Abstract
Malaria transmission in northern Zambia has increased in the past decade, despite malaria control activities. Evidence-based intervention strategies are needed to effectively reduce malaria transmission. Zambia’s National Malaria Control Centre conducted targeted indoor residual spraying (IRS) in Nchelenge District, Luapula Province, from 2014 to 2016 using the organophosphate insecticide pirimiphos-methyl. An evaluation of the IRS campaign was conducted by the Southern Africa International Centers of Excellence for Malaria Research using actively detected malaria cases in bimonthly household surveys carried out from April 2012 to July 2017. Changes in malaria parasite prevalence after IRS were assessed by season using Poisson regression models with robust standard errors, controlling for clustering of participants in households and demographic, geographical, and climatological covariates. In targeted areas, parasite prevalence declined approximately 25% during the rainy season following IRS with pirimiphos-methyl but did not decline during the dry season or in the overall study area. Within targeted areas, parasite prevalence declined in unsprayed households, suggesting both direct and indirect effects of IRS. The moderate decrease in parasite prevalence within sprayed areas indicates that IRS with pirimiphos-methyl is an effective malaria control measure, but a more comprehensive package of interventions is needed to effectively reduce the malaria burden in this setting.
Keywords: indoor residual spraying, malaria, targeted indoor residual spraying, vector control, Zambia
Malaria remains the leading nonneonatal cause of child mortality in Zambia, despite significant progress in malaria control (1, 2). Numbers of inpatient malaria cases and deaths in Zambia declined by approximately two thirds between 2000 and 2008 following implementation of universal access to rapid diagnostic tests (RDTs), artemisinin combination therapy, and long-lasting insecticide-treated nets (LLINs), as well as expanded indoor residual spraying (IRS) (3). However, these achievements were not distributed equally across the country, and there has been a resurgence of cases in recent years (4). The World Health Organization estimated that there were 3.1 million malaria cases and 7,000 malaria deaths in Zambia in 2016, an increase of almost 1 million cases since 2010 (1, 2). This increase occurred disproportionately in northern Zambia, where malaria parasite prevalence has increased nearly every year since 2009 (1, 4–6). In Luapula Province, parasite prevalence over 50% has been reported among children under age 5 years, while Lusaka and southern Zambia have maintained parasite prevalence between <1% and 14% in children this age (3, 6). This heterogeneity in malaria control under the same intervention policy, with reversal of progress in northern Zambia, indicates a need to develop and evaluate new intervention strategies for different epidemiologic settings.
Vector control is a key priority for Zambia’s national malaria control strategy (1, 7, 8). In combination with other interventions, IRS has been successful and cost-effective because of the indoor resting and biting behavior of Anopheles funestus and Anopheles gambiae species, the major malaria vectors in the country (3, 9–11). However, increasing resistance to organochlorine, pyrethroid, and carbamate insecticides has reduced the efficacy of IRS in Zambia (6, 9). In response, a novel formulation of the organophosphate pirimiphos-methyl (Actellic 300CS; Syngenta AG, Basel, Switzerland) underwent sensitivity testing in 2013 and was found to be 100% effective against the malaria vectors in Luapula Province (12, 13).
In 2014, Zambia’s National Malaria Control Centre (NMCC) implemented an IRS campaign using pirimiphos-methyl in high-burden districts in northern and central Zambia. The standard strategy for IRS is to spray all eligible households in a district; however, the increased cost of this novel insecticide introduced resource constraints, and the NMCC elected to use a targeted IRS approach in an effort to maximize the impact of the intervention (14). Targeted IRS is an emerging strategy in low-resource settings (15). The approach focuses spraying activities on transmission hot spots in order to concentrate resources on households that make the greatest contribution to local transmission (15). The proposed advantages of this strategy include cost savings and logistical ease.
Although the need for targeted malaria control interventions has been discussed broadly (16–18), the body of published literature on targeted IRS is sparse. The World Health Organization recommends using targeted IRS in areas of low endemicity for residual foci of transmission, and several studies have evaluated this strategy (19–25). Use of targeted IRS in mesoendemic valleys in Burundi resulted in substantial reductions in parasite prevalence and vector densities in targeted areas (20, 21), and targeted IRS in western Kenya produced similar beneficial results (22–24). In northwestern Tanzania, IRS targeted to epidemic-prone villages was associated with reduced malaria prevalence in both targeted and neighboring villages (25). However, to our knowledge, studies have not previously been conducted in high-transmission settings.
As interest in targeted interventions increases, research is needed to determine the effectiveness of this strategy. The increase in malaria transmission in northern Zambia despite active malaria control highlights the need for evidence-based intervention strategies. Our objective in this study was to evaluate the impact of 3 consecutive years of targeted IRS on individual malaria prevalence in Nchelenge District, Luapula Province, a high-transmission area in northern Zambia.
METHODS
NMCC targeted IRS campaign
The NMCC identified 40 high-burden districts in 5 provinces in Zambia, and subdistrict areas were targeted for IRS with pirimiphos-methyl (14, 26). Methods for selecting targeted areas were developed by the Africa Indoor Residual Spraying Project and the Lusaka-based nongovernmental organization Akros, and are described in detail elsewhere (14, 15, 27). In brief, all structures in selected districts were enumerated using publicly available satellite images. A 50-m buffer was drawn around each structure, and contiguous buffers were joined to create operationally manageable clusters of at least 25 households. Clusters were ranked by predicted malaria burden, which was based on population density and malaria incidence in the nearest rural health center, and high-ranking clusters were selected for IRS. Clusters with fewer than 25 households were excluded. Spraying was initiated in October 2014 with the goal of at least 85% coverage (14). The NMCC repeated this strategy in 2015 and 2016 in fewer districts, but in larger targeted areas within selected districts (28, 29). Distribution of LLINs continued in antenatal and vaccination clinics during this time (8).
Study site
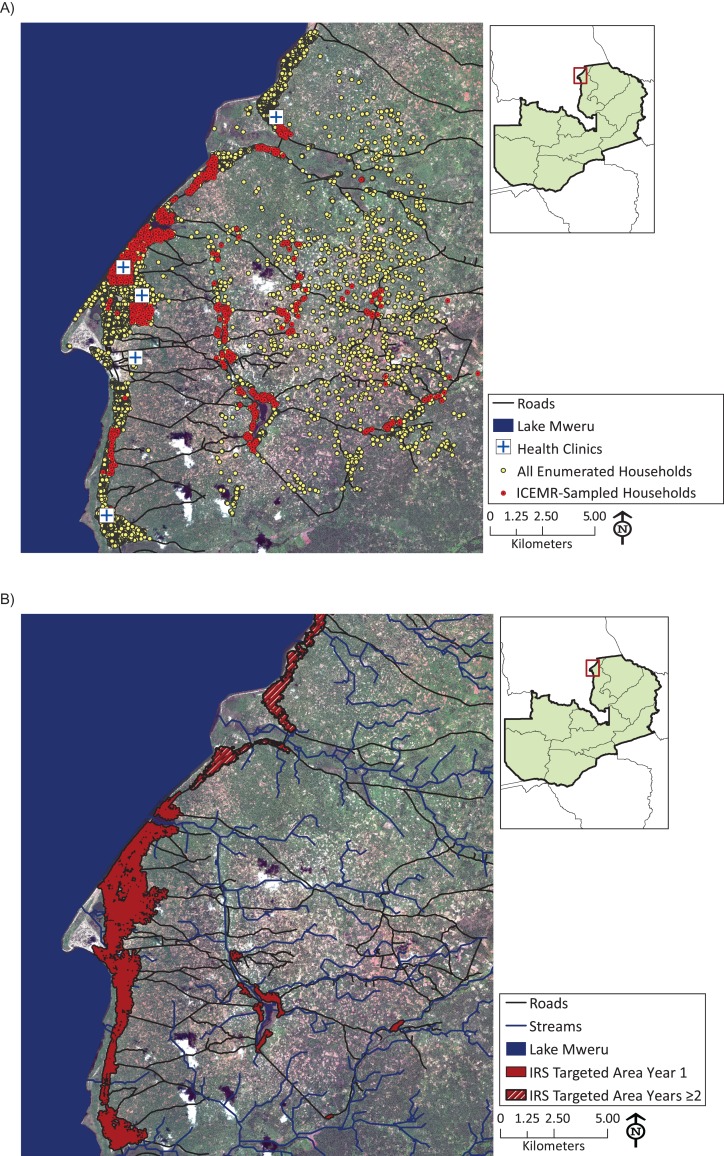
Nchelenge District is a surveillance site for the Southern Africa International Centers of Excellence for Malaria Research (ICEMR) and was among the districts in Zambia selected for targeted IRS. The district is located in the marshlands of the Luapula River along the banks of Lake Mweru, which forms the border with the Democratic Republic of the Congo (Figure 1A). There are approximately 150,000 residents in the district, with an average of 4.7 people per household (30). There is a single rainy season in the region from October to April.
Figure 1.
A) Households sampled and enumerated by the Southern Africa International Centers of Excellence for Malaria Research (ICEMR) in Nchelenge District, Zambia, April 2012–July 2017. B) Areas in Nchelenge District targeted for indoor residual spraying (IRS) in year 1 (2014) and years 2 and 3 (2015 and 2016) of an IRS campaign.
The predominant malaria vectors in Nchelenge District are An. gambiae sensu stricto (s.s.) and An. funestus s.s., and the distribution of these vectors varies across small spatial scales (31, 32). An. funestus is the predominant vector throughout the year, and its abundance peaks during the dry season, whereas An. gambiae abundance peaks during the rainy season in lakeside areas (31, 32). The differing ecology of these vectors and the suitability of local breeding sites supports year-round holoendemic malaria transmission with 2 annual peaks. Since 2012, malaria prevalence as assessed by RDT has averaged 70% in school-age children (ages 5–16 years), and the entomological inoculation rate is approximately 140 infective bites per year (33, 34). Parasite prevalence in the region has increased during the past decade, despite the distribution of LLINs and annual IRS campaigns using pyrethroid (2008–2010) and carbamate (2011–2012) insecticides (6, 8).
Data collection
The Southern Africa ICEMR has conducted active household-based surveillance in Nchelenge District since April 2012 (5). Households in the study area were enumerated using QuickBird satellite images (Digital Global Services, Inc., Denver, Colorado). A 1- × 1-km grid was overlaid on the study area, and grid quadrants were randomly selected. Households were randomly selected within each quadrant using population proportional to size sampling. Every other month, 25 new cross-sectional households were selected to measure trends in parasite prevalence. In alternating months, 25 households were visited longitudinally 6 times per year to measure trends in malaria incidence and were then replaced with a new longitudinal cohort. Household selection was independent of the IRS intervention.
At each study visit, household coordinates were recorded, and a questionnaire was administered to each consenting household member aged 16 years or older and to guardians of children under age 16 years. Participants’ temperatures were taken using a digital ear thermometer, and a blood sample was collected by finger prick for hemoglobin testing and Plasmodium falciparum histidine-rich protein 2–based RDT (SD Bioline; Standard Diagnostics, Yongin, South Korea). All participants positive for P. falciparum by RDT were offered treatment with Coartem (Novartis International AG, Basel, Switzerland). Additional details on the study methods are provided elsewhere (31, 32, 35). Ethical approval was obtained from the Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland) and the Tropical Diseases Research Centre (Ndola, Zambia).
Data management
Data collected electronically from participating households were uploaded into REDCap secure file-sharing software (36). Data collected included sex, age (categorized as <5, 5–16, or >16 years), reported use of LLINs, type of household flooring (natural vs. finished), type of household water source (open vs. protected), head-of-household school attendance, head-of-household employment status, type of roof (thatch vs. metal), and type of household eaves (open vs. closed). Participants were defined to have a fever if their tympanic temperature exceeded 38°C (100.4°F). Anemia was determined using World Health Organization criteria for age and sex (37).
Locations of participating households were plotted in ArcGIS 10.2 (ESRI, Redlands, California). Population density at the location of each household was calculated as the number of other households within 500 m of the home. Geographical variables for roads, stream networks, elevation, slope, and normalized difference vegetation index were created from previously developed geolocated files (35). Streams were categorized using the Strahler classification system, in which 2 small category 1 streams join to form a category 2 stream, and so on (38). Distances to Lake Mweru, health centers, roads, and category 1–4 streams were calculated for each household. Residence in the area targeted for spraying in each year was determined using shapefiles provided by Akros (15).
Climatological data were collected from a HOBO Micro Station (Onset Computer Corporation, Bourne, Massachusetts) located at the field office and through use of an online tool, the African Flood and Drought Monitor (39, 40). Climatological variables included rainfall (in mm/day), evaporation (in mm/day), minimum and maximum daily temperature (in °C), wind speed (in m/second), streamflow (in m3/second), and percent soil moisture. The start and end of the rainy season each year were defined as the first and last weeks in which average rainfall exceeded 1 mm/day.
Statistical analysis
Data for this analysis were collected between April 2012 and July 2017. The primary comparison of interest was the change in parasite prevalence after (vs. before) the implementation of targeted IRS with pirimiphos-methyl. Participant data were included from all visits to cross-sectional households and the first visit to longitudinal households in order to measure individual parasite prevalence. Repeat visits to longitudinal households were excluded.
Data were analyzed using STATA 13.1 (StataCorp LLC, College Station, Texas) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). Regression models were fit by season using the Poisson estimation of the binomial distribution with robust standard errors, which can directly estimate the prevalence rate ratio (PRR) (41–44). Separate models were fit to estimate the change in parasite prevalence at the district level and within targeted areas only. The unit of analysis was the individual study participant, and generalized estimating equations were used to account for clustering of participants within households (45, 46).
Bivariate comparisons were conducted for all variables, and covariates significant at the P = 0.1 level or identified as relevant in the literature were retained for multivariate analyses. Roof type and eave type were not included in multivariate analyses, since they were measured in a subsample of households. A cross-correlation approach was adapted to identify the most etiologically relevant time period for climatological variables (47, 48). The mean values for each climate variable (e.g., rainfall) were calculated at intervals of 1–12 weeks and lags of 1–12 weeks prior to each day of data collection. A preliminary list of the most predictive climate variables was identified by season using random forest algorithms, which can handle a large number of collinear covariates (49, 50). Final model selection was conducted by stepwise regression and Akaike information criterion optimization methods using all relevant variables (51, 52).
For models restricted to targeted areas, a secondary analysis was conducted to investigate the indirect effects of the IRS intervention. Participants in targeted areas were stratified by self-reported history of household IRS, and the change in parasite prevalence after (vs. before) the intervention was determined separately for individuals in sprayed and unsprayed households.
A difference-in-differences analysis was conducted to directly compare the change in parasite prevalence between targeted and untargeted areas. The value of an interaction term in this model (before IRS vs. after IRS × targeted areas vs. untargeted areas) was interpreted as the ratio of the change in parasite prevalence in targeted areas to the change in untargeted areas, or the ratio of risk ratios.
RESULTS
Characteristics of targeted IRS
Targeted IRS with pirimiphos-methyl started in Nchelenge District between September 26 and October 20 each year and continued for 7–10 weeks (14, 28, 29). Targeted areas were primarily located in the periurban lakeside region, because most inland regions did not have a sufficient population density to be eligible (Figure 1B). The number of targeted households in Nchelenge District increased from 18,315 in 2014 to approximately 26,000 in 2015 and 2016 (28, 29).
Study population
A total of 3,309 individuals residing in 1,025 households participated in the study between April 2012 and July 2017, including 2,446 participants in targeted areas and 863 participants in untargeted areas. Approximately 45% of participants were male, 19% were younger than age 5 years, and 34% were school age (ages 5–16 years). At the time of the study visit, 60% of participants were anemic and 2% had a fever. Average parasite prevalence as assessed by RDT was 49.8%. Demographic and clinical characteristics of participants before and after the IRS intervention are provided in Table 1.
Table 1.
Demographic and Clinical Characteristics of Participants in Sprayed and Unsprayed Areas Before and After Indoor Residual Spraying With Pirimiphos-Methyl (n = 3,309), Nchelenge District, Zambia, 2012–2017
| Participant Characteristic | IRS Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sprayed Areas | Unsprayed Areas | |||||||||
| Pre-IRS (n = 1,281) | Post-IRS (n = 1,165) | P Valuea | Pre-IRS (n = 353) | Post-IRS (n = 510) | P Valuea | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| Demographic variables | ||||||||||
| Male sex | 592 | 46.2 | 514 | 44.1 | 0.3 | 160 | 45.3 | 216 | 41.4 | 0.2 |
| Age <5 years | 278 | 21.7 | 181 | 15.5 | <0.001 | 78 | 22.1 | 107 | 20.5 | 0.6 |
| Age 5–16 years | 425 | 33.2 | 422 | 36.2 | 0.1 | 115 | 32.6 | 160 | 30.7 | 0.5 |
| Sleeping under a bed net | 671 | 52.4 | 652 | 56.0 | 0.08 | 210 | 59.5 | 349 | 70.0 | 0.02 |
| Dirt floor in home | 1,134 | 89.1 | 918 | 79.3 | <0.001 | 347 | 99.7 | 501 | 96.0 | 0.001 |
| Open water source | 474 | 37.0 | 437 | 37.7 | 0.7 | 322 | 91.2 | 396 | 75.9 | <0.001 |
| HOH primary schooling only | 799 | 62.6 | 811 | 70.1 | <0.001 | 252 | 71.4 | 412 | 79.2 | 0.008 |
| HOH permanently employed | 96 | 7.5 | 63 | 5.4 | 0.04 | 30 | 8.5 | 12 | 2.3 | <0.001 |
| Clinical results | ||||||||||
| RDT-positive | 643 | 50.2 | 534 | 45.8 | 0.03 | 172 | 48.7 | 303 | 58.1 | 0.007 |
| Use of Coartemb in past month | 294 | 23.0 | 200 | 17.2 | <0.001 | 68 | 19.3 | 98 | 18.8 | 0.9 |
| Feverc | 37 | 2.9 | 15 | 1.3 | 0.006 | 7 | 2.0 | 11 | 2.1 | 0.9 |
| Anemiad | 674 | 52.6 | 766 | 65.8 | <0.001 | 196 | 55.5 | 368 | 80.5 | <0.001 |
Abbreviations: HOH, head of household; IRS, indoor residual spraying; RDT, rapid diagnostic test.
a χ2 analyses.
b Novartis International AG, Basel, Switzerland.
c Fever was defined as tympanic temperature exceeding 38°C (100.4°F).
d Anemia was determined using World Health Organization criteria for age and sex (37).
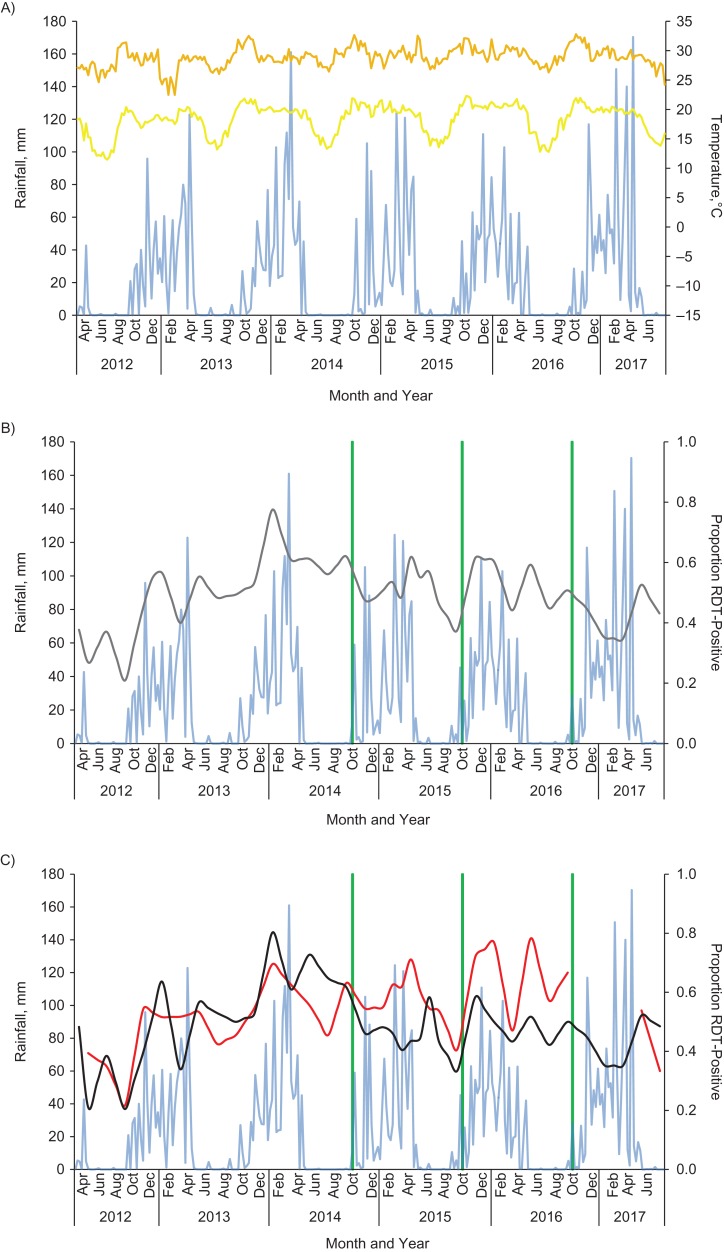
There were 2 annual peaks in parasite prevalence. Overall parasite prevalence ranged from 20% to 80% in targeted areas and from 22% to 78% in untargeted areas (Figure 2). Because of sampling irregularities, no households from untargeted areas participated from November 2016 to March 2017. Over the 3 years of IRS, 54% of households in targeted areas reported that they were sprayed, corresponding to 55% of participants.
Figure 2.
Time series (by month) of weather patterns (A), Plasmodium falciparum parasite prevalence in the overall study area (B), and parasite prevalence in sprayed areas versus unsprayed areas (C), Nchelenge District, Zambia, 2012–2017. Blue, rainfall; yellow, minimum temperature; orange, maximum temperature; green, indoor residual spraying intervention; gray, overall proportion of participants RDT-positive; black, proportion of participants RDT-positive in sprayed areas; red, proportion of participants RDT-positive in unsprayed areas. RDT, rapid diagnostic test.
Impact of targeted IRS on parasite prevalence
In unadjusted χ2 analyses, the odds of prevalent parasitemia decreased 16% in targeted areas after IRS but increased 54% in untargeted areas over this same time period (Table 1). There was also a 66% decrease in fever and a 30% decrease in history of malaria medication use in targeted areas, but there was a 73% increase in anemia. In unadjusted bivariate Poisson models, the change in parasite prevalence was not statistically significant in targeted areas or in the overall study area (see Web Table 1, available at https://academic.oup.com/aje).
In final adjusted regression models for the whole study area, there was no significant change in parasite prevalence following IRS with pirimiphos-methyl in either the rainy season (PRR = 0.89, 95% confidence interval (CI): 0.79, 1.01) or the dry season (PRR = 0.91, 95% CI: 0.80, 1.04) (Table 2).
Table 2.
Impact of Targeted Indoor Residual Spraying With Pirimiphos-Methyl on Malaria Parasite Prevalence in the Entire Study Area, by Season (n = 3,311), Nchelenge District, Zambia, 2012–2017a
| Participant Characteristic | Season | |||||
|---|---|---|---|---|---|---|
| Rainy Season | Dry Season | |||||
| PRR | 95% CI | P Value | PRR | 95% CI | P Value | |
| Post-IRS period (vs. pre-IRS period) | 0.89 | 0.79, 1.01 | 0.07 | 0.91 | 0.80, 1.04 | 0.2 |
| Male sex | 1.17 | 1.07, 1.27 | <0.001 | |||
| Age <5 years | 1.78 | 1.56, 2.03 | <0.001 | 1.88 | 1.61, 2.20 | <0.001 |
| Age 5–16 years | 2.16 | 1.92, 2.42 | <0.001 | 2.32 | 2.02, 2.67 | <0.001 |
| Sleeping under a bed net | 0.82 | 0.74, 0.91 | <0.001 | 0.87 | 0.78, 0.97 | 0.01 |
| HOH primary schooling only | 1.13 | 1.00, 1.27 | 0.04 | 1.17 | 0.97, 1.29 | 0.1 |
| No. of HHs within 500 m (per 100 HH) | 0.92 | 0.90, 0.95 | <0.001 | 0.95 | 0.92, 0.98 | 0.003 |
| Elevation, m (per 10 m) | 0.88 | 0.84, 0.92 | <0.001 | 0.90 | 0.85, 0.95 | <0.001 |
| Distance from health clinics, km | 1.06 | 1.03, 1.09 | <0.001 | 1.07 | 1.02, 1.12 | 0.006 |
| Distance from roads, m (per 100 m) | 0.96 | 0.93, 0.98 | 0.001 | |||
| Distance from category 3 streams, km | 0.85 | 0.72, 1.01 | 0.07 | |||
| Lagged rainfall, mm (per 10 mm)b | 1.19 | 0.98, 1.44 | 0.08 | |||
| Lagged minimum temperature, °Cc | 1.09 | 1.03, 1.15 | 0.002 | |||
| Lagged maximum temperature, °Cd | 1.17 | 1.09, 1.17 | <0.001 | |||
| Lagged streamflow, m3/second (per 1,000 m3/second)e | 0.90 | 0.83, 0.97 | 0.005 | |||
Abbreviations: CI, confidence interval; HH, household; HOH, head of household; IRS, indoor residual spraying; PRR, prevalence rate ratio.
a Multivariate Poisson models using robust standard errors and with responses clustered by household using generalized estimating equations.
b Lag = 1–5 weeks.
c Lag = 2–5 weeks.
d Lag = 3–8 weeks.
e Lag = 4–6 weeks.
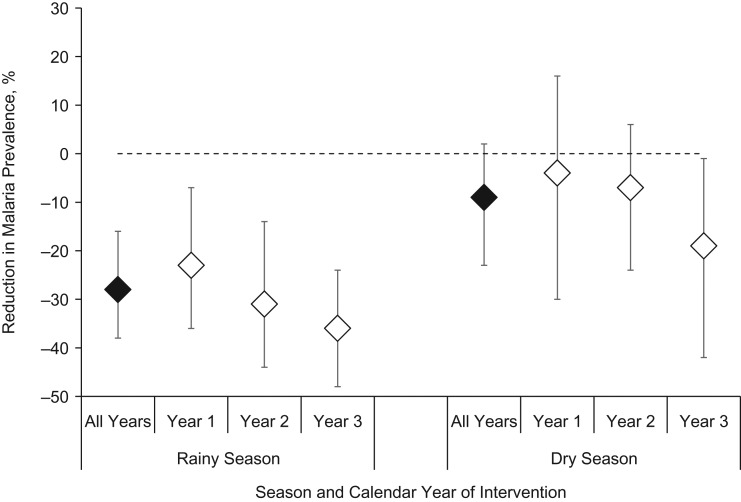
In final adjusted regression models restricted to targeted areas, rainy season parasite prevalence declined 28% following IRS with pirimiphos-methyl (PRR = 0.72, 95% CI: 0.62, 0.84), but there was no change in parasite prevalence during the dry season (PRR = 0.91, 95% CI: 0.80, 1.05) (Table 3). There was no significant difference year-to-year; however, there was a nonsignificant trend toward a greater impact of IRS with each subsequent year (Figure 3). The inclusion of climate variables in multivariate models substantially increased the magnitude of the association and the precision of the point estimate (Web Figure 1).
Figure 3.
Reduction in Plasmodium falciparum parasite prevalence after indoor residual spraying (IRS) in comparison with the pre-IRS time period, by year, Nchelenge District, Zambia, 2012–2017. Year 1, 2014; year 2, 2015; year 3, 2016. Bars, 95% confidence intervals.
Within targeted areas, households were stratified by self-reported history of IRS. Rainy season parasite prevalence declined 33% among participants in sprayed households (PRR = 0.67, 95% CI: 0.63, 0.87) and also declined 26% in unsprayed households (PRR = 0.74, 95% CI: 0.63, 0.87), indicating both a direct and indirect effect of IRS. There was no significant change in dry season parasite prevalence in either sprayed (PRR = 0.88, 95% CI: 0.74, 1.05) or unsprayed (PRR = 0.98, 95% CI: 0.83, 1.15) households within targeted areas.
In the difference-in-differences analysis for the rainy season, the interaction term denoting the ratio of risk ratios was 0.77 (95% CI: 0.62, 0.95), indicating that there was a 23% larger decrease in parasite prevalence in targeted areas compared with untargeted areas. For the dry season, the interaction term was not statistically significant (PRR = 0.83, 95% CI: 0.65, 1.05).
Impact of covariates
The strongest risk factors for prevalent parasitemia were male sex, being of school age (5–16 years), age under 5 years, and having a head of household with only primary education (Table 3). The strongest protective factors were sleeping under a bed net and increased household elevation. Residence in inland rural areas was associated with increased malaria risk, with inland areas having lower population density and greater distance from Lake Mweru and health centers. Proximity to category 1 streams was associated with increased malaria risk in the dry season. In unadjusted models, closed household eaves and metal roof type were associated with lower malaria prevalence (Web Table 1).
Table 3.
Impact of Targeted Indoor Residual Spraying With Pirimiphos-Methyl on Malaria Parasite Prevalence Within Targeted Areas, by Season (n = 2,446), Nchelenge District, Zambia, 2012–2017a
| Participant Characteristic | Season | |||||
|---|---|---|---|---|---|---|
| Rainy Season | Dry Season | |||||
| PRR | 95% CI | P Value | PRR | 95% CI | P Value | |
| Post-IRS period (vs. pre-IRS period) | 0.72 | 0.62, 0.84 | <0.001 | 0.91 | 0.80, 1.05 | 0.2 |
| Male sex | 1.16 | 1.05, 1.29 | 0.004 | |||
| Age <5 years | 1.70 | 1.44, 2.01 | <0.001 | 1.88 | 1.61, 2.20 | <0.001 |
| Age 5–16 years | 2.12 | 1.84, 2.45 | <0.001 | 2.32 | 2.02, 2.67 | <0.001 |
| Sleeping under a bed net | 0.75 | 0.66, 0.86 | <0.001 | 0.87 | 0.78, 0.97 | 0.01 |
| HOH primary schooling only | 1.16 | 1.01, 1.34 | 0.04 | 1.17 | 0.97, 1.29 | 0.1 |
| No. of HHs within 500 m (per 100 HH) | 0.92 | 0.89, 0.95 | <0.001 | 0.95 | 0.92, 0.98 | 0.003 |
| Elevation, m (per 10 m) | 0.88 | 0.83, 0.93 | <0.001 | 0.90 | 0.85, 0.95 | <0.001 |
| Distance from Lake Mweru, km | 1.04 | 1.02, 1.07 | 0.001 | 0.95 | 0.91, 0.99 | 0.02 |
| Distance from health clinics, km | 1.07 | 1.02, 1.12 | 0.006 | |||
| Distance from category 1 streams, km | 0.85 | 0.72, 1.01 | 0.07 | |||
| Lagged rainfall, mm (per 10 mm)b | 1.24 | 1.06, 1.45 | 0.007 | |||
| Lagged minimum temperature, °Cc | 1.15 | 1.08, 1.22 | <0.001 | |||
| Lagged maximum temperature, °Cd | 1.17 | 1.09, 1.17 | <0.001 | |||
| Lagged streamflow, m3/second (per 1,000 m3/second)e | 0.90 | 0.83, 0.97 | 0.005 | |||
Abbreviations: CI, confidence interval; HH, household; HOH, head of household; IRS, indoor residual spraying; PRR, prevalence rate ratio.
a Multivariate Poisson models using robust standard errors and with responses clustered by household using generalized estimating equations.
b Lag = 3–5 weeks.
c Lag = 2–5 weeks.
d Lag = 3–8 weeks.
e Lag = 4–6 weeks.
The climate variables that best predicted malaria risk were lagged rainfall, minimum and maximum temperature, and streamflow, with time lags of 2–8 weeks (Table 3). During the rainy season, there was a 24% increase in malaria risk for each additional 10 mm of rainfall lagged 3–5 weeks (PRR = 1.24, 95% CI: 1.06, 1.45) and a 15% increase in risk for each 1°C increase in minimum temperature lagged 2–5 weeks (PRR = 1.15, 95% CI: 1.08, 1.22). During the dry season, risk increased 17% for each 1°C increase in maximum temperature lagged 3–8 weeks (PRR = 1.17, 95% CI: 1.09, 1.17), and risk decreased 10% for each 1,000 m3/second increase in streamflow lagged 4–6 weeks (PRR = 0.90, 95% CI: 0.83, 0.97).
DISCUSSION
Parasite prevalence in Nchelenge District, Zambia, declined by approximately 25% after IRS with pirimiphos-methyl, but this observation was only seen within targeted areas and only during the 6 months after the intervention. This reduction in parasite prevalence corresponds to a large number of cases averted in this high-transmission region. However, the magnitude of the decline was not as large as anticipated given the scale of the intervention and the efficacy of the insecticide used. Several potential explanations for the lower-than-expected impact are presented below.
Nchelenge District is a particularly challenging setting for malaria control. The region experiences year-round transmission and high entomological inoculation rates, with a predominance of asymptomatic infections resulting in a large untreated parasite reservoir. A lack of paved roads and frequent flooding create barriers to malaria control in remote rural areas. Vector density is high, and studies at other sites have indicated that An. funestus and An. gambiae may have outdoor biting behavior, which could reduce the effectiveness of indoor vector control activities (53–55).
Given these challenges, there are limitations of the current IRS strategy to reduce disease burden in this setting. Although targeted IRS may be effective and economical in regions of Zambia with more moderate transmission, the high level of baseline transmission in Nchelenge District may preclude targeting as an appropriate strategy. The proximity of independently high-transmission untargeted areas may have reduced the impact of the intervention in targeted areas because of movement of people and vectors. Studies have also shown that this formulation of pirimiphos-methyl produces only 5–8 months of insecticidal activity, with a shorter duration of efficacy on the natural or mud walls common in this region (12, 29, 56–58). Therefore, a single application of the insecticide at the start of the rainy season would not be expected to affect dry-season transmission 6 months later. Furthermore, only 54% of households in targeted areas reported receiving IRS—well below the coverage goal of 85% (14).
Zambia has a national goal of malaria elimination by 2021 (8). This objective will be difficult to achieve in Nchelenge District with the current rate of progress, and a substantial increase in resources and intervention coverage will be needed. In high-transmission areas, vectorial capacity must be reduced substantially to interrupt transmission (54, 59, 60). Mathematical models have demonstrated that in order to achieve this, IRS must be performed consistently at high coverage, twice yearly, and for multiple years to have a considerable impact on parasite prevalence in high-transmission areas (61, 62). This combination of interventions could reduce prevalence to 10% (62).
Because of these factors, IRS should continue to be used in Nchelenge District as one of a suite of malaria control activities, but spraying should ideally be conducted twice yearly with at least 85% coverage across the district to have a substantial impact on malaria burden. The significant reduction in parasite prevalence in targeted areas under the current strategy indicates that IRS can be an important part of a vector control strategy and that much larger gains could be made if interventions were scaled up and coverage was increased. The observation of indirect effects of IRS suggests that the impact might increase disproportionately at high levels of coverage. In keeping with Zambia’s current policy, monitoring for insecticide resistance should continue in order to ensure that IRS remains effective (7, 8).
This evaluation had several strengths. The study included a long time series of epidemiologic data, which allowed for investigation of seasonality, interannual variation, and long-term temporal trends. The long time series allowed for the inclusion of climatological covariates, which are often omitted from analyses because of a lack of appropriate multiyear data with which to parameterize models. In this analysis, the impact of the intervention was attenuated when climate variables were not included, indicating the importance of climatological data to evaluate interventions over time. The use of active surveillance data also allowed for a sensitive evaluation of the intervention. Because of the high prevalence of asymptomatic malaria in this population, passively collected data might not accurately represent changes in transmission.
This study had several limitations. Because the overall ICEMR study was designed for long-term surveillance and not for evaluation of a specific intervention, there was limited statistical power to investigate the impact of this IRS campaign at finer spatial and temporal scales. Targeted IRS was conducted programmatically throughout northeastern Zambia and was not conducted as a trial, so there were no areas using a different IRS strategy for comparison. The use of cross-sectional parasite prevalence data rather than malaria incidence data was another limitation, since prevalence is a less direct indicator of recent malaria transmission. However, the long time series of data collected postintervention should have mitigated the difference in metrics, as reduced malaria transmission will ultimately result in reduced prevalence. Prevalence is also a useful indicator of population disease burden and therefore provides a programmatically relevant metric of intervention effectiveness.
Overall, IRS continues to be an important element of vector control. Three years of targeted IRS with pirimiphos-methyl was associated with significant reductions in malaria parasite prevalence within intervention areas in Nchelenge District, but declines were not as large as anticipated because of a variety of structural and logistical challenges. Substantial financial and logistical investments must be made in this region to interrupt malaria transmission, including twice-yearly IRS with high coverage across the district.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Marisa A. Hast, Tamaki Kobayashi, Timothy Shields, Justin Lessler, William J. Moss); Tropical Diseases Research Centre, Ndola, Zambia (Mike Chaponda, Mbanga Muleba, Jean-Bertin Kabuya, James Lupiya, Modest Mulenga); Department of Molecular Microbiology and Immunology and Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Jennifer C. Stevenson, Douglas E. Norris, William J. Moss); and Macha Research Trust, Macha, Zambia (Jennifer C. Stevenson).
This work was supported by funds from the National Institutes of Health (Southern and Central Africa International Centers of Excellence in Malaria Research grant 3U19AI089680), the Bloomberg Philanthropies, and the Johns Hopkins Malaria Research Institute.
We thank Dr. Jessie Pinchoff, Dr. Tom Louis, Dr. Kelly Searle, Anton Kvit, and Dr. Keri Calkins for contributing materials and support for spatial and statistical analysis. We thank the study and field teams at the Tropical Diseases Research Centre for their work across 5 years of data collection. We are grateful to the National Malaria Elimination Centre, to the district health office in the Zambian Ministry of Health, and to the communities of Nchelenge District, Zambia, for their participation.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- ICEMR
International Centers of Excellence for Malaria Research
- IRS
indoor residual spraying
- LLIN
long-lasting insecticide-treated net
- NMCC
National Malaria Control Centre
- PRR
prevalence rate ratio
- RDT
rapid diagnostic test
REFERENCES
- 1. World Health Organization World Malaria Report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2. Institute for Health Metrics and Evaluation GBD Compare | Viz Hub. Global—both sexes, all ages, 2017, DALYs. 2015. http://vizhub.healthdata.org/gbd-compare. Accessed December 4, 2017.
- 3. Masaninga F, Chanda E, Chanda-Kapata P, et al. Review of the malaria epidemiology and trends in Zambia. Asian Pac J Trop Biomed. 2013;3(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamuliwo M, Chanda E, Haque U, et al. The changing burden of malaria and association with vector control interventions in Zambia using district-level surveillance data, 2006–2011. Malar J. 2013;12:Article 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mharakurwa S, Thuma PE, Norris DE, et al. Malaria epidemiology and control in Southern Africa. Acta Trop. 2012;121(3):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mukonka VM, Chanda E, Haque U, et al. High burden of malaria following scale-up of control interventions in Nchelenge District, Luapula Province, Zambia. Malar J. 2014;13:Article 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Malaria Control Programme, Ministry of Health, Republic of Zambia National Malaria Control Programme Strategic Plan for FY 2011–2015 Lusaka, Zambia: Zambia Ministry of Health; 2011. http://www.nationalplanningcycles.org/sites/default/files/country_docs/Zambia/zambia_malaria_nsp_2011-2015_.pdf. Accessed December 12, 2017.
- 8. President’s Malaria Initiative President’s Malaria Initiative: Zambia—Malaria Operational Plan FY 2018 Bethesda, MD: Abt Associates, Inc.; 2017. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-2018/fy-2018-zambia-malaria-operational-plan.pdf?sfvrsn=7. Accessed December 7, 2017.
- 9. Chanda E, Hemingway J, Kleinschmidt I, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS One. 2011;6(9):e24336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zahar AR. Vector Bionomics in the Epidemiology and Control of Malaria. Geneva, Switzerland: World Health Organization; 1985. [Google Scholar]
- 11. Yukich JO, Lengeler C, Tediosi F, et al. Costs and consequences of large-scale vector control for malaria. Malar J. 2008;7:Article 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chanda E, Chanda J, Kandyata A, et al. Efficacy of ACTELLIC 300 CS, pirimiphos methyl, for indoor residual spraying in areas of high vector resistance to pyrethroids and carbamates in Zambia. J Med Entomol. 2013;50(6):1275–1281. [DOI] [PubMed] [Google Scholar]
- 13. Choi KS, Christian R, Nardini L, et al. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:Article 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. President’s Malaria Initiative PMI | Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Four. Zambia 2014 End of Spray Report Bethesda, MD: Abt Associates Inc.; 2015. https://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/zambia-end-of-spray-report-2014-indoor-residual-spraying-irs-2-task-order-four-africa-irs-airs-project.pdf?sfvrsn=4. Accessed March 7, 2017.
- 15. Pinchoff J, Larsen DA, Renn S, et al. Targeting indoor residual spraying for malaria using epidemiological data: a case study of the Zambia experience. Malar J. 2016;15:Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78(12):1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 17. Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9(1):e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dolgin E. Targeting hotspots of transmission promises to reduce malaria. Nat Med. 2010;16(10):1055. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission, Control and Elimination. 2nd ed Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 20. Protopopoff N, Van Bortel W, Marcotty T, et al. Spatial targeted vector control in the highlands of Burundi and its impact on malaria transmission. Malar J. 2007;6:Article 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Protopopoff N, Van Bortel W, Marcotty T, et al. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am J Trop Med Hyg. 2008;79(1):12–18. [PubMed] [Google Scholar]
- 22. Zhou G, Githeko AK, Minakawa N, et al. Community-wide benefits of targeted indoor residual spray for malaria control in the western Kenya highland. Malar J. 2010;9:Article 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulambalah CS, Siamba DN, Ngeiywa MM, et al. Targeted indoor insecticide and malaria control in the western highlands Kenya. J Infect Dis Immun. 2011;3(3):50–58. [Google Scholar]
- 24. Bousema T, Stresman G, Baidjoe AY, et al. The impact of hotspot-targeted interventions on malaria transmission in Rachuonyo South District in the western Kenyan highlands: a cluster-randomized controlled trial. PLoS Med. 2016;13(4):e1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mashauri FM, Kinung’hi SM, Kaatano GM, et al. Impact of indoor residual spraying of lambda-cyhalothrin on malaria prevalence and anemia in an epidemic-prone district of Muleba, north-western Tanzania. Am J Trop Med Hyg. 2013;88(5):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musonda PT, Acquaye A, Chandonait P. Zambia Supplemental Environmental Assessment for Indoor Residual Spraying for Malaria Control 2015–2000 Lusaka, Zambia: The PMI AIRS Project, Abt Associates, Inc.; 2015. https://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/zambia-irs-supplemental-environmental-assessment-2015-2020.pdf. Accessed March 7, 2017.
- 27. Kamanga A, Renn S, Pollard D, et al. Open-source satellite enumeration to map households: planning and targeting indoor residual spraying for malaria. Malar J. 2015;14:Article 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. President’s Malaria Initiative PMI | Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Six. Zambia 2015 End of Spray Report Bethesda, MD: Abt Associates, Inc.; 2016. https://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/zambia-end-of-spray-report-2015-indoor-residual-spraying-(irs-2)-task-order-six-africa-irs-(airs)-project.pdf?sfvrsn=4. Accessed December 8, 2017.
- 29. President’s Malaria Initiative . PMI | Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Six. Zambia 2016 End of Spray Report Bethesda, MD: Abt Associates, Inc.; 2017. https://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/zambia-end-of-spray-report-2016-indoor-residual-spraying-irs-2-task-order-six-africa-irs-airs-project.pdf. Accessed December 8, 2017.
- 30. Central Statistical Office, Republic of Zambia Zambia 2010 Census of Population and Housing: National Analytical Report Lusaka, Zambia: Zambia Central Statistical Office; 2011. https://www.zamstats.gov.zm/phocadownload/2010_Census/2010%20Census%20of%20Population%20National%20Analytical%20Report.pdf. Accessed January 15, 2018.
- 31. Das S, Muleba M, Stevenson JC, et al. Habitat partitioning of malaria vectors in Nchelenge District, Zambia. Am J Trop Med Hyg. 2016;94(6):1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevenson JC, Pinchoff J, Muleba M, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for vector control. Parasit Vectors. 2016;9:Article 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinchoff J, Chaponda M, Shields TM, et al. Individual and household level risk factors associated with malaria in Nchelenge District, a region with perennial transmission: a serial cross-sectional study from 2012 to 2015. PLoS One. 2016;11(6):e0156717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moss WJ, Dorsey G, Mueller I, et al. Malaria epidemiology and control within the International Centers of Excellence for Malaria Research. Am J Trop Med Hyg. 2015;93(3 suppl):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinchoff J, Chaponda M, Shields T, et al. Predictive malaria risk and uncertainty mapping in Nchelenge District, Zambia: evidence of widespread, persistent risk and implications for targeted interventions. Am J Trop Med Hyg. 2015;93(6):1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 38. Tarboton D, Bras R, Rodriguez-Iturbe I. On the extraction of channel networks from digital elevation data. Hydrol Process. 1991;5(1):81–100. [Google Scholar]
- 39. Sheffield J, Wood EF, Chaney N, et al. A drought monitoring and forecasting system for sub-Sahara African water resources and food security. Bull Am Meteorol Soc. 2014;95(6):861–882. [Google Scholar]
- 40. Princeton Climate Analytics African Flood and Drought Monitor. Version 1.2. https://platform.princetonclimate.com/PCA_Platform/afdmLanding.html. Accessed September 1, 2017.
- 41. Zou GY. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 42. Skov T, Deddens J, Petersen MR, et al. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27(1):91–95. [DOI] [PubMed] [Google Scholar]
- 43. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65(7):501–506. [DOI] [PubMed] [Google Scholar]
- 45. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 46. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 47. Kvit A. The Effect of Drought Associated Indicators on Malaria in the Choma District of Zambia [master’s thesis]. Baltimore, MD: Bloomberg School of Public Health, Johns Hopkins University; 2017.
- 48. Curriero FC, Shone SM, Glass GE. Cross correlation maps: a tool for visualizing and modeling time lagged associations. Vector Borne Zoonotic Dis. 2005;5(3):267–275. [DOI] [PubMed] [Google Scholar]
- 49. Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 50. Liaw A, Wiener M. Classification and regression by randomForest. R News 2002;2(3):18–22. [Google Scholar]
- 51. Yamashita K, Kamimura R. A stepwise AIC method for variable selection in linear regression. Commun Stat Theory Methods. 2007;36(13):2395–2403. [Google Scholar]
- 52. Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–220. [Google Scholar]
- 53. Killeen GF. A second chance to tackle African malaria vector mosquitoes that avoid houses and don’t take drugs. Am J Trop Med Hyg. 2013;88(5):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:Article 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kabbale FG, Akol AM, Kaddu JB, et al. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District, Uganda. Parasit Vectors. 2013;6:Article 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haji KA, Thawer NG, Khatib BO, et al. Efficacy, persistence and vector susceptibility to pirimiphos-methyl (Actellic 300CS) insecticide for indoor residual spraying in Zanzibar. Parasit Vectors. 2015;8:Article 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mashauri FM, Manjurano A, Kinung’hi S, et al. Indoor residual spraying with micro-encapsulated pirimiphos-methyl (Actellic® 300CS) against malaria vectors in the Lake Victoria basin, Tanzania. PLoS One. 2017;12(5):e0176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yewhalaw D, Balkew M, Shililu J, et al. Determination of the residual efficacy of carbamate and organophosphate insecticides used for indoor residual spraying for malaria control in Ethiopia. Malar J. 2017;16:Article 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61(1):109–113. [DOI] [PubMed] [Google Scholar]
- 60. MacDonald G. The Epidemiology and Control of Malaria. London, United Kingdom: Oxford University Press; 1957. [Google Scholar]
- 61. Kolaczinski K, Kolaczinski J, Kilian A, et al. Extension of indoor residual spraying for malaria control into high transmission settings in Africa. Trans R Soc Trop Med Hyg. 2007;101(9):852–853. [DOI] [PubMed] [Google Scholar]
- 62. Griffin JT, Hollingsworth TD, Okell LC, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7(8):e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.