Abstract
Whether persons without prevalent cardiovascular disease (CVD) but elevated levels of high-sensitivity cardiac troponin T (hs-cTnT) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) are at high risk of infection is unknown. Using 1996–2013 data from the Atherosclerosis Risk in Communities Study, we estimated hazard ratios for incident hospitalization with infection in relation to plasma hs-cTnT and NT-proBNP concentrations among participants without prevalent CVD and contrasted them with hazard ratios for persons with prevalent CVD (coronary heart disease, heart failure, or stroke). In a multivariable Cox model, prevalent CVD was significantly associated with risk of hospitalization with infection (hazard ratio (HR) = 1.31, 95% confidence interval (CI): 1.19, 1.45). Among participants without prevalent CVD, hs-cTnT and NT-proBNP were independently associated with infection risk in a graded fashion (e.g., HR = 1.44 (95% CI: 1.24, 1.69) for hs-cTnT ≥14 ng/L and HR = 1.28 (95% CI: 1.14, 1.44) for hs-cTnT 9–13 ng/L vs. <3 ng/L; HR = 1.57 (95% CI: 1.35, 1.81) for NT-proBNP ≥248.1 pg/mL and HR = 1.19 (95% CI: 1.06, 1.34) for NT-proBNP 137.2–248.0 pg/mL vs. <48.1 pg/mL). The 15-year cumulative incidences of hospitalization with infection were similar for participants with prevalent CVD and participants who did not have prevalent CVD but had hs-cTnT ≥14 ng/L or NT-proBNP ≥248.1 pg/mL. Thus, hs-cTnT and NT-proBNP were independently associated with infection risk. Persons without CVD but with elevated hs-cTnT or NT-proBNP levels should be recognized to have similar infection risks as persons with prevalent CVD.
Keywords: cardiovascular disease, high-sensitivity cardiac troponin T, hospitalization, infection, N-terminal pro-B-type natriuretic peptide
Infectious diseases are a major cause of hospitalization (1), posing a significant social and economic burden (2). Cardiovascular disease (CVD), such as myocardial infarction, stroke, and heart failure, is an important risk factor for many infections (3–7), and several infection prevention programs target patients with CVD (8, 9). However, reasons why CVD increases infection risk are not well understood.
Higher risk of infection among persons with CVD may be partly due to shared risk factors for both conditions or unique clinical characteristics of CVD patients. For example, several comorbid conditions, such as diabetes and frailty, can increase the risks of both CVD and infection. Additionally, frequent clinic visits among patients with CVD may increase both risk of infection and the chance of being diagnosed with infection. However, there is a body of evidence indicating that the pathogenesis of atherosclerosis (10), myocardial damage, or heart failure may affect immune response (e.g., up-regulated T-cell activity, activated toll-like receptor signaling, complement activation) (11). These pathophysiological changes could precede any clinical diagnosis of CVD (12, 13) and may contribute to increased susceptibility to infection among adults with subclinical CVD.
Cardiac markers such as high-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are known to reflect cardiac damage (14) and overload (15), respectively. If these cardiac biomarkers were to demonstrate dose-response relationships with the risk of infection, especially among persons without a clinical history of CVD, this would further support a pathophysiological link between the process of CVD development and the occurrence of infectious diseases. In addition, quantification of the associations of these cardiac biomarkers with infection will have implications for risk-centered preventive approaches for some infectious diseases (e.g., pneumococcal vaccination) (16).
We explored the incidence of hospitalization with infection according to CVD status (i.e., prevalent CVD and levels of hs-cTnT and NT-proBNP for persons without prevalent CVD) using data from a large community-based cohort study, the Atherosclerosis Risk in Communities (ARIC) Study. We also investigated whether these markers can help identify persons who are without CVD but at high infection risk.
METHODS
Study population
The ARIC Study is a prospective cohort study of 15,792 persons aged 45–64 years in 1987–1989 (visit 1) from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; selected suburbs of Minneapolis, Minnesota; and Washington County, Maryland (17). Subsequent triennial follow-up visits were conducted in 1990–1992 (visit 2), 1993–1995 (visit 3), and 1996–1998 (visit 4). Plasma hs-cTnT and NT-proBNP concentrations were measured in blood samples collected at visit 4; these values were used as the baseline levels for the present study. Of 11,656 ARIC participants at visit 4, we excluded persons with a history of prior hospitalization with infection (n = 1,264), missing hs-cTnT or NT-proBNP data (n = 403), race other than white or African-American (n = 31), and missing covariate data (n = 490). We also excluded persons with end-stage renal disease (n = 20) or an estimated glomerular filtration rate less than 30 mL/minute/1.73 m2 (n = 45), since levels of hs-cTnT and NT-proBNP could be elevated merely because of kidney dysfunction (18) and severely reduced kidney function is an established risk factor for infection (19–22). After these exclusions, 9,403 participants were included in the present study. Written informed consent for the ARIC examination was obtained from all participants, and the institutional review board at each study site approved the study protocol.
Exposures: prevalent CVD and cardiac markers
Prevalent CVD was defined as a history of coronary heart disease, stroke, heart failure, or atrial fibrillation at visit 4. Coronary heart disease and stroke were defined as self-reported history at visit 1 or physician-adjudicated definite or probable myocardial infarction, including silent myocardial infarction detected by an abnormal Q wave at study visits, coronary revascularization hospitalization, or definite or probable stroke between visit 1 and visit 4, on the basis of active surveillance of community hospitals and annual telephone interviews with participants or their proxies. Heart failure was defined as self-reported history of heart failure at visit 1 based on the Gothenburg criteria, including dyspnea symptoms and any clinical history of cardiac disease (23), or hospitalization with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 428 (heart failure) between visits 1 and 4. The positive predictive value of using the ICD-9-CM code for diagnosis of heart failure was high (93%) (24). Cases of atrial fibrillation were identified through electrocardiographic examinations performed at study visits, as well as hospitalization with ICD-9-CM code 427.31 (atrial fibrillation) (25).
According to the standard protocol, blood samples were collected at visit 4 and stored at −70°C until they were used for the assay (26). Using the frozen plasma samples, hs-cTnT and NT-proBNP concentrations were analyzed in the ARIC Central Chemistry Laboratory at the University of Minnesota between 2010 and 2011. Plasma hs-cTnT level was measured using a novel sensitive assay, the Roche Elecsys T immunoassay (Roche Diagnostics, Indianapolis, Indiana), on a Cobas e411 analyzer (Roche Diagnostics) with a limit of blank of 3 ng/L. Coefficients of variation were 6.0% at a mean concentration of 25 ng/L and <10% below 14 ng/L. Plasma NT-proBNP level was measured using the Elecys proBNP II immunoassay (Roche Diagnostics) on a Cobas e411 analyzer with an assay limit of detection of 5 pg/mL. The coefficient of variation was 5.4% at a concentration of 133 pg/mL.
Outcomes
The primary outcome was incident hospitalization with infection. In ARIC, all hospitalizations were identified through annual telephone calls to participants, as well as by obtaining discharge lists from local hospitals. For all identified hospitalizations, ARIC staff obtained hospital discharge information, including ICD-9-CM codes. Hospitalization with infection was defined as discharge with any of the infection-related ICD-9-CM codes used for defining infection in a national survey (27) (see Web Table 1, available at https://academic.oup.com/aje). For primary analysis, we defined infection by ICD-9-CM codes regardless of their diagnostic position. Participants who did not develop the primary outcome were removed when they died, were lost to follow-up, or were administratively censored on December 31, 2013. The secondary outcome was mortality risk related to hospitalization with infection, which was defined as death occurring during hospitalization with infection or within 30 days postdischarge (28).
Covariates
All covariates were assessed at visit 4, except for years of education, which was assessed at visit 1. Age, sex, race, smoking status, alcohol consumption, and years of education were self-reported. Hypertension was defined as use of an antihypertensive drug, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Diabetes was defined as use of an antidiabetic drug, a self-reported physician’s diagnosis of diabetes, fasting glucose concentration ≥126 mg/dL, or random glucose concentration ≥200 mg/dL. Histories of cancer and chronic obstructive pulmonary disease were based on relevant hospital ICD-9-CM codes prior to visit 4 (ICD-9-CM codes 140–165, 170–176, 179–209, and 235–239 for cancer and ICD-9-CM codes 490–492, 494, and 496 for chronic obstructive pulmonary disease). Abnormal liver function was defined as a history of hospitalization with liver cirrhosis (ICD-9-CM codes 571 and 456.1) or aspartate transaminase and alanine transaminase levels greater than 3 times the upper limit of normal (29, 30). We also included the need for help with chores or shopping as an indicator of frailty (31). Incident CVD during follow-up was defined as an adjudicated coronary heart disease or stroke event or hospitalization for heart failure. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (32). Urinary albumin:creatinine ratio was calculated from urinary albumin and creatinine levels. High-sensitivity C-reactive protein level was measured using the immunoturbidimetric CRP-Latex (II) high-sensitivity assay (Denka Seiken, Tokyo, Japan) on a Hitachi 911 chemistry analyzer (Hitachi High Technologies America, Inc., Schaumburg, Illinois).
Statistical analysis
Baseline characteristics were determined according to CVD status at baseline (prevalent CVD or not). Among persons without prevalent CVD, baseline characteristics were determined for those with hs-cTnT levels of <3 ng/L vs. ≥3 ng/L and NT-proBNP levels of <48.1 pg/mL vs. ≥48.1 pg/mL to match the percentile used for the hs-cTnT cutoff.
Crude incidence rates of hospitalization with infection and 95% confidence intervals were estimated using Poisson regression models. Adjusted hazard ratios were estimated using Cox proportional hazards models. To confirm the association of CVD with risk of infection, we first estimated the hazard ratio associated with prevalent CVD as compared with no prevalent CVD in the overall population. Next, among persons without prevalent CVD, we assessed the associations of hs-cTnT and NT-proBNP concentrations with risk of infection. Levels of hs-cTnT and NT-proBNP were treated as categorical variables as well as continuous variables. In the categorical analysis, hs-cTnT level was categorized into 5 groups—<3, 4–5, 6–8, 9–13, and ≥14 ng/L—according to previous literature (33, 34). NT-proBNP level was categorized into 5 groups according to the percentiles for the 5 groups of hs-cTnT (<48.1, 48.1–80.1, 80.2–137.1, 137.2–248.0, and ≥248.1 pg/mL). The lowest level of each cardiac biomarker (hs-cTnT and NT-proBNP) served as the reference category. In the continuous analysis, data on hs-cTnT and NT-proBNP levels were log-transformed. The multivariable models adjusted for age, sex, race, body mass index (weight (kg)/height (m)2), smoking status (ever smoking), alcohol consumption (ever drinking), duration of education (<12 years vs. ≥12 years), frailty, high-sensitivity C-reactive protein level, estimated glomerular filtration rate, urinary albumin:creatinine ratio, medication use (aspirin, statins, loop diuretics, and anticoagulants), and medical history (hypertension, diabetes, cancer, chronic obstructive pulmonary disease, and abnormal liver function).
We performed a few sensitivity analyses. First, we additionally accounted for incident CVD during follow-up (i.e., censoring persons with incident CVD during follow-up, treating incident CVD as a competing event, and adjusting for incident CVD as a time-varying variable), since elevated levels of hs-cTnT and NT-proBNP are associated with incident CVD (35, 36). Second, we restricted our analysis to hospitalizations with infection listed in the first position on the discharge diagnosis form to capture cases with infection as the main cause of hospitalization. Third, since the assay for hs-cTnT was less reliable below the level of 5 ng/L, we performed a sensitivity analysis using hs-cTnT <5 ng/L as the reference level. Fourth, to account for possible undiagnosed CVD at baseline, we excluded persons who were diagnosed with incident CVD within the first 3 years of follow-up. Fourth, to more rigorously control for CVD risk at baseline, we further adjusted for traditional CVD risk factors (i.e., systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol). In addition, we assessed potential interaction by age (<65 years vs. ≥65 years), sex (male vs. female), race (white vs. African-American), diabetes status (yes vs. no), and chronic kidney disease as defined by estimated glomerular filtration rate less than 60 mL/minute/1.73 m2 or urinary albumin:creatinine ratio ≥30 mg/g (yes vs. no) using log-likelihood tests. Finally, we analyzed 4 major infection outcomes separately: (37) pneumonia (ICD-9-CM codes 480–486), kidney and urinary tract infections (ICD-9-CM codes 590, 590.0–590.4, 597, 598, 599.0, 601, 604, 607, and 608), bloodstream infections (ICD-9-CM codes 038 and 790.7), and cellulitis (ICD-9-CM codes 681 and 682).
To evaluate the discriminative ability of hs-cTnT and NT-proBNP for predicting risk of hospitalization with infection among persons without prevalent CVD, we computed the Harrell’s C statistic in multivariable Cox models with and without inclusion of hs-cTnT and NT-proBNP as continuous variables. A 2-sided P value less than 0.05 was considered statistically significant. All of the statistical analyses were performed using Stata, version 13 (StataCorp LLC, College Station, Texas).
RESULTS
Baseline characteristics
In the overall sample (n = 9,403), the mean age was 63 years; 44% of participants were male, and 22% were African-American (Table 1). The prevalence of CVD at baseline was 11% (n = 1,066). Among persons without prevalent CVD (n = 8,337), those with higher levels of hs-cTnT and NT-proBNP tended to be older and hypertensive, to have a lower estimated glomerular filtration rate and a higher urinary albumin:creatinine ratio, and to take aspirin and anticoagulants. These patterns were more evident among persons with prevalent CVD.
Table 1.
Baseline Characteristics of Participants in a Study of the Associations of High-Sensitivity Cardiac Troponin and Natriuretic Peptide With Risk of Hospitalization with Infection, by Cardiovascular Disease Status, ARIC Study, 1996–1998a
| Characteristic | Overall Sample (n = 9,403) | Persons With Prevalent CVD at Baseline (n = 1,066) | Persons Without Prevalent CVD at Baseline (n = 8,337) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hs-cTnT Concentration | NT-proBNP Concentration | |||||||||||||||||
| <3 ng/L (n = 3,401) | ≥3 ng/L (n = 4,936) | <48.1 pg/mL (n = 3,400) | ≥48.1 pg/mL (n = 4,937) | |||||||||||||||
| No.b | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Age, years | 62.6 (5.6) | 63.5 (5.6)c | 60.7 (5.1) | 63.5 (5.6)d | 60.9 (5.2) | 63.4 (5.6)e | ||||||||||||
| Male sex | 4,096 | 43.6 | 651c | 61.1 | 759 | 22.3 | 2,686d | 1,870 | 55.0 | 1,575e | 31.9 | |||||||
| Black race | 2,075 | 22.1 | 221 | 20.7 | 728 | 21.4 | 1,126d | 54.4 | 1,025 | 30.1 | 829e | 16.8 | ||||||
| Body mass indexf | 28.7 (5.5) | 29.0 (5.4)c | 28.1 (5.4) | 22.8 | 29.0 (5.4)d | 29.3 (5.2) | 28.2 (5.6)e | |||||||||||
| Ever smokingg | 5,426 | 57.7 | 752c | 70.5 | 1,895 | 55.7 | 2,779d | 5.4 | 1,972 | 58.0 | 2,702e | 54.7 | ||||||
| Ever consuming alcoholg | 7,463 | 79.4 | 872 | 81.8 | 2,701 | 79.4 | 3,890d | 56.3 | 2,746 | 80.8 | 3,845 | 77.9 | ||||||
| ≥12 years of education | 7,680 | 81.7 | 789c | 74.0 | 2,909 | 85.5 | 3,982 | 78.8 | 2,810 | 82.6 | 4,081 | 82.7 | ||||||
| Needing help with chores/shopping | 346 | 3.7 | 91c | 8.5 | 79 | 2.3 | 176 | 3.6 | 97 | 2.9 | 158 | 3.2 | ||||||
| Heart rate, beats/minute | 65.7 (9.5) | 64.7 (10.1)c | 66.3 (8.8) | 65.6 (9.7)d | 67.2 (9.2) | 65.0 (9.4)e | ||||||||||||
| Systolic blood pressure, mm Hg | 127.4 (18.9) | 130.0 (19.8)c | 124.5 (17.9) | 128.9 (19.1)d | 123.5 (16.1) | 129.6 (20.0)e | ||||||||||||
| Diastolic blood pressure, mm Hg | 71.2 (10.3) | 69.9 (11.6) | 70.8 (9.8) | 71.7 (10.3)d | 72.0 (9.3) | 70.9 (10.6)e | ||||||||||||
| Laboratory tests | ||||||||||||||||||
| eGFR, mL/minute/1.73 m2 | 86.4 (15.9) | 81.3 (17.5)c | 90.0 (14.4) | 85.0 (16.1)d | 90.4 (14.8) | 84.7 (15.7)e | ||||||||||||
| Urinary albumin:creatinine ratio, mg/g | 22.4 (168.3) | 53.8 (271.2)c | 9.1 (64.2) | 24.8 (186.9)d | 10.4 (72.0) | 23.9 (185.0)e | ||||||||||||
| High-sensitivity C-reactive protein, mg/dL | 4.3 (6.3) | 5.0 (7.3)c | 4.5 (6.0) | 4.0 (6.3)d | 3.8 (5.0) | 4.5 (6.9)e | ||||||||||||
| Total cholesterol, mmol/L | 5.2 (0.9) | 5.0 (1.0)c | 5.3 (0.9) | 5.2 (0.9)d | 5.3 (1.0) | 5.2 (0.9)e | ||||||||||||
| HDL cholesterol, mmol/L | 1.3 (0.4) | 1.2 (0.4)c | 1.4 (0.4) | 1.3 (0.4)d | 1.2 (0.4) | 1.4 (0.4)e | ||||||||||||
| hs-cTnTh, ng/L | 5.0 (3.0–8.0) | 7.0 (4.0–12.0)c | 3.0 (3.0–3.0) | 7.0 (5.0–9.0)d | 4.0 (3.0–7.0) | 4.0 (3.0–8.0)e | ||||||||||||
| NT-proBNPh, pg/mL | 65.8 (32.5–126.1) | 138.1 (61.3–312.2)c | 59.6 (30.0–106.5) | 62.5 (30.8–120.3)d | 25.8 (14.6–36.5) | 101.6 (70.3–156.8)e | ||||||||||||
| Any electrocardiographic abnormality | 293 | 3.1 | 103c | 9.7 | 56 | 1.6 | 134d | 2.7 | 41 | 1.2 | 149e | 3.0 | ||||||
| Left ventricular hypertrophyi | 229 | 2.4 | 51c | 4.8 | 54 | 1.6 | 124d | 2.5 | 39 | 1.1 | 139e | 2.8 | ||||||
| Major Q wave abnormality | 48 | 0.5 | 38c | 3.6 | 2 | 0.1 | 8d | 0.2 | 1 | 0.0 | 9e | 0.2 | ||||||
| Minor Q wave abnormality | 23 | 0.2 | 21c | 2.0 | 0 | 0.0 | 2 | 0.0 | 1 | 0.0 | 1 | 0.0 | ||||||
| Medication use | ||||||||||||||||||
| Aspirin | 5,266 | 56.0 | 828c | 77.7 | 1,809 | 53.2 | 2,629d | 53.3 | 1,755 | 51.6 | 2,683e | 54.3 | ||||||
| Statins | 1,022 | 10.9 | 316c | 29.6 | 280 | 8.2 | 426 | 8.6 | 303 | 8.9 | 403 | 8.2 | ||||||
| Loop diuretics | 304 | 3.2 | 156c | 14.6 | 38 | 1.1 | 110d | 2.2 | 44 | 1.3 | 104e | 2.1 | ||||||
| Anticoagulants | 148 | 1.6 | 109c | 10.2 | 9 | 0.3 | 30 | 0.6 | 13 | 0.4 | 26 | 0.5 | ||||||
| Medical history | ||||||||||||||||||
| Hypertension | 3,449 | 36.7 | 610c | 57.2 | 990 | 29.1 | 1,849d | 37.5 | 1,077 | 31.7 | 1,762e | 35.7 | ||||||
| Diabetes | 1,475 | 15.7 | 282c | 26.5 | 323 | 9.5 | 870d | 17.6 | 577 | 17.0 | 616e | 12.5 | ||||||
| Cancer | 389 | 4.1 | 53 | 5.0 | 125 | 3.7 | 211 | 4.3 | 124 | 3.6 | 212 | 4.3 | ||||||
| COPD | 140 | 1.5 | 65c | 6.1 | 25 | 0.7 | 50 | 1.0 | 28 | 0.8 | 47 | 1.0 | ||||||
| Abnormal liver function | 15 | 0.2 | 5c | 0.5 | 2 | 0.1 | 8 | 0.2 | 3 | 0.1 | 7 | 0.1 | ||||||
Abbreviations: ARIC, Atherosclerosis Risk in Communities; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SD, standard deviation.
a P values were based χ2, analysis of variance, or Kruskal-Wallis tests.
b Number of participants.
c P < 0.05 for comparison with persons without prevalent CVD at baseline.
d P < 0.05 for comparison with persons with hs-cTnT <3 ng/L.
e P < 0.05 for comparison with persons with NT-proBNP <48.1 pg/mL.
f Body mass index was calculated as weight (kg)/height (m)2.
g Smoking status and alcohol consumption were based on the questionnaire items “Have you ever smoked cigarettes?” and “Have you ever consumed alcoholic beverages?”.
h Values are expressed as median (interquartile range).
i Left ventricular hypertrophy was defined as the sum of the R wave in aVL and the S wave in V3 exceeding 20 mm (women) or 28 mm (men).
Associations of hs-cTnT and NT-proBNP with overall incident hospitalization with infection
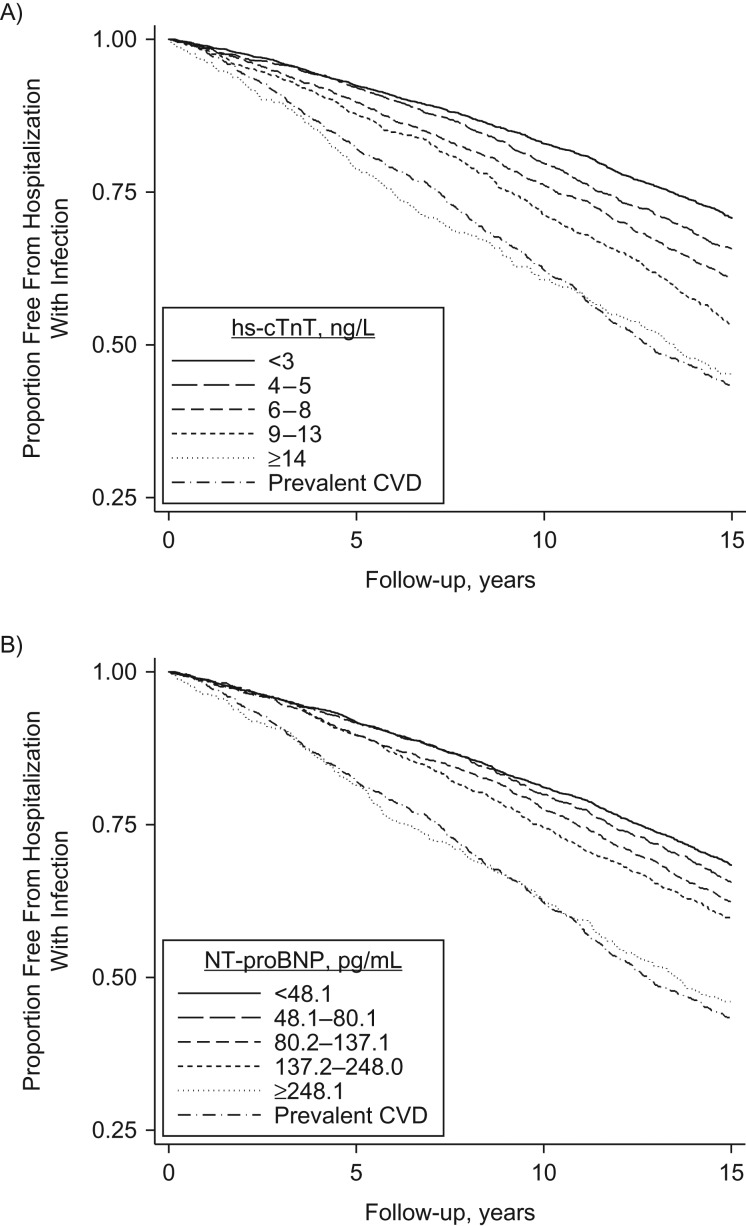
During follow-up (median, 15.2 years), 3,763 participants (576 with prevalent CVD and 3,187 without prevalent CVD) had an incident hospitalization with infection. The 15-year cumulative incidence of hospitalization with infection was highest (57%) for persons with prevalent CVD (Figure 1). However, the 15-year cumulative incidence among participants without prevalent CVD who were in the highest categories of hs-cTnT (≥14 ng/L) and NT-proBNP (≥248.1 pg/mL) level was nearly the same as that for persons with prevalent CVD (55% and 57%, respectively). There was a dose-response relationship across the remaining 4 groups of hs-cTnT and NT-proBNP level among participants without prevalent CVD. Similar patterns were observed when we estimated the cumulative incidence of hospitalization with infection while accounting for death as a competing risk event (Web Figure 1).
Figure 1.
Kaplan-Meier curves for the proportion of participants who were free from hospitalization with infection among persons with prevalent cardiovascular disease (CVD) and persons without prevalent CVD, by category of high-sensitivity cardiac troponin T (hs-cTnT) concentration (A) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration (B), ARIC Study, 1996–2013. ARIC, Atherosclerosis Risk in Communities.
In multivariable Cox analyses, persons with prevalent CVD had a 31% higher risk of hospitalization with infection than those without prevalent CVD (hazard ratio (HR) = 1.31, 95% confidence interval (CI): 1.19, 1.45) (Table 2). Among participants without prevalent CVD, higher levels of hs-cTnT were associated with higher risk of infection in a graded fashion (HR = 1.10 (95% CI: 0.99, 1.21) for 4–5 ng/L, 1.14 (95% CI: 1.03, 1.26) for 6–8 ng/L, 1.28 (95% CI: 1.14, 1.44) for 9–13 ng/L, and 1.44 (95% CI: 1.24, 1.69) for ≥14 ng/L, as compared with <3 ng/L). A similar graded pattern was observed for NT-proBNP (HR = 1.04 (95% CI: 0.94, 1.15) for 48.1–80.1 pg/mL, 1.15 (95% CI: 1.04, 1.27) for 80.2–137.1 pg/mL, 1.19 (95% CI: 1.06, 1.34) for 137.2–248.0 pg/mL, and 1.57 (95% CI: 1.35, 1.81) for ≥248.1 pg/mL, as compared with <48.1 pg/mL).
Table 2.
Crude Incidence Rates and Adjusted Hazard Ratios for Hospitalization With Infection, by Cardiovascular Disease Status, ARIC Study, 1996–2013
| Exposure Status | No. of Participants | No. of Events | Incidence Rate per 1,000 Person-Years | 95% CI | Adjusted Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Overall sample | ||||||
| No prevalent CVD at baseline | 8,337 | 3,187 | 29.9 | 28.9, 31.0 | 1.00 | Referent |
| Prevalent CVD at baseline | 1,066 | 576 | 53.4 | 49.2, 57.9 | 1.31 | 1.19, 1.45 |
| Persons without prevalent CVD at baseline | ||||||
| hs-cTnT concentration | ||||||
| Categorical variable, ng/L | ||||||
| <3 | 3,401 | 1,114 | 24.1 | 22.7, 25.5 | 1.00 | Referent |
| 4–5 | 1,717 | 647 | 28.8 | 26.7, 31.1 | 1.10 | 0.99, 1.21 |
| 6–8 | 1,687 | 683 | 32.5 | 30.2, 35.1 | 1.14 | 1.03, 1.26 |
| 9–13 | 1,028 | 480 | 40.3 | 36.9, 44.1 | 1.28 | 1.14, 1.44 |
| ≥14 | 504 | 263 | 52.8 | 46.8, 59.6 | 1.44 | 1.24, 1.69 |
| Continuous variable, per log(hs-cTnT) | 1.21 | 1.13, 1.29 | ||||
| NT-proBNP concentration | ||||||
| Categorical variable, pg/mL | ||||||
| <48.1 | 3,378 | 1,172 | 26.1 | 24.6, 27.6 | 1.00 | Referent |
| 48.1–80.1 | 1,712 | 630 | 28.3 | 26.2, 30.6 | 1.04 | 0.94, 1.15 |
| 80.2–137.1 | 1,691 | 677 | 31.5 | 29.3, 34.0 | 1.15 | 1.04, 1.27 |
| 137.2–248.0 | 1,034 | 433 | 34.5 | 31.4, 37.9 | 1.19 | 1.06, 1.34 |
| ≥248.1 | 522 | 275 | 51.2 | 45.5, 57.7 | 1.57 | 1.35, 1.81 |
| Continuous variable, per log(NT-proBNP) | 1.09 | 1.05, 1.13 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; CVD, cardiovascular disease; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
The association was consistent after accounting for incident CVD during follow-up (Web Table 2). When hs-cTnT level <5 ng/L was used as the reference category, the association with hs-cTnT was consistent (HR = 1.13 (95% CI: 1.04, 1.24) for 5–8 ng/L, 1.27 (95% CI: 1.13, 1.42) for 9–13 ng/L, and 1.46 (1.26, 1.70) for ≥14 ng/L) (Web Table 3). The association was consistent, with similar hazard ratios, when we excluded the 211 persons diagnosed with incident CVD within the first 3 years of follow-up (per log increase, hazard ratios were 1.22 (95% CI: 1.13, 1.31) for hs-cTnT and 1.08 (95% CI: 1.04, 1.12) for NT-proBNP) or further adjusted for traditional CVD risk factors (systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol) (per log increase, hazard ratios were 1.22 (95% CI: 1.14, 1.31) for hs-cTnT and 1.09 (95% CI: 1.05, 1.13) for NT-proBNP). The associations were consistent when we analyzed the 1,664 cases of hospitalization that had infection as the primary diagnosis (Web Table 4). In subgroup analyses, there was no significant interaction with any of the subgrouping variables for either hs-cTnT or NT-proBNP (all P values for interaction > 0.05) (Web Table 5). When we explored participants with prevalent CVD, the hazard ratios for hospitalization with infection per log increase were 1.20 (95% CI: 1.05, 1.38) for hs-cTnT and 1.14 (95% CI: 1.05, 1.23) for NT-proBNP.
Incident hospitalization with specific types of infection
The associations were consistent across 4 major types of infection: pneumonia, urinary tract infection, bloodstream infection, and cellulitis (Table 3). Risk was 28%–40% higher across the 4 types of infection for prevalent CVD as compared with no prevalent CVD. Among participants without prevalent CVD, both hs-cTnT and NT-proBNP (log-transformed) showed consistent and significant positive associations with all types of infection examined.
Table 3.
Adjusted Hazard Ratios for Type-Specific Risk of Incident Hospitalization With Infection, by Cardiovascular Disease Status, ARIC Study, 1996–2013
| Type of Infection | Overall Sample (n = 9,403) | Participants Without Prevalent CVD at Baseline (n = 8,337) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Events | Prevalent CVD at Baseline (No vs. Yes) | No. of Events | hs-cTnT Concentration, per log(hs-cTnT) | NT-proBNP Concentration, per log(NT-proBNP) | ||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Pneumonia | 1,150 | 1.40 | 1.18, 1.66 | 929 | 1.32 | 1.17, 1.49 | 1.08 | 1.01, 1.16 |
| Urinary tract infection | 1,137 | 1.31 | 1.10, 1.57 | 964 | 1.23 | 1.09, 1.39 | 1.14 | 1.06, 1.22 |
| Bloodstream infection | 670 | 1.28 | 1.02, 1.61 | 554 | 1.34 | 1.15, 1.56 | 1.13 | 1.04, 1.24 |
| Cellulitis | 416 | 1.31 | 0.99, 1.74 | 339 | 1.56 | 1.29, 1.88 | 1.19 | 1.06, 1.34 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Mortality risk related to hospitalization with infection
When assessing deaths that occurred during hospitalization with infection or within 30 days after discharge (666 deaths), the association was consistent (Web Table 6). In the overall sample, prevalent CVD was associated with 57% higher infection-related mortality than no prevalent CVD (HR = 1.57, 95% CI: 1.27, 1.93). Among participants without prevalent CVD, the hazard ratio for the highest category compared with the lowest was approximately 2 for hs-cTnT and NT-proBNP.
Discriminative ability of hs-cTnT and NT-proBNP to predict infection
The addition of prevalent CVD to the base model to predict infection (C = 0.6441) significantly improved the C statistic (ΔC statistic: 0.0027, 95% CI: 0.0011, 0.0042) (Table 4). Among participants without prevalent CVD, the addition of hs-cTnT or NT-proBNP significantly improved the C statistic (ΔC statistic: 0.0041 (95% CI: 0.0021, 0.0061) for hs-cTnT and 0.0037 (95% CI: 0.0015, 0.0058) for NT-proBNP). When both hs-cTnT and NT-proBNP were included in the model, we observed a further improvement in risk discrimination, with a C-statistic change of 0.0063 (95% CI: 0.0036, 0.0090).
Table 4.
Discriminative Ability of High-Sensitivity Cardiac Troponin T and N-Terminal Pro-B-Type Natriuretic Peptide to Predict Risk of Hospitalization With Infection Among Persons Without Prevalent Cardiovascular Disease, ARIC Study, 1996–2013
| Exposure Status | C Statistic | Difference From Base Model | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Overall sample (n = 9,403) | ||||
| Base modela | 0.6414 | 0 | Referent | |
| + prevalent CVD at baseline | 0.6441 | 0.0027 | 0.0011, 0.0042 | <0.001 |
| Participants without prevalent CVD at baseline (n = 8,337) | ||||
| Base modela | 0.6407 | 0 | Referent | |
| + log hs-cTnT only | 0.6448 | 0.0041 | 0.0021, 0.0061 | <0.001 |
| + log NT-proBNP only | 0.6444 | 0.0037 | 0.0015, 0.0058 | 0.001 |
| + log hs-cTnT/ NT-proBNP | 0.6470 | 0.0063 | 0.0036, 0.0090 | <0.001 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro- B-type natriuretic peptide.
a The base model adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, duration of education (<12 years vs. ≥12 years), frailty, high-sensitivity C-reactive protein, estimated glomerular filtration rate, urinary albumin:creatinine ratio, medication use (aspirin, anticoagulants, and statins), and medical history (hypertension, diabetes, cancer, chronic obstructive pulmonary disease, and abnormal liver function).
DISCUSSION
In this biracial community cohort, participants without prevalent CVD who had higher levels of hs-cTnT and NT-proBNP had greater risks of hospitalization with infection that increased in a graded fashion, and those with an hs-cTnT concentration greater than or equal to 14 ng/L or an NT-proBNP concentration greater than or equal to 248.1 pg/mL had a similar infection risk as persons with prevalent CVD. Among persons without prevalent CVD, those with mildly elevated levels of hs-cTnT (6–13 ng/L) and NT-proBNP (80.2–248.0 pg/mL) also had significantly higher risks of infection in comparison with the reference categories.
Previous studies showing an increased risk of infection among persons with prevalent CVD may have been influenced by shared risk factors for both conditions (e.g., diabetes, frailty) and unique clinical characteristics of patients (e.g., a higher chance of diagnosis with infection through frequent health-care encounters) (3–7). To our knowledge, this is the first study to have specifically evaluated the associations of hs-cTnT and NT-proBNP with risk of infection. We observed a dose-response relationship between these cardiac markers and infection risk at a range lower than their clinical cutoffs for diagnosing myocardial infarction or acute heart failure (i.e., <14 ng/L or <300 pg/mL). Additionally, the associations remained similar after rigorously accounting for incident CVD during follow-up and in a series of sensitivity analyses. Our findings further support a link between the pathophysiology of CVD and infection risk.
Although we excluded participants with prevalent CVD from the analyses of hs-cTnT and NT-proBNP, it is possible that some persons with high levels of hs-cTnT and NT-proBNP had undiagnosed CVD (38, 39). In this regard, we confirmed the consistent association after excluding persons diagnosed with incident CVD within the first 3 years of follow-up. Moreover, the prospective design of the ARIC Study, with repeated visits, allowed us to have a window of approximately 9 years from visit 1 (study initiation) to visit 4 (baseline of the current study) in which to identify prevalent CVD on the basis of active surveillance and annual telephone interviews.
While our study cannot confirm causality, several potential mechanisms linking hs-cTnT and NT-proBNP to risk of infection deserve some discussion. Atherosclerosis is characterized by a chronic condition of low-grade inflammation and involves the dysregulation of immune cells (10). In addition, myocardial damage and volume overload could induce an inflammatory response (11). Furthermore, the complement pathway, which plays an integral part in the innate immune system, is dysregulated in patients with chronic heart failure (40). Importantly, it is well-known that the pathophysiological process of CVD is a continuum (12, 13), and thus persons without clinical manifestations of CVD but with elevated hs-cTnT and NT-proBNP levels might have subclinical abnormalities of the cardiovascular system and accompanying alterations of the immune system. In addition, it is possible that these 2 cardiac markers, as markers of end-organ damage, may reflect overall wellness.
Although addition of hs-cTnT and NT-proBNP to the models significantly improved the prediction of hospitalization with infection, we note the modest C statistic value of approximately 0.65. The high proportion of persons with hospitalization with infection (i.e., approximately 40%) may have made the discriminative ability of the models less efficient, since many participants developed an infection event regardless of the risk. Moreover, some unique aspects of infection as a communicable disease may be relevant, as opposed to noncommunicable diseases such as CVD and diabetes. Specifically, a pathogen is essential for development of infection, and thus there would be a limit to the prediction of infection risk based on host factors only. Nonetheless, it is important to recognize that persons who are free of CVD but have high levels of hs-cTnT and NT-proBNP are at high risk of hospitalization with infection.
The present study may have some clinical and pathophysiological implications. Since the improvement in infection risk discrimination through the addition of hs-cTnT and NT-proBNP was modest, our results may not support newly measuring these cardiac markers specifically for classification of risk of infection. However, according to a growing body of evidence, some investigators suggest utilizing hs-cTnT and NT-proBNP levels for classifying the risk of incident CVD (39, 41). Thus, when these cardiac biomarkers have already been measured, they can simultaneously help identify persons at greater risk of infection and persons who may benefit from infection prevention policies (42–44). In this context, it is worth noting that persons without prevalent CVD but with hs-cTnT or NT-proBNP levels above clinical thresholds (e.g., ≥14 ng/L or ≥300 pg/mL, respectively) (45, 46) had risks similar to those of persons with prevalent CVD (Figure 1). Additionally, although researchers are still debating why hs-cTnT is detected in general populations without any signs/symptoms of CVD (47), it may be important for health-care providers to search for undiagnosed heart disease in persons with elevated levels of hs-cTnT and NT-proBNP.
Several limitations of this study should be acknowledged. First, our outcome ascertainment relied on discharge ICD-9-CM codes, which might have led to some misclassification. However, previous studies have found that ICD-9-CM infection codes were valid for identifying hospital infection outcomes (48, 49). This approach also resulted in missing infections that did not require hospitalization. Second, we included coronary heart disease, stroke, and heart failure requiring hospitalization as examples of prevalent CVD. Although this is a common definition of prevalent CVD (36, 50, 51), it is important that other CVD subtypes were not included. Third, although we did our best to account for incident CVD during follow-up and investigated infection as the primary hospitalization diagnosis, we cannot completely deny the possibility that differential misclassification of infection diagnoses in persons at higher CVD risk influenced our estimates. Fourth, there is a possibility of residual confounding, even though we accounted for several major risk factors for infection (4, 19). Future studies are warranted to explore pathophysiological mechanisms linking elevated levels of cardiac markers to the risk of infection. Fifth, it is uncertain whether our findings are applicable to cardiac markers measured in acute-care settings to rule some cardiac diseases in or out. Finally, our study population was restricted to persons aged 53–75 years and to persons of white or African-American race; our results may not be fully generalizable to other age or racial groups.
In conclusion, among persons without prevalent CVD, higher plasma hs-cTnT and NT-proBNP levels were associated with higher risk of infection. Persons without CVD but with clinically elevated levels of hs-cTnT or NT-proBNP should be recognized as a population at high risk of infection, similar to those with prevalent CVD. These findings support a link between the pathophysiology of CVD and infection.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Junichi Ishigami, Josef Coresh, Elizabeth Selvin, Kunihiro Matsushita); Department of Medicine, Baylor College of Medicine, Houston, Texas (Ron C. Hoogeveen, Christie M. Ballantyne); and Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Aaron R. Folsom).
J.I. was supported by National Institutes of Health (NIH) grant T32HL007024 from the National Heart, Lung, and Blood Institute (NHLBI). E.S. was supported by NIH grants K24DK106414 and R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases. This research was also supported by NIH grant R01HL134320 awarded to C.M.B. and E.S. by the NHLBI. The Atherosclerosis Risk in Communities Study is performed as a collaborative study supported by NHLBI contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C.
We thank the staff and participants of the Atherosclerosis Risk in Communities Study for their important contributions. Reagents for the high-sensitivity cardiac troponin T and NT-proBNP assays were donated by Roche Diagnostics Corporation (Indianapolis, Indiana).
The sponsors played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
K.M. received nonfinancial support from Roche Diagnostics outside of the work.
Abbreviations
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- hs-cTnT
high-sensitivity cardiac troponin T
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
REFERENCES
- 1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott RD., II The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention Atlanta, GA: Centers for Disease Control and Prevention; 2009. http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed January 24, 2016.
- 3. Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J. 2008;31(6):1274–1284. [DOI] [PubMed] [Google Scholar]
- 4. Jackson ML, Nelson JC, Jackson LA. Risk factors for community-acquired pneumonia in immunocompetent seniors. J Am Geriatr Soc. 2009;57(5):882–888. [DOI] [PubMed] [Google Scholar]
- 5. Mor A, Thomsen RW, Ulrichsen SP, et al. Chronic heart failure and risk of hospitalization with pneumonia: a population-based study. Eur J Intern Med. 2013;24(4):349–353. [DOI] [PubMed] [Google Scholar]
- 6. van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24. [DOI] [PubMed] [Google Scholar]
- 7. Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1(1):ofu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–819. [PubMed] [Google Scholar]
- 9. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2016–17 influenza season. MMWR Recomm Rep. 2016;65(5):1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng X, Yang J, Dong M, et al. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016;13(3):167–179. [DOI] [PubMed] [Google Scholar]
- 11. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 13. Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. [DOI] [PubMed] [Google Scholar]
- 14. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. [DOI] [PubMed] [Google Scholar]
- 15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195–203. [DOI] [PubMed] [Google Scholar]
- 16. Ishigami J, Padula WV, Grams ME, et al. Cost-effectiveness of pneumococcal vaccination among patients with CKD in the United States [published online ahead of print March 19, 2019]. Am J Kidney Dis. (doi: 10.1053/j.ajkd.2019.01.025). [DOI] [PubMed] [Google Scholar]
- 17. The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18. Freda BJ, Tang WH, Van Lente F, et al. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40(12):2065–2071. [DOI] [PubMed] [Google Scholar]
- 19. Ishigami J, Grams ME, Chang AR, et al. CKD and risk for hospitalization with infection: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2017;69(6):752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishigami J, Matsushita K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol. 2019;23(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017;12(9):1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. United States Renal Data System US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2015. https://www.usrds.org/2015/view/Default.aspx. Accessed April 17, 2019.
- 23. Matsushita K, Blecker S, Pazin-Filho A, et al. The association of hemoglobin A1c with incident heart failure among people without diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2010;59(8):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) Study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal SK, Avery CL, Ballantyne CM, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the Atherosclerosis Risk in Communities Study. Clin Chem. 2011;57(6):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. United States Renal Data System ESRD analytical methods. http://www.usrds.org/2014/view/v2_00_appx.aspx. Accessed February 14, 2016.
- 28. Metersky ML, Tate JP, Fine MJ, et al. Temporal trends in outcomes of older patients with pneumonia. Arch Intern Med. 2000;160(22):3385–3391. [DOI] [PubMed] [Google Scholar]
- 29. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. [DOI] [PubMed] [Google Scholar]
- 30. Ishigami J, Grams ME, Naik RP, et al. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the Atherosclerosis Risk in Communities (ARIC) Study. Clin J Am Soc Nephrol. 2016;11(10):1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piggott DA, Muzaale AD, Varadhan R, et al. Frailty and cause-specific hospitalization among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci. 2017;72(3):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Folsom AR, Yao L, Alonso A, et al. Circulating biomarkers and abdominal aortic aneurysm incidence: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;132(7):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McEvoy JW, Chen Y, Nambi V, et al. High-sensitivity cardiac troponin T and risk of hypertension. Circulation. 2015;132(9):825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Astor BC, Yi S, Hiremath L, et al. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: the African American Study of Kidney Disease and Hypertension (AASK). Circulation. 2008;117(13):1685–1692. [DOI] [PubMed] [Google Scholar]
- 37. Christensen KL, Holman RC, Steiner CA, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49(7):1025–1035. [DOI] [PubMed] [Google Scholar]
- 38. Mukoyama M, Nakao K, Saito Y, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;323(11):757–758. [DOI] [PubMed] [Google Scholar]
- 39. Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shahini N, Michelsen AE, Nilsson PH, et al. The alternative complement pathway is dysregulated in patients with chronic heart failure. Sci Rep. 2017;7:42532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–1720. [DOI] [PubMed] [Google Scholar]
- 43. Ducel G, Fabry J, Nicolle L, eds. Prevention of Hospital-Acquired Infections: A Practical Guide. 2nd ed Geneva, Switzerland: World Health Organization; 2002. http://apps.who.int/medicinedocs/documents/s16355e/s16355e.pdf. Accessed May 05, 2018. [Google Scholar]
- 44. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. [DOI] [PubMed] [Google Scholar]
- 45. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. 2014;35(35):2383–2431. [DOI] [PubMed] [Google Scholar]
- 46. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 47. Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56(14):1071–1078. [DOI] [PubMed] [Google Scholar]
- 48. Schneeweiss S, Robicsek A, Scranton R, et al. Veteran’s Affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. [DOI] [PubMed] [Google Scholar]
- 49. Drahos J, Vanwormer JJ, Greenlee RT, et al. Accuracy of ICD-9-CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol. 2013;23(5):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. [DOI] [PubMed] [Google Scholar]
- 51. Doust JA, Pietrzak E, Dobson A, et al. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330(7492):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.