Abstract
Background
Ischaemia-reperfusion injury (IRI) is a major obstacle during liver transplantation and resection surgeries for cancer, with a need for effective and safe drugs to reduce IRI. Zinc preconditioning has been shown to protect against liver IRI in a partial (70%) ischaemia model. However, its efficacy against a clinically relevant Pringle manoeuvre that results in global liver ischaemia (100%) is unknown.
Aims
The aim of this study was to test the efficacy of zinc preconditioning in a rat model of global liver ischaemia.
Methods
Rats were preconditioned via subcutaneous injection of 10 mg/kg of ZnCl2, 24 h and 4 h before ischaemia. Total liver ischaemia (100%) was induced by placing a clamp across the portal triad for 30 min. Liver injury was assessed by serum alanine transaminase (ALT) and aspartate transaminase (AST) levels in blood taken before ischaemia (baseline) and at 1, 2, 4, 24, 48, 72, 96 and 120 hours after ischaemia. Animals were culled after 7 days, and the harvested livers were histologically analysed.
Results
On a two-way repeated-measures analysis of variance, there was a statistically significant (p = 0.025) difference in the mean ALT levels between saline- and ZnCl2-treated groups. Specifically at 24 h after ischaemia, the ALT (341 ± 99 U/L) and AST (606 ± 78 U/L) in the zinc-treated group were significantly less than the ALT (2863 ± 828 U/L) and AST (3591 ± 948 U/L) values in the saline-treated group. Zinc significantly reduced neutrophil infiltration and necrosis compared with the saline control.
Conclusion
Zinc preconditioning reduces the overall hepatocellular damage from IRI. These results lay the foundation to assess the benefit of zinc preconditioning for clinical applications.
Keywords: ischaemia, reperfusion, injury, zinc, liver
List of abbreviations: ALT, Alanine Transaminase; ANOVA, Analysis of Variance; AST, Aspartate Transaminase; IRI, Ischaemia-Reperfusion Injury
Over the years, liver transplantation and resection surgeries have significantly increased the quality of life and survival of patients with liver cancer and chronic liver disease. However, both surgeries involve a period of ischaemia and reperfusion to the liver where the blood supply to the liver is stopped and then restored. Such an event causes an ischaemia-reperfusion injury (IRI) to the liver, which can be harmful and in some cases fatal to the patient.1, 2 Various treatments to ameliorate IRI have been studied extensively, but there are currently none that have been able to be transitioned into clinical practice.3, 4 Therefore, the development of strategies to prevent or reduce IRI is an important research goal.
There have been various pharmacological and surgical treatments that have been trialled for IRI with some early positive results in animal experiments.5 However, subsequent human clinical trials have failed to show benefit.6 There may be many reasons for this lack of successful translation, and recent reviews have explored the possible contributing factors such as different anatomy and physiology between humans and animals.6, 7 Therefore, there is a pressing need to find an agent or technique that can successfully prevent or reduce IRI in humans.
Zinc is an essential biometal that has been studied in the treatment for IRI in several organs.8 Zinc preconditioning has been shown to protect against renal IRI in vitro and in a small animal rat9 and a preclinical large sheep animal model.10 In the liver, using a partial (70%) ischaemia model, hepatic IRI induced by 45 min of ischaemia followed by 6 h of reperfusion in rats could be attenuated by dihydrolipoyl histidinate zinc complex11 or zinc sulphate.12 In humans, 70% occlusion is rarely practical. The Pringle manoeuvre, where the portal triad is occluded which induces 100% global ischaemia of liver, is used when selective clamping is not technically achievable because of certain tumour locations or when surgical access is difficult.13 Previously, it has been shown that there are differences in the protective effect of certain drugs against liver IRI injury depending on whether the 70% partial ischaemia vs 100% global ischaemia model is used (e.g., ATP-MgCl2).7, 14, 15 Therefore, it is important to confirm the efficacy of zinc preconditioning in a 100% global liver ischaemia model before any clinical trials can begin. Furthermore, reperfusion injury consists of two phases: an early phase mediated by oxidative stress predominantly via reactive oxygen species (ROS) generation and a later phase 6–24 h after reperfusion, which consists of inflammatory mediators that can cause direct tissue damage. This late phase of liver IRI has not been previously investigated. Both the studies showing zinc to be protective were concluded based on early reperfusion times of only 60 min12 and 6 h.11
Therefore, the aim of this study was to investigate the protective effect of zinc in a clinically relevant rat model of 100% liver ischaemia and reperfusion duration of one week, followed by histological analysis of the liver.
Materials and methods
Animal ethics and care
The present study (Ethics ID A2017/05472) was approved by the Austin Health Animal Ethics Committee in accordance with the guidelines laid down by the National Health and Medical Research Council of Australia's code of practice for the care and use of animals for experimental purposes.
Randomisation and blinding regime
Specifically, animals undergoing ischaemia were randomised to one of two experimental groups (saline vs zinc preconditioning). To reduce bias against the outcomes, the investigators remained blinded to experimental settings such that an investigator independent of the surgical procedure was responsible for zinc preconditioning, day-to-day monitoring of animals and blood sampling. A sole investigator was designated to perform the surgeries, who also remained blinded to the treatment groups. The investigators at the Clinical Trials Department at Austin Health who performed the serum analysis of alanine transaminase/aspartate transaminase (ALT/AST) and the pathologist who analysed the liver samples remained blinded to the treatment groups.
Animals
Male Sprague Dawley rats aged 14–16 weeks and weighing 200–300g were acquired from the Animal Resource Centre in Western Australia. Rats were housed in caged boxes (up to 2 animals per cage) containing clean bedding. Animals were allowed 5 days to be accustomed to their new surroundings. Animals were kept in a temperature-controlled environment around 22–24 °C with a 12-h light-dark cycle and were allowed water and rat pellets ad libitum. Animals were fasted on the morning of the surgery to avoid dilatation of the stomach with food during surgery.
Animal grouping and preconditioning with zinc
Animals were randomly allocated to saline control and zinc treatment groups. Specifically, there were four rats in the control group and five rats in the zinc group. Animals had baseline blood taken before the surgery and were weighed. Subcutaneous injection of 0.1 mg/kg of buprenorphine was given preoperatively, 6–8 h after operation and the day after surgery for pain relief. The treatment group was given subcutaneous injection of 10 mg/kg of zinc chloride (ZnCl2) 24 h and 4 h before the surgery, while the control group was given normal saline via subcutaneous injection. Eighty milligrams/millilitre of concentrated ZnCl2 solution was diluted with normal saline to make a 10-mg/ml solution before it was injected subcutaneously into the rat, and the final injection volume ranged from 0.25 to 0.35 ml depending on the weight of rat.
Induction of liver IRI and surgical procedure
Once anaesthetised, a roll of paper towel was placed underneath the rat to elevate the abdomen and present the liver for easy dissection. Laparotomy was carried out through a vertical midline skin incision. The retractor was used to allow a better view of the liver and surrounding organs. Fine-tipped forceps and wet cotton buds were used to isolate the portal vein, bile duct and hepatic artery by gently retracting the liver up and retracting the duodenum down. Curved nontoothed forceps were then passed underneath the portal triad. The forceps were then used to grasp a silk suture that was looped around the portal triad to allow access for the clamp and isolation of the portal triad. Total liver ischaemia (100%) was induced by placing a clamp across the portal triad for 30 min. While the clamp was placed across the portal triad, a gauze pad soaked with sterile saline was used to cover the incision wound to prevent drying out of the organs. After 30 min, the clamp and the silk suture were carefully removed. Five millilitres of warmed saline was administered into the peritoneal cavity to counter any blood or fluid losses. The liver and intestines were observed for a few minutes to ensure adequate reperfusion before the wound was sutured. Animals were monitored and weighed daily after surgery to determine the weight change after surgery.16 Weight change was expressed as a percentage of the presurgery weight.
Liver function test
Blood samples (400 μL) were collected from the tail vein of the rats into Microtainer tubes 1, 2, 4, 24, 48, 72, 96 and 120 h after reperfusion. Blood samples were centrifuged at 4 °C and analysed by the Clinical Trials Department, Austin Pathology, Heidelberg, Australia. ALT and AST were measured using an automated Roche Cobas c 702 analyser (Roche Diagnostics, Mannheim Distribution, USA) via the electrochemical test method according to the International Federation of Clinical Chemistry and Laboratory Medicine.17
Histological investigations
At the completion of the experiment, the livers were harvested, fixed in 10% formalin for 24 h and then stored in 70% alcohol. The haematoxylin and eosin–stained liver samples were histologically and semiquantitatively analysed for liver damage by an anatomical pathologist blinded to the treatment group. The assessment was conducted according to a previously devised method of evaluating neutrophil infiltration and hepatic necrosis after ischaemia, which was described by Suzuki et al.18 Both neutrophil infiltration and hepatic necrosis were scored from 0 to 4, as shown in Table 1.
Table 1.
Histological Score of Rat Livers After 30 min of Ischaemia Followed by 7 Days of Reperfusion.
| Group | Neutrophilic infiltration score (0–4) | Average score | Criteria for assessing neutrophil infiltration |
|---|---|---|---|
| Saline control (n = 5) | 2 | 1.8 | Neutrophil infiltration expressed as neutrophil count per 50 high-power fields 0 = <15 (none/scant) 1 = 16 to 50 (minimal) 2 = 51 to 100 (mild) 3 = 101 to 250 (moderate) 4 = >250 (severe) |
| 2 | |||
| 1 | |||
| 2 | |||
| 2 | |||
| Zinc (10 mg/kg) (n = 5) |
1 | 0.6 (p = 0.016) |
|
| 1 | |||
| 0 | |||
| 0 | |||
| 1 |
| Group | Liver necrosis score (0–4) | Average score | Criteria for assessing liver necrosis |
|---|---|---|---|
| Saline control (n = 5) | 3 | 2 | Liver necrosis assessed as the degree of hepatocellular necrosis 0 = None 1 = Single-cell apoptosis 2 = Lobular confluent necrosis <30% 3 = Lobular confluent necrosis 31–60% 4 = Lobular confluent necrosis >60% |
| 2 | |||
| 1 | |||
| 2 | |||
| 2 | |||
| Zinc (10 mg/kg) (n = 5) | 1 | 0.8 (p = 0.012) | |
| 1 | |||
| 1 | |||
| 1 | |||
| 0 |
A grading scale of 0–4, as outlined by Suzuki et al,18 was used for the histopathological analysis of neutrophil infiltration and necrosis in the liver.
Statistical methods
Results are expressed as mean ± standard error mean unless otherwise stated. Statistics were analysed using SigmaPlot, version 12.0, (Systat Software, Inc, Chicago, IL, USA). A two-way repeated-measures analysis of variance (ANOVA) was used to evaluate the differences in liver function over time between the groups as described previously.19 One-way ANOVA was carried out to analyse differences between different time points in the same group. Student t-tests were performed to study differences between two groups at each individual time point. Where the data were not normally distributed, the Mann-Whitney rank-sum test was used.
Results
Mortality after 30 min of global liver ischaemia
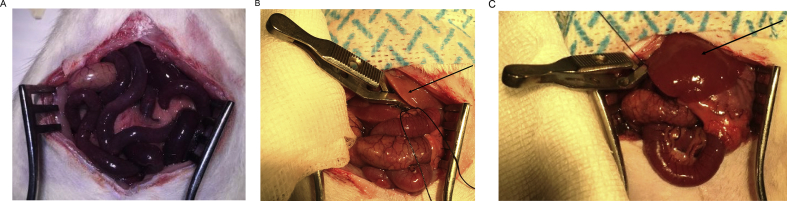
Previous articles have reported a 50–80% mortality rate in rats from 30 min of occlusion of the portal triad.20, 21 Early in the present study, marked splanchnic congestion with significant oedema and swelling of the small intestines was observed. Necropsy revealed extensive intestinal ischaemia (Figure 1A). The most likely cause of this ischaemia and necrosis was due to thrombosis in the portal triad and damage caused by congestion in the bowel upon release of the portal triad, similar to the findings of the previous studies.22
Figure 1.
The appearance of the abdominal cavity after 30 min of clamping/ischaemia. (A) The appearance of the bowel with oedema and signs of vascular distress of the animal that did not survive beyond 4 h after ischaemia. (B) The appearance of the bowel and liver of the rat that had adequate ischaemia. Intense venous congestion of the splanchnic is present; however, the pale colour of the liver upon the placement of the clamp demonstrates adequate ischaemia (see the arrow). (C) The appearance of the bowel and liver of the rat that had inadequate ischaemia. Intense venous congestion of the splanchnic is present. However, the failure of the liver to become pale (see the arrow) upon the placement of the clamp as compared with the liver seen in (B) demonstrates inadequate ischaemia. This particular animal was therefore excluded from the study.
The portal triad is very sensitive to movement, and therefore, fixing the clamp into position is a very crucial step in the surgery. Our optimised surgical technique with extremely gentle handling of the portal triad and careful manipulation of the surrounding vessels with minimal twisting or pulling of these delicate vessels (Figure 1B and C) avoided any further deaths in the animals. This allowed us to create a stable and reproducible global ischaemia model, which is clinically more relevant than the partial ischaemia model as it closely replicates the Pringle manoeuvre used in the clinical setting.23
Zinc preconditioning reduces the rise in serum ALT and AST levels
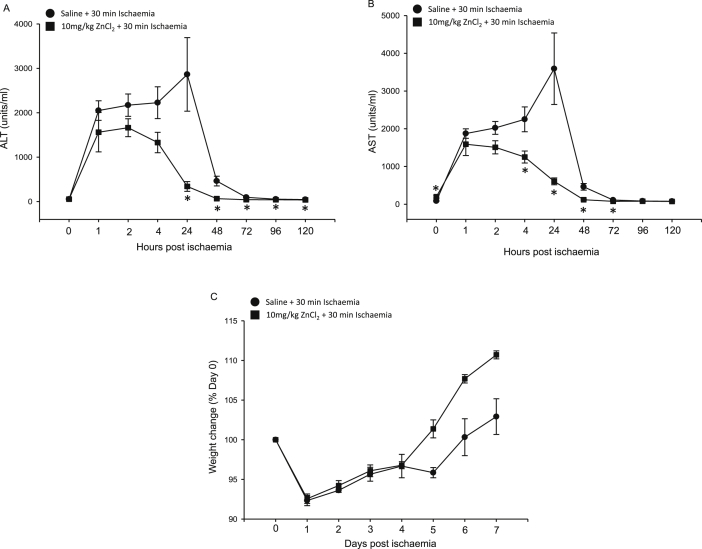
As seen in Figure 2A and B, one-way repeated-measures ANOVA established a statistically significant difference in serum ALT (p = 0.013) and AST (p = 0.005) values in the saline-treated group from baseline to day 4, confirming reduced liver function after 30 min of liver ischaemia in our model. Significantly, the peak for ALT and AST levels in the saline control group was at 24 h after ischaemia. On a two-way repeated-measures ANOVA comparing the saline-treated group with the 10-mg/kg ZnCl2 group, there was a statistically significant difference in the mean values of ALT (p = 0.025) concentrations between treatment groups; however, AST levels failed to reach significance (p = 0.06). Student t-tests were performed to determine differences between two groups (saline vs 10 mg/kg of ZnCl2) at each individual time point. The ALT and AST values for zinc-treated groups were significantly lower than those for the control groups from 24 h onwards, as seen in Figure 2A and B, respectively. However, the difference in ALT values between the zinc and control group was most marked at 24 h (341 ± 99 U/L vs 2863 ± 828 U/L, p = 0.011). Similarly, the difference in AST values between the zinc and control group was most marked at 24 h (606 ± 78 U/L vs 3591 ± 948 U/L, p = 0.01). ALT values at 72, 96 and 120 hours for the saline-treated group were 96 ± 15, 54 ± 4 and 46 ± 1, respectively. In the zinc-treated group, the statistically significant lower ALT values at 72, 96 and 120 hours were 42 ± 3 (P = 0.014), 41 ± 3 (P = 0.031) and 38 ± 2 (P = 0.01), respectively. Moreover, ALT and AST levels in zinc-treated groups returned to preischaemic levels by 72 h, compared with 96 h required by the saline-treated control, suggesting early recovery prompted by zinc preconditioning.
Figure 2.
Zinc preconditioning reduced the increase in serum AST and ALT levels after liver IRI. Serum ALT (A) and AST (B) concentrations (mean ± SEM [units/L]) in rats treated with saline (control) (●) and 10 mg/kg of ZnCl2 (▪). The rise in serum ALT and AST levels was less in rats preconditioned with 10 mg/kg of ZnCl2 than in the saline control group. (C) Mean percentage weight change from baseline weight between zinc treatment and control groups. ZnCl2 preconditioning improves the health of rats after IRI. Weight loss was observed in both the groups after liver IRI; however, the rats preconditioned with 10 mg/kg of ZnCl2 appeared to have an earlier return to weight gain than the saline control group rats, although these changes were not statistically significant. *p < 0.05 vs saline-treated control. AST, aspartate transaminase; ALT, alanine transaminase; SEM, standard error of mean; IRI, ischaemia-reperfusion injury.
Zinc preconditioning improves the health of animals' post-IRI
Zinc and control groups had a similar amount of weight loss early after surgery (day 1–4), as seen in Figure 2C. However, zinc-treated groups showed earlier return to baseline weight with the restoration of original weight 5 days after ischaemia compared with 6 days after ischaemia in the control group. Moreover, there was a trend that zinc-treated groups also showed greater weight gain at day 7 than control groups with 10% weight gain from baseline found in zinc-treated groups compared with only 3% in control groups.
Zinc administration reduced the necrosis, neutrophil infiltration and inflammation after liver IRI
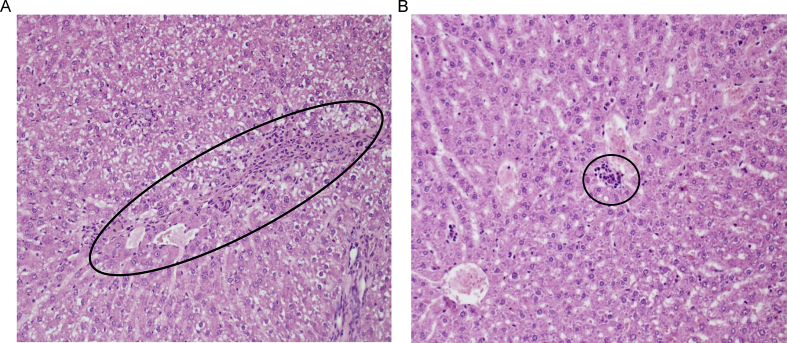
The grading systems of histologic tissue damage adopted by Suzuki et al.18 were used to assess liver architecture one week after the 30-min ischaemic injury. The livers from the group treated with ZnCl2 had significantly reduced liver damage (Table 1 and Figure 3). Zinc protected against the histological hallmarks of liver injury as the zinc-treated liver showed lower necrosis and less neutrophil infiltration than the control, as seen in Table 1. Histologically, zone 3 is located around the central veins, which makes it oxygen-poor and more vulnerable to IRI.24 IRI caused marked confluent necrosis in zone 3 of the liver with focal bridging, and neutrophilic infiltrates in control groups. Figure 3A and B shows the representative photomicrographs of the liver after 30 min of ischaemia and 7 days after reperfusion. The saline-treated control liver (Figure 3A) demonstrates extensive hepatocellular necrosis and vacuolisation with loss of sinusoidal architecture, whereas the liver from the zinc-preconditioned rat (Figure 3B) demonstrates almost no necrosis with only mild vacuolisation. Zinc significantly reduced neutrophil infiltration and inflammation in liver tissue as shown in Table 1. Therefore, from our histopathological analysis, zinc-treated groups had less inflammation and hepatocellular injury than control groups.
Figure 3.
Zinc preconditioning reduces liver necrosis and inflammation. Blinded histopathological examination of the liver by H&E staining 7 days after 30-min ischaemia. (A) An H&E-stained section of the liver from a rat treated with saline displays centrilobular confluent necrosis and inflammation in the liver (circled). (B) An H&E-stained section of the liver from a rat treated with 10 mg/kg of ZnCl2 displays a near-normal tissue architecture and a smaller area (circled) of necrosis and inflammation than the control group. H&E, haematoxylin and eosin.
Discussion
The present study used a global liver ischaemia model unlike most previously published literature which used a partial ischaemia model.7 The 70% partial ischaemia model was first described by Yamauchi et al. and involved the occlusion of the hepatic artery, portal vein and bile duct of the median and left lobes of the liver.25 The purported benefit of partial occlusion is to preserve blood flow through the right and caudate lobes and reduce the venous congestion in the bowels.26 In our study, a complete liver ischaemia model was chosen to mimic Pringle manoeuvre widely used in the clinical setting. Furthermore, some previous rodent studies have used ischaemia times of more than 30 min, resulting in greater than 75% necrosis and severely impaired liver function.11, 12 These do not represent the clinical situation in the context of liver surgery.27 Therefore, it has been suggested that rodent models of hepatic IRI should not exceed 30 min of liver ischaemia, and this formed the basis of our study.27
Owing to the anatomical variation in the blood vessels, one of the complications while inducing complete liver ischaemia could be insufficient ischaemia in some animals.28 In rats that had an accessory branch from the left gastric artery to the left lobe of the liver, clamping of the portal triad did not induce significant ischaemia in that lobe of the liver. This was evidenced by the failure of the left lobe of the liver to become pale after occlusion of the portal triad, as seen in Figure 1C. When this occurred, the rat was excluded from the study as total liver ischaemia was not achieved, and the extremely low liver transaminase enzyme levels and scant hepatocellular damage from the histological assessment supported this.
Injury due to ischaemia and reperfusion is characterised by direct damage during the ischaemia phase and then two phases of damage during the reperfusion phase: the early phase of oxidative stress mediated mainly by ROS generation and then the later phase 6–24 h after reperfusion that consists of inflammatory mediators which can cause direct tissue damage. Previous studies showing the protective benefit of zinc were based on early reperfusion times of only 60 min12 and 6 h.11 In the present study, within the first 2 h after 100% ischaemia, zinc did not show any significant reduction in AST and ALT levels. The early equivalent increase in AST and ALT levels in zinc- and saline-treated groups after ischaemia suggests that there was minimal variation in the surgical technique used to induce injury between the two groups. However, 4 h after ischaemia, there was a significant difference in AST and ALT levels between control and zinc groups. We conclude that zinc cannot protect against the immediate effects of a lack of blood flow and oxygen, and therefore, zinc did not significantly decrease early AST and ALT. However, it may be that zinc protects against the reperfusion injury rather than the ischaemic insult.
In comparison with the study by Mard et al12 which found that zinc reduced the AST and ALT levels within 60 min after ischaemia, our study failed to show any such reduction in AST and ALT levels between zinc and saline control group at early time points. Their study used a partial ischaemia model which despite 45 min of ischaemia produced a peak AST increase of only 200 units/mL from a baseline of ∼40 units/mL and peak ALT increase to mere 60 units/mL from a baseline of 20 units/ml. Therefore, such a discrepancy between the study by Mard et al12 and ours could be due to the difference in the degree of IRI.
In contrast to our 10-mg/kg ZnCl2 dose being effective against renal IRI9 and was able to stimulate gastrin gene expression in vivo,29 the 5-mg/kg dose used in the study by Mard et al12 was ineffective in both scenarios. It could be that lower concentrations of zinc could be effective against a lesser injury as in the case of the study by Mard et al.12 Another difference between the study by Mard et al12 and the present study is the use of a two-dose regimen of 10-mg/kg dose of ZnCl2 given subcutaneously compared with 5-dose regimen in the study by Mard et al12 comprising 5 mg/kg of ZnSO4 given intraperitoneally. From a patient and hospital, administrative perspective, the two-dose regimen used in the present study can be easily translated to the clinic compared with a longer five-dose intraperitoneal regimen as in the study by Mard et al.12 Furthermore, ZnCl2 and ZnSO4 are the most widely used inorganic salts; a study comparing the influence of the seven different counterions on the cytotoxicity of zinc concluded ZnSO4 to be the most toxic salt, particularly at low concentrations.30 This observation suggests that ZnCl2 used in the present study could be the preferred choice for clinical application.
It has been suggested that some cytoprotective drugs may only postpone and not avoid an injury, and therefore, a robust approach would be to analyse the protective effect by repeatedly measuring the outcome metrics over time, i.e., AST/ALT in the case of liver IRI.31 Furthermore, although a single–time point assessment may indicate early protection, it may miss delayed toxicity of an agent. For example, although the rats treated with 10 mg/kg and 30 mg/kg of ZnCl2 reduced the creatinine and urea rise compared with the saline control after 60 min of renal ischaemia, histological analysis of renal tissue 7 days after renal IRI concluded that 30-mg/kg ZnCl2–treated kidneys displayed a reduction in renal protection.9 Both the previous studies analysed the protective effect of zinc at early time points of only 6 h11 and 60 min after reperfusion.12 Therefore, our study has now rectified this limitation by demonstrating the protective effect of zinc in a repeated-measures model simulating a clinical scenario.
An interesting observation in the present study was the preischaemic baseline elevated AST levels in zinc-treated animals compared with the control; however, no such elevation was observed in the ALT level after zinc treatment. It is known that AST is found in various tissues including liver, cardiac and skeletal muscle, while ALT is mainly expressed by the liver cells and is a relatively specific indicator of liver damage.32 First, if zinc was toxic to the liver, it would have resulted in an increase in both AST and ALT. Second, postischaemia zinc preconditioning would not have resulted in reduced levels of AST and ALT compared with saline-treated animals. Furthermore, a high AST level can mean damage to another organ such as heart or kidneys. However, as zinc preconditioning previously protected against renal IRI,9 renal toxicity can be ruled out particularly at 10-mg/kg ZnCl2 dose used in the present study. Similar to previously published studies,8, 9, 10 in the present study, no ill effects were observed in animals treated with zinc. The concentration of zinc in the injection solution (10 mg/ml) used in the present study was 73.5 mM. Various studies have shown zinc to be toxic at millimolar concentration.33, 34, 35 Therefore, it may be that the high baseline AST level after zinc administration itself could be due to the localised injury at the site of subcutaneous injection.
The aspect of extrapolation of drug dose from animal studies is important before starting clinical studies in humans. It has been suggested that larger animals have lower metabolic rates and that physiological process of larger animals is slower.36 Furthermore, the larger the size of the species is, the smaller the required drug dose is on a weight basis. Based on the body surface area, pharmacokinetics and physiological time, the human dose equivalent deduced from the present rat study would be 1.62mg of ZnCl2/kg.36
Histological analysis of liver samples collected on day 7 after liver IRI showed greater liver damage in the control group than in the zinc-treated group. Specifically, zinc preconditioning decreased the amount of inflammation and necrosis after IRI. Most preclinical animal studies and human clinical trials define liver injury based on the increase in serum ALT or AST. In the present study, although the AST and ALT levels had returned to the baseline level by 96 h after IRI in the control groups, the histological analysis determined a significant amount of liver damage at 7 days after ischaemia. This observation suggested that AST/ALT, although useful markers in quantifying liver IRI immediately within 24–96 h after ischaemia, may not detect histological damage beyond that and the potential for long-term liver impairment. It is known that severe IRI influences long-term liver transplant outcomes. On multivariate analysis, peak ALT within the first week after transplantation predicted 1-year graft survival (P < 0.05); however, the prediction was much stronger based on histological analysis on liver biopsy.37 Taken together, our study has highlighted that such discordance between AST/ALT values and histologic liver damage could fail to uncover the long-term protective effect of an agent being tested if outcomes are based on merely the levels of ALT/AST within 1–96 h after IRI.
In summary, zinc preconditioning reduces the overall hepatocellular damage from IRI and results in greater recovery and weight gain than in control groups. Moreover, this study used a complete ischaemia model that has clinical ramifications and also showed it to be a reproducible and safe ischaemia model. The result of this study has laid the foundation to investigate the protective effect of zinc preconditioning on liver IRI in humans.
Author contributions
E.C., M.N. and O.P. performed the experiments and analysed the data; L.J. performed the histological investigations; D.B., J.I. and O.P. designed and coordinated the research; E.C., J.I. and O.P. wrote the manuscript. All authors edited the manuscript.
Conflict of interest
The authors have none to declare.
Acknowledgements
The authors thank the following people for their assistance in this project: Lakmie Gunarathne, Dr Chandana Herath, Eleanor Hunt, Josh Lorimer, Hayley Sleep and Cleo Christodoulou.
Funding
This work was in part supported by the Austin Medical Research Foundation (AMRF) and by University of Melbourne.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.07.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Eltzschig H.K., Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Junnarkar S.P., Tapuria N., Dutt N., Fuller B., Seifalian A.M., Davidson B.R. Bucillamine improves hepatic microcirculation and reduces hepatocellular injury after liver warm ischaemia-reperfusion injury. HPB : Off J Int Hepato Pancreato Biliary Assoc. 2009;11:264–273. doi: 10.1111/j.1477-2574.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W., Chen J., Meng Y., Chen Z., Yang J. Novel targets for treating ischemia-reperfusion injury in the liver. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazato P.C.G., Victorino J.P., Fina C.F. Liver ischemia and reperfusion injury. Pathophysiology and new horizons in preconditioning and therapy. Acta Cir Bras. 2018;33:723–735. doi: 10.1590/s0102-865020180080000008. [DOI] [PubMed] [Google Scholar]
- 5.Selzner N., Rudiger H., Graf R., Clavien P.A. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 6.Andreani P., Hoti E., de la Serna S. Ischaemic preconditioning of the graft in adult living related right lobe liver transplantation: impact on ischaemia-reperfusion injury and clinical relevance. HPB. 2010;12:439–446. doi: 10.1111/j.1477-2574.2010.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendes-Braz M., Elias-Miró M., Jiménez-Castro M.B., Casillas-Ramírez A., Ramalho F.S., Peralta C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J Biomed Biotechnol. 2012;2012:298657. doi: 10.1155/2012/298657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagulova G., Yue Y., Moreyra A., Boutjdir M., Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 9.Rao K., Sethi K., Ischia J. Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Kane D., Gibson L., May C.N. Zinc preconditioning protects against renal ischaemia reperfusion injury in a preclinical sheep large animal model. Biometals. 2018;31:821–834. doi: 10.1007/s10534-018-0125-3. [DOI] [PubMed] [Google Scholar]
- 11.Masuda T., Iwashita Y., Hagiwara S. Dihydrolipoyl histidinate zinc complex, a new antioxidant, attenuates hepatic ischemia-reperfusion injury in rats. J Gastroenterol Hepatol. 2011;26:1652–1658. doi: 10.1111/j.1440-1746.2011.06773.x. [DOI] [PubMed] [Google Scholar]
- 12.Mard S.A., Akbari G., Dianat M., Mansouri E. Protective effects of crocin and zinc sulfate on hepatic ischemia-reperfusion injury in rats: a comparative experimental model study. Biomed Pharmacother. 2017;96:48–55. doi: 10.1016/j.biopha.2017.09.123. [DOI] [PubMed] [Google Scholar]
- 13.Chouillard E.K., Gumbs A.A., Cherqui D. Vascular clamping in liver surgery: physiology, indications and techniques. Ann Surg Innovat Res. 2010;4:2. doi: 10.1186/1750-1164-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudry I.H., Clemens M.G., Ohkawa M., Schleck S., Baue A.E. Restoration of hepatocellular function and blood flow following hepatic ischemia with ATP-MgCl2. Adv Shock Res. 1982;8:177–186. [PubMed] [Google Scholar]
- 15.Hasselgren P.O., Jennische E., Fornander J., Hellman A. No beneficial effect of ATP-MgCl2 on impaired transmembrane potential and protein synthesis in liver ischemia. Acta Chir Scand. 1982;148:601–607. [PubMed] [Google Scholar]
- 16.Brennan M.P., Sinusas A.J., Horvath T.L., Collins J.G., Harding M.J. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim. 2009;38:87–93. doi: 10.1038/laban0309-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmeyer H.U., Horder M., Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1) J Clin Chem Clin Biochem. 1986;24:497–510. [PubMed] [Google Scholar]
- 18.Suzuki S., Toledo-Pereyra L.H., Rodriguez F.J., Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Jacques F., El-Hamamsy I., Fortier A. Acute renal failure following lung transplantation: risk factors, mortality, and long-term consequences. Eur J Cardiothorac Surg. 2012;41:193–199. doi: 10.1016/j.ejcts.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes H.M.P., Serigiolle L.C., Rodrigues D.A.B., Lopes C.M., Studart SdV., Leme P.L.S. Unfeasible experimental model of normothermic hepatic ischemia and reperfusion in rats using the pringle maneuver. Arquivos Brasileiros de Cirurgia Digestiva : ABCD = Braz Arch Dig Surg. 2014;27:196–200. doi: 10.1590/S0102-67202014000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato L.T., Poggetti R.S., Fontes B. Evaluation of the mortality rate caused by different periods of selective portal vein occlusion in rats. Acta Cir Bras. 2007;22:279–284. doi: 10.1590/s0102-86502007000400009. [DOI] [PubMed] [Google Scholar]
- 22.Simsek A., Yagci G., Zeybek N. Effects of portal triad occlusion on left-sided colonic anastomosis. Int Surg. 2002;87:25–30. [PubMed] [Google Scholar]
- 23.Man K., Fan S.T., Ng I.O. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533–539. doi: 10.1001/archsurg.134.5.533. [DOI] [PubMed] [Google Scholar]
- 24.Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ (Can Med Assoc J) 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamauchi H., Baca I., Mittmann U., Geisen H.P., Salzer M. Postischemic liver damage in rats: effect of some therapeutic interventions on survival rate. Tohoku J Exp Med. 1982;138:63–70. doi: 10.1620/tjem.138.63. [DOI] [PubMed] [Google Scholar]
- 26.Marta M.S., Amine Z.M., Susagna P.A. Activation of peroxisome proliferator-activated receptor-α inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia–reperfusion. Hepatology. 2008;47:461–472. doi: 10.1002/hep.21935. [DOI] [PubMed] [Google Scholar]
- 27.Olthof P.B., van Golen R.F., Meijer B. Warm ischemia time-dependent variation in liver damage, inflammation, and function in hepatic ischemia/reperfusion injury. Biochim Biophys Acta, Mol Basis Dis. 2017;1863:375–385. doi: 10.1016/j.bbadis.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Li C.-H., Chen Y.-W., Chen Y.-L. Preserving low perfusion during surgical liver blood inflow control prevents hepatic microcirculatory dysfunction and irreversible hepatocyte injury in rats. Sci Rep. 2015;5:14406. doi: 10.1038/srep14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall K.M., Laval M., Estacio O. Activation by zinc of the human gastrin gene promoter in colon cancer cells in vitro and in vivo. Metallomics. 2015;7:1390–1398. doi: 10.1039/c5mt00147a. [DOI] [PubMed] [Google Scholar]
- 30.Pavlica S., Gaunitz F., Gebhardt R. Comparative in vitro toxicity of seven zinc-salts towards neuronal PC12 cells. Toxicol In Vitro. 2009;23:653–659. doi: 10.1016/j.tiv.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Schaller B. Nova Science Publishers; New York: 2004. Cerebral Ischemic Tolerance : From Animal Models to Clinical Relevance. [Google Scholar]
- 32.Panteghini M. Aspartate aminotransferase isoenzymes. Clin Biochem. 1990;23:311–319. doi: 10.1016/0009-9120(90)80062-n. [DOI] [PubMed] [Google Scholar]
- 33.Haase H., Hebel S., Engelhardt G., Rink L. The biochemical effects of extracellular Zn(2+) and other metal ions are severely affected by their speciation in cell culture media. Metallomics. 2015;7:102–111. doi: 10.1039/c4mt00206g. [DOI] [PubMed] [Google Scholar]
- 34.Wetherell D., Baldwin G.S., Shulkes A., Bolton D., Ischia J., Patel O. Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of HIF1alpha. Oncotarget. 2018;9:8463–8477. doi: 10.18632/oncotarget.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bozym R.A., Chimienti F., Giblin L.J. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp Biol Med (Maywood) 2010;235:741–750. doi: 10.1258/ebm.2010.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali J.M., Davies S.E., Brais R.J. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transplant. 2015;21:487–499. doi: 10.1002/lt.24072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.