Abstract
Aim:
The Clinical Application of DecisionDx-UM Gene Expression Assay Results study aimed to evaluate the clinical utility of the prognostic 15-gene expression profile (15-GEP) test for uveal melanoma (UM) patients in a large, prospective multicenter cohort.
Patients & methods:
Nine centers prospectively enrolled 138 UM patients clinically tested with the 15-GEP. Physician-recommended specialty referrals and metastatic surveillance regimens were collected.
Results:
A total of 93% of high-risk class 2 patients were referred to medical oncology for follow-up, compared with 51% of class 1 patients. A majority (62%) of class 2 patients were recommended overall high-intensity metastatic surveillance, while 85% of class 1 patients were recommended low-intensity metastatic surveillance.
Conclusion:
Treatment plan recommendations for UM patients are aligned with GEP-informed metastatic risk, consistent with prior studies.
Keywords: : DecisionDx-UM, gene expression profiling, imaging, ocular melanoma, surveillance, uveal melanoma
Practice points.
The multicenter Clinical Application of DecisionDx-UM Gene Expression Assay Results study provides prospective validation of the clinical utility of the 15-gene expression profile test in directing metastatic surveillance imaging, follow-up and referral patterns.
Medical oncology referral was more common for high-risk class 2 patients compared with class 1 patients (p < 0.001).
Class 2 patients were significantly more likely to have their metastatic surveillance managed by medical oncology compared with class 1 patients (p < 0.001).
Physicians recommended significantly different metastatic surveillance regimens for class 1 versus class 2 patients in accordance with metastatic risk (p < 0.001) for frequency of abdominal imaging, chest imaging and/or liver function testing.
Uveal melanoma (UM) is the most common primary cancer of the eye in adults. Although successful control of the primary tumor is achieved in a majority of UM patients, 30–50% of patients will experience distant metastases and die of their disease [1]. In these patients, it is presumed that undetectable micrometastases develop early, before treatment of the primary tumor occurs [2]. For this reason, many UM patients were historically managed with high-intensity surveillance, including frequent imaging and laboratory tests, with the goal of detecting early metastatic events. Since becoming clinically available in 2010, widespread adoption of the prognostic 15-gene expression profile (15-GEP) test, commercially available in USA as DecisionDx-UM, has allowed physicians to develop metastatic surveillance plans based on a patient’s individual metastatic risk [3,4]. The 15-GEP test accurately identifies patients who are at low risk (class 1) or high risk (class 2) of metastasis based on the biology of the primary tumor [5].
The accuracy of the 15-GEP test has been demonstrated in both retrospective and prospective studies [4–10]. The test has been shown to be the strongest independent predictor of both metastasis free survival and melanoma specific survival in multivariate analysis when compared with clinicopathologic features and monosomy 3 [4,5,9,10]. Importantly, three published clinical utility studies (two retrospective and one prospective) have shown that administration of the 15-GEP leads to significant differences in clinical management between patients with class 1 and class 2 results [3,4,11]. Here, we provide the results of a second prospective registry study. The purpose of this multicenter effort was to prospectively evaluate patterns of physician referral and metastatic surveillance regimens for UM patients who received a 15-GEP test result, and to compare management plans between class 1 and class 2 patients.
Methods
Patient enrollment
The Clinical Application of DecisionDx-UM Gene Expression Assay Results (CLEAR II) registry study was a prospective, multicenter registry study that enrolled patients within USA who were ≥18 and <90 years old, and who had prognostic 15-GEP testing performed as part of their routine clinical care at the time of radiation or enucleation. Eligible patients were diagnosed with UM between March 2018 and February 2019. Written, informed consent was obtained from all enrolled patients after IRB approval at participating centers. Upon receipt of patients’ 15-GEP test result, physicians entered their treatment plan recommendations for specialty referrals and frequencies of metastatic surveillance imaging and lab testing into a secure web-based case report form.
Study design
This study was designed using assumptions based on two previously published prospective clinical utility studies of DecisionDx-UM, and the rates of high- and low-intensity surveillance that were observed for class 1 and 2 patients in those studies [3,4]. Sample size calculations indicated that 58 patients were needed to show a ≥40% difference in management (defined as a statistical difference in the frequency of interventions) between class 1 and class 2 patients (80% power and alpha = 0.05). Patient demographics and management decisions including follow-up, imaging modality and imaging frequency were collected.
Tumor sample acquisition & processing for 15-GEP analysis
UM primary tumor specimens were collected by either fine needle aspiration biopsy or from formalin-fixed paraffin-embedded tumor tissue. Samples were submitted to Castle Biosciences laboratory (AZ, USA) for 15-GEP testing. Sample processing and analysis by 15-GEP were performed as previously described [4,12].
Surveillance categorization
Chest imaging was defined as any imaging modality that included visualization of the chest (chest x-ray, MRI of chest/abdomen/pelvis, computed tomography (CT) of chest/abdomen/pelvis and PET-CT). Abdominal imaging was defined as any imaging modality that included visualization of the abdomen (liver ultrasound, MRI of chest/abdomen/pelvis, MRI of abdomen only, CT of chest/abdomen/pelvis, CT of abdomen only and PET-CT). Overall intensity of metastatic surveillance was categorized in alignment with current National Comprehensive Cancer Network (NCCN) guidelines regarding frequency of surveillance for class 1 versus class 2 patients. Specifically, a low-intensity regimen was defined as any imaging and/or liver function testing occurring every 6–12 months, and a high-intensity regimen was defined as any imaging and/or liver function testing occurring every 3–4 months.
Statistical analyses
Continuous variables are reported as mean ± standard deviation. Categorical variables are described using frequencies and percentages. The p-values were calculated using either Pearson Chi-square test, two-tailed Student’s t-test or Fisher’s exact test. A p < 0.05 was considered significant.
Results
Patient enrollment & characteristics
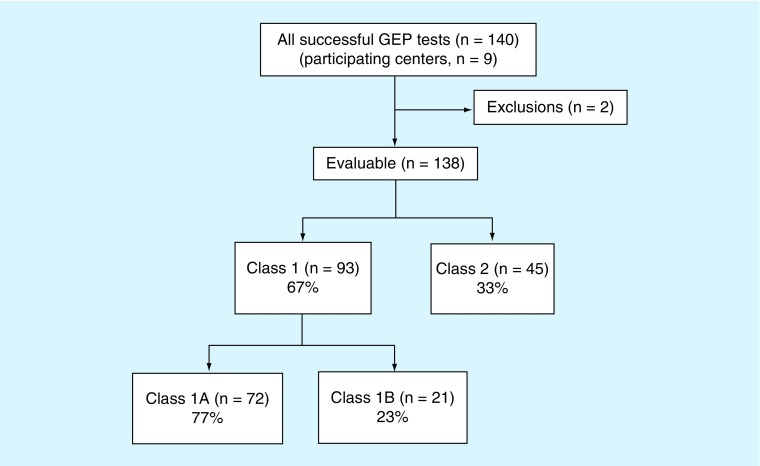
Enrollment of patients into the CLEAR II registry is outlined in Figure 1. A total of 140 patients were prospectively enrolled from nine centers across the USA. Of these 140 patients, two did not meet the inclusion criteria (one due to age, and the other due to receipt of 15-GEP test results prior to the study start date), and were therefore excluded from analysis, leaving 138 evaluable patients. There were 93 (67%) class 1 tumors (72 class 1A [52%], 21 class 1B [15%]) and 45 (33%) class 2 tumors. Patient demographics and physician referral patterns are summarized in Table 1. The cohort was 43% female and 57% male, with no significant gender difference between class 1 and class 2 frequencies. Median age of the cohort was 65 years (range: 25–89 years), although patients with a class 2 result were on an average 10 years older than those with a class 1 result (p = 0.0001). Of all enrolled patients, 113 (82%) were referred to the participating study doctor by an ophthalmologist. Moreover, 17 (12%) patients were referred to the participating study doctor by an optometrist, three (2%) by a primary care physician and five (4%) by a physician of unknown specialty.
Figure 1. . Clinical Application of DecisionDx-UM Gene Expression Assay Results registry enrollment.
A total of 140 recently diagnosed UM patients were prospectively enrolled into the registry from nine centers across the USA. Of these 140 patients, two did not meet the inclusion criteria and were excluded from analysis, leaving 138 evaluable patients. The 15-GEP test identified 93 (67%) class 1 tumors and 45 (33%) class 2 tumors. Of the class 1 tumors, 72 (77%) were class 1A and 21 (23%) were class 1B.
15-GEP: 15-gene expression profile; UM: Uveal melanoma.
Table 1. . Clinical Application of DecisionDx-UM Gene Expression Assay Results registry patient characteristics.
| Class 1 (n = 93) |
Class 2 (n = 45) |
Combined (n = 138) |
p-value | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 41 (44%) | 18 (40%) | 59 (43%) | NS‡ |
| Male | 52 (56%) | 27 (60%) | 79 (57%) | |
| Age at diagnosis (years) | ||||
| Mean | 59 ± 15 | 69 ± 12 | 62 ± 15 | 0.0001§ |
| Median | 60 | 70 | 65 | |
| Range | 25–83 | 42–89 | 25–89 | |
| Race, n (%) | ||||
| Caucasian | 91 (98%) | 44 (98%) | 135 (98%) | NS‡ |
| Unknown | Two (2%) | One (2%) | Three (2%) | |
| Specialty of primary physician†, n (%) | ||||
| Ophthalmologist | 78 (86%) | 35 (81%) | 113 (82%) | NS‡ |
| Optometrist | Ten (11%) | Seven (16%) | 17 (12%) | NS‡ |
| Primary care | Two (2%) | One (2%) | Three (2%) | NS‡ |
| Other/unknown | Three (3%) | Two (4%) | Five (4%) | NS‡ |
Specialty of physician recommending patient for UM work-up.
Fisher’s exact test.
Student’s t-test.
NS: Not significant; UM: Uveal melanoma.
Referral patterns & follow-up
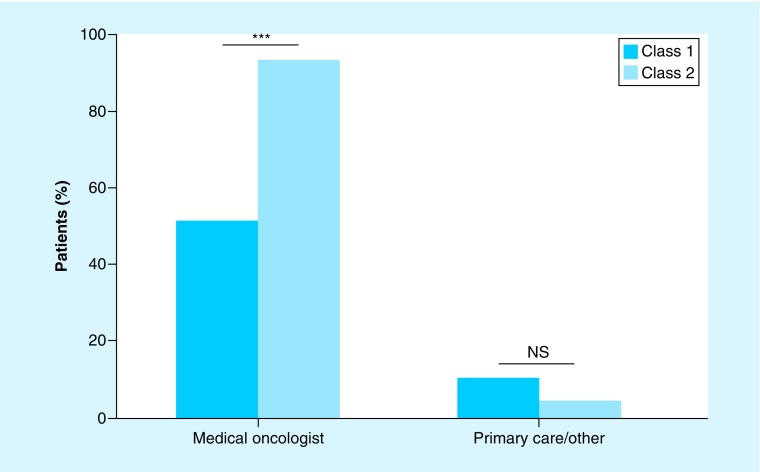
Following receipt of the 15-GEP test result, 44 (98%) class 2 patients received a referral from ophthalmology to another provider, while 55 (59%) class 1 patients received a referral (p < 0.0001). A total of 42 (93%) class 2 patients were referred to medical oncology, compared with 47 (51%) class 1 patients (Figure 2; p < 0.0001). Referrals to a primary care physician or other specialty were infrequent, accounting for only two (4%) class 2 patients and nine (10%) class 1 patients. In terms of the specialty responsible for ordering metastatic surveillance imaging, medical oncology was responsible for prescribing surveillance for 44% of class 1 patients, compared with 76% of class 2 patients.
Figure 2. . Physician referral patterns post-gene expression profile test results.
Bar graph summarizing post-GEP physician referrals for low-risk (class 1) and high-risk (class 2) UM patients.
***p < 0.001.
GEP: Gene expression profile; NS: Not significant; UM: Uveal melanoma.
Physician recommendations for chest imaging, abdominal imaging & liver function testing
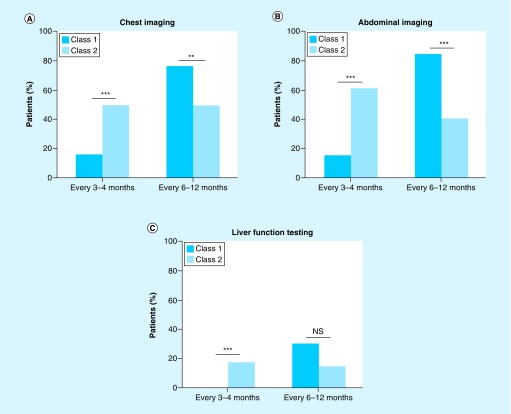
Physicians recommended more frequent metastatic surveillance screening for class 2 patients as compared with class 1 patients in accordance with their significantly higher metastatic risk (Figure 3). The class 2 patients were 3.3-times more likely than class 1 patients to receive a recommendation for chest imaging every 3–4 months, while class 1 patients were 1.5-times more likely than class 2 patients to receive a recommendation for chest imaging every 6–12 months (Figure 3A; p < 0.001 and p = 0.002, respectively). Similarly, class 2 patients were 4.3-times more likely than class 1 patients to receive a recommendation for abdominal imaging every 3–4 months, while class 1 patients were 2.1-times more likely than class 2 patients to receive a recommendation for abdominal imaging every 6–12 months (Figure 3B; p < 0.001 for both). Liver function testing was not recommended for most patients, regardless of their GEP class, with only 27 (29%) class 1 patients and 13 (29%) class 2 patients receiving this recommendation. However, when recommended, class 2 patients were more likely to receive the recommendation for more frequent testing compared with class 1 patients (Figure 3C; p = 0.0003).
Figure 3. . Physician recommendations for metastatic surveillance regimens post-gene expression profile test results.
Bar graphs summarizing the recommended frequencies for class 1 and class 2 patients to receive (A) chest imaging, (B) abdominal imaging, and (C) liver function testing.
**p < 0.01; ***p < 0.001.
NS: Not significant.
Imaging modalities recommended for 15-GEP tested class 1 & class 2 patients
Surveillance by ultrasound, x-ray, CT, and/or MRI was recommended for the majority of patients. The type of imaging recommended varied based upon GEP class. The class 1 patients were significantly more likely to receive a recommendation for ultrasound imaging, while class 2 patients were more likely to receive a recommendation for MRI (p = 0.006 and p < 0.0001, respectively; Supplementary Table 1). The frequency of imaging for each modality also differed between class 1 and 2 patients, with class 2 patients receiving recommendations for more frequent screening by x-ray, CT and MRI compared with class 1 patients (Supplementary Figure 1).
Clinical impact of 15-GEP test results on patient management decisions
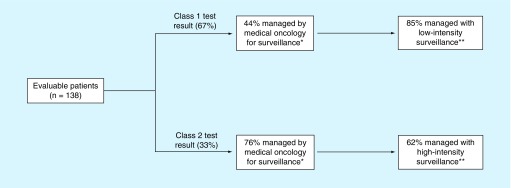
Physician recommendations for metastatic surveillance were categorized into low-intensity and high-intensity based on the frequency of recommended screening by imaging and/or liver function testing, as described in the methods. Clinical management decisions following receipt of 15-GEP test results are summarized in Figure 4. The class 2 patients were 4.1-times more likely to be followed with high-intensity surveillance compared with class 1 patients (p < 0.0001). Furthermore, patients with a class 2 test result were 1.7-times more likely to be managed by medical oncology for metastatic surveillance compared with those with a class 1 result (p = 0.0005).
Figure 4. . Clinical management decisions associated with 15-gene expression profile test results.
Schematic showing differential management of 15-GEP tested class 1 versus class 2 patients. High-intensity surveillance is defined as any imaging and/or liver functions tests occurring every 3–4 months. Low-intensity surveillance is defined as any imaging and/or liver function tests occurring every 6–12 months.
Low-intensity surveillance is defined as any imaging modality and/or liver function tests occurring every 6–12 months.
High-intensity surveillance is defined as any imaging modality and/or liver function tests occurring every 3–4 months.
*p = 0.0005 (Fisher’s exact test); **p < 0.0001 (Fisher’s exact test).
15-GEP: 15-gene expression profile.
Discussion
This prospective, multicenter study is the largest study to date evaluating the continued clinical utility of the prognostic 15-GEP test for UM patients. The sample size of this study (n = 138 patients) is more than double the minimum number needed to show a statistically significant difference in management between class 1 and 2 patients (calculated n = 58). Currently, the primary clinical use of the 15-GEP test is in risk-stratifying patients for surveillance imaging to identify metastatic disease, as there is no proven adjuvant therapy to reduce risk of spread in patients identified to be at high risk due to class 2 test results. This is important because detection of metastasis when tumor burden is low allows for earlier surgical resection, which has been shown to improve survival in UM patients [13,14]. In this study, we found that patients stratified into high risk (class 2) were managed differently with regards to surveillance imaging than patients with low risk (class 1). The majority of patients with a class 2 test result were referred to medical oncology and had metastatic surveillance prescribed by medical oncology, while significantly fewer of these high-risk patients were referred to primary care physicians for metastatic surveillance. Further, patients with a class 2 result were significantly more likely to receive a recommendation for frequent (three or four times a year) abdominal imaging, chest imaging and/or liver function testing compared with class 1 patients. These findings are in alignment with current NCCN guidelines, which recommend that class 2 patients be followed, with surveillance imaging every 3–6 months, and that class 1 patients be followed, with surveillance imaging every 6–12 months [15]. It is to be noted that the 15-GEP is not included in clinical guidelines outside of the USA where it is not commercially available [16]. Thus, other predictors of metastatic risk such as clinicopathologic features of the tumor, cytology and chromosome 3 status, are commonly used in Europe and elsewhere for risk stratification of UM patients [17].
While this study was not designed to evaluate patient outcomes, and is, therefore, limited by the lack of survival data for the reported cohort, previous studies documenting the association of 15-GEP results with patient outcomes have been published [3–9]. The results presented here are consistent with three previously published studies documenting the impact of 15-GEP on UM patient management (Table 2) [3,4,11]. Aaberg et al. [3] conducted a retrospective chart review of 88 Medicare beneficiaries clinically tested with the 15-GEP. Analysis of physician-recommended metastatic surveillance regimens revealed that all class 1 patients were followed with low-intensity (one to two times per year) surveillance, while all class 2 patients were followed with high-intensity (two to four times per year) surveillance. Additionally, more class 2 patients were referred to medical oncology for follow-up and/or were referred for adjuvant treatment protocols. A 2016 study by Plasseraud et al. [4] reported the interim analysis of the first prospective, multicenter study (CLEAR Registry) to track treatment plan recommendations as well as clinical outcomes for UM patients with 15-GEP results. Median follow-up time was 2.6 years for class 1, and 2.0 years for class 2 patients. This study also reported significant differences in clinical management of 15-GEP tested class 1 and class 2 patients, and showed that these differences were risk-adjusted, as class 1 patients had a significantly higher rate of metastasis free survival (100% at 3 years of follow-up) compared with class 2 patients (63% at 3 years of follow-up). Recently, a retrospective chart review was conducted by Davanzo et al. [11] to document patients’ adherence to recommendations regarding systemic surveillance during the first 2 years after primary intervention. In this study, patients were found to be more likely to adhere to surveillance recommendations if they were class 2, and it was suggested that this was perhaps because they were more likely to be referred to medical oncology for follow-up.
Table 2. . Summary of clinical utility studies in uveal melanoma patients who received a DDx-UM test result.
| Study (year) | Study design (n) | Patient population | Outcomes measured | Class 1 | Class 2 | Major findings | Study limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Aaberg (2014) | Retrospective, chart review, multicenter (n = 88) | Clinically tested Medicare beneficiaries with no evidence of metastatic disease | Treatment plan recommendations (surveillance regimens, physician referrals regarding adjunctive treatment) | 48 (55%) | 40 (45%) | High-intensity surveillance recommended for 100% of class 2 patients; 0% of class 1 patients | Focused only on Medicare patients | [3] |
| Plasseraud (2016) (CLEAR registry interim analysis) |
Prospective, multicenter (n = 70) | Clinically tested patients with no evidence of metastatic disease | Treatment plan recommendations (surveillance regimens, treatment referral patterns) and clinical outcomes | 37 (53%) | 33 (47%) | High-intensity surveillance maintained for 100% of class 2 patients; 19% of class 1 patients | Limited time of follow-up | [4] |
| Davanzo (2019) | Retrospective, chart review, single center (n = 107; 68 with GEP testing) | Consecutively diagnosed patients with no evidence of metastatic disease | Adherence to recommendations regarding systemic surveillance for first 2 years after primary intervention | 31 (29%); 39 (36%) with unknown risk | 37 (35%) | Class 1 patients are 9.5–9.8-times less likely to have expected surveillance performed compared with class 2 patients | Low-risk group combined patients with unknown risk (no GEP performed) with GEP class 1 | [11] |
| Current study (CLEAR II registry study) | Prospective, multicenter (n = 138; n from power calculation = 58) |
Clinically tested patients with no evidence of metastatic disease | Treatment plan recommendations (surveillance regimens, physician referrals) | 93 (67%) | 45 (33%) | High-frequency surveillance recommended for 62% of class 2 patients; 15% of class 1 patients | Study does not include patient outcomes |
CLEAR: Clinical Application of DecisionDx-UM Gene Expression Assay Result; GEP: Gene expression profile.
It is important to note that systemic surveillance for UM patients, while recommended in NCCN guidelines, remains controversial as there is currently no curative therapy for metastatic UM. Consequently, there is no consensus regarding which imaging modality/modalities and what frequency is best for surveillance of presumed subclinical micrometastasis in UM patients [18]. In the current study, fewer than 30% of all patients were recommended liver function testing, the utility of which has not been demonstrated to aid in early detection of hepatic metastasis [19]. On the other hand, surveillance via a form of systemic imaging (x-ray, hepatic ultrasound, CT or MRI) was recommended for a majority of both class 1 and class 2 patients, and has been shown to aid in the detection of subclinical metastases in primary UM patients [18,20]. Together, these data indicate that physicians are using an evidence-based approach to guide decision making regarding metastatic surveillance of UM patients, and that distinguishing class 1 from class 2 tumors impacts patient care.
Conclusion
The findings from the CLEAR II study show that treatment plan recommendations for UM patients are appropriately aligned with the calculated metastatic risk predicted by the 15-GEP test, consistent with results from previously published studies documenting the impact of the test on UM patient management.
Acknowledgments
The authors would like to acknowledge TM Aaberg, T Tsai, Y Shildkrot, K Covington, K Oelschlager, and the clinical research team at Castle Biosciences for their appreciable contributions to this study.
Footnotes
Data sharing statement
The registry data reported in this manuscript are available within the article, and additional information from the study (e.g., study protocol) is available upon reasonable request.
Financial & competing interests disclosure
Financial support/sponsorship for this study was provided by Castle Biosciences, Inc. The following authors are/were employees of Castle at the time of study enrollment: KM Plasseraud, KM Alsina and FA Monzon. AC Schefler has provided consulting services for Castle Biosciences. This work was also supported by the National Eye Institute grant NIH/NEI 5K08EY027464-02 [AB Daniels] and by a Career Development Award from the Research to Prevent Blindness Foundation [AB Daniels]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Written, informed consent was obtained from all enrolled patients after IRB approval of the study at participating centers.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 44(11), 4651–4659 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Nichols EE, Richmond A, Daniels AB. Micrometastatic dormancy in uveal melanoma: a comprehensive review of the evidence, mechanisms, and implications for future adjuvant therapies. Int. Ophthalmol. Clin. 57(1), 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin. Ophthalmol. 8, 2449–2460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Medical chart review of 88 Medicare beneficiaries demonstrates that the 15-gene expression profile (15-GEP) test directs differential surveillance and treatment plans in accordance with metastatic risk.
- 4.Plasseraud KM, Cook RW, Tsai T. et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J. Oncol. 2016, 5325762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Prospective registry that reports risk-tailored metastatic surveillance imaging following the 15-GEP result for 70 class 1 and 2 patients by four independent physicians.
- 5.Onken MD, Worley LA, Char DH. et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 119(8), 1596–1603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A total of 12 ocular oncology centers participated in this prospective study of 446 patients, which validates the accuracy of the 15-GEP test in identifying uveal melanoma (UM) patients who have a low risk (class 1) or high risk (class 2) of metastasis.
- 6.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J. Mol. Diagn. 12(4), 461–468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell MC, Char DH, Cole TB. et al. Uveal melanoma: molecular pattern, clinical features, and radiation response. Am. J. Ophthalmol. 154(2), 227.e2–232.e2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch. Clin. Exp. Ophthalmol. 252(1), 131–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am. J. Ophthalmol. 162, 20.e1–27.e1 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 134(7), 734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davanzo JM, Binkley EM, Bena JF, Singh AD. Risk-stratified systemic surveillance in uveal melanoma. Br. J. Ophthalmol. 103(12), 1868–1871 (2019). [DOI] [PubMed] [Google Scholar]; • 15-GEP tested high-risk (class 2) UM patients are significantly more likely to have expected surveillance performed compared with low-risk (class 1) patients.
- 12.Plasseraud KM, Wilkinson JK, Oelschlager KM. et al. Gene expression profiling in uveal melanoma: technical reliability and correlation of molecular class with pathologic characteristics. Diagn. Pathol. 12(1), 59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez D, Wetherill C, Cheong J. et al. The Liverpool uveal melanoma liver metastases pathway: outcome following liver resection. J. Surg. Oncol. 109(6), 542–547 (2014). [DOI] [PubMed] [Google Scholar]; • High-risk UM patients eligible for surgical intervention show significantly improved survival compared with palliative care/chemotherapy.
- 14.Piperno-Neumann S, Servois V, Mariani P. et al. Prospective study of surveillance testing for metastasis in 100 high-risk uveal melanoma patients. J. Fr. Ophtalmol. 38(6), 526–534 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Coit D, Thompson JA, Albertini M. et al. Uveal melanoma NCCN guidelines National Comprehensive Cancer Network (2019). https://www.nccn.org/professionals/physician_gls/default.aspx#site ; •• Gene expression profiling is included as a prognostic method to determine metastatic risk, and risk-tailored surveillance regimens are recommended based on a class 1A, 1B and 2 result (with recommendations for increased frequency with increasing class risk).
- 16.Nathan P, Cohen V, Coupland S. et al. Uveal Melanoma UK National Guidelines. Eur. J. Cancer 51(16), 2404–2412 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Damato B, Eleuteri A, Taktak AFG, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog. Retin. Eye Res. 30(5), 285–295 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Bellerive C, Ouellet E, Kamaya A, Singh AD. Liver imaging techniques: recognition of uveal melanoma metastases. Ocul. Oncol. Pathol. 4(4), 254–260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouriaux F, Diorio C, Bergeron D, Berchi C, Rousseau A. Liver function testing is not helpful for early diagnosis of metastatic uveal melanoma. Ophthalmology 119(8), 1590–1595 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Choudhary MM, Gupta A, Bena J, Emch T, Singh AD. Hepatic ultrasonography for surveillance in patients with uveal melanoma. JAMA Ophthalmol. 134(2), 174–180 (2016). [DOI] [PubMed] [Google Scholar]