Abstract
Tuberculosis (TB) has been a leading infectious cause of death worldwide for much of human history, with 1.6 million deaths estimated in 2017. The Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health has played an important role in understanding and responding to TB, and it has made particularly substantial contributions to prevention of TB with chemoprophylaxis. TB preventive therapy is highly efficacious in the prevention of TB disease, yet it remains underutilized by TB programs worldwide despite strong evidence to support its use in high-risk groups, such as people living with HIV and household contacts, including those under 5 years of age. We review the evidence for TB preventive therapy and discuss the future of TB prevention.
Keywords: prevention, TB preventive therapy (TPT), tuberculosis
The Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health has made substantial contributions to tuberculosis (TB) research and public health practice over the past century. Dr. Wade Hampton Frost, the first chair of the Department and later dean of the School, used TB mortality as a model to develop longitudinal cohort analysis, a significant contribution to the field of epidemiology (1). Dr. George Comstock conducted landmark studies of TB community dynamics, bacillus Calmette-Guérin vaccination, and preventive therapy using isoniazid in Alaskan natives. As the impact of the human immunodeficiency virus (HIV) epidemic on TB incidence emerged as an important public health problem, faculty and students in the Department used data from large cohort studies involving people living with HIV (PLHIV), including the Multicenter AIDS Cohort Study (MACS), the AIDS Linked to Intravenous Experience (ALIVE) study, and the Women’s Interagency HIV Study (WIHS), to understand how TB infection affects the natural history of HIV/acquired immune deficiency syndrome (AIDS), to define tuberculin skin test (TST) thresholds for TB infection in PLHIV, and to evaluate the long-term effectiveness of TB preventive therapy (TPT) in PLHIV (2–8). Dr. Richard Chaisson, at Johns Hopkins Center for Tuberculosis Research, led the Consortium to Respond Effectively to the AIDS/TB Epidemic, which evaluated strategies to improve TB/HIV control in high-burden settings. This group of clinical trials contributed to our understanding of TPT efficacy in high-risk populations (9–11). More recently, the Department of Epidemiology’s TB prevention research has focused on modeling the impact (12) and economic evaluation of TPT regimens (13–15), as well as defining strategies for implementation among PLHLIV and young children (16–20). Although the range of TB research topics carried out in the Department is broad, we focus in this review on TB prevention, an area where the contribution is considerable.
BACKGROUND
TB is the leading infectious cause of death in the world. An estimated 1.7 billion people, or a quarter of the world’s population, are currently infected with Mycobacterium tuberculosis and are at risk of developing this disease, which has scourged society for thousands of years (21). In the modern era, TB disproportionately affects vulnerable populations, including young children and PLHIV. Young children are particularly vulnerable given their increased risk of progression to TB disease, including TB meningitis and miliary TB, but also due to diagnostic challenges that contributed to the 1 million cases of childhood TB and 230,000 child deaths in 2017 (22). TB is the leading cause of death among PLHIV, who are 20 times more likely to develop active TB disease than those without HIV disease (23).
Latent tuberculosis is an infection with M. tuberculosis organisms that are contained by host defenses but maintain the capacity to replicate and cause disease in the future (24). TB preventive therapy prevents reactivation of latent TB infection (LTBI), thereby averting progression to TB disease. In low-burden settings, TPT is recommended for treatment of LTBI and recent TB exposure in high-risk groups. In high-burden settings, TPT is recommended for populations with a high risk of progression to TB disease, including PLHIV and young children, independent of their TB infection status. Given the immense size of the latent TB reservoir, treating and preventing reactivation of LTBI will be necessary to achieve the TB elimination goals set forth by the END TB Strategy for 2035 and 2050 (21). However, treating almost 2 billion people is not feasible. Thus, identification and treatment of those most at risk of reactivation is needed.
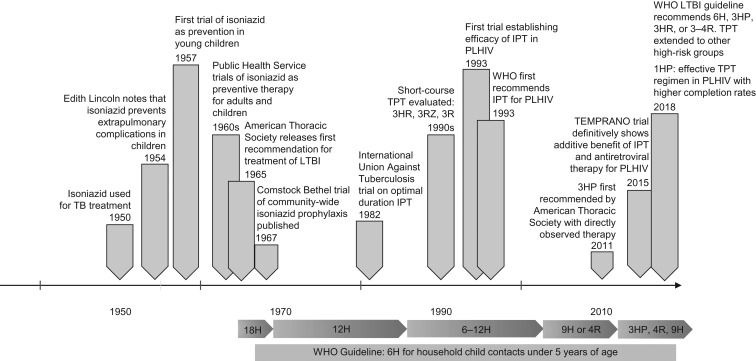
Herein we describe the history of treatment for TB prevention (Figure 1), naming the breakthroughs and champions of these treatments and treatment strategies, the barriers that have prevented widescale uptake of these effective treatments, and future possibilities as these treatments have become shorter, safer, and more effective.
Figure 1.
A history of tuberculosis preventive therapy (TPT). 18H, 12H, 9H, and 6H refer to 18, 12, 9, and 6 months of daily isoniazid, respectively, and 3R and 4R refer to 3 and 4 months of daily rifampin, respectively. 1HP, 1 month of daily rifapentine and isoniazid; 3HP, 3 months of weekly rifapentine and isoniazid; 3HR, 3 months of daily isoniazid and rifampin; 3RZ, 3 months of daily rifampin and pyrazinamide; IPT, isoniazid preventive therapy; LTBI, latent tuberculosis infection; PLHIV, people living with human immunodeficiency virus; TB, tuberculosis; WHO, World Health Organization.
Detecting latent TB infection
Both the TST and the interferon gamma release assays, including the T-SPOT (Oxford Immunotec Ltd., Abingdon, United Kingdom) and QuantiFERON-TB Gold (Cellestis/a Qiagen company, Valencia, California), measure infection with M. tuberculosis by detecting the memory T-cell response. Importantly, these tests do not detect infection directly—they infer the presence of dormant bacteria. These tests suffer from imperfect sensitivity and specificity due to the need for a functioning host immune system (TST and interferon gamma release assays), cross-reaction with bacillus Calmette-Guérin vaccination and other environmental nontuberculous mycobacteria (TST), logistical issues including tuberculin shortages (TST), and costly, labor-intensive laboratory processing (interferon gamma release assays). The search is ongoing for a biomarker that would not only detect an immune response but also predict progression to TB disease (25). This would allow a more feasible approach to treating LTBI and ending the TB epidemic.
Risk groups
Risk factors for progression to TB disease include young age (<5 years), HIV infection, close contact with an infectious TB case, immunocompromising medications including corticosteroids and tumor necrosis factor–α inhibitors, tobacco exposure, poorly controlled diabetes, and chronic renal failure. Worldwide, the World Health Organization (WHO) has focused on providing TPT to 2 groups with the highest risk of TB disease: PLHIV and household child contacts under 5 years of age. In 2018, the World Health Organization expanded its focus to all household contacts and other high-risk groups, including initiation of anti–tumor necrosis factor antibody therapy, receipt of dialysis, preparation for solid organ or hematologic transplant, and silicosis (26).
A HISTORY OF TB PREVENTIVE THERAPY
With the introduction of isoniazid as treatment for tuberculosis in the 1950s, Dr. Edith Lincoln noted that the use of isoniazid prevented common complications in young children, including TB meningitis (27). This important observation led to the hypothesis that isoniazid might be useful not only as treatment but also as prevention for tuberculosis disease. The Public Health Service initiated a controlled clinical trial of isoniazid for treatment of primary TB disease in young children to prevent TB meningitis and other complications (28, 29). Given this trial’s success in preventing immediate extrapulmonary complications of the child’s TB disease, the Public Health Service, led by Dr. Comstock, conducted several trials in the 1950s to assess the efficacy of isoniazid for the prevention of TB in different patient populations (30, 31). In the mid-1950s Dr. Comstock led one of the first cluster-randomized trials of community-wide isoniazid prophylaxis in Bethel, Alaska, where 1% of the population developed active TB disease each year. In the study’s first year, a 69% reduction in TB incidence was observed; this protection lasted at least 5 years. At that time, Dr. Comstock and his colleagues decided to treat all households with isoniazid to provide community-wide protection against TB. This decision was particularly remarkable given that there were few ethical standards for activities following trial completion at that time. With over 7,000 participants, the efficacy of isoniazid monotherapy for TB prevention was established—and estimated to be >60% over 2 years or longer, without a significant difference between 6 and 12 months of therapy (32, 33).
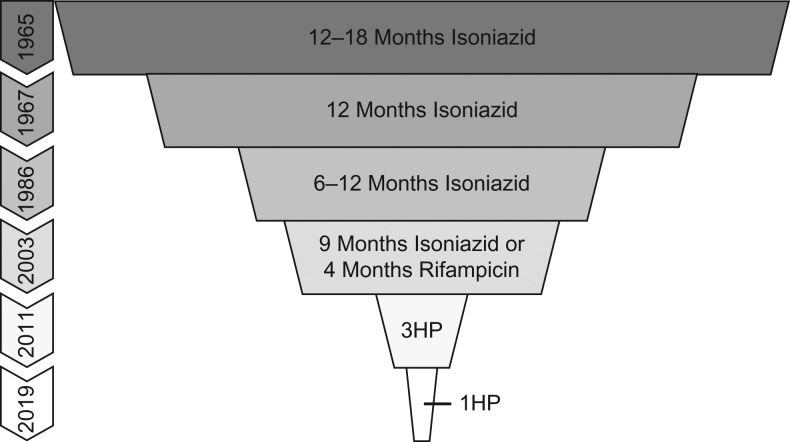
Isoniazid Preventive Therapy: 18 to 9 Months’ Duration
The first official recommendation for isoniazid preventive therapy (IPT) came in 1965 when the American Thoracic Society recommended 12–18 months of isoniazid for TST-positive patients (Figure 2) (34). Based on Dr. Comstock’s and others’ work, 12 months of IPT became the recommended treatment duration in 1967, followed by scale-up of IPT for all TST-positive persons. From long-term follow-up of the Public Health Service trials and the subsequent scale-up of IPT, several important findings were noted. First, the protection of isoniazid in the prevention of TB appeared durable. Second, adherence waned over time, with a rising number of early discontinuations. Data from these early discontinuations in Comstock’s community-wide prophylaxis trial in Bethel led him to hypothesize that as little as 6 months of therapy might be sufficient to prevent progression to TB disease. Third, isoniazid resistance was infrequent and not considered to be relevant, a finding that was verified in subsequent trials (35).
Figure 2.
Duration of tuberculosis preventive therapy over time, as recommended by the American Thoracic Society. Abbreviations: 1HP, 1 month of daily rifapentine and isoniazid; 3HP, 3 months of weekly rifapentine and isoniazid.
Despite the rarity of hepatitis among participants in the Public Health Service trials, clinically significant hepatitis occurred in nearly 1% of patients, with 2 associated deaths, during its widespread use in Washington DC in 1970 (36). More case reports followed, weakening the enthusiasm for widespread use of isoniazid as TB prevention. The US Public Health Service began a surveillance study of nearly 14,000 persons in 21 health departments from 1971 to 1972. The study was stopped in 1972 due to 8 hepatotoxicity-related deaths among study participants, 7 of which occurred in Baltimore city. These findings led to the recommendation for routine monitoring in high-risk populations, including people over age 35 and those who drink alcohol daily (37, 38). Dr. Comstock later noted a simultaneous increase in deaths attributed to cirrhotic liver disease in Baltimore city, suggesting a separate etiology for surveillance study deaths (39, 40). Subsequent studies showed that serious liver disease could be avoided with careful monitoring and early isoniazid discontinuation (40).
To improve adherence, completion rates, and safety, researchers began to study the optimal duration of IPT. In 1982, the International Union Against Tuberculosis Committee on Prophylaxis published a placebo-controlled clinical trial comparing 12, 24, or 52 weeks of isoniazid for the prevention of TB disease. In the intention-to-treat analysis, the 24-week and 52-week regimens were similarly efficacious (65% vs 75%) (41). The per-protocol analysis, including only those who reported good adherence and completed therapy, showed 52 weeks was more effective than 24 weeks of IPT (93% vs 69%) in preventing progression to TB disease (42). Dr. Comstock used these data and data from the Bethel trial to argue that the optimal duration of IPT to maximize efficacy and support adherence is 9 months (42). Ultimately this led to the 2000 American Thoracic Society and Center for Disease Control’s recommendation for 9 months of isoniazid as the preferred regimen (43).
Short-course TB preventive therapy
In the 1990s, trials began to evaluate the efficacy and safety of shorter TB prevention regimens, including 3 months of daily rifampin and pyrazinamide, 3 months of daily rifampin, and 3 months of daily isoniazid and rifampin (44–48). While highly effective, 3 cases of severe hepatotoxicity, with 2 deaths, were observed with 3 months of daily rifampin/pyrazinamide, leading to diminished interest in this regimen (49). A Hong Kong Study in men with silicosis compared 3 months of daily rifampin, 3 months of daily rifampin and isoniazid, and 6 months of isoniazid. Interestingly, 3 months of daily rifampin was superior to 6 months of isoniazid while 3 months of daily isoniazid and rifampin was not, suggesting possible antagonism by isoniazid. These data contributed to a recommendation in the United States in 2003 of 4 months of rifampin as an alternative to 9 months of isoniazid (50). Neither 3 months of daily isoniazid and rifampin nor 3 months of daily rifampin/pyrazinamide were recommended.
A decade later, in 2011, a 12-dose, once-weekly regimen of rifapentine and isoniazid (3HP) was shown to be noninferior to 9 months of daily isoniazid (51). This regimen had superior completion rates and rare adverse events, including hepatotoxicity. In 2015 and 2016, similar results were reported for PLHIV and children aged 2 years or older (52, 53). In 2018, it was shown that 4 months of daily rifampin was noninferior to 9 months of daily isoniazid, again with lower hepatotoxicity and higher completion rates in both adults and children (54, 55). In 2019, the Brief Rifapentine-Isoniazid Evaluation for TB Prevention (BRIEF-TB) trial showed 1 month of daily rifapentine and isoniazid (1HP) to be noninferior to 9 months of isoniazid for TB prevention in PLHIV who had either LTBI or were living in a high-burden setting (56). This study also showed rare adverse events, minimal hepatotoxicity, and superior completion rates. While this regimen requires further study in HIV-uninfected persons with LTBI, children, and pregnant women, it shows tremendous promise to shorten therapy from what was originally 18 months to 4 weeks (56).
TB PREVENTION AND HIV
Interest in TB prevention was renewed in the 1980s with the emergence of HIV-related TB. Among PLHIV, TB was quickly identified as a common opportunistic infection and has remained a leading cause of death for the last 30 years. As early as 1993, studies began to show TPT’s efficacy in preventing TB and delaying mortality in PLHIV in high-TB-incidence settings (45, 57). Continued concerns with toxicity, the durability of protection, drug resistance, and adherence prevented the widespread uptake of TPT despite the first World Health Organization recommendation supporting IPT in PLHIV in 1993 (58). With the introduction of combination antiretroviral therapy (ART) in 1990s and its subsequent scale-up, observational studies began to demonstrate a reduced TB incidence; however, unlike many other opportunistic infections, TB incidence remained high (59–62). In Rio de Janeiro, ecological studies found no difference in the incidence of TB before and after ART (62). The role of IPT in reducing TB incidence among PLHIV who were both ART-eligible and ART-ineligible became the focus of both observational studies and clinical trials. Using medical records and a retrospective cohort design, researchers at Johns Hopkins showed that the combination of IPT and ART in PLHIV significantly reduced TB incidence compared with patients taking ART alone, IPT alone, or neither ART or IPT (63). Importantly, this was independent of CD4 count; those with early and advanced HIV disease benefited from IPT. A similar analysis found a 90% reduction in TB incidence among PLHIV receiving both ART and IPT in South Africa (64), suggesting that IPT might assist with TB control in high-burden settings.
In higher burden settings, observational studies suggested IPT’s benefit might last only 6–18 months (65, 66). It remains unclear whether this poor durability was due to reinfection with M. tuberculosis or inadequate duration of IPT among immunocompromised individuals. Questions remained about the optimal timing of IPT, the appropriate treatment duration, and whether evidence of TB infection should be required for treatment, given its toxicity and duration.
A randomized controlled trial of 36 versus 6 months of isoniazid was conducted in Botswana and showed a 43% reduction in TB incidence with 36 versus 6 months of IPT (67). ART, which was not randomized, also reduced TB incidence by 50% and had an additive effect with IPT. The benefit of IPT was pronounced among those participants who had evidence of TB infection. Durability became a concern because TB incidence started to rise 200 days after completing IPT. The authors attributed this rise in TB disease to reinfection and not poor durability of IPT, citing prior work and known clustering and reinfection rates in Botswana at the time (68–70).
Concurrently, researchers at Johns Hopkins conducted a clinical trial to compare efficacy, safety, and durability of 3HP, 3 months of daily isoniazid and rifampin, and continuous isoniazid to 6 months of isoniazid for TB prevention among PLHIV not yet ART-eligible in South Africa, with high rates of both HIV and TB transmission (71). All 3 regimens had comparable efficacy. The only clinically significant safety concern included nonfatal hepatotoxicity with continuous isoniazid. Failure to demonstrate superiority of continuous isoniazid, as was seen in the Botswana study, might have been due to poor long-term adherence due to adverse events. Importantly, shorter rifamycin-based regimens, including both 3HP and 3 months of daily isoniazid and rifampin, had improved completion rates without evidence for selection of drug-resistant strains.
In the early 2000s, the Gates Foundation provided $80 million to the Johns Hopkins Center for TB Research for the Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE) to determine the impact of new interventions to control the TB and HIV co-epidemics. The TB/HIV in Rio (THRio) study was a pragmatic cluster-randomized trial to evaluate the effectiveness of a TB prevention package including both TST and IPT (9). Despite real-world barriers to implementation, including poor adherence to HIV care, IPT was shown to significantly reduce the incidence of TB and death by 24% among PLHIV who also had TB infection in Brazil. The Thibela study measured the effect of mass screening and treatment among South African gold miners on cluster-level TB incidence 12 months after the intervention (10). They found substantial reductions in TB incidence when participants were receiving preventive therapy; however, this protection was rapidly reduced once preventive therapy was complete. In this high-burden setting, the durability of IPT’s benefit was limited. This might have been due to poor population-level ART coverage, leaving a substantial portion of the population at risk of TB disease, reinfection given extraordinarily high rates of TB transmission in South African mines, or poor durability.
The independent effect of IPT on TB incidence among PLHIV who were on ART remained unclear. In 2014, investigators at the University of Cape Town demonstrated a 37% reduced TB incidence among PLHIV on ART among those with and without evidence of TB infection during a placebo-controlled randomized trial of 12 months of IPT (72). They also showed that the number needed to harm was 4 times that of the number needed to treat, indicating a favorable risk-to-benefit ratio. There was an increase in TB incidence after discontinuation of IPT, attributed to reinfection in this high-TB-incidence setting, ultimately leading to a recommendation supporting long-term use of IPT in all PLHIV in moderate- or high-incidence settings.
The TEMPRANO study was planned with a 2-by-2 factorial design to better understand the separate effects of early ART and IPT (73). Because most of the studies discussed above were conducted in an era when ART was provided only to those who were severely immunocompromised, the added benefit of IPT in the treat-all era was questioned. This study clearly established a survival benefit of IPT that was independent of ART, known TB infection, and CD4 count. Importantly, this benefit lasted over 5 years without signs of waning, demonstrating strong durability of IPT. Independent of early ART, IPT lowered the incidence of severe illness or death by 35% (73) and mortality in PLHIV by 37% (74).
TPT durability
The durability of preventive therapy has long been a concern. The early US Public Health Service’s initial trials showed strong durability of IPT, with protection lasting decades (30, 75). This durability came into question when studying TPT in PLHIV in high-burden settings where the force of infection likely contributes to observed TB cases shortly after TPT completion due to exogenous reinfection (10, 67). In lower-burden settings, including the United States and Brazil, where reinfection was less likely, durability has been shown to be robust (11). In South Africa, continuous isoniazid was as effective at preventing TB as shorter regimens but was poorly tolerated (71). Modeling studies suggested that rifamycin-based regimens, while shorter, might be more curative of latent infection, providing hope that 3HP or 1HP could provide more durable protection (76). The Evaluation of the Effect of 3HP versus Periodic 3HP versus 6H in HIV-Positive Individuals (WHIP3TB; ClinicalTrials.gov: NCT02980016) is comparing periodic 3HP with a single round of 3HP to assess the need for repeat courses of TPT for high-risk populations in areas of high transmission and likely reinfection.
Global implementation of TPT among PLHIV and household contacts less than 5 years old
Despite decades of recommendation, TPT remains poorly implemented worldwide. The World Health Organization estimates that only 23% of household, child TB contacts under 5 years of age received TPT in 2017 (22). Coverage for PLHIV was similarly poor, ranging from 1% to 53% in high-burden countries among newly diagnosed PLHIV (22). For both children and adults, losses early in the cascade of care were the most significant, although every step in the care cascade needs intervention and improvement to reach the World Health Organization goal of initiating TPT among 90% of eligible candidates (22, 77, 78). Health-system, health-care worker, and patient-related barriers contribute to poor implementation (79, 80). Despite strong evidence to the contrary, continued questions about survival benefits, fear of enabling drug resistance, apprehension regarding hepatotoxicity, and concerns about poor durability of protection continue to hinder TPT implementation and have resulted in millions of deaths worldwide (81). Innovative, cost-effective models of care including community-based and integrated facility-based models of care, alongside improved strategies for active case finding, are needed to address this complex set of barriers to ensure successful implementation of TPT and ultimately reduce TB incidence (12, 82).
PREVENTION OF DRUG-RESISTANT TB
The emergence of drug-resistant TB has become a challenge for TB prevention. In 2017, there were over 500,000 drug-resistant TB cases worldwide. Multidrug-resistant TB, defined as TB resistant to both rifampin and isoniazid, leaves no currently recommended preventive regimens likely to be efficacious among contacts of these cases. The Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB; ClinicalTrials.gov: NCT03568383) trial will compare 6 months of delaminid, a new nitro-dihydro-imidazooxazole anti-tuberculosis agent, with 6 months of isoniazid for household contacts of multidrug-resistant TB patients.
THE FUTURE OF TB PREVENTION
Despite the summary of evidence presented above and World Health Organization recommendations for IPT in PLHIV since 1993, implementation of IPT for PLHIV has remained extremely poor in high-burden countries. New, short-course regimens might improve uptake and completion, but these regimens must be coupled with innovative, well-funded implementation approaches to ensure coverage of high-risk populations. Who receives treatment for latent TB has been questioned since isoniazid’s preventive effects were first noted in the 1950s. Currently, we target epidemiologically at-risk populations, but this approach needs further specificity. Biomarkers that predict progression to TB disease might be one answer, and research in this direction is growing. 1HP shows tremendous promise for TB prevention in PLHIV in high-burden settings, but additional research is needed to demonstrate this regimen’s efficacy in HIV-uninfected populations, children, and pregnant women (56). In addition to 1HP and 3HP, mouse studies have shown promise for a 1-time intramuscular injection of bedaquiline for preventing both drug-sensitive and drug-resistant TB (83). Until biomarkers help identify most at-risk populations and 1-time medications are available, we need to continue to target populations epidemiologically and develop innovative strategies to find and treat them.
ACKNOWLEDGMENTS
Author affiliations: Department of Pediatrics, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Nicole Salazar-Austin); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (David W. Dowdy, Richard E. Chaisson, Jonathan E. Golub); and Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Richard E. Chaisson, Jonathan E. Golub).
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K23HD096973 to N.S.-A.) and the National Institutes of Health (grant P30AI094189 to R.E.C.).
Conflict of interest: none declared.
Abbreviations
- 1HP
1 month of daily isoniazid and rifapentine
- 3HP
3 months of weekly isoniazid and rifapentine
- AIDS
acquired immune deficiency virus
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- IPT
isoniazid preventive therapy
- LTBI
latent tuberculosis infection
- PLHIV
people living with human immunodeficiency virus
- TB
tuberculosis
- TPT
tuberculosis preventive therapy
- TST
tuberculin skin test.
REFERENCES
- 1. Comstock GW. Cohort analysis: W.H. Frost’s contributions to the epidemiology of tuberculosis and chronic disease. Soz Praventivmed. 2001;46(1):7–12. [DOI] [PubMed] [Google Scholar]
- 2. López-Gatell H, Cole SR, Hessol NA, et al. Effect of tuberculosis on the survival of women infected with human immunodeficiency virus. Am J Epidemiol. 2007;165(10):1134–1142. [DOI] [PubMed] [Google Scholar]
- 3. López-Gatell H, Cole SR, Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22(14):1869–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golub JE, Astemborski J, Ahmed M, et al. Long-term effectiveness of diagnosing and treating latent tuberculosis infection in a cohort of HIV-infected and at-risk injection drug users. J Acquir Immune Defic Syndr. 2008;49(5):532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadaphal P, Astemborski J, Graham NM, et al. Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with Mycobacterium tuberculosis. Clin Infect Dis. 2001;33(10):1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manoff SB, Farzadegan H, Muñoz A, et al. The effect of latent Mycobacterium tuberculosis infection on human immunodeficiency virus (HIV) disease progression and HIV RNA load among injecting drug users. J Infect Dis. 1996;174(2):299–308. [DOI] [PubMed] [Google Scholar]
- 7. Graham NM, Nelson KE, Solomon L, et al. Prevalence of tuberculin positivity and skin test anergy in HIV-1-seropositive and -seronegative intravenous drug users. JAMA. 1992;267(3):369–373. [PubMed] [Google Scholar]
- 8. Sterling TR, Lau B, Zhang J, et al. Risk factors for tuberculosis after highly active antiretroviral therapy initiation in the United States and Canada: implications for tuberculosis screening. J Infect Dis. 2011;204(6):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13(10):852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370(4):301–310. [DOI] [PubMed] [Google Scholar]
- 11. Golub JE, Cohn S, Saraceni V, et al. Long-term protection from isoniazid preventive therapy for tuberculosis in HIV-infected patients in a medium-burden tuberculosis setting: the TB/HIV in Rio (THRio) study. Clin Infect Dis. 2015;60(4):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowdy DW, Golub JE, Saraceni V, et al. Impact of isoniazid preventive therapy for HIV-infected adults in Rio de Janeiro, Brazil: an epidemiological model. J Acquir Immune Defic Syndr. 2014;66(5):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HY, Hanrahan CF, Martinson N, et al. Cost-effectiveness of universal isoniazid preventive therapy among HIV-infected pregnant women in South Africa. Int J Tuberc Lung Dis. 2018;22(12):1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azadi M, Bishai DM, Dowdy DW, et al. Cost-effectiveness of tuberculosis screening and isoniazid treatment in the TB/HIV in Rio (THRio) Study. Int J Tuberc Lung Dis. 2014;18(12):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson KT, Churchyard GJ, Sohn H, et al. Cost-effectiveness of preventive therapy for tuberculosis with isoniazid and rifapentine versus isoniazid alone in high-burden settings. Clin Infect Dis. 2018;67(7):1072–1078. [DOI] [PubMed] [Google Scholar]
- 16. Kim HY, Dowdy DW, Martinson NA, et al. Maternal priorities for preventive therapy among HIV-positive pregnant women before and after delivery in South Africa: a best-worst scaling survey. J Int AIDS Soc. 2018;21(7):e25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim HY, Dowdy DW, Martinson NA, et al. Maternal motivation to take preventive therapy in antepartum and postpartum among HIV-positive pregnant women in South Africa: a choice experiment. AIDS Behav. 2019;23(7):1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim HY, Hanrahan CF, Dowdy DW, et al. The effect of partner HIV status on motivation to take antiretroviral and isoniazid preventive therapies: a conjoint analysis. AIDS Care. 2018;30(10):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Little KM, Khundi M, Barnes GL, et al. Predictors of isoniazid preventive therapy completion among adults newly diagnosed with HIV in rural Malawi. Int J Tuberc Lung Dis. 2018;22(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salazar-Austin N, Cohn S, Barnes GL, et al. Improving TPT uptake: a cluster-randomized trial of symptom-based versus tuberculin skin test-based screening of household tuberculosis contacts less than 5 years of age [published online ahead of print May 24, 2019]. Clin Infect Dis. (doi: 10.1093/cid/ciz436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization Global Tuberculosis Report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 23. World Health Organization Tuberculosis Fact Sheet: HIV-Associated Tuberculosis. 2018. https://www.who.int/tb/areas-of-work/tb-hiv/tbhiv_factsheet.pdf?ua=1. Accessed February 13, 2018.
- 24. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372(22):2127–2135. [DOI] [PubMed] [Google Scholar]
- 25. Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387(10035):2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Geneva, Switzerland: World Health Organization; 2018. [PubMed] [Google Scholar]
- 27. Lincoln EM. The effect of antimicrobial therapy on the prognosis of primary tuberculosis in children. Am Rev Tuberc. 1954;69(5):682–689. [DOI] [PubMed] [Google Scholar]
- 28. Mount FW, Ferebee SH. Preventive effects of isoniazid in the treatment of primary tuberculosis in children. N Engl J Med. 1961;265(15):713–721. [DOI] [PubMed] [Google Scholar]
- 29. Ferebee S, Mount FW, Anastasiades A. Prophylactic effects of isoniazid on primary tuberculosis in children; a preliminary report. Am Rev Tuberc. 1957;76(6):942–963. [DOI] [PubMed] [Google Scholar]
- 30. Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95(6):935–943. [DOI] [PubMed] [Google Scholar]
- 31. Ferebee SH, Mount FW. Tuberculosis morbidity in a controlled trial of the prophylactic use of isoniazid among household contacts. Am Rev Respir Dis. 1962;85:490–510. [DOI] [PubMed] [Google Scholar]
- 32. Smieja MJ, Marchetti CA, Cook DJ, et al. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;(2):CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayieko J, Abuogi L, Simchowitz B, et al. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis. 2014;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Runyon EH. Preventive treatment in tuberculosis: a statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis. 1965;91:297–298. [DOI] [PubMed] [Google Scholar]
- 35. Balcells ME, Thomas SL, Godfrey-Faussett P, et al. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12(5):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention Summary of the report of the ad hoc advisory committee on isoniazid and liver disease. MMWR Morb Mortal Wkly Rep. 1970;20(26):231–234. [Google Scholar]
- 37. Bass JB Jr., Farer LS, Hopewell PC, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149(5):1359–1374. [DOI] [PubMed] [Google Scholar]
- 38. Kopanoff DE, Snider DE Jr., Caras GJ. Isoniazid-related hepatitis: a US Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117(6):991–1001. [DOI] [PubMed] [Google Scholar]
- 39. Comstock GW. Prevention of tuberculosis among tuberculin reactors: maximizing benefits, minimizing risks. JAMA. 1986;256(19):2729–2730. [PubMed] [Google Scholar]
- 40. Dash LA, Comstock GW, Flynn JP. Isoniazid preventive therapy: retrospect and prospect. Am Rev Respir Dis. 1980;121(6):1039–1044. [DOI] [PubMed] [Google Scholar]
- 41. International Union Against Tuberculosis Committee on Prophylaxis Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982;60(4):555–564. [PMC free article] [PubMed] [Google Scholar]
- 42. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3(10):847–850. [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention Update: fatal and severe liver injuries associated with rifampin and pyrazinamide for latent tuberculosis infection, and revisions in American Thoracic Society/CDC recommendations—United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50(34):733–735. [PubMed] [Google Scholar]
- 44. Halsey NA, Coberly JS, Desormeaux J, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351(9105):786–792. [DOI] [PubMed] [Google Scholar]
- 45. Whalen CC, Johnson JL, Okwera A, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda–Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337(12):801–808. [DOI] [PubMed] [Google Scholar]
- 46. Gordin F, Chaisson RE, Matts JP, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. Terry Beirn Community Programs for Clinical Research on AIDS, the Adult AIDS Clinical Trials Group, the Pan American Health Organization, and the Centers for Disease Control and Prevention Study Group. JAMA. 2000;283(11):1445–1450. [DOI] [PubMed] [Google Scholar]
- 47. Mwinga A, Hosp M, Godfrey-Faussett P, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12(18):2447–2457. [DOI] [PubMed] [Google Scholar]
- 48. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1992;145(1):36–41. [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection—New York and Georgia, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(15):289–291. [PubMed] [Google Scholar]
- 50. Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. [DOI] [PubMed] [Google Scholar]
- 51. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. [DOI] [PubMed] [Google Scholar]
- 52. Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169(3):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sterling TR, Scott NA, Miro JM, et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379(5):440–453. [DOI] [PubMed] [Google Scholar]
- 55. Diallo T, Adjobimey M, Ruslami R, et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med. 2018;379(5):454–463. [DOI] [PubMed] [Google Scholar]
- 56. Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380(11):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pape JW, Jean SS, Ho JL, et al. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342(8866):268–272. [DOI] [PubMed] [Google Scholar]
- 58. Tuberculosis preventive therapy in HIV-infected individuals. A joint statement of the WHO Tuberculosis Programme and the Global Programme on AIDS, and the International Union Against Tuberculosis and Lung Disease (IUATLD), Wkly Epidemiol Rec. 1993;68(49):361–364. [PubMed] [Google Scholar]
- 59. Jones JL, Hanson DL, Dworkin MS, et al. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4(11):1026–1031. [PubMed] [Google Scholar]
- 60. Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. [DOI] [PubMed] [Google Scholar]
- 61. Santoro-Lopes G, de Pinho AM, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34(4):543–546. [DOI] [PubMed] [Google Scholar]
- 62. Pacheco AG, Durovni B, Cavalcante SC, et al. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS One. 2008;3(9):e3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21(11):1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23(5):631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quigley MA, Mwinga A, Hosp M, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15(2):215–222. [DOI] [PubMed] [Google Scholar]
- 66. Johnson JL, Okwera A, Hom DL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15(16):2137–2147. [DOI] [PubMed] [Google Scholar]
- 67. Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–1598. [DOI] [PubMed] [Google Scholar]
- 68. Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171(12):1430–1435. [DOI] [PubMed] [Google Scholar]
- 69. Easterbrook PJ, Gibson A, Murad S, et al. High rates of clustering of strains causing tuberculosis in Harare, Zimbabwe: a molecular epidemiological study. J Clin Microbiol. 2004;42(10):4536–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lockman S, Sheppard JD, Braden CR, et al. Molecular and conventional epidemiology of Mycobacterium tuberculosis in Botswana: a population-based prospective study of 301 pulmonary tuberculosis patients. J Clin Microbiol. 2001;39(3):1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384(9944):682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. TEMPRANO ANRS Study Group, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. [DOI] [PubMed] [Google Scholar]
- 74. Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5(11):e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 75. Hsu KH. Thirty years after isoniazid: its impact on tuberculosis in children and adolescents. JAMA. 1984;251(10):1283–1285. [DOI] [PubMed] [Google Scholar]
- 76. Houben RM, Sumner T, Grant AD, et al. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci U S A. 2014;111(14):5325–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Alsdurf H, Hill PC, Matteelli A, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278. [DOI] [PubMed] [Google Scholar]
- 78. Salazar-Austin N, Cohn S, Barnes GL, et al. A cluster-randomized trial of symptom-based versus TST-based screening of pediatric household TB contacts to improve IPT uptake. Paper presented at: CROI 2018 Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, March 4–7, 2018.
- 79. Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24(suppl 5):S57–S65. [DOI] [PubMed] [Google Scholar]
- 80. Szkwarko D, Hirsch-Moverman Y, Du Plessis L, et al. Child contact management in high tuberculosis burden countries: a mixed-methods systematic review. PLoS One. 2017;12(8):e0182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chaisson RE, Golub JE. Preventing tuberculosis in people with HIV—no more excuses. Lancet Glob Health. 2017;5(11):e1048–e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kendall EA, Azman AS, Maartens G, et al. Projected population-wide impact of antiretroviral therapy-linked isoniazid preventive therapy in a high-burden setting. AIDS. 2019;33(3):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kaushik A, Ammerman NC, Tyagi S, et al. Activity of a long-acting injectable bedaquiline formulation in a paucibacillary mouse model of latent tuberculosis infection. Antimicrob Agents Chemother. 2019;63(4). [DOI] [PMC free article] [PubMed] [Google Scholar]