 402–406, 2010. [DOI] [PubMed] [Google Scholar]
- 61. Hahn C , Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol : 53–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hussain MU. Micro-RNAs (miRNAs): genomic organisation, biogenesis and mode of action. Cell Tissue Res : 409–413, 2012. [DOI] [PubMed] [Google Scholar]
- 63. Ikezoe T , Takeuchi A , Taniguchi A , Togitani K , Yokoyama A. Recombinant human soluble thrombomodulin counteracts capillary leakage associated with engraftment syndrome. Bone Marrow Transplant : 616–618, 2011. [DOI] [PubMed] [Google Scholar]
- 64. Inomata M , Ishihara Y , Matsuyama T , Imamura T , Maruyama I , Noguchi T , Matsushita K. Degradation of vascular endothelial thrombomodulin by arginine- and lysine-specific cysteine proteases from Porphyromonas gingivalis. J Periodontol : 1511–1517, 2009. [DOI] [PubMed] [Google Scholar]
- 65. Irani K. Crippling of Krüppel (like factor 2) by bad flow portends a miRky day for endothelial function. Circulation : 541–543, 2011. [DOI] [PubMed] [Google Scholar]
- 66. Isermann B , Hendrickson SB , Zogg M , Wing M , Cummiskey M , Kisanuki YY , Yanagisawa M , Weiler H. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest : 537–546, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ishibazawa A , Nagaoka T , Takahashi T , Yamamoto K , Kamiya A , Ando J , Yoshida A. Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci : 8496–8504, 2011. [DOI] [PubMed] [Google Scholar]
- 68. Ishii H , Tezuka T , Ishikawa H , Takada K , Oida K , Horie S. Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of RARbeta-RXRalpha heterodimers and Sp1 and Sp3 to their binding sequences in the TM promoter. Blood : 4765–4774, 2003. [DOI] [PubMed] [Google Scholar]
- 69. Ito T , Maruyama I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost : 168–173, 2011. [DOI] [PubMed] [Google Scholar]
- 70. Jackson DE , Tetaz TJ , Salem HH , Mitchell CA. Purification and characterization of two forms of soluble thrombomodulin from human urine. Eur J Biochem : 1079–1087, 1994. [DOI] [PubMed] [Google Scholar]
- 71. Joner M , Nakazawa G , Finn AV , Quee SC , Coleman L , Acampado E , Wilson PS , Skorija K , Cheng Q , Xu X , Gold HK , Kolodgie FD , Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol : 333–342, 2008. [DOI] [PubMed] [Google Scholar]
- 72. Joyce DE , Gelbert L , Ciaccia A , DeHoff B , Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem : 11199–11203, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Jun P , Huangqing C , Xiaoheng L , Ruheng L , Xiaohong Z. Effects of shear stress on protein C activation, EPCR expression and TM expression in endothelial cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi : 303–309, 2009. [PubMed] [Google Scholar]
- 74. Kapur NK , Bian C , Lin E , Deming CB , Sperry JL , Hansen BS , Kakouros N , Rade JJ. Inhibition of transforming growth factor-β restores endothelial thromboresistance in vein grafts. J Vasc Surg : 1117–1123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karakas M , Baumert J , Herder C , Rottbauer W , Meisinger C , Koenig W , Thorand B. Soluble thrombomodulin in coronary heart disease: lack of an association in the MONICA/KORA case-cohort study. J Thromb Haemost : 1078–1080, 2011. [DOI] [PubMed] [Google Scholar]
- 76. Kawano N , Yoshida S , Ono N , Himeji D , Nagahiro Y , Sayaka Kawano Yamashita K , Ikeda N , Uezono S , Ochiai H , Kawano F , Kikuchi I , Ishikawa F , Shimoda K , Ueda A , Akashi K. Clinical features and outcomes of 35 disseminated intravascular coagulation cases treated with recombinant human soluble thrombomodulin at a single institution. J Clin Exp Hematop : 101–107, 2011. [DOI] [PubMed] [Google Scholar]
- 77. Kobayashi H , Sadakata H , Suzuki K , She MY , Shibata S , Terao T. Thrombomodulin release from umbilical endothelial cells initiated by preeclampsia plasma-induced neutrophil activation. Obstet Gynecol : 425–430, 1998. [DOI] [PubMed] [Google Scholar]
- 78. Kokame K , Zheng X , Sadler JE. Activation of thrombin-activable fibrinolysis inhibitor requires epidermal growth factor-like domain 3 of thrombomodulin and is inhibited competitively by protein C. J Biol Chem : 12135–12139, 1998. [DOI] [PubMed] [Google Scholar]
- 79. Koyama T , Parkinson JF , Sié P , Bang NU , Müller-Berghaus G , Preissner KT. Different glycoforms of human thrombomodulin. Their glycosaminoglycan-dependent modulatory effects on thrombin inactivation by heparin cofactor II and antithrombin III. Eur J Biochem : 563–570, 1991. [DOI] [PubMed] [Google Scholar]
- 80. Krukemyer JJ , Talbert RL. Lovastatin: a new cholesterol-lowering agent. Pharmacotherapy : 198–210, 1987. [DOI] [PubMed] [Google Scholar]
- 81. Kumar A , Hoffman TA , Dericco J , Naqvi A , Jain MK , Irani K. Transcriptional repression of Kruppel like factor-2 by the adaptor protein p66shc. FASEB J : 4344–4352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leung LL , Nishimura T , Myles T. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI). Adv Exp Med Biol : 61–69, 2008. [PubMed] [Google Scholar]
- 83. Li D , Yang P , Xiong Q , Song X , Yang X , Liu L , Yuan W , Rui YC. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens : 1646–1654, 2010. [DOI] [PubMed] [Google Scholar]
- 84. Li JM , Singh MJ , Itani M , Vasiliu C , Hendricks G , Baker SP , Hale JE , Rohrer MJ , Cutler BS , Nelson PR. Recombinant human thrombomodulin inhibits arterial neointimal hyperplasia after balloon injury. J Vasc Surg : 1074–1083, 2004. [DOI] [PubMed] [Google Scholar]
- 85. Li YH , Hsieh CY , Wang DL , Chung HC , Liu SL , Chao TH , Shi GY , Wu HL. Remodeling of carotid arteries is associated with increased expression of thrombomodulin in a mouse transverse aortic constriction model. Thromb Haemost : 658–664, 2007. [PubMed] [Google Scholar]
- 86. Light DR , Glaser CB , Betts M , Blasko E , Campbell E , Clarke JH , McCaman M , McLean K , Nagashima M , Parkinson JF , Rumennik G , Young T , Morser J. The interaction of thrombomodulin with Ca2+. Eur J Biochem : 522–533, 1999. [DOI] [PubMed] [Google Scholar]
- 87. Lin JH , McLean K , Morser J , Young TA , Wydro RM , Andrews WH , Light DR. Modulation of glycosaminoglycan addition in naturally expressed and recombinant human thrombomodulin. J Biol Chem : 25021–25030, 1994. [PubMed] [Google Scholar]
- 88. Lin PY , Shen HC , Chen CJ , Wu SE , Kao HL , Huang JH , Wang DL , Chen SC. The inhibition in tumor necrosis factor-alpha-induced attenuation in endothelial thrombomodulin expression by carvedilol is mediated by nuclear factor-kappaB and reactive oxygen species. J Thromb Thrombolysis : 52–59, 2010. [DOI] [PubMed] [Google Scholar]
- 89. Lin SJ , Hsieh FY , Chen YH , Lin CC , Kuan II , Wang SH , Wu CC , Chien HF , Lin FY , Chen YL. Atorvastatin induces thrombomodulin expression in the aorta of cholesterol-fed rabbits and in TNFalpha-treated human aortic endothelial cells. Histol Histopathol : 1147–1159, 2009. [DOI] [PubMed] [Google Scholar]
- 90. Lin SM , Wang YM , Lin HC , Lee KY , Huang CD , Liu CY , Wang CH , Kuo HP. Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med : 683–689, 2008. [DOI] [PubMed] [Google Scholar]
- 91. Liu ZH , Wei R , Wu YP , Lisman T , Wang ZX , Han JJ , Ren DL , Chen B , Xia ZL , Chen B , Zhu Z , Zhang Y , Cui X , Hu HT , de Groot PG , Xu WB. Elevated plasma tissue-type plasminogen activator (t-PA) and soluble thrombomodulin in patients suffering from severe acute respiratory syndrome (SARS) as a possible index for prognosis and treatment strategy. Biomed Environ Sci : 260–264, 2005. [PubMed] [Google Scholar]
- 92. Lohi O , Urban S , Freeman M. Diverse substrate recognition mechanisms for rhomboids; thrombomodulin is cleaved by Mammalian rhomboids. Curr Biol : 236–241, 2004. [DOI] [PubMed] [Google Scholar]
- 93. MacGregor IR , Perrie AM , Donnelly SC , Haslett C. Modulation of human endothelial thrombomodulin by neutrophils and their release products. Am J Respir Crit Care Med : 47–52, 1997. [DOI] [PubMed] [Google Scholar]
- 94. Maillard C , Berruyer M , Serre CM , Amiral J , Dechavanne M , Delmas PD. Thrombomodulin is synthesized by osteoblasts, stimulated by 1,25-(OH)2D3 and activates protein C at their cell membrane. Endocrinology : 668–674, 1993. [DOI] [PubMed] [Google Scholar]
- 95. Malek AM , Jackman R , Rosenberg RD , Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res : 852–860, 1994. [DOI] [PubMed] [Google Scholar]
- 96. Mao SS , Holahan MA , Bailey C , Wu G , Colussi D , Carroll SS , Cook JJ. Demonstration of enhanced endogenous fibrinolysis in thrombin activatable fibrinolysis inhibitor-deficient mice. Blood Coagul Fibrinolysis : 407–415, 2005. [DOI] [PubMed] [Google Scholar]
- 97. Marchetti M , Vignoli A , Bani MR , Balducci D , Barbui T , Falanga A. All-trans retinoic acid modulates microvascular endothelial cell hemostatic properties. Haematologica : 895–905, 2003. [PubMed] [Google Scholar]
- 98. Maruyama I , Majerus PW. The turnover of thrombin-thrombomodulin complex in cultured human umbilical vein endothelial cells and A549 lung cancer cells. Endocytosis and degradation of thrombin. J Biol Chem : 15432–15438, 1985. [PubMed] [Google Scholar]
- 99. Matsusaki M , Omichi M , Maruyama I , Akashi M. Physical adsorption of human thrombomodulin (ART-123) onto polymeric biomaterials for developing an antithrombogenic blood-contacting material. J Biomed Mater Res A : 1–9, 2008. [DOI] [PubMed] [Google Scholar]
- 100. Matsuyama T , Izumi Y , Shibatate K , Yotsumoto Y , Obama H , Uemura M , Maruyama I , Sueda T. Expression and activity of thrombomodulin in human gingival epithelium: in vivo and in vitro studies. J Periodontal Res : 146–157, 2000. [DOI] [PubMed] [Google Scholar]
- 101. Matsuyama T , Tokuda M , Izumi Y. Significance of thrombomodulin release from gingival epithelial cells in periodontitis patients. J Periodontal Res : 379–385, 2008. [DOI] [PubMed] [Google Scholar]
- 102. Menschikowski M , Hagelgans A , Eisenhofer G , Tiebel O , Siegert G. Reducing agents induce thrombomodulin shedding in human endothelial cells. Thromb Res : e88–e93, 2010. [DOI] [PubMed] [Google Scholar]
- 103. Michiels C. Endothelial cell functions. J Cell Physiol : 430–443, 2003. [DOI] [PubMed] [Google Scholar]
- 104. Mishra N , Vercauteren E , Develter J , Bammens R , Declerck PJ , Gils A. Identification and characterisation of monoclonal antibodies that impair the activation of human thrombin activatable fibrinolysis inhibitor through different mechanisms. Thromb Haemost : 90–101, 2011. [DOI] [PubMed] [Google Scholar]
- 105. Mok CC , Poon WL , Lai JP , Wong CK , Chiu SM , Wong CK , Lun SW , Ko GT , Lam CW , Lam CS. Metabolic syndrome, endothelial injury, and subclinical atherosclerosis in patients with systemic lupus erythematosus. Scand J Rheumatol : 42–49, 2010. [DOI] [PubMed] [Google Scholar]
- 106. Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets : 421–431, 2012. [DOI] [PubMed] [Google Scholar]
- 107. Motley ED , Eguchi K , Patterson MM , Palmer PD , Suzuki H , Eguchi S. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension : 577–583, 2007. [DOI] [PubMed] [Google Scholar]
- 108. Nakano M , Furutani M , Hiraishi S , Ishii H. Characterization of soluble thrombomodulin fragments in human urine. Thromb Haemost : 331–337, 1998. [PubMed] [Google Scholar]
- 109. Nakashima M , Uematsu T , Umemura K , Maruyama I , Tsuruta K. A novel recombinant soluble human thrombomodulin, ART-123, activates the protein C pathway in healthy male volunteers. J Clin Pharmacol : 540–544, 1998. [DOI] [PubMed] [Google Scholar]
- 110. Nan B , Yang H , Yan S , Lin PH , Lumsden AB , Yao Q , Chen C. C-reactive protein decreases expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Surgery : 212–222, 2005. [DOI] [PubMed] [Google Scholar]
- 111. Nara H , Okamoto H , Minota S , Yoshio T. Mouse monoclonal anti-human thrombomodulin antibodies bind to and activate endothelial cells through NF-kappaB activation in vitro. Arthritis Rheum : 1629–1637, 2006. [DOI] [PubMed] [Google Scholar]
- 112. Nesheim M , Wang W , Boffa M , Nagashima M , Morser J , Bajzar L. Thrombin, thrombomodulin and TAFI in the molecular link between coagulation and fibrinolysis. Thromb Haemost : 386–391, 1997. [PubMed] [Google Scholar]
- 113. Nomura E , Kohriyama T , Kozuka K , Kajikawa H , Nakamura S , Matsumoto M. Significance of serum soluble thrombomodulin level in acute cerebral infarction. Eur J Neurol : 329–334, 2004. [DOI] [PubMed] [Google Scholar]
- 114. Ogawa S , Shreeniwas R , Brett J , Clauss M , Furie M , Stern DM. The effect of hypoxia on capillary endothelial cell function: modulation of barrier and coagulant function. Br J Haematol : 517–524, 1990. [DOI] [PubMed] [Google Scholar]
- 115. Ogawa Y , Yamakawa K , Ogura H , Kiguchi T , Mohri T , Nakamori Y , Kuwagata Y , Shimazu T , Hamasaki T , Fujimi S. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg : 1150–1157, 2012. [DOI] [PubMed] [Google Scholar]
- 116. Ogura H , Tanaka H , Koh T , Fujita K , Fujimi S , Nakamori Y , Hosotsubo H , Kuwagata Y , Shimazu T , Sugimoto H. Enhanced production of endothelial microparticles with increased binding to leukocytes in patients with severe systemic inflammatory response syndrome. J Trauma : 823–831, 2004. [DOI] [PubMed] [Google Scholar]
- 117. Oida K , Takai H , Maeda H , Takahashi S , Tamai T , Nakai T , Miyabo S , Ishii H. Plasma thrombomodulin concentration in diabetes mellitus. Diabetes Res Clin Pract : 193–196, 1990. [DOI] [PubMed] [Google Scholar]
- 118. Omichi M , Matsusaki M , Kato S , Maruyama I , Akashi M. Enhancement of the blood compatibility of dialyzer membranes by the physical adsorption of human thrombomodulin (ART-123). J Biomed Mater Res B Appl Biomater : 291–297, 2010. [DOI] [PubMed] [Google Scholar]
- 119. Pawlak K , Myśliwiec M , Pawlak D. Kynurenine pathway—a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci : 196–203, 2010. [DOI] [PubMed] [Google Scholar]
- 120. Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol : 329–341, 1999. [DOI] [PubMed] [Google Scholar]
- 121. Qin B , Yang H , Xiao B. Role of microRNAs in endothelial inflammation and senescence. Mol Biol Rep : 4509–4518, 2012. [DOI] [PubMed] [Google Scholar]
- 122. Rajashekhar G , Gupta A , Marin A , Friedrich J , Willuweit A , Berg DT , Cramer MS , Sandusky GE , Sutton TA , Basile DP , Grinnell BW , Clauss M. Soluble thrombomodulin reduces inflammation and prevents microalbuminuria induced by chronic endothelial activation in transgenic mice. Am J Physiol Renal Physiol : F703–F712, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ran XZ , Ran X , Zong ZW , Liu DQ , Xiang GM , Su YP , Zheng HE. Protective effect of atorvastatin on radiation-induced vascular endothelial cell injury in vitro. J Radiat Res : 527–533, 2010. [DOI] [PubMed] [Google Scholar]
- 124. Redl H , Schlag G , Schiesser A , Davies J. Thrombomodulin release in baboon sepsis: its dependence on the dose of Escherichia coli and the presence of tumor necrosis factor. J Infect Dis : 1522–1527, 1995. [DOI] [PubMed] [Google Scholar]
- 125. Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases. IUBMB Life : 390–396, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Riewald M , Petrovan RJ , Donner A , Ruf W. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. J Endotoxin Res : 317–321, 2003. [DOI] [PubMed] [Google Scholar]
- 127. Rijneveld AW , Weijer S , Florquin S , Esmon CT , Meijers JC , Speelman P , Reitsma PH , Ten Cate H , van der Poll T. Thrombomodulin mutant mice with a strongly reduced capacity to generate activated protein C have an unaltered pulmonary immune response to respiratory pathogens and lipopolysaccharide. Blood : 1702–1709, 2004. [DOI] [PubMed] [Google Scholar]
- 128. Rossi J , Jonak P , Rouleau L , Danielczak L , Tardif JC , Leask RL. Differential response of endothelial cells to simvastatin when conditioned with steady, non-reversing pulsatile or oscillating shear stress. Ann Biomed Eng : 402–413, 2011. [DOI] [PubMed] [Google Scholar]
- 129. Rossi J , Rouleau L , Tardif JC , Leask RL. Effect of simvastatin on Kruppel-like factor2, endothelial nitric oxide synthase and thrombomodulin expression in endothelial cells under shear stress. Life Sci : 92–99, 2010. [DOI] [PubMed] [Google Scholar]
- 130. Rousseau A , Favier R , Van Dreden P. Elevated circulating soluble thrombomodulin activity, tissue factor activity and circulating procoagulant phospholipids: new and useful markers for pre-eclampsia? Eur J Obstet Gynecol Reprod Biol : 46–49, 2009. [DOI] [PubMed] [Google Scholar]
- 131. Ryang YM , Dang J , Kipp M , Petersen KU , Fahlenkamp AV , Gempt J , Wesp D , Rossaint R , Beyer C , Coburn M. Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci : 113, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Saito H , Maruyama I , Shimazaki S , Yamamoto Y , Aikawa N , Ohno R , Hirayama A , Matsuda T , Asakura H , Nakashima M , Aoki N. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost : 31–41, 2007. [DOI] [PubMed] [Google Scholar]
- 133. Sakai M , Ikezoe T , Bandobashi K , Yokoyama A. Successful treatment of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus with recombinant human soluble thrombomodulin. Thromb Res : e392–e393, 2010. [DOI] [PubMed] [Google Scholar]
- 134. Salomaa V , Matei C , Aleksic N , Sansores-Garcia L , Folsom AR , Juneja H , Chambless LE , Wu KK. Soluble thrombomodulin as a predictor of incident coronary heart disease and symptomless carotid artery atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) Study: a case-cohort study. Lancet : 1729–1734, 1999. [DOI] [PubMed] [Google Scholar]
- 135. Satta N , Freyssinet JM , Toti F. The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br J Haematol : 534–542, 1997. [DOI] [PubMed] [Google Scholar]
- 136. Séguin C , Abid MR , Spokes KC , Aird WC. Thrombin downregulates thrombomodulin expression and activity in primary human endothelial cells. Endothelium : 143–148, 2008. [DOI] [PubMed] [Google Scholar]
- 137. Sen-Banerjee S , Mir S , Lin Z , Hamik A , Atkins GB , Das H , Banerjee P , Kumar A , Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation : 720–726, 2005. [DOI] [PubMed] [Google Scholar]
- 138. Senet P , Peyri N , Berard M , Dubertret L , Boffa MC. Thrombomodulin, a functional surface protein on human keratinocytes, is regulated by retinoic acid. Arch Dermatol Res : 151–157, 1997. [DOI] [PubMed] [Google Scholar]
- 139. Sharfuddin AA , Sandoval RM , Berg DT , McDougal GE , Campos SB , Phillips CL , Jones BE , Gupta A , Grinnell BW , Molitoris BA. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol : 524–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shi CS , Shi GY , Chang YS , Han HS , Kuo CH , Liu C , Huang HC , Chang YJ , Chen PS , Wu HL. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation : 1627–1636, 2005. [DOI] [PubMed] [Google Scholar]
- 141. Shi J , Wang J , Zheng H , Ling W , Joseph J , Li D , Mehta JL , Ponnappan U , Lin P , Fink LM , Hauer-Jensen M. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis : 575–585, 2003. [DOI] [PubMed] [Google Scholar]
- 142. Soff GA , Jackman RW , Rosenberg RD. Expression of thrombomodulin by smooth muscle cells in culture: different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood : 515–8, 1991. [PubMed] [Google Scholar]
- 143. Sohn RH , Deming CB , Johns DC , Champion HC , Bian C , Gardner K , Rade JJ. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood : 3910–3917, 2005. [DOI] [PubMed] [Google Scholar]
- 144. Sperry JL , Deming CB , Bian C , Walinsky PL , Kass DA , Kolodgie FD , Virmani R , Kim AY , Rade JJ. Wall tension is a potent negative regulator of in vivo thrombomodulin expression. Circ Res : 41–47, 2003. [DOI] [PubMed] [Google Scholar]
- 145. Stanková J , Rola-Pleszczynski M , D'Orléans-Juste P. Endothelin 1 and thrombin synergistically stimulate IL-6 mRNA expression and protein production in human umbilical vein endothelial cells. J Cardiovasc Pharmacol : S505–S507, 1995. [PubMed] [Google Scholar]
- 146. Staszel T , Zapała B , Polus A , Sadakierska-Chudy A , Kieć-Wilk B , Stȩpień E , Wybrańska I , Chojnacka M , Dembińska-Kieć A. Role of microRNAs in endothelial cell pathophysiology Pol Arch Med Wewn : 361–366, 2011. [PubMed] [Google Scholar]
- 147. Stearns-Kurosawa DJ , Kurosawa S , Mollica JS , Ferrell GL , Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA : 10212–10216, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stites WE , Froude JW. Does the oxidation of methionine in thrombomodulin contribute to the hypercoaguable state of smokers and diabetics? Med Hypotheses : 811–821, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Su EJ , Geyer M , Wahl M , Mann K , Ginsburg D , Brohmann H , Petersen KU , Lawrence DA. The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J Thromb Haemost : 1174–1182, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Suarez Y , Fernandez-Hernando C , Pober JS , Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res : 1164–1173, 2007. [DOI] [PubMed] [Google Scholar]
- 151. Sun X , Icli B , Wara AK , Belkin N , He S , Kobzik L , Hunninghake GM , Vera MP , Blackwell TS , Baron RM , Feinberg MW. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest : 473–490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Suzuki K , Kusumoto H , Deyashiki Y , Nishioka J , Maruyama I , Zushi M , Kawahara S , Honda G , Yamamoto S , Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J : 1891–1897, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Suzuki K , Nichioka J , Hayaoni T , Kosaka Y. Functionally active thrombomodulin is present in human platelets. J Biochem : 628–632, 1988. [DOI] [PubMed] [Google Scholar]
- 154. Takada Y , Shinkai F , Kondo S , Yamamoto S , Tsuboi H , Korenaga R , Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun : 1345–1352, 1994. [DOI] [PubMed] [Google Scholar]
- 155. Takagi T , Taguchi O , Toda M , Ruiz DB , Bernabe PG , D'Alessandro-Gabazza CN , Miyake Y , Kobayashi T , Aoki S , Chiba F , Yano Y , Conway EM , Munesue S , Yamamoto Y , Yamamoto H , Suzuki K , Takei Y , Morser J , Gabazza EC. Inhibition of allergic bronchial asthma by thrombomodulin is mediated by dendritic cells. Am J Respir Crit Care Med : 31–42, 2011. [DOI] [PubMed] [Google Scholar]
- 156. Takeda N , Maemura K , Horie S , Oishi K , Imai Y , Harada T , Saito T , Shiga T , Amiya E , Manabe I , Ishida N , Nagai R. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem : 32561–32567, 2007. [DOI] [PubMed] [Google Scholar]
- 157. Takemoto M , Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol : 1712–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 158. Taylan A , Sari I , Kozaci DL , Yildiz Y , Bilge S , Coker I , Maltas S , Gunay N , Akkoc N. Evaluation of various endothelial biomarkers in ankylosing spondylitis. Clin Rheumatol : 23–28, 2012. [DOI] [PubMed] [Google Scholar]
- 159. Tazawa R , Hirosawa S , Suzuki K , Hirokawa K , Aoki N. Functional characterization of the 5′-regulatory region of the human thrombomodulin gene. J Biochem : 600–606, 1993. [DOI] [PubMed] [Google Scholar]
- 160. Thorand B , Baumert J , Herder C , Meisinger C , Koenig W. Soluble thrombomodulin as a predictor of type 2 diabetes: results from the MONICA/KORA Augsburg case-cohort study, 1984– . Diabetologia : 545–548, 2007. [DOI] [PubMed] [Google Scholar]
- 161. Tineli RA , Viaro F , Dalio MB , Reis GS , Basseto S , Vicente WV , Rodrigues AJ , Evora PR. Mechanical forces and human saphenous veins: coronary artery bypass graft implications. Rev Bras Cir Cardiovasc : 87–95, 2007. [DOI] [PubMed] [Google Scholar]
- 162. Tonooka K , Ito H , Shibata T , Ozaki S. Recombinant human soluble thrombomodulin for treatment of thrombotic microangiopathy associated with lupus nephritis. J Rheumatol : 1766, 2012. [DOI] [PubMed] [Google Scholar]
- 163. Traub O , Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol : 677–685, 1998. [DOI] [PubMed] [Google Scholar]
- 164. Tsai CS , Tsai YT , Lin CY , Lin TC , Huang GS , Hong GJ , Lin FY. Expression of thrombomodulin on monocytes is associated with early outcomes in patients with coronary artery bypass graft surgery. Shock : 31–39, 2010. [DOI] [PubMed] [Google Scholar]
- 165. Urban M , Wojtkielewicz K , Głowińska B , Peczyńska J. Soluble thrombomodulin: a molecular marker of endothelial cell injury in children and adolescents with obesity. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw : 73–77, 2005. [PubMed] [Google Scholar]
- 166. Uszyński M , Sztenc S , Zekanowska E , Uszyński W. Thrombomodulin in human gestational tissues: placenta, fetal membranes and myometrium. Adv Med Sci : 312–315, 2006. [PubMed] [Google Scholar]
- 167. Van de Wouwer M , Conway EM. Novel functions of thrombomodulin in inflammation. Crit Care Med : S254–S261, 2004. [DOI] [PubMed] [Google Scholar]
- 168. van Iersel T , Stroissnig H , Giesen P , Wemer J , Wilhelm-Ogunbiyi K. Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb Haemost : 302–312, 2011. [DOI] [PubMed] [Google Scholar]
- 169. Vasa-Nicotera M , Chen H , Tucci P , Yang AL , Saintigny G , Menghini R , Mahè C , Agostini M , Knight RA , Melino G , Federici M. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis : 326–330, 2011. [DOI] [PubMed] [Google Scholar]
- 170. Voellenkle C , Rooij Jv Guffanti A , Brini E , Fasanaro P , Isaia E , Croft L , David M , Capogrossi MC , Moles A , Felsani A , Martelli F. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA : 472–484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wakabayashi H , Natsuka S , Honda M , Naotsuka M , Ito Y , Kajihara J , Hase S. Structural analysis of the sugar chains of human urinary thrombomodulin. J Biochem : 543–552, 2001. [DOI] [PubMed] [Google Scholar]
- 172. Walsh TG , Murphy RP , Fitzpatrick P , Rochfort KD , Guinan AF , Murphy A , Cummins PM. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol : 3053–3063, 2011. [DOI] [PubMed] [Google Scholar]
- 173. Wang HJ , Lu TL , Huang H , Huang HC. Paclitaxel induces thrombomodulin downregulation in human aortic endothelial cells. Tex Heart Inst J : 20–26, 2011. [PMC free article] [PubMed] [Google Scholar]
- 174. Wang JH , Thampatty BP. An introductory review of cell mechanobiology. Biomechan Model Mechanobiol : 1–16, 2006. [DOI] [PubMed] [Google Scholar]
- 175. Wang W , Nagashima M , Schneider M , Morser J , Nesheim M. Elements of the primary structure of thrombomodulin required for efficient thrombin-activable fibrinolysis inhibitor activation. J Biol Chem : 22942–22947, 2000. [DOI] [PubMed] [Google Scholar]
- 176. Wang YX , Wu C , Vincelette J , Martin-McNulty B , Alexander S , Larsen B , Light DR , McLean K. Amplified anticoagulant activity of tissue factor-targeted thrombomodulin: in-vivo validation of a tissue factor-neutralizing antibody fused to soluble thrombomodulin. Thromb Haemost : 317–324, 2006. [DOI] [PubMed] [Google Scholar]
- 177. Wei HJ , Li YH , Shi GY , Liu SL , Chang PC , Kuo CH , Wu HL. Thrombomodulin domains attenuate atherosclerosis by inhibiting thrombin-induced endothelial cell activation. Cardiovasc Res : 317–327, 2011. [DOI] [PubMed] [Google Scholar]
- 178. Welters I , Menges T , Ballesteros M , Knothe C , Ruwoldt R , Görlach G , Hempelmann G. Thrombin generation and activation of the thrombomodulin protein C system in open heart surgery depend on the underlying cardiac disease. Thromb Res : 1–9, 1998. [DOI] [PubMed] [Google Scholar]
- 179. Wen DZ , Dittman WA , Ye RD , Deaven LL , Majerus PW , Sadler JE. Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry : 4350–4357, 1987. [DOI] [PubMed] [Google Scholar]
- 180. Wong G , Li JM , Hendricks G , Eslami MH , Rohrer MJ , Cutler BS. Inhibition of experimental neointimal hyperplasia by recombinant human thrombomodulin coated ePTFE stent grafts. J Vasc Surg : 608–615, 2008. [DOI] [PubMed] [Google Scholar]
- 181. Wood MJ , Becvar LA , Prieto JH , Melacini G , Komives EA. NMR structures reveal how oxidation inactivates thrombomodulin. Biochemistry : 11932–11942, 2003. [DOI] [PubMed] [Google Scholar]
- 182. Wood MJ , Helena Prieto J , Komives EA. Structural and functional consequences of methionine oxidation in thrombomodulin. Biochim Biophys Acta : 141–147, 2005. [DOI] [PubMed] [Google Scholar]
- 183. Wood SC , Tang X , Tesfamariam B. Paclitaxel potentiates inflammatory cytokine-induced prothrombotic molecules in endothelial cells. J Cardiovasc Pharmacol : 276–285, 2010. [DOI] [PubMed] [Google Scholar]
- 184. Wu HL , Lin CI , Huang YL , Chen PS , Kuo CH , Chen MS , Wu GC , Shi GY , Yang HY , Lee H. Lysophosphatidic acid stimulates thrombomodulin lectin-like domain shedding in human endothelial cells. Biochem Biophys Res Commun : 162–168, 2008. [DOI] [PubMed] [Google Scholar]
- 185. Wu KK. Soluble thrombomodulin and coronary heart disease. Curr Opin Lipidol : 373–375, 2003. [DOI] [PubMed] [Google Scholar]
- 186. Wu MH , Kouchi Y , Onuki Y , Shi Q , Yoshida H , Kaplan S , Viggers RF , Ghali R , Sauvage LR. Effect of differential shear stress on platelet aggregation, surface thrombosis, and endothelialization of bilateral carotid-femoral grafts in the dog. J Vasc Surg : 382–390, 1995. [DOI] [PubMed] [Google Scholar]
- 187. Wu W , Xiao H , Laguna-Fernandez A , Villarreal G , Wang KC , Geary GG , Zhang Y , Wang WC , Huang HD , Zhou J , Li YS , Chien S , Garcia-Cardena G , Shyy JY. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation : 633–641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yao A , Wang J , Fink LM , Hardin JW , Hauer-Jensen M. Molecular cloning and sequence analysis of the 5′-flanking region of the Sprague-Dawley rat thrombomodulin gene. DNA Seq : 55–60, 1999. [DOI] [PubMed] [Google Scholar]
- 189. Ye J , Esmon CT , Johnson AE. The chondroitin sulfate moiety of thrombomodulin binds a second molecule of thrombin. J Biol Chem : 2373–2379, 1993. [PubMed] [Google Scholar]
- 190. Yerkovich ST , Roponen M , Smith ME , McKenna K , Bosco A , Subrata LS , Mamessier E , Wikström ME , Le Souef P , Sly PD , Holt PG , Upham JW. Allergen-enhanced thrombomodulin (blood dendritic cell antigen 3, CD141) expression on dendritic cells is associated with a TH2-skewed immune response. J Allergy Clin Immunol : 209–216, 2009. [DOI] [PubMed] [Google Scholar]
- 191. Yin R , Bao W , Xing Y , Xi T , Gou S. MiR-19b-1 inhibits angiogenesis by blocking cell cycle progression of endothelial cells. Biochem Biophys Res Commun : 771–776, 2012. [DOI] [PubMed] [Google Scholar]
- 192. Zaitsev S , Kowalska MA , Neyman M , Carnemolla R , Tliba S , Ding BS , Stonestrom A , Spitzer D , Atkinson JP , Poncz M , Cines DB , Esmon CT , Muzykantov VR. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood : 4779–4785, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhao L , Buckman B , Seto M , Morser J , Nagashima M. Mutations in the substrate binding site of thrombin-activatable fibrinolysis inhibitor (TAFI) alter its substrate specificity. J Biol Chem : 32359–32366, 2003. [DOI] [PubMed] [Google Scholar]
- 194. Zycinska K , Wardyn KA , Zielonka TM , Krupa R , Lukas W. Clinical implications of serum thrombomodulin in PR3-ANCA-associated vasculitis. Eur J Med Res : 268–270, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]