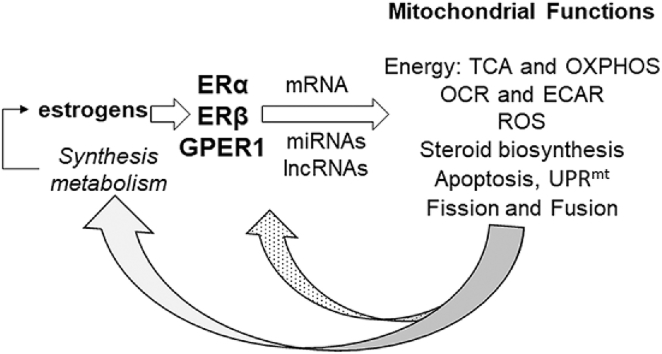
Abstract
Sex-based differences in human disease are caused in part by the levels of endogenous sex steroid hormones which regulate mitochondrial metabolism. This review updates a previous review on how estrogens regulate metabolism and mitochondrial function that was published in 2017. Estrogens are produced by ovaries and adrenals, and in lesser amounts by adipose, breast stromal, and brain tissues. At the cellular level, the mechanisms by which estrogens regulate diverse cellular functions including reproduction and behavior is by binding to estrogen receptors α, β (ERα and ERβ) and G-protein coupled ER (GPER1). ERα and ERβ are transcription factors that bind genomic and mitochondrial DNA to regulate gene transcription. A small proportion of ERα and ERβ interact with plasma membrane-associated signaling proteins to activate intracellular signaling cascades that ultimately alter transcriptional responses, including mitochondrial morphology and function. Although the mechanisms and targets by which estrogens act directly and indirectly to regulate mitochondrial function are not fully elucidated, it is clear that estradiol regulates mitochondrial metabolism and morphology via nuclear and mitochondrial-mediated events, including stimulation of nuclear respiratory factor-1 (NRF-1) transcription that will be reviewed here. NRF-1 is a transcription factor that interacts with coactivators including peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α) to regulate nuclear-encoded mitochondrial genes. One NRF-1 target is TFAM that binds mtDNA to regulate its transcription. Nuclear-encoded miRNA and lncRNA regulate mtDNA-encoded and nuclear-encoded transcripts that regulate mitochondrial function, thus acting as anterograde signals. Other estrogen-regulated mitochondrial activities including bioenergetics, oxygen consumption rate (OCR), and extracellular acidification (ECAR), are reviewed.
Keywords: Estrogen, Estrogen receptor, GPER1, Mitochondria, NRF-1, Sex-differences
Graphical abstract
Abbreviations
- AF-2
Activation function-2
- AD
Alzheimer's disease
- AR
androgen receptor
- AHR
arylhydrocarbon receptor
- BPA
Bisphenol A
- ChIP
chromatin immunoprecipitation
- CREB
cAMP Response Element-Binding Protein
- DILI
drug-induced liver injury
- EDC
endocrine disrupting chemical
- EGFR
epidermal growth factor receptor
- E2
estradiol
- eRNA
enhancer RNA
- E1
estrone
- E3
estriol
- ERα and ERβ
estrogen receptors α, β
- ERE
estrogen response element
- ER
endoplasmic reticulum
- ETC
electron transport chain
- EIF2AK2
eukaryotic translation initiation factor 2-α kinase 2
- ECAR
extracellular acidification rate
- FXR
farnesoid-X-receptor
- GPER1
G-protein coupled ER
- GR
glucocorticoid receptor
- JNK2
C-Jun N-Terminal Kinase 2
- LBD
ligand binding domain
- ncRNA
long non-coding RNAs
- MPA
Medroxyprogesterone acetate
- miRNA
microRNAs
- MRE
miRNA response element
- mtDNA
mitochondrial genome
- (MNX) mouse model
Mitochondrial-Nuclear-eXchange
- UPRmt
mitochondrial unfolded protein response
- MERKO
muscle-specific Esr1 (ERα) ERα knockout
- NK
natural killer
- NRF-1
nuclear respiratory factor-1
- ncRNA
non-coding RNA
- NB
neuroblastoma
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- PPARA
peroxisome proliferator activated receptor α
- PGC-1α
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- PHB
prohibitin
- PKA
protein kinase A
- PM
plasma membrane
- PKC
protein kinase C
- PNPase
polynucleotide phosphorylase
- ROS
reactive oxygen species
- RTKs
receptor tyrosine kinases
- RARα, RARγ
retinoic acid receptors α and γ
- RXR
retinoid X receptor
- 4-OHT
4-hydroxytamoxifen
- SERM
selective estrogen receptor modulator
- STAT
signal transducer and activator of transcription
- SIRT1
sirtuin 1
- SIRT3
sirtuin 3
- SLE
systemic lupus erythematosus
- TFAM
transcription factor A, mitochondrial
- TR
thyroid receptor
- T1D and T2D
Type 1 and Type 2 diabetes
1. Introduction
The history of the discovery and characterization of estradiol (E2) was recently published by Evan Simpson and Richard J. Santen [1]. Drs. Simpson [2] and Santen [3] are noted for their work on estrogens, reproduction, aromatase, and the clinical use of aromatase inhibitors for the treatment of postmenopausal breast cancer patients with estrogen receptor α (ERα) positive primary tumors. Estrone (E1) and estriol (E3) were first isolated in 1930-31 from the urine of pregnant women by Edward A. Doisy [4]. E2 was later isolated by Dr. Doisy from pig follicular fluid [4]. Subsequent discoveries of E2 metabolism, tissue-specific uptake, cellular activities, E2-dependent changes in the subcellular distribution of ERα [5], cloning of ERα from MCF-7 human breast cancer cells [6], and the discovery and cloning of ERβ [7] have enriched our understanding of estrogen action. Studies of the genomic binding distribution of ERα [[8], [9], [10], [11], [12], [13]] and ERβ [14] in chromatin immunoprecipitation (ChIP) studies revealed the diversity of genes regulated by these receptors in response to E2. “Omics” approaches have identified cell-specific estrogen-regulated transcriptomes of mRNA and all the ncRNAs (reviewed in Ref. [15]).
At this point, astute readers will note that the use of the word estrogen is not synonymous with E2. That is because there are three primary estrogens with E2 considered to be the most potent estrogen, i.e., E2 has the highest affinity for estrogen receptor α (ERα) [16] and is the predominant estrogen in circulation in premenopausal women (high pM to nM range). The lower ERα affinity estrogens are E1 and E3. E2 is synthesized in the ovary whereas E1 is synthesized from androstenedione in the adrenal cortex and E3 is primarily from the placenta, although each can be synthesized from androgenic precursors depending on the tissue expression of aromatase (CYP19) [17,18]. Obese and overweight postmenopausal women have higher levels of circulating estrogens produced by adipose tissue compared with lean women [19].
Estrogens bind ERα and ERβ that are conserved nuclear receptors (NR) showing high identity within the DNA binding and ligand binding domains, but differences in the amino acid (aa) composition of N terminus [20]. In addition to the full length ERα and ERβ, each subtype has numerous splice variants [21,22]. More recent studies identified a ‘hot spot’ within the ligand binding domain (LBD) of ERα where mutations activate the receptor independent of ligand binding to drive aromatase-resistant metastatic breast cancer [23,24].
E2 forms hydrogen bonds within the ligand binding pocket in the LBD of both ERα and ERβ resulting in activation of activation function 2 (AF-2) [25]. E2- ERα and ERβ binding within the cytoplasm causing conformational changes in the receptor resulting in dissociation of the receptor from chaperone proteins, e.g., Hsp90, Hsp40, p23, hsp70, allowing receptor dimerization [26]. This also exposes the receptors' nuclear localization domain, thus allowing its nuclear localization, although some proportion of each receptor subtype is in the nucleus, and bound to DNA, in the absence of ligand [27,28]. ERα and ERβ bind directly with high affinity to a DNA sequence that is an inverted repeat called the estrogen response element (ERE), i.e., 5′-AGGTCAnnnTGACCT-3’ [29]. EREs are located throughout the genome with their accessibility regulated by chromatin structure. ERα and ERβ binding to EREs facilitates chromatin looping bringing DNA-bound proteins and their interacting proteins into proximity to increase RNA pol II recruitment to promoter sequences and stimulate its conversion to an elongation complex [30]. ERα and ERβ were identified in mitochondria of various cell types where they bind mtDNA (reviewed in Ref. [31]). ERα also interacts indirectly to nuclear DNA by its direct (protein:protein) interaction with other DNA-bound transcription factors, e.g., SP1 [32], AP-1 [33], and RUNX1 [9]. This mechanism of ERα activation of transcription is called “tethering”. In addition to ligand binding, ERα is regulated by phosphorylation that can activate the receptor independent of ligand binding (reviewed in Refs. [34,35]). Recent studies show the cistrome of phospho-ser118-ERα is enriched for EREs and also for association with Grainyhead Like Transcription Factor 2 (GRHL2), a transcription factor best known as a tumor suppressor [36], which acts like a pioneer factor [37].
The conformational changes elicited upon E2 binding to ERα facilitates the recruitment of coactivators and chromatin remodeling complexes that interact with the C-terminus of the receptors via helix 12 of the LBD in a ligand-dependent manner [38]. ERα is a component of a pre-formed “MegaTrans complex” of 1–2 MDa which includes the pioneering factor FOXA1 that can remodel chromatin and retinoic acid receptors α and γ (RARα, RARγ), STAT1, GATA3, and AP2 [39]. The MegaTrans complex is required for activation of enhancer RNA (eRNA) transcription and recruitment of coactivators, including p300 and Mediator (MED1), forming “super enhancers” that span large chromatin domains, are co-occupied by multiple transcription factors and Mediator, and drive high cell-type specific gene transcription programs [39,40]. Acute enhancer activation by E2 results in eRNA-mediated ribonucleoprotein (RNP) assembly of the MegaTrans complex that has properties of phase separated condensates that are required for cooperative interactions to bring genomic loci into proximity which increases local cofactor retention time [41].
E2 also activates GPER1 (previously called GPR30 and GPER) which is a seven trans-membrane G-protein coupled receptor (GPCR) that localizes to the plasma membrane (PM) and also shows intracellular localization in a tissue-specific manner [42]. GPER1 shares structural homology with chemokine GPCRs, but is not activated by cytokines [43]. Ligand binding and activation of GPER1 rapidly stimulates intracellular signaling pathways in a cell-specific manner including activation of adenylate cyclase, epidermal growth factor receptor (EGFR), mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK), insulin-like growth factor receptor (IGFR), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), and [Ca+2]i (reviewed in Refs. [44,45]). GPER1 (GPR30) was reported to localize to the nucleus of C2C12 murine skeletal muscle cells [46]. E2-GPER1 interaction activates adenyl cyclase thus increasing intracellular cAMP production and protein kinase A (PKA) signaling [47], and activates epidermal growth factor receptor (EGFR) [48]. GPER1 is also activated by 4-hydroxytamoxifen (4-OHT), an active metabolite of tamoxifen a selective estrogen receptor modulator (SERM) used in breast cancer therapy [49]. Additional ligands that activate GPER1 include Bisphenol A (BPA), an endocrine disrupting chemical (EDC) [50]and E3 [42]. GPER1 is the target of synthetic agonists (G-1) and antagonists (G-15 and G-36) produced for experimental studies [51]. GPER1 has also been identified as an aldosterone receptor [[52], [53], [54], [55]], although this is disputed [56]. A recent report identified the first ERα/ERβ selective ligand (AB-1) that does not activate GPER1 [57]. Although GPER1 knockout mice have no reproductive phenotype, they are insulin resistant, obese, and have cardiovascular dysfunction (reviewed in Refs. [44,47]). GPER1 knockout mice showed increased cardiac oxidative stress and damage, increased 4-hydroxynonenal and 8-hydroxy-2′-deoxyguanosine (8-oxo-DG) staining, and increased oxidative stress-related genes, e.g., Gstk1, Ucp3, and Sod2 [58]. Recent studies in Wistar rats demonstrated a sexual dimorphic role of GPER1 in regulating body weight in early postnatal life [59]. GPER1 expression is widespread in the central nervous system (CNS) and contributes to spatial memory, anxiety, social memory, and lordosis behavior in mice (reviewed in Ref. [60]). These data indicate that estrogens regulate normal function in the nervous, immune, skeletal, and cardiovascular systems, adipocytes, liver, pancreas, and kidney by activating GPER1 (reviewed in Refs. [44,47]).
In addition to their genomic activity, ~ 5–10% of ERα and ERβ are localized to the PM [61]. ERα is palmitoylated and interacts with PM-associated caveolin-1 and signaling proteins to activate intracellular signaling cascades that ultimately alter transcriptional responses [62]. The small ubiquitous redox-active protein Memo (MEMO1) is overexpressed in breast tumors and conveys migratory signals from receptor tyrosine kinases (RTKs), e.g., epidermal growth factor receptor 2 (HER2), and promotes rapid ERα and ERβ phosphorylation [63]. This membrane-initiated E2-signaling can occur within seconds (changes in calcium flux [64]) or minutes (altered kinase cascades, e.g., ERK1/2 [65]) and cooperates with nuclear ERα and ERβ transcriptional programming in a cell-specific manner [62]. The use of transgenic and knockout ERα mouse models has revealed that the protective effect of E2 in tissues including adipose tissues (white and brown), heart, liver, pancreas, and skeletal muscle require nuclear ERα whereas membrane-ERα mediates some of the endothelial/vascular effects of E2 [66].
EDCs can interfere with any aspect of hormone action including altering synthesis, release, transport, receptor binding, metabolism, or clearance [67,68]. A subclass of EDCs are termed “metabolism disrupting chemicals” (MDCs) that can alter any aspect of metabolism [69]. Further, there is crosstalk between EDCs and MDCs in terms of interaction with ERα, ERβ, and GPER1 and their signaling pathways. In a study testing 8000 EDCs in vitro, ~11% disrupted the mitochondrial membrane potential, although further studies are clearly needed to understand potential impacts for human health and fundamental mechanisms [70]. The effects of some MDCs in mitochondrial bioenergetics was recently reviewed [71].
2. Sex differences in disease
A number of tissues, diseases, and cancers, in addition to those expected: gonads, breast, and prostate; show sex differences. There is currently great interest in understanding these sex-based differences in disease with the goal of personalizing treatment. The National Institutes of Health requires every grant application to address “sex as a biological variable”. The list of diseases showing sex differences is far too long to include here, but examples include hypertension [72], ischemic stroke and myocardial infarction [73], and neurodegenerative and neuropsychiatric diseases [74]. Each of these diseases are associated with mitochondrial dysfunction. Clinical and knockout mouse studies have provided evidence that the protective effects of estrogens in vascular and metabolic protection are mediated by ERα′s AF-2 domain by both nuclear and PM-initiated signaling (reviewed in Ref. [66]). Other differences in disease prevalence that are associated with higher E2 levels in premenopausal women include both Type 1 and Type 2 diabetes (T1D and T2D) where premenopausal women show lower incidence of both metabolic disorders whereas postmenopausal women with T1D and T2D show higher development of cardiovascular disease and end stage kidney disease than men [75]. Mitochondrial dysfunction is associated with insulin-resistance [76] and multiple endocrine disorders [77]. Mechanisms for sex differences include not only estrogens and androgens, but also sex chromosome-encoded genes (reviewed tin [78,79]). Here, I will focus on estrogens and their role in metabolism and disease.
It is well established from rodent studies and observations in humans that E2 increases fat oxidation, inhibits lipogenesis, and has a host of regulatory functions on the cells of the immune system, e.g., B cells, T cells, natural killer (NK) cells, neutrophils, and macrophages [80]. Over 75% of autoimmune disorders show higher prevalence in females and recent studies demonstrated a direct role for T cell ERα expression in the development of T-cell dependent colitis in mice and reduced T cell proliferation [81]. The role for estrogens and ERα in systemic autoimmune disease, e.g., systemic lupus erythematosus (SLE) involves B cells and T cells has been reviewed [82].
E2 acts centrally and systemically to regulate energy balance and metabolism. Sex differences in neurodegenerative diseases suggest a protective role for estrogens in some diseases, e.g., there is a higher incidence of Parkinson's disease in men and a role for loss of estrogen-protection at menopause is associated with a higher prevalence and severity of Parkinson's disease as well as Alzheimer's disease (AD) in women [83]. Mitochondrial dysfunction plays a role in these diseases [84,85]. E2 is produced in brain, specifically in the hippocampus, amygdala, and preoptic area, in both female and male rats [86]. The mechanisms of action of E2 in the brain are identical to those in peripheral tissues including both genomic and non-genomic ERα and ERβ (reviewed in Ref. [87]).
A recent report examined mitochondrial function in peripheral mononuclear blood cells (PBMCs) from healthy male and female volunteers (avg. age 30 and 31, respectively) [88]. PBMCs from females showed higher mitochondrial mass, higher Complex I, I + II, IV, and electron transport compared to males; however, no difference in ATP production were detected. The mitochondrial brain metabolite N-acetylaspartate (NAA) was also significantly higher in females compared to males; however, there was no significant correlation between individual parameters in PBMCs and brain NAA. Nonetheless, the authors conclude that measuring mitochondrial function in PBMCs could be a useful surrogate marker to examine differences in mitochondrial function in older adults and determine if this could be an AD marker [88].
Hepatocellular cancer (HCC) has a higher incidence in males than females where estrogens have protective effects against the initiation and progression of HCC [89]. Sex-dependent differences in liver include the expression of hepatic cytochrome P450 enzymes as well as transcription factors (TF) including ERα, arylhydrocarbon receptor (AHR), peroxisome proliferator activated receptor α (PPARA), and farnesoid-X-receptor (FXR) leading to differences in drug responses and metabolism in men and women [19,90,91]. Non-alcoholic fatty liver disease (NAFLD) is higher in men than premenopausal women, but increases in postmenopausal women [91]. Drug-induced liver injury (DILI) also shows sex differences with 41 drugs showing female-dominant DILI only in premenopausal women [92]. Interestingly, drugs with female-biased DILI show a higher prevalence of mitochondrial liability, reactive metabolite formation and higher transporter inhibition potential [92]. An immune-mediated DILI model in BALB/c mice showed that females had higher production pro-inflammatory hepatic cytokines (IL-6) than males and had more severe hepatitis suggesting that E2 and IL-6 may be responsible for reducing protective regulatory T-cells [93]; however, no measure of mitochondrial function was included in this study.
3. Mitochondria: structure and function
The mitochondrion is a densely packed, dynamic organelle of bacterial ancestry and endosymbiotic origin [94]. Mitochondria sustain life by converting metabolites from dietary fuels to ATP, CO2, and H2O, producing heat in the process, and by enabling stress adaptation for survival. An average mitochondrial is ~1 μm and changes its shape constantly by fusion and fission to form a dynamic network that interacts with the endoplasmic reticulum (ER) at points called MAM (mitochondria-associated membranes) for the transfer of Ca++, lipids, and other signals [95]. Fission and fusion are regulated by nutrient availability and metabolic demand [96]. Mitochondrial fusion is required for removal of damaged mitochondria by autophagosomes (mitophagy) whereas fission is necessary for the distribution of mitochondrial DNA during cell division [97]. Mitochondria are commonly considered the cellular powerhouse because the enzyme complexes I–V of the electron transport chain (ETC), with complex V being T1F0 (ATPase) reside as supramolecular complexes in the inner mitochondrial membrane producing ATP by oxidative phosphorylation of reduced substrates (NADH and FADH2). In addition, mitochondrial metabolic multienzyme complexes (metabolons) are dynamically regulated [98,99]. Of the 80 proteins in complexes I–V, 13 are encoded by the mitochondrial genome (mtDNA) [100]. The OXPHOS-ETC complexes interact with each other forming supercomplexes referred to as ‘respirasome’ and ‘respiratory megacomplexes’, depending on the exact stoichiometry [101]. The regulation of this process is not yet completely elucidated.
Nuclear-encoded mitochondrial transcription factors TFAM (transcription factor A, mitochondrial) and TFB (mitochondrial transcription factor B, encoded byTFB1M and TFB2M), regulate mtDNA transcription [102] (Fig. 1). These and other nuclear-to-mitochondria anterograde signals regulate mitochondrial function including bioenergetics, biogenesis, mitophagy, and fission/fusion. Within each cell, mitochondria are heterogeneous and mitochondria from different tissues display diversity in terms of fuel preference, protein composition, and metabolic functions [94]. mtDNA is oocyte-derived, so inherited mt disorders follow maternal inheritance [94]. Paternal mitochondrial transmission is a rare occurrence [103].
Fig. 1.
Examples of anterograde and retrograde signaling. Anterograde signals from the nucleus to the mitochondria include the nuclear-encoded transcription factors (TFs), including TFAM and TFB that bind the mtDNA, and nuclear receptors (NRs). Other nuclear-encoded proteins such as SIRT3, a deacetylase that is important for regulating subunits of the ETC, among other targets are important for regulating mitochondrial metabolism. Nuclear encoded miRNAs and lncRNAs are also transported into mitochondria. Retrograde signals from mitochondria that regulate nuclear function include calcium and ROS. In addition, mtDNA-encoded lncRNAs have been reported in the nucleus.
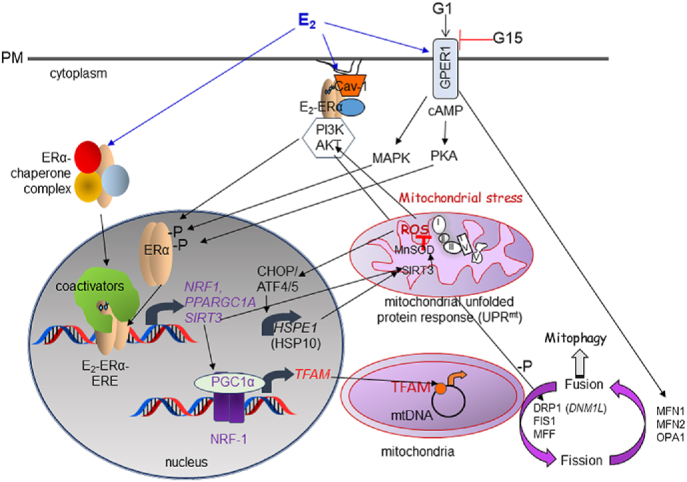
During the production of ATP, the transport of electrons also generates reactive oxygen species (ROS) that damage macromolecules including mtDNA, proteins, and lipids. Mitochondrial dysfunction contributes to age-related degeneration and disease in tissues including neurons and vestibulocochlear hair cells resulting in hearing loss [104], retina leading to age-related macular degeneration (AMD) [105], muscle causing weakness and falls [106], heart [107], liver and adipose [108], and brain [109]. Estrogens and androgens protect mitochondria against degenerative effects of aging in a tissue-specific manner by activation of their respective receptors [108]. ROS contributes to mitochondrial stress and protein misfolding. Misfolded proteins and aggregates accumulate in the inner mitochondrial membrane space (IMS) and the mitochondrial matrix which leads to activation of the mitochondrial unfolded protein response (UPRmt). UPRmt responsive pathways include antioxidant response enzymes, oxidative phosphorylation (OXPHOS), mitophagy, and mitochondrial biogenesis (reviewed in Ref. [110]). Activation of UPRmt in initiates mitochondrial-nuclear crosstalk (retrograde signaling) for the maintenance of cellular homeostasis, and some mtDNA mutations may perturb crosstalk signaling [111]. UPRmt stimulates dsRNA-activated protein kinase (PKR), eukaryotic translation initiation factor 2-α kinase 2 (EIF2AK2), and C-Jun N-Terminal Kinase 2 (JNK2) to activate JUN which increases the transcription CHOP/ATF4/5 which then increases UPRmt responsive genes [112]. In addition, activation of UPRmt activates AKT which phosphorylates ser167 of ERα that results in ligand-independent transcriptional ERα activation, thus increasing nuclear respiratory factor-1 (NRF-1, NRF1) [112]. E2-ERα also regulates UPRmt [110,112,113]. Recent studies demonstrate that breast cancer cells co-opt “mitohormesis”, a process of increased basal UPRmt and decreased oxidative stress leading to increased invasion and metastasis and worse survival of breast cancer patients with the UPRmt gene signature [114]. E2-ERα increases sirtuin 3 (SIRT3) transcription and SIRT3 localizes to mitochondria were it attenuates ROS by deacetylation of manganese superoxide dismutase (MnSOD, SOD2) and interacts with FOXO3A to activate its translocation to the nucleus to upregulate the expression of the genes encoding PGC-1α, a coregulator required for NRF-1 and PPAR-regulated transcription [115,116], and MnSOD [117].
Retrograde signals originating in the mitochondria in response to oxidative stress, ROS, increased intracellular calcium ([Ca+2]), reduced ATP, acetyl CoA, and an imbalance in the NADH/NAD + ratio disrupt sirtuin 1 (SIRT1), protein kinase C (PKC), and NFκB signaling pathways leading to changes in the expression and activity of NRF-1, peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α), and TFAM [111] (Fig. 2). Mitoprotective outcomes elicited during UPRmt include increase in 26S proteasome, LC3B, Hsp10, Hsp90, CLPP, LonP, and activation of SOD1, SOD2, and catalase [118].
Fig. 2.
Mechanisms of E2 regulation of cellular and mitochondrial responses. Shown are ERα and GPER1. The mitochondrial unfolded protein response (UPRmt) includes retrograde signaling from mitochondria to the nucleus via CHOP and ROS. ROS activation of AKT results in phosphorylation and activation of ERα. ERα increases NRF-1 which, in turn, increases TFAM that stimulates mtDNA transcription. Mitochondria are in a dynamic network and, as described in the text, GPER1 was shown to increase MFN2 and MFNS while reducing FIS1, suggesting a role of GPER1 in stimulating fusion.
In addition to energy production, mitochondria are the site of synthesis for all steroid hormones [119], including E2 in the ovarian granulosa cells [120]. Apoptotic signals result in permeabilization of the mitochondrial outer membrane and the release of cytochrome c initiating activation of a caspase cascade leading to cell death [121]. E2 inhibits apoptosis by a variety of mechanisms (reviewed in Refs. [110,122]). Under mitochondrial stress, mitochondria also produce and secrete “mitokines”, e.g., humanin, an exercise-responsive peptide encoded by the MT-RNR2 gene in mtDNA, and fibroblast growth factor 21 (FGF-21) that regulates energy metabolism [123].
The impact of mtDNA genome on metabolic function in mice has been examined using Mitochondrial-Nuclear-eXchange (MNX) mouse models in which C57BL/6J and C3H/HeN mouse strains have reciprocally exchanged mtDNA [124]. There are 2 non-synonymous mutations in protein coding subunits in the C57 versysC3H mtDNA (subunit 3 in complex I and subunit 3 in complex IV), with the C57 mtDNA linked to greater organelle economy and oxidant production relative to the C3H mtDNA [124]. Recent studies demonstrated that C57BL/6J mtDNA protects against high-fat diet-induced obesity and alters the expression of a higher number of genes in adipose tissue compared to C3H/HeN mtDNA, although the mechanisms is unknown [125].
Sex-specific differences in mitochondria and mitochondrial function in different organs, mostly from rodents, has been reviewed [126]. For example, human females have higher intracellular lipid content and show enhanced protection against oxidative stress in skeletal muscle and female rodents have higher ETC transport, ATP production, and mitochondrial biogenesis in the brain [126]. A recent study reported that the influence of sex on gene expression and mitochondrial metabolism in adipose tissue depended on the strain in a study of 100 inbred strains of mice [127].
Another recent review summarized sex differences in human skeletal muscle [128]. Interestingly, skeletal muscle is among the most sex-divergent tissues showing ~3000 gene expression differences between females and males at an average age of 27 ± 3 yrs [129]. Many mitochondrial function genes were found to be differently expressed including PPARGC1A (PGC-1α), correlating with known sex difference in muscle fiber compositions with females having a higher percent of type I fibers with a more oxidative phenotype [129]. The differences in gene expression between the skeletal muscles of men and women is mediated in part by epigenetic changes including differences in DNA methylation, histone modification, and miRNA expression [128].
4. Non-coding RNAs in mitochondrial function
Nuclear-encoded microRNAs (miRNA) and long noncoding RNAs (lncRNAs) can regulate anterograde signaling by translocation into mitochondria and the mtDNA genome encodes miRNA and lncRNAs that can act as retrograde signals [130] (Fig. 1). miRNAs generally inhibit their target gene's translation by binding to a miRNA response element (MRE) in the 3′UTR of the target mRNA transcript within the RNA induced silencing complex (RISC) [131,132]. lncRNAs are defined as non-coding RNAs of >200 nucleotides that show tissue-specific expression and interact with DNA, RNA, and proteins to regulate gene expression, chromatin modification and dynamics, protein complex assembly, splicing, and translation [133]. Another type of non-coding RNA are circular-RNAs (circRNAs) that generally arise during splicing of the mRNA transcript and are localized to the cytoplasm where they, like lncRNAs, act as ‘sponges’ for miRNAs, blocking their function [15]. A recent study profiling the subcellular distribution of circRNAs did not find circRNAs in mitochondria from HepG2 cells [134]; thus, based on this and the lack of other studies on circRNA and mitochondria in PubMed, I will not discuss circRNAs.
Nuclear-encoded miRNA regulate mtDNA-encoded transcripts. The term ‘mitomiRs’ refers to miRNAs functioning in mitochondria, whether nuclear- or mtDNA-encoded [135]. For example miR-214 targets mtND6 and mtND4I in renal tubular cells [136]. miR-214 was upregulated in response to insults that mimic chronic kidney disease in mice, e.g., ischemia-reperfusion injury, and resulted in a decrease in mtDNA copy number and OCR [136]. RNA seq of an isolated mitochondrial fraction from human tongue squamous cell carcinoma cells identified 57 mitochondria-enriched miRNAs, including miR-2392 that downregulated mtDNA-encoded ND2, ND4, ND5, CYTB, and COX1 and shifted cells to increased lactate production [137].
Examples of other nuclear-encoded miRNAs reported to be imported into mitochondria include miR-34, miR-181c-5p, miR-146a-5p, miR-1, miR-378, and miR-21 (reviewed in Refs. [[138], [139], [140]]). miRNAs are imported into mitochondria by interaction with AGO2 and polynucleotide phosphorylase (PNPase) [141]. PNPase is increased in arterial tissue from diabetic human patients and this increase was correlated with higher mitochondrial miR-378 in these samples, in db/db mice, and in cell culture studies [142]. The identity and roles of miRNAs in mitochondrial biology have recently been reviewed [139].
miRNAs also target nuclear and mtDNA-encoded transcripts for degradation. NRF1 is directly targeted by miR-504 [143] and mouse Nrf1 is a direct target of miR-378 which is upregulated in fatty livers of mice and humans [144]. Other examples of proteins important in mitochondrial function that are regulated by miRNA include these paired examples: miR-494-TRAM; miR-696-PGC-1α; and miR-23-COX4 and PGC-1α (reviewed in Ref. [140]). We reported that miR-29b-1 and miR-29a directly target ATP5G1, a subunits of ATP synthase (Complex V in OXPHOS), and ATPIF1 that encodes ATPase Inhibitory Factor I that limits ATP depletion when the mitochondrial membrane potential falls below that required for ATP synthesis [145].
Mitochondrial-DNA encoded miRNAs, including miR-1974, miR-1977, and miR-1978 in humans, have been reported (reviewed in Ref. [135]).
Nuclear-encoded lncRNAs: RMRP, RPPH1, and MALAT were reported to act as anterograde signals between the nucleus and mitochondria in HepG2 cells [146]. Additional lncRNAs that regulate mitochondrial function include SAMMSON, UCA1, RMRP, HOTAIR, TUG1, linc-p21 (TP53COR1), and MEG3 were recently reviewed [147]. MEG3 is a tumor suppressor that is downregulated in endocrine-related cancers, including breast prostate, and endometrial cancer [148]. There is cross-talk between mitochondrial function and the expression of nuclear-encoded lncRNAs as exemplified by the observation that various mitochondrial stressors, e.g., FCCP, oligomycin, rotenone, and doxycycline, increased expression of the lncRNA NEAT1, resulting in increased elongated nuclear paraspeckles and mRNA sequestration of nuclear-encoded mitochondrial proteins in HeLa cells [149]. The lncRNA HOTAIR is important for mitochondrial function in HeLa cells [150]. NEAT1 is overexpressed in breast tumors and appears to play an oncogenic role in breast cancer (reviewed in Ref. [15]), but its involvement in mitochondrial dysfunction in breast tumorigenesis and metastasis is unknown. The lncRNA CEROX1 sponges miR-488-3p resulting in increased transcript expression of 8 subunits of OXPHOS Complex I and increased mitochondrial respiration [151]. RNA binding proteins including HuR, GRSF1, PPR, PNPAse, SLIRP, and SHARP may be involved in lncRNA transport into mitochondria [152]. One lncRNA, LINC00116, was recently reported to encode an evolutionarily conserved 56 aa peptide called MTLN that is detected in mitochondria where its deletion reduced OXPHOS Complex I activity [153].
Seven lncRNAs are derived from mtDNA [130] and are called “mitolncRNAs” [139]. Several are chimeric lncRNAs containing nucleotides of mtDNA: LIPCAR, SncmtRNA, ASncmtRNA-1, and ASncmtRNA-2. ASncmtRNA-1 and ASncmtRNA-2 are present in mitochondria and nuclei, suggesting a possible role in retrograde signaling [130].
5. E2 stimulated NRF-1 transcription
Mitochondrial function can be regulated by E2 activation of genomic ERα and ERβ which stimulate transcription of NRF1 and by binding to mtDNA to promote mtDNA transcription (reviewed in Refs. [122,154]) (Fig. 2). NRF-1 promotes transcription of TFAM that binds and upregulates mtDNA-encoded genes [116,155]. The transcription of nuclear-encoded ETC proteins, e.g., mitochondrial ATP synthase subunit E and COVII, are increased by E2 via NRF-1 (reviewed in Ref. [156]). NRF-1 binds to its DNA response element as a dimer and interacts with coactivators PGC-1α, PGC-1β, and PRC to regulate target gene transcription depending on the cell type [115]. Whether mitochondrial ERα and ERβ play direct roles on mtDNA gene transcription and mitochondrial function appears to depend on the cell type, consistent with cell-type specific localization. Doris Germain's group has demonstrated that retrograde signaling via ROS-AKT pathway activation in response to UPRmt activates ERα and increases NRF-1 signaling (reviewed in Refs. [118,[157], [158], [159]]). The role of estrogens on mitochondria function has been reviewed ([122,154,[160], [161], [162], [163]]). However the protective effects of estrogens in mitochondria remain to be fully elucidated (reviewed in Ref. [164]).
In addition to its role in regulating mitochondrial genes, NRF-1 has been demonstrated to have oncogenic activity in breast cancer cell lines and to increase breast cancer stem cell properties in stably transformed, NRF-1-overexpressing MCF-10A breast epithelial cells [165,166]. The NRF-1-overexpressing MCF-10A cells had higher mammosphere formation than the parental MCF-10A cells and had increased expression of pluripotency markers SOX2, NANOG, and OCT4 and the mesenchymal stem cell marker CXCR4 [165]. These authors did not examine the impact of NRF-1 overexpression on redox-sensitive events or mitochondrial bioenergetics in this paper. Knockdown of endogenous NRF1 in MDA-MB-231 TNBC cells reduced spheroid formation and tumor growth in mammary fat pads and lung metastasis in immunodeficient mice [166]. Overexpression of NRF-1 in MCF-10A cells reduced basal OCR, but increased mitochondrial reserve whereas NRF-1 knockdown in MDA-MB-231 cells decreased basal OCR and mitochondrial reserve [166].
6. Nuclear transcription factors within mitochondria
Nuclear transcription factors also localize within mitochondria. TFs identified in mitochondria include the recently annotated JUNB [167] as well as NFκB, p53, cAMP Response Element-Binding Protein (CREB), signal transducer and activator of transcription (STAT)-1, STAT3, and STAT5, Interferon Regulatory Factor 3 (IRF3), and myocyte-specific enhancer factor-2D (MEF-2d) that may also regulate to mtDNA transcription (reviewed in Ref. [168]). ERα and ERβ were identified within mitochondria and were reported to regulate mtDNA transcription (reviewed in Ref. [31]). E2 has been reported to increase redox signaling in MCF-7 cells containing ERα [169]. This process is considered part of the oncogenic process in breast cancer and involves E2 activation of AKT signaling leading to NRF-1 activation [170]. E2 rapidly increased transient ERα localization to mitochondria in MCF-7 breast cancer cells and stimulated ERα-MnSOD direct interaction as detected by confocal imaging and co-immunoprecipitation experiments [171]. The ERα mitochondrial localization and ERα-MnSOD interaction was blocked by fulvestrant, suggesting the ERα AF-2 conformation is important for these interactions. The rapid (increased by 15 min and gone by 60 min) E2-induced migration of ERα to mitochondria in MCF-7 cells is considered a non-genomic response E2 increased MnSOD acetylation of K68, resulting in inhibition of MnSOD activity. The E2-ERα-MnSOD interaction was reported to block MnSOD-SIRT3 interaction, increasing superoxide and activating mTORC2 [171].
Mitochondria contain other nuclear receptors (NR), i.e., thyroid receptor (TR), androgen receptor (AR), retinoid X receptor (RXR), RARs, glucocorticoid receptor (GR), and peroxisome proliferator activated receptor gamma (PPARG, PPARγ2) (reviewed in Ref. [172]). ERβ was identified in human heart mitochondrial proteins [173]; however, no independent confirmation of this finding has been reported. A recent study reported that low levels of mitochondrial ERβ (mitoERβ) were associated with increased breast tumor recurrence [174]. Transfection of MCF-7 breast cancer cells with GST-ERβ followed by GST-pulldown identified HSPA9 (heat shock 70 kDa protein, mitochondrial; also called GRP75) associated with ERβ. MALDI-TOF mass spectrometry identified ERβ and HSPA9 in a purified complex and knockdown and overexpression studies showed that HSPA9 translocates ERβ into MCF-7 mitochondria. Transfection of MDA-MB-231 triple negative breast cancer (TNBC) cells with a mitochondria-targeted ERβ expression vector reduced cell proliferation, invasion, and migration in vitro and tumor formation in vivo. Previous studies have implicated ERβ as a tumor suppressor in breast and other cancers [[175], [176], [177], [178], [179], [180]]. Another group identified higher ERβ in mitochondrial fractions from ectopic endometrial tissues versus normal uterine myoma or non-lesion controls [181]. Given the uncertainty about the specificity of some ERβ antibodies [182,183], further studies of the localization and activity of ERβ in mitochondria are warranted.
The naturally-occurring ERα splice variants ERα36 and ERα46 result from differential promoter usage and splicing, resulting in truncated forms of ERα lacking the N-terminal A and B domains that constitute AF-1 [184]. ERα36 also lacks the F-domain at the C-terminus of full-length ERα66 and has a truncated LBD [184]. ERα36 was reported to localize primarily in mitochondria in human uterine leiomoma (UtLM) and smooth muscle cell lines and interacts with prohibitin (PHB) [185]. A recent report from the same investigators showed that BPA increased ERα36 expression in the UtLM cells and that ERα36 activated MAPK signaling, increased Src and EGFR phosphorylation and mitochondrial localization [186].
7. Estrogens regulate mitochondrial bioenergetics
The development of the Seahorse Bioscience Extracellular Flux Analyzer has allowed investigation of the effect of estrogens, SERMs, and other potential ER ligands on mitochondrial bioenergetics in live cells in real time and to define the impact of these perturbations on oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), a surrogate measure of lactate production and glycolysis [187,188]. We reported that E2 (10 nM) stimulated baseline OCR and ECAR in MCF-7 and T47D luminal A (ERα+) breast cancer cells and stimulated ATP-linked OCR with no effect on maximal mitochondrial reserve capacity, suggesting that E2 does not affect tolerance to cellular stress in these cell lines [189,190]. An earlier report demonstrated that 10 nM E2 increased ECAR and decreased OCR in primary human stromal endometrial cells, but no mechanisms were examined [191]. Medroxyprogesterone acetate (MPA) inhibits E2 potentiation of rat primary hippocampal neuron and glia mitochondrial respiratory reserve capacity in vitro [192], but no studies identifying mechanisms were included. A recent report demonstrated that knockout of ERα in CD4+ T cells reduced the mitochondrial reserve capacity, suggesting that ERα regulates mitochondrial metabolism in T cells [81].
My search of PubMed and the Agilent Publications database for articles examining the effect of estrogens on mitochondrial bioenergetics identified relatively few reports. The ERβ-selective agonist DPN partially restored basal and maximum OCR in primary rat hippocampal neurons against synthetic amyloid β oligomer (Aβ1–42) treatment-induced suppression of mitochondrial OCR [193]. One group found that overexpression of ERα in SK-N-BE(2) MYCN-amplified (MNA) neuroblastoma (NB) cells repressed xenograft tumor growth downregulated many processed linked to NB tumorigenesis [194]. Glycolysis (measured as ECAR in the Seahorse Bioanalyzer), maximal glycolytic capacity, and the glycolytic reserve were all significantly reduced in cells overexpressing ERα and treatment with E2 and nerve growth factor had no additional effect on any of these parameters in the NB cells [194]. Likewise, baseline OCR, ATP-linked OCR, and mitochondrial reserve were creased in the ERα overexpressing SK-N-BE(2) MNA NB cells, mediated in part by suppression of utilization of fatty acids. Overexpression of mitochondrial-targeting sequence tagged ERβ in primary human endometriotic cells increased basal OCR and mitochondrial reserve [181]. Knockdown of ERβ decreased the expression of NRF1, TFAM, MT-CO1, and MT-ATP6 transcripts in the endometriotic cells and increased anti-apoptotic protein BCL-2, thus “rescuing the cells form oxidative stress-induced mitochondrial-apoptosis” [181].
Studies in muscle-specific Esr1 (ERα) ERα knockout (MERKO) female mice showed that these mice had impaired glucose homeostasis, increased adiposity coupled with aberrant mitochondrial morphology, increased ROS, impaired mitochondrial fission, impaired calcium handling and ATP production, although difference in muscle size of maximum force [195]. These data implicate a critical role for ERα muscle mitochondrial function. ERα was reduced in the muscle of women with metabolic syndrome [195]. Transmission electron microscopy revealed elongated, hyperfused mitochondria with increased inactive DRP1 phosphorylated on the inhibitory SER 637 residue. They also observed an increase in Rcan1, an inhibitor of calcineurin which results in impaired mitophagy, increased ROS, inflammation, and insulin-resistance [195]. E2-ERα is also required for maintaining the satellite cell number (muscle stem cells) in the muscle of female rodents and humans [196]. Indeed, whole body ERα knockout (Esr1−/−) mice show decreased muscle fatty acid oxidation and increased overall adiposity while muscle-specific ERα knockout mice showed impaired mtDNA replication, mitophagy, autophagy, impaired insulin signaling (including glucose disposal), increased H2O2, superoxide, lipid accumulation and inflammation [197]. The authors noted the importance of identifying ways or therapeutics to modulate tissue-specific ERα-regulated pathways that will regulate energy balance and glucose homeostasis, specifically in postmenopausal women [197].
E2 replacement in ovariectomized female mice improved mitochondrial complex I (CI) activity and decreed H2O2 in skeletal muscle but increased CI-mediated H2O2 production and decreased OXPHOS capacity in liver [198]. The authors stated that the “mechanism(s) behind tissue specificity of E2 action on mitochondrial function remains unknown” [198]. While transcriptomic profiling has identified miRNAs regulating glycolysis and oxidative metabolism in male mouse muscle fibers [199], the role of estrogens in regulation of these miRNAs is not yet known. Interestingly, studies examining miRNAs in skeletal muscle of homozygotic twins with discordant use of hormone-replacement therapy (HRT) identified miR-182, miR-233, and miR-142-3p targeting IGF-1R and FOXO3A [200] and inflammatory signaling [201]. This group of investigators also identified E2-regulation of muscle energy pathways in HRT users [202,203].
8. GPER1 regulates mitochondrial respiration and function
When GPER1 was overexpressed in MCF-7 cells, it localized in the endoplasmic reticulum and inhibited cell growth while increasing intracellular cAMP and stimulating autophagy [204]. On the other hand, E2 activation of GPER1 suppressed mitophagy of ATDC5 chondrocytes in vitro by stimulating the PI3K/AKT-mTOR pathway [205]. GPER1 overexpression decreased basal OCR and ECAR while increasing the maximal respiratory rate and reserve capacity. GPER1 overexpression increased the levels of mitofusion 1 (MFN1) and 2 (MFN2) and Parkin (PRKN) mRNA expression while reducing mitochondrial fission 1 protein (FIS1), implicating a role for GPER1 in regulating mitochondrial fission/fusion and thus increasing mitophagy [204]. Parkin associates with TFAM to increase mitochondrial transcription [206]. E2 and receptor subtype-specific agonists (PPT, DPN, and G1) stimulated basal OCR and mitochondrial reserve capacity via activation of ERα, ERβ, and GPER1, respectively in 3T3-L1 adiopocytes [207]. GPER1 agonist G1 increased ERα phosphorylation on ser 118, and indicator of receptor AF-1 activation, and both E2 and G1 increased the expression of mouse Ppargc1a, Ppargc1b, and Nrf1 in mouse white adipose tissue explants [207]. The authors proposed a model that E2 activation of GPER1 in adipocytes activates adenylate cyclase PKA leading to CREB and ERα phosphorylation and activation of the expression of genes that stimulate mitochondrial function and protect adipocytes against IL6-induced mitochondrial function [207]. However, gene knockout studies will be required to validate these conclusions that were based on the use of chemical inhibitors.
In vivo studies showed that i.p. injection of GPER1 agonist G1 (10 μg/kg for two weeks) in 16-month old, ovariectomized, Sprague-Dawley rats had antidepressant- and anxiolytic-effects in the rats [208]. Examination of the hippocampal function in the rats showed that G1 increased mitochondrial function, SOD1, ERα, GPER1, and UCP2 protein levels, while reducing Bcl-2 (BCL2) protein expression [208]. The effect of estrogens on anxiety and depression in rodents and humans is considered to by mediated by ERα, ERβ, and GPER1 on neuronal signaling networks and pathways [209].
9. Estradiol regulates mitochondrial dynamics: fusion and fission
As stated previously, mitochondria form an interconnected network governed by signals regulating mitochondrial fusion and fission. DRP1 is a GTPase that is differentially regulated by phosphorylation at specific residues which regulate its movement from the cytoplasm to the mitochondria where GTP hydrolysis enables membrane constriction and scission [210]. E2 increased the mRNA transcript levels of MFN1, MFN2, OPA1, and DRP1 while decreasing FIS1 with 4 h of treatment of MCF-7 cells and these transcriptional responses were inhibited by the antiestrogen fulvestrant (ICI 182,780) [211]. The authors reported that E2 induced mitochondrial fusion in MCF-7 cells, decreased expression of OXPHOS complex proteins, and increased ATP levels. These investigators reported similar findings in E2-treated T47D cells and observed that overexpression of ERβ in T47D cells increased OXPHOS complex proteins and decreased fission while increasing fusion [212]. In contrast, E2-activation of ERα in MCF-7 cells increased Drp1 phosphorylation at ser616 to induce Drp1 activity resulting in mitochondrial fission [213]. The mechanism required ERα, since knockdown blocked E2 induced Drp1 phosphorylation, but whether this was mediated by non-genomic activation of AKT or by upregulation of a gene that affected this response was not evaluated.
10. Conclusions
Sex differences in health and disease are mediated by the levels of endogenous estrogens and androgens as well as by genes encoded by sex chromosomes. Many of the sex-dependent differences in diseases include altered cellular metabolism. E2 and other estrogens and synthetic GPER1 agonists regulate mitochondrial bioenergetics, fusion, and fission. Estrogens regulate the expression of genes, including miRNAs and lncRNAs, that regulate mitochondrial functions: metabolism, OXPHOS, apoptosis, UPRmt, fission, and fusion. The mechanism for these events involve binding of E2 and other estrogens to ERα and ERβ to regulate nuclear gene transcription and PM-initiated signaling cascades. In addition, estrogens, as well as EDCs, activate GPER1 which also regulates intracellular signaling events, including by cross-talk with EGFR. NRs including ERα and ERβ, as well as AR, RXR, RARs, GR, and PPARγ2, have been observed within mitochondria. NRF-1 is a key target of nuclear ERα and ERβ-mediated transcriptional activation in response to E2. NRF-1 is a regulator of nuclear-encoded mitochondrial genes including TFAM that binds mtDNA and regulates its transcription. Nuclear-encoded miRNA and lncRNA regulate mtDNA-encoded as well as nuclear-encoded transcripts thus functioning as anterograde signals. mtDNA-encoded lncRNAs may play a role in retrograde signaling. Since there are cell-specific pathways for each mechanism of estrogen regulation of mitochondrial function, further research is needed to delineate these mechanisms.
Declaration of competing interest
The author declares no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101435.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Simpson E., Santen R.J. Celebrating 75 years of oestradiol. J. Mol. Endocrinol. 2015;55:T1–T20. doi: 10.1530/JME-15-0128. [DOI] [PubMed] [Google Scholar]
- 2.Editorial: centennial celebration - an interview with professor evan Simpson on hormones and cancer. Mol. Endocrinol. 2016;30:1013–1014. doi: 10.1210/me.2016-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The endocrine society 2006 laureate awards. J. Clin. Endocrinol. Metab. 2006;91:3243–3254. doi: 10.1210/jcem.97.8.zeg2932. [DOI] [PubMed] [Google Scholar]
- 4.Simoni R.D., Hill R.L., Vaughan M. The discovery of estrone, estriol, and estradiol and the biochemical study of reproduction. The work of Edward Adelbert Doisy. J. Biol. Chem. 2002;277:e17. [Google Scholar]
- 5.Jensen E.V., DeSombre E.R. Estrogen-receptor interaction. Science. 1973;182:126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- 6.Walter P., Green S., Greene G.L., Krust A., Birnert J.M., Jeltsch J.M., Staub A., Jensen E., Scrace G., Waterfield M., Chambon P. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.-A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welboren W.-J., van Driel M.A., Janssen-Megens E.M., van Heeringen S.J., Sweep F.C.G.J., Span P.N., Stunnenberg H.G. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28:1418–1428. doi: 10.1038/emboj.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stender J.D., Kim K., Charn T.H., Komm B., Chang K.C.N., Kraus W.L., Benner C., Glass C.K., Katzenellenbogen B.S. Genome-Wide analysis of estrogen receptor {alpha} DNA binding and tethering mechanisms identifies Runx1 as a novel tethering factor in receptor-mediated transcriptional activation. Mol. Cell Biol. 2010;30:3943–3955. doi: 10.1128/MCB.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt S.C., Li L., Grimm S.A., Chen Y., Liu L., Li Y., Bushel P.R., Fargo D., Korach K.S. Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq. Mol. Endocrinol. 2012;26:887–898. doi: 10.1210/me.2011-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caizzi L., Ferrero G., Cutrupi S., Cordero F., Ballaré C., Miano V., Reineri S., Ricci L., Friard O., Testori A., Corà D., Caselle M., Di Croce L., De Bortoli M. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:4892–4897. doi: 10.1073/pnas.1315445111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll J.S., Liu X.S., Brodsky A.S., Li W., Meyer C.A., Szary A.J., Eeckhoute J., Shao W., Hestermann E.V., Geistlinger T.R., Fox E.A., Silver P.A., Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Carroll J.S., Meyer C.A., Song J., Li W., Geistlinger T.R., Eeckhoute J., Brodsky A.S., Keeton E.K., Fertuck K.C., Hall G.F., Wang Q., Bekiranov S., Sementchenko V., Fox E.A., Silver P.A., Gingeras T.R., Liu X.S., Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 14.Madak-Erdogan Z., Charn T.H., Jiang Y., Liu E.T., Katzenellenbogen J.A., Katzenellenbogen B.S. Integrative genomics of gene and metabolic regulation by estrogen receptors alpha and beta, and their coregulators. Mol. Syst. Biol. 2013;9:676. doi: 10.1038/msb.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinge C.M. Non-coding RNAs in breast cancer: intracellular and intercellular communication. Non-coding RNA. 2018;4:40. doi: 10.3390/ncrna4040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper G.G., Carlsson B., Grandien J., Enmark E., Haggblad J., Nilsson S., Gustafsson J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 17.Klinge C.M., Clark B.J., Prough R.A. Dehydroepiandrosterone research: past, current, and future. Vitam. Horm. 2018;108:1–28. doi: 10.1016/bs.vh.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Prough R.A., Clark B.J., Klinge C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016;56:R139–R155. doi: 10.1530/JME-16-0013. [DOI] [PubMed] [Google Scholar]
- 19.Chen K.L., Madak-Erdogan Z. Estrogens and female liver health. Steroids. 2018;133:38–43. doi: 10.1016/j.steroids.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson J.-A. Historical overview of nuclear receptors. J. Steroid Biochem. Mol. Biol. 2016;157:3–6. doi: 10.1016/j.jsbmb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Herynk M.H., Fuqua S.A.W. Estrogen receptor mutations in human disease. Endocr. Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 22.Lee M.-T., Ouyang B., Ho S.-M., Leung Y.-K. Differential expression of estrogen receptor beta isoforms in prostate cancer through interplay between transcriptional and translational regulation. Mol. Cell. Endocrinol. 2013;376:125–135. doi: 10.1016/j.mce.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toy W., Shen Y., Won H., Green B., Sakr R.A., Will M., Li Z., Gala K., Fanning S., King T.A., Hudis C., Chen D., Taran T., Hortobagyi G., Greene G., Berger M., Baselga J., Chandarlapaty S. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toy W., Weir H., Razavi P., Lawson M., Goeppert A.U., Mazzola A.M., Smith A., Wilson J., Morrow C., Wong W.L., De Stanchina E., Carlson K.E., Martin T.S., Uddin S., Li Z., Fanning S., Katzenellenbogen J.A., Greene G., Baselga J., Chandarlapaty S. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Canc. Discov. 2017;7:277–287. doi: 10.1158/2159-8290.CD-15-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanišić V., Lonard D.M., O'Malley B.W. Chapter 9 - modulation of steroid hormone receptor activity. In: Martini L., editor. vol. 181. Elsevier; 2010. pp. 153–176. (Progress in Brain Research). [DOI] [PubMed] [Google Scholar]
- 26.Smith D.F., Toft D.O. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol. Endocrinol. 2008;22:2229–2240. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G., Yanamala N., Lathrop K.L., Zhang L., Klein-Seetharaman J., Srinivas H. Ligand-independent antiapoptotic function of estrogen Receptor-{beta} in lung cancer cells. Mol. Endocrinol. 2010;24:1737–1747. doi: 10.1210/me.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardamone M.D., Bardella C., Gutierrez A., Di Croce L., Rosenfeld M.G., Di Renzo M.F., De Bortoli M. ERalpha as ligand-independent activator of CDH-1 regulates determination and maintenance of epithelial morphology in breast cancer cells. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:7420–7425. doi: 10.1073/pnas.0903033106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klinge C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flach K.D., Zwart W. The first decade of estrogen receptor cistromics in breast cancer. J. Endocrinol. 2016;229:R43–R56. doi: 10.1530/JOE-16-0003. [DOI] [PubMed] [Google Scholar]
- 31.Klinge C.M. Estrogens regulate life and death in mitochondria. J. Bioenerg. Biomembr. 2017;49:307–324. doi: 10.1007/s10863-017-9704-1. [DOI] [PubMed] [Google Scholar]
- 32.Dong L., Wang W., Wang F., Stoner M., Reed J.C., Harigai M., Samudio I., Kladde M.P., Vyhlidal C., Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J. Biol. Chem. 1999;274:32099–32107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- 33.Paech K., Webb P., Kuiper G.G., Nilsson S., Gustafsson J., Kushner P.J., Scanlan T.S. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 34.Lannigan D.A. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 35.Anbalagan M., Rowan B.G. Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Mol. Cell. Endocrinol. 2015;418(Part 3):264–272. doi: 10.1016/j.mce.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Reese R.M., Harrison M.M., Alarid E.T. Grainyhead-like protein 2: the emerging role in hormone-dependent cancers and epigenetics. Endocrinology. 2019;160:1275–1288. doi: 10.1210/en.2019-00213. [DOI] [PubMed] [Google Scholar]
- 37.Helzer K.T., Szatkowski Ozers M., Meyer M.B., Benkusky N.A., Solodin N., Reese R.M., Warren C.L., Pike J.W., Alarid E.T. The phosphorylated estrogen receptor α (ER) cistrome identifies a subset of active enhancers enriched for direct ER-DNA binding and the transcription factor GRHL2. Mol. Cell Biol. 2019;39 doi: 10.1128/MCB.00417-18. e00417-00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lonard D.M., O'Malley B.W. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat. Rev. Endocrinol. 2012;8:598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., Merkurjev D., Yang F., Li W., Oh S., Friedman Meyer J., Song X., Zhang F., Ma Q., Ohgi Kenneth A., Krones A., Rosenfeld Michael G. Enhancer activation requires trans-recruitment of a mega transcription factor complex. Cell. 2014;159:358–373. doi: 10.1016/j.cell.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whyte Warren A., Orlando David A., Hnisz D., Abraham Brian J., Lin Charles Y., Kagey Michael H., Rahl Peter B., Lee Tong I., Young Richard A. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair S.J., Yang L., Meluzzi D., Oh S., Yang F., Friedman M.J., Wang S., Suter T., Alshareedah I., Gamliel A., Ma Q., Zhang J., Hu Y., Tan Y., Ohgi K.A., Jayani R.S., Banerjee P.R., Aggarwal A.K., Rosenfeld M.G. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat. Struct. Mol. Biol. 2019;26:193–203. doi: 10.1038/s41594-019-0190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaudet H.M., Cheng S.B., Christensen E.M., Filardo E.J. The G-protein-coupled estrogen receptor, GPER: the inside and inside-out story. Mol. Cell. Endocrinol. 2015;418:207–219. doi: 10.1016/j.mce.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Prossnitz E.R., Arterburn J.B. International union of basic and clinical pharmacology. XCVII. G protein-coupled estrogen receptor and its pharmacologic modulators. Pharmacol. Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prossnitz E.R., Hathaway H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol. 2015;153:114–126. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prossnitz E.R., Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronda A.C., Boland R.L. Intracellular distribution and involvement of GPR30 in the actions of E2 on C2C12 cells. J. Cell. Biochem. 2016;117:793–805. doi: 10.1002/jcb.25369. [DOI] [PubMed] [Google Scholar]
- 47.Barton M., Prossnitz E.R. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol. Metabol. 2015;26:185–192. doi: 10.1016/j.tem.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prossnitz E.R., Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maggiolini M., Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 50.Prins G.S., Patisaul H.B., Belcher S.M., Vandenberg L.N. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin. Pharmacol. Toxicol. 2019;125(Suppl 3):14–31. doi: 10.1111/bcpt.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosano C., Ponassi M., Santolla M.F., Pisano A., Felli L., Vivacqua A., Maggiolini M., Lappano R. Macromolecular modelling and docking simulations for the discovery of selective GPER ligands. AAPS J. 2016;18:41–46. doi: 10.1208/s12248-015-9844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gros R., Ding Q., Sklar L.A., Prossnitz E.E., Arterburn J.B., Chorazyczewski J., Feldman R.D. GPR30 expression is required for the mineralocorticoid receptor–independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gros R., Ding Q., Liu B., Chorazyczewski J., Feldman R.D. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am. J. Physiol. Cell Physiol. 2013;304:C532–C540. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- 54.Broughton B.R.S., Brait V.H., Kim H.A., Lee S., Chu H.X., Gardiner-Mann C.V., Guida E., Evans M.A., Miller A.A., Arumugam T.V., Drummond G.R., Sobey C.G. Sex-dependent effects of G protein–coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45:835–841. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- 55.Feldman R.D., Ding Q., Hussain Y., Limbird L.E., Pickering J.G., Gros R. Aldosterone mediates metastatic spread of renal cancer via the G protein–coupled estrogen receptor (GPER) Faseb. J. 2016;30:2086–2096. doi: 10.1096/fj.15-275552. [DOI] [PubMed] [Google Scholar]
- 56.Cheng S.B., Dong J., Pang Y., LaRocca J., Hixon M., Thomas P., Filardo E.J. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol. Cell. Endocrinol. 2014;382:950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Revankar C.M., Bologa C.G., Pepermans R.A., Sharma G., Petrie W.K., Alcon S.N., Field A.S., Ramesh C., Parker M.A., Savchuk N.P., Sklar L.A., Hathaway H.J., Arterburn J.B., Oprea T.I., Prossnitz E.R. A selective ligand for estrogen receptor proteins discriminates rapid and genomic signaling. Cell Chem. Biol. 2019;26:1692–1702. doi: 10.1016/j.chembiol.2019.10.009. e1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Sun X., Lin M.S., Ferrario C.M., Van Remmen H., Groban L. G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl. Res. 2018;199:39–51. doi: 10.1016/j.trsl.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carrillo B., Collado P., Díaz F., Chowen J.A., Grassi D., Pinos H. Blocking of estradiol receptors ERα, ERβ and GPER during development, differentially alters energy metabolism in male and female rats. Neuroscience. 2020;426:59–68. doi: 10.1016/j.neuroscience.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Hadjimarkou M.M., Vasudevan N. GPER1/GPR30 in the brain: crosstalk with classical estrogen receptors and implications for behavior. J. Steroid Biochem. Mol. Biol. 2018;176:57–64. doi: 10.1016/j.jsbmb.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Gustafsson K.L., Farman H., Henning P., Lionikaite V., Moverare-Skrtic S., Wu J., Ryberg H., Koskela A., Gustafsson J.A., Tuukkanen J., Levin E.R., Ohlsson C., Lagerquist M.K. The role of membrane ERalpha signaling in bone and other major estrogen responsive tissues. Sci. Rep. 2016;6 doi: 10.1038/srep29473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levin E.R. Membrane estrogen receptors signal to determine transcription factor function. Steroids. 2018;132:1–4. doi: 10.1016/j.steroids.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Frei A., MacDonald G., Lund I., Gustafsson J.A., Hynes N.E., Nalvarte I. Memo interacts with c-Src to control Estrogen Receptor alpha sub-cellular localization. Oncotarget. 2016;7:56170–56182. doi: 10.18632/oncotarget.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson C.S., Bulayeva N.N., Wozniak A.L., Finnerty C.C. Signaling from the membrane via membrane estrogen receptor-[alpha]: estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70:364–371. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Watson C.S., Jeng Y.-J., Kochukov M.Y. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. Faseb. J. 2008;22:3328–3336. doi: 10.1096/fj.08-107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gourdy P., Guillaume M., Fontaine C., Adlanmerini M., Montagner A., Laurell H., Lenfant F., Arnal J.-F. Estrogen receptor subcellular localization and cardiometabolism. Mol. Metabol. 2018;15:56–69. doi: 10.1016/j.molmet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zoeller R.T., Brown T.R., Doan L.L., Gore A.C., Skakkebaek N.E., Soto A.M., Woodruff T.J., Vom Saal F.S. Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zoeller R.T., Bergman A., Becher G., Bjerregaard P., Bornman R., Brandt I., Iguchi T., Jobling S., Kidd K.A., Kortenkamp A., Skakkebaek N.E., Toppari J., Vandenberg L.N. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ. Health. 2014;13:118. doi: 10.1186/1476-069X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., Nadal A., Palanza P., Panzica G., Sargis R., Vandenberg L.N., vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Messerlian C., Martinez R.M., Hauser R., Baccarelli A.A. 'Omics' and endocrine-disrupting chemicals - new paths forward. Nat. Rev. Endocrinol. 2017;13:740–748. doi: 10.1038/nrendo.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marroqui L., Tuduri E., Alonso-Magdalena P., Quesada I., Nadal A., dos Santos R.S. Mitochondria as a target of endocrine-disrupting chemicals: implications for type 2 diabetes. J. Endocrinol. 2018;239:R27–R45. doi: 10.1530/JOE-18-0362. [DOI] [PubMed] [Google Scholar]
- 72.Beale A.L., Kaye D.M., Marques F.Z. The role of the gut microbiome in sex differences in arterial pressure. Biol. Sex Differ. 2019;10:22. doi: 10.1186/s13293-019-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Link J.C., Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu. Rev. Nutr. 2017;37:225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearse R.V., Young-Pearse T.L. Lost in translational biology: understanding sex differences to inform studies of diseases of the nervous system. Brain Res. 2019;1722 doi: 10.1016/j.brainres.2019.146352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepard B.D. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am. J. Physiol. Ren. Physiol. 2019 doi: 10.1152/ajprenal.00249.2019. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez-Franquesa A., Patti M.E. Insulin resistance and mitochondrial dysfunction. Adv. Exp. Med. Biol. 2017;982:465–520. doi: 10.1007/978-3-319-55330-6_25. [DOI] [PubMed] [Google Scholar]
- 77.Chow J., Rahman J., Achermann J.C., Dattani M.T., Rahman S. Mitochondrial disease and endocrine dysfunction. Nat. Rev. Endocrinol. 2017;13:92–104. doi: 10.1038/nrendo.2016.151. [DOI] [PubMed] [Google Scholar]
- 78.Balaton B.P., Dixon-McDougall T., Peeters S.B., Brown C.J. The eXceptional nature of the X chromosome. Hum. Mol. Genet. 2018;27:R242–R249. doi: 10.1093/hmg/ddy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maan A.A., Eales J., Akbarov A., Rowland J., Xu X., Jobling M.A., Charchar F.J., Tomaszewski M. The Y chromosome: a blueprint for men's health? Eur. J. Hum. Genet. 2017;25:1181. doi: 10.1038/ejhg.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henstridge D.C., Abildgaard J., Lindegaard B., Febbraio M.A. Metabolic control and sex: a focus on inflammatory-linked mediators. Br. J. Pharmacol. 2019;176:4193–4207. doi: 10.1111/bph.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohammad I., Starskaia I., Nagy T., Guo J., Yatkin E., Väänänen K., Watford W.T., Chen Z. Estrogen receptor α contributes to T cell–mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aap9415. [DOI] [PubMed] [Google Scholar]
- 82.Moulton V.R. Sex hormones in acquired immunity and autoimmune disease. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zárate S., Stevnsner T., Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front. Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grimm A., Friedland K., Eckert A. Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology. 2016;17:281–296. doi: 10.1007/s10522-015-9618-4. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen M., Wong Y.C., Ysselstein D., Severino A., Krainc D. Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson's disease. Trends Neurosci. 2019;42:140–149. doi: 10.1016/j.tins.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J., Gibbs R.B. Detection of estradiol in rat brain tissues: contribution of local versus systemic production. Psychoneuroendocrinology. 2019;102:84–94. doi: 10.1016/j.psyneuen.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 87.Balthazart J., Choleris E., Remage-Healey L. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav. 2018;99:1–8. doi: 10.1016/j.yhbeh.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silaidos C., Pilatus U., Grewal R., Matura S., Lienerth B., Pantel J., Eckert G.P. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018;9:34. doi: 10.1186/s13293-018-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Xu A., Jia S., Huang J. Recent advances in the molecular mechanism of sex disparity in hepatocellular carcinoma. Oncol. Lett. 2019;17:4222–4228. doi: 10.3892/ol.2019.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cederbaum A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi: 10.1016/j.redox.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skubic C., Drakulić Ž., Rozman D. Personalized therapy when tackling nonalcoholic fatty liver disease: a focus on sex, genes, and drugs. Expet Opin. Drug Metabol. Toxicol. 2018;14:831–841. doi: 10.1080/17425255.2018.1492552. [DOI] [PubMed] [Google Scholar]
- 92.George N., Chen M., Yuen N., Hunt C.M., Suzuki A. Interplay of gender, age and drug properties on reporting frequency of drug-induced liver injury. Regul. Toxicol. Pharmacol. 2018;94:101–107. doi: 10.1016/j.yrtph.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 93.Cho J., Kim L., Li Z., Rose N.R., Talor M.V., Njoku D.B. Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for tregs, estrogen, and IL-6. PloS One. 2013;8 doi: 10.1371/journal.pone.0061186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vafai S.B., Mootha V.K. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 95.López-Crisosto C., Bravo-Sagua R., Rodriguez-Peña M., Mera C., Castro P.F., Quest A.F.G., Rothermel B.A., Cifuentes M., Lavandero S. ER-to-mitochondria miscommunication and metabolic diseases. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2015;1852:2096–2105. doi: 10.1016/j.bbadis.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 96.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metabol. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Ong S.B., Hausenloy D.J. Mitochondrial morphology and cardiovascular disease. Cardiovasc. Res. 2010;88:16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Islam M.M., Wallin R., Wynn R.M., Conway M., Fujii H., Mobley J.A., Chuang D.T., Hutson S.M. A novel branched-chain amino acid metabolon: protein-protein interactions in a supramolecular complex. J. Biol. Chem. 2007;282:11893–11903. doi: 10.1074/jbc.M700198200. [DOI] [PubMed] [Google Scholar]
- 99.Srere P.A. Macromolecular interactions: tracing the roots. Trends Biochem. Sci. 2000;25:150–153. doi: 10.1016/s0968-0004(00)01550-4. [DOI] [PubMed] [Google Scholar]
- 100.Chen J.-Q., Yager J.D., Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim. Biophys. Acta Mol. Cell Res. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Signes A., Fernandez-Vizarra E. Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem. 2018;62:255–270. doi: 10.1042/EBC20170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scarpulla R.C. Nuclear control of respiratory gene expression in mammalian cells. J. Cell. Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 103.Rius R., Cowley M.J., Riley L., Puttick C., Thorburn D.R., Christodoulou J. Biparental inheritance of mitochondrial DNA in humans is not a common phenomenon. Genet. Med. 2019;21:2823–2826. doi: 10.1038/s41436-019-0568-0. [DOI] [PubMed] [Google Scholar]
- 104.Moos W.H., Faller D.V., Glavas I.P., Harpp D.N., Irwin M.H., Kanara I., Pinkert C.A., Powers W.R., Steliou K., Vavvas D.G., Kodukula K. A new approach to treating neurodegenerative otologic disorders. Biores Open Access. 2018;7:107–115. doi: 10.1089/biores.2018.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lefevere E., Toft-Kehler A.K., Vohra R., Kolko M., Moons L., Van Hove I. Mitochondrial dysfunction underlying outer retinal diseases. Mitochondrion. 2017;36:66–76. doi: 10.1016/j.mito.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 106.Larsson L., Degens H., Li M., Salviati L., Lee Yi, Thompson W., Kirkland J.L., Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Picca A., Mankowski R.T., Burman J.L., Donisi L., Kim J.-S., Marzetti E., Leeuwenburgh C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 2018;15:543–554. doi: 10.1038/s41569-018-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.López-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing Dev. 2017;162:108–121. doi: 10.1016/j.mad.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Lane R.K., Hilsabeck T., Rea S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta Bioenerg. 2015;1847:1387–1400. doi: 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Germain D. Chapter five - sirtuins and the estrogen receptor as regulators of the mammalian mitochondrial UPR in cancer and aging. In: Kenneth D.T., Paul B.F., editors. vol. 130. Academic Press; 2016. pp. 211–256. (Advances in Cancer Research). [DOI] [PubMed] [Google Scholar]
- 111.Horan M.P., Cooper D.N. The emergence of the mitochondrial genome as a partial regulator of nuclear function is providing new insights into the genetic mechanisms underlying age-related complex disease. Hum. Genet. 2014;133:435–458. doi: 10.1007/s00439-013-1402-4. [DOI] [PubMed] [Google Scholar]
- 112.Jovaisaite V., Mouchiroud L., Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 2013;217:137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papa L., Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J. Cell Sci. 2011;124:1396–1402. doi: 10.1242/jcs.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kenny T.C., Craig A.J., Villanueva A., Germain D. Mitohormesis primes tumor invasion and metastasis. Cell Rep. 2019;27:2292–2303. doi: 10.1016/j.celrep.2019.04.095. e2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scarpulla R.C. Nucleus-encoded regulators of mitochondrial function: integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim. Biophys. Acta Gene Regul. Mech. 2012;1819:1088–1097. doi: 10.1016/j.bbagrm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scarpulla R.C., Vega R.B., Kelly D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metabol. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lejri I., Grimm A., Eckert A. Mitochondria, estrogen and female brain aging. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gomez M., Germain D. Cross talk between SOD1 and the mitochondrial UPR in cancer and neurodegeneration. Mol. Cell. Neurosci. 2019;98:12–18. doi: 10.1016/j.mcn.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Midzak A., Papadopoulos V. Adrenal mitochondria and steroidogenesis: from individual proteins to functional protein assemblies. Front. Endocrinol. 2016;7 doi: 10.3389/fendo.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amsterdam A., Keren-Tal I., Aharoni D., Dantes A., Land-Bracha A., Rimon E., Sasson R., Hirsh L. Steroidogenesis and apoptosis in the mammalian ovary. Steroids. 2003;68:861–867. doi: 10.1016/j.steroids.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 121.Kim H., Tu H.-C., Ren D., Takeuchi O., Jeffers J.R., Zambetti G.P., Hsieh J.J.D., Cheng E.H.Y. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klinge C.M. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008;105:1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Picard M., McEwen B.S., Epel E.S., Sandi C. An energetic view of stress: focus on mitochondria. Front. Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fetterman J.L., Zelickson B.R., Johnson L.W., Moellering D.R., Westbrook D.G., Pompilius M., Sammy M.J., Johnson M., Dunham-Snary K.J., Cao X., Bradley W.E., Zhang J., Wei C.-C., Chacko B., Schurr T.G., Kesterson R.A., Dell’italia L.J., Darley-Usmar V.M., Welch D.R., Ballinger S.W. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem. J. 2013;455:157–167. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dunham-Snary K.J., Sandel M.W., Sammy M.J., Westbrook D.G., Xiao R., McMonigle R.J., Ratcliffe W.F., Penn A., Young M.E., Ballinger S.W. Mitochondrial – nuclear genetic interaction modulates whole body metabolism, adiposity and gene expression in vivo. EBioMedicine. 2018;36:316–328. doi: 10.1016/j.ebiom.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ventura-Clapier R., Moulin M., Piquereau J., Lemaire C., Mericskay M., Veksler V., Garnier A. Mitochondria: a central target for sex differences in pathologies. Clin. Sci. (Lond.) 2017;131:803–822. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- 127.Norheim F., Hasin-Brumshtein Y., Vergnes L., Chella Krishnan K., Pan C., Seldin M.M., Hui S.T., Mehrabian M., Zhou Z., Gupta S., Parks B.W., Walch A., Reue K., Hofmann S.M., Arnold A.P., Lusis A.J. Gene-by-Sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metabol. 2019;29:932–949. doi: 10.1016/j.cmet.2018.12.013. e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Landen S., Voisin S., Craig J.M., McGee S.L., Lamon S., Eynon N. Genetic and epigenetic sex-specific adaptations to endurance exercise. Epigenetics. 2019;14:523–535. doi: 10.1080/15592294.2019.1603961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lindholm M.E., Huss M., Solnestam B.W., Kjellqvist S., Lundeberg J., Sundberg C.J. The human skeletal muscle transcriptome: sex differences, alternative splicing, and tissue homogeneity assessed with RNA sequencing. Faseb. J. 2014;28:4571–4581. doi: 10.1096/fj.14-255000. [DOI] [PubMed] [Google Scholar]
- 130.De Paepe B., Lefever S., Mestdagh P. How long noncoding RNAs enforce their will on mitochondrial activity: regulation of mitochondrial respiration, reactive oxygen species production, apoptosis, and metabolic reprogramming in cancer. Curr. Genet. 2018;64:163–172. doi: 10.1007/s00294-017-0744-1. [DOI] [PubMed] [Google Scholar]
- 131.Leung A.K.L. The whereabouts of microRNA actions: cytoplasm and beyond. Trends Cell Biol. 2015;25:601–610. doi: 10.1016/j.tcb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang J.X., Rastetter R.H., Wilhelm D. Non-coding RNAs: an introduction. Adv. Exp. Med. Biol. 2016;886:13–32. doi: 10.1007/978-94-017-7417-8_2. [DOI] [PubMed] [Google Scholar]
- 133.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J., Zhang X., Li C., Yue L., Ding N., Riordan T., Yang L., Li Y., Jen C., Lin S., Zhou D., Chen F. Circular RNA profiling provides insights into their subcellular distribution and molecular characteristics in HepG2 cells. RNA Biol. 2019;16:220–232. doi: 10.1080/15476286.2019.1565284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duarte F.V., Palmeira C.M., Rolo A.P. The emerging role of MitomiRs in the pathophysiology of human disease. Adv. Exp. Med. Biol. 2015;888:123–154. doi: 10.1007/978-3-319-22671-2_8. [DOI] [PubMed] [Google Scholar]
- 136.Bai M., Chen H., Ding D., Song R., Lin J., Zhang Y., Guo Y., Chen S., Ding G., Zhang Y., Jia Z., Huang S., He J.C., Yang L., Zhang A. MicroRNA-214 promotes chronic kidney disease by disrupting mitochondrial oxidative phosphorylation. Kidney Int. 2019;95:1389–1404. doi: 10.1016/j.kint.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 137.Fan S., Tian T., Chen W., Lv X., Lei X., Zhang H., Sun S., Cai L., Pan G., He L., Ou Z., Lin X., Wang X., Perez M.F., Tu Z., Ferrone S., Tannous B.A., Li J. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Canc. Res. 2019;79:1069–1084. doi: 10.1158/0008-5472.CAN-18-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim K.M., Noh J.H., Abdelmohsen K., Gorospe M. Mitochondrial noncoding RNA transport. BMB Rep. 2017;50:164–174. doi: 10.5483/BMBRep.2017.50.4.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vendramin R., Marine J.C., Leucci E. Non-coding RNAs: the dark side of nuclear-mitochondrial communication. EMBO J. 2017;36:1123–1133. doi: 10.15252/embj.201695546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soledad R.B., Charles S., Samarjit D. The secret messages between mitochondria and nucleus in muscle cell biology. Arch. Biochem. Biophys. 2019;666:52–62. doi: 10.1016/j.abb.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hathaway Q.A., Pinti M.V., Durr A.J., Waris S., Shepherd D.L., Hollander J.M. Regulating microRNA expression: at the heart of diabetes mellitus and the mitochondrion. Am. J. Physiol. Heart Circ. Physiol. 2017;314:H293–H310. doi: 10.1152/ajpheart.00520.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shepherd D.L., Hathaway Q.A., Pinti M.V., Nichols C.E., Durr A.J., Sreekumar S., Hughes K.M., Stine S.M., Martinez I., Hollander J.M. Exploring the mitochondrial microRNA import pathway through Polynucleotide Phosphorylase (PNPase) J. Mol. Cell. Cardiol. 2017;110:15–25. doi: 10.1016/j.yjmcc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao L., Tang M., Hu Z., Yan B., Pi W., Li Z., Zhang J., Zhang L., Jiang W., Li G., Qiu Y., Hu F., Liu F., Lu J., Chen X., Xiao L., Xu Z., Tao Y., Yang L., Bode A.M., Dong Z., Zhou J., Fan J., Sun L., Cao Y. miR-504 mediated down-regulation of nuclear respiratory factor 1 leads to radio-resistance in nasopharyngeal carcinoma. Oncotarget. 2015;6:15995–16018. doi: 10.18632/oncotarget.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang T., Zhao X., Steer C.J., Yan G., Song G. A negative feedback loop between microRNA-378 and Nrf1 promotes the development of hepatosteatosis in mice treated with a high fat diet. Metabolism. 2018;85:183–191. doi: 10.1016/j.metabol.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muluhngwi P., Alizadeh-Rad N., Vittitow S.L., Kalbfleisch T.S., Klinge C.M. The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells. Sci. Rep. 2017;7:5205. doi: 10.1038/s41598-017-05727-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao Y., Liu S., Zhou L., Li X., Meng Y., Li Y., Li L., Jiao B., Bai L., Yu Y., Zhang S., Li W., Hoffman A.R., Hu J.F., Cui J. Aberrant shuttling of long noncoding RNAs during the mitochondria-nuclear crosstalk in hepatocellular carcinoma cells. Am. J. Canc. Res. 2019;9:999–1008. [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao Y., Sun L., Wang R.R., Hu J.-F., Cui J. The effects of mitochondria-associated long noncoding RNAs in cancer mitochondria: new players in an old arena. Crit. Rev. Oncol. Hematol. 2018;131:76–82. doi: 10.1016/j.critrevonc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 148.Klinge C.M. Non-coding RNAs: long non-coding RNAs and microRNAs in endocrine-related cancers. Endocr. Relat. Canc. 2018;25:R259–R282. doi: 10.1530/ERC-17-0548. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Hu S.-B., Wang M.-R., Yao R.-W., Wu D., Yang L., Chen L.-L. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat. Cell Biol. 2018;20:1145–1158. doi: 10.1038/s41556-018-0204-2. [DOI] [PubMed] [Google Scholar]
- 150.Zheng P., Xiong Q., Wu Y., Chen Y., Chen Z., Fleming J., Gao D., Bi L., Ge F. Quantitative proteomics analysis reveals novel insights into mechanisms of action of long noncoding RNA hox transcript antisense intergenic RNA (HOTAIR) in HeLa cells. Mol. Cell. Proteomics. 2015;14:1447–1463. doi: 10.1074/mcp.M114.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sirey T.M., Roberts K., Haerty W., Bedoya-Reina O., Rogatti-Granados S., Tan J.Y., Li N., Heather L.C., Carter R.N., Cooper S., Finch A.J., Wills J., Morton N.M., Marques A.C., Ponting C.P. The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. eLife. 2019;8 doi: 10.7554/eLife.45051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong Y., Yoshitomi T., Hu J.F., Cui J. Long noncoding RNAs coordinate functions between mitochondria and the nucleus. Epigenet. Chromatin. 2017;10:41. doi: 10.1186/s13072-017-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chugunova A., Loseva E., Mazin P., Mitina A., Navalayeu T., Bilan D., Vishnyakova P., Marey M., Golovina A., Serebryakova M., Pletnev P., Rubtsova M., Mair W., Vanyushkina A., Khaitovich P., Belousov V., Vysokikh M., Sergiev P., Dontsova O. LINC00116 codes for a mitochondrial peptide linking respiration and lipid metabolism. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116:4940–4945. doi: 10.1073/pnas.1809105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen J.-Q., Cammarata P.R., Baines C.P., Yager J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta Mol. Cell Res. 2009;1793:1540–1570. doi: 10.1016/j.bbamcr.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scarpulla R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 156.Chen J.Q., Brown T.R., Yager J.D. Mechanisms of hormone carcinogenesis: evolution of views, role of mitochondria. Adv. Exp. Med. Biol. 2008;630:1–18. [PubMed] [Google Scholar]
- 157.Germain D. Toward the identification and the targeting of key players of the mitochondrial unfolded protein response (UPRmt) in cancer. J. Bioenerg. Biomembr. 2017;49 doi: 10.1007/s10863-017-9715-y. 291-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kenny T.C., Germain D. From discovery of the CHOP axis and targeting ClpP to the identification of additional axes of the UPRmt driven by the estrogen receptor and SIRT3. J. Bioenerg. Biomembr. 2017;49:297–305. doi: 10.1007/s10863-017-9722-z. [DOI] [PubMed] [Google Scholar]
- 159.Kenny T.C., Manfredi G., Germain D. The mitochondrial unfolded protein response as a non-oncogene addiction to support adaptation to stress during transformation in cancer and beyond. Front. Oncol. 2017;7:159. doi: 10.3389/fonc.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Simpkins J.W., Yi K.D., Yang S.-H., Dykens J.A. Mitochondrial mechanisms of estrogen neuroprotection. Biochim. Biophys. Acta Gen. Subj. 2010;1800:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jia G., Aroor A.R., Sowers J.R. Chapter nine - estrogen and mitochondria function in cardiorenal metabolic syndrome. In: Heinz D.O., editor. ume 127. Academic Press; 2014. pp. 229–249. (Progress in Molecular Biology and Translational Science). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carson J.A., Manolagas S.C. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67–78. doi: 10.1016/j.bone.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yao J., Brinton R.D. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer's disease. In: Elias K.M., Mary L.M., editors. vol. 64. Academic Press; 2012. pp. 327–371. (Advances in Pharmacology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hara Y., Waters E.M., McEwen B.S., Morrison J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015;95:785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Das K.J., Felty Q., Poppiti R., Jackson M.R., Roy D. Nuclear respiratory factor 1 acting as an oncoprotein drives estrogen-induced breast carcinogenesis. Cells. 2018;7 doi: 10.3390/cells7120234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou Y., Xu Z., Quan D., Zhang F., Zhang H., Xiao T., Hou S., Qiao H., Harismendy O., Wang J.Y.J., Suo G. Nuclear respiratory factor 1 promotes spheroid survival and mesenchymal transition in mammary epithelial cells. Oncogene. 2018;37:6152–6165. doi: 10.1038/s41388-018-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu F., Lössl P., Rabbitts B.M., Balaban R.S., Heck A.J.R. The interactome of intact mitochondria by cross-linking mass spectrometry provides evidence for coexisting respiratory supercomplexes. Mol. Cell. Proteomics. 2018;17:216–232. doi: 10.1074/mcp.RA117.000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sepuri N.B.V., Tammineni P., Mohammed F., Paripati A. Springer Berlin Heidelberg; Berlin, Heidelberg: 2016. Nuclear Transcription Factors in the Mitochondria: A New Paradigm in Fine-Tuning Mitochondrial Metabolism; pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 169.Kumari Kanchan R., Tripathi C., Singh Baghel K., Kumar Dwivedi S., Kumar B., Sanyal S., Sharma S., Mitra K., Garg V., Singh K., Sultana S., Kamal Tripathi R., Kumar Rath S., Bhadauria S. Estrogen receptor potentiates mTORC2 signaling in breast cancer cells by upregulating superoxide anions. Free Radic. Biol. Med. 2012;53:1929–1941. doi: 10.1016/j.freeradbiomed.2012.08.595. [DOI] [PubMed] [Google Scholar]
- 170.Okoh V.O., Garba N.A., Penney R.B., Das J., Deoraj A., Singh K.P., Sarkar S., Felty Q., Yoo C., Jackson R.M., Roy D. Redox signalling to nuclear regulatory proteins by reactive oxygen species contributes to oestrogen-induced growth of breast cancer cells. Br. J. Canc. 2015;112:1687–1702. doi: 10.1038/bjc.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lone M.U.D., Baghel K.S., Kanchan R.K., Shrivastava R., Malik S.A., Tewari B.N., Tripathi C., Negi M.P.S., Garg V.K., Sharma M., Bhatt M.L.B., Bhadauria S. Physical interaction of estrogen receptor with MnSOD: implication in mitochondrial O2.- upregulation and mTORC2 potentiation in estrogen-responsive breast cancer cells. Oncogene. 2017;36:1829–1839. doi: 10.1038/onc.2016.346. [DOI] [PubMed] [Google Scholar]
- 172.Psarra A.-M.G., Sekeris C.E. Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochim. Biophys. Acta Bioenerg. 2009;1787:431–436. doi: 10.1016/j.bbabio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 173.Yang S.-H., Prokai L., Simpkins J.W. Correspondence regarding Schwend and Gustafsson, “False positives in MALDI-TOF detection of ER[beta] in mitochondria”. Biochem. Biophys. Res. Commun. 2006;345:917–918. doi: 10.1016/j.bbrc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 174.Song I.S., Jeong Y.J., Jeong S.H., Kim J.E., Han J., Kim T.H., Jang S.W. Modulation of mitochondrial ERbeta expression inhibits triple-negative breast cancer tumor progression by activating mitochondrial function. Cell. Physiol. Biochem. 2019;52:468–485. doi: 10.33594/000000034. [DOI] [PubMed] [Google Scholar]
- 175.Thomas C., Gustafsson J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Canc. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 176.Thomas C., Rajapaksa G., Nikolos F., Hao R., Katchy A., McCollum C.W., Bondesson M., Quinlan P., Thompson A., Krishnamurthy S., Esteva F.J., Gustafsson J.A. ERbeta1 represses basal-like breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012;14:R148. doi: 10.1186/bcr3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cotrim C.Z., Fabris V., Doria M.L., Lindberg K., Gustafsson J.A., Amado F., Lanari C., Helguero L.A. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390–2402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 178.Dey P., Barros R.P.A., Warner M., Ström A., Gustafsson J.-Å. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J. Mol. Endocrinol. 2013;51:T61–T74. doi: 10.1530/JME-13-0150. [DOI] [PubMed] [Google Scholar]
- 179.Bado I., Nikolos F., Rajapaksa G., Gustafsson J.A., Thomas C. ERbeta decreases the invasiveness of triple-negative breast cancer cells by regulating mutant p53 oncogenic function. Oncotarget. 2016;7:13599–13611. doi: 10.18632/oncotarget.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nikolos F., Thomas C., Bado I., Gustafsson J.-Å. ERβ sensitizes NSCLC to chemotherapy by regulating DNA damage response. Mol. Canc. Res. 2018;16:233–242. doi: 10.1158/1541-7786.MCR-17-0201. [DOI] [PubMed] [Google Scholar]
- 181.Liao T.-L., Lee Y.-C., Tzeng C.-R., Wang Y.-P., Chang H.-Y., Lin Y.-F., Kao S.-H. Mitochondrial translocation of estrogen receptor β affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free Radic. Biol. Med. 2019;134:359–373. doi: 10.1016/j.freeradbiomed.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 182.Andersson S., Sundberg M., Pristovsek N., Ibrahim A., Jonsson P., Katona B., Clausson C.-M., Zieba A., Ramström M., Söderberg O., Williams C., Asplund A. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun. 2017;8 doi: 10.1038/ncomms15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nelson A.W., Groen A.J., Miller J.L., Warren A.Y., Holmes K.A., Tarulli G.A., Tilley W.D., Katzenellenbogen B.S., Hawse J.R., Gnanapragasam V.J., Carroll J.S. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol. Cell. Endocrinol. 2017;440:138–150. doi: 10.1016/j.mce.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Z.-Y., Yin L. Estrogen receptor alpha-36 (ER-α36): a new player in human breast cancer. Mol. Cell. Endocrinol. 2015;418:193–206. doi: 10.1016/j.mce.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 185.Yan Y., Yu L., Castro L., Dixon D. ERα36, a variant of estrogen receptor α, is predominantly localized in mitochondria of human uterine smooth muscle and leiomyoma cells. PloS One. 2017;12 doi: 10.1371/journal.pone.0186078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu L., Das P., Vall A.J., Yan Y., Gao X., Sifre M.I., Bortner C.D., Castro L., Kissling G.E., Moore A.B., Dixon D. Bisphenol A induces human uterine leiomyoma cell proliferation through membrane-associated ERα36 via nongenomic signaling pathways. Mol. Cell. Endocrinol. 2019;484:59–68. doi: 10.1016/j.mce.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell'Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L., Chatham J.C., Hill B.G., Zhang J., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Radde B.N., Ivanova M.M., Mai H.X., Salabei J.K., Hill B.G., Klinge C.M. Bioenergetic differences between MCF-7 and T47D breast cancer cells and their regulation by estradiol and tamoxifen. Biochem. J. 2015;465:49–61. doi: 10.1042/BJ20131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Radde B.N., Ivanova M.M., Mai H.X., Alizadeh-Rad N., Piell K., Van Hoose P., Cole M.P., Muluhngwi P., Kalbfleisch T.S., Rouchka E.C., Hill B.G., Klinge C.M. Nuclear respiratory factor-1 and bioenergetics in tamoxifen-resistant breast cancer cells. Exp. Cell Res. 2016;347:222–231. doi: 10.1016/j.yexcr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Salama S.A., Mohammad M.A., Diaz-Arrastia C.R., Kamel M.W., Kilic G.S., Ndofor B.T., Abdel-Baki M.S., Theiler S.K. Estradiol-17beta upregulates Pyruvate kinase M2 expression to co-activate estrogen receptor-alpha and to integrate metabolic reprogramming with the mitogenic response in endometrial cells. J. Clin. Endocrinol. Metab. 2014;90:3790–3799. doi: 10.1210/jc.2013-2639. [DOI] [PubMed] [Google Scholar]
- 192.Irwin R.W., Yao J., Ahmed S.S., Hamilton R.T., Cadenas E., Brinton R.D. Medroxyprogesterone acetate antagonizes estrogen up-regulation of brain mitochondrial function. Endocrinology. 2011;152:556–567. doi: 10.1210/en.2010-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sarkar S., Jun S., Simpkins J.W. Estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERβ, AKAP and Drp1. Brain Res. 2015;1616:101–111. doi: 10.1016/j.brainres.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dzieran J., Rodriguez Garcia A., Westermark U.K., Henley A.B., Eyre Sánchez E., Träger C., Johansson H.J., Lehtiö J., Arsenian-Henriksson M. MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:E1229–E1238. doi: 10.1073/pnas.1710901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ribas V., Drew B.G., Zhou Z., Phun J., Kalajian N.Y., Soleymani T., Daraei P., Widjaja K., Wanagat J., de Aguiar Vallim T.Q., Fluitt A.H., Bensinger S., Le T., Radu C., Whitelegge J.P., Beaven S.W., Tontonoz P., Lusis A.J., Parks B.W., Vergnes L., Reue K., Singh H., Bopassa J.C., Toro L., Stefani E., Watt M.J., Schenk S., Akerstrom T., Kelly M., Pedersen B.K., Hewitt S.C., Korach K.S., Hevener A.L. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 2016;8:334–354. doi: 10.1126/scitranslmed.aad3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Collins B.C., Arpke R.W., Larson A.A., Baumann C.W., Xie N., Cabelka C.A., Nash N.L., Juppi H.-K., Laakkonen E.K., Sipilä S., Kovanen V., Spangenburg E.E., Kyba M., Lowe D.A. Estrogen regulates the satellite cell compartment in females. Cell Rep. 2019;28:368–381. doi: 10.1016/j.celrep.2019.06.025. e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hevener A.L., Zhou Z., Moore T.M., Drew B.G., Ribas V. The impact of ERalpha action on muscle metabolism and insulin sensitivity - strong enough for a man, made for a woman. Mol. Metabol. 2018;15:20–34. doi: 10.1016/j.molmet.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Torres M.J., Ryan T.E., Lin C.-T., Zeczycki T.N., Neufer P.D. Impact of 17β-estradiol on complex I kinetics and H2O2 production in liver and skeletal muscle mitochondria. J. Biol. Chem. 2018;293:16889–16898. doi: 10.1074/jbc.RA118.005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chemello F., Grespi F., Zulian A., Cancellara P., Hebert-Chatelain E., Martini P., Bean C., Alessio E., Buson L., Bazzega M., Armani A., Sandri M., Ferrazza R., Laveder P., Guella G., Reggiani C., Romualdi C., Bernardi P., Scorrano L., Cagnin S., Lanfranchi G. Transcriptomic analysis of single isolated myofibers identifies miR-27a-3p and miR-142-3p as regulators of metabolism in skeletal muscle. Cell Rep. 2019;26:3784–3797. doi: 10.1016/j.celrep.2019.02.105. e3788. [DOI] [PubMed] [Google Scholar]
- 200.Olivieri F., Ahtiainen M., Lazzarini R., Pollanen E., Capri M., Lorenzi M., Fulgenzi G., Albertini M.C., Salvioli S., Alen M.J., Kujala U.M., Borghetti G., Babini L., Kaprio J., Sipila S., Franceschi C., Kovanen V., Procopio A.D. Hormone replacement therapy enhances IGF-1 signaling in skeletal muscle by diminishing miR-182 and miR-223 expressions: a study on postmenopausal monozygotic twin pairs. Aging Cell. 2014;13:850–861. doi: 10.1111/acel.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kangas R., Törmäkangas T., Fey V., Pursiheimo J., Miinalainen I., Alen M., Kaprio J., Sipilä S., Säämänen A.-M., Kovanen V., Laakkonen E.K. Aging and serum exomiR content in women-effects of estrogenic hormone replacement therapy. Sci. Rep. 2017;7 doi: 10.1038/srep42702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Laakkonen E.K., Soliymani R., Lalowski M. Estrogen regulates muscle bioenergetic signaling. Aging (N Y) 2018;10:160–161. doi: 10.18632/aging.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Laakkonen E.K., Soliymani R., Karvinen S., Kaprio J., Kujala U.M., Baumann M., Sipila S., Kovanen V., Lalowski M. Estrogenic regulation of skeletal muscle proteome: a study of premenopausal women and postmenopausal MZ cotwins discordant for hormonal therapy. Aging Cell. 2017;16:1276–1287. doi: 10.1111/acel.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wegner M.-S., Gruber L., Schömel N., Trautmann S., Brachtendorf S., Fuhrmann D., Schreiber Y., Olesch C., Brüne B., Geisslinger G., Grösch S. GPER1 influences cellular homeostasis and cytostatic drug resistance via influencing long chain ceramide synthesis in breast cancer cells. Int. J. Biochem. Cell Biol. 2019;112:95–106. doi: 10.1016/j.biocel.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 205.Fan D.X., Yang X.H., Li Y.N., Guo L. 17beta-Estradiol on the expression of G-protein coupled estrogen receptor (GPER/GPR30) mitophagy, and the PI3K/akt signaling pathway in ATDC5 chondrocytes in vitro. Med. Sci. Mon. 2018;24:1936–1947. doi: 10.12659/MSM.909365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kuroda Y., Mitsui T., Kunishige M., Shono M., Akaike M., Azuma H. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum. Mol. Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- 207.Bauzá-Thorbrügge M., Rodríguez-Cuenca S., Vidal-Puig A., Galmés-Pascual B.M., Sbert-Roig M., Gianotti M., Lladó I., Proenza A.M. GPER and ERα mediate estradiol enhancement of mitochondrial function in inflamed adipocytes through a PKA dependent mechanism. J. Steroid Biochem. Mol. Biol. 2019;185:256–267. doi: 10.1016/j.jsbmb.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 208.Wang J., Yu R., Han Q.-Q., Huang H.-J., Wang Y.-L., Li H.-Y., Wang H.-M., Chen X.-R., Ma S.-L., Yu J. G-1 exhibit antidepressant effect, increase of hippocampal ERs expression and improve hippocampal redox status in aged female rats. Behav. Brain Res. 2019;359:845–852. doi: 10.1016/j.bbr.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 209.Borrow A.P., Handa R.J. Chapter two - estrogen receptors modulation of anxiety-like behavior. In: Litwack G., editor. vol. 103. Academic Press; 2017. pp. 27–52. (Vitamins and Hormones). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lima A.R., Santos L., Correia M., Soares P., Sobrinho-Simoes M., Melo M., Maximo V. Dynamin-related protein 1 at the crossroads of cancer. Genes. 2018;9:115. doi: 10.3390/genes9020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sastre-Serra J., Nadal-Serrano M., Pons D.G., Roca P., Oliver J. Mitochondrial dynamics is affected by 17β-estradiol in the MCF-7 breast cancer cell line. Effects on fusion and fission related genes. Int. J. Biochem. Cell Biol. 2012;44:1901–1905. doi: 10.1016/j.biocel.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 212.Sastre-Serra J., Nadal-Serrano M., Pons D.G., Roca P., Oliver J. The over-expression of ERbeta modifies estradiol effects on mitochondrial dynamics in breast cancer cell line. Int. J. Biochem. Cell Biol. 2013;45:1509–1515. doi: 10.1016/j.biocel.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 213.Oo P.S., Yamaguchi Y., Sawaguchi A., Tin Htwe Kyaw M., Choijookhuu N., Noor Ali M., Srisowanna N., Hino S.I., Hishikawa Y. Estrogen regulates mitochondrial morphology through phosphorylation of dynamin-related protein 1 in MCF7 human breast cancer cells. Acta Histochem. Cytoc. 2018;51:21–31. doi: 10.1267/ahc.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.