Abstract
HIV associated neurocognitive disorders (HAND) continue to affect a large proportion of persons living with HIV despite effective viral suppression with combined antiretroviral therapy (cART). Importantly, milder versions of HAND have become more prevalent. The pathogenesis of HAND in the era of cART appears to be multifactorial with contributions from central nervous system (CNS) damage that occur prior to starting cART, chronic immune activation, cART neurotoxicity, and various age-related comorbidities (i.e. cardiovascular and cerebrovascular disease, Diabetes, hyperlipidemia). Individuals with HIV may experience premature aging, which could also contribute to cognitive impairment. Likewise, degenerative disorders aside from HAND increase with age and there is evidence of shared pathology between HAND and other neurodegenerative diseases, such as Alzheimer’s disease, which can occur with or without co-existing HAND. Given the aforementioned complex interactions associated with HIV, cognitive impairment, and aging, it is important to consider an age-appropriate differential diagnosis for HAND as the HIV positive population continues to grow older. These factors make the accuracy and reliability of the diagnosis of mild forms of HAND in an aging population of HIV-infected individuals challenging. The complexity of current diagnosis of mild HAND also highlights the need to develop reliable biomarkers. Ultimately, the identification of a set of specific biomarkers will be required to achieve early and accurate diagnosis, which will be necessary assuming specific treatments for HAND are developed.
INTRODUCTION
The number of individuals living with HIV continues to increase, primarily due to the effectiveness of combination anti-retroviral therapy (cART). In this aging population of cART HIV-infected individuals, increasing numbers of people are diagnosed with HIV associated neurocognitive disorders (HAND). Studies examining the prevalence of HAND have shown variable results (e.g. CHARTER cohort estimates 52%, MACS cohort estimates 33%), possibly due to differences in the burden of comorbidities between studies [1, 2]. Cognitive dysfunction also affects the general population due to various etiologies frequently associated with aging, such as neurodegenerative disorders (e.g. Alzheimer’s disease; AD), long-standing substance abuse, and other medical co-morbidities like poorly controlled diabetes and cerebrovascular disease. Fifteen percent of uninfected older (>65) adults have mild cognitive impairment (MCI) [3]. Since most HAND cases in individuals taking cART are mild (see below), one challenge is to differentiate MCI related to these numerous comorbidities from mild cognitive dysfunction due to HAND. In addition, it is unclear how these comorbidities interact with HAND. Therefore, a further challenge in the field is to determine whether these frequent comorbidities in the older HIV population result in earlier onset of cognitive dysfunction and/or accelerate HAND. In this article, we will review HAND, list various etiologies for cognitive decline that can be associated with aging, highlighting AD, and discuss how they relate to HAND. We will also propose important avenues for research in aging HIV-infected individuals.
HIV-associated neurocognitive disorders (HAND)-
According to the Antinori et al. (AKA Frascati) criteria, HAND includes three separate diagnoses related to the degree of cognitive dysfunction: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HIV associated dementia (HAD) [4] (Table 1). ANI and MND include in their definition having at least one standard deviation below the mean in no less than two cognitive domains (i.e. language, attention, working memory, learning, information processing, sensory perception, motor skills) [5]. In a broader sense and particularly important in aging HIV-infected individuals, these mild forms of HAND can be considered comparable to MCI. MCI is also characterized by changes in cognition seen on neuropsychological testing (NPT), but without any significant functional impairment. MCI is further characterized into categories of amnestic vs non-amnestic and single-domain vs. multi-domain [6]. Differentiating mild forms of HAND from MCI related to other potential comorbidities (e.g., AD) is one of the major challenges in the field of NeuroHIV. Differentiating mild HAND from MCI from any cause will require the identification of sensitive and specific biomarkers for HAND (see below). The clinical phenotype of HAND can be inconsistent. There is evidence that ANI and MND can eventually progress to HAD [7], the most severe form of HAND; however, there is also support that a subset of patients can improve, while the majority remain stable [2, 8]. HAD is associated with significant morbidity and relatively rapid progression to death [9]. Approximately 35 to 50% of the almost 40 million HIV-infected individuals worldwide will develop HAND with or without cART [5]. Therefore, another major challenge for NeuroHIV investigators is to develop novel treatments for HAND with the intent of reversing mild forms and preventing the development of HAD, which is probably more refractory to treatment [10].
Table 1.
HIV-associated neurocognitive disorders (HAND) and mild cognitive impairment (MCI)
| HIV-associated neurocognitive disorder (HAND) [4] | Mild Cognitive Impairment (MCI) [3] |
|---|---|
| Asymptomatic neurocognitive impairment (ANI) • Cognitive impairment in at least two domains at least 1 SD below the mean.* • No impairment of everyday functioning • Does not meet criteria for dementia diagnosis |
• Cognitive impairment in one or more domains (i.e. focal or multifocal cognitive impairment). • Minimal impairment of everyday functioning • Does not meet criteria for dementia diagnosis |
| Mild neurocognitive disorder (MND) • Cognitive impairment in at least two domains at least 1 SD below the mean.* • Mild impairment in everyday functioning • Does not meet criteria for dementia diagnosis |
|
| HIV-1 associated dementia (HAD) • Cognitive impairment in at least two domains at least 2 SD below the mean.* • Marked impairment in everyday functioning |
NPT must survey at least the following cognitive abilities: verbal/language, attention/working memory, abstraction/executive, memory (learning, recall), speed of information processing, sensory-perceptual, motor skills
HAND has remained prevalent regardless of systemic HIV RNA suppression [2, 11]. In the pre-cART era the majority of HAND cases were characterized by more severe impairment or HAD; however, with improved treatment there has been a shift to mild forms [1, 2]. There remains controversy as to the ideal way to diagnose ANI and MND, the milder versions of HAND, which have become increasingly prevalent [12]. The current criteria are over ten years old and a limitation relating to differentiating HAND from other neurodegenerative conditions is a lack of specific NPT criteria for these disorders, which results in low specificity. As it stands, many investigators depend on lengthy neuropsychological batteries that are often difficult to implement in clinical/routine practice where it can be more difficult to diagnose subtle cognitive abnormalities using shorter screening tools [13, 14]. Another issue is the relative lack of normative data when investigating HAND as many studies use normal controls which are inadequately matched in areas such as ethnicity, gender, and education [12, 15]. Obtaining country/culturally-based normative data is also important as there is evidence of significant variation in neuropsychological testing between different regions and cultures [15]. Lastly, current criteria do not provide objective measures to address functional aspects of HAND diagnosis, which may be important when differentiating ANI from MND. This can subsequently lead to under- or over-estimations of ANI or MND depending on one’s insight into patient limitations or self-perceptions[16]. Therefore, functional scales are needed for more accurate diagnosis. Altogether, improved identification of specific neuropsychological patterns of cognitive dysfunction in HAND (e.g., compared to comorbidities like AD) in addition to emphasis on functional, behavioral, affective, motor, and/or MRI findings could aid in more accurate diagnosis and differentiation from other potential etiologies [16, 17].
Pathogenesis-
Although some investigators believe HAND in the cART era occurs primarily through systemic mechanisms or that mild forms are more related to comorbidities directly affecting the brain, it is generally accepted that all forms of HAND are related to the presence of HIV within the brain [9]. HIV enters the CNS early following infection, principally via monocytes and spreads primarily to intra-parenchymal mononuclear phagocytes and astrocytes [18]. CNS HIV can then act as a reservoir for latent infection. Neurotoxins (i.e. cytokines, chemokines, free radicals) are released within the CNS due to the presence of HIV-infected cells and this subsequently leads to an inflammatory cascade resulting in degeneration of neurons [18]. HIV itself may cause neurotoxicity via gp120 and Tat whereby the neurotoxic effects of glutamate are enhanced, contributing to HAND pathogenesis [19–21]. Therefore, cognitive impairment occurs due to virus exposure in brain and subsequent inflammation-induced neuronal dysfunction leading eventually to neuronal death [22, 23].
After cART initiation, peripheral viral load is usually reduced to non-detectable HIV RNA levels; however, there is poor CNS penetrance of most ARVs and brain infection continues [9]. Continued inflammation during cART occurs, albeit lesser than those with untreated HIV [24–26]. Peripheral reservoirs of HIV DNA within CD4+ T cells and mononuclear phagocytes can persist despite cART, and while these reservoirs have been linked to HAD, their role in the mild HAND (ANI and MND) is less clear [27]. Altogether, inadequate systemic viral suppression (i.e. duration of untreated HIV) is more strongly associated with HAND since it probably is a marker of prolonged inflammation and the extent of neurodegeneration [11, 22].
Despite the aforementioned associations, the exact underlying neuropathology for mild HAND remains uncertain. These forms can occur in the setting of undetectable plasma HIV RNA. Some argue that the concept of ongoing viral replication in the CNS, inflammation, and neurodegeneration as the underlying pathology for HAND in the era of cART is no longer applicable and should be reassessed [28]. It is possible that HIV-related brain injury occurs primarily from activated cells in the systemic compartment which then migrate into the CNS to cause neurodegeneration; but it seems more likely that brain injury results from HIV reservoirs within the brain and resultant chronic CNS inflammation separate from the systemic compartment [29]. Additionally, with the shift of predominantly HAD to ANI and MND in the cART era, it is possible that there may be differences in the pathogenesis between the different subtypes of HAND, namely ANI/MND from HAD. Nevertheless, there is evidence of brain inflammation in the cART era, particularly microglial activation, and this almost certainly contributes to pathogenesis [30, 31].
Although some recent pathological studies suggest very low or even absent CNS HIV in cART patients, most NeuroHIV investigators believe that HIV probably enters the CNS early in most if not all cases, cART does not penetrate the CNS well and almost certainly does not eradicate CNS HIV, and that CNS HIV persists [32–34]. What seems most clear is that there is ongoing neuroinflammation in HAND regardless of cART and that novel therapies that address brain pathogenic mechanisms are needed to reverse mild forms of HAND and prevent HAD.
HIV-RELATED RISK FACTORS ASSOCIATED WITH HAND-
There have been various risk factors associated with HAND (Table 2). Importantly, many of these increase in prevalence with age. The longer time between infection and cART initiation, the higher likelihood of severe cognitive impairment [22]. Duration of disease, regardless of treatment, is also a risk factor for neurocognitive decline and while early cART has significantly reduced the incidence of HAD, progression from ANI to MND can be evident with disease duration [7, 35]. Likewise, compared to HIV patients with normal cognition, those with ANI are two to six times more likely to develop MND or HAD [7]. Duration of infection is associated with deficits in information processing speed, episodic memory, and executive function irrespective of chronological age [36]. When controlling for duration of disease, there is a greater effect of aging on episodic memory and motor function with the more advanced stages of HIV infection (i.e. AIDS). The cognitive domains implicated by the Multicenter AIDS Cohort study suggest damage to the hippocampus and basal ganglia regions and this has been separately assessed in neuroimaging studies showing greater than expected age-related atrophy in these regions [37, 38]. Additionally, Cystique et al. (2017) showed white matter DTI measures were nearly normal between virally-suppressed HIV+ and HIV− individuals, except for those with neurocognitive impairment and longer HIV duration [39]. In addition to length of infection and timing of treatment, having a lower cognitive reserve may also contribute to the development of HAND [40, 41].
Table 2.
Risk factors and comorbidities in HAND (Smail and Brew 2018)
| Risk Factors and Comorbidities in HAND [18] |
|---|
| Low CD4+ |
| Longer duration of infection |
| Hx of AIDs defining illness |
| High plasma HIV viral load |
| HIV-related infections |
| cART-related toxicity |
| Alcohol or drug abuse |
| Metabolic (e.g. anemia, low hematocrit, thrombocytopenia, renal failure) |
| Cardiovascular and cerebrovascular disease |
| Previous immune deficiency |
| Diabetes, hyperlipidemia, carotid atherosclerosis |
| Epilepsy |
| Depressive symptoms [8, 65] |
| Lower education or cognitive reserve |
| Hepatitis C [135] |
Another risk factor for cognitive impairment is history of a depleted CD4+ cell count (i.e. <200); a higher CD4 nadir is associated with lower odds of cognitive impairment [42]. Even slightly impaired CD4+ counts with values up to 350 have been associated with higher risk of cognitive disorders [43]. Pfefferbaum et al. showed slower rates of brain atrophy with increasing CD4+ counts [38].
As mentioned above, cART has positive effects on the development of HAND. However, there is evidence that some antiretrovirals (ARVs) are known to be associated with neurotoxicity and cognitive impairment [44–46]. The exact underlying etiology for the neurotoxic effects has not been fully elucidated; however, mitochondrial toxicity appears to be a contributor [45].
Aging, Comorbidities, and HAND-
HIV infected individuals have earlier onset of non-infectious age-related comorbidities such as hypertension, cerebrovascular disease, diabetes, and end-renal failure resulting in an advanced biological age [47–49]. Aging itself is characterized by cellular damage over time; however, the pathogenesis of early aging in individuals living with HIV remains uncertain. The association of premature aging with HIV infection may be due to a combination of effects from the virus itself as well as ARVs. Proposed mechanisms for early aging in HIV include higher levels of systemic chronic inflammation, macromolecular damage, mitochondrial damage and oxidative stress, altered metabolism, premature aging of the immune system, and more extensive atherosclerosis [48, 50, 51]. The cholinergic and dopaminergic systems are adversely affected by aging and associated with cognitive changes [52, 53], and are implicated in HAND pathogenesis [54].
When considering premature aging, it remains unclear if HIV potentiates or accelerates the risk for cognitive impairment [55, 56]. For example, both aging and HIV can result in mitochondrial dysfunction through slightly different mechanisms- a reduced ability to mitigate reactive oxygen species in aging and increased reactive oxygen species production in HIV [48]. While there remain conflicting studies as to whether older age and HIV interact to create an increased risk of cognitive impairment, the preponderance of data suggest that premature cognitive aging likely occurs among those with HIV [57–63]. However, examining the relationship between HAND and aging is often difficult to interpret due to the presence of comorbidities. Age-associated comorbidities including cardiovascular disease and metabolic factors (i.e. DM, renal failure), are determinants for cognitive impairment in HIV+ men, further highlighting the likelihood of multifactorial pathology contributing to HAND [64].
Here we briefly mention several comorbidities and/or risk factors associated with HAND and related to aging; however, due to space constraints, will focus a more in-depth review to MCI and AD. HIV has been associated with mood disorders and depressive symptoms are predictive of HAND [8, 65]. Evidence suggests that there is an additional impact from traumatic brain injury (TBI) over HIV alone with significantly more impairment in working memory and executive functioning in HIV-infected individuals with a history of TBI [66]. This is important to consider given that TBI was a reported comorbidity in nearly 1/5th of the study participants from the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Study group [1]. This is also consistent with our HIV cohort where approximately 20% of HIV-infected veterans at the Atlanta VA have a history of TBI (unpublished data). Specific drugs of abuse that have been examined among the HIV-positive population include cocaine, methamphetamines, and marijuana. Evidence suggests that cognitive impairment is worse in this subset of patients [67–69]. Vascular disease is accelerated in HIV patients and traditional vascular risk factors such as diabetes and hyperlipidemia are also risk factors for HAND [70]. This raises the question of how much shared pathology occurs between HAND (especially mild HAND) and vascular cognitive impairment [71]. Lastly, next to AD, Parkinson’s disease (PD) is one of the more common neurodegenerative diseases. PD can be a diagnostic consideration since parkinsonian features are seen in HIV+ individuals, but also as a comorbidity may interact with HAND pathogenesis [72, 73]. Neurotoxicity to the dopaminergic systems have been implicated in both HIV and PD pathology [74].
Mild Cognitive Impairment
MCI is generally considered an intermediate between the cognitive changes seen in normal aging and those seen in dementia. Although definitions of dementia vary to some degree with respect to severity of symptoms, most clinicians would agree that cognitive dysfunction has progressed to the point that activities of daily living are significantly impaired. These patients are not employable and are dependent on others for some critical aspects of daily life.
A study supporting the theory of accelerated cognitive aging examined HIV+ persons older than 50 and found they are over seven times more likely to have MCI compared to HIV-negative counterparts [75]. It was concluded that HIV infection may increase the likelihood of developing non-HIV associated dementias with increasing age. In clinical practice, however, it is difficult to differentiate those with MCI who will go on to develop dementia. Furthermore, of those with MCI who will develop dementia, determining the dementia type is difficult (e.g., AD). Although with AD specialized testing can aid in diagnosis (see next section); however, this highlights the importance of maintaining a broad differential diagnosis during the evaluation of cognitive impairment among persons living with HIV as they may be more vulnerable to neurodegenerative pathology. Treatments that reverse or stop the progression of cognitive impairment will necessarily need to target mild cognitive impairment before it progresses to dementia. Mild cognitive dysfunction likely represents relatively more physiological, reversible dysfunction of neurons than dementia. In demented patients’ neuronal death is more prominent and therefore will be relatively refractory to novel treatments that address pathogenic mechanisms.
Alzheimer’s disease and HAND-
The interaction between AD and HAND is unclear. Their simultaneous presence may accelerate the disease course. In addition, they may share pathological features such as increased association with APOE epsilon4 (ApoE4) and amyloid deposition. ApoE4 is known to be a risk factor for AD and has also been shown to have an association with HAND. In HIV+ individuals, having at least one ApoE4 allele is associated with decreased cognitive performance (i.e. executive functioning, attention/working memory, fluency, memory) and brain atrophy [76, 77]. Likewise, among older HIV+ individuals, ApoE4 has been suggested to be an independent risk factor for HAD [78]. However, there are conflicting studies with some showing individuals with HAD being more likely to be ApoE4 carriers and others finding no relationship between the two variables [79–82]. In general, a correlation between cognitive impairment and ApoE4 has been more consistently shown in older HIV+ individuals [76, 78, 83].
With regard to brain amyloid-beta (Aβ) deposition, a critical component in AD pathology, higher amounts have been seen among HIV+ individuals (compared to HIV-negative individuals) and in association with long-term survival with HIV [78, 84, 85]. Long term presence of HIV in the brain results in neuronal damage and cognitive impairment may be due to the inadequate clearance of proteins such as Aβ [86]. Tat, for example, has been implicated in Aβ accumulation because it inhibits the Aβ-degrading enzyme neprilysin, which prevents Aβ accumulation [87]. Likewise, HIV protein gp120 is involved in amyloid accumulation through the release of inflammatory cytokines (TNFα, IL-1β) from infected microglia, which eventually results in increased cleavage of amyloid precursor protein (APP) leading to Aβ accumulation [88]. Aging and secondary effects of cART have also been implicated as contributing to amyloid deposition in HIV+ patients [84].
Differentiating HAND from AD is especially important to avoid a delayed AD diagnosis, which has specific treatment. AD is typically more unremitting in progression compared to HAND, which has a more fluctuant course. Mild HAND patients may exhibit cognitive recovery, decline, or more commonly a static course [2, 8, 89, 90]. Therefore, lack of progression or reversal of impairment may be more indicative of HAND. While there is overlap between AD and HAND on NPT, the neurocognitive profile in HAND is classically characterized by dysfunction in retrieval, slowness, attention, executive dysfunction, and focal cognitive deficits. AD tends to have more predominant dysfunction in learning and consolidation, visual spatial, semantic memory, and more often with a global cognitive deficit [17]. There is also the possibility, especially above the age of 65, that both AD and HAND co-exist, with features of both [91]. Successful treatments must be initiated at an early stage where accurate diagnosis, at least in our present state, is difficult or impossible. There are, nevertheless, some features on NPT that can support a diagnosis of ANI or MND as opposed to MCI leading to AD. For example, MCI leading to AD would be supported by the presence of memory impairment +/− deficits in language, executive function, and visuospatial skills [92]. Despite these NPTs that can suggest a specific diagnosis, uncertainty will remain, and this is where additional testing must be used to increase the accuracy of diagnosis.
Cerebrospinal fluid (CSF) biomarkers for AD include Aβ (decreased) and tau (increased) and these have helped significantly to improve diagnostic accuracy; however, among HIV patients these changes may be less specific given that these markers have also shown patterns of alteration among other inflammatory conditions [91]. While reports vary among those living with HIV, they tend to suggest low to normal levels of amyloid. T-tau and p-tau reports have shown both elevated and normal levels [85, 93–95]. Our own data suggest that CSF Aβ and p-Tau are decreased in mild HAND patients (William Hu et al. unpublished). It should be emphasized that the patterns of Aβ and tau appear to differ between AD and HAND; for example, it is the combination of Aβ and tau that is most sensitive for the diagnosis of AD, and so while there may be some degree of shared pathology, there is also evidence to suggest two distinct pathogenic processes [96]. These differences in relationships of Tau and Aβ in HAND and AD CSF analyses may ultimately provide biomarkers that enable differentiation between the two diseases, particularly early when MCI can be confused with ANI and MND. Future, larger studies should clarify the significance of these important biomarkers in HAND patients.
CSF neurofilament light chain (NFL), a measure of CNS axonal injury, has been examined in HAND and AD [97, 98]. Amongst those with AD, there is evidence to suggest NFL is associated with accelerated cognitive decline [98–100]. Among those with HIV who are neurologically asymptomatic, ART-induced viral suppression is associated with normal or slightly elevated CSF NFL, but higher among those with HAD [93]. NFL, in addition to other potential biomarkers for HAND, is discussed further in the next section.
Subtle differences in diagnostic imaging may help in differentiating HAND from AD. In AD, for example, cortical atrophy in the temporal-parietal regions can become prominent early, while the motor regions on MRI tend to be spared until later in the disease course (Figure 1) [101]. In HAND, many patients will have cortical atrophy plus periventricular white matter hyperintensities, which can potentially distinguish it from AD. However, these findings in general are non-specific in the aging population, differences between AD and HAND imaging can be quite subtle (Figure 1), and other MRI measures need to be developed. Characteristic findings on PET can be extremely helpful in ruling-in AD pathology. The presence of a negative PET suggests pathology other than AD [17]. Unfortunately, PET is not always readily assessible and its use in clinical practice is limited by access and cost.
Figure 1-.
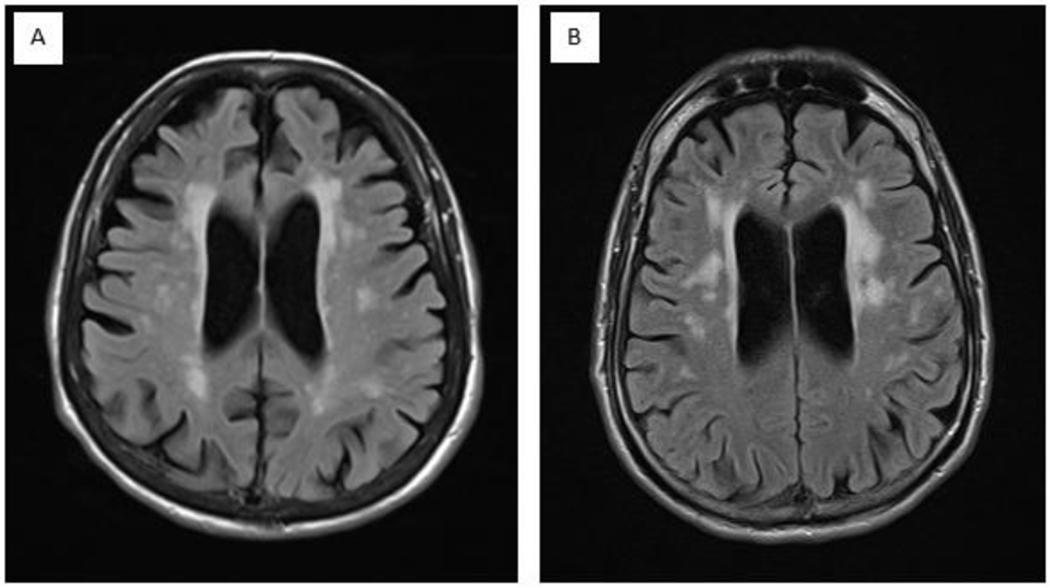
A. Axial T2 FLAIR image of an Alzheimer’s disease patient demonstrating pronounced cortical atrophy; B. Axial T2 FLAIR image of an HAND patient with less pronounced cortical atrophy though mild hydrocephalus ex vacuo can still be appreciated.
BIOMARKERS AND HAND-
Identifying biomarkers that are specific for HAND and associated comorbidities are critical to making an early and accurate diagnosis, especially since mild HAND and MCI are difficult to differentiate. Identifying disease specific biomarkers will also direct investigators towards novel treatments. In addition to identifying disease specific biomarkers, it will be equally important to identify shared mechanisms of disease pathogenesis so that HAND and its comorbidities can be treated simultaneously.
Studies suggest an association between increased HIV DNA in circulating monocytes and HAND [102, 103]. HIV DNA levels in peripheral blood mononuclear cells were not associated with overall neurocognitive performance or HAND in chronically infected HIV+ adults on long-term suppressive cART. Nonetheless, an increase in HIV DNA levels was associated with a decline in neurocognitive functions and progression to HAND [29]. Additional markers of monocyte activation have also been examined in relation to cognitive impairment. These include sCD163, sCD14, CD16, and loss of CCR2 [104–110]. These biomarkers may have implications for treatment as evidenced by a study showing increased cognitive performance in 15 HIV subjects treated with maraviroc (CCR5 antagonist) leading to CD16-expressing monocyte declines [111]. Finding reliable markers of mononuclear phagocyte activation in the brain could be immensely helpful, not only in diagnosis but as markers of treatment effects. Brain mononuclear phagocyte activity remains one of the key components in HAND pathogenesis[31, 112].
NFL is a marker of neuronal damage within the CNS and has been shown to be altered with advancing age and in a variety of neurodegenerative diseases to include AD, multiple sclerosis, and amyotrophic lateral sclerosis [113–115]. In HIV, NFL levels are altered regardless of the presence/absence of cognitive impairment, though levels have been shown to be relatively higher among those with HAD [97, 107, 116–118]. Therefore, future efforts should focus on whether there is a cutoff for serum NFL levels in HAND patients, particularly those with mild HAND. Since increased NFL is found in many brain disorders, ultimately it may serve more as a biomarker for treatment response.
Another potential marker of cognitive impairment in HAND is interferon-alpha (IFNα). IFNα correlated with HAD in the pre-cART era [119]; importantly, Anderson et. al. examined CSF IFNα levels and cognitive impairment in HIV+ individuals with milder impairment (as opposed to HAD). It was determined that IFNα levels negatively correlated with composite NP-8 scores suggesting that IFNα levels continue to play a role in HAND pathogenesis despite adequate viral suppression in the cART era [120].
There is evidence that the intestinal immune system and gut flora are altered in HIV infected individuals. For example, HIV-related changes in the microbiome have been associated with increased microbial translocation, monocyte stimulation, and measures of innate and adaptive immune activation [121, 122]. In a group of AIDS patients, plasma lipopolysaccharide (LPS), a marker of microbial translocation, was shown to be higher in HAD patients, presumably due to LPS-induced monocyte activation and trafficking into the brain [123]. It is unclear whether a similar relationship exists for the milder forms of HAND and this is yet another area of ongoing research [124]. A summary of the aforementioned biomarkers in addition to select other blood and CSF markers with an emphasis on those related to neuronal injury and immune activation are listed in Table 3; however, it should be emphasized that there are no universally accepted single or set of biomarkers for HAND diagnosis available at this time. Finally, it should be emphasized that our discussion and Table 3 only includes a sampling of biomarkers studied in HAND, as it is beyond the scope of this review to provide an exhaustive compendium.
Table 3 –
Potential Biomarkers for HAND
| BLOOD | CSF |
|---|---|
| Peripheral monocyte HIV DNA [29, 102, 103] | Neurofilament light (NFL) [97, 116] |
| CD16+ monocytes [109, 111] | t-tau [93] |
| sCD163 [106] | sAPPβ [93] |
| sCD14 [105] | Human prion protein (PRPc) [142] |
| CCR2 [108] | IL-8 [143] |
| Specific plasma microRNAs [136] | Monocyte chemotactic protein- 1 (MCP-1) [143] |
| Neurofilament light (NFL) [118] | |
| Osteopontin [137] | Induced protein- 10 (IP-10) [143] |
| IFNα-2b [138] | Granulocyte colony-stimulating factor (G-CSF) [143] |
| IL-6 [138] | |
| IL-2 [138] | IFN-α [120] |
| TNFα [139] | Neopterin [144, 145] |
| Lipopolysaccharide (LPS) [123] | Osteopontin [146] |
| Neuron-derived exosomes (NDEs) [140] | Glutamine [147] |
| Ceruloplasmin [148] | |
| Haptoglobin [148] | |
| Vascular endothelial growth factor (VEGF) [148] | |
| Galectin-9 (Gal-9) [149] |
Brain atrophy is more prominent in HAND. HIV may accelerate brain aging, presumably due to neurodegeneration [36]. Cognitive dysfunction in HAND is classically thought to occur in a subcortical pattern, especially early on, with destruction in connectivity, processing and association areas [4, 22]; however, in the cART era there is increasing evidence that the majority of cognitive impairment seen in HAND arises from cortical dysfunction [125]. Cortical atrophy in the prefrontal and parietal cortices, in particular, have been implicated in cognitive dysfunction in persons living with HIV, further complicating attempts to differentiate HAND from AD [125]. Structural findings on MRI can include cerebral atrophy, white matter volume loss, and basal ganglia volume loss [38, 126]. White matter changes occur both as diffuse and discrete lesions, and are likely to be influenced by HIV-associated small vessel disease [18]. MR spectroscopy can detect changes indicative of inflammation and neural injury and there have been changes associated with cognitive impairment in HIV [127]. Differences in fMRI patterns between HIV+ individuals with and without HAND have also been established [128–132] and this remains an expanding area of research. Finally, a PET study looking at altered translocator protein (TSPO; a marker of microglial activation) found increased binding in the hippocampus, amygdala, and thalamus correlating to poorer cognitive performance [133]. PET and MRI show significant promise for identifying biomarkers specific for HAND and other associated comorbidities.
CONCLUSION
In the current era of cART, it has become increasingly important to reassess and characterize the pathogenesis of HAND and how it is impacted by aging given that our HIV population receiving cART is living longer. There is a higher proportion of incident infections with HIV occurring in persons greater than 50 years and it is estimated that more than 50% of those living with HIV will be older than age 50 by the year 2020 [134]. Roughly 50% of these HIV-infected patients on cART will develop HAND and so novel treatments used in combination with cART need to be developed. Importantly, these adjunctive [to cART] treatments must be investigated and used in mild HAND patients to prevent deterioration to HAD, a condition less amenable to therapy. However, HAND patients are susceptible to common age-related and other comorbidities common to the HIV population that affect cognition. These comorbidities complicate the early diagnosis of mild HAND and emphasize the need to identify biomarkers. Biomarkers must be disease specific for diagnosis and treatment trials to demonstrate reversal or stabilization of HAND; but in addition, identifying biomarkers that elucidate disease mechanisms common to both HAND and its comorbidities may allow novel treatments to be developed that address pathways common to multiple diseases causing cognitive dysfunction. This is important because older HIV-infected patients are likely to have comorbidities affecting cognition [in addition to HAND] and treating multiple conditions with shared pathways using single therapies is highly preferable.
References-
- 1.Heaton RK, et al. , HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 2010. 75(23): p. 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacktor N, et al. , Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology, 2016. 86(4): p. 334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, et al. , Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology, 2018. 90(3): p. 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, et al. , Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 2007. 69(18): p. 1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumbaugh JA and Tyor W, HIV-associated neurocognitive disorders: Five new things. Neurol Clin Pract, 2015. 5(3): p. 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC, et al. , Mild cognitive impairment: a concept in evolution. J Intern Med, 2014. 275(3): p. 214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant I, et al. , Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology, 2014. 82(23): p. 2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton RK, et al. , Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis, 2015. 60(3): p. 473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McArthur JC, et al. , Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol, 2010. 67(6): p. 699–714. [DOI] [PubMed] [Google Scholar]
- 10.Tyor WR and Bimonte-Nelson H, A mouse model of HIV-associated neurocognitive disorders: a brain-behavior approach to discover disease mechanisms and novel treatments. J Neurovirol, 2018. 24(2): p. 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton RK, et al. , HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 2011. 17(1): p. 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nightingale S, et al. , Controversies in HIV-associated neurocognitive disorders. Lancet Neurol, 2014. 13(11): p. 1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipursky AR, et al. , Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS, 2013. 27(15): p. 2385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joska JA, et al. , A Comparison of Five Brief Screening Tools for HIV-Associated Neurocognitive Disorders in the USA and South Africa. AIDS Behav, 2016. 20(8): p. 1621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson K, et al. , International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J Neurovirol, 2016. 22(4): p. 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiao S, et al. , Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retroviruses, 2013. 29(6): p. 949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milanini B and Valcour V, Differentiating HIV-Associated Neurocognitive Disorders From Alzheimer’s Disease: an Emerging Issue in Geriatric NeuroHIV. Curr HIV/AIDS Rep, 2017. 14(4): p. 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smail RC and Brew BJ, HIV-associated neurocognitive disorder. Handb Clin Neurol, 2018. 152: p. 75–97. [DOI] [PubMed] [Google Scholar]
- 19.Haughey NJ, et al. , HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem, 2001. 78(3): p. 457–67. [DOI] [PubMed] [Google Scholar]
- 20.Melendez RI, et al. , Decreased glial and synaptic glutamate uptake in the striatum of HIV-1 gp120 transgenic mice. J Neurovirol, 2016. 22(3): p. 358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst T, et al. , Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging, 2010. 32(5): p. 1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamkwalala A and Newhouse P, Mechanisms of Cognitive Aging in the HIV-Positive Adult. Curr Behav Neurosci Rep, 2017. 4(3): p. 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koneru R, et al. , Reversing interferon-alpha neurotoxicity in a HIV-associated neurocognitive disorder mouse model. AIDS, 2018. 32(11): p. 1403–1411. [DOI] [PubMed] [Google Scholar]
- 24.Abdulle S, et al. , Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. AIDS, 2002. 16(16): p. 2145–9. [DOI] [PubMed] [Google Scholar]
- 25.Harezlak J, et al. , Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS, 2011. 25(5): p. 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desplats P, et al. , Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology, 2013. 80(15): p. 1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valcour VG, Shiramizu BT, and Shikuma CM, HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol, 2010. 87(4): p. 621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelman BB, Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr HIV/AIDS Rep, 2015. 12(2): p. 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cysique LA, et al. , Peripheral blood mononuclear cells HIV DNA levels impact intermittently on neurocognition. PLoS One, 2015. 10(4): p. e0120488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazzi E, et al. , Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res, 2014. 12(2): p. 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginsberg SD, et al. , Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann Neurol, 2018. 83(2): p. 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koneru R, Olive MF, and Tyor WR, Combined antiretroviral therapy reduces brain viral load and pathological features of HIV encephalitis in a mouse model. J Neurovirol, 2014. 20(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 33.Dahl V, et al. , Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS, 2014. 28(15): p. 2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fois AF and Brew BJ, The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr HIV/AIDS Rep, 2015. 12(2): p. 299–303. [DOI] [PubMed] [Google Scholar]
- 35.Baldewicz TT, et al. , Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS Behav, 2004. 8(3): p. 345–55. [DOI] [PubMed] [Google Scholar]
- 36.Goodkin K, et al. , Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV, 2017. 4(9): p. e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holt JL, Kraft-Terry SD, and Chang L, Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol, 2012. 18(4): p. 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfefferbaum A, et al. , Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging, 2014. 35(7): p. 1755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cysique LA, et al. , White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J Neurovirol, 2017. 23(4): p. 539–547. [DOI] [PubMed] [Google Scholar]
- 40.Underwood J and Winston A, Guidelines for Evaluation and Management of Cognitive Disorders in HIV-Positive Individuals. Curr HIV/AIDS Rep, 2016. 13(5): p. 235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milanini B, et al. , Cognitive reserve and neuropsychological functioning in older HIV-infected people. J Neurovirol, 2016. 22(5): p. 575–583. [DOI] [PubMed] [Google Scholar]
- 42.Ellis RJ, et al. , CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS, 2011. 25(14): p. 1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaskaran K, et al. , Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol, 2008. 63(2): p. 213–21. [DOI] [PubMed] [Google Scholar]
- 44.Nightingale S and Winston A, Measuring and managing cognitive impairment in HIV. AIDS, 2017. 31 Suppl 2: p. S165–S172. [DOI] [PubMed] [Google Scholar]
- 45.Ciavatta VT, et al. , In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem Res, 2017. 42(11): p. 3220–3232. [DOI] [PubMed] [Google Scholar]
- 46.Robertson KR, et al. , Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology, 2010. 74(16): p. 1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guaraldi G, et al. , Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis, 2011. 53(11): p. 1120–6. [DOI] [PubMed] [Google Scholar]
- 48.Lagathu C, et al. , Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS, 2017. 31 Suppl 2: p. S105–S119. [DOI] [PubMed] [Google Scholar]
- 49.Pathai S, et al. , Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci, 2014. 69(7): p. 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks SG, Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med, 2009. 17(4): p. 118–23. [PubMed] [Google Scholar]
- 51.Grunfeld C, et al. , Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS, 2009. 23(14): p. 1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dumas JA and Newhouse PA, The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav, 2011. 99(2): p. 254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anglade P, et al. , Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol, 1997. 12(3): p. 603–10. [PubMed] [Google Scholar]
- 54.Ipser JC, et al. , HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc, 2015. 21(3): p. 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wendelken LA and Valcour V, Impact of HIV and aging on neuropsychological function. J Neurovirol, 2012. 18(4): p. 256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciccarelli N, et al. , Effect of aging and human immunodeficiency virus infection on cognitive abilities. J Am Geriatr Soc, 2012. 60(11): p. 2048–55. [DOI] [PubMed] [Google Scholar]
- 57.Becker JT, et al. , Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS, 2004. 18 Suppl 1: p. S11–8. [PubMed] [Google Scholar]
- 58.Iudicello JE, et al. , Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. J Clin Exp Neuropsychol, 2012. 34(5): p. 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valcour V, et al. , The Effects of Age and HIV on Neuropsychological Performance. J Int Neuropsychol Soc, 2011. 17(1): p. 190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cysique LA, et al. , HIV and age do not substantially interact in HIV-associated neurocognitive impairment. J Neuropsychiatry Clin Neurosci, 2011. 23(1): p. 83–9. [DOI] [PubMed] [Google Scholar]
- 61.Cohen RA, Seider TR, and Navia B, HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther, 2015. 7(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole JH, et al. , Increased brain-predicted aging in treated HIV disease. Neurology, 2017. 88(14): p. 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levine AJ, et al. , Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol, 2016. 22(3): p. 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schouten J, et al. , Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS, 2016. 30(7): p. 1027–38. [DOI] [PubMed] [Google Scholar]
- 65.Malaspina L, et al. , Successful cognitive aging in persons living with HIV infection. J Neurovirol, 2011. 17(1): p. 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin K, et al. , Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. J Clin Exp Neuropsychol, 2011. 33(3): p. 326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rippeth JD, et al. , Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc, 2004. 10(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 68.Cristiani SA, Pukay-Martin ND, and Bornstein RA, Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci, 2004. 16(3): p. 330–5. [DOI] [PubMed] [Google Scholar]
- 69.Cai Y, et al. , Multiple Faceted Roles of Cocaine in Potentiation of HAND. Curr HIV Res, 2016. 14(5): p. 412–416. [DOI] [PubMed] [Google Scholar]
- 70.Fabbiani M, et al. , Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med, 2013. 14(3): p. 136–44. [DOI] [PubMed] [Google Scholar]
- 71.Su T, et al. , White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS, 2016. 30(15): p. 2329–39. [DOI] [PubMed] [Google Scholar]
- 72.Valcour V, et al. , Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol, 2008. 14(5): p. 362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeVaughn S, et al. , Aging with HIV-1 Infection: Motor Functions, Cognition, and Attention--A Comparison with Parkinson’s Disease. Neuropsychol Rev, 2015. 25(4): p. 424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar AM, et al. , Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol, 2009. 15(3): p. 257–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheppard DP, et al. , Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol, 2015. 21(5): p. 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wendelken LA, et al. , ApoE epsilon4 Is Associated With Cognition, Brain Integrity, and Atrophy in HIV Over Age 60. J Acquir Immune Defic Syndr, 2016. 73(4): p. 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang L, et al. , Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology, 2014. 82(24): p. 2213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valcour V, et al. , Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol, 2004. 157(1–2): p. 197–202. [DOI] [PubMed] [Google Scholar]
- 79.Valcour V, Shiramizu B, and Shikuma C, Frequency of apolipoprotein E4 among older compared with younger HIV patients: support for detrimental effect of E4 on survival. Proc Natl Acad Sci U S A, 2008. 105(41): p. E66; author reply E67–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spector SA, et al. , APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS, 2010. 24(10): p. 1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joska JA, et al. , Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol, 2010. 16(5): p. 377–83. [DOI] [PubMed] [Google Scholar]
- 82.Morgan EE, et al. , Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol, 2013. 19(2): p. 150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nir TM, et al. , Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp, 2014. 35(3): p. 975–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green DA, et al. , Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS, 2005. 19(4): p. 407–11. [DOI] [PubMed] [Google Scholar]
- 85.Clifford DB, et al. , CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology, 2009. 73(23): p. 1982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Achim CL, et al. , Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol, 2009. 4(2): p. 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rempel HC and Pulliam L, HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS, 2005. 19(2): p. 127–35. [DOI] [PubMed] [Google Scholar]
- 88.Ortega M and Ances BM, Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol, 2014. 9(4): p. 483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woods SP, et al. , Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev, 2009. 19(2): p. 152–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brouillette MJ, et al. , Identifying Neurocognitive Decline at 36 Months among HIV-Positive Participants in the CHARTER Cohort Using Group-Based Trajectory Analysis. PLoS One, 2016. 11(5): p. e0155766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turner RS, et al. , An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst), 2016. 4: p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen RC, Mild cognitive impairment as a diagnostic entity. J Intern Med, 2004. 256(3): p. 183–94. [DOI] [PubMed] [Google Scholar]
- 93.Peterson J, et al. , Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One, 2014. 9(12): p. e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brew BJ, et al. , CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology, 2005. 65(9): p. 1490–2. [DOI] [PubMed] [Google Scholar]
- 95.Calcagno A, et al. , Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. J Neurovirol, 2016. 22(1): p. 88–92. [DOI] [PubMed] [Google Scholar]
- 96.Molinuevo JL, et al. , The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement, 2014. 10(6): p. 808–17. [DOI] [PubMed] [Google Scholar]
- 97.Peluso MJ, et al. , Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis, 2013. 207(11): p. 1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zetterberg H, et al. , Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol, 2016. 73(1): p. 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mattsson N, et al. , Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol, 2017. 74(5): p. 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewczuk P, et al. , Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther, 2018. 10(1): p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pini L, et al. , Brain atrophy in Alzheimer’s Disease and aging. Ageing Res Rev, 2016. 30: p. 25–48. [DOI] [PubMed] [Google Scholar]
- 102.Valcour VG, et al. , HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One, 2013. 8(7): p. e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Oliveira MF, et al. , Circulating HIV DNA Correlates With Neurocognitive Impairment in Older HIV-infected Adults on Suppressive ART. Sci Rep, 2015. 5: p. 17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryan LA, et al. , Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis, 2001. 184(6): p. 699–706. [DOI] [PubMed] [Google Scholar]
- 105.Lyons JL, et al. , Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr, 2011. 57(5): p. 371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burdo TH, et al. , Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS, 2013. 27(9): p. 1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGuire JL, et al. , Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol, 2015. 21(4): p. 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ndhlovu LC, et al. , Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naive HIV-infected Thais. J Neuroimmunol, 2015. 288: p. 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kusao I, et al. , Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci, 2012. 24(1): p. 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carroll A and Brew B, HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res, 2017. 6: p. 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ndhlovu LC, et al. , Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol, 2014. 20(6): p. 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Glass JD, et al. , Clinical-neuropathologic correlation in HIV-associated dementia. Neurology, 1993. 43(11): p. 2230–7. [DOI] [PubMed] [Google Scholar]
- 113.Norgren N, Rosengren L, and Stigbrand T, Elevated neurofilament levels in neurological diseases. Brain Res, 2003. 987(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 114.Malmestrom C, et al. , Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology, 2003. 61(12): p. 1720–5. [DOI] [PubMed] [Google Scholar]
- 115.Rosengren LE, et al. , Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem, 1996. 67(5): p. 2013–8. [DOI] [PubMed] [Google Scholar]
- 116.Abdulle S, et al. , CSF neurofilament protein (NFL) -- a marker of active HIV-related neurodegeneration. J Neurol, 2007. 254(8): p. 1026–32. [DOI] [PubMed] [Google Scholar]
- 117.Jessen Krut J, et al. , Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One, 2014. 9(2): p. e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gisslen M, et al. , Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine, 2016. 3: p. 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rho MB, et al. , A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun, 1995. 9(4): p. 366–77. [DOI] [PubMed] [Google Scholar]
- 120.Anderson AM, et al. , Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-Infected individuals. J Neurovirol, 2017. 23(1): p. 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brenchley JM, et al. , Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med, 2006. 12(12): p. 1365–71. [DOI] [PubMed] [Google Scholar]
- 122.Vassallo M, et al. , The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J, 2012. 9: p. 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ancuta P, et al. , Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One, 2008. 3(6): p. e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vassallo M, et al. , Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study. J Neurovirol, 2013. 19(4): p. 376–82. [DOI] [PubMed] [Google Scholar]
- 125.Guha A, et al. , Topographies of Cortical and Subcortical Volume Loss in HIV and Aging in the cART Era. J Acquir Immune Defic Syndr, 2016. 73(4): p. 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stout JC, et al. , Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol, 1998. 55(2): p. 161–8. [DOI] [PubMed] [Google Scholar]
- 127.Chang L, et al. , A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage, 2004. 23(4): p. 1336–47. [DOI] [PubMed] [Google Scholar]
- 128.Ann HW, et al. , Characteristics of Resting-State Functional Connectivity in HIV-Associated Neurocognitive Disorder. PLoS One, 2016. 11(4): p. e0153493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plessis SD, et al. , HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS, 2014. 28(6): p. 803–11. [DOI] [PubMed] [Google Scholar]
- 130.du Plessis L, et al. , Resting-state functional magnetic resonance imaging in clade C HIV: within-group association with neurocognitive function. J Neurovirol, 2017. 23(6): p. 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ventura N, et al. , Increased posterior cingulate cortex efficiency may predict cognitive impairment in asymptomatic HIV patients. Neuroradiol J, 2018. 31(4): p. 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaganti JR, et al. , Functional Connectivity in Virally Suppressed Patients with HIV-Associated Neurocognitive Disorder: A Resting-State Analysis. AJNR Am J Neuroradiol, 2017. 38(8): p. 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vera JH, et al. , Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology, 2016. 86(15): p. 1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brooks JT, et al. , HIV infection and older Americans: the public health perspective. Am J Public Health, 2012. 102(8): p. 1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ciccarelli N, et al. , Comparison of cognitive performance in HIV or HCV mono-infected and HIV-HCV co-infected patients. Infection, 2013. 41(6): p. 1103–9. [DOI] [PubMed] [Google Scholar]
- 136.Asahchop EL, et al. , Plasma microRNA profiling predicts HIV-associated neurocognitive disorder. AIDS, 2016. 30(13): p. 2021–31. [DOI] [PubMed] [Google Scholar]
- 137.Brown A, et al. , Osteopontin enhances HIV replication and is increased in the brain and cerebrospinal fluid of HIV-infected individuals. J Neurovirol, 2011. 17(4): p. 382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cassol E, et al. , Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol, 2013. 8(5): p. 1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sevigny JJ, et al. , Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology, 2004. 63(11): p. 2084–90. [DOI] [PubMed] [Google Scholar]
- 140.Pulliam L, et al. , Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamat A, et al. , Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr, 2012. 60(3): p. 234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Megra B, et al. , Protease resistant protein cellular isoform (PrP(c)) as a biomarker: clues into the pathogenesis of HAND. J Neuroimmune Pharmacol, 2013. 8(5): p. 1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yuan L, et al. , Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol, 2013. 19(2): p. 144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hagberg L, et al. , Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther, 2010. 7: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eden A, et al. , Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS One, 2016. 11(6): p. e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burdo TH, Ellis RJ, and Fox HS, Osteopontin is increased in HIV-associated dementia. J Infect Dis, 2008. 198(5): p. 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dickens AM, et al. , Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS, 2015. 29(5): p. 559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kallianpur AR, et al. , Cerebrospinal Fluid Ceruloplasmin, Haptoglobin, and Vascular Endothelial Growth Factor Are Associated with Neurocognitive Impairment in Adults with HIV Infection. Mol Neurobiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Premeaux TA, et al. , Elevated cerebrospinal fluid Galectin-9 is associated with central nervous system immune activation and poor cognitive performance in older HIV-infected individuals. J Neurovirol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


