Abstract
Hemophilia A (HA) is an X-linked bleeding disorder due to deficiencies in coagulation factor VIII (FVIII). The major complication of current protein-based therapies is the development of neutralizing anti-FVIII antibodies, termed inhibitors, that block the hemostatic effect of therapeutic FVIII. Inhibitors develop in about 20–30% of people with severe HA, but the risk is dependent on the interaction between environmental and genetic factors, including the underlying F8 gene mutation. Recently, multiple clinical trials evaluating adeno-associated viral (AAV) vector liver-directed gene therapy for HA have reported promising results of therapeutically relevant to curative FVIII levels. The inclusion criteria for most trials prevented enrollment of subjects with a history of inhibitors. However, preclinical data from small and large animal models of HA with inhibitors suggests that liver-directed gene therapy can in fact eradicate pre-existing anti-FVIII antibodies, induce immune tolerance, and provide long-term therapeutic FVIII expression to prevent bleeding. Herein, we review the accumulating evidence that continuous uninterrupted expression of FVIII and other transgenes after liver-directed AAV gene therapy can bias the immune system toward immune tolerance induction, discuss the current understanding of the immunological mechanisms of this process, and outline questions that will need to be addressed to translate this strategy to clinical trials.
Keywords: hemophilia A, inhibitors, adeno-associated virus, gene therapy, anti-drug antibodies, immune tolerance
Introduction
Hemophilia A (HA) is an X-linked bleeding disorder due to inherited deficiency in coagulation factor VIII (FVIII) activity (1, 2). Until recently, treatment in the developed world involved the intravenous administration of FVIII concentrates to treat or prevent bleeding. Recently, a FVIII-mimetic that is delivered subcutaneously, emicizumab, has been approved as prophylactic treatment for HA to prevent bleeding (3–6). Emicizumab is the first approved non-factor therapy for HA, but several others are in clinical development (7, 8). Other novel treatments for HA in clinical studies include several gene therapy approaches (9–11).
The major complication of treatment with FVIII concentrates is the development of neutralizing anti-FVIII antibodies, termed inhibitors, which substantially increase the mortality and morbidity of HA (12–19). Inhibitors are clinically quantified in Bethesda Units (BU), where 1 BU neutralizes 50% of normal FVIII activity. High-titer inhibitors greater than 5 BU generally prevent administered FVIII from having a therapeutic effect (20). On a research basis, anti-FVIII antibodies can also be quantified by immunosorbent assays that measure both non-neutralizing and neutralizing antibodies. Clinically, decreased FVIII recovery (expected peak level after FVIII protein administration) and half-life are also used as markers for non-neutralizing antibodies and evidence of an anti-FVIII immune response even in the absence of measurable Bethesda titers. Bypassing agents, which circumvent the inhibitor to provide hemostasis, are required to treat or prevent bleeding in high-titer inhibitor patients (21, 22). Due to their mechanism of action, emicizumab and other non-factor therapies are also effective in the presence of high-titer inhibitors, though they are currently only studied to prevent bleeding (5).
Inhibitors develop in about 20–30% of patients with severe hemophilia A (<1% normal FVIII activity) and in about 10% in patients with non-severe hemophilia A (1–40% normal FVIII activity) (23, 24). The risk of inhibitor development for the former is highest during their initial FVIII exposure days (25), while it is relatively constant for the latter (23). Both genetic and environmental factors influence the risk of inhibitor development (25–29). A major genetic determinant of inhibitor risk is the underlying hemophilia-causing FVIII-gene (F8) mutation (28). Patients with F8 mutations resulting in the expression of some FVIII cross-reactive material (CRM), such as missense or small in-frame deletions or insertions, are less likely to develop inhibitors while CRM-negative patients with large F8 deletions are more likely to develop inhibitors (28). Environmental factors include the manufacturing process and type of factor product, timing of first factor exposure, factor dosage, and clinical situations that result in immunological “danger signals” (24–26, 29).
Treatment of HA patients with inhibitors includes the prevention and treatment of bleeds (20, 30) and, historically, eradication of the inhibitor via the immune tolerance induction (ITI) regimens (30–33). ITI is the frequent regular infusion of FVIII concentrates over extended period-of-time (often years) with a goal of sufficiently decreasing the inhibitor to allow for the use of therapeutic FVIII, as nothing provides as effective hemostasis as FVIII in the absence of an inhibitor (20). The frequency and the dose of FVIII in ITI remain debatable, but the dosing regimens of daily or every-other-day from the International ITI Study (33) are often used (20, 30). However, ITI is only successful in about 60% of patients (32, 33). The underlying mechanism is likely to be peripheral immune tolerance induction where the activity of anti-FVIII immune cells is suppressed through tolerogenic interactions in the periphery, rather than central immune tolerance where the anti-FVIII immune cells are eliminated prior to leaving either the thymus or bone marrow.
The recent advent of emicizumab, which provides significantly improved bleeding prophylaxis compared to other bypassing agents (5, 6), has raised the question of whether inhibitor eradication remains necessary in the management of inhibitor patients (34, 35). Though the clinical consensus to this question is still forming, many experts continue to recommend ITI for new inhibitors (36) given the ongoing concerns about thrombotic complications in inhibitor patients on emicizumab receiving high cumulative doses of the bypassing agent activated Prothrombin Complex Concentrates for break-through bleeding (5, 37, 38). Long-term follow up is needed to define the “real world” safety and efficacy of indefinite emicizumab compared standard ITI.
The limited success rate of current ITI approaches has driven the pre-clinical investigations of several novel ITI strategies (39), including gene therapy approaches (40). Multiple adeno-associated viral (AAV) vector gene therapies for HA without inhibitors are in clinical development, as summarized in Table 1 (9–11). These drugs all direct the therapeutic FVIII-gene to hepatocytes expression. Though the goal of these studies is to achieve durable therapeutically relevant FVIII levels, emerging preclinical data suggest the liver-directed gene therapy can utilize the liver tolerance effect (41) to induce immune tolerance to the transgene-product (40, 42, 43). Here we review the preclinical data supporting the hypothesis that AAV liver-directed gene therapy can induce immune tolerance to FVIII and present the open questions that need to be considered when translating this approach to clinical trials.
TABLE 1.
Current FVIII AAV liver-directed gene therapy products for HA in clinical development.
| Name | Sponsor | Vector serotype | Transgene‡ | Manu-facturing | Phase | FVIII range (% normal) | ClinicalTrials.gov Identifier |
| Valoctocogene | Biomarin | AAV5 | FVIII-SQ | Baculovirus/Insect cells | 3 | 19–164 | NCT03370913 |
| roxaparvovec (BMN-270) | NCT03392974 | ||||||
| SPK-8011 | Spark | LK03 | FVIII-SQ | Plasmid/Mammalian cells | 1/2 | <5–49 | NCT03003533 |
| SPK-8016 | Spark | NA | FVIII-SQ | Plasmid/Mammalian cells | 1/2 | NA | NCT03734588 |
| AAV2/8-HLP-FVIII-V3 | UCL | AAV8 | FVIII-V3 | Plasmid/Mammalian cells | 1/2 | 6–69 | NCT03001830 |
| SB-525 | Sangamo | AAV6 | FVIII-SQ | Baculovirus/Insect cells | 1/2 | 4–150 | NCT03061201 |
| SHP654 (BAX888) | Shire | AAV8 | FVIII-SQ | Plasmid/Mammalian cells | 1/2 | NA | NCT03370172 |
| BAY 2599023 (DTX201) | Bayer | AAVhu37 | FVIII-SQ | Plasmid/Mammalian cells | 1/2 | 5–17 | NCT03588299 |
‡See (69) and reference therein for details about FVIII variants used in gene therapy. NA, not available; UCL, University College of London.
The Liver Tolerance Effect in Liver-Directed Gene Therapy
The Liver Tolerance Effect
The liver is a tolerogenic organ (41, 42, 44–46). Its specialized immune system limits immune reactivity against the constant flux of digested food-products as well as antigens from commensal microorganisms, while simultaneously safeguarding against gastrointestinal pathogens. From a therapeutic perspective, the liver tolerance effect was first recognized in studies of an outbred porcine liver transplant model where some allografts achieved long-term survival without immunosuppression (47); similar results were subsequently reported in other in vivo transplant models (48, 49). Moreover in animal studies, liver allotransplants also promote the immunological tolerance to other organ allografts from the same donor (47), and tolerance to renal and small bowel transplants is enhanced if the venous blood drainage of the grafts is through the portal system (50). Clinically, immunosuppression can be safely withdrawn in about 20% of liver-transplant patients (51), which is not achievable in other solid organ transplant patients.
The liver tolerance effect is also exploited by hepatotropic pathogens (44–46, 52). The Plasmodium species responsible for malaria initially target the liver after being delivered by an infected mosquito and then mature and replicate sheltered within hepatocytes before being released back into the blood stream. Malaria remains one of the most deadly human pathogens responsible for millions of annual deaths and has resisted effective vaccination strategies to date. Likewise, the protolerogenic environment of the liver likely impedes an effective adaptive anti-viral response in chronic infections by hepatitis B and hepatitis C virus (HCV) (52). Viral hepatitis remains a major source of morbidity and mortality especially in the developing world and in people with hemophilia (53, 54).
The Liver Tolerance Effect in Liver-Directed Gene Therapy for Hemophilia B
The liver tolerance effect can also be exploited therapeutically by liver-directed gene therapy to induce immune tolerance to the transgene product (40, 42, 43). This was first demonstrated in HB mice that were tolerized to human (h) factor IX (FIX) after AAV gene therapy (55), but has subsequently been demonstrated in several other disease models including HA, which is discussed in detail below. In naïve HB mice, administration of liver-directed AAV (55) or lentiviral (LV) (56) vectors encoding hFIX induces immune tolerance that is resistant to subsequent hFIX immunizations. Interestingly, induction of immune tolerance with LV vectors requires the use of genome regulatory elements that prevented transgene expression in immune cells (56), suggesting that the tolerogenic bias of liver-directed gene therapy can be overwhelmed by non-liver transgene expression. In addition to induction of immune tolerance in naïve HB mice, both liver-directed AAV and LV hFIX gene therapy can also eradicate preexisting anti-hFIX antibodies and induce immune tolerance in hFIX-immunized HB mice (57, 58).
Similar outcomes have also been observed in canine models of severe HB. Colonies of naturally occurring HB dogs with distinct F9 mutations provide highly informative models for evaluating immune responses to therapeutic canine (c) FIX. HB dogs with a F9 null mutation are inhibitor-prone and typically develop an inhibitor after a single administration of cFIX concentrate (59), while HB dogs with a F9 missense mutation are non-inhibitor-prone and very rarely develop inhibitors against cFIX, usually only in the context of a pro-inflammatory stimulus (60, 61). The use of cFIX protein, canine plasma, and cFIX encoding vectors allows the immune response to be interrogated in a species-specific manner.
No evidence of an anti-cFIX immune response has been reported after AAV (59, 62–65) or LV (66) liver-directed gene transfer with cFIX in 11 and 3 non-inhibitor-prone HB dogs, respectively, though only 1 dog has been subsequently challenged with canine plasma for bleeding (64). Moreover, AAV liver-directed gene therapy with cFIX successfully tolerized 5 out 6 naïve inhibitor-prone dogs that typically form inhibitors after cFIX protein exposure (59, 67). In this study, the immune tolerance was demonstrated to be maintained despite subsequent cFIX protein exposures in all 4 dogs evaluated, while the 5th animal was not challenged (67, 68). The singe naïve HB dog that developed an inhibitor notably had a hemolytic anemia associated with liver iron-loading and consequentially liver-fibrosis, both of which were seen on liver histology at necropsy (59). The course of this single naïve inhibitor-prone dog that was not tolerized after AAV liver-directed gene therapy with cFIX may be the exception-that-proves-the-rule as his unrelated liver pathology likely disrupted the liver tolerance effect.
Liver-directed AAV gene therapy has also eradicated preexisting anti-cFIX antibodies and induced immune tolerance in an inhibitor-prone HB dog (Wiley) (67). In this study, an inhibitor-prone HB dog was previously exposed to hFIX and developed an anti-hFIX response with cross-reactive neutralization of cFIX activity. AAV liver-directed gene transfer with the hyperactive cFIX variant, Padua (69, 70), resulted in eradication of the anti-cFIX antibodies and disappearance of the anti-hFIX neutralization within 3 months of vector administration, though a minimal residual non-neutralizing anti-hFIX response remained detectable years after vector administration (67). The persistence of non-neutralizing anti-hFIX antibodies suggests that the immune tolerance induced after liver-directed AAV gene therapy is transgene specific. Nonetheless, this dog remained tolerant to cFIX even after exposure to cFIX-WT with over 3 years of ongoing observations (VRA, unpublished data).
Immune Mechanisms of the Liver Tolerance Effect
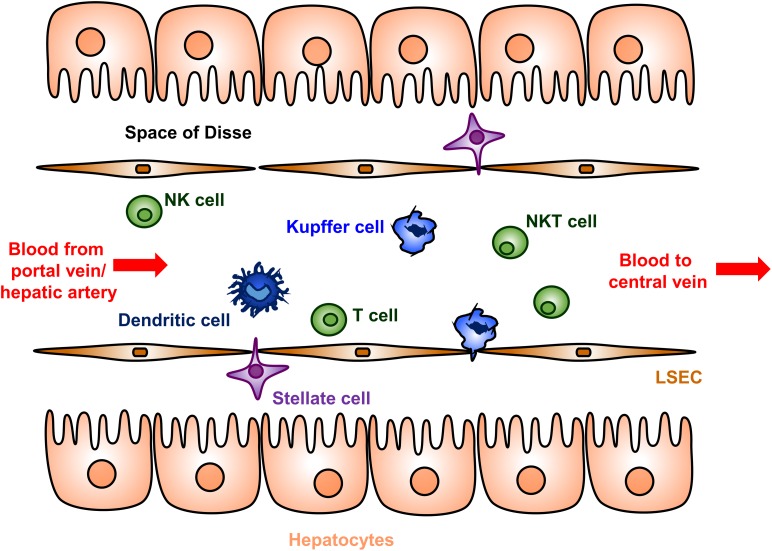
Several complementary immunological mechanisms underlying the liver tolerance effect have been described [reviewed in detail in (41, 42, 44, 45, 71–73)]. The liver microenvironment contains unique populations of both antigen presenting cells and suppressor and effector T cells (Figure 1). Hepatic antigen presenting cells include conventional types such as dendritic cells, but also unique non-conventional antigen presenting cells that include liver sinusoidal endothelial cells (LSECs), Kupffer cells, stellate cells, and hepatocytes. Presentation of antigens by these specialized hepatic antigen presenting cells typically results in diminished effector T cell activity and/or increased suppressor activity of regulator T cells (Tregs), both of which promote immune tolerance.
FIGURE 1.
Cellular anatomy of the liver sinusoid. Blood enters the liver from the portal vein and hepatic artery, flows through a network of sinusoids schematically represented here, and then exits via the hepatic central vein. The sinusoids are lined by a fenestrated layer of specialized liver sinusoidal endothelial cells (LSECs), which are the endogenous site of most FVIII secretion. The LSECs shield the hepatocytes from direct sinusoidal blood flow by creating the Space of Disse, which contains the stellate cells. Dendritic cells, Kupffer cells, T cells, natural killer (NK) cells, and natural killer T (NKT) cells are abundantly present in the sinusoidal lumen. Hepatic antigen presenting cells include dendritic cells, Kupffer cells, LSECs, stellate cells, and hepatocytes. The hepatocyte microvilli can interact with luminal T cells.
Interactions between these hepatic antigen presenting cells and effector T cells often results in functional inhibition or cell death of the effector T cell. Low-expression of major histocompatibility complex (MHC) and co-stimulatory molecules on several types of hepatic antigen presenting cells contributes to abortive activation and result in T cell anergy in an antigen-specific manner (42, 73, 74). The absent production of the costimulatory cytokine IL-12 by Kupffer and dendritic cells also contributes to this process. Additionally, hepatic antigen presenting cells actively express a number of molecules that suppress effector T cell activity. Both Kupffer cells and hepatic dendritic cells produce indoleamine 2,3 dioxygenase (IDO) and prostaglandin E2, which inhibit T cell proliferation through distinct pathways (42, 74). Dendritic cells, LSECs and AAV transduced hepatocytes express Fas-L (CD95L), which allows for the direct deletion of effector T cells (42).
The liver microenvironment also promotes Treg activation and proliferation. Abundant Tregs are noted in liver allografts in mouse models and their depletion result in a loss of tolerance (75). Secretion of the suppressor cytokine IL-10 by LSECs, Kupffer cells, and hepatic dendritic cells promote Treg proliferation as well as conversion of effector T cells to Treg (42, 74). The decrease in the inhibitor titer after AAV gene therapy in the HB dog with a pre-existing inhibitor (Wiley) was associated the increasing IL-10 levels (67). Hepatocyte production of transforming growth factor-β similarly promotes Treg proliferation (76).
Immune Tolerance Induction After Liver-Directed Gene Therapy in Preclinical Hemophilia a Models
Immune Tolerance Induction in Naïve Hemophilia A Animal Models
Immunocompetent HA mice generally develop a xenoprotein immune response against hFVIII or cFVIII after exposures via protein administration or gene therapy (77, 78), and typically not against mouse (m) FVIII (79, 80). As such, preclinical efficacy studies of non-murine FVIII gene therapy have typically utilized immunosuppression or HA mice crossed with an immunodeficient model (81, 82).
However, AAV-liver directed gene therapy with hFVIII has been demonstrated to be able to induce immune tolerance that is resistant to subsequent protein challenges in HA mice (83). The ability of this approach to induce tolerance to hFVIII was dependent on both the HA strain background and the expressed FVIII level (83, 84). Immune tolerance to hFVIII in HA mice increased with higher hFVIII levels after AAV gene therapy, achieved either through codon optimization (83) or higher vector doses (84), though the controls were likely at sub-therapeutic hFVIII levels (<1% normal).
In contrast to the murine model, outbred severe HA dogs predictably develop inhibitors against cFVIII (85–88). These dogs have a cF8 mutation analogous to the human F8 intron-22 inversion, the most common causative severe HA mutation in patients (89, 90). Intriguingly, the risk of inhibitor development in these HA dogs is in part inherited through yet undefined non-cF8 genes as the propensity of inhibitor development is increased in the progeny of certain outside breeders (86, 88). About 30% of these inhibitor-prone HA dogs, either at the colony at Queens University (QU) or University of North Carolina at Chapel Hill (UNC-CH) colony, develop anti-cFVIII inhibitors (86, 91).
None of the 15 non-inhibitor-prone HA dogs that have received cFVIII AAV-liver-directed gene therapy without immunosuppression have made an anti-cFVIII immune response (92–94). At least 6 of these animals were subsequently challenged with recombinant cFVIII protein (25 U/kg weekly for 4 weeks) 1–5 years after vector administration and did not display an anti-FVIII immune response. Recovery of the administered cFVIII protein was similar to that of naïve dogs, which confirmed complete tolerance to cFVIII (94).
However, 2 out of 9 inhibitor-prone HA dogs developed an inhibitor after cFVIII AAV-liver-directed gene therapy (91, 94, 95). A QU-housed HA dog Junior developed an inhibitor 2 weeks after administration of an AAV2 vector via the portal-vein with a peak titer of 9 BU at 4 weeks that became undetectable by week 9 (91). Similarly, an UNC-CH inhibitor-prone HA dog L51 developed an inhibitor within a week of intravenous administration of an AAV8 vector that peaked at 2.5 BU and became undetectable by week 7 (94). Notably, the eradication of L51’s inhibitor was stringently demonstrated to be immune tolerance by the lack of recurrent anti-cFVIII antibodies and expected recovery of challenges of recombinant cFVIII protein (25 U/kg weekly for 4 weeks) (94). Though limited by the small numbers, the experience of Junior and L51 suggest that AAV-liver-directed gene therapy can induce stringent immune tolerance to transgene-FVIII in large outbred models of HA.
However, the liver tolerance effect after liver-directed gene therapy in HA dogs can be disrupted in the setting of hepatotoxicity (96), similar to what was discussed above for HB dog models (59). In an early study using an adenoviral vector (rather than AAV), all 4 QU HA dogs that received liver-directed cFVIII gene therapy developed inhibitors in the setting of an early (0–4 weeks), presumably adenovirus-induced, hepatotoxicity manifested by over a 20-fold increase in liver enzymes (96). Both the increase in liver enzymes and the inhibitor titer appeared to be vector dose dependent with the 2 dogs that received the lower vector dose only developing low titer inhibitors (peak < 2 BU) that disappeared within 4 weeks; there was also no recurrence of the inhibitor after administration of canine FVIII cryoprecipitate (20 U/kg) in these 2 low-dose dogs (96). Though this study was conducted with a vector that is no longer translationally relevant for HA, the observations about the immune response in setting of acute hepatotoxicity may be germane for other gene therapy approaches.
Consistent with this hypothesis that hepatotoxicity can interfere with the pro-tolerogenic effects of liver gene therapy is the subsequent observation that none of the 3 the QU HA dogs that received liver-directed cFVIII gene therapy with a less toxic helper-dependent adenovirus vector concomitantly with steroid immunosuppression developed an anti-cFVIII immune response (97). A fourth dog in this study received cFVIII gene therapy under-the-control of a ubiquitous CMV promoter and did develop a high-titer inhibitor (peak 150 BU 1 week after vector administration) (97). This observation suggests that non-liver expressed cFVIII can potentially interfere with immune tolerance from liver-expressed transgene and is consistent with the observations from HB (56) and Pompe (98, 99) disease models.
The cumulative data from both small and large animal HA models indicates that liver-directed gene therapy, especially with AAV vectors that have minimal hepatoxicity in pre-clinical models, can induce immune tolerance in naïve animals to FVIII. Moreover, if anti-FVIII antibodies develop, the continued transgene expressed FVIII can eradicate the inhibitors and, in a limited number of examples, induce immune tolerance that is resistant to subsequent protein challenges.
Inhibitor Eradication and Immune Tolerance Induction in Hemophilia A Inhibitor Models With Pre-existing Anti-FVIII Immune Response
The likely more challenging and clinically relevant scenario is the eradication of a pre-existing anti-FVIII immune response. We have previously reported on the successful immune tolerance induction in 4 inhibitor-prone HA dogs with pre-existing anti-cFVIII antibodies after AAV liver-directed gene therapy (Table 2) (100). In this study, 3 HA dogs from UNC-CH with peak historical inhibitor titers from 4 to 12 BU received cFVIII AAV8 liver-directed gene therapy. Notably, the anti-FVIII IgG and inhibiter titers in all 3 dogs disappeared by 5 weeks after vector administration (100).
TABLE 2.
Summary of inhibitor eradication in hemophilia A dogs following cFVIII AAV liver-directed gene therapy.
|
Pre-gene therapy |
Titer at vector (BU) |
Post-gene therapy |
||||||
| Dog | Age (year) | Weight (kg) | Inhibitor duration (wks) | Peak titer (BU) | Peak titer (BU) | Time to eradication (wks) | cFVIII activity (%)‡ | |
| K01 | 1.7 | 20.1 | 32 | 12 | 3 | 7 | 5 | 1.5 |
| K03 | 1 | 19.3 | 28 | 12 | 3 | 3 | 4 | 8.0 |
| L44 | 0.7 | 16.0 | 28 | 4.5 | 2.2 | 2.2 | 4 | 1.5 |
| Wembley | 4.9 | 16.5 | 96 | 3.6 | 3.5 | 216 | 80 | >1.5† |
Data from (100). †VRA, unpublished observation. ‡Plateau level after inhibitor eradication.
The fourth inhibitor-prone HA dog (Wembley from QU) with pre-existing anti-cFVIII antibodies treated similarly with cFVIII AAV8. At the time of vector administration, his cFVIII-inhibitor was 4 BU, which was similar to his peak titer. After vector administration, Wembley also had a large anamnestic response with dramatic increases in his anti-cFVIII IgG and inhibiter titer that peaked at 216 BU. However, both these parameters decreased until they became undetectable at about 80 weeks after vector administration (VRA, unpublished observation).
The post-gene therapy cFVIII activity after inhibitor eradications was between 1.5 and 8% of normal in these 4 dogs (100) (and VRA, unpublished observation). The immune tolerance of all 4 of these dogs was maintained despite multiple challenges with recombinant cFVIII protein that displayed typical pharmacokinetics in an ongoing study (100) (and VRA, unpublished observation). Though Wembley was tolerized to cFVIII after cFVIII AAV liver-directed gene therapy, he never fully tolerized to hFVIII, his triggering antigen (VRA, unpublished observation). Similar to the above discussion for the HB dog models (67), Wembley’s course suggests that the immune tolerance of AAV liver-directed gene therapy is transgene specific. To date, cFVIII inhibitors have been eradicated in 6 HA dogs (2 dogs naïve discussed in section “Immune Tolerance Induction in Naïve Hemophilia A Animal Models” and 4 dogs with pre-existing inhibitors discussed in section “Inhibitor Eradication and Immune Tolerance Induction in Hemophilia A Inhibitor Models With Pre-existing Anti-FVIII Immune Response”) by cFVIII AAV liver-directed gene therapy, which also provided stringent immune tolerance and long-term therapeutically relevant cFVIII levels. This limited data suggests that AAV liver-directed gene therapy has translational potential for people with HA and inhibitors.
Translational and Clinical Considerations
Overview of Current Clinical Development of Liver-Directed Gene Therapy for Hemophilia A
It is an exciting time for gene therapy for HA with multiple liver-directed AAV-based products in clinical trials reporting therapeutically relevant to curative levels of FVIII (Table 1) (9–11). These current products are the result of decades of experimentation developing the necessary technologies and protocols to achieve these end points (101–104). These AAV drugs differ in their vector serotype, FVIII transgene, and manufacturing process. A variety of naturally occurring and bioengineered vector serotypes are being tested (105). Because the full-length FVIII gene exceeds the ∼4.7 kb packaging capacity of AAV vectors, all current approaches rely on B-domain deleted FVIII variants, either the standard FVIII-SQ (106) as is used in B-domain deleted protein products (e.g., Xyntha and Pfizer) or an engineered B-domain deleted variant (FVIII-V3) that is associated with increased FVIII expression (107). All transgenes have been codon-optimized, though likely by distinct algorithms.
To date, all trials stringently limit subjects at risk for inhibitor development by including only subjects with no history of inhibitors and more than 150 FVIII exposures, as inhibitors rarely develop after 50 exposure days in severe HA (25). However, a current study (NCT03734588) enrolling subjects meeting these inclusion criteria is described as a dose-finding Part 1 of a planned two part clinical development strategy focused on inhibitor patients.
No major sustained safety concerns have been reported in these studies, though several immunological obstacles to the AAV vector capsid continue to limit efficacy and widespread enrollment (108, 109). The long-term durability also remains an open question with trials reporting therapeutically relevant FVIII levels out to 3 years after vector administration, albeit with declining levels (110). Nonetheless, the success of these studies raises the question whether these drugs or similar products could be harnessed to induce immune tolerance in HA inhibitor patients by exploiting the liver tolerance effects (41, 42, 44–46).
There are several obstacles that need to be address to define the role of AAV liver gene therapy for HA complicated by the presence of inhibitors to FVIII. The experience with preclinical HA inhibitor models, especially the canine data, support the potential translation of AAV liver-directed gene therapy for people with HA and inhibitors. However, therapeutic translation of this approach will require several yet unresolved issues to be considered and specific preclinical studies designed to address unanswered questions (Table 3). Preclinical studies using the immunocompetent HA dog models that naturally develop inhibitors when exposed to cFIII in a species-specific manner will likely be the most informative.
TABLE 3.
Translational considerations of AAV liver gene therapy for immune tolerance induction.
| Goals | Potential solutions | |
| Hepatotoxicity: transient increase in liver enzymes | Limit AAV-capsid mediated cellular response or AAV-associated transient transaminitis of unknown origin | Lowering the vector dose and/or immunosuppression |
| Immunosuppression regimens | To prevent or to control ongoing liver toxicity and/or loss of transgene expression | Transient oral steroid, mycophenolate mofetil, tracrolimus, rapamycin (alone or in combination)* Avoid intense immunosuppression at the time of AAV administration |
| Expression of the transgene outside the liver | Optimize transgene expression Avoid inadvertent non-hepatocyte tissue with increased risk of immune response | Use of promoter and regulatory elements highly active in hepatocyte |
| Optimized FVIII function by developing variants of the transgene | Lowering the therapeutic vector dose with increased biological activity without detrimental immunogenicity | Systematic screening for FVIII variants and testing in both in vitro and in vivo studies |
*Partial list of immunosuppressive drugs evaluated in AAV liver gene therapy [reviewed in (136)].
Hepatotoxicity and Immunosuppression
Foremost, is the potential detrimental role of hepatotoxicity in successful immune tolerance induction. As discussed above, preclinical studies suggest that the tolerogenic bias of liver-directed gene therapy can be disrupted in the setting of hepatotoxicity. The hepatotoxicity in these disparate canine studies was secondary to either the specific vector employed (96) or underlying liver disease in the dog model studied (59). There are several liver pathologies that occur in HA patients receiving AAV liver-directed gene therapy that may impact the liver tolerance effect.
Though rates of viral iatrogenic infections including HCV have thankfully plummeted with improved blood donor screening, highly effective virucidal procedures, and the use of recombinant FVIII products, about a third of young men with severe HA have a history of HCV infection, while the rate in older men exceeds 90% (53). Historically, about 20–30% of HCV infected patients eventually developed end-stage liver disease, though the recent approval of highly effective oral anti-viral regimens will likely radically reverse this trajectory (54). Asymptomatic liver damage from chronic HCV infection theoretically could impair the liver-tolerance effect after AAV liver-directed gene therapy. Current AAV liver-directed gene therapy clinical trials exclude patients with active HCV or clinical evidence of liver disease. Moreover, there were no appreciable differences in the clinical course of 3 out of 9 published HA subjects that received AAV5-FVIII (valoctocogene roxaparvovec, BMN 270) that had a history of resolved HCV infection (111); similarly, there has been no appreciable difference in the clinical course of HB subjects with a history of HCV receiving AAV FIX gene therapy (112–114). Other liver diseases that occur in the HA population such as non-alcoholic fatty liver disease pose the same theoretical risk of potentially disrupting the liver tolerance effect. Preclinical studies may help inform on this concern, but stringent liver disease exclusion criteria will probably be advisable in initial clinical studies with inhibitor patients.
To date, some subjects in all AAV liver-directed gene therapy trials for HA or HB have demonstrated hepatotoxicity after vector administration as evidenced by asymptomatic elevated liver enzymes [reviewed in (7, 104)]. At least 2 distinct mechanisms likely contribute to these observations. In products utilizing an AAV2, AAV8, or similar serotype vectors manufactured in mammalian cells, a well described anti-AAV capsid cellular immune response can target transduced hepatocytes leading to loss of transgene expression, which can often, though not always, be controlled with immunosuppression with steroids (108, 109, 112–116). In products utilizing AAV5 vectors manufactured in insect cells, elevated liver enzymes have also been reported, but do not appear to be associated with loss of transgene or evidence of cellular immunity (104, 111, 117); the etiology of this latter hepatotoxicity is still being investigated. Studies of both these hepatotoxicities have been hampered by lack of preclinical models that fully recapitulate the clinical observations (118–122). The potential adverse role either of these hepatotoxicities could have on inducing immune tolerance after AAV liver-directed gene therapy is unknown. However, as both hepatoxicities appear to be vector-dose dependent, avoiding them by using the lowest effective vector dose is likely sensible. The potential immunological consequence of lowering the transgene FVIII level is discussed below in section “FVIII Level and Variant Transgenes.”
The use of immunosuppression in AAV liver-directed gene therapy for HA and inhibitors will require specific preclinical studies. In HA dogs with pre-existing inhibitors, there is an early increase in CD25 + FOXP3 + CD4 + cells, assumed to be Treg cells, within the first few days after cFVIII AAV liver-directed gene therapy that is associated with the decline in anti-cFVIII antibodies that is not observed in HA dogs without inhibitors receiving similar therapy (40, 100). However, intense immunosuppression with the anti-CD25 antibody daclizumab around vector delivery increases the anti-hFIX immune response in non-human primates (NHP) receiving hFIX AAV liver-directed gene therapy and is associated with a decrease in Treg cells (123). The rationale of this study was that daclizimab could potentially decrease effector T cell activity to limit the anti-AAV capsid immune response; however, daclizumab also depleted Treg cells leading to an unanticipated increase immunity against the transgene hFIX.
These findings are not restricted to anti-CD25 antibodies. More recently we uncovered that rabbit antitymoglobulin (ATG) may also be detrimental if administered around vector delivery (BSJ and VRA, unpublished data, manuscript in preparation). Combined, this limited data does suggest that there is an early critical time-period around vector administration where Treg cell expansion may be important for immune tolerance induction. As such, immunosuppression around the time of AAV vector delivery needs to be thoughtfully considered. Reassuringly, steroids promote antigen-specific immune tolerance HA mice against concomitantly administered hFVIII protein, likely through Treg-dependent mechanisms (124). However, more intense immunosuppression therapy needs to be stringently tested in large animal models to avoid unanticipated increased immunogenicity. Ongoing mechanistic studies evaluating cFVIII AAV gene therapy in HA dogs with pre-existing inhibitors may better inform on this issue.
There is also a concern about the potential for liver toxicity due to ectopic expression of FVIII in hepatocytes after gene therapy due to specific features of FVIII secretion (125–128). However, though 2 studies in HA mice did find evidence of the unfolded protein response (UPR) after AAV liver-directed gene therapy, there was no correlation between FVIII inhibitor formation and UPR markers (126, 127). Ectopic expression of human FVIII in mouse megakaryocytes is also associated with megakaryocyte apoptosis (129). However, the theoretical concern of UPR-induced hepatotoxicity further supports the initial use of the lowest effective vector dose.
Non-liver Transgene Expression
Preclinical data in HA (97) and other disease models (56, 98, 99) highlight that non-liver expression of the transgene can disrupt the pro-tolerogenic effects of liver expression. Most preclinical and clinical studies for AAV liver-directed gene therapy have utilized liver-specific promoters based on the seminal work by Dr. Kathy Ponder (130); the prototypical expression cassette contains sequences from the truncated apolipoprotein E (ApoE) hepatic control region (HCR) and the alpha-1-anti-trypsin (A1AT) promoter. Though this construct mostly limits non-liver transgene expression, we are not aware of studies that have rigorously quantified the non-liver transgene expression in a preclinical model. The non-liver expression of a particular AAV product is a function of both the transduction efficiency of the vector to other tissues and the promoter efficiency in these tissues. Recent work using a new, highly sensitive experimental system demonstrated much broader AAV transduction as well as low level and/or transient transgene expression than previously appreciated (131). This study highlights the importance of preclinical studies in immune competent animal models with preexisting inhibitors to FVIII to specifically evaluate the efficacy of immune tolerance induction of a particular vector before moving into clinical studies.
FVIII Level and Variant Transgenes
Studies in both HA (83, 84) and HB (55) mice have concluded that increasing transgene levels after AAV liver-directed gene therapy promote immune tolerance. In non-severe HA patients, increasing FVIII levels are associated with decreased bleeding frequency (132). However, as discussed above, these potential benefits should be weighed against complications that also likely increase with increasing vector dose that potentially could interfere with the liver tolerance effect, including AAV-associated hepatotoxicity and UPR in transduced hepatocytes.
By definition, antigen expression is required for immune tolerance induction. In the randomized International-ITI trial, higher and more frequent FVIII dosing was associated with a more rapid immune tolerance induction, but the lower and less frequent dose demonstrated similar overall success rate (33). The sustained cFVIII levels of the 6 HA dogs described above that had inhibitor eradication and immune tolerance induction with cFVIII AAV liver gene therapy ranged from 0.5 to 8% of normal (91, 94, 100). This canine data suggest that transgene FVIII levels in the low-mild range is sufficient for immune tolerance induction.
The use of variant FVIII transgene also needs to be carefully considered. Studies in HA and HB dogs suggest that the immune tolerance after AAV liver-directed gene therapy is transgene specific; gene therapy with cFVIII or cFIX transgenes resulted in immune tolerance toward the canine orthologs, while the anti-hFVIII or anti-FIX response persisted (67, 100). However, in this HB dog study, the transgene was the single-amino acid substituted hyperactive variant cFVIII-Padua (R338L) (69, 70) and the animal was also fully tolerized to wild-type (WT) cFIX as evidenced by the lack of neutralizing or non-neutralizing anti-cFIX-WT antibodies despite protein challenges with cFIX-WT (67) (and VRA, unpublished observation). This suggests that though the immune tolerance is transgene specific, there can be cross-tolerance between highly similar transgenes, though the degree of similarity required is undefined. The degree of similarity between variant FVIII transgenes (69) and available protein FVIII products, therefore, should be considered when designing gene therapy products for inhibitor patients.
The concern is that if the inhibitor patient tolerized only to a variant-FVIII transgene that was immunological distinct from available FVIII protein products, these FVIII protein products could be ineffective if needed for breakthrough bleeding or surgery. This would likely be salient for novel FVIII variants with multiple modifications outside the FVIII B-domain, which are likely more immunogenic than modifications restricted to the B-domain; all current FVIII transgenes in Table 1 differ only in their B-domain replacement linker (69). If a patient is tolerized to a B-domain deleted FVIII transgene after gene therapy, the clinical decision making about using alternative FVIII protein products such as extended-half-life products would be similar to switching products after ITI with FVIII protein. Preclinical studies in inhibitor dogs using the canine orthologs of FVIII variants could evaluate this scenario for specific FVIII variants, as we did for the FIX-Padua (67, 133, 134).
Non-factor Replacement for Hemostatic Prophylaxis and AAV Gene Therapy
Emicizumab and other non-factor therapies offer the potential for better and more convenient hemostatic prophylaxis for HA patients with inhibitors (3–6), including those undergoing ITI (135). Though the experience with emicizumab and FVIII protein ITI is currently limited (135), we would anticipate that a combination of a non-factor therapy hemostatic prophylaxis and AAV FVIII gene therapy ITI would be similarly attractive. This approach would have the advantage of limiting bleeds and thus exposure to non-transgene expressed FVIII or bypassing agents. Furthermore, the use of emicizumab would allow for both inhibitor titer and transgene FVIII levels to be closely monitored.
Conclusion
Several AAV liver-directed gene therapy products for HA are moving through the clinical development pipeline (Table 1). To date, most clinical trials have mitigated the risk of inhibitor development by selecting only those subjects with heavy exposures to FVIII protein. Preclinical studies in a limited number of HA dogs (91, 94, 100) and other preclinical disease models (40, 42, 43) support the concept that AAV liver-directed gene therapy can harness the unique pro-tolerogenic properties of the liver, termed the liver tolerance effect (41), to induce peripheral immune tolerance to transgene FVIII. The potential advantages of our proposed AAV-mediated approach compared to standard ITI are summarized in Table 4. The ideal AAV FVIII vector for gene therapy ITI could be dosed low enough to avoid hepatotoxicities while still providing long-term hemostatic FVIII levels that, at a minimum, compare favorably to the hemostatic effect of emicizumab. Any planned immunosuppression must be evaluated in an immunocompetent animal model to determine if the specific regimen would negatively interfere with the immune tolerance induction to FVIII. This therapy would have dual benefits of eradicating the anti-FVIII antibodies while also providing continuous therapeutically relevant FVIII.
TABLE 4.
Comparison of AAV-liver gene therapy versus protein-based FVIII for immune tolerance induction (ITI).
| AAV-mediated | Protein-based | |
| Administration/frequency | IV/single injection | IV/3–7 days per week for years |
| Central vein catheterization | Not needed | Usually |
| Target population | Older children (>13 yo), likely similar to adult liver | Any age |
| Eligibility | No or low neutralizing antibodies titers to the vector capsid | All patients |
| Compliance | 100% | <80% |
| Prophylaxis after inhibitor eradication | Endogenous expression of FVIII | FVIII replacement 2–3 times/week, indefinitely |
| Reversible in the event of allergic/anaphylaxis | No | Yes |
| Immunosuppression | May be needed to prevent/overcome cellular responses triggered by the vector capsid | Only in cases that failed multiple ITI attempts |
| Long-term complications | Potential insertional mutagenesis | Not applicable |
| Rates of success | No clinical data available. Preclinical studies in canine models showed high rates | 60% in patients of good risk factors |
| Economic burden | High | High |
| Post ITI: maintenance of immune tolerance | None | FVIII protein 2–3 times/week |
Author Contributions
BS-J and VA conceived, drafted, and reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Heart, Lung, and Blood Institute (1K08HL140078 to BS-J, R01HL137335, and 1U54HL142012 to VA).
References
- 1.Peyvandi F, Garagiola I, Young G.The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. (2016) 388:187–97. 10.1016/S0140-6736(15)01123-X [DOI] [PubMed] [Google Scholar]
- 2.Konkle BA, Huston H, Nakaya Fletcher S. Hemophilia A: GeneReviews((R)). Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. editors Seattle, WA: University of Washington; (1993–2020). [Google Scholar]
- 3.Yada K, Nogami K.Spotlight on emicizumab in the management of hemophilia A: patient selection and special considerations. J Blood Med. (2019) 10:171–81. 10.2147/JBM.S175952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahlangu J, Oldenburg J, Paz-Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. (2018) 379:811–22. 10.1056/NEJMoa1803550 [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. (2017) 377:809–18. 10.1056/NEJMoa1703068 [DOI] [PubMed] [Google Scholar]
- 6.Young G, Liesner R, Chang T, Sidonio R, Oldenburg J, Jiménez-Yuste V, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. (2019) 134:2127–38. 10.1182/blood.2019001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arruda VR, Doshi BS, Samelson-Jones BJ.Novel approaches to hemophilia therapy: successes and challenges. Blood. (2017) 130:2251–6. 10.1182/blood-2017-08-742312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan MU, Sidonio R, Pipe SW.Novel therapeutics for hemophilia and other bleeding disorders. Blood. (2018) 132:23–30. 10.1182/blood-2017-09-743385 [DOI] [PubMed] [Google Scholar]
- 9.Peyvandi F, Garagiola I.Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia. Haemophilia. (2019) 25:738–46. 10.1111/hae.13816 [DOI] [PubMed] [Google Scholar]
- 10.Perrin GQ, Herzog RW, Markusic DM.Update on clinical gene therapy for hemophilia. Blood. (2019) 133:407–14. 10.1182/blood-2018-07-82072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollomp KL, Doshi BS, Arruda VR.Gene therapy for hemophilia: progress to date and challenges moving forward. Transfus Apher Sci. (2019) 58:602–12. 10.1016/j.transci.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 12.Walsh CE, Soucie JM, Miller CH. United States Hemophilia Treatment Center Network Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. (2015) 90:400–5. 10.1002/ajh.23957 [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt CL, Loomans JI, van Velzen AS, Peters M, Mauser-Bunschoten EP, Schwaab R, et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost. (2015) 13:1217–25. 10.1111/jth.12990 [DOI] [PubMed] [Google Scholar]
- 14.Darby SC, Keeling DM, Spooner RJ, Wan Kan S, Giangrande PL, Collins PW, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977-99. J Thromb Haemost. (2004) 2:1047–54. [DOI] [PubMed] [Google Scholar]
- 15.Donfield SM, Lynn HS, Lail AE, Hoots WK, Berntorp E, Gomperts ED, et al. Delays in maturation among adolescents with hemophilia and a history of inhibitors. Blood. (2007) 110:3656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witmer C, Presley R, Kulkarni R, Soucie JM, Manno CS, Raffini L.Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. (2011) 152:211–6. 10.1111/j.1365-2141.2010.08469.x [DOI] [PubMed] [Google Scholar]
- 17.Hoots WK.Arthropathy in inhibitor patients: differences in the joint status. Semin Hematol. (2008) 45(2 Suppl. 1):S42–9. 10.1053/j.seminhematol.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM.Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. (2012) 18:268–75. 10.1111/j.1365-2516.2011.02692.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindvall K, von Mackensen S, Elmståhl S, Khair K, Stain AM, Ljung R, et al. Increased burden on caregivers of having a child with haemophilia complicated by inhibitors. Pediatr Blood Cancer. (2014) 61:706–11. 10.1002/pbc.24856 [DOI] [PubMed] [Google Scholar]
- 20.Kempton CL, Meeks SL.Toward optimal therapy for inhibitors in hemophilia. Blood. (2014) 124:3365–72. 10.1182/blood-2014-05-577643 [DOI] [PubMed] [Google Scholar]
- 21.Leissinger C, Gringeri A, Antmen B, Berntorp E, Biasoli C, Carpenter S, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med. (2011) 365:1684–92. 10.1056/NEJMoa1104435 [DOI] [PubMed] [Google Scholar]
- 22.Konkle BA, Ebbesen LS, Erhardtsen E, Bianco RP, Lissitchkov T, Rusen L, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. (2007) 5:1904–13. [DOI] [PubMed] [Google Scholar]
- 23.Eckhardt CL, van Velzen AS, Peters M, Astermark J, Brons PP, Castaman G, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. (2013) 122:1954–62. 10.1182/blood-2013-02-483263 [DOI] [PubMed] [Google Scholar]
- 24.Peyvandi F, Mannucci PM, Garagiola I, El-Beshlawy A, Elalfy M, Ramanan V, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. (2016) 374:2054–64. 10.1056/NEJMoa1516437 [DOI] [PubMed] [Google Scholar]
- 25.Gouw SC, van der Bom JG, Marijke van den Berg H.Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. (2007) 109:4648–54. [DOI] [PubMed] [Google Scholar]
- 26.Astermark J.FVIII inhibitors: pathogenesis and avoidance. Blood. (2015) 125:2045–51. 10.1182/blood-2014-08-535328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gouw SC, van den Berg HM, Oldenburg J, Astermark J, de Groot PG, Margaglione M, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. (2012) 119:2922–34. 10.1182/blood-2011-09-379453 [DOI] [PubMed] [Google Scholar]
- 28.Oldenburg J, Pavlova A.Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia. (2006) 12(Suppl. 6):15–22. [DOI] [PubMed] [Google Scholar]
- 29.Garagiola I, Palla R, Peyvandi F.Risk factors for inhibitor development in severe hemophilia A. Thromb Res. (2018) 168:20–7. 10.1016/j.thromres.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 30.Young G.How I treat children with haemophilia and inhibitors. Br J Haematol. (2019) 186:400–8. 10.1111/bjh.15942 [DOI] [PubMed] [Google Scholar]
- 31.Brackmann HH, Gormsen J.Massive factor-VIII infusion in haemophiliac with factor-VIII inhibitor, high responder. Lancet. (1977) 2:933. [DOI] [PubMed] [Google Scholar]
- 32.DiMichele DM.Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol. (2012) 159:123–34. 10.1111/bjh.12028 [DOI] [PubMed] [Google Scholar]
- 33.Hay CR, DiMichele DM. International Immune Tolerance Study The principal results of the International immune tolerance study: a randomized dose comparison. Blood. (2012) 119:1335–44. 10.1182/blood-2011-08-369132 [DOI] [PubMed] [Google Scholar]
- 34.Young G.Implementing emicizumab in hemophilia inhibitor management: emicizumab should be prescribed after tolerance. Blood Adv. (2018) 2:2780–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Quellec S, Negrier C.Emicizumab should be prescribed independent of immune tolerance induction. Blood Adv. (2018) 2:2783–6. 10.1136/bmjopen-2017-020904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carcao M, Escuriola-Ettingshausen C, Santagostino E, Oldenburg J, Liesner R, Nolan B, et al. The changing face of immune tolerance induction in haemophilia A with the advent of emicizumab. Haemophilia. (2019) 25:676–84. 10.1111/hae.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makris M, Iorio A, Lenting PJ.Emicizumab and thrombosis: the story so far. J Thromb Haemost. (2019) 17:1269–72. [DOI] [PubMed] [Google Scholar]
- 38.Aledort LM.Deaths associated with emicizumab in patients with hemophilia A. N Engl J Med. (2019) 381:1878–9. [DOI] [PubMed] [Google Scholar]
- 39.Sherman A, Biswas M, Herzog RW.Innovative approaches for immune tolerance to factor VIII in the treatment of hemophilia A. Front Immunol. (2017) 8:1604 10.3389/fimmu.2017.01604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arruda VR, Samelson-Jones BJ.Gene therapy for immune tolerance induction in hemophilia with inhibitors. J Thromb Haemost. (2016) 14:1121–34. 10.1111/jth.13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiegs G, Lohse AW.Immune tolerance: what is unique about the liver. J Autoimmun. (2010) 34:1–6. 10.1016/j.jaut.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 42.Keeler GD, Markusic DM, Hoffman BE.Liver induced transgene tolerance with AAV vectors. Cell Immunol. (2019) 342:103728. 10.1016/j.cellimm.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sack BK, Herzog RW, Terhorst C, Markusic DM.Development of gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev. (2014) 1:14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crispe IN.Hepatic T cells and liver tolerance. Nat Rev Immunol. (2003) 3:51–62. [DOI] [PubMed] [Google Scholar]
- 45.Crispe IN.Immune tolerance in liver disease. Hepatology. (2014) 60:2109–17. 10.1002/hep.27254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant CR, Liberal R.Liver immunology: how to reconcile tolerance with autoimmunity. Clin Res Hepatol Gastroenterol. (2017) 41:6–16. 10.1016/j.clinre.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 47.Calne R, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. (1969) 223:472. [DOI] [PubMed] [Google Scholar]
- 48.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE.Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. (1994) 19:916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houssin D, Gigou M, Franco D, Bismuth H, Charpentier B, Lang P, et al. Specific transplantation tolerance induced by spontaneously tolerated liver allograft in inbred strains of rats. Transplantation. (1980) 29:418. [DOI] [PubMed] [Google Scholar]
- 50.Gorczynski RM, Chan Z, Chung S, Cohen Z, Levy G, Sullivan B, et al. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation. (1994) 58:816–20. [PubMed] [Google Scholar]
- 51.Lerut J, Sanchez−Fueyo A.An appraisal of tolerance in liver transplantation. Am J Transplant. (2006) 6:1774–80. [DOI] [PubMed] [Google Scholar]
- 52.Protzer U, Maini MK, Knolle PA.Living in the liver: hepatic infections. Nat Rev Immunol. (2012) 12:201–13. 10.1038/nri3169 [DOI] [PubMed] [Google Scholar]
- 53.Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM. Us Hemophilia Treatment Center Network. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. (2016) 127:3073–81. 10.1182/blood-2015-10-675140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makris M, Konkle BA.Hepatitis C in haemophilia: time for treatment for all. Haemophilia. (2017) 23:180–1. [DOI] [PubMed] [Google Scholar]
- 55.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. (2003) 111:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. (2007) 110:4144–52. [DOI] [PubMed] [Google Scholar]
- 57.Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. (2013) 5:1698–709. 10.1002/emmm.201302859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annoni A, Cantore A, Della Valle P, Goudy K, Akbarpour M, Russo F, et al. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med. (2013) 5:1684–97. 10.1002/emmm.201302857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. (2002) 99:2670–6. [DOI] [PubMed] [Google Scholar]
- 60.Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. (2010) 115:4678–88. 10.1182/blood-2009-12-261156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. (2005) 105:3458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. (2005) 105:3079–86. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM.Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. (2000) 1:154–8. [DOI] [PubMed] [Google Scholar]
- 64.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. (1999) 5:64–70. [DOI] [PubMed] [Google Scholar]
- 65.Harding TC, Koprivnikar KE, Tu GH, Zayek N, Lew S, Subramanian A, et al. Intravenous administration of an AAV-2 vector for the expression of factor IX in mice and a dog model of hemophilia B. Gene Ther. (2004) 11:204–13. [DOI] [PubMed] [Google Scholar]
- 66.Cantore A, Ranzani M, Bartholomae CC, Volpin M, Valle PD, Sanvito F, et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci Transl Med. (2015) 7:277ra28. 10.1126/scitranslmed.aaa1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crudele JM, Finn JD, Siner JI, Martin NB, Niemeyer GP, Zhou S, et al. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. (2015) 125:1553–61. 10.1182/blood-2014-07-588194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. (2009) 113:797–806. 10.1182/blood-2008-10-181479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samelson-Jones BJ, Arruda VR.Protein-engineered coagulation factors for hemophilia gene therapy. Mol Ther Methods Clin Dev. (2019) 12:184–201. 10.1016/j.omtm.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simioni P, Tormene D, Tognin G, Gavasso S, Bulato C, Iacobelli NP, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. (2009) 361:1671–5. 10.1056/NEJMoa0904377 [DOI] [PubMed] [Google Scholar]
- 71.Moris D, Lu L, Qian S.Mechanisms of liver-induced tolerance. Curr Opin Organ Transplant. (2017) 22:71–8. 10.1097/MOT.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 72.Doherty DG.Antigen-specific immune tolerance in the liver. Nat Biomed Eng. (2019) 3:763–5. [DOI] [PubMed] [Google Scholar]
- 73.Horst AK, Neumann K, Diehl L, Tiegs G.Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. (2016) 13:277–92. 10.1038/cmi.2015.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomson AW, Knolle PA.Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. (2010) 10:753–66. 10.1038/nri2858 [DOI] [PubMed] [Google Scholar]
- 75.Li W, Carper K, Zheng XX, Kuhr CS, Reyes JD, Liang Y, et al. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. (2006) 38:3205–6. [DOI] [PubMed] [Google Scholar]
- 76.Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. (2008) 118:3403–10. 10.1172/JCI32132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian J, Borovok M, Bi L, Kazazian HH, Jr, Hoyer LW.Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost. (1999) 81:240–4. [PubMed] [Google Scholar]
- 78.Scallan CD, Liu T, Parker AE, Patarroyo-White SL, Chen H, Jiang H, et al. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. (2003) 102:3919–26. [DOI] [PubMed] [Google Scholar]
- 79.Sarkar R, Xiao W, Kazazian HH., Jr. A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J Thromb Haemost. (2003) 1:220–6. [DOI] [PubMed] [Google Scholar]
- 80.Doering C, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P.Expression and characterization of recombinant murine factor VIII. Thromb Haemost. (2002) 88:450–8. [PubMed] [Google Scholar]
- 81.Ishiwata A, Mimuro J, Mizukami H, Kashiwakura Y, Takano K, Ohmori T, et al. Liver-restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII-deficient mice. J Gene Med. (2009) 11:1020–9. 10.1002/jgm.1391 [DOI] [PubMed] [Google Scholar]
- 82.Siner JI, Samelson-Jones BJ, Crudele JM, French RA, Lee BJ, Zhou S, et al. Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models. JCI Insight. (2016) 1:e89371. 10.1172/jci.insight.89371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ, et al. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One. (2012) 7:e37671. 10.1371/journal.pone.0037671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lytle AM, Brown HC, Paik NY, Knight KA, Wright JF, Spencer HT, et al. Effects of FVIII immunity on hepatocyte and hematopoietic stem cell-directed gene therapy of murine hemophilia A. Mol Ther Methods Clin Dev. (2016) 3:15056. 10.1038/mtm.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabatino DE, Nichols TC, Merricks E, Bellinger DA, Herzog RW, Monahan PE.Animal models of hemophilia. Prog Mol Biol Transl Sci. (2012) 105:151–209. 10.1016/B978-0-12-394596-9.00006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nichols TC, Hough C, Agersø H, Ezban M, Lillicrap D.Canine models of inherited bleeding disorders in the development of coagulation assays, novel protein replacement and gene therapies. J Thromb Haemost. (2016) 14:894–905. 10.1111/jth.13301 [DOI] [PubMed] [Google Scholar]
- 87.Giles AR, Tinlin S, Hoogendoorn H, Greenwood P, Greenwood R.Development of factor VIII:C antibodies in dogs with hemophilia A (factor VIII:C deficiency). Blood. (1984) 63:451–6. [PubMed] [Google Scholar]
- 88.Tinlin S, Webster S, Giles AR.The development of homologous (canine/anti-canine) antibodies in dogs with haemophilia A (factor VIII deficiency): a ten-year longitudinal study. Thromb Haemost. (1993) 69:21–4. [PubMed] [Google Scholar]
- 89.Hough C, Kamisue S, Cameron C, Notley C, Tinlin S, Giles A, et al. Aberrant splicing and premature termination of transcription of the FVIII gene as a cause of severe canine hemophilia A: similarities with the intron 22 inversion mutation in human hemophilia. Thromb Haemost. (2002) 87:659–65. [PubMed] [Google Scholar]
- 90.Lozier JN, Dutra A, Pak E, Zhou N, Zheng Z, Nichols TC, et al. The Chapel hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci USA. (2002) 99:12991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scallan CD, Lillicrap D, Jiang H, Qian X, Patarroyo-White SL, Parker AE, et al. Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector. Blood. (2003) 102:2031–7. [DOI] [PubMed] [Google Scholar]
- 92.Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B, et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther. (2010) 18:1907–16. 10.1038/mt.2010.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. (2006) 17:427–39. [DOI] [PubMed] [Google Scholar]
- 94.Sabatino DE, Lange AM, Altynova ES, Sarkar R, Zhou S, Merricks EP, et al. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. (2011) 19:442–9. 10.1038/mt.2010.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. (2006) 108: 107–15. [DOI] [PubMed] [Google Scholar]
- 96.Gallo-Penn AM, Shirley PS, Andrews JL, Tinlin S, Webster S, Cameron C, et al. Systemic delivery of an adenoviral vector encoding canine factor VIII results in short-term phenotypic correction, inhibitor development, and biphasic liver toxicity in hemophilia A dogs. Blood. (2001) 97:107–13. [DOI] [PubMed] [Google Scholar]
- 97.Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D.Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. (2004) 103:804–10. [DOI] [PubMed] [Google Scholar]
- 98.Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. (2005) 12:876–84. [DOI] [PubMed] [Google Scholar]
- 99.Zhang P, Sun B, Osada T, Rodriguiz R, Yang XY, Luo X, et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum Gene Ther. (2012) 23:460–72. 10.1089/hum.2011.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. (2010) 116:5842–8. 10.1182/blood-2010-06-288001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lheriteau E, Davidoff AM, Nathwani AC.Haemophilia gene therapy: progress and challenges. Blood Rev. (2015) 29:321–8. 10.1016/j.blre.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 102.Rogers GL, Herzog RW.Gene therapy for hemophilia. Front Biosci (Landmark Ed). (2015) 20:556–603. 10.2741/4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.High KA.The gene therapy journey for hemophilia: are we there yet? Hematology Am Soc Hematol Educ Program. (2012) 2012:375–81. 10.1182/asheducation-2012.1.375 [DOI] [PubMed] [Google Scholar]
- 104.Doshi BS, Arruda VR.Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol. (2018) 9:273–93. 10.1177/2040620718791933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang D, Tai PWL, Gao G.Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. (2019) 18:358–78. 10.1038/s41573-019-0012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lind P, Larsson K, Spira J, Sydow-Bäckman M, Almstedt A, Gray E, et al. Novel forms of B-domain deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur J Biochem. (1995) 232:19–27. [DOI] [PubMed] [Google Scholar]
- 107.McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. (2013) 121:3335–44. 10.1182/blood-2012-10-462200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mingozzi F, High KA.Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu Rev Virol. (2017) 4:511–34. 10.1146/annurev-virology-101416-041936 [DOI] [PubMed] [Google Scholar]
- 109.Ertl HCJ, High KA.Impact of AAV capsid-specific T-Cell responses on design and outcome of clinical gene transfer trials with recombinant adeno-associated viral vectors: an evolving controversy. Hum Gene Ther. (2017) 28:328–37. 10.1089/hum.2016.172 [DOI] [PubMed] [Google Scholar]
- 110.Pasi KJ, Rangarajan S, Mitchell N, Lester W, Symington E, Madan B, et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene therapy for hemophilia A. N Engl J Med. (2020) 382:29–40. 10.1056/NEJMoa1908490 [DOI] [PubMed] [Google Scholar]
- 111.Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-Factor VIII gene transfer in severe hemophilia A. N Engl J Med. (2017) 377:2519–30. 10.1056/NEJMoa1708483 [DOI] [PubMed] [Google Scholar]
- 112.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. (2006) 12:342–7. [DOI] [PubMed] [Google Scholar]
- 113.George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B Gene therapy with a high-specific-activity factor IX Variant. N Engl J Med. (2017) 377:2215–27. 10.1056/NEJMoa1708538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. (2011) 365:2357–65. 10.1056/NEJMoa1108046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. (2014) 371:1994–2004. 10.1056/NEJMoa1407309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. (2007) 13:419–22. [DOI] [PubMed] [Google Scholar]
- 117.Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. (2018) 131:1022–31. 10.1182/blood-2017-09-804419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. (2013) 121:2224–33. 10.1182/blood-2012-10-460733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Tuyishime S, Wu TL, Giles-Davis W, Zhou D, Xiao W, et al. Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice. Mol Ther. (2011) 19:536–46. 10.1038/mt.2010.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li C, Hirsch M, DiPrimio N, Asokan A, Goudy K, Tisch R, et al. Cytotoxic-T-lymphocyte-mediated elimination of target cells transduced with engineered adeno-associated virus type 2 vector in vivo. J Virol. (2009) 83:6817–24. 10.1128/JVI.00278-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li C, Goudy K, Hirsch M, Asokan A, Fan Y, Alexander J, et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci USA. (2009) 106:10770–4. 10.1073/pnas.0902269106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM.Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. (2007) 18:185–94. [DOI] [PubMed] [Google Scholar]
- 123.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. (2007) 110:2334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Georgescu MT, Moorehead PC, van Velzen AS, Nesbitt K, Reipert BM, Steinitz KN, et al. Dexamethasone promotes durable factor VIII-specific tolerance in hemophilia A mice via thymic mechanisms. Haematologica. (2018) 103:1403–13. 10.3324/haematol.2018.189852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc.Natl.Acad.Sci.USA. (2008) 105:18525–30. 10.1073/pnas.0809677105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lange AM, Altynova ES, Nguyen GN, Sabatino DE.Overexpression of factor VIII after AAV delivery is transiently associated with cellular stress in hemophilia A mice. Mol Ther Methods Clin Dev. (2016) 3:16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zolotukhin I, Markusic DM, Palaschak B, Hoffman BE, Srikanthan MA, Herzog RW.Potential for cellular stress response to hepatic factor VIII expression from AAV vector. Mol Ther Methods Clin Dev. (2016) 3: 16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Staber JM, Pollpeter MJ, Anderson CG, Burrascano M, Cooney AL, Sinn PL, et al. Long-term correction of hemophilia A mice following lentiviral mediated delivery of an optimized canine factor VIII gene. Gene Ther. (2017) 24:742–8. 10.1038/gt.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greene TK, Lyde RB, Bailey SC, Lambert MP, Zhai L, Sabatino DE, et al. Apoptotic effects of platelet factor VIII on megakaryopoiesis: implications for a modified human FVIII for platelet-based gene therapy. J Thromb Haemost. (2014) 12:2102–12. 10.1111/jth.12749 [DOI] [PubMed] [Google Scholar]
- 130.Okuyama T, Huber RM, Bowling W, Pearline R, Kennedy SC, Flye MW, et al. Liver-directed gene therapy: a retroviral vector with a complete LTR and the ApoE enhancer-alpha 1-antitrypsin promoter dramatically increases expression of human alpha 1-antitrypsin in vivo. Hum Gene Ther. (1996) 7:637–45. [DOI] [PubMed] [Google Scholar]
- 131.Lang JF, Toulmin SA, Brida KL, Eisenlohr LC, Davidson BL.Standard screening methods underreport AAV-mediated transduction and gene editing. Nat Commun. (2019) 10:3415. 10.1038/s41467-019-11321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, Schutgens RE, Biesma DH, Grobbee DE, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. (2011) 17:849–53. 10.1111/j.1365-2516.2011.02539.x [DOI] [PubMed] [Google Scholar]
- 133.Finn JD, Nichols TC, Svoronos N, Merricks EP, Bellenger DA, Zhou S, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. (2012) 120:4521–3. 10.1182/blood-2012-06-440123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.French RA, Samelson-Jones BJ, Niemeyer GP, Lothrop CD, Jr., Merricks EP, Nichols TC, et al. Complete correction of hemophilia B phenotype by FIX-Padua skeletal muscle gene therapy in an inhibitor-prone dog model. Blood Adv. (2018) 2:505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Batsuli G, Zimowski KL, Tickle K, Meeks SL, Sidonio RF., Jr. Immune tolerance induction in paediatric patients with haemophilia A and inhibitors receiving emicizumab prophylaxis. Haemophilia. (2019) 25:789–96. 10.1111/hae.13819 [DOI] [PubMed] [Google Scholar]
- 136.Arruda VR, Favaro P, Finn JD.Strategies to modulate immune responses: a new frontier for gene therapy. Mol Ther. (2009) 17:1492–503. 10.1038/mt.2009.150 [DOI] [PMC free article] [PubMed] [Google Scholar]