Abstract
Sex differences in diseases involving oxidative and proteolytic stress are common, including greater ischemic heart disease, Parkinson disease and stroke in men, and greater Alzheimer disease in women. Sex differences are also observed in stress response of cells and tissues, where female cells are generally more resistant to heat and oxidative stress-induced cell death. Studies implicate beneficial effects of estrogen, as well as cell-autonomous effects including superior mitochondrial function and increased expression of stress response genes in female cells relative to male cells. The p53 and forkhead box (FOX)-family genes, heat shock proteins (HSPs), and the apoptosis and autophagy pathways appear particularly important in mediating sex differences in stress response.
Keywords: Oxidative stress, Proteostasis, Heat shock, Sex differences, Sexual dimorphism, Sexual antagonistic pleiotropy
Graphical abstract
1. Definitions of sex differences
Sex differences refers to all differences between male and female, including metabolism, gene expression, dosage compensation, cellular differentiation, anatomical structures, behaviors, stress responses and life span. The related term sexual dimorphism has traditionally been used to refer to morphological differences between the sexes, but more recently has also been used to refer to all sex differences. Sexual differentiation refers to the development and maintenance of sex differences. Mechanisms for sexual differentiation vary across species, and include inheritance of sex chromosome, inheritance of sex-regulatory alleles, and environmental inputs.
2. Mechanisms of sexual differentiation and dosage compensation
A common mechanism for sex determination is inheritance of sex chromosomes. For example, in Drosophila and human, X/X determines female and X/Y determines male (Fig. 1). In contrast, birds use a ZW system, where females are Z/W and males are Z/Z. In C. elegans, X/X determines the hermaphrodite, and X/O (presence of only one X chromosome) determines the male. In other species, such as the copepod Tigriopus californicus, there are no specific sex chromosomes, and sex is determined by the inheritance of multiple male-promoting and female-promoting segregating alleles, located on multiple chromosomes [1]. Finally, in many species, environmental inputs play a regulatory role in sex determination. For example, temperature dependent sex determination is often observed in reptiles and fish [2].
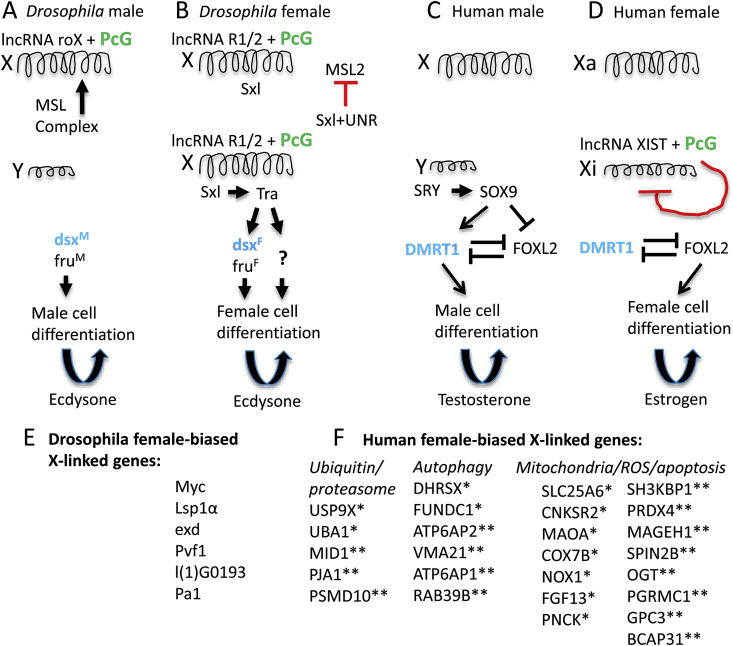
Fig. 1.
Sex determination and dosage compensation in Drosophila and human. The sex determination and dosage compensation pathways are outlined for Drosophila and human, with an emphasis on similarities. LncRNAs and the conserved PcG proteins (indicated in green font) function to regulate X-linked gene expression and dosage compensation in both species. Similarly, the conserved doublesex-related transcription factor (indicated in blue font) promotes male cell differentiation in both species. (A) In Drosophila males, the male-specific-lethal complex (MSL) interacts with lncRNA roX and PcG proteins to activate gene expression along the X chromosome, thereby contributing to dosage compensation. The double-sex (dsx) and fruitless (fru) gene transcripts splice in the default male mode, thereby yielding the male isoform of each transcription factor. These male isoforms of Dsx and Fru then direct male somatic cell differentiation. The steroid hormone ecdysone further regulates spermatogenesis. (B) In Drosophila females, the lncRNAs R1 and R2 interact with PcG proteins to activate expression of the Sex-lethal gene (Sxl). Sxl protein interacts with partner UNR to inhibit the translation of the male-specific lethal (MSL) subunit MSL2 (as indicated by red t-bar). Because the MSL complex is not formed, it does not activate X chromosome gene expression in the female, and this limited X chromosome gene expression contributes to dosage compensation. Sxl protein regulates splicing of the transformer (tra) gene, and the resultant Tra protein in turn regulates splicing of dsx and fru gene transcripts in the female mode, to produce the female isoform of each transcription factor. These female isoforms of Dsx and Fru then direct female somatic cell differentiation. Tra protein also acts through an unknown mechanism (indicated by question mark) to regulate additional gene expression and female somatic cell differentiation. The steroid hormone ecdysone further regulates oogenesis. (C) In human males, the SRY gene on the Y chromosome activates expression of the SOX9 gene. SOX9 activates DMRT1 and inhibits FOXL2, thereby shifting the balance in activity between these two mutually-antagonistic factors towards DMRT1. DMRT1 promotes male cell differentiation. Male cell differentiation yields greater production of male-biased hormones, including testosterone, which further promotes male cell differentiation and spermatogenesis. (D) In human females, the lncRNA Xist interacts with PcG proteins to inactivate gene expression in cis along one X chromosome (Xi, as indicated by curved red t-bar). On the other X chromosome, Xist is not expressed, and gene expression remains active (Xa). The balance of activity between DMRT1 and FOXL2 favors FOXL2, which in turn promotes female cell differentiation. Female cell differentiation yields greater production of female-biased hormones, including estrogens, which further promotes female cell differentiation and oogenesis. (E) In Drosophila, several X-linked genes show incomplete dosage compensation and female-biased expression. (F) In human females, numerous X-linked genes “escape” from X-inactivation to varying degree, resulting in female-biased expression (indicated by single asterisk). Additional X-linked genes show female-biased expression in certain studies, that may or may not be related to the X-inactivation mechanism (indicated by double asterisk). The genes presented are limited to examples that fall under the general functional categories “Ubiquitin/proteasome”, “Autophagy” and “Mitochondria/ROS/apoptosis”. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Species with sex chromosomes have a different gene dosage between the sexes. For example, human and Drosophila females have two copies of each X chromosome gene, whereas males of each species have only one copy. These species exhibit dosage compensation mechanisms to equalize gene expression levels between the two sexes, and therefore dosage compensation is one aspect of sexual differentiation (Fig. 1). Recent studies reveal that dosage compensation mechanisms have several similarities across species, including regulation by a master switch gene (X-inactive specific transcript, XIST, in human, and Sex-lethal in Drosophila), that is in the on-state in females, and in the off-state in males [3]. These switch genes regulate pathways that limit X chromosome gene expression in the female. In Drosophila, the switch gene on/off state also regulates other aspects of sexual differentiation, including morphological differences.
Traditionally, in humans and other mammals, female differentiation was thought to be the default state, while male differentiation was determined by the presence of the sex-determining region Y (SRY) gene, located on the Y chromosome. However, recent studies reveal that human sex determination is regulated at the level of the cell by the balance of transcription factors, including the doublesex- and mab-3 related transcription factor 1 (DMRT1) and the SRY-related HMG-box 9 (SOX9) transcription factor (both male-promoting), and the forkhead box protein L2 (FOXL2) transcription factor (female-promoting). These factors continue to be required to maintain cellular sexual identify in the adult [4,5]. In females, several factors favor FOXL2 activity, whereas in males the powerful SRY modifier favors DMRT1 and SOX9 activity. Notably, DMRT1-related genes are involved in male sex determination across multiple species, including Drosophila (doublesex), C. elegans (male abnormal 3, Mab-3), and Zebrafish (Dmrt1) [6], consistent with partial conservation of sex determination mechanisms [3,7]. Intriguingly, the stress response gene growth arrest and DNA-damage-inducible protein GADD45 gamma (GADD45G) is required for male sexual differentiation in mice, through activation of the oxidative stress-response factor p38 mitogen-activated protein kinase (p38MAPK). P38MAPK activates transcription factor GATA4, which is required for expression of SRY [8,9]. Consistent with these observations, the mouse gene mitogen-activated protein kinase kinase 4 (MAP3K4) is required for SRY activation and male sexual differentiation [10,11]. These data implicate greater oxidative stress levels in male cells as a contributor to mammalian male sex determination, through activation of MAPK signaling; conceivably this may be related to mother's curse, as discussed below.
Several studies reveal how genotypic and environmental ques can converge in sex determination. In many species with defined sex chromosomes, environmental temperature acts as the predominant determinant of sex, and again this involves tipping the balance of transcription factor activities [12,13]. For example, in the turtle, Dmrt1 expression is a primary determinant of sex. Turtle Dmrt1 is highly expressed at male-producing temperatures (~26 °C), and shows lower expression at female-producing temperatures (~32 °C) [14]. Conversely, alterations in genotype can sometimes override temperature determination of sex. In the turtle, downregulation of lysine demethylase 6B (KDM6B) was shown to suppress Dmrt1, resulting in female determination (albeit in embryos subjected to male-favored temperatures) [15]. Finally, studies with P. vitticeps provided the first demonstration of a naturally occurring sex reversal, which in turn enabled the experimental transition from a primarily genotypic to a temperature-dependent sex determination system [16], illustrating the evolutionary fluidity between these sex determination mechanisms.
In each of the species, once sex is determined (at least partly) at the level of individual cells, these cells produce hormones, including steroid and steroid-like hormones, that further promote differentiation of both male and female (Fig. 1). These differences in gene expression and hormone levels between the sexes are found to contribute to sex-differences in stress response, including a general female advantage in cellular stress resistance.
3. Sex chromosome gene dosage and X-linked gene mutations
Sex chromosome gene dosage has several implications for sex differences in stress response. In species where males have only one X chromosome, any X-linked mutations will be expressed (the “unguarded X″) [17,18], whereas in females, the second X chromosome may contribute a wild-type copy of the gene and the phenotype will not be expressed. The preferential expression of X-linked mutations in males might possibly contribute to less effective stress response in males.
4. Sex chromosome gene dosage and X-linked gene expression
In both humans and rodents, numerous X-linked genes “escape” from X-inactivation to varying degrees, leading to increased expression of these genes in the female relative to the male [[19], [20], [21]]. Several of the human genes that escape X-inactivation encode proteins involved in the regulation of protein turnover, autophagy, redox state and apoptosis, and are potentially relevant to sex differences in stress response (Fig. 1). For example, ubiquitin-like modifier activating enzyme 1 (UBA1) catalyzes the first step in ubiquitination, resulting in the targeting of proteins for degradation by the proteasome. UBA1 is responsible for the majority of protein ubiquitination in humans [22]. Studies in cultured human cells indicate that dehydrogenase/reductase (short-chain dehydrogenase family) X-linked (DHRSX) is a positive regulator of starvation-induced autophagy [23]. Similarly, FUN14 domain containing 1 (FUNDC) is an outer mitochondrial membrane protein that marks mitochondria for destruction by autophagy (mitophagy) [24]. FUNDC-regulated mitophagy is reported to protect mammalian nerve cells from hypoxia-induced apoptotic cell death, and to protect cardiomyocytes from ischemia/reperfusion induced apoptotic cell death [25,26]. FUNDC is also reported to interact with heat shock cognate 71 kDa protein (HSC70) to facilitate import of cytosolic proteins into the mitochondrial matrix, where they are subsequently degraded by the Lon peptidase 1 mitochondrial (LONP1) protease [27]. Solute carrier family 25 member 6 (SLC25A6) is a subunit of the mitochondrial ATP/ADP transporter, which is implicated in regulation of apoptosis and the etiology of muscular dystrophy and cancer [28]. Fibroblast growth factor 13 (FGF13) encodes an intracellular growth factor, which is implicated in the sensing of heat, as well as enhancing axonal mitochondrial function and regeneration after spinal cord injury [29,30]. X-linked genes have also been implicated in sex-differences in immune response. Cluster of differentiation 40 ligand (CD40LG) shows variable escape from X-inactivation, and encodes a ligand for the CD40 receptor involved in activation of T-cells and B-cells. O-GlcNAc transferase (OGT) has been reported to show a female bias in expression [21]; OGT targets include p53, HSPs, and several pro-inflammatory regulators [31]. CD40LG and OGT are implicated in conferring a beneficial immune advantage to women relative to men, but may also be involved in the increased incidence of autoimmune disorders in women [32], and conceivably might contribute to other female-biased diseases involving immune response, such as Alzheimer disease. Also among the X-linked genes in humans are two that encode key stress response proteins: Glucose-6-phosphate dehydrogenase (G6PD) is a primary producer of reducing equivalents required for oxidative stress resistance, and X-linked inhibitor of apoptosis protein (XIAP) is an inhibitor of apoptosis. Whereas these two genes have not been identified as escaping from X-inactivation, increased expression of G6PD is implicated in the greater resistance of female mouse pre-implantation embryos to heat stress relative to male embryos [33], and XIAP is implicated in conferring increased resistance to ischemic brain injury in female mice relative to male mice [33,34].
The Y chromosome is implicated in regulation of stress response in several ways. In rodents, SRY has been found to directly regulate expression of catecholamine pathway genes in the brain, and is implicated in the preferential “fight-or-flight” stress response in males [35]. SRY is upregulated in rodent models and human cell culture models of Parkinson disease, and experimental downregulation of SRY decreased the male bias in neurodegeneration phenotype in a rat model of Parkinson disease [36]. Also in mice, the Y chromosome was protective against hypoxia-induced pulmonary hypertension, independent of gonadal hormones [37].
Escape from dosage compensation is also observed in Drosophila, where several genes show female-biased expression proportional to the number of X chromosomes (Fig. 1). For example, the conserved gene Myc is not dosage compensated, and its greater expression in females leads to greater cell growth and contributes to the larger size of females relative to males [38].
Finally, as a result of sexual differentiation, numerous genes on the autosomes are expressed in a sex-biased manner in humans and Drosophila, and are likely to contribute to sex differences in stress resistance [[39], [40], [41], [42]].
5. Sexual antagonistic pleiotropy (SAP) and mother's curse
In humans, gene alleles segregating in the population are thought to contribute to disease, including sex-biased diseases such as cancer [43] and neurodegenerative disease [44,45]. These deleterious alleles are thought to be maintained in the population in part due to sex-specific selective pressures [[7], [46]]. Gene alleles can be selected for and maintained in the population because they have a benefit for one sex, despite the fact that the same allele may have a deleterious effect in the other sex; therefore, these are genes that exhibit sexual antagonistic pleiotropy (SAP). Consistent with this idea, segregating alleles of certain stress response genes show sex-specific effects in humans. For example, a reduced-activity allele of p53 was associated with increased life span in women but not men [47]. Similarly, an allele of the p53 regulatory factor gene mouse double minute 2 homolog (MDM2) increased cancer in women not men, whereas in p53 mutation carriers, the p53 PIN3 polymorphism increased cancer in men but not women [48,49]. Similarly, the FOXO-family gene FOXO3 is an important regulator of stress response, and several segregating alleles of FOXO3 are associated with longevity in humans [50,51].
The gene composition of the sex chromosomes illustrates sex-specific selection. For example, in organisms with X/Y sex determination, such as humans and Drosophila, the Y chromosome is inherited only through the male, and therefore Y chromosome genes are optimized only for function in the male. Consequently, Y chromosome genes are typically involved in male-specific functions such as spermatogenesis and male sex determination [52]. In contrast, X chromosome genes are expected to be under greater female selection. Because females have two X chromosome and males have only one, X-linked genes spend twice as much time under selection in females relative to males. Consistent with this, the X chromosome is enriched for genes that are highly expressed in females in Drosophila, nematodes and mice [[53], [54], [55]]. In Drosophila, genes that regulate oogenesis and sex determination are over-represented on the X chromosome, whereas genes involved in spermatogenesis and apoptosis are under-represented, consistent with sex differences in selection for gametogenesis and apoptosis regulation [7].
One special example of sexual antagonistic pleiotropy involves heterogametic species (with egg and sperm), where the mitochondrial genome is preferentially inherited from the mother through the egg. This means that natural selection can only act in females to optimize mitochondrial gene functions and mitochondrial-nuclear gene interactions. As a result, the mitochondria are expected to be less optimized for function in the male relative to the female, and mitochondrial gene alleles are expected to accumulate that may be preferentially detrimental to the male (called the Frank and Hurst hypothesis, or “mother's curse”) [56,57]. The resultant sub-optimal function of mitochondria in males may contribute to the increased oxidative stress markers observed in males, as well as sex differences in stress response and apoptosis. Natural selection is expected to act in the male to select for nuclear alleles that can compensate for the male-harming mitochondrial alleles, and in the next generation, these compensatory nuclear alleles may in turn be relatively detrimental to females [7,58]. Therefore, uniparental mitochondrial transmission can potentially lead to maintenance of mitochondrial and nuclear gene alleles with sexual antagonistic pleiotropy. Nuclear and mitochondrial genes with sexual antagonistic pleiotropy may contribute to sex-differences in stress response.
6. Sex ratios and deleterious SAP allele frequencies
The forces discussed above can potentially lead to selection for alleles that reduce the viability of one sex, or that inhibit the sexual differentiation of one sex, leading to a distortion in the typical 1:1 sex ratio. However, there is an opposing selection for maintenance of the 1:1 sex ratio (Fisher's principle) [59,60]. For example, if females become greater in frequency, males will have access to more mates, resulting in greater male fitness. As a result, there will be selection for parents that produce a greater number of male progeny, thereby selecting against alleles that reduce male viability and/or male differentiation. The same principles apply if males become in excess, and therefore selection tends to stabilize near a 1:1 ratio of male to female. This selection can act on both nuclear and mitochondrial alleles in females, but can act only on nuclear alleles in males. Therefore, because males are required for reproduction, Fisher's principle creates a limit to the extent that selection in females can favor male-harming alleles [7].
7. Definitions of stress resistance and stress adaptation
Stress resistance is revealed by the relative survival of cells or organisms subjected to stress. Stress resistance can also be defined as the relative maintenance of homeostatic functions of cells and organisms upon stress. In contrast, stress adaptation (adaptive homeostasis) refers to the ability of a moderate stress to protect cells and organisms from a subsequent toxic stress, as well as the ability of exercise to favor future exercise endurance [61,62]. Adaptation to oxidative stress has been extensively characterized in cultured mammalian cells, and involves Nuclear factor E2-related factor 2 (Nrf2)-dependent upregulation of proteasome subunits and the 20S proteasome [63], as well as increased activity of the mitochondrial Lon protease [64]; these mechanisms have also been shown to function in oxidative stress adaptation at the organismal level in Drosophila and C. elegans [65,66]. Heat stress toxicity involves protein denaturation and aggregation, as well as oxidative stress [67].
8. Sex differences in organismal stress resistance and stress adaptation
In humans, women live longer than men [68], and this female life span advantage is also observed during severe famines and epidemics [69], consistent with a female advantage in stress response. In contrast, women are generally more sensitive to the acute detrimental effects of heat and cold stress [70]. This relative sensitivity is largely attributed to sex differences in body mass, body fat, surface area, circulating blood volume and sweat production. However, training under heat conditions leads to acclimatization (adaptive homeostasis), with women acclimatizing more quickly and effectively than men [71,72]. Humans also show sex differences in the therapeutic response and toxicity to chemotherapies. Differences in liver metabolism, female cyclic levels of sex hormones, and drug bioavailability all impact patient response [73], with women more prone to tissue-specific toxicities, especially with agents with shorter half-life, such as steroids [74].
In mice, females were more resistant to exertional heat stress than were males, and this was associated with greater corticosteroid levels in the females under stress [75]. Female mice also showed improved survival relative to male mice upon posttraumatic abdominal sepsis [76]. Female rats show improved cardiac protection compared to males following trauma-induced hemorrhage, associated with estrogen-favored upregulation of cardiac-specific HSPs [77]. Likewise, in a model of ischemia/reperfusion, female rats showed higher basal and post-ischemia levels of HSP72 (an inducible form of HSP70) [78], suggestive of greater protection against subsequent acute oxidative stress [79] which may contribute to female-favored post-ischemic survival [80]. Examination of rat ‘fast twitch’ muscle (vastus lateralis) showed higher basal levels in males of small HSPs (HSP25 and αβ-crystallin), commonly associated with muscle repair, with no impact upon HSP70 expression levels [81]. Rodents also show acclimatization to heat stress, and analysis of tissues indicates this is associated with increased basal HSF activation and HSP70 expression, increased expression of DNA repair genes, hypoxia-inducible factor 1-alpha (HIF1A) and ion channels, and altered histone acetylation [82]. Studies of cultured rat cardiomyocytes show that estrogen indirectly upregulates expression of HSP72 by sequentially activating the expression of transcription factor nuclear factor-κB (NF-κB) and heat shock transcription factor-1 (HSF-1) [83]. In contrast, a study of male and female rats subjected to heat stress found that cardiac tissue isolated from the male rats had higher levels of HSP72 compared to females, suggesting an inhibitory role of estrogen on activation of the heat shock response under these conditions [84]. Together, these findings highlight the nuanced interplay between sex, sex-hormones, and tissue variability in regulating the heat shock response pathway.
9. Estrogen and testosterone regulate cellular oxidative and proteotoxic stress resistance
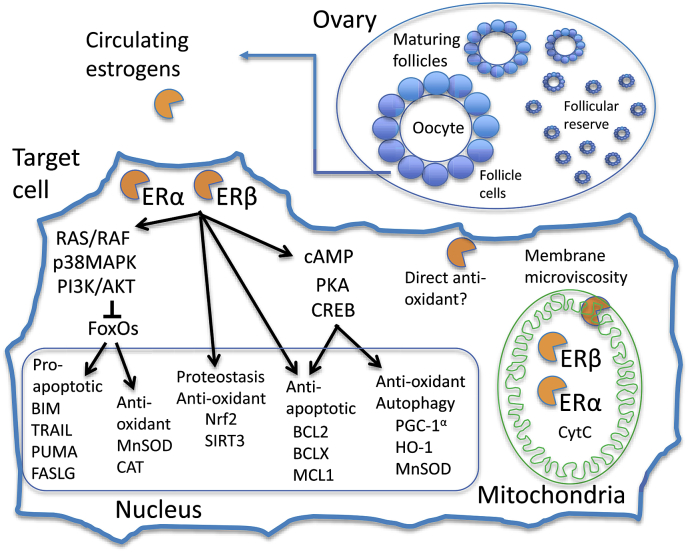
In women, the primary source of circulating estrogens is the somatic follicle cells of maturing oocyte follicles in the ovary (Fig. 2) [85]. The most active of these circulating estrogens is 17β-estradiol, referred to here as estrogen. Estrogen binds to estrogen receptors ERα and ERβ in target cells, and the activated ERs then promote several physiological responses that can increase resistance to oxidative and proteotoxic stress. Both oxidative stress and proteotoxic stress cause cell death by activating programmed cell death (apoptosis) pathways, and therefore inhibiting apoptosis is an important mechanism for protecting cells and tissues from these stresses [86]. In the cytoplasm, ERs can activate the cAMP/protein kinase A (PKA) signaling cascade to activate the cAMP response element-binding protein (CREB) transcription factor. CREB in turn can induce expression of several antioxidant proteins, including the mitochondrial superoxide dismutase MnSOD, heme-oxygenase-1 (HO-1), and the global regulator of autophagy and oxidative stress response peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [[87], [88], [89]]. The ERs can also act in cytoplasm to activate the p38 MAPK/phosphoinositide 3-kinase (PI3K)/RAC-alpha serine/threonine-protein kinase (AKT) signaling cascade, which inhibits activity of FOXO family transcription factors, including FOXO3a, thereby reducing expression of pro-apoptotic FOXO targets such as Bcl-2-like protein 11 (BIM), p53 upregulated modulator of apoptosis (PUMA) and Fas ligand (FASLG) [90,91]. However, FOXO targets also include genes encoding the anti-oxidant enzymes manganese-dependent superoxide dismutase (MnSOD) and catalase (CAT), and therefore FOXO activity has context-dependent effects on oxidative stress resistance [92,93]. The ERs are themselves transcription factors that act in the nucleus to activate expression of anti-apoptotic genes, including the B-cell lymphoma 2 (BCL2)-family members BCL2, BCLX and induced myeloid leukemia cell differentiation protein Mcl-1 (MCL1) [[94], [95], [96], [97]]. Both ERα and ERβ are also present within the mitochondria, where they bind to mitochondrial DNA and ETC subunits, and mediate reduced ROS production in response to estrogen [[98], [99], [100]].
Fig. 2.
Cellular targets for estrogen function in response to oxidative and proteotoxic stress. In the ovary, the somatic follicle cells of maturing oocyte follicles produce the majority of circulating estrogens, of which 17β-estradiol is the most active, and here referred to as estrogen. In the target cell, estrogen binds to receptors ERα and ERβ, and the activated receptors directly activate membrane and cytoplasmic signaling cascades. These include the rat sarcoma (RAS)/rapidly accelerated fibrosarcoma (RAF)/p38MAPK/PI3K/AKT cascade, which inactivates FOXO-family transcription factors, and the cAMP/PKA/CREB cascade which activates the CREB transcription factor. In addition, there is significant cross-talk between these signaling cascades and others regulated by estrogen, including p53, JNK and extracellular signal-regulated kinase (ERK). In the nucleus, ERα, ERβ, FOXO and CREB transcription factors activate expression of genes involved in regulation of apoptosis, proteostasis, redox state, and autophagy. Activated ERα and ERβ also translocate to the mitochondria where they activate expression of mitochondrial-encoded genes including cytochrome C, as well as interacting with components of the ETC. Estrogen also interacts directly with the mitochondrial membrane to modulate membrane microviscosity, and has been reported to act directly as an antioxidant to detoxify lipid peroxyradicals. Please see text for additional details.
Estrogen acts through ERα to upregulate expression of nuclear factor E2-related factor 2 (Nrf2), which is a key transcriptional activator of proteostasis and oxidative stress response genes [[101], [102], [103]]. These Nrf2 targets include multiple proteasome subunits, the autophagy regulator p62, the mitochondrial LON protease, NAD-dependent deacetylase sirtuin-3 mitochondrial (SIRT3), peroxidase and glutathione peroxidase [104,105]. Activated ERβ also transcriptionally upregulates expression of SIRT3 [106]. SIRT3 protein translocates to the mitochondria where it activates MnSOD enzyme to increase detoxification of superoxide radicals [107].
Estrogen itself can also have direct effects in the mammalian cell. Estrogen is reported to directly interact with muscle mitochondria to modulate mitochondrial membrane microviscosity, optimize bioenergetic function, and reduce the potential for hydrogen peroxide release [108]. Under homeostatic conditions estrogen-activated ERα can promote insulin sensitivity through AKT/FOXO signaling [[109], [110], [111]], however, under conditions of abnormally high estrogen levels (hyperestrogenemia), estrogen can directly bind and inhibit the insulin receptor and may promote insulin resistance [112]. Finally, estrogen has been reported to act directly as an antioxidant by donating a hydrogen atom from its phenolic hydroxyl group to lipid peroxyradicals, however the relevance of this activity in vivo remains unclear [113].
In male mammals, the circulating steroid testosterone is produced by the testes. In target cells, testosterone binds to the androgen receptor (AR) and modulates signaling pathways at the cell membrane and in the cytoplasm, including the MAPK/PI3K/AKT signaling cascade [114]. AR also acts in the nucleus as a transcription factor, and is localized to the mitochondria [115,116]. In response to oxidative stress, activated AR can be either anti-apoptotic or pro-apoptotic depending on the cellular context [117,118], and beneficial effects may be preferentially observed in male cells [119]. Testosterone is also reported to favor cell survival through direct effects on the mitochondrial membrane [120]. One possibility is that one or more of the sex-specific genetic mechanisms discussed above (sex chromosome composition, dosage compensation, mother's curse, Fisher's principle, etc.) are required to create a permissive cellular environment for the respective sex steroids to exhibit a beneficial effect. Finally, whereas both estrogen and testosterone can have beneficial effects in the short term, they may also promote aging-related loss of stress resistance by activating insulin/insulin-like growth factor signaling (IIS) and target of rapamycin (TOR) signaling, thereby inhibiting mitochondrial turnover and long-term mitochondrial maintenance [58].
10. Sex differences in the regulation of autophagy
Autophagy is differentially regulated by sex, and this contributes to sex differences in stress response [121,122]. Autophagy (macroautophagy) is a cellular “self-eating” process that degrades large protein aggregates, bulk cytoplasm and damaged organelles, including ribosomes and mitochondria [123,124]. These materials become enveloped by a double membrane (the autophagosome) which subsequently fuses with the lysosome to introduce degradative enzymes. Rodents show sex differences in autophagy markers and autophagy levels that vary by tissue [125,126], and increased autophagy markers were observed in male rat heart and liver [127]. Autophagy is also differentially regulated by sex in cancer [128]. Cancer in humans is associated with cachexia, involving loss of fat and muscle tissue, and this effect is greater in males. In a mouse cancer model, cardiac atrophy was associated with increased autophagy, whereas in females, estrogen signaling inhibited cardiac autophagy and atrophy. In rodent models of iron-induced brain injury, estrogen signaling through receptor ERα suppressed autophagy, leading to less severe brain injury in females relative to males [129,130]. Whereas autophagy is generally thought to be protective under normal conditions [123,124], the data indicate that upon specific stress, estrogen inhibition of autophagy limits damage in females relative to males.
11. Sex differences in invertebrate stress response
In Drosophila melanogaster, virgin females show a survival advantage relative to males for most stresses tested, including heat stress, oxidative stress (dietary paraquat and H2O2), ionizing radiation (IR) stress and starvation stress [66,[131], [132], [133], [134], [135], [136], [137], [138]]. The conserved stress response genes p53 and foxo show sex differences in activity in Drosophila. Under normal conditions, and upon IR stress, p53 favored the survival of males but was detrimental to the survival of females [138]. The foxo target genes l(2)efl and 4E-BP were expressed at higher level in males relative to females, and this preferential male expression required functional foxo [139], suggesting greater foxo transcription factor activity in males. Finally, modulating the sexual differentiation of the Drosophila fat-body tissue in the head, using targeted expression of transformer (tra), caused sex-reversals in the patterns of locomotor behaviors and heart rate in response to paraquat [140].
Adaptation to stress also shows sex differences in Drosophila. For example, heat stress adaptation was greater in males relative to females [132,141]. Certain stress adaptations were observed only in one sex in Drosophila. For example, Drosophila females, but not males, could adapt to hydrogen peroxide stress [65], whereas Drosophila males, but not females, could adapt to paraquat stress [66]. Notably, RNAi-mediated inhibition of expression of the proteasome subunit gene prosβ1 eliminated adaptation to hydrogen peroxide in the female, indicating the importance of the 20S Proteasome [65]. Strikingly, Drosophila was found to have sex-specific expression of protein isoforms of the mitochondrial Lon protease that correlated with sex-specific stress adaptation [66]. RNAi-mediated inhibition of expression of Lon eliminated hydrogen peroxide adaptation in the female, and also eliminated adaptation to paraquat stress in the male, revealing a requirement for Lon for oxidative stress adaptation in each sex. Forced over-expression of the female-specific transcript of the gene tra transforms males in pseudo-females, and this produced the female-specific pattern of Lon isoform expression and conferred adaptation to hydrogen peroxide stress. In turn, RNAi-mediated inhibition of tra expression transforms females into pseudo-males, and this produced the male pattern of Lon protein isoform expression and conferred adaptation to paraquat stress [66]. These results demonstrate that the Drosophila sex determination pathway regulates sex-specific expression of Lon protein isoforms, as well as sex-specific patterns of oxidative stress adaptation. Several genes have been implicated as being required for IR stress adaptation in Drosophila, including GADD45, p53, HSF, and foxo, and notably, NAD-dependent deacetylase sirtuin 2 (Sir2) appeared preferentially required in females, and c-Jun N-terminal kinase (bsk or JNK) preferentially required in males [142,143].
C. elegans also shows sex differences in stress resistance. In the C. elegans Bristol N2 strain, males in isolation live longer than females, and males also show greater survival upon long-term anoxia [144,145]. In C. elegans hermaphrodites, adaptation to heat stress was associated with increased expression of the small heat shock protein HSP16, and required HSF, DAF-16/Foxo, the autophagy pathway, and the C. elegans phosphatase and tensin (PTEN) homolog DAF-18, which is a conserved negative regulator of insulin-like signaling [[146], [147], [148]].
The copepod Tigriopus californicus lacks canonical sex chromosomes, and sex is determined by multiple segregating autosomal alleles [1]. T. californicus showed greater stress resistance in females for a variety of stressors, including heat stress, salinity, copper and bisphenol A (BPA) [149,150]; however, stress adaptation has not yet been tested.
12. Sex differences in cellular stress responses and stress adaptation
In mammals, stress responses are regulated in part by sex differences in the levels of steroid hormones, including estrogen, which is greater in females, and testosterone, which is greater in males, through the cellular mechanisms discussed above. In humans, women are found to have reduced markers of oxidative stress relative to men, consistent with greater generation of oxidative stress in men [151]. Estrogen is implicated in this female advantage, and up-regulation of glutathione metabolism by estrogen has been suggested as a possible factor in the greater resistance of human female newborns to diseases associated with oxidative stress [152]. Estrogen is reported to favor stress resistance in both neural and muscle tissues, including the heart and skeletal muscle, in part through its ability to inhibit apoptosis [[153], [154], [155]]. For example, in cultured rat cardiomyocytes subjected to hypoxia/reoxygenation stress, estrogen favored survival by inhibiting phosphorylation of p53 by p38alpha MAPK, and by inhibiting p53 translocation to the mitochondria [156]. As discussed above, estrogen can also be beneficial upon acute stress through inhibition of autophagy. Testosterone is also reported to favor stress resistance and to be neuroprotective upon ischemic stroke [157]. However, testosterone is also reported to exacerbate oxidative stress in certain models [151]. One possible contribution to the conflicting results observed with testosterone is that beneficial effects may be preferentially observed when using male cells [119,157]. Both estrogen and testosterone are implicated in activation of MAPK/PI3K/AKT/TOR signaling pathways, which promote growth and sexual differentiation, but also inhibit autophagy. Hormonal inhibition of autophagy can be beneficial to health by limiting loss of cells in bone and other tissues, as discussed above, but may also decrease mitochondrial turnover important for longevity [58,121,158,159].
Women are more resistant to ischemic stroke than are men, and this sex difference is reproduced in mouse models [160]. Mouse studies show that circulating gonadal estrogen is protective, but that the second X chromosome has a detrimental effect that becomes evident after reproductive senescence [161]. Female brains have more favorable antioxidant status, whereas male brains show greater oxidative stress upon injury. In a mouse model of ischemic brain injury, male cells die through a caspase-independent, poly [ADP-ribose] polymerase (PARP)-dependent programmed cell death pathway called parthanatos [162], whereas female cells die through a caspase-dependent pathway. These studies suggest the potential for sex-specific interventions in ischemic disease [160].
The p53 transcription factors is a conserved regulator of stress responses and apoptosis across species, and is implicated in mediating sex differences in stress response and cell survival [118,163]. P53 promotes apoptosis in part through regulation of mitochondrial ROS production and mitochondrial membrane integrity. Mitochondria are key regulators of apoptosis in response to stress, through production of pro-apoptotic reactive oxygen and nitrogen species, and through the release of pro-apoptotic factors, including cytochrome C, allograft inflammatory factor 1 (AIF1) and endonuclease G mitochondrial (EndoG) [86]. For example, in mammalian fetal germ cells, male cells underwent p53-dependent apoptosis upon low-level IR stress, whereas female cells underwent apoptosis only with higher-level IR stress, and this apoptosis was p53-independent [164].
A major difference between the sexes is longevity. Females typically outlive males in several species, including Drosophila and humans [7,18,165,166]. In contrast, in birds, where the female is the heteromorphic sex (Z/W), males are generally longer lived. Genetic and dietary manipulations of life span often show sex-specific or sex-biased effects in various species [3,7,[167], [168], [169], [170]]. However how sex-determination pathways modulate survival is difficult to test. In Drosophila, adult-specific over-expression of several sexual differentiation genes decreased both male and female life span [171], and as discussed above, Drosophila stress adaptation is regulated in a sex-specific manner by tra. A differentiated germ-line has been found to modulate life span in Drosophila, C. elegans and mice [[171], [172], [173]]. In C. elegans, inactivation of the sex-determining gene tra-1 led to shortened lifespan, whereas its overexpression resulted in increased survival that was dependent upon the stress-responsive transcription factor DAF-16 (the orthologue of mammalian FOXO) [174]. Dietary restriction (DR), which can be considered a metabolic stress, increases life span preferentially in females in both C. elegans and Drosophila [[175], [176], [177]]. Taken together, these findings support the conclusion that the sexual differentiation pathway modulates both stress resistance and life span across species.
13. Sex differences in disease involving oxidative and proteotoxic stress
Women are more resistant than men to several diseases involving oxidative stress, including ischemic heart disease [[178], [179], [180]] and ischemic stroke [[181], [182], [183]]. Estrogen is implicated in mediating much of this advantage, however, as discussed above, studies with cultured cells indicate that cell-autonomous mechanisms also contribute to the resistance of female cells to oxidative stress-induced apoptosis (Fig. 3).
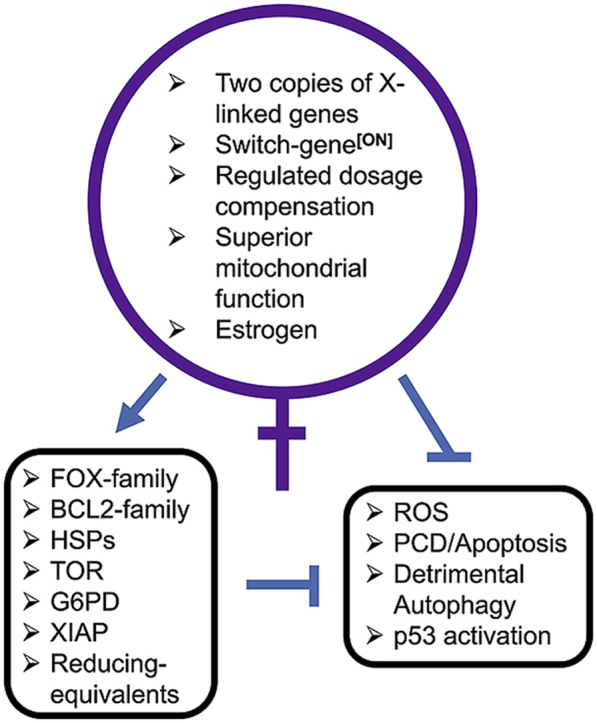
Fig. 3.
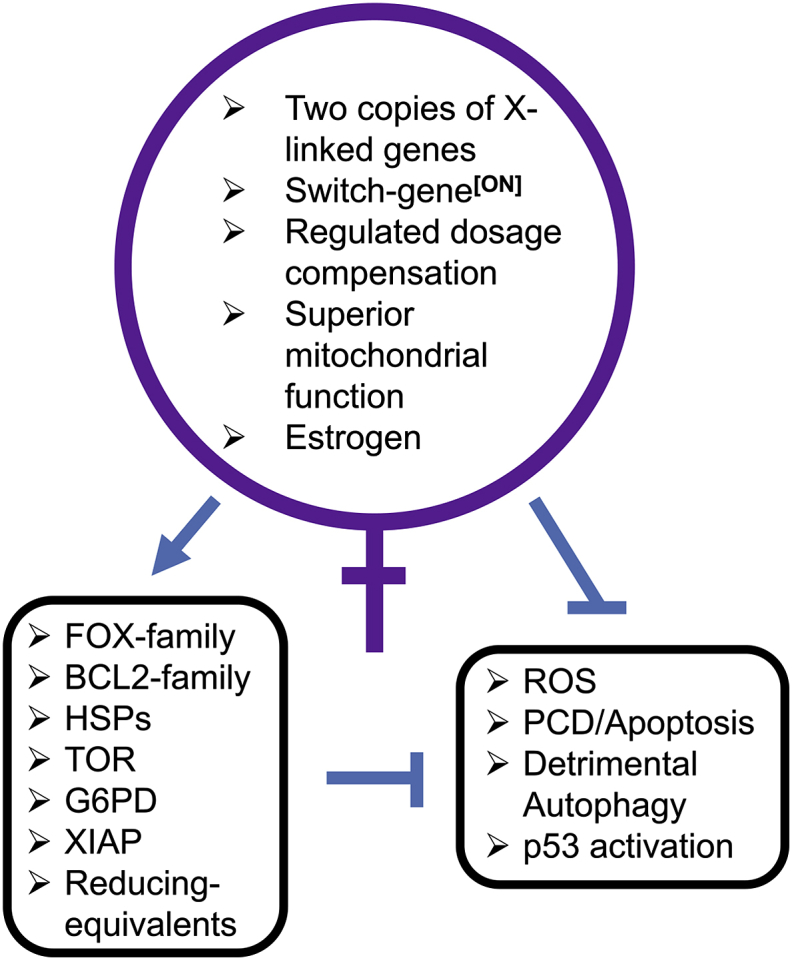
Summary of mammalian female advantage in cellular stress resistance. The major genetic and physiological features that contribute to the human female advantage in resistance to oxidative and proteotoxic stress are diagrammed. Arrow indicates activation, and t-bars indicate suppression. Please see text for additional details.
Alzheimer disease involves the abnormal accumulation and aggregation of amyloid protein in the brain [184], and preferentially affects women relative to men [185,186]. The precise causes remain unclear; however, several mechanisms have been implicated. These include age-related decrease in mitochondrial function [187], metabolic aging [188], age-related decrease in autophagic clearance of protein aggregates [189,190], decreased brain vascular function and blood flow [191,192], and maladaptive immune/inflammatory response [193,194]. The immune/inflammatory component may be particularly relevant to the sex differences and is potentially related to the female propensity towards maladaptive immune response and autoimmune disease discussed above.
Parkinson disease also involves the abnormal accumulation and aggregation of protein in the brain, and preferentially causes the malfunction and loss of dopaminergic neurons. Men are twice as likely to develop Parkinson disease relative to women, however, women experience faster progression of the disease and greater mortality [195]. Estrogen has been implicated in the female resistance to disease, as well as the greater mitochondrial electron transport chain function and reduced levels of mitochondrial damage and ROS production in women relative to men.
Finally, amyotropic lateral sclerosis (ALS) is associated with superoxide dismutase 1 (SOD1) mutations in familial ALS [196]. ALS preferentially affects males, and the ALS-associated mutant form of SOD1 (G93A) was preferentially toxic to male cells when expressed in cultured rat neurospheres [197].
14. Summary/conclusions
Sex differences in oxidative and proteotoxic stress response are common, and are related to sex differences in the incidence and severity of diseases involving oxidative and proteotoxic stress. Mechanisms for sex differences in stress response involve both cell non-autonomous effects (hormones), as well as cell-autonomous effects including sex chromosome gene expression (Fig. 3). In the future, it will be important to further investigate these mechanisms, as a guide for the development of sex-based interventions for human disease.
Acknowledgements
JT was supported by NIH/NIA grant 5R01AG057741.
KJAD was supported by NIH grant AG052374.
KJAD and LCDP were supported by NIH grant ES003598 to KJAD.
LCDP was supported by the NIH Grant Fi2GM123963.
Abbreviations used
- ALS
amyotropic lateral sclerosis
- Dmrt1
doublesex- and mab-3-related transcription factor 1
- HSF
heat shock factor
- HSP
heat shock protein
- SAP
sexual antagonistic pleiotropy
- MAPK
mitogen-activated protein kinase
- PCD
programmed cell death
- lncRNA
long non-coding RNA
- PcG
polycomb-group
- ROS
reactive oxygen species
- IIS
insulin/insulin-like growth factor signaling
- ETC
electron transport chain
- IR
ionizing radiation
- PARP
poly [ADP-ribose] polymerase
References
- 1.Alexander H.J., Richardson J.M., Edmands S., Anholt B.R. Sex without sex chromosomes: genetic architecture of multiple loci independently segregating to determine sex ratios in the copepod Tigriopus californicus. J. Evol. Biol. 2015;28:2196–2207. doi: 10.1111/jeb.12743. [DOI] [PubMed] [Google Scholar]
- 2.Georges A., Holleley C.E. How does temperature determine sex? Science. 2018;360:601–602. doi: 10.1126/science.aat5993. [DOI] [PubMed] [Google Scholar]
- 3.Tower J. Sex-specific gene expression and life span regulation. Trends Endocrinol. Metabol. 2017;28:735–747. doi: 10.1016/j.tem.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashamboo A., McElreavey K. Mechanism of sex determination in humans: insights from disorders of sex development. Sex Dev. 2016;10:313–325. doi: 10.1159/000452637. [DOI] [PubMed] [Google Scholar]
- 5.Huang S., Ye L., Chen H. Sex determination and maintenance: the role of DMRT1 and FOXL2. Asian J. Androl. 2017;19(6):619–624. doi: 10.4103/1008-682X.194420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webster K.A., Schach U., Ordaz A., Steinfeld J.S., Draper B.W., Siegfried K.R. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017;422:33–46. doi: 10.1016/j.ydbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tower J. Sex-specific regulation of aging and apoptosis. Mech. Ageing Dev. 2006;127:705–718. doi: 10.1016/j.mad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Gierl M.S., Gruhn W.H., von Seggern A., Maltry N., Niehrs C. GADD45G functions in male sex determination by promoting p38 signaling and Sry expression. Dev. Cell. 2012;23:1032–1042. doi: 10.1016/j.devcel.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Warr N., Carre G.A., Siggers P., Faleato J.V., Brixey R., Pope M., Bogani D., Childers M., Wells S., Scudamore C.L., Tedesco M., del Barco Barrantes I., Nebreda A.R., Trainor P.A., Greenfield A. Gadd45gamma and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev. Cell. 2012;23:1020–1031. doi: 10.1016/j.devcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogani D., Siggers P., Brixey R., Warr N., Beddow S., Edwards J., Williams D., Wilhelm D., Koopman P., Flavell R.A., Chi H., Ostrer H., Wells S., Cheeseman M., Greenfield A. Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warr N., Siggers P., Carre G.A., Bogani D., Brixey R., Akiyoshi M., Tachibana M., Teboul L., Wells S., Sanderson J., Greenfield A. Transgenic expression of Map3k4 rescues T-associated sex reversal (Tas) in mice. Hum. Mol. Genet. 2014;23:3035–3044. doi: 10.1093/hmg/ddu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mork L., Czerwinski M., Capel B. Predetermination of sexual fate in a turtle with temperature-dependent sex determination. Dev. Biol. 2014;386:264–271. doi: 10.1016/j.ydbio.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Radder R.S., Quinn A.E., Georges A., Sarre S.D., Shine R. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol. Lett. 2007;4:176–178. doi: 10.1098/rsbl.2007.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettlewell J.R., Raymond C.S., Zarkower D. Temperature‐dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- 15.Ge C., Ye J., Weber C., Sun W., Zhang H., Zhou Y., Cai C., Qian G., Capel B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science. 2018;360:645–648. doi: 10.1126/science.aap8328. [DOI] [PubMed] [Google Scholar]
- 16.Holleley C.E., O'Meally D., Sarre S.D., Marshall Graves J.A., Ezaz T., Matsubara K., Azad B., Zhang X., Georges A. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature. 2015;523:79. doi: 10.1038/nature14574. [DOI] [PubMed] [Google Scholar]
- 17.Maklakov A.A., Lummaa V. Evolution of sex differences in lifespan and aging: causes and constraints. Bioessays. 2013;35:717–724. doi: 10.1002/bies.201300021. [DOI] [PubMed] [Google Scholar]
- 18.Tower J., Arbeitman M. The genetics of gender and life span. J. Biol. 2009;8:38. doi: 10.1186/jbiol141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berletch J.B., Ma W., Yang F., Shendure J., Noble W.S., Disteche C.M., Deng X. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotton A.M., Price E.M., Jones M.J., Balaton B.P., Kobor M.S., Brown C.J. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum. Mol. Genet. 2015;24:1528–1539. doi: 10.1093/hmg/ddu564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tukiainen T., Villani A.C., Yen A., Rivas M.A., Marshall J.L., Satija R., Aguirre M., Gauthier L., Fleharty M., Kirby A., Cummings B.B., Castel S.E., Karczewski K.J., Aguet F., Byrnes A., Consortium, G.T., Laboratory, D.A., Coordinating Center -Analysis Working, G., Statistical Methods groups-Analysis Working, G., Enhancing, G.g., Fund, N.I.H.C., Nih/Nci, Nih/Nhgri, Nih/Nimh, Nih/Nida, Biospecimen Collection Source Site, N., Biospecimen Collection Source Site, R., Biospecimen Core Resource, V., Brain Bank Repository-University of Miami Brain Endowment, B., Leidos Biomedical-Project, M., Study, E., Genome Browser Data, I., Visualization, E.B.I., Genome Browser Data, I., Visualization-Ucsc Genomics Institute, U.o.C.S.C., Lappalainen, T., Regev, A., Ardlie, K.G., Hacohen, N., MacArthur, D.G. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. [Google Scholar]
- 22.Groen E.J.N., Gillingwater T.H. UBA1: at the crossroads of ubiquitin homeostasis and neurodegeneration. Trends Mol. Med. 2015;21:622–632. doi: 10.1016/j.molmed.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G., Luo Y., Li G., Wang L., Na D., Wu X., Zhang Y., Mo X., Wang L. DHRSX, a novel non-classical secretory protein associated with starvation induced autophagy. Int. J. Med. Sci. 2014;11:962–970. doi: 10.7150/ijms.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Liu L., Zhu Y., Chen Q. Mitochondria organize the cellular proteostatic response and promote cellular senescence. Cell Stress. 2019;3:110–114. doi: 10.15698/cst2019.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., Huang L., Xue P., Li B., Wang X., Jin H., Wang J., Yang F., Liu P., Zhu Y., Sui S., Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D., Hu S., Ren J., Cao F., Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Xue Y., Xu X., Wang G., Liu Y., Wu H., Li W., Wang Y., Chen Z., Zhang W., Zhu Y., Ji W., Xu T., Liu L., Chen Q. A mitochondrial FUNDC1/HSC70 interaction organizes the proteostatic stress response at the risk of cell morbidity. EMBO J. 2019;38 doi: 10.15252/embj.201798786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemencon B., Babot M., Trezeguet V. The mitochondrial ADP/ATP carrier (SLC25 family): pathological implications of its dysfunction. Mol. Aspect. Med. 2013;34:485–493. doi: 10.1016/j.mam.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Wang Q., Wang H., Wu Y., Yin J., Chen J., Zheng Z., Jiang T., Xie L., Wu F., Zhang H., Li X., Xu H., Xiao J. Lentivirus mediating FGF13 enhances axon regeneration after spinal cord injury by stabilizing microtubule and improving mitochondrial function. J. Neurotrauma. 2018;35:548–559. doi: 10.1089/neu.2017.5205. [DOI] [PubMed] [Google Scholar]
- 30.Yang L., Dong F., Yang Q., Yang P.F., Wu R., Wu Q.F., Wu D., Li C.L., Zhong Y.Q., Lu Y.J., Cheng X., Xu F.Q., Chen L., Bao L., Zhang X. FGF13 selectively regulates heat nociception by interacting with Nav1.7. Neuron. 2017;93:806–821 e9. doi: 10.1016/j.neuron.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Xie M., Men L., Du J. O-GlcNAcylation in immunity and inflammation: an intricate system (Review) Int. J. Mol. Med. 2019;44:363–374. doi: 10.3892/ijmm.2019.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Syrett C.M., Kramer M.C., Basu A., Atchison M.L., Anguera M.C. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2029–E2038. doi: 10.1073/pnas.1520113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Crespo M., Ramirez M.A., Fernandez-Gonzalez R., Rizos D., Lonergan P., Pintado B., Gutierrez-Adan A. Differential sensitivity of male and female mouse embryos to oxidative induced heat-stress is mediated by glucose-6-phosphate dehydrogenase gene expression. Mol. Reprod. Dev. 2005;72:502–510. doi: 10.1002/mrd.20366. [DOI] [PubMed] [Google Scholar]
- 34.Siegel C., Li J., Liu F., Benashski S.E., McCullough L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czech D.P., Lee J., Sim H., Parish C.L., Vilain E., Harley V.R. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J. Neurochem. 2012;122:260–271. doi: 10.1111/j.1471-4159.2012.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Pinares-Garcia P., Loke H., Ham S., Vilain E., Harley V.R. Proc Natl Acad Sci U S A; 2019. Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umar S., Cunningham C.M., Itoh Y., Moazeni S., Vaillancourt M., Sarji S., Centala A., Arnold A.P., Eghbali M. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2018;197:952–955. doi: 10.1164/rccm.201707-1345LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathews K.W., Cavegn M., Zwicky M. Sexual Dimorphism of Body Size Is Controlled by Dosage of the X-Chromosomal Gene Myc and by the Sex-Determining Gene tra in Drosophila. Genetics. 2017;205:1215–1228. doi: 10.1534/genetics.116.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin R.M., Schielzeth H., Friberg U. Autosomal and X-linked additive genetic variation for lifespan and aging: comparisons within and between the sexes in Drosophila melanogaster. G3: Genes, Genomes, Genetics. 2016;6:3903–3911. doi: 10.1534/g3.116.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heid I.M., Jackson A.U., Randall J.C., Winkler T.W., Qi L., Steinthorsdottir V., Thorleifsson G., Zillikens M.C., Speliotes E.K., Mägi R. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010;42:949. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinn J.L., Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Trabzuni D., Ramasamy A., Imran S., Walker R., Smith C., Weale M.E., Hardy J., Ryten M., Consortium N.A.B.E. Widespread sex differences in gene expression and splicing in the adult human brain. Nat. Commun. 2013;4:2771. doi: 10.1038/ncomms3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun T., Plutynski A., Ward S., Rubin J.B. An integrative view on sex differences in brain tumors. Cell. Mol. Life Sci. 2015;72:3323–3342. doi: 10.1007/s00018-015-1930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delamarre A., Meissner W.G. Epidemiology, environmental risk factors and genetics of Parkinson's disease. Presse Med. 2017;46:175–181. doi: 10.1016/j.lpm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Narayan P., Dragunow M. Alzheimer's disease and histone code alterations. Adv. Exp. Med. Biol. 2017;978:321–336. doi: 10.1007/978-3-319-53889-1_17. [DOI] [PubMed] [Google Scholar]
- 46.Poissant J., Wilson A.J., Coltman D.W. Sex‐specific genetic variance and the evolution of sexual dimorphism: a systematic review of cross‐sex genetic correlations. Evolution. 2010;64:97–107. doi: 10.1111/j.1558-5646.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 47.Gross S., Immel U.D., Klintschar M., Bartel F. Germline genetics of the p53 pathway affect longevity in a gender specific manner. Curr. Aging Sci. 2014;7:91–100. doi: 10.2174/1874609807666140321150751. [DOI] [PubMed] [Google Scholar]
- 48.Fang S., Krahe R., Bachinski L.L., Zhang B., Amos C.I., Strong L.C. Sex-specific effect of the TP53 PIN3 polymorphism on cancer risk in a cohort study of TP53 germline mutation carriers. Hum. Genet. 2011;130:789–794. doi: 10.1007/s00439-011-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang S., Krahe R., Lozano G., Han Y., Chen W., Post S.M., Zhang B., Wilson C.D., Bachinski L.L., Strong L.C., Amos C.I. Effects of MDM2, MDM4 and TP53 codon 72 polymorphisms on cancer risk in a cohort study of carriers of TP53 germline mutations. PloS One. 2010;5 doi: 10.1371/journal.pone.0010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davy P.M.C., Allsopp R.C., Donlon T.A., Morris B.J., Willcox D.C., Willcox B.J. FOXO3 and exceptional longevity: insights from Hydra to humans. Curr. Top. Dev. Biol. 2018;127:193–212. doi: 10.1016/bs.ctdb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanese P., Forte G., Disciglio V., Grossi V., Simone C. FOXO3 on the road to longevity: lessons from SNPs and chromatin hubs. Comput. Struct. Biotechnol. J. 2019;17:737–745. doi: 10.1016/j.csbj.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charlesworth D., Charlesworth B. Sex chromosomes: evolution of the weird and wonderful. Curr. Biol. 2005;15:R129–R131. doi: 10.1016/j.cub.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Albritton S.E., Kranz A.L., Rao P., Kramer M., Dieterich C., Ercan S. Sex-biased gene expression and evolution of the x chromosome in nematodes. Genetics. 2014;197:865–883. doi: 10.1534/genetics.114.163311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliver B., Parisi M. Battle of the xs. Bioessays. 2004;26:543–548. doi: 10.1002/bies.20034. [DOI] [PubMed] [Google Scholar]
- 55.Reinius B., Johansson M.M., Radomska K.J., Morrow E.H., Pandey G.K., Kanduri C., Sandberg R., Williams R.W., Jazin E. Abundance of female-biased and paucity of male-biased somatically expressed genes on the mouse X-chromosome. BMC Genom. 2012;13:607. doi: 10.1186/1471-2164-13-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank S.A., Hurst L.D. Mitochondria and male disease. Nature. 1996;383:224. doi: 10.1038/383224a0. [DOI] [PubMed] [Google Scholar]
- 57.Gemmell N.J., Metcalf V.J., Allendorf F.W. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Tower J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch. Biochem. Biophys. 2015;576:17–31. doi: 10.1016/j.abb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carvalho A.B., Sampaio M.C., Varandas F.R., Klaczko L.B. An experimental demonstration of Fisher's principle: evolution of sexual proportion by natural selection. Genetics. 1998;148:719–731. doi: 10.1093/genetics/148.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uller T., Pen I., Wapstra E., Beukeboom L.W., Komdeur J. The evolution of sex ratios and sex-determining systems. Trends Ecol. Evol. 2007;22:292–297. doi: 10.1016/j.tree.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Davies K.J. Adaptive homeostasis. Mol. Aspect. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies K.J.A. Cardiovascular adaptive homeostasis in exercise. Front. Physiol. 2018;9:369. doi: 10.3389/fphys.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickering A.M., Linder R.A., Zhang H., Forman H.J., Davies K.J. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngo J.K., Pomatto L.C., Davies K.J. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pickering A.M., Staab T.A., Tower J., Sieburth D.S., Davies K.J. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative-stress adaptation in mammals, C. elegans and D. melanogaster. J. Exp. Biol. 2012;216(Pt 4):543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pomatto L.C., Carney C., Shen B., Wong S., Halaszynski K., Salomon M.P., Davies K.J., Tower J. The mitochondrial Lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr. Biol. 2017;27:1–15. doi: 10.1016/j.cub.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tower J. Heat shock proteins and Drosophila aging. Exp. Gerontol. 2011;46:355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Austad S.N., Fischer K.E. Sex differences in lifespan. Cell Metabol. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zarulli V., Barthold Jones J.A., Oksuzyan A., Lindahl-Jacobsen R., Christensen K., Vaupel J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E832–E840. doi: 10.1073/pnas.1701535115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burse R.L. Sex differences in human thermoregulatory response to heat and cold stress. Hum. Factors. 1979;21:687–699. doi: 10.1177/001872087912210606. [DOI] [PubMed] [Google Scholar]
- 71.Horstman D.H., Christensen E. Acclimatization to dry heat: active men vs. active women. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982;52:825–831. doi: 10.1152/jappl.1982.52.4.825. [DOI] [PubMed] [Google Scholar]
- 72.Periard J.D., Racinais S., Sawka M.N. Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand. J. Med. Sci. Sports. 2015;25(Suppl 1):20–38. doi: 10.1111/sms.12408. [DOI] [PubMed] [Google Scholar]
- 73.Schmetzer O., Florcken A. Sex differences in the drug therapy for oncologic diseases. Handb. Exp. Pharmacol. 2012:411–442. doi: 10.1007/978-3-642-30726-3_19. [DOI] [PubMed] [Google Scholar]
- 74.Schmetzer O., Flörcken A. Springer; 2013. Sex Differences in the Drug Therapy for Oncologic Diseases, Sex and Gender Differences in Pharmacology; pp. 411–442. [DOI] [PubMed] [Google Scholar]
- 75.Garcia C.K., Mattingly A.J., Robinson G.P., Laitano O., King M.A., Dineen S.M., Leon L.R., Clanton T.L. Sex-dependent responses to exertional heat stroke in mice. J. Appl. Physiol. 2018;1985(125):841–849. doi: 10.1152/japplphysiol.00220.2018. [DOI] [PubMed] [Google Scholar]
- 76.Wehrenpfennig P., Drechsler S., Weixelbaumer K.M., Bahrami S., Osuchowski M.F. Mouse model of posttraumatic abdominal sepsis: survival advantage of females over males does not depend on the cecum size. Eur. Surg. Res. 2014;52:83–89. doi: 10.1159/000362543. [DOI] [PubMed] [Google Scholar]
- 77.Yu H.-P., Shimizu T., Choudhry M.A., Hsieh Y.-C., Suzuki T., Bland K.I., Chaudry I.H. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-β agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J. Mol. Cell. Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Fekete A., Vannay Á., Vér Á., Rusai K., Müller V., Reusz G., Tulassay T., Szabó A.J. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2006;291:F806–F811. doi: 10.1152/ajprenal.00080.2006. [DOI] [PubMed] [Google Scholar]
- 79.Hosszu A., Antal Z., Veres-Szekely A., Lenart L., Balogh D.B., Szkibinszkij E., Illesy L., Hodrea J., Banki N.F., Wagner L., Vannay A., Szabo A.J., Fekete A. The role of Sigma-1 receptor in sex-specific heat shock response in an experimental rat model of renal ischaemia/reperfusion injury. Transpl. Int. 2018;31:1268–1278. doi: 10.1111/tri.13293. [DOI] [PubMed] [Google Scholar]
- 80.Müller V., Losonczy G., Heemann U., Vannay Á., Fekete A., Reusz G., Tulassay T., Szabó A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62:1364–1371. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 81.Romani W.A., Russ D.W. Acute effects of sex-specific sex hormones on heat shock proteins in fast muscle of male and female rats. Eur. J. Appl. Physiol. 2013;113:2503–2510. doi: 10.1007/s00421-013-2686-8. [DOI] [PubMed] [Google Scholar]
- 82.Horowitz M. Epigenetics and cytoprotection with heat acclimation. J. Appl. Physiol. 2016;1985(120):702–710. doi: 10.1152/japplphysiol.00552.2015. [DOI] [PubMed] [Google Scholar]
- 83.Stice J.P., Chen L., Kim S.C., Jung J.S., Tran A.L., Liu T.T., Knowlton A.A. 17beta-Estradiol, aging, inflammation, and the stress response in the female heart. Endocrinology. 2011;152:1589–1598. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shinohara T., Takahashi N., Ooie T., Ichinose M., Hara M., Yonemochi H., Saikawa T., Yoshimatsu H. Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. J. Mol. Cell. Cardiol. 2004;37:1053–1061. doi: 10.1016/j.yjmcc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Zhou J., Peng X., Mei S. Autophagy in ovarian follicular development and atresia. Int. J. Biol. Sci. 2019;15:726–737. doi: 10.7150/ijbs.30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tower J. Programmed cell death in aging. Ageing Res. Rev. 2015;23:90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., Spiegelman B., Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 88.Kim H.P., Roe J.H., Chock P.B., Yim M.B. Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. J. Biol. Chem. 1999;274:37455–37460. doi: 10.1074/jbc.274.52.37455. [DOI] [PubMed] [Google Scholar]
- 89.Sakamoto K., Karelina K., Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 92.Fasano C., Disciglio V., Bertora S., Lepore Signorile M., Simone C. 2019. FOXO3a from the nucleus to the mitochondria: a round trip in cellular stress response. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 94.Behl C. Oestrogen as a neuroprotective hormone. Nat. Rev. Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 95.Pike C.J. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J. Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 96.Schacter J.L., Henson E.S., Gibson S.B. Estrogen regulation of anti-apoptotic Bcl-2 family member Mcl-1 expression in breast cancer cells. PloS One. 2014;9 doi: 10.1371/journal.pone.0100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singer C.A., Rogers K.L., Dorsa D.M. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 98.Chen J.Q., Eshete M., Alworth W.L., Yager J.D. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements. J. Cell. Biochem. 2004;93:358–373. doi: 10.1002/jcb.20178. [DOI] [PubMed] [Google Scholar]
- 99.Lejri I., Grimm A., Eckert A. Mitochondria, estrogen and female brain aging. Front. Aging Neurosci. 2018;10:124. doi: 10.3389/fnagi.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Razmara A., Sunday L., Stirone C., Wang X.B., Krause D.N., Duckles S.P., Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Therapeut. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fao L., Mota S.I., Rego A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer's and Parkinson's diseases. Ageing Res. Rev. 2019;54:100942. doi: 10.1016/j.arr.2019.100942. [DOI] [PubMed] [Google Scholar]
- 102.Pajares M., Cuadrado A., Rojo A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017;11:543–553. doi: 10.1016/j.redox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pomatto L.C.D., Tower J., Davies K.J.A. Sexual dimorphism and aging differentially regulate adaptive homeostasis. J Gerontol A Biol Sci Med Sci. 2018;73:141–149. doi: 10.1093/gerona/glx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bota D.A., Davies K.J. Mitochondrial Lon protease in human disease and aging: including an etiologic classification of Lon-related diseases and disorders. Free Radic. Biol. Med. 2016;100:188–198. doi: 10.1016/j.freeradbiomed.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oh J.Y., Choi G.E., Lee H.J., Jung Y.H., Chae C.W., Kim J.S., Lee C.K., Han H.J. 17beta-Estradiol protects mesenchymal stem cells against high glucose-induced mitochondrial oxidants production via Nrf2/Sirt3/MnSOD signaling. Free Radic. Biol. Med. 2019;130:328–342. doi: 10.1016/j.freeradbiomed.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Panza S., Santoro M., De Amicis F., Morelli C., Passarelli V., D'Aquila P., Giordano F., Cione E., Passarino G., Bellizzi D., Aquila S. Estradiol via estrogen receptor beta influences ROS levels through the transcriptional regulation of SIRT3 in human seminoma TCam-2 cells. Tumour Biol. 2017;39 doi: 10.1177/1010428317701642. 1010428317701642. [DOI] [PubMed] [Google Scholar]
- 107.Shi H., Deng H.X., Gius D., Schumacker P.T., Surmeier D.J., Ma Y.C. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum. Mol. Genet. 2017;26:1915–1926. doi: 10.1093/hmg/ddx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torres M.J., Kew K.A., Ryan T.E., Pennington E.R., Lin C.T., Buddo K.A., Fix A.M., Smith C.A., Gilliam L.A., Karvinen S., Lowe D.A., Spangenburg E.E., Zeczycki T.N., Shaikh S.R., Neufer P.D. 17beta-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metabol. 2018;27:167–179 e7. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupte A.A., Pownall H.J., Hamilton D.J. Estrogen: an emerging regulator of insulin action and mitochondrial function. J. Diabetes. Res. 2015;2015:916585. doi: 10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamilton D.J., Minze L.J., Kumar T., Cao T.N., Lyon C.J., Geiger P.C., Hsueh W.A., Gupte A.A. Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Phys. Rep. 2016;4 doi: 10.14814/phy2.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan H., Yang W., Zhou F., Li X., Pan Q., Shen Z., Han G., Newell-Fugate A., Tian Y., Majeti R., Liu W., Xu Y., Wu C., Allred K., Allred C., Sun Y., Guo S. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes. 2019;68:291–304. doi: 10.2337/db18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Root-Bernstein R., Podufaly A., Dillon P.F. Estradiol binds to insulin and insulin receptor decreasing insulin binding in vitro. Front Endocrinol (Lausanne) 2014;5:118. doi: 10.3389/fendo.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santanam N., Shern-Brewer R., McClatchey R., Castellano P.Z., Murphy A.A., Voelkel S., Parthasarathy S. Estradiol as an antioxidant: incompatible with its physiological concentrations and function. J. Lipid Res. 1998;39:2111–2118. [PubMed] [Google Scholar]
- 114.Serra C., Sandor N.L., Jang H., Lee D., Toraldo G., Guarneri T., Wong S., Zhang A., Guo W., Jasuja R., Bhasin S. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology. 2013;154:4594–4606. doi: 10.1210/en.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lucas-Herald A.K., Alves-Lopes R., Montezano A.C., Ahmed S.F., Touyz R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clin. Sci. (Lond.) 2017;131:1405–1418. doi: 10.1042/CS20170090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vasconsuelo A., Pronsato L., Ronda A.C., Boland R., Milanesi L. Role of 17beta-estradiol and testosterone in apoptosis. Steroids. 2011;76:1223–1231. doi: 10.1016/j.steroids.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Pierdominici M., Ortona E., Franconi F., Caprio M., Straface E., Malorni W. Gender specific aspects of cell death in the cardiovascular system. Curr. Pharmaceut. Des. 2011;17:1046–1055. doi: 10.2174/138161211795656891. [DOI] [PubMed] [Google Scholar]
- 118.Pronsato L., Milanesi L., Vasconsuelo A., La Colla A. Testosterone modulates FoxO3a and p53-related genes to protect C2C12 skeletal muscle cells against apoptosis. Steroids. 2017;124:35–45. doi: 10.1016/j.steroids.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 119.Palomar-Morales M., Morimoto S., Mendoza-Rodriguez C.A., Cerbon M.A. The protective effect of testosterone on streptozotocin-induced apoptosis in beta cells is sex specific. Pancreas. 2010;39:193–200. doi: 10.1097/MPA.0b013e3181c156d9. [DOI] [PubMed] [Google Scholar]
- 120.Er F., Michels G., Gassanov N., Rivero F., Hoppe U.C. Testosterone induces cytoprotection by activating ATP-sensitive K+ channels in the cardiac mitochondrial inner membrane. Circulation. 2004;110:3100–3107. doi: 10.1161/01.CIR.0000146900.84943.E0. [DOI] [PubMed] [Google Scholar]
- 121.Lista P., Straface E., Brunelleschi S., Franconi F., Malorni W. On the role of autophagy in human diseases: a gender perspective. J. Cell Mol. Med. 2011;15:1443–1457. doi: 10.1111/j.1582-4934.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei Y., Huang J. Role of estrogen and its receptors mediated-autophagy in cell fate and human diseases. J. Steroid Biochem. Mol. Biol. 2019;191:105380. doi: 10.1016/j.jsbmb.2019.105380. [DOI] [PubMed] [Google Scholar]
- 123.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F., Choi A.M., Chu C.T., Codogno P., Colombo M.I., Cuervo A.M., Debnath J., Deretic V., Dikic I., Eskelinen E.L., Fimia G.M., Fulda S., Gewirtz D.A., Green D.R., Hansen M., Harper J.W., Jaattela M., Johansen T., Juhasz G., Kimmelman A.C., Kraft C., Ktistakis N.T., Kumar S., Levine B., Lopez-Otin C., Madeo F., Martens S., Martinez J., Melendez A., Mizushima N., Munz C., Murphy L.O., Penninger J.M., Piacentini M., Reggiori F., Rubinsztein D.C., Ryan K.M., Santambrogio L., Scorrano L., Simon A.K., Simon H.U., Simonsen A., Tavernarakis N., Tooze S.A., Yoshimori T., Yuan J., Yue Z., Zhong Q., Kroemer G. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schneider J.L., Cuervo A.M. Autophagy and human disease: emerging themes. Curr. Opin. Genet. Dev. 2014;26:16–23. doi: 10.1016/j.gde.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olivan S., Calvo A.C., Manzano R., Zaragoza P., Osta R. Sex differences in constitutive autophagy. BioMed Res. Int. 2014;2014:652817. doi: 10.1155/2014/652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vega-Naredo I., Caballero B., Sierra V., Huidobro-Fernandez C., de Gonzalo-Calvo D., Garcia-Macia M., Tolivia D., Rodriguez-Colunga M.J., Coto-Montes A. Sexual dimorphism of autophagy in Syrian hamster Harderian gland culminates in a holocrine secretion in female glands. Autophagy. 2009;5:1004–1017. doi: 10.4161/auto.5.7.9610. [DOI] [PubMed] [Google Scholar]
- 127.Campesi I., Straface E., Occhioni S., Montella A., Franconi F. Protein oxidation seems to be linked to constitutive autophagy: a sex study. Life Sci. 2013;93:145–152. doi: 10.1016/j.lfs.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Cosper P.F., Leinwand L.A. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Canc. Res. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen C.W., Chen T.Y., Tsai K.L., Lin C.L., Yokoyama K.K., Lee W.S., Chiueh C.C., Hsu C. Inhibition of autophagy as a therapeutic strategy of iron-induced brain injury after hemorrhage. Autophagy. 2012;8:1510–1520. doi: 10.4161/auto.21289. [DOI] [PubMed] [Google Scholar]
- 130.Wang L.F., Yokoyama K.K., Chen T.Y., Hsiao H.W., Chiang P.C., Hsieh Y.C., Lo S., Hsu C. Male-specific alleviation of iron-induced striatal injury by inhibition of autophagy. PloS One. 2015;10 doi: 10.1371/journal.pone.0131224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chandegra B., Tang J.L.Y., Chi H., Alic N. Sexually dimorphic effects of dietary sugar on lifespan, feeding and starvation resistance in Drosophila. Aging (Albany NY) 2017;9:2521–2528. doi: 10.18632/aging.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Folk D.G., Zwollo P., Rand D.M., Gilchrist G.W. Selection on knockdown performance in Drosophila melanogaster impacts thermotolerance and heat-shock response differently in females and males. J. Exp. Biol. 2006;209:3964–3973. doi: 10.1242/jeb.02463. [DOI] [PubMed] [Google Scholar]
- 133.Hercus M.J., Loeschcke V., Rattan S.I. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi: 10.1023/a:1024197806855. [DOI] [PubMed] [Google Scholar]
- 134.Lashmanova E., Zemskaya N., Proshkina E., Kudryavtseva A., Volosnikova M., Marusich E., Leonov S., Zhavoronkov A., Moskalev A. The evaluation of geroprotective effects of selected flavonoids in Drosophila melanogaster and Caenorhabditis elegans. Front. Pharmacol. 2017;8:884. doi: 10.3389/fphar.2017.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matzkin L.M., Watts T.D., Markow T.A. Evolution of stress resistance in Drosophila: interspecific variation in tolerance to desiccation and starvation. Funct. Ecol. 2009;23:521–527. [Google Scholar]
- 136.Rush B., Sandver S., Bruer J., Roche R., Wells M., Giebultowicz J. Mating increases starvation resistance and decreases oxidative stress resistance in Drosophila melanogaster females. Aging Cell. 2007;6:723–726. doi: 10.1111/j.1474-9726.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 137.Service P.M. The effect of mating status on lifespan, egg laying, and starvation resistance in Drosophila melanogaster in relation to selection on longevity. J. Insect Physiol. 1989;35:447–452. [Google Scholar]
- 138.Waskar M., Landis G.N., Shen J., Curtis C., Tozer K., Abdueva D., Skvortsov D., Tavare S., Tower J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY) 2009;1:903–936. doi: 10.18632/aging.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen J., Tower J. Drosophila foxo acts in males to cause sexual-dimorphism in tissue-specific p53 life span effects. Exp. Gerontol. 2010;45:97–105. doi: 10.1016/j.exger.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Argue K.J., Neckameyer W.S. Altering the sex determination pathway in Drosophila fat body modifies sex-specific stress responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R82–R92. doi: 10.1152/ajpregu.00003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khazaeli A.A., Tatar M., Pletcher S.D., Curtsinger J.W. Heat-induced longevity extension in Drosophila. I. Heat treatment, mortality, and thermotolerance. J Gerontol A Biol Sci Med Sci. 1997;52:B48–B52. doi: 10.1093/gerona/52a.1.b48. [DOI] [PubMed] [Google Scholar]
- 142.Moskalev A., Plyusnina E., Shaposhnikov M., Shilova L., Kazachenok A., Zhavoronkov A. The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle. 2012;11:4222–4241. doi: 10.4161/cc.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moskalev A.A., Plyusnina E.N., Shaposhnikov M.V. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 2011;12:253–263. doi: 10.1007/s10522-011-9320-0. [DOI] [PubMed] [Google Scholar]
- 144.McCulloch D., Gems D. Evolution of male longevity bias in nematodes. Aging Cell. 2003;2:165–173. doi: 10.1046/j.1474-9728.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 145.Mendenhall A.R., LeBlanc M.G., Mohan D.P., Padilla P.A. Reduction in ovulation or male sex phenotype increases long-term anoxia survival in a daf-16-independent manner in Caenorhabditis elegans. Physiol. Genom. 2009;36:167–178. doi: 10.1152/physiolgenomics.90278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cypser J.R., Tedesco P., Johnson T.E. Hormesis and aging in Caenorhabditis elegans. Exp. Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumsta C., Chang J.T., Schmalz J., Hansen M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017;8:14337. doi: 10.1038/ncomms14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker G.A., Lithgow G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 149.Foley H.B., Sun P.Y., Ramirez R., So B.K., Venkataraman Y.R., Nixon E.N., Davies K.J.A., Edmands S. Sex-specific stress tolerance, proteolysis, and lifespan in the invertebrate Tigriopus californicus. Exp. Gerontol. 2019;119:146–156. doi: 10.1016/j.exger.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li N., Arief N., Edmands S. Effects of oxidative stress on sex-specific gene expression in the copepod Tigriopus californicus revealed by single individual RNA-seq. Comp. Biochem. Physiol. Genom. Proteonomics. 2019;31:100608. doi: 10.1016/j.cbd.2019.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tenkorang M.A., Snyder B., Cunningham R.L. Sex-related differences in oxidative stress and neurodegeneration. Steroids. 2018;133:21–27. doi: 10.1016/j.steroids.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lavoie J.C., Tremblay A. Sex-specificity of oxidative stress in newborns leading to a personalized antioxidant nutritive strategy. Antioxidants (Basel) 2018;7 doi: 10.3390/antiox7040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.La Colla A., Pronsato L., Milanesi L., Vasconsuelo A. 17beta-Estradiol and testosterone in sarcopenia: role of satellite cells. Ageing Res. Rev. 2015;24:166–177. doi: 10.1016/j.arr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 154.Luo T., Kim J.K. The role of estrogen and estrogen receptors on cardiomyocytes: an overview. Can. J. Cardiol. 2016;32:1017–1025. doi: 10.1016/j.cjca.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ventura-Clapier R., Dworatzek E., Seeland U., Kararigas G., Arnal J.F., Brunelleschi S., Carpenter T.C., Erdmann J., Franconi F., Giannetta E., Glezerman M., Hofmann S.M., Junien C., Katai M., Kublickiene K., Konig I.R., Majdic G., Malorni W., Mieth C., Miller V.M., Reynolds R.M., Shimokawa H., Tannenbaum C., D'Ursi A.M., Regitz-Zagrosek V. Sex in basic research: concepts in the cardiovascular field. Cardiovasc. Res. 2017;113:711–724. doi: 10.1093/cvr/cvx066. [DOI] [PubMed] [Google Scholar]
- 156.Liu H., Pedram A., Kim J.K. Oestrogen prevents cardiomyocyte apoptosis by suppressing p38alpha-mediated activation of p53 and by down-regulating p53 inhibition on p38beta. Cardiovasc. Res. 2011;89:119–128. doi: 10.1093/cvr/cvq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Farajdokht F., Farhoudi M., Majdi A., Zamanlu M., Sadigh-Eteghad S., Vahedi S., Mahmoudi J. Testosterone may hold therapeutic promise for the treatment of ischemic stroke in aging: a closer look at laboratory findings. Adv. Pharmaceut. Bull. 2019;9:48–55. doi: 10.15171/apb.2019.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palikaras K., Lionaki E., Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 159.Park J., Shin H., Song H., Lim H.J. Autophagic regulation in steroid hormone-responsive systems. Steroids. 2016;115:177–181. doi: 10.1016/j.steroids.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 160.Chauhan A., Moser H., McCullough L.D. Sex differences in ischaemic stroke: potential cellular mechanisms. Clin. Sci. (Lond.) 2017;131:533–552. doi: 10.1042/CS20160841. [DOI] [PubMed] [Google Scholar]
- 161.McCullough L.D., Mirza M.A., Xu Y., Bentivegna K., Steffens E.B., Ritzel R., Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 2016;8:1432–1441. doi: 10.18632/aging.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hagberg H., Wilson M.A., Matsushita H., Zhu C., Lange M., Gustavsson M., Poitras M.F., Dawson T.M., Dawson V.L., Northington F., Johnston M.V. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 163.La Colla A., Vasconsuelo A., Milanesi L., Pronsato L. 17beta-Estradiol protects skeletal myoblasts from apoptosis through p53, bcl-2, and FoxO families. J. Cell. Biochem. 2017;118:104–115. doi: 10.1002/jcb.25616. [DOI] [PubMed] [Google Scholar]
- 164.Guerquin M.J., Duquenne C., Coffigny H., Rouiller-Fabre V., Lambrot R., Bakalska M., Frydman R., Habert R., Livera G. Sex-specific differences in fetal germ cell apoptosis induced by ionizing radiation. Hum. Reprod. 2009;24:670–678. doi: 10.1093/humrep/den410. [DOI] [PubMed] [Google Scholar]
- 165.Beltrán-Sánchez H., Finch C.E., Crimmins E.M. Twentieth century surge of excess adult male mortality. Proc. Natl. Acad. Sci. U. A. S. 2015;112:8993–8998. doi: 10.1073/pnas.1421942112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tower J., Landis G.N., Shen J., Choi R., Fan Y., Lee D., Song J. Mifepristone/RU486 acts in Drosophila melanogaster females to counteract the life span-shortening and pro-inflammatory effects of male Sex Peptide. Biogerontology. 2017;18:413–427. doi: 10.1007/s10522-017-9703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Austad S.N., Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2015;62:40–46. doi: 10.1159/000381472. [DOI] [PubMed] [Google Scholar]
- 168.Burger J.M., Promislow D.E. Sex-specific effects of interventions that extend fly life span. Sci. Aging Knowl. Environ. 2004;2004:pe30. doi: 10.1126/sageke.2004.28.pe30. [DOI] [PubMed] [Google Scholar]
- 169.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 170.Strong R., Miller R.A., Antebi A., Astle C.M., Bogue M., Denzel M.S., Fernandez E., Flurkey K., Hamilton K.L., Lamming D.W. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shen J., Ford D., Landis G.N., Tower J. Identifying sexual differentiation genes that affect Drosophila life span. BMC Geriatr. 2009;9:56. doi: 10.1186/1471-2318-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsin H., Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 173.Mason J.B., Cargill S.L., Anderson G.B., Carey J.R. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64:1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hotzi B., Kosztelnik M., Hargitai B., Takács-Vellai K., Barna J., Bördén K., Málnási-Csizmadia A., Lippai M., Ortutay C., Bacquet C., Pasparaki A., Arányi T., Tavernarakis N., Vellai T. Sex-specific regulation of aging in Caenorhabditis elegans. Aging Cell. 2018;17 doi: 10.1111/acel.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Honjoh S., Ihara A., Kajiwara Y., Yamamoto T., Nishida E. The sexual dimorphism of dietary restriction responsiveness in Caenorhabditis elegans. Cell Rep. 2017;21:3646–3652. doi: 10.1016/j.celrep.2017.11.108. [DOI] [PubMed] [Google Scholar]
- 176.Shen J., Landis G.N., Tower J. Multiple metazoan life-span interventions exhibit a sex-specific strehler-mildvan inverse relationship between initial mortality rate and age-dependent mortality rate acceleration. J Gerontol A Biol Sci Med Sci. 2017;72:44–53. doi: 10.1093/gerona/glw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zajitschek F., Zajitschek S.R., Friberg U., Maklakov A.A. Interactive effects of sex, social environment, dietary restriction, and methionine on survival and reproduction in fruit flies. Age (Dordr) 2013;35:1193–1204. doi: 10.1007/s11357-012-9445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Crea F., Battipaglia I., Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. 2015;241:157–168. doi: 10.1016/j.atherosclerosis.2015.04.802. [DOI] [PubMed] [Google Scholar]
- 179.Ostadal B., Ostadal P. Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. Br. J. Pharmacol. 2014;171:541–554. doi: 10.1111/bph.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yahagi K., Davis H.R., Arbustini E., Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 181.Girijala R.L., Sohrabji F., Bush R.L. Sex differences in stroke: review of current knowledge and evidence. Vasc. Med. 2017;22:135–145. doi: 10.1177/1358863X16668263. [DOI] [PubMed] [Google Scholar]
- 182.Haast R.A., Gustafson D.R., Kiliaan A.J. Sex differences in stroke. J. Cerebr. Blood Flow Metabol. 2012;32:2100–2107. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seifert H.A., Benedek G., Liang J., Nguyen H., Kent G., Vandenbark A.A., Saugstad J.A., Offner H. Sex differences in regulatory cells in experimental stroke. Cell. Immunol. 2017;318:49–54. doi: 10.1016/j.cellimm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Walsh D.M., Selkoe D.J. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat. Rev. Neurosci. 2016;17:251. doi: 10.1038/nrn.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mazure C.M., Swendsen J. Sex differences in Alzheimer's disease and other dementias. Lancet Neurol. 2016;15:451. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pike C.J. Sex and the development of Alzheimer's disease. J. Neurosci. Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tobore T.O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer's disease. Neurol. Sci. 2019;40:1527–1540. doi: 10.1007/s10072-019-03863-x. [DOI] [PubMed] [Google Scholar]
- 188.Zhao L., Mao Z., Woody S.K., Brinton R.D. Sex differences in metabolic aging of the brain: insights into female susceptibility to Alzheimer's disease. Neurobiol. Aging. 2016;42:69–79. doi: 10.1016/j.neurobiolaging.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu Y.-B., Zou Y., Huang Y., Zhang Y.-F., Lourenco G.F., Chen S.-D., Halliday G.M., Wang G., Ren R.-J. ROCK1 is associated with Alzheimer's Disease-specific plaques, as well as enhances autophagosome formation but not autophagic Aβ clearance. Front. Cell. Neurosci. 2016;10:253. doi: 10.3389/fncel.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Q., Liu Y., Sun M. Autophagy and Alzheimer's disease. Cell. Mol. Neurobiol. 2017;37:377–388. doi: 10.1007/s10571-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Daulatzai M.A. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J. Neurosci. Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- 192.Kisler K., Nelson A.R., Montagne A., Zlokovic B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017;18:419. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Minhas P.S., Liu L., Moon P.K., Mhatre S.D., Wang Q., Coronado M., Contrepois K., Joshi A.U., Dove C.G., Kvalheim G. DE NOVO NAD+ synthesis rescues neuroinflammation and ALZHEIMER’S disease phenotypes: MODULATING IMMUNE CELL RESPONSES AND MITOCHONDRIAL BIOENERGETICS. Alzheimer's & Dementia. The Journal of the Alzheimer's Association. 2018;14:P1403–P1404. [Google Scholar]
- 194.Sa A.C.C., Madsen H., Brown J.R. Shared molecular signatures across neurodegenerative diseases and herpes virus infections highlights potential mechanisms for maladaptive innate immune responses. Sci. Rep. 2019;9:8795. doi: 10.1038/s41598-019-45129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cerri S., Mus L., Blandini F. Parkinson's disease in women and men: what's the difference? J. Parkinsons Dis. 2019;9:501–515. doi: 10.3233/JPD-191683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kaur S.J., McKeown S.R., Rashid S. Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene. 2016;577:109–118. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 197.Li R., Strykowski R., Meyer M., Mulcrone P., Krakora D., Suzuki M. Male-specific differences in proliferation, neurogenesis, and sensitivity to oxidative stress in neural progenitor cells derived from a rat model of ALS. PloS One. 2012;7 doi: 10.1371/journal.pone.0048581. [DOI] [PMC free article] [PubMed] [Google Scholar]