Abstract
Autoimmune diseases are characterized by circulating antibodies and immune complexes directed against self-tissues that result in both systemic and organ-specific inflammation and pathology. Most autoimmune diseases occur more often in women than men. One exception is myocarditis, which is an inflammation of the myocardium that is typically caused by viral infections. Sex differences in the immune response and the role of the sex hormones estrogen and testosterone are well established based on animal models of autoimmune viral myocarditis as well as in mitochondrial function leading to reactive oxygen species production. RNA viruses like coxsackievirus B3, the primary cause of myocarditis in the US, activate the inflammasome through mitochondrial antiviral signaling protein located on the mitochondrial outer membrane. Toll-like receptor 4 and the inflammasome are the primary signaling pathways that increase inflammation during myocarditis, which is increased by testosterone. This review describes what is known about sex differences in inflammation, redox biology and mitochondrial function in the male-dominant autoimmune disease myocarditis and highlights gaps in the literature and future directions.
Keywords: ROS, Myocarditis, Macrophage, NLRP3 inflammasome, TLR4, TNF
Highlights
-
•
Main source of immune and tissue cell ROS are mitochondria.
-
•
ERα in mitochondria mediate protective effect of estrogen in cardiovascular disease.
-
•
RNA viruses that cause myocarditis activate the inflammasome through MAVS.
-
•
Testosterone increases TLR4/inflammasome in myocarditis in male mice.
-
•
TLR4/inflammasome increases mitochondrial ROS.
Abbreviations
- APCs
antigen presenting cells
- AR
androgen receptor
- ARE
androgen response elements
- CVB3
coxsackievirus group B3
- DAMPs
danger-associated molecular patterns
- DCM
dilated cardiomyopathy
- DCs
dendritic cells
- DPI
diphenyleneiodonium
- DRP1
dynamin-related protein 1
- EAM
experimental autoimmune myocarditis
- ECSIT
evolutionarily conserved signaling intermediate in Toll pathways
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- EREs
estrogen response elements
- ETC
electron transport chain
- GSH
glutathione
- GPx
glutathione peroxidase
- HLA
human leukocyte antigen
- ICAM
intercellular adhesion molecule
- IFNγ
interferon-γ
- Ig
immunoglobulin
- IL
interleukin
- LPS
lipopolysaccharide
- MAVS
mitochondrial antiviral signaling protein
- MCs
mast cells
- MHC
major histocompatibility complex
- mtROS
mitochondrial ROS
- NK
natural killer
- NLRs
nucleotide binding domain-leucine rich repeat proteins
- NLRP3
NLR pyrin domain containing 3
- NOX
NADPH oxidase
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- ROS
reactive oxygen species
- RSV
respiratory syncytial virus
- SASP
senescence-associated secretory phenotype
- SOD
superoxide dismutase
- Th
T helper
- Tim-3
T cell Ig mucin
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRAF6
TNF-associated factor 6
- Treg
regulatory T cells
1. Introduction
An autoimmune disease is defined as a disease where autoimmunity causes the disease or plays a major role in its pathogenesis and is generally based on the presence of autoantibodies and/or autoreactive T cells which culminate in the breakdown of self-tolerance [1]. The presence of autoantibodies in the sera of patients continues to be a hallmark for identifying and diagnosing an autoimmune disease. However, other factors like systemic- or tissue-specific dysfunction or metabolic dysfunction, histological evidence of inflammation, biomarkers, human leukocyte antigen (HLA) testing (HLA is the human designation for major histocompatibility complex (MHC) proteins which present antigens on the surface of cells), and assays to test the presence of autoreactive T cells are also used in the diagnosis of disease. A seminal characteristic of autoimmune diseases is that they display marked sex differences with most occurring more often in women than men such as rheumatic autoimmune diseases like rheumatoid arthritis and Sjögren's syndrome [2,3]. Far fewer autoimmune diseases are more common in men like type I diabetes and myocarditis. Sex differences in the immune response are responsible for many of the characteristic features of autoimmune diseases like elevated circulating autoantibodies and immune complex deposition resulting in tissue damage and organ-specific autoinflammation. Immune-mediated reactive oxygen species (ROS) production is generated following viral and bacterial infections as well as from tissue damage. Infections and chemicals have long been postulated to play a role in the pathogenesis and/or exacerbation of autoimmune diseases [4]. Immune signaling pathways that are associated with ROS generation include tumor necrosis factor (TNF) and the NLRP3 (NLR pyrin domain containing 3)/caspase-1 inflammasome. TNF induces pyroptosis, which is a form of cell death characterized by caspase-1-dependent formation of plasma membrane pores leading to the release of the proinflammatory cytokines interleukin (IL)-1β and IL-18, ROS generation and cell lysis. The pro-forms of IL-1β and IL-18 are induced by Toll-like receptor (TLR)4 and activated by enzymatic cleavage from caspase-1 [[5], [6], [7]]. Sex hormones, estrogen in particular, are known to regulate ROS production in mitochondria [8,9]. This review describes what is known about sex differences in inflammation, redox biology and mitochondrial function in the male-dominant autoimmune disease myocarditis and highlights gaps in the literature and future directions.
2. Sex differences in autoimmune disease
One of the earliest epidemiology studies to report sex differences in autoimmune diseases was by Beeson's group in 1994 [10]. Jacobson et al. later reported in 1997 that women were at 2.7 times greater risk of developing one of 24 autoimmune diseases and that nearly 80% of all patients with autoimmune diseases from 1965 to 1995 in the US were women [11]. A more recent assessment of sex differences in autoimmune diseases by Hayter and Cook came to a similar conclusion as the Jacobson's group (Table 1) [3]. The top 5 most prevalent autoimmune diseases according to Hayter and Cook are rheumatoid arthritis, Hashimoto's thyroiditis, celiac disease, Graves' disease, and type I diabetes; however, the most prevalent autoimmune diseases are not necessarily those with the highest sex bias (Table 1). Thyroiditis (19:1), Sjögren's syndrome (16:1) and scleroderma/systemic sclerosis (12:1) have been reported to have high female to male sex ratios, although individual studies vary [12]. Inflammatory conditions that occur more frequently in men like cardiovascular diseases (i.e., atherosclerosis and dilated cardiomyopathy), many cancers (i.e., lung, liver, stomach) and male-dominant autoimmune diseases (i.e., type I diabetes and myocarditis) are leading causes of death in men [[13], [14], [15], [16], [17]]. In contrast, inflammatory diseases that occur more often in women tend to be chronic with lower mortality like most autoimmune diseases, allergy and asthma [16,18].
Table 1.
Prevalence and sex ratio of well-known autoimmune diseases.a
| Autoimmune Disease | Prevalence (per 105) | Ratio of women to men (% women) |
|---|---|---|
| Rheumatoid arthritis | 860 | 3:1 (75%) |
| Hashimoto's thyroiditis | 792 | 19:1 (95%) |
| Celiac disease | 750 | 1.3:1 (57%) |
| Graves' disease | 629 | 7:1 (88%) |
| Type 1 diabetes | 480 | 1:1.2 (45%) |
| Multiple sclerosis | 58 | 2:1 (64%) |
| Systemic lupus erythematosus | 32 | 7:1 (88%) |
| Ulcerative colitis | 30 | 2:1 (65%) |
| Crohn's disease | 25 | 1:1.4 (40%) |
| Scleroderma | 24 | 12:1 (92%) |
| Autoimmune hepatitis type 1 | 17 | 4:1 (78%) |
| Sjögren's syndrome | 14 | 16:1 (94%) |
| Myositis | 5 | 2:1 (67%) |
| Myocarditis | – | 1:3.5 (29%) |
Ref. 3, 44.
3. Sex differences in inflammation in autoimmune disease
Effective immunity depends on a coordinated immune response that is influenced by genetic, environmental and hormonal effects that alter the response to pathogens or damage in a sex-specific manner [19]. The major steroid hormones that have been studied in the immune response are estrogens, testosterone and progesterone. These so-called sex hormones bind to specific cell membrane and/or nuclear-associated receptors on/in immune cells and influence gene expression. Estrogen receptor alpha (ERα) and beta (ERβ) and the androgen receptor (AR) are sequestered in the cytoplasm bound to heat shock proteins. When activated by ligand, they bind directly to estrogen response elements (EREs) or androgen response elements (ARE) in the promoter region of specific genes like the interferon (IFN)γ gene or indirectly activate gene transcription by binding transcription factors such as NFκB [20,21]. Sex steroids also alter immune cell function via non-nuclear membrane expression of hormone receptors. Signaling through membrane hormone receptors is more rapid than nuclear receptors and can result in both gene transcription through initiation of MAPK, ERK and other kinase signal pathways or in non-transcriptional signals via calcium flux and activation of glutamate receptors [[21], [22], [23], [24]].
The immune system has been divided into innate and adaptive arms with the former responding rapidly (minutes/hours) to “foreign” non-self antigens while the adaptive immune response generally requires one to two weeks to develop after exposure to the initiating agent for a primary immune response. Innate immunity responds to classes of microbes like viruses, bacteria and parasites by recognizing pathogen-associated molecular patterns (PAMPs) while the adaptive immune response responds to specific short sequences of amino acids from microbes. The innate immune response also responds to self-derived danger-associated molecular patterns (DAMPs) like ATP, cholesterol crystals, and oxidized mitochondrial DNA, for example, or foreign-derived particles like alum, silica, aluminium hydroxide and nanoparticles [25]. In this manner the adaptive immune response depends on the innate immune response to provide “direction” [26]. PAMPs and/or DAMPs that bind pattern recognition receptors (PRRs) like TLRs drive the adaptive T and B cell immune response in three basic directions reflected as T helper (Th) 1, Th2 and Th17-type immune responses and antibody isotypes (i.e., human immunoglobulin (Ig)G1- Th1, human IgG2- Th2). T helper cells are often delineated by the major type of cytokine produced- IFNγ from Th1 cells, interleukin (IL)-4 from Th2 and IL-17A from Th17 cells. Cytokines and other mediators released from Th cells are needed to clear infections. For example, IFNγ released from Th1 cells activates cytolytic CD8 T and natural killer (NK) cells to reduce viral infections, IL-4-type responses from Th2 cells clear parasitic infections via mast cell and eosinophil activation, and IL-17A from Th17 cells promotes neutrophil and macrophage responses to bacteria [27]. Once the pathogen appears to be effectively controled by the immune response, regulatory T cells (Treg) inhibit Th responses to regain immunological homeostasis [28]. Tregs comprise up to 10% of peripheral CD4+ T cells in naive mice and humans, express CD25 (IL-2 receptor α chain), are important negative modulators of the immune response, and are particularly important in preventing autoimmune responses [29,30]. The description of the immune response into three basic arms including Th1, Th2 and Th17 that are regulated by Treg is an oversimplification of the immune response, but each of these primary Th responses has been described to be important in specific autoimmune diseases [31,32].
Reviewing the literature regarding sex differences in the immune response can be confusing due to the many conflicting reports on the effect of sex hormones on immune cell subtypes. This is because the immune response is entirely context dependent; that is, the context depends on the cell type, tissue where inflammation occurs, genetic background, initiating antigen, dose of the antigen, age of the cell/animal, etc. Importantly, the type of immune response that will be generated to any particular antigen is strongly influenced by biological sex (i.e., circulating sex hormone levels, cellular sex hormone receptors, sex hormone receptor ratio on/in cells). Although sex hormone receptors are typically expressed intracellularly, in immune cells expression can also be found on the cell surface where they are able to interact as part of the immunological synapse during antigen presentation [21]. Most reports in the literature do not take these factors into account when summarizing the effects of sex hormones on immune cells/responses.
Sex hormone receptors are located on/in many cells of the immune system including T cells, B cells, monocytes, macrophages, dendritic cells (DCs), and mast cells (MCs) in humans and rodents [21]. However, only monocyte/macrophages and mast cells have both nuclear and membrane ERs and ARs [21]. Aside from their role in allergy, MCs act as antigen presenting cells (APCs) particularly at potential sites of foreign antigen entry to the host like the skin, gut, vasculature and peritoneum [33,34]. Estrogen has a well-known ability to increase antibody responses to vaccines, infections and autoantigens by activating B cells, while androgens have the opposite effect [4,35,36]. However, depending on the context, estrogen can either promote inflammation by enhancing Th1 and/or Th17 responses via transcriptional activation of NFκB or increase the regulatory arm of the adaptive immune response by promoting tolerogenic DCs, anti-inflammatory/regulatory Th2-type immune responses, and T regulatory and regulatory macrophage cell populations [[37], [38], [39]]. Estrogen and progesterone are known to enhance Treg cell activation [40]. However, Treg predominantly express ERβ rather than ERα that has a nuclear rather than cell surface localization [21]. The immune cell responses to testosterone are more poorly characterized.
3.1. Sex differences in inflammation in a male-dominant autoimmune disease-myocarditis
Myocarditis is an example of an autoimmune disease that occurs more often in men than women (Table 1). Several clinical trials and registries of myocarditis report a male to female sex ratio between 1.5:1 and 1.7:1 [[41], [42], [43]]. A recent study of around 300 patients with myocarditis found a sex ratio of 3.5:1 male to female [44]. Sex differences in myocarditis can be explained not only by sex hormone receptor expression on/in immune cells but also by their expression on cardiac tissues. Genomic and membrane signaling via ERα/β, AR, and aromatase, the enzyme that converts androgens to estrogens, are expressed on and within vascular endothelial cells, vascular smooth muscle cells, cardiac fibroblasts and cardiomyocytes in humans and rodents [13,15,45,46]. Additionally, sex differences exist in ER and AR levels on various cell types. For example, females express higher levels of ERs in their arteries [46]. 17β-estradiol has been shown to prevent apoptosis in cardiac myocytes, ROS-induced cardiac damage, and prevent cardiac hypertrophy and fibrosis [45]. Testosterone levels have been reported to be higher in the heart of men than women [47]. Importantly, underlying sex differences in tissue physiology, in this case in the heart, are likely to influence the immune response to infection and injury.
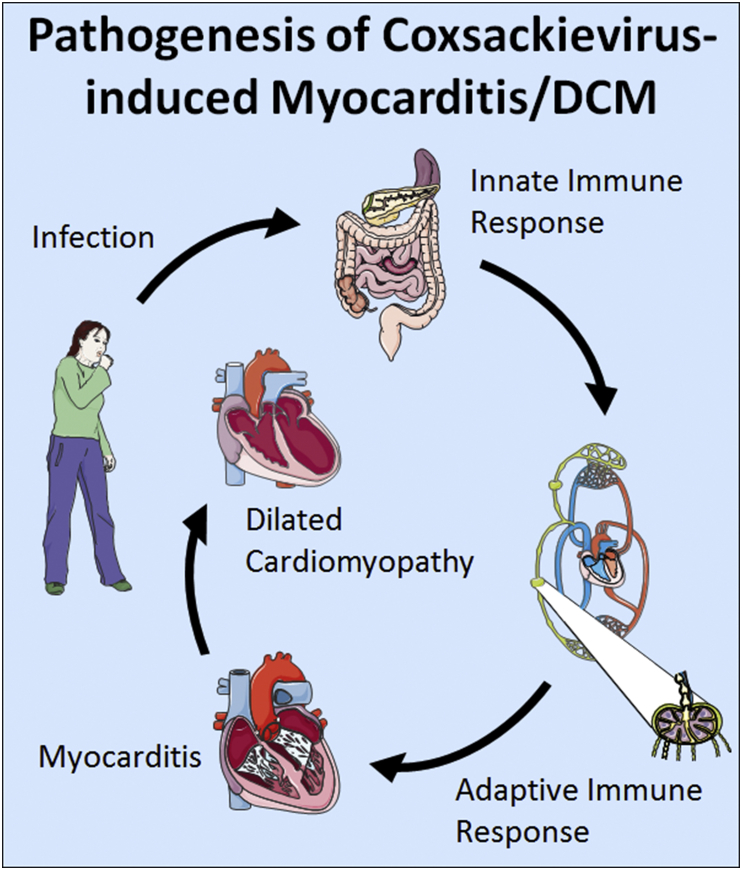
Myocarditis is a leading cause of sudden heart failure in young adults worldwide and can progress to dilated cardiomyopathy (DCM) and chronic heart failure [48]. Viral infections are the most common cause of myocarditis in developed countries where endomyocardial biopsies have been used to detect inflammation and viruses in patients with myocarditis (Fig. 1) [49,50]. Enteroviruses, which include coxsackievirus group B3 (CVB3), are most often associated with myocarditis in the US, where disease occurs most frequently in Whites [49].
Fig. 1.
Proposed pathogenesis of coxsackievirus-induced myocarditis/DCM: After infection by fecal-oral transmission, coxsackievirus enters the gut where it replicates. When innate antiviral and self-damage immune signaling is initiated, dendritic cells that have captured viral antigen travel to the lymph nodes to activate T and B cells. Around day 2–3 after infection coxsackievirus causes viremia which allows it access to the heart. Activation of resident cardiac mast cell and macrophage populations increase monocyte trafficking. During peak myocarditis in males (around 10 days after infection) macrophages can comprise nearly 80% of the infiltrate in the heart with smaller numbers of T and B cells. After clearance of active viral infection around day 12–14 after infection immune cells in the heart quickly disappear. The immune response to the viral infection activates remodeling genes during myocarditis that over time can lead to cardiac remodeling, fibrosis and cardiac dilatation. Patients are at risk of sudden cardiac death during myocarditis and those that survive can develop dilated cardiomyopathy and chronic heart failure that may require a heart transplant.
TLR4 is a unique PRR in that it responds to bacterial lipopolysaccharide (LPS), viruses and damaged self-tissue [51]. TLR4 is most well-known for its recognition of LPS by means of the accessory protein, MD-2 [52,53]. Interestingly, certain viral infections, such as respiratory syncytial virus (RSV), have been found to interact with MD-2 to activate TLR4 [54]. Other viruses like Ebola and Dengue virus bind cellular membranes and transiently activate TLR4 [[55], [56], [57]]. Damaged self-proteins like HMGB1, which is a nuclear protein that binds to DNA and can be released during viral infections or other causes of tissue damage, are known to activate TLR4 [58,59]. Whether CVB3 activates TLR4 directly has not been investigated, but RNA viruses like CVB3 are known to activate the inflammasome via mitochondrial antiviral signaling protein (MAVS) [60,61].
TLR4 mRNA expression has been found to be higher in patients with biopsy-proven myocarditis and DCM than controls and strongly correlates with enteroviral RNA levels in the heart [62,63]. Although sex differences were not examined in the study, nearly 75% of the patients were men. Animal models that recapitulate myocarditis include those that inject virus only, inject virus plus damaged heart proteins or classic autoimmune models that use cardiac myosin/peptide and complete Freund's adjuvant to induce disease [64,65]. Susceptibility to adjuvant-induced experimental autoimmune myocarditis (EAM) or DCM following acute myocarditis requires using white background mice (i.e., A/J, BALB/c) [[66], [67], [68]], mouse strains that have more MCs expressing TLR4 [33,34,69].
Male mice with CVB3 autoimmune myocarditis develop more severe myocardial inflammation than females (Fig. 2). In males, this is characterized by elevated expression of TLR4 on MCs and macrophages and a dominant IFNγ-associated Th1-type immune response with Th1-associated IgG2a anti-CVB3 antibodies (as well as autoantibodies against cardiac myosin). In contrast, females develop an IL-4-associated Th2-type immune response with elevated regulatory T cell populations that express FoxP3, T cell Ig mucin (Tim-3) and CTLA4 [33,[69], [70], [71], [72]]. Furthermore, patients with clinically suspected myocarditis or DCM were found to have higher IgG3 autoantibody levels that correlated to poor cardiac function and higher IFNγ levels [73]. Although IFNγ levels were higher in the hearts of male mice with myocarditis, and IL-4 elevated in females, no sex difference was observed in IL-17A levels in the heart [69]. However, the dominant IFNγ/Th1 response found in male mice with myocarditis was not due to classic IL-12/STAT4-induced IFNγ production but likely due to elevated IL-18 levels [74]. IL-18 was originally named IFNγ-inducing factor and its ability to strongly upregulate IFNγ levels mimic a true Th1-type immune response. Male BALB/c mice deficient in TLR4 had less myocarditis as well as reduced IL-1β and IL-18 levels in the heart, indicating the importance of TLR4 signaling and these cytokines in promoting disease [75]. Recently, blocking IL-1β was found to reduce acute myocarditis and progression from myocarditis to DCM in CVB3 myocarditis in mice [76]. Furthermore, testosterone increases IL-1β levels in the heart during autoimmune CVB3 myocarditis [68].
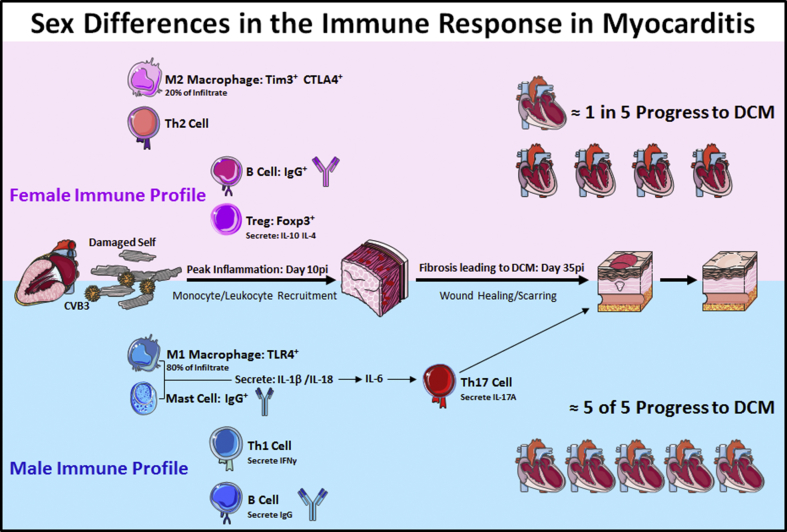
Fig. 2.
Sex differences in the immune response in myocarditis/DCM: Females (top, pink) have low levels of cardiac inflammation during myocarditis due to an elevated anti-inflammatory immune response that is characterized by higher numbers of Th2-type immune cells, an M2 (anti-inflammatory) macrophage phenotype, greater numbers of Foxp3+ regulatory T cells, and higher levels of anti-inflammatory cytokines like IL-4 and IL-10. In contrast, males (bottom, blue) produce a robust proinflammatory immune response during myocarditis that is characterized by mast cell activation, greater numbers of macrophages that express a more M1 phenotype, and elevated proinflammatory cytokines that release IL-1β, IL-18, and IL-6 that stimulates Th17 cells, which promote remodeling and fibrosis that leads to DCM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Redox biology and mitochondria
The main source of cellular ROS such as superoxide and hydrogen peroxide are from mitochondria and immune-mediated NADPH oxidase (NOX). Immune-mediated ROS production from phagocytic monocyte burst has been studied primarily in relation to bacterial infections. Previously ROS produced from the electron transport chain (ETC) of mitochondria were considered to be an accidental and destructive by-product of oxidative metabolism. It is increasingly recognized that mitochondrial ROS (mtROS) is important in mounting an effective immune response to infection and damage and that immune-mediated and mitochondrial-mediated regulation of ROS are inter-related [77,78]. However, high levels of ROS can cause irreversible damage to DNA, lipids and proteins and so ROS levels act as key signaling molecules regulating cell function in health and disease [79].
During bacterial infections, monocytes engulf bacteria in phagosomes that fuse with lysosomes to form phagolysosomes where NOX (i.e., NOX2) generates superoxide which is converted to hydrogen peroxide by superoxide dismutase (SOD) and further to hypochlorous acid by myeloperoxidase [80,81]. Excess ROS is eliminated from mitochondria by the three scavenging enzymes SOD1-3: SOD1 is located in the mitochondrial intermembrane space and in the cytosol, SOD2 in the mitochondrial matrix, and SOD3 in the extracellular matrix [82,83]. During bacterial infections mitochondria are recruited to phagosomes to produce ROS following TLR activation. One immune pathway that has long been recognized as central to the pathogenesis of autoimmune disease [84] that is also important for mtROS production is TNF-mediated signaling. TLR activation in macrophages has been shown to activate TNF-associated factor 6 (TRAF6) translocation to mitochondria where it facilitates mtROS production by mediated ubiquitination of evolutionarily conserved signaling intermediate in Toll pathways (ECSIT) and subsequent interference of complex 1 assembly [77,81]. Mycobacterium tuberculosis infections have been found to activate mtROS via the TNF-induced RIP1/RIP3 pathway. Interestingly, this pathway may promote activation of mtROS in EAM which uses M. tuberculosis in complete Freund's adjuvant to induce myocarditis [65]. Although viral infections are typically associated with causing myocarditis, a number of bacterial infections have been proposed as possible etiologies for myocarditis including M. tuberculosis [4]. Another immune pathway that is known to activate mtROS in macrophages infected with Listeria monocytogenes is IFNγ [85].
Macrophages are phagocytes that change their phenotype based on the local environment, especially in response to cytokines [86]. Important in innate immune responses, macrophages also contribute to acute and chronic pathology. For example, in male BALB/c mice with CVB3 myocarditis, macrophages comprise up to 80% of the infiltrate [87]. Macrophages are not only responsive to cytokines, but also to the redox state. Differences in macrophage phenotype have been associated with distinct energy production profiles and ROS generation states of the cell [88]. M1 macrophages, associated with Th1 responses, are induced primarily by IFNγ, secrete proinflammatory cytokines such as IL-12, TNFα, IL-1β and IL-6, and are defined metabolically by storage of fatty acid, decreased OXPHOS and increased NO production [[89], [90], [91]]. In contrast, Th2-associated M2 macrophages are activated by IL-4 and IL-13, secrete anti-inflammatory cytokines including IL-10, TGFβ, and have increased OXPHOS and fatty acids that undergo β-oxidation [[92], [93], [94]].
The role of endothelial cells in the immune response is typically discussed in the context of cell recruitment where the endothelium acts as a “port of entry” where immune cells are drawn by binding integrins (e.g., ICAMs) following a chemokine gradient after diapedesis through the endothelial layer [95]. Endothelial cells effect immune cell recruitment by releasing cytokines including IL-1, IL-6, and TNFα [96], leading to upregulation of intercellular adhesion molecule (ICAM)-1 [97]. Mitochondrial dysfunction leads to cellular senescence primarily through the release of ROS by ETC electron leakage and subsequent superoxide spillage into the cytoplasm in a process that induces a senescence-associated secretory phenotype (SASP) [98]. Mitochondrial ROS is primarily derived from the activity of ETC complex I and II [99]. The generated ROS can cause nuclear DNA damage of telomeres leading to senescence [98]. Due to intracellular stress in this state of cell-cycle arrest, DAMPs such as mitochondrial DNA initiate NLRP3 inflammasome signaling and caspase-1 activation [100]. Interestingly, it has been observed that a lower NAD+/NADH ratio in cells can lead to a distinct SASP that lacks the IL-1 signaling arm [101]. The exact relationship of this process to the NLRP3 inflammasome is not entirely understood, but NLRP3 has been found to be a key feature of endothelial cell SASP during long-term, low-grade inflammation in the context of aging [100].
One of the primary immune pathways that activate mtROS following infection is the inflammasome [25]. The inflammasome senses infections and cellular damage via nucleotide binding domain-leucine rich repeat proteins (NLRs) that recognize PAMPs like double-stranded DNA and DAMPS like mitochondrial DNA, ATP and histones and activate the cysteine protease caspase-1 which is able to cleave the pro-forms of IL-1β and IL-18 that are induced by TLR4 activation [25,81]. The NLRP3 inflammasome is activated by fungi, helminths, protozoa, bacteria and viruses. Importantly, mtROS also activates the NLRP3 inflammasome. MAVS is a mitochondrial membrane protein that activates an IFN response and the NLRP3 inflammasome following viral infections [25]. Interestingly, mitofusins, which are outer mitochondrial membrane GTPases involved in mitochondrial fusion, have been reported to be necessary for NLRP3 activation during viral infection [102]. Consistent with this, several viruses are known to induce mitochondrial fission and mitophagy which disrupt MAVS and NLRP3 antiviral signaling, respectively [103,104].
There are several ways that mitochondrial- and cell-derived sources of ROS activate immune signaling pathways within immune and non-immune cells [88]. Immune activation and cell death can be inhibited via antioxidant systems that convert free radicals and oxidized side-chain residues into more inert states preventing cellular, DNA and mitochondrial DNA damage [105,106]. Thioredoxin and glutathione are two crucial and well-studied antioxidant systems that convert dangerous free radical substrates into non-reactive products in the form of oxidized cysteine residues and hydrogen peroxide derived from SODs in the cytosol and cellular compartments [[105], [106], [107], [108], [109], [110]]. Man-made antioxidant compounds can also mitigate cellular damage and are an increasingly studied area that holds promise to reduce ROS-mediated local or systemic inflammation [111,112].
5. Sex differences in redox biology and mitochondria in myocarditis
Clinical evidence that redox biology could be important in myocarditis comes from a study by Nimata et al. which found that thioredoxin, a redox-regulating protein, was upregulated in cardiac biopsies of patients with myocarditis or DCM [113]. Thioredoxin has two isoforms Trx1 and Trx2, which are the nuclear/cytosolic and mitochondrial localizing isoforms, respectively. The primary function of thioredoxin is to facilitate the reduction of cysteine residues on oxidized proteins by cysteine thiol-disulfide exchange [109,110]. This replacement is possible due to the conserved CXXC motif on thioredoxin which allows the transfer of reduced cysteine residues [106]. Thioredoxin then receives the oxidized cysteine residues from its substrate that are reduced by thioredoxin reductase in a reaction that requires NADPH [114]. Although the specific isoform of thioredoxin was not specified in biopsies from patients with myocarditis or DCM, areas of the biopsies with active necrosis reflective of DNA damage had the highest expression of thioredoxin measured using immunohistochemistry [113]. Increased levels of antioxidant expression in damaged tissue can be interpreted as a reparative response to initial injury from cytotoxic ROS [113].
When discussing the contribution of ROS to disease pathology, it is important to consider tissue-specific generation of ROS. In the case of myocarditis, ROS has been observed to play roles in cardiac myocyte damage, apoptosis and inflammation [111,112,115]. CVB3 infection of HeLa or Hep-2 cells was found to produce a massive amount of ROS [111], which was reversed using the anti-oxidant nitronyl nitroxyl derivatives pyrrolidine dithiocarbamate and nitronyl nitroxide derivative, respectively [115]. Nox4 expression in the heart of male BALB/c mice increased during cardiac stress and pressure overload in a model of transverse aortic constriction demonstrating a role for Nox4 during cardiomyocyte strain. [116]. During CVB3-induced myocarditis, diphenyleneiodonium (DPI) was used to decrease apoptosis and was found to inhibit a Nox4-induced increase in ROS [112]. This finding is interesting because DPI is typically thought to be a non-specific inhibitor of Nox. Another possible explanation for the decrease in Nox4 expression with DPI could be that a reduction in ROS by Nox4 led to less ROS signaling during the innate immune response which decreased cardiac inflammation [112]. In this case, the inhibitory effect of DPI on Nox4 was likely primarily affecting immune cell Nox4 expression, which contributes more to ROS generation in myocarditis than cardiomyocytes [117].
A mechanism by which cells protect themselves from ROS is via the antioxidant, glutathione (GSH) [105,107,108,118]. The primary purpose of glutathione is to convert peroxides, such as those produced by SOD, into water. In short, the reduction of peroxides via GSH is catalyzed by the selenium-dependent enzyme glutathione peroxidase (GPx) resulting in a water molecule being formed along with GSH in its oxidized form [105,107,108,118]. The ratio of the oxidized and reduced forms of GSH can be used as a general measure of cellular oxidative stress where healthy cells have about 90% of GSH in the reduced form [105,107,108,118]. In one study, GPx-1 was found to play a crucial role in protecting mice from viral-induced myocarditis. GPx-1 knock-out and wild type mice were infected with CVB3/0, a non-pathogenic viral serotype of coxsackievirus [119]. In addition to having more severe cardiac inflammation, the GPx-1 knock-out mice produced nearly 5-fold less CVB3/0 antibodies compared to wild type animals [119]. Another study measured the reduced form of GSH in BALB/c mice after CVB3 infection and found that the level of GSH decreased over time coinciding with cardiomyocyte apoptosis [120]. GSH also plays an important role in maintaining lipids and lipid-membranes after oxidative damage [121]. Oxidative damage to lipids can cause disruption of homeostatic conditions of cellular membranes and compartments [122,123]. GSH acts as a conjugating factor via glutathione-s-transferase to remove byproducts of lipid peroxidation due to free radical oxidative degradation [124]. In the same experiment by Beck et al. above, it was found that the GPX-1 knock-out mice exposed to CVB3/0 had increased lipid peroxidation compared to WT mice [119]. These studies suggest an important role for the antioxidant glutathione in myocarditis. However, there has been limited research in this area.
In a mouse model of CVB3-induced myocarditis in C57BL/6 mice, viral infection was found to increase complex I and III activity in the heart during myocarditis, which was associated with elevated oxidative stress (i.e., ROS production) [125]. In a highly lethal model of CVB3 myocarditis induced by virus alone (i.e., no injection of heart antigens and so not an autoimmune model) where 50% of BALB/c male mice die by day 6 pi, the NLRP3 inflammasome was upregulated at day 7 pi but activation was reversed following treatment with caspase-1 or IL-1β inhibitors resulting in improved heart function and lower mortality [126]. Interestingly, the medicinal plant manassantin B was found to exert antiviral activity to CVB3 infection by promoting mtROS production in cell culture and TNFα, IFNγ and IL-6 levels from sera during the innate immune response in immunologically immature 5 week old female BALB/c mice [127]. Importantly, CVB3 was found to localize to mitochondria in cell culture, trigger dynamin-related protein 1 (DRP1)-mediated fragmentation, induce mitophagy (autophagy to destroy damaged mitochondria), and allow virus and proviral microRNA to escape in mitochondrial extracellular microvesicles [128,129]. Almost all of these studies examined male mice. Thus, there is a paucity of data examining sex differences in redox biology and mitochondrial function during myocarditis.
Mitochondria exhibit strong sex- and tissue-specificity (reviewed in Ref. [130]). Because mitochondria are maternally inherited, except for some genetic situations [131], in men nuclear genes inherited from the father are important in regulating the function of maternally inherited mitochondria [132]. Mitochondria are a major component of cardiac cells which are estimated to contain between 7000–10,000 mitochondria per cell [133]. Mitochondrial-related gene expression has been shown to depend on sex and age [9,134]. Mitochondrial localizing genes that are upregulated in the heart of females and downregulated in males include MTHFD1, ACP1, IFITM3, and CBX5 [13]. Additionally, it was found that genes related to energy metabolism tend to be more downregulated in males compared to females (i.e., ACADL, PPARGC1, PDK4, COX5A, SLC25A20, PFKB1, and MLYCD) [13]. These results from Regitz-Zargrosek et al. suggest a role for sex hormone signaling in mitochondrial dynamics and cellular redox biology[13], which needs further investigation. Sex differences in mitochondrial function in the heart are summarized in a comprehensive review by Ventura-Clapier et al. [135]. The findings summarize the published literature and indicate that females have less mitochondrial content, higher mitochondrial efficiency and differentiation, higher glutamate/malate-stimulated respiration, higher ADP/oxygen ratios, lower ROS production, lower calcium uptake, and higher calcium retention [[136], [137], [138], [139], [140], [141]]. These sex differences suggest a role for ER signaling in mitochondria [135].
Sex hormone receptor signaling is known to alter mitochondrial dynamics by localizing to mitochondria and modulating expression of nuclear genes that localize to the mitochondria such as DRP1 and mitofusin [[142], [143], [144]]. Androgen receptors have been shown to localize to and modulate mitochondria in select cell types, such as sperm cells, but not cardiomyocytes [143,144]. In contrast, the effect of estrogen on mitochondria of cardiomyocytes has been well studied [9]. Animal studies of estrogen and estrogen-related receptors have shown that estrogen is required to maintain normal, healthy cardiac function by regulating mitochondrial processes [145,146]. When activated, estrogen-related receptors increase expression of genes involved in fatty acid oxidation, respiratory chain activity and mitochondrial dynamics [[145], [146], [147]]. Estrogen increases expression of genes involved in both mitochondrial fission and fusion, but overall favors mitochondrial fusion in cardiomyocytes, which is a healthy phenotype for cardiomyocytes compared to other cell types where fusion can be deleterious [145,146,148,149]. Studies investigating mitochondrial morphology in the heart show that female mice have a population of mitochondria that are larger in size compared to males, which tend to be much smaller in size [150].
Women are protected from many cardiovascular diseases that lead to heart failure including coronary artery disease, myocardial infarct, myocarditis and DCM [13,15,151,152]. ERα expression in mitochondria is believed to mediate the protective effect of estrogen in reducing cardiovascular disease prior to menopause [130,147]; however, no studies have examined the role of biological sex on mitochondrial function in myocarditis. One study reported sex-specific differences in autophagy-related genes during CVB3 myocarditis in mice and in men with CVB3-associated cardiomyopathy compared to healthy control men [153]. Mitochondria not only generate energy for cells in the form of ATP (>90% for the heart), but they also function in steroid hormone synthesis, which is used in a paracrine and autocrine manner in immune and tissue cells [130,147]. Additionally, sex hormones are known to regulate mitochondrial function in a sex- and tissue-specific manner. Estrogens, acting via ERs are able to increase the expression of both nuclear and mitochondrial genomes. In mitochondria, ERs have been shown to bind to mitochondrial DNA. In cells like cardiomyocytes, mitochondria are an essential source of ROS. The literature consistently reports that cardiomyocytes from female animals have lower numbers of mitochondria that are larger because they are fused, produce less ROS and have a greater antioxidant capacity than males [130,154,155]. The effect of estrogen to increase mitochondrial fusion in cardiomyocytes contributes to decreased oxidative stress in females [[145], [146], [147]] and may explain, at least in part, why women have greater protection from cardiovascular disease and heart failure than men.
Although no studies have investigated whether sex differences in mitochondrial function contribute to sex differences in myocarditis, several extrapolations can be made (Fig. 3). The increased Th1, M1 and IFNγ response in male mice with myocarditis suggests that damaging ROS levels could be elevated in males compared to females [69,74]. This is also suggested by the higher expression of TLR4, IL-1β, IL-18 and the inflammasome in the heart and on immune cells from male mice with myocarditis compared to females [69,75], a pathway that is known to increase ROS production. We also know that women and female mice are protected from heart failure in myocarditis after viral infection suggesting that estrogen could be mediating this effect through cardiac and/or immune cell mitochondria [44]. Furthermore, we and others have shown that estrogen mediates these protective effects in female mice during viral myocarditis with an anti-inflammatory/regulatory Th2, macrophage and T regulatory cell immune response [69,[156], [157], [158]].
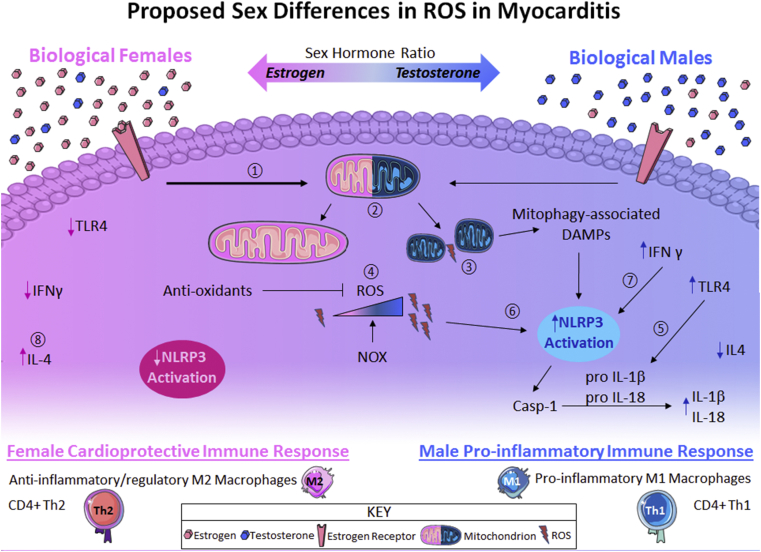
Fig. 3.
Proposed model of sex differences in ROS during myocarditis.① Increased ER activation due to higher estrogen in females leads to ② a profusion phenotype during myocarditis, whereas lower ER signaling in males during myocarditis leads to ③ increased mitochondrial fission. ④ Profusion mitochondrial phenotype in females decreases ROS while anti-oxidants neutralize excess ROS produced by NOX. In males, mitochondrial fission contributes to higher ROS while also releasing mitophagy-associated DAMPs (as mitochondrial fusion can be a precursor to mitophagy under cellular stress conditions such as infection) which can activate the inflammasome. ⑤ Increased TLR4 signaling in males compared to females leads to higher proIL-1β and proIL-18 which are cleaved by activated caspase-1 ⑥ to the active forms IL-1β and IL-18. ⑦ More inflammasome activation occurs in males due to increased intracellular ROS with higher IFNγ signaling compared to females. ⑧ Elevated IL-4 in females promotes a cardioprotective immune response comprised of anti-inflammatory Th2 CD4+ T cells and regulatory M2 macrophages while in males elevated IL-18 and IFNγ promote a proinflammatory immune phenotype resulting in M1 macrophages and Th1 CD4+ T cells.
6. Conclusions, gaps in research and future directions
The effect of sex hormones is tissue/organ specific and affected by sex and age. This is also true for mitochondria. Thus, generalizing the effect of estrogen and testosterone on mitochondrial function and redox/ROS production from experiments examining cardiomyocytes in culture and/or normal or diseased heart tissue may not be true for other cells and tissues. Aside from age, sex differences in mitochondrial function may also be influenced by race (i.e., genetic background), environmental influences that cause disease like viral and bacterial infections or tissue damage leading to autoimmunity caused by infections or toxins/chemicals. TLRs and the NLRP3 inflammasome strongly regulate gene profiles that influence the pathogenesis of disease and autoimmune diseases in particular and are also strongly influenced by sex hormones. For these reasons it will be important to report the effect of sex hormones on disease, mitochondrial function, and redox biology based on the sex, age and race of the patient group. Thus, for any given disease, men and women need to be analyzed separately with a further stratification using age 50 as a cutoff as a surrogate for menopause if clinical data on menopause are missing.
This review has focused on a male-dominant viral-induced autoimmune model of myocarditis, where a great deal is known about sex differences in the immunopathogenesis of disease and in the role of mitochondrial ROS in cardiomyocytes. However, more information on the effect of sex hormones on mitochondrial ROS and redox biology are needed for myocarditis and DCM. Additionally, further investigation should be conducted on the effect of sex hormones on mitochondrial ROS for female-dominant autoimmune diseases. Understanding these effects in the context of age will be critically important because most autoimmune diseases in men and women occur either before or after menopause/50 years of age [44,159,160]. What impact does age of both host and immune cells have on mitochondrial function, ROS production, protection against infection and the pathogenesis of autoimmune diseases? Interestingly, myocarditis occurs most often in men under age 50 and the risk of heart failure from myocarditis disappears at the age of 50 raising the question of what happens to mitochondria at this age to protect the heart of men [44]. Additionally, more research is needed to understand the interplay between immune-mediated ROS vs. mitochondrial ROS within immune cells and cardiomyocytes or other cardiac cells. Insight into these and other fundamental questions related to how immune cells interact with pathogens at the site of infection will have broad application to autoimmune diseases that have long been postulated to be caused by viral or bacterial infections [4]. Little information exists on the role of viral infection in mediating redox function in mitochondria and even less information exists on how sex differences might alter redox immune signaling. Insights into how infections alter mitochondrial function may be gained not only using animal models but also clinical data gleaned from patients with mitochondrial mutations that affect mitochondrial function following infection [161].
Funding
This work was funded in part by the National Institutes of Health [grant numbers R21 AI145356, R21 AI152318, and NIH training grant TL1 TR002380] and the Mayo Clinic Center for Regenerative Medicine.
Disclosures
The authors declare that they do not have financial or other conflicts of interest.
Authorship
All authors made substantial contributions to the conception and design of the article, drafting or revising the article for critically important intellectual content and approved the final version of the article.
Declaration of competing interest
The authors declare that they do not have financial or other conflicts of interest. All authors made substantial contributions to the conception and design of the article, drafting or revising the article for critically important intellectual content and approved the final version of the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101482.
Contributor Information
Damian N. Di Florio, Email: DiFlorio.Damian@mayo.edu.
Jon Sin, Email: Jon.Sin@cshs.org.
Michael J. Coronado, Email: coronamj@whitman.edu.
Paldeep S. Atwal, Email: dra@atwalclinic.com.
DeLisa Fairweather, Email: Fairweather.DeLisa@mayo.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rose N.R., Mackay I.R. Elsevier Academic Press; Amsterdam: 2014. The Autoimmune Diseases. [Google Scholar]
- 2.Fairweather D., Frisancho-Kiss S., Rose N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008;173(3):600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayter S.M., Cook M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 2012;11(10):754–765. doi: 10.1016/j.autrev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Fairweather D., Petri M.A., Coronado M.J., Cooper L.T. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expet Rev. Clin. Immunol. 2012;8(3):269–284. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pei P., Yao X., Jiang L., Qiu T., Wang N., Yang L., Gao N., Wang Z., Yang G., Liu X., Liu S., Jia X., Tao Y., Wei S., Sun X. Inorganic arsenic induces pyroptosis and pancreatic beta cells dysfunction through stimulating the IRE1alpha/TNF-alpha pathway and protective effect of taurine. Food Chem. Toxicol. 2019;125:392–402. doi: 10.1016/j.fct.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Zeng C., Wang R., Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int. J. Biol. Sci. 2019;15(7):1345–1357. doi: 10.7150/ijbs.33568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi X., Yang Y., Ma J., Chen Q., Zeng Y., Li J., Chen L., Li Y. MiR-130a alleviated high-glucose induced retinal pigment epithelium (RPE) death by modulating TNF-alpha/SOD1/ROS cascade mediated pyroptosis. Biomed. Pharmacother. 2020;125:109924. doi: 10.1016/j.biopha.2020.109924. [DOI] [PubMed] [Google Scholar]
- 8.Liao T.L., Lee Y.C., Tzeng C.R., Wang Y.P., Chang H.Y., Lin Y.F., Kao S.H. Mitochondrial translocation of estrogen receptor beta affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free Radic. Biol. Med. 2019;134:359–373. doi: 10.1016/j.freeradbiomed.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Ventura-Clapier R., Piquereau J., Veksler V., Garnier A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front. Endocrinol. 2019;10:557. doi: 10.3389/fendo.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeson P.B. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 1994;96(5):457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 12.Purnamawati K., Ong J.A., Deshpande S., Tan W.K., Masurkar N., Low J.K., Drum C.L. The importance of sex stratification in autoimmune disease biomarker research: a systematic review. Front. Immunol. 2018;9:1208. doi: 10.3389/fimmu.2018.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regitz-Zagrosek V., Oertelt-Prigione S., Seeland U., Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ. J. 2010;74(7):1265–1273. doi: 10.1253/circj.cj-10-0196. [DOI] [PubMed] [Google Scholar]
- 14.Dorak M.T., Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front. Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairweather D., Cooper L.T., Jr., Blauwet L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013;38(1):7–46. doi: 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultheiss H.P., Fairweather D., Caforio A.L.P., Escher F., Hershberger R.E., Lipshultz S.E., Liu P.P., Matsumori A., Mazzanti A., McMurray J., Priori S.G. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5(1):32. doi: 10.1038/s41572-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend E.A., Miller V.M., Prakash Y.S. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun. Rev. 2012;11(6–7):A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Fox H.S., Bond B.L., Parslow T.G. Estrogen regulates the IFN-gamma promoter. J. Immunol. 1991;146(12):4362–4367. [PubMed] [Google Scholar]
- 21.Buskiewicz I.A., Huber S.A., Fairweather D. Sex Differences in Physiology. Academic Press; Amsterdam: 2016. Sex hormone receptor expression in the immune system; pp. 45–60. [Google Scholar]
- 22.Benten W.P., Lieberherr M., Stamm O., Wrehlke C., Guo Z., Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol. Biol. Cell. 1999;10(10):3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benten W.P., Lieberherr M., Giese G., Wrehlke C., Stamm O., Sekeris C.E., Mossmann H., Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. Faseb. J. 1999;13(1):123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 24.Schlegel A., Wang C., Katzenellenbogen B.S., Pestell R.G., Lisanti M.P. Caveolin-1 potentiates estrogen receptor alpha (ERalpha) signaling. caveolin-1 drives ligand-independent nuclear translocation and activation of ERalpha. J. Biol. Chem. 1999;274(47):33551–33556. doi: 10.1074/jbc.274.47.33551. [DOI] [PubMed] [Google Scholar]
- 25.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairweather D., Frisancho-Kiss S., Rose N.R. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev. Med. Virol. 2005;15(1):17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 27.Sun B., Zhang Y. Overview of orchestration of CD4+ T cell subsets in immune responses. Adv. Exp. Med. Biol. 2014;841:1–13. doi: 10.1007/978-94-017-9487-9_1. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020. Feb 4 doi: 10.1146/annurev-immunol-042718-041717. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Robinson D.P., Huber S.A., Moussawi M., Roberts B., Teuscher C., Watkins R., Arnold A.P., Klein S.L. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinecker C., Goschl L., Bonelli M. Treg cells in health and autoimmune diseases: new insights from single cell analysis. J. Autoimmun. 2019:102376. doi: 10.1016/j.jaut.2019.102376. [DOI] [PubMed] [Google Scholar]
- 31.Dube S.R., Fairweather D., Pearson W.S., Felitti V.J., Anda R.F., Croft J.B. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 2009;71(2):243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda K., Takeuchi Y., Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019;41(3):283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 33.Fairweather D., Frisancho-Kiss S., Gatewood S., Njoku D., Steele R., Barrett M., Rose N.R. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37(2):131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 34.Frisancho-Kiss S., Nyland J.F., Davis S.E., Barrett M.A., Gatewood S.J., Njoku D.B., Cihakova D., Silbergeld E.K., Rose N.R., Fairweather D. Cutting Edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006;176(11):6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 35.Cook I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29–30):3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 36.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 37.Evans M.J., Eckert A., Lai K., Adelman S.J., Harnish D.C. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ. Res. 2001;89(9):823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 38.Papenfuss T.L., Powell N.D., McClain M.A., Bedarf A., Singh A., Gienapp I.E., Shawler T., Whitacre C.C. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 2011;186(6):3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 40.Aristimuno C., Teijeiro R., Valor L., Alonso B., Tejera-Alhambra M., de Andres C., Minarro D.O., Lopez-Lazareno N., Faure F., Sanchez-Ramon S. Sex-hormone receptors pattern on regulatory T-cells: clinical implications for multiple sclerosis. Clin. Exp. Med. 2012;12(4):247–255. doi: 10.1007/s10238-011-0172-3. [DOI] [PubMed] [Google Scholar]
- 41.Mason J.W., O'Connell J.B., Herskowitz A., Rose N.R., McManus B.M., Billingham M.E., Moon T.E. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995;333(5):269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 42.Magnani J.W., Danik H.J., Dec G.W., Jr., DiSalvo T.G. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am. Heart J. 2006;151(2):463–470. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 43.Caforio A.L., Calabrese F., Angelini A., Tona F., Vinci A., Bottaro S., Ramondo A., Carturan E., Iliceto S., Thiene G., Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007;28(11):1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 44.Coronado M.J., Bruno K.A., Blauwet L.A., Tschope C., Cunningham M.W., Pankuweit S., van Linthout S., Jeon E.S., McNamara D.M., Krejci J., Bienertova-Vasku J., Douglass E.J., Abston E.D., Bucek A., Frisancho J.A., Greenaway M.S., Hill A.R., Schultheiss H.P., Cooper L.T., Jr., Fairweather D. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. 2019;8(2) doi: 10.1161/JAHA.118.008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regitz-Zagrosek V., Becher E., Mahmoodzadeh S., Schubert C. Sex steroid hormones. In: Bader M., editor. Molecular Mechanisms to Novel Therapuetics. Wiley-Blackwell; Weinheim, Germany: 2008. pp. 39–64. [Google Scholar]
- 46.Vitale C., Mendelsohn M.E., Rosano G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009;6(8):532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 47.Deslypere J.P., Vermeulen A. Influence of age on steroid concentrations in skin and striated muscle in women and in cardiac muscle and lung tissue in men. J. Clin. Endocrinol. Metab. 1985;61(4):648–653. doi: 10.1210/jcem-61-4-648. [DOI] [PubMed] [Google Scholar]
- 48.Elamm C., Fairweather D., Cooper L.T. Pathogenesis and diagnosis of myocarditis. Heart. 2012;98(11):835–840. doi: 10.1136/heartjnl-2012-301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauschinger M., Phan M.D., Doerner A., Kuehl U., Schwimmbeck P.L., Poller W., Kandolf R., Schultheiss H.P. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation. 1999;99(7):889–895. doi: 10.1161/01.cir.99.7.889. [DOI] [PubMed] [Google Scholar]
- 50.Tschope C., Bock C.T., Kasner M., Noutsias M., Westermann D., Schwimmbeck P.L., Pauschinger M., Poller W.C., Kuhl U., Kandolf R., Schultheiss H.P. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111(7):879–886. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 51.Olejnik J., Hume A.J., Muhlberger E. Toll-like receptor 4 in acute viral infection: too much of a good thing. PLoS Pathog. 2018;14(12) doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuzmich N.N., Sivak K.V., Chubarev V.N., Porozov Y.B., Savateeva-Lyubimova T.N., Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 2017;5(4) doi: 10.3390/vaccines5040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rallabhandi P., Phillips R.L., Boukhvalova M.S., Pletneva L.M., Shirey K.A., Gioannini T.L., Weiss J.P., Chow J.C., Hawkins L.D., Vogel S.N., Blanco J.C. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. mBio. 2012;3(4) doi: 10.1128/mBio.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akey D.L., Brown W.C., Dutta S., Konwerski J., Jose J., Jurkiw T.J., DelProposto J., Ogata C.M., Skiniotis G., Kuhn R.J., Smith J.L. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science. 2014;343(6173):881–885. doi: 10.1126/science.1247749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iampietro M., Younan P., Nishida A., Dutta M., Lubaki N.M., Santos R.I., Koup R.A., Katze M.G., Bukreyev A. Ebola virus glycoprotein directly triggers T lymphocyte death despite of the lack of infection. PLoS Pathog. 2017;13(5) doi: 10.1371/journal.ppat.1006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Escudero-Perez B., Volchkova V.A., Dolnik O., Lawrence P., Volchkov V.E. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog. 2014;10(11) doi: 10.1371/journal.ppat.1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsung A., Klune J.R., Zhang X., Jeyabalan G., Cao Z., Peng X., Stolz D.B., Geller D.A., Rosengart M.R., Billiar T.R. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koh W.U., Kim J., Lee J., Song G.W., Hwang G.S., Tak E., Song J.G. Remote ischemic preconditioning and diazoxide protect from hepatic ischemic reperfusion injury by inhibiting HMGB1-induced TLR4/MyD88/NF-kappaB signaling. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20235899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts B.J., Dragon J.A., Moussawi M., Huber S.A. Sex-specific signaling through Toll-like receptors 2 and 4 contributes to survival outcome of coxsackievirus B3 infection in C57Bl/6 mice. Biol. Sex Differ. 2012;3(1):25. doi: 10.1186/2042-6410-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukherjee A., Morosky S.A., Delorme-Axford E., Dybdahl-Sissoko N., Oberste M.S., Wang T., Coyne C.B. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011;7(3) doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satoh M., Nakamura M., Akatsu T., Iwasaka J., Shimoda Y., Segawa I., Hiramori K. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci (Lond). 2003;104(6):577–584. doi: 10.1042/CS20020263. [DOI] [PubMed] [Google Scholar]
- 63.Satoh M., Nakamura M., Akatsu T., Shimoda Y., Segawa I., Hiramori K. Toll-like receptor 4 is expressed with enteroviral replication in myocardium from patients with dilated cardiomyopathy. Lab. Invest. 2004;84(2):173–181. doi: 10.1038/labinvest.3700031. [DOI] [PubMed] [Google Scholar]
- 64.Fairweather D., Stafford K.A., Sung Y.K. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 2012;24(4):401–407. doi: 10.1097/BOR.0b013e328353372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myers J.M., Fairweather D., Huber S.A., Cunningham M.W. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr Protoc Immunol. 2013;((Chapter 15):Unit 15.14):1–51. doi: 10.1002/0471142735.im1514s101. (Chapter 15):Unit 15.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afanasyeva M., Wang Y., Kaya Z., Park S., Zilliox M.J., Schofield B.H., Hill S.L., Rose N.R. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am. J. Pathol. 2001;159(1):193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huber S.A. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med. Microbiol. Immunol. 2005;194(3):121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 68.Coronado M.J., Brandt J.E., Kim E., Bucek A., Bedja D., Abston E.D., Shin J., Gabrielson K.L., Mitzner W., Fairweather D. Testosterone and interleukin-1beta increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am. J. Physiol. Heart Circ. Physiol. 2012;302(8):H1726–H1736. doi: 10.1152/ajpheart.00783.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frisancho-Kiss S., Davis S.E., Nyland J.F., Frisancho J.A., Cihakova D., Barrett M.A., Rose N.R., Fairweather D. Cutting Edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178(11):6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 70.Huber S.A., Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 1994;68(8):5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fairweather D., Kaya Z., Shellam G.R., Lawson C.M., Rose N.R. From infection to autoimmunity. J. Autoimmun. 2001;16(3):175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 72.Huber S.A. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378(2):292–298. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warraich R.S., Noutsias M., Kazak I., Seeberg B., Dunn M.J., Schultheiss H.P., Yacoub M.H., Kuhl U. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am. Heart J. 2002;143(6):1076–1084. doi: 10.1067/mhj.2002.124406. [DOI] [PubMed] [Google Scholar]
- 74.Frisancho-Kiss S., Nyland J.F., Davis S.E., Frisancho J.A., Barrett M.A., Rose N.R., Fairweather D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006;1126(1):139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Fairweather D., Yusung S., Frisancho S., Barrett M., Gatewood S., Steele R., Rose N.R. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003;170(9):4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 76.Kraft L., Erdenesukh T., Sauter M., Tschope C., Klingel K. Blocking the IL-1beta signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res. Cardiol. 2019;114(2):11. doi: 10.1007/s00395-019-0719-0. [DOI] [PubMed] [Google Scholar]
- 77.West A.P., Shadel G.S., Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garaude J. Reprogramming of mitochondrial metabolism by innate immunity. Curr. Opin. Immunol. 2019;56:17–23. doi: 10.1016/j.coi.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 79.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al Ghouleh I., Khoo N.K., Knaus U.G., Griendling K.K., Touyz R.M., Thannickal V.J., Barchowsky A., Nauseef W.M., Kelley E.E., Bauer P.M., Darley-Usmar V., Shiva S., Cifuentes-Pagano E., Freeman B.A., Gladwin M.T., Pagano P.J. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011;51(7):1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawamata H., Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxidants Redox Signal. 2010;13(9):1375–1384. doi: 10.1089/ars.2010.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karnati S., Luers G., Pfreimer S., Baumgart-Vogt E. Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes. Histochem. Cell Biol. 2013;140(2):105–117. doi: 10.1007/s00418-013-1099-4. [DOI] [PubMed] [Google Scholar]
- 84.Davignon J.L., Rauwel B., Degboe Y., Constantin A., Boyer J.F., Kruglov A., Cantagrel A. Modulation of T-cell responses by anti-tumor necrosis factor treatments in rheumatoid arthritis: a review. Arthritis Res. Ther. 2018;20(1):229. doi: 10.1186/s13075-018-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonoda J., Laganiere J., Mehl I.R., Barish G.D., Chong L.W., Li X., Scheffler I.E., Mock D.C., Bataille A.R., Robert F., Lee C.H., Giguere V., Evans R.M. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21(15):1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bracamonte-Baran W., Cihakova D. Cardiac autoimmunity: myocarditis. Adv. Exp. Med. Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Root-Bernstein R., Fairweather D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J. Theor. Biol. 2015;375:101–123. doi: 10.1016/j.jtbi.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Angajala A., Lim S., Phillips J.B., Kim J.H., Yates C., You Z., Tan M. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front. Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jha A.K., Huang S.C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K.M., Ashall J., Everts B., Pearce E.J., Driggers E.M., Artyomov M.N. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Kong M.J., Han S.J., Kim J.I., Park J.W., Park K.M. Mitochondrial NADP(+)-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells. Cell Death Dis. 2018;9(5):488. doi: 10.1038/s41419-018-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., Zheng L., Gardet A., Tong Z., Jany S.S., Corr S.C., Haneklaus M., Caffrey B.E., Pierce K., Walmsley S., Beasley F.C., Cummins E., Nizet V., Whyte M., Taylor C.T., Lin H., Masters S.L., Gottlieb E., Kelly V.P., Clish C., Auron P.E., Xavier R.J., O'Neill L.A. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan Z., Xie N., Cui H., Moellering D.R., Abraham E., Thannickal V.J., Liu G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J. Immunol. 2015;194(12):6082–6089. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He C., Carter A.B. The metabolic prospective and redox regulation of macrophage polarization. J. Clin. Cell. Immunol. 2015;6(6) doi: 10.4172/2155-9899.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 96.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi S.J., Piao S., Nagar H., Jung S.B., Kim S., Lee I., Kim S.M., Song H.J., Shin N., Kim D.W., Irani K., Jeon B.H., Park J.W., Kim C.S. Isocitrate dehydrogenase 2 deficiency induces endothelial inflammation via p66sh-mediated mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2018;503(3):1805–1811. doi: 10.1016/j.bbrc.2018.07.117. [DOI] [PubMed] [Google Scholar]
- 98.Kirkland J.L., Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114(3):524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chapman J., Fielder E., Passos J.F. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 2019;593(13):1566–1579. doi: 10.1002/1873-3468.13498. [DOI] [PubMed] [Google Scholar]
- 101.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A., Gerencser A.A., Verdin E., Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23(2):303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ichinohe T., Yamazaki T., Koshiba T., Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. U. S. A. 2013;110(44):17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castanier C., Garcin D., Vazquez A., Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11(2):133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J., Ma C., Long F., Yang D., Liu X., Hu Y., Wu C., Wang B., Wang M., Chen Y., Liu G., Moynagh P.N., Zhou J., Peng T., Yang S. Parkin impairs antiviral immunity by suppressing the mitochondrial reactive oxygen species-Nlrp3 axis and antiviral inflammation. iScience. 2019;16:468–484. doi: 10.1016/j.isci.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meister A., Anderson M.E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura H., Nakamura K., Yodoi J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 107.Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263(33):17205–17208. [PubMed] [Google Scholar]
- 108.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holmgren A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 1989;264(24):13963–13966. [PubMed] [Google Scholar]
- 110.Nordberg J., Arner E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 111.Si X., McManus B.M., Zhang J., Yuan J., Cheung C., Esfandiarei M., Suarez A., Morgan A., Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J. Virol. 2005;79(13):8014–8023. doi: 10.1128/JVI.79.13.8014-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chi J., Yu S., Liu C., Zhao X., Zhong J., Liang Y., Ta N., Yin X., Zhao D. Nox4-dependent ROS production is involved in CVB3-induced myocardial apoptosis. Biochem. Biophys. Res. Commun. 2018;503(3):1641–1644. doi: 10.1016/j.bbrc.2018.07.093. [DOI] [PubMed] [Google Scholar]
- 113.Nimata M., Kishimoto C., Shioji K., Ishizaki K., Kitaguchi S., Hashimoto T., Nagata N., Kawai C. Upregulation of redox-regulating protein, thioredoxin, in endomyocardial biopsy samples of patients with myocarditis and cardiomyopathies. Mol. Cell. Biochem. 2003;248(1–2):193–196. doi: 10.1023/a:1024156923322. [DOI] [PubMed] [Google Scholar]
- 114.Nagarajan N., Oka S., Sadoshima J. Modulation of signaling mechanisms in the heart by thioredoxin 1. Free Radic. Biol. Med. 2017;109:125–131. doi: 10.1016/j.freeradbiomed.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H., Gao P., Jing L., Qin X., Sun X. The heart-protective mechanism of nitronyl nitroxide radicals on murine viral myocarditis induced by CVB3. Biochimie. 2012;94(9):1951–1959. doi: 10.1016/j.biochi.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Zhang M., Brewer A.C., Schroder K., Santos C.X., Grieve D.J., Wang M., Anilkumar N., Yu B., Dong X., Walker S.J., Brandes R.P., Shah A.M. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(42):18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tada Y., Suzuki J. Oxidative stress and myocarditis. Curr. Pharmaceut. Des. 2016;22(4):450–471. doi: 10.2174/1381612822666151222160559. [DOI] [PubMed] [Google Scholar]
- 118.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 119.Beck M.A., Esworthy R.S., Ho Y.S., Chu F.F. Glutathione peroxidase protects mice from viral-induced myocarditis. Faseb. J. 1998;12(12):1143–1149. doi: 10.1096/fasebj.12.12.1143. [DOI] [PubMed] [Google Scholar]
- 120.Kyto V., Lapatto R., Lakkisto P., Saraste A., Voipio-Pulkki L.M., Vuorinen T., Pulkki K. Glutathione depletion and cardiomyocyte apoptosis in viral myocarditis. Eur. J. Clin. Invest. 2004;34(3):167–175. doi: 10.1111/j.1365-2362.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 121.Fernandes A.P., Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxidants Redox Signal. 2004;6(1):63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 122.Li S., Zheng M.Q., Rozanski G.J. Glutathione homeostasis in ventricular myocytes from rat hearts with chronic myocardial infarction. Exp. Physiol. 2009;94(7):815–824. doi: 10.1113/expphysiol.2008.046201. [DOI] [PubMed] [Google Scholar]
- 123.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 124.Mieyal J.J., Gallogly M.M., Qanungo S., Sabens E.A., Shelton M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxidants Redox Signal. 2008;10(11):1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebermann L., Wika S., Klumpe I., Hammer E., Klingel K., Lassner D., Volker U., Erben U., Zeichhardt H., Schultheiss H.P., Dorner A. The mitochondrial respiratory chain has a critical role in the antiviral process in Coxsackievirus B3-induced myocarditis. Lab. Invest. 2012;92(1):125–134. doi: 10.1038/labinvest.2011.145. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y., Gao B., Xiong S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am. J. Physiol. Heart Circ. Physiol. 2014;307(10):H1438–H1447. doi: 10.1152/ajpheart.00441.2014. [DOI] [PubMed] [Google Scholar]
- 127.Song J.H., Ahn J.H., Kim S.R., Cho S., Hong E.H., Kwon B.E., Kim D.E., Choi M., Choi H.J., Cha Y., Chang S.Y., Ko H.J. Manassantin B shows antiviral activity against coxsackievirus B3 infection by activation of the STING/TBK-1/IRF3 signalling pathway. Sci. Rep. 2019;9(1):9413. doi: 10.1038/s41598-019-45868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sin J., McIntyre L., Stotland A., Feuer R., Gottlieb R.A. Coxsackievirus B escapes the infected cell in ejected mitophagosomes. J. Virol. 2017;91(24) doi: 10.1128/JVI.01347-17. e01347-01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Germano J.F., Sawaged S., Saadaeijahromi H., Andres A.M., Feuer R., Gottlieb R.A., Sin J. Coxsackievirus B infection induces the extracellular release of miR-590-5p, a proviral microRNA. Virology. 2019;529:169–176. doi: 10.1016/j.virol.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ventura-Clapier R., Dworatzek E., Seeland U., Kararigas G., Arnal J.F., Brunelleschi S., Carpenter T.C., Erdmann J., Franconi F., Giannetta E., Glezerman M., Hofmann S.M., Junien C., Katai M., Kublickiene K., Konig I.R., Majdic G., Malorni W., Mieth C., Miller V.M., Reynolds R.M., Shimokawa H., Tannenbaum C., D'Ursi A.M., Regitz-Zagrosek V. Sex in basic research: concepts in the cardiovascular field. Cardiovasc. Res. 2017;113(7):711–724. doi: 10.1093/cvr/cvx066. [DOI] [PubMed] [Google Scholar]
- 131.Luo S., Valencia C.A., Zhang J., Lee N.C., Slone J., Gui B., Wang X., Li Z., Dell S., Brown J., Chen S.M., Chien Y.H., Hwu W.L., Fan P.C., Wong L.J., Atwal P.S., Huang T. Biparental inheritance of mitochondrial DNA in humans. Proc. Natl. Acad. Sci. U. S. A. 2018;115(51):13039–13044. doi: 10.1073/pnas.1810946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mittwoch U. The elusive action of sex-determining genes: mitochondria to the rescue? J. Theor. Biol. 2004;228(3):359–365. doi: 10.1016/j.jtbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 133.Fernandez-Vizarra E., Enriquez J.A., Perez-Martos A., Montoya J., Fernandez-Silva P. Tissue-specific differences in mitochondrial activity and biogenesis. Mitochondrion. 2011;11(1):207–213. doi: 10.1016/j.mito.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 134.Mayne B.T., Bianco-Miotto T., Buckberry S., Breen J., Clifton V., Shoubridge C., Roberts C.T. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front. Genet. 2016;7:183. doi: 10.3389/fgene.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ventura-Clapier R., Moulin M., Piquereau J., Lemaire C., Mericskay M., Veksler V., Garnier A. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond). 2017;131(9):803–822. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- 136.Chweih H., Castilho R.F., Figueira T.R. Tissue and sex specificities in Ca2+ handling by isolated mitochondria in conditions avoiding the permeability transition. Exp. Physiol. 2015;100(9):1073–1092. doi: 10.1113/EP085248. [DOI] [PubMed] [Google Scholar]
- 137.Colom B., Alcolea M.P., Valle A., Oliver J., Roca P., Garcia-Palmer F.J. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell. Physiol. Biochem. 2007;19(1–4):205–212. doi: 10.1159/000099208. [DOI] [PubMed] [Google Scholar]
- 138.Colom B., Oliver J., Roca P., Garcia-Palmer F.J. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc. Res. 2007;74(3):456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 139.Ribeiro R.F., Jr., Ronconi K.S., Morra E.A., Do Val Lima P.R., Porto M.L., Vassallo D.V., Figueiredo S.G., Stefanon I. Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations. Mol. Cell. Biochem. 2016;419(1–2):41–51. doi: 10.1007/s11010-016-2748-4. [DOI] [PubMed] [Google Scholar]
- 140.Lagranha C.J., Deschamps A., Aponte A., Steenbergen C., Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010;106(11):1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arieli Y., Gursahani H., Eaton M.M., Hernandez L.A., Schaefer S. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J. Mol. Cell. Cardiol. 2004;37(2):507–513. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 142.Chen J.Q., Yager J.D. Estrogen's effects on mitochondrial gene expression: mechanisms and potential contributions to estrogen carcinogenesis. Ann. N. Y. Acad. Sci. 2004;1028:258–272. doi: 10.1196/annals.1322.030. [DOI] [PubMed] [Google Scholar]
- 143.Solakidi S., Psarra A.M., Nikolaropoulos S., Sekeris C.E. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum. Reprod. 2005;20(12):3481–3487. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- 144.Psarra A.M., Sekeris C.E. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life. 2008;60(4):210–223. doi: 10.1002/iub.37. [DOI] [PubMed] [Google Scholar]
- 145.Chen Y., Liu Y., Dorn G.W., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 2011;109(12):1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang T., McDonald C., Petrenko N.B., Leblanc M., Wang T., Giguere V., Evans R.M., Patel V.V., Pei L. Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function. Mol. Cell Biol. 2015;35(7):1281–1298. doi: 10.1128/MCB.01156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rattanasopa C., Phungphong S., Wattanapermpool J., Bupha-Intr T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 2015;147:1–9. doi: 10.1016/j.jsbmb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 148.Capllonch-Amer G., Llado I., Proenza A.M., Garcia-Palmer F.J., Gianotti M. Opposite effects of 17-beta estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J. Mol. Endocrinol. 2014;52(2):203–214. doi: 10.1530/JME-13-0201. [DOI] [PubMed] [Google Scholar]
- 149.Coronado M., Fajardo G., Nguyen K., Zhao M., Kooiker K., Jung G., Hu D.Q., Reddy S., Sandoval E., Stotland A., Gottlieb R.A., Bernstein D. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circ. Res. 2018;122(2):282–295. doi: 10.1161/CIRCRESAHA.117.310725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dworatzek E., Mahmoodzadeh S., Schubert C., Westphal C., Leber J., Kusch A., Kararigas G., Fliegner D., Moulin M., Ventura-Clapier R., Gustafsson J.A., Davidson M.M., Dragun D., Regitz-Zagrosek V. Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovasc. Res. 2014;102(3):418–428. doi: 10.1093/cvr/cvu065. [DOI] [PubMed] [Google Scholar]
- 151.Greiten L.E., Holditch S.J., Arunachalam S.P., Miller V.M. Should there be sex-specific criteria for the diagnosis and treatment of heart failure? J Cardiovasc Transl Res. 2014;7(2):139–155. doi: 10.1007/s12265-013-9514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 153.Koenig A., Sateriale A., Budd R.C., Huber S.A., Buskiewicz I.A. The role of sex differences in autophagy in the heart during coxsackievirus B3-induced myocarditis. J Cardiovasc Transl Res. 2014;7(2):182–191. doi: 10.1007/s12265-013-9525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malorni W., Campesi I., Straface E., Vella S., Franconi F. Redox features of the cell: a gender perspective. Antioxidants Redox Signal. 2007;9(11):1779–1801. doi: 10.1089/ars.2007.1596. [DOI] [PubMed] [Google Scholar]
- 155.Khalifa A.R., Abdel-Rahman E.A., Mahmoud A.M., Ali M.H., Noureldin M., Saber S.H., Mohsen M., Ali S.S. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep. 2017;5(6) doi: 10.14814/phy2.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huber S.A., Kupperman J., Newell M.K. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J. Virol. 1999;73(6):4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frisancho-Kiss S., Coronado M.J., Frisancho J.A., Lau V.M., Rose N.R., Klein S.L., Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav. Immun. 2009;23(5):649–657. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koenig A., Buskiewicz I., Huber S.A. Age-associated changes in estrogen receptor ratios correlate with increased female susceptibility to coxsackievirus B3-induced myocarditis. Front. Immunol. 2017;8:1585. doi: 10.3389/fimmu.2017.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brandt J.E., Priori R., Valesini G., Fairweather D. Sex differences in Sjogren's syndrome: a comprehensive review of immune mechanisms. Biol. Sex Differ. 2015;6:19. doi: 10.1186/s13293-015-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fairweather D. Autoimmune skin diseases: role of sex hormones, vitamin D and menopause. In: Farage M.A., Miller K.W., Fugate-Woods N., Mailbach H.I., editors. Skin, Mucosa and Menopause: Management of Clinical Issues. Springer; Heidelberg: 2015. pp. 359–381. [Google Scholar]
- 161.Kapnick S.M., Pacheco S.E., McGuire P.J. The emerging role of immune dysfunction in mitochondrial diseases as a paradigm for understanding immunometabolism. Metabolism. 2018;81:97–112. doi: 10.1016/j.metabol.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.