Abstract
Immune-checkpoint inhibitors (ICIs) are approved in the first-line and third-line settings for patients with extensive-stage or relapsed small-cell lung cancer (SCLC), respectively. In the first-line setting, the addition of the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab to chemotherapy improves overall survival (OS). In patients with relapsed disease, data from nonrandomized trials have revealed promising responses, although a significant improvement in OS over that obtained with conventional chemotherapy was not achieved in a randomized trial in this setting. Substantial research interest exists in identifying predictive biomarkers that could guide the use of ICIs in patients with SCLC. PD-L1 expression is typically low or absent in SCLC, which has precluded its use as a predictive biomarker. Tumour mutational burden might have some predictive value, although blood-based measures of tumour mutational burden did not have predictive value in patients receiving atezolizumab plus chemotherapy in the first-line setting. After three decades, ICIs have finally enabled an improvement in OS for patients with SCLC; however, a substantial amount of research remains to be done, including identifying the optimal therapeutic strategy and predictive biomarkers. In this Review, we describe the available data on clinical efficacy, the emerging evidence regarding biomarkers and ongoing clinical trials using ICIs and other immunotherapies in patients with SCLC.
Small-cell lung cancer (SCLC) accounts for ~15% of all lung cancers and ~30,000 deaths in the USA annually1. Owing to the elusive pathophysiology of the disease, the poor prognosis of patients and minimal improvement in the effectiveness of therapies over the past decades, SCLC is a US National Cancer Institute-designated recalcitrant malignancy2.
With the FDA approval of carboplatin, etoposide and the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab as a first-line therapy, and the anti-programmed cell death protein 1 (PD-1) antibodies nivolumab and pembrolizumab as monotherapies in the third-line setting, immune-checkpoint inhibitors (ICIs) have entered the treatment armamentarium for patients with SCLC. These approvals are an important advance for patients with SCLC, whose treatment strategies and clinical outcomes had remained unchanged for decades. To date, however, no consistent predictive biomarkers that can accurately guide the use of ICIs in patients with SCLC have been identified; response rates in the first-line setting remain consistent at 60–65% with or without ICI3. Despite the fact that SCLC is known to have a relatively high tumour mutational burden (TMB; median ~8 mutations per megabase (mut/Mb))4, histological examinations of tumour material demonstrate that <20% of SCLCs express PD-L1 in >1% of tumour cells5–8. The use of TMB alone as a predictor of benefit from ICIs has shown early promise in patients with relapsed SCLC receiving combinations of ICIs7, although blood-based methods of TMB quantification (bTMB) have not demonstrated clear predictive value in patients receiving chemotherapy plus an ICI in the first-line setting3.
SCLC is difficult to study clinically owing to a paucity of substantive tumour specimens. This issue arises because surgical resection is rarely a therapeutic option, leading to a reliance on diagnostic biopsy samples, which are often small and necrotic. Furthermore, repeat biopsy samples are rarely obtained at times of disease progression. The fundamental questions involving the use of ICIs in patients with SCLC remain how to better understand the paradox of a high TMB4, generally low or absent PD-L1 expression5,9 and lower than expected responses rates compared with those of other solid tumours with a similar median TMB10, even when PD-L1 expression is detectable11,12. Furthermore, ongoing trials involving novel immune-based treatment strategies are assessing whether ICIs will ultimately prove to be the most successful therapeutic strategy, or whether other novel immunotherapeutic approaches will offer greater levels of benefit (such as chimeric antigen receptor (CAR) T cells, or bispecific T cell engagers (BiTEs)). In this Review, we describe the available clinical data, biomarker evaluations and ongoing clinical trials involving ICIs and other immunotherapies in patients with SCLC.
Immunobiology and subtypes
Tumour specimens obtained through the standard-of-care management of patients with SCLC are often sparse; therefore, large-cohort studies that include analysis of such samples have been limited. However, several observations have led to the application of ICIs in patients with SCLC and highlight areas for future study. SCLCs have a high median TMB4, and the observed associations between TMB assessed in tumour material and responsiveness to ICIs across multiple tumour types10, as well as in non-small-cell lung cancer (NSCLC) specifically13, led to the successful application of ICIs in combination with chemotherapy in patients with SCLC3. The working hypothesis regarding why combination chemotherapy and ICIs has proved to be the most successful strategy to date is that chemotherapy administration in this generally chemosensitive disease results in increased presentation of tumour-associated antigens, resulting in increased T cell priming and amplification of the cytotoxic T cell response. Similar observations have been made preclinically in mouse models of mesothelioma14–16.
PD-L1 expression in >1% of tumour cells is present in only a minority (~20%) of SCLC specimens5–8. Retrospective studies of samples obtained from patients with SCLC before the introduction of ICIs have demonstrated a better prognosis in those with high counts of tumour-infiltrating lymphocytes (TILs)17–20. Each retrospective analysis was performed in samples from a slightly different patient population and revealed different associations. The presence of CD8+ stromal TILs was associated with superior progression-free survival (PFS) and overall survival (OS) (both P < 0.05) in patients with pulmonary neuroendocrine tumours of any histology, 59.1% of whom had SCLC18. The presence of suppressive FOXP3+ regulatory T cells was found to be associated with a better prognosis in patients with limited-stage (stage I–III) SCLC (LS-SCLC) (HR 0.37, 95% CI 0.17–0.81; P = 0.013)17. The presence of CD45RO+ memory T cells in SCLC brain metastases was associated with prolonged median OS (11 months versus 5 months; P = 0.007)19. Finally, a subset of patients with SCLC with neurological paraneoplastic syndromes (PNS) had greater levels of tumour T cell infiltration (P = 0.033) and numerically longer PFS and OS durations than those with endocrinological PNS or no PNS20. In a cohort of 102 patients with both LS-SCLC and extensive-stage SCLC (ES-SCLC), investigators observed a statistically significant correlation between PD-L1 expression and having limited-stage disease (in 85.4% versus 62.3%; P = 0.011). In this same cohort, PD-L1 expression was found to be independently predictive of a favourable outcome in the ES-SCLC cohort (median OS 9.2 months versus 5.4 months; P = 0.037)21. Collectively, these data suggest an association between immune infiltration and improved outcomes in patients with this disease.
TILs have been observed in SCLC specimens with no detectable PD-L1 expression20, suggesting that alternative immune checkpoints might also be clinically relevant. However, data from studies designed to investigate the presence or absence of alternative, potentially clinically important immune checkpoints in SCLC, such as LAG3, TIM3, TIGIT, OX40 and ICOS, are currently unavailable. A better understanding of the immune microenvironment is an important area of unmet need in the immunobiology of SCLC.
An overarching goal of research designed to further our understanding of the SCLC immune microenvironment is to enable immunological characteristics to be integrated with the findings of the substantial preclinical efforts to define distinct molecular subtypes of SCLC. Outstanding work from several independent groups, using various platforms including analysis of tumour samples from patients22–24, patient-derived xenografts23, patient-derived cell lines23–27 and mouse models28–30, suggests that SCLCs can be divided into four primary, molecularly defined subtypes. These subtypes can be defined by unique transcriptional factor expression profiles overlaid with RNA sequencing profiles31: SCLC-A, defined by a high level of achaete-scute homologue 1 (ASCL-1) expression; SCLC-N, defined by a high level of neurogenic differentiation factor 1 (NEUROD1) expression; SCLC-Y, defined by expression of the transcriptional co-activator YAP1; and SCLC-P, defined by POU domain, class 2, transcription factor 3 (POU2F3) expression. Future studies should seek to both define the relationship between these SCLC subtypes and immunobiological features and consider prospective clinical trial designs with selection for specific molecular subtypes.
Clinical outcomes
First-line therapy.
The characteristic chemosensitivity of most SCLCs in the first-line setting results in substantial amounts of tumour cell death and neoantigen release, theoretically making ICIs in combination with cytotoxic chemotherapy an attractive strategy32. Results from three phase III trials exploring the efficacy of this approach have been reported.
In a phase III, placebo-controlled randomized controlled trial (RCT), the efficacy of ipilimumab, a monoclonal anti-cytotoxic T lymphocyte protein 4 (CTLA-4) antibody, in combination with etoposide plus either cisplatin or carboplatin, was compared with placebo plus this combination of chemotherapies in patients with ES-SCLC (defined as not confined within one hemithorax of the lungs, with evidence of regional lymph node metastasis). Patients initially received two cycles of chemotherapy and were then randomized to receive two cycles of ipilimumab (10 mg/kg) plus chemotherapy or placebo plus chemotherapy, followed by two additional cycles of ipilimumab or placebo. A total of 954 patients were treated: 478 in the ipilimumab arm and 476 in the control arm. Median OS was not significantly improved in the ipilimumab arm (11.0 months versus 10.9 months; HR 0.94, 95% CI 0.81–1.09; P = 0.38), although a modest statistically significant improvement in median PFS was observed with the addition of ipilimumab to chemotherapy (4.6 months versus 4.4 months; HR 0.85, 95% CI 0.75–0.97; P = 0.016). The objective response rate (ORR), defined as a partial or complete response, was not different between the two treatment arms (62% in both groups)33 (TABLE 1).
Table 1 |.
Outcomes of immunotherapy trials in small-cell lung cancer
| Trial | Treatment arms (n) | ORR (%) | PFS outcomes | OS outcomes | Grade ≥3 adverse events (%) |
|---|---|---|---|---|---|
| Upfront | |||||
| IMPower 133 (2018)3 | Chemotherapya + atezolizumab (201) vs chemotherapya (202) | 60 vs 64 | Median PFS 5.2 months vs 4.3 months; 1-year PFS 12.6% vs 5.4% | Median OS 12.3 months vs 10.3 months (HR 0.70, 95% CI 0.54–0.91, P = 0.007); 1-year OS 51.7% vs 38.2% | 56.6 vs 56.1 |
| CASPIAN (2019)36 | Chemotherapya + durvalumab (268) vs chemotherapya (269) | 79.5 vs 70.3 | Median PFS 5.1 months vs 5.4 months (HR 0.78, 95% CI 0.65–0.94); 1-year PFS 17.5% vs 4.7% | Median OS 13 months vs 10.3 months (HR 0.73, 95% CI 0.59–0.91, P = 0.0047); 1-year OS 5 3.7% vs 39.8% | 62 vs 62 |
| Reck et al. (2016)33 | Chemotherapya + ipilimumab (478) vs chemotherapya (476) | 62 vs 62 | Median PFS 4.6 months vs 4.4 months (HR 0.85, 95% CI 0.75–0.97, P = 0.016) | Median OS 11 months versus 10.9 months (HR 0.94, 95% CI 0.81–1.09, P = 0.38); 1-year OS 40% vs 40% | 48 vs 45 |
| First-line maintenance | |||||
| Cadgeel et at. (2018)9 | Chemotherapya followed by pembrolizumab (45) | 14.7 | Median PFS 1.4 months; 1-year PFS 13% | Median OS 9.6 months; 1-year OS 37% | Grade ≥3 NR; grade 5:4% |
| CheckMate 451 (2019)41 | Chemotherapya followed by nivolumab + ipilimumab (279) vs nivolumab (280) vs placebo (275) | NR | Median PFS 1.7 months (HR 0.72,95% CI 0.60–0.87)b vs 1.9 months (HR 0.67, 95% CI 0.56–0.81)b vs 1.4 months | Median OS 9.2 months (HR 0.92, 95% CI 0.75–1.12, P = 0.37)b vs 10.4 months (HR 0.84, 95% CI 0.69—1.02)b vs 9.6 months; 1-year OS 41% vs 44% vs 40% | 52 vs 12 vs 8 |
| Second line or later | |||||
| CheckMate 032 (2016)5 | Nivolumab (98) | 10 | 1.4 months; 1-year PFS 11% | 4.4 months; 1-year OS 33% | 13 |
| Nivolumab 1 mg/kg + ipilimumab 3 mg/kg (61) | 23 | 2.6 months; 1-year PFS 19% | 7.7 months; 1-year OS 43% | 30 | |
| Nivolumab 3 mg/kg + ipilimumab 1 mg/kg (54) | 19 | 1.4 months; 1-year PFS NR | 6 months; 1-year OS 35% | 19 | |
| CheckMate 032 (2019)42 | Nivolumab (109) | 11.9 | 1.4 months; 1-year PFS NR | 5.6 months; 1-year OS 28.3% | 11.9 |
| CheckMate 331 (2018)43 | Nivolumab (284) vs chemotherapyc (285) | NR | 1.5 months vs 3.8 months (HR 1.41, 95% CI 1.18–1.69); 1-year PFS 11% vs 10% | 7.5 months vs 8.4 months (HR 0.86, 95% CI 0.72–1.04, P = 0.11); 1-year OS 37% vs 34% | 14 vs 73 |
| KEYNOTE-028 (2017)d11 | Pembrolizumab (24) | 33 | 1.9 months; 1-year PFS 23.8% | 9.7 months; 1-year OS 37.7% | 4.2 |
| KEYNOTE-158 (2018)44 | Pembrolizumab (107) | 18.7 | 2 months | 9.1 months | Grade ≥3 NR; grade 5: 0.9% |
| IFCT-1603 (2019)45 | Atezolizumab (49) vs chemotherapye (24) | 2 vs 10 | 1.4 months vs 4.3 months | 9.5 months vs 8.7 months (HR 0.84, 95% CI 0.45–1.58, P = 0.60); 1-year OS 42.5% vs NR | 4.2 vs NR (at least 33.3) |
NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Etoposide plus a platinum-containing agent.
Comparison with placebo.
Topotecan or amrubicin.
Enrolled patients with programmed cell death 1 ligand 1 ≥1%.
Amrubicin or initial chemotherapy.
In the IMPower 133 trial3, the efficacy of atezolizumab in combination with carboplatin and etoposide was assessed in patients with ES-SCLC. In this phase III, placebo-controlled RCT, investigators enrolled a total of 403 patients: 201 in the chemotherapy plus atezolizumab arm and 202 in the chemotherapy plus placebo arm. The combination of chemotherapy plus atezolizumab resulted in a significant improvement in median OS (12.3 months versus 10.3 months; HR 0.70, 95% CI 0.54–0.91; P = 0.007). A statistically significant improvement in median PFS was also observed with upfront atezolizumab plus chemotherapy (5.2 months versus 4.3 months; HR 0.77, 95% CI 0.62–0.96; P = 0.02). No statistically significant difference in ORR was observed between the two groups (60% in patients who received atezolizumab versus 64% in the chemotherapy-alone group). The separation of the OS curves at ~8 months in this study suggests a divergence at this approximate time point, implying that only a subset of patients derive additional survival benefit from the addition of this ICI to chemotherapy3. Updated OS data presented at the 2019 ESMO Congress confirmed the superior efficacy of atezolizumab plus chemotherapy (18-month OS 34% versus 21%)34 (TABLE 1).
In September 2019, investigators from the CASPIAN trial reported on the efficacy of the anti-PD-L1 antibody durvalumab in combination with etoposide plus either cisplatin or carboplatin in patients with treatment-naive ES-SCLC35. In this open-label, randomized, phase III trial, 268 patients received durvalumab plus chemotherapy and 269 patients received chemotherapy alone. Similar to the IMPower 133 study, median OS was significantly improved in the durvalumab arm compared with the chemotherapy-only arm (13.0 months versus 10.3 months; HR 0.73, 95% CI 0.59–0.91; P = 0.0047). Investigator-assessed ORR was also improved in the durvalumab arm (79.5% versus 70.3%, respectively)36 (TABLE 1). Key differences between the CASPIAN and IMPower 133 trials include the protocol of CASPIAN allowing the use of cisplatin in the chemotherapy back-bone, although a previous meta-analysis of data from patients with ES-SCLC revealed no improvement in OS for patients receiving cisplatin compared with carboplatin37. Furthermore, CASPIAN was an unblinded study and patients in the durvalumab group were not allowed to undergo prophylactic cranial irradiation, while patients enrolled in the IMPower 133 trial were blinded to study therapy and prophylactic cranial irradiation was permitted. A similar difference in OS was observed between the different treatment groups in CASPIAN compared with IMPower 133, although how the site of disease progression, specifically with regard to central nervous system disease, differs between the two treatment arms would be interesting to note.
Regardless of these minor differences, these two trials have almost identical survival data and these results corroborate the use of upfront chemotherapy plus an ICI targeting PD-1 or PD-L1 as a successful treatment strategy for patients with ES-SCLC. Approximately 10% of patients with SCLC will develop PNS1, with neurological PNS thought to be autoimmune sequelae38. Importantly, given the potential for activation of autoimmunity, no significant increase in PNS or other grade 3 or 4 adverse events was observed in patients receiving chemotherapy plus an ICI in either IMPower 133 or CASPIAN3,34,36. Moreover, the incidence and type of other treatment-related adverse events and treatment discontinuation were similar to that observed when ICIs have been combined with chemotherapy in patients with NSCLC39,40.
First-line maintenance monotherapy or combination therapy.
The efficacy of maintenance with single-agent pembrolizumab after induction therapy with a platinum-containing agent and etoposide has been evaluated in patients with ES-SCLC in a single-arm, phase II study involving 45 patients without disease progression after 4–6 cycles of chemotherapy. The study required that patients began maintenance pembrolizumab within 8 weeks of completion of chemotherapy, with a median time to initiation of 5 weeks. The median PFS was disappointing at 1.4 months, with a 1-year PFS of 13%. The median OS was 9.6 months, with a 1-year OS of 37%. Most patients (n = 34) had measurable disease at the start of maintenance pembrolizumab, and the ORR of 14.7% is similar to that reported for patients with SCLC receiving ICIs as monotherapy in the second-line or third-line setting9 (TABLE 1).
ICIs targeting PD-1 or PD-L1 have also been evaluated in combination with ipilimumab as maintenance therapies in patients with SCLC. In CheckMate 451, a placebo-controlled, phase III RCT, investigators evaluated the efficacy of nivolumab plus ipilimumab (n = 279) and nivolumab monotherapy (n = 280) as maintenance therapy after induction chemotherapy, compared with placebo alone during the maintenance period (n = 275), in patients with ES-SCLC. In this study, neither the ICI combination nor nivolumab monotherapy improved median OS versus placebo (median OS 9.2 months and 10.4 months, respectively, versus 9.6 months). Both groups receiving ICIs had modest statistically nonsignificant improvements in median PFS relative to placebo (median PFS 1.7 months and 1.9 months, respectively, versus 1.4 months; P = 0.72 and P = 0.67)41 (TABLE 1). Interestingly, an improvement in OS emerged in a subgroup analysis of data from patients receiving maintenance nivolumab within 5 weeks of completing chemotherapy. This observation could reflect a difference in the mechanism of action of nivolumab (based on the hypothesis that larger numbers of tumour-associated antigens are likely to be available nearer to completion of chemotherapy) or selection bias (in that earlier initiation of ICI might be a surrogate for better clinical outcomes). Nonetheless, the negative outcomes of this phase III RCT, and the successes with upfront use of ICIs plus chemotherapy with subsequent maintenance ICI, have superseded ICI monotherapy as a maintenance strategy following chemotherapy in patients with ES-SCLC. Importantly, the utility of both upfront and maintenance ICIs in patients with LS-SCLC following concurrent chemoradiotherapy remains under investigation (NCT03703297, NCT03585998, NCT02046733, NCT03540420 and NCT03811002).
Second-line or later monotherapy.
ICI monotherapy with nivolumab or pembrolizumab is FDA-approved for patients with advanced-stage SCLC, independent of PD-L1 status, as a third-line or later-line therapy. Nivolumab was approved for this indication based on data from CheckMate 032, in which investigators evaluated the efficacy of nivolumab monotherapy versus nivolumab plus ipilimumab in patients with disease progression on platinum-based chemotherapy, regardless of PD-L1 expression. Among the 98 patients in the nivolumab monotherapy arm, 41% were treated in the second line and 56% in the third line or fourth line. Patients receiving nivolumab monotherapy had an ORR of 10%, a median PFS duration of 1.4 months, a 1-year PFS of 11%, a median OS duration of 4.4 months and a 1-year OS of 33%5. In a later publication42, data from 109 patients enrolled in CheckMate 032 who received nivolumab in the third-line or later-line setting were reported. Among this cohort, 71.6% were treated in the third line with an ORR of 11.9%, a median PFS duration of 1.4 months, a median OS duration of 5.6 months and a 1-year OS of 28.3%42.
The efficacy of nivolumab monotherapy has been compared with that of chemotherapy (with topotecan or amrubicin) in a phase III RCT involving 569 patients with relapsed SCLC following platinum-based first-line therapy (CheckMate 331). The primary OS end point of this trial was not met (median OS 7.5 months and 8.4 months in the nivolumab and chemotherapy arms, respectively; HR 0.86, 95% CI 0.72–1.04). Median OS was 7 months versus 5.7 months among patients with platinum-resistant or refractory disease in the nivolumab versus chemotherapy arms; in patients with platinum-sensitive disease (defined as disease relapse >90 days after completion of induction therapy), median OS was 7.6 months versus 11.1 months43 (TABLE 1).
Data on the efficacy of pembrolizumab that led to FDA approval included both the KEYNOTE-028 and the KEYNOTE-158 trials. In KEYNOTE-028, only patients with a tumour cell, immune infiltrate and stromal summative PD-L1 combined positive score (CPS) ≥1% were included. Among 24 patients with relapsed SCLC, 12.5% of whom were receiving pembrolizumab in the second line and 50% in the third line of therapy, the ORR was 33%. Median PFS was 1.9 months, 1-year PFS was 23.8%, median OS was 9.7 months and the 1-year OS was 37.7%11. In KEYNOTE-158, involving 107 patients with relapsed SCLC, 79% received pembrolizumab in the second-line or third-line setting and 47% of patients had PD-L1-negative tumours, with an ORR of 18.7% (35.7% and 6.0% in the PD-L1-positive and PD-L1-negative subgroups, respectively). Similar to KEYNOTE-028, median PFS was 2 months and median OS was 9.1 months44 (TABLE 1).
In a phase II RCT, the efficacy of atezolizumab monotherapy was compared with that of chemotherapy (with either topotecan or platinum rechallenge) in the second line in patients with relapsed SCLC, without selection for PD-L1 expression. Among 73 patients randomized, 49 received atezolizumab and 24 received chemotherapy. Overall, 64% of the patients had platinum-sensitive disease (defined as disease progression ≥90 days after completion of induction chemotherapy). Results from this trial, published in January 2019, revealed no significant difference in median OS (9.5 months versus 8.7 months; HR 0.84, 95% CI 0.45–1.58; P = 0.60)45. Furthermore, median PFS was statistically inferior in patients who received atezolizumab (1.4 months versus 4.3 months in patients receiving chemotherapy; adjusted HR 2.26, 95% CI 1.3–3.9; P = 0.004). ORRs were low in both groups: 2.3% in patients receiving atezolizumab and 10% in those receiving chemotherapy45 (TABLE 1).
In summary, both nivolumab43 and atezolizumab45 have failed to improve OS compared with standard chemotherapy in RCTs involving patients with relapsed SCLC requiring second-line therapy. FDA approval of ICI monotherapy, with either nivolumab or pembrolizumab, has been granted only in the third-line or later setting based on ORRs of 10–30% in single-arm studies11,42,44. However, with the approval of chemotherapy plus atezolizumab in the first-line setting, ICI monotherapy is unlikely to be a widely used treatment strategy. Importantly, the clinical implications, in terms of response rate and duration of response, of receiving an anti-PD-L1 antibody in the first line followed by an anti-PD-1 antibody in the third line remain unclear.
Second-line or later combination therapy.
The efficacy of nivolumab plus ipilimumab in patients with relapsed SCLC was assessed in the second line in CheckMate 032. Two different dose combinations of nivolumab plus ipilimumab were assessed in the initial nonrandomized component of this trial: nivolumab 1 mg/kg plus ipilimumab 3 mg/kg and nivolumab 3 mg/kg plus ipilimumab 1 mg/kg. Patients received four cycles of either combination followed by nivolumab monotherapy until disease progression. Among 61 patients with relapsed SCLC in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg arm, the ORR was 23% with a median PFS of 2.6 months and a median OS of 7.7 months. Among the 53 patients with relapsed SCLC in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg arm, the ORR was 19% with a median PFS duration of 1.4 months and a median OS duration of 6 months5 (TABLE 1). Numerically higher response rates were seen in patients with platinum-sensitive disease and in patients who had received only one previous line of therapy. PD-L1 expression was assessable in 69% of patients enrolled (148 of 216); 17% and 5% of specimens were PD-L1 ≥1% and ≥5%, respectively. Responses were seen regardless of PD-L1 expression46. On the basis of the more promising results from the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg arm, this combination proceeded to investigation in the randomized component of CheckMate 032, in which the outcomes of these patients were compared with those receiving nivolumab monotherapy.
Tumour parameters as biomarkers
Tumour and immune cell PD-L1 expression.
Expression of PD-L1 on tumour and immune cells has been evaluated as a predictive biomarker of response to ICIs in the first-line upfront and maintenance settings and in the second-line or later setting in patients with SCLC (FIG. 1; TABLE 2). At the 2019 ESMO Congress, data on tumour and immune cell PD-L1 expression from the IMPower 133 trial were reported. Of the 403 patients enrolled, 137 had evaluable tumour material, thus reflecting the difficulties in obtaining biopsy material from this population. Of these 137 evaluated tumours, 129 (94.2%) had <1% PD-L1 expression on tumour cells and 68 (49.6%) had <1% PD-L1 expression on immune cells when analysed using immunohistochemistry with the VENTANA SP263 antibody. In a subgroup analysis of data from patients with evaluable tumour specimens, no significant improvement in OS with chemotherapy plus atezolizumab compared with chemotherapy plus placebo was detected among those with ≥1% or ≥5% PD-L1 expression on tumour cells or immune cells. In patients with both tumour and immune cell PD-L1 expression <1%, a statistically significant improvement in OS was observed in those receiving chemotherapy plus atezolizumab versus chemotherapy plus placebo (median OS 10.2 months versus 8.3 months, respectively; HR 0.51, 95% CI 0.30–0.89)34 (TABLE 2). These inconsistent findings suggest that PD-L1 expression is not predictive of OS in patients with SCLC receiving chemotherapy in combination with an ICI.
Fig. 1 |. Predictive biomarkers of response and/or survival in patients receiving immune-checkpoint inhibitors for small-cell lung cancer.
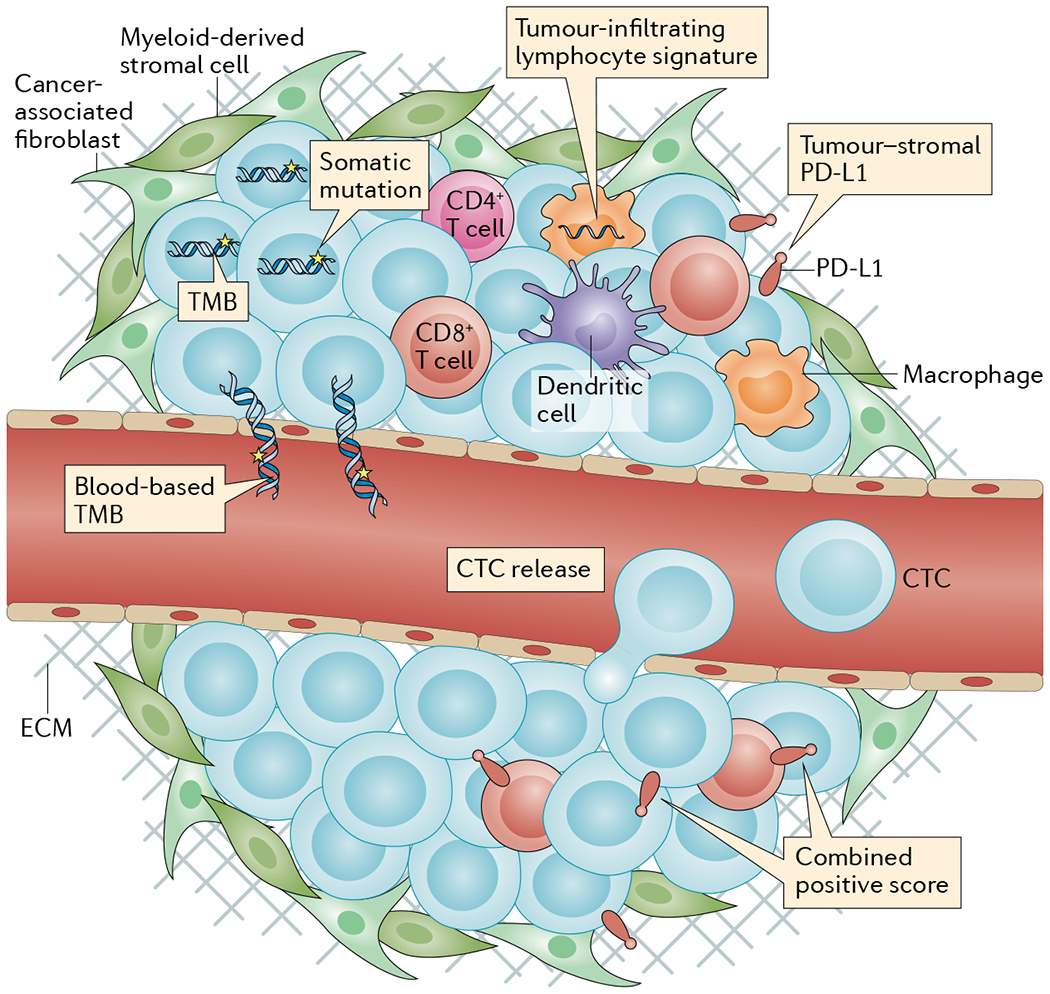
Various tumour-based and/or blood-based assays have been evaluated for their ability to predict clinical benefit from immune-checkpoint inhibitors in patients with small-cell lung cancer. Biomarkers that are thought to continue to hold potential clinical predictive value include tumour mutational burden (TMB) and tumour-infiltrating lymphocyte RNA expression. Biomarkers that are thought to not hold predictive value based on data from larger analyses include tumour programmed cell death 1 ligand 1 (PD-L1) expression and blood-based TMB. Biomarkers that have only been evaluated in very small numbers of patients include circulating tumour cells (CTCs), combined tumour plus tumour-infiltrating immune cell PD-L1 expression, and PD-L1 expression at the tumour–stromal interface. ECM, extracellular matrix.
Table 2 |.
Predictive biomarkers evaluated in patients with small-cell lung cancer receiving immune-checkpoint inhibitors
| Biomarker | ORR | Median PFS | Median OS |
|---|---|---|---|
| Upfront | |||
| Blood-based TMB | NE | NE | Different blood-based TMB subgroups derive similar levels of benefit from addition of atezolizumab to chemotherapy: ≥10 mut/Mb HR 0.70; >10 mut/Mb HR 0.68; <16 mut/Mb HR 0.71; ≥16 mut/Mb HR 0.63 (REF.3) |
| Tumour or immune cell PD-L1 expression | NE | NE | Patients with PD-L1 <1% derived the highest level of benefit from addition of atezolizumab to chemotherapy (HR 0.51) compared with ≥1% (HR 0.87), <5% (HR 0.77) and ≥5% (HR0.60)34 |
| First-line maintenance | |||
| Tumour PD-L1 expression | Median PFS 11 months among 3 patients with PD-L1-positive tumours who received pembrolizumab9 | NE | NE |
| Tumour–stromal interface PD-L1 expression | ORR 37.5% in 9 patients with PD-L1-positive stroma vs 8.3% in 12 patients with PD-L1-negative stroma receiving pembrolizumab9 | Median PFS 6.5 months in 9 patients with PD-L1-positive stroma vs 1.3 months in 12 patients with PD-L1-negative stroma receiving pembrolizumab9 | Median OS 12.8 months in 9 patients with PD-L1-positive stroma vs 6.5 months in 12 patients with PD-L1-negative stroma receiving pembrolizumab9 |
| CTCs | NE | Not predictive (n = 37)9 | Not predictive (n = 37)9 |
| Second line or later | |||
| Tumour PD-L1 expression | Not predictive5,7 | NE | NE |
| PD-L1 CPS | ORR 35.7% in 42 patients with PD-L1-positive tumours vs 6% in 50 patients with PD-L1-negative tumours receiving pembrolizumab44 | Median PFS 2.1 months in 42 patients with PD-L1-positive tumours vs 1.9 months in 50 patients with PD-L1-negative tumours receiving pembrolizumab | Median OS 14.6 months in 42 patients with PD-L1-positive tumours vs 7.7 months in 50 patients with PD-L1-negative tumours receiving pembrolizumab44 |
| TMB | ORRs 21.3% and 46.2% in patients in the highest TMB tertile receiving nivolumab or ipilimumab plus nivolumab versus 6.8% and 16.0% and 4.8% and 22.2% in the medium and low TMB tertiles, respectively7,48 | Median PFS 1.3, 1.3 and 1.4 months, and 1.5, 1.3 and 7.8 months in the low, medium and high TMB tertiles in response to nivolumab or nivolumab plus ipilimumab, respectively7 | Median OS 3.1, 3.9 and 5.4 months, and 3.4, 3.6 and 22 months in the low, medium and high TMB tertiles in response to nivolumab or nivolumab plus ipilimumab, respectively7 |
| TIL signature | T cell-inflamed GEP associated with superior ORR in patients with solid tumours receiving pembrolizumab including SCLC (P = 0.012)48 | T cell-inflamed GEP associated with longer PFS in patients with solid tumours receiving pembrolizumab including SCLC (P = 0.017)48 | NE |
CPS, combined positive score; CTC, circulating tumour cell; GEP, gene-expression profile; mut/Mb, mutations per megabase; NE, not evaluated; ORR, objective response rate; OS, overall survival; PD-L1, programmed cell death 1 ligand 1; PFS, progression-free survival; SCLC, small-cell lung cancer; TIL, tumour-infiltrating lymphocyte; TMB, tumour mutational burden.
In the aforementioned single-arm, phase II study investigating the performance of first-line maintenance pembrolizumab in patients with ES-SCLC, Gadgeel and colleagues9 retrieved pretreatment archival specimens and used the DAKO 22C3 anti-PD-L1 antibody to evaluate PD-L1 expression. Tumours were only considered assessable for PD-L1 positivity if at least 50 viable tumour cells or five PD-L1-positive tumour cells could be identified. In this study, 30 out of 45 specimens (66%) were evaluable, of which three (10%) had PD-L1 expression ≥1%. Among these three patients, two responded to therapy and the third patient had no measurable disease at study entry. The median PFS among these three patients was 11 months (range 10–13 months). The ORR and median PFS of the 27 patients with evaluable tumours and no detectable PD-L1 expression were not reported9.
PD-L1 expression had also been evaluated in patients receiving ICI in the second-line or later relapsed setting. In CheckMate 032, investigators evaluated tumour PD-L1 expression using the 28-8 pharmDx antibody47 in pretreatment tumour specimens obtained within 3 months of beginning ICI treatment from patients who received no other anticancer therapies in the 3-month period prior to commencing ICIs. In total, 148 tumour samples were obtained, and acceptable samples were defined as those containing ≥100 evaluable tumour cells. Among patients in the nivolumab monotherapy arm, the ORR was 38% (3 of 8) if PD-L1 was ≥1%, 28% (12 of 43) if PD-L1 was <1% and 24% (6 of 25) in those with nonevaluable tumours. In the nivolumab 1 mg/kg and ipilimumab 3 mg/kg arm, ORRs in these subgroups were 33% (2 of 6), 36% (8 of 22) and 33% (6 of 18), respectively. In comparison, ORRs were 60% (3 of 5), 24% (7 of 29) and 15% (2 of 13), respectively, among these subgroups in the nivolumab 3 mg/kg and ipilimumab 1 mg/kg arm5. An updated analysis of data from CheckMate 032 indicates no statistically significant associations between tumour PD-L1 expression and ORR among the 109 patients included in the nonrandomized population who received either nivolumab or nivolumab plus ipilimumab7.
Following the IFCT-1603 phase II RCT, in which patients received atezolizumab or chemotherapy in the second line, investigators evaluated tumour PD-L1 expression in archived tumour specimens using the SP-142 assay. However, only 1 of 53 evaluable specimens had >1% tumour PD-L1 expression, thus precluding evaluations of predictive value45.
Combined PD-L1-positive score.
The predictive value of CPS has been evaluated in patients with relapsed SCLC, but not in those with treatment-naive disease or in those receiving ICIs as first-line maintenance therapy (FIG. 1; TABLE 2). In KEYNOTE-028, involving patients with relapsed ES-SCLC, a CPS of ≥1% determined by analysis of either a fresh or archived pretreatment tumour specimen was an inclusion criterion for treatment with pembrolizumab11. PD-L1 was assessed using the 22C3 antibody47 in specimens containing ≥50 viable tumour cells. These patients had an ORR of 33% (8 of 24)11.
KEYNOTE-158, a trial investigating the efficacy of pembrolizumab monotherapy, included patients with a CPS as low as 0% (CPS was evaluated in the same way as in KEYNOTE-028). Patients were stratified into two arms: CPS ≥1% (n = 42) and CPS <1% (n = 50). ORRs were 35.7% versus 6%, 1-year OS was 53.1% versus 30.7% and the median OS duration was 14.6 months versus 7.7 months44. The ORR was 27% (4 of 15) among patients with unknown PD-L1 status.
Owing to the low overall level of tumour cell PD-L1 expression in SCLCs, expression of this immune checkpoint is unlikely to have predictive value regarding the effectiveness of ICIs. In a broader evaluation of PD-L1 expression inclusive of tumour cells, stromal cells and infiltrating immune cells, the CPS seemed to have predictive value in KEYNOTE-158, although replication of this finding is needed in order to demonstrate the further potential of this composite score as a predictive biomarker in patients with SCLC who are eligible for ICIs. The likelihood of PD-L1 CPS becoming a predictive biomarker to guide the use of the combination of chemotherapy with ICI in the first-line setting is low.
PD-L1 expression at the tumour–stromal interface.
The role of PD-L1 at the tumour–stromal interface as a predictive biomarker has only been evaluated in the first-line maintenance setting (FIG. 1; TABLE 2). In patients with ES-SCLC who had a response or stable disease following induction chemotherapy, Gadgeel and colleagues retrieved pretreatment archival specimens and observed PD-L1 expression at the tumour–stromal interface in 40% of patients (8 of 20). Comparisons of the outcomes of the eight patients with PD-L1 expression at the tumour–stromal interface versus those of the 12 without such expression revealed ORRs of 37.5% versus 8.3%, with median PFS durations of 6.5 months versus 1.3 months and median OS durations of 12.8 months versus 7.6 months9.
Tumour T cell-inflamed gene-expression signature.
The presence of a T cell-inflamed gene-expression signature (RNA-based TIL signature) has only been reported as a predictive biomarker of response to ICIs in patients with relapsed SCLC (FIG. 1; TABLE 2). An analysis of tumour material from patients with solid tumours of various histologies who received pembrolizumab in KEYNOTE-028, including eight patients with SCLC, showed that a TIL signature was correlated with ORR and median PFS48. This TIL signature was based on an 18-gene RNA expression platform49,50 but, similar to TMB in this analysis, making specific conclusions regarding the predictive utility of this signature in patients with SCLC is difficult owing to the limited number of patients.
Circulating tumour cells.
Circulating tumour cells (CTCs) are detectable in blood samples from patients with ES-SCLC who are receiving maintenance pembrolizumab following a response to first-line chemotherapy (TABLE 2). Gadgeel and colleagues reported that 51% of patients (19 of 37) had detectable CTCs at treatment initiation, and found no correlation between baseline CTC count or changes in CTC count during therapy and median PFS or OS9 (TABLE 2). The predictive value of PD-L1 expression at the tumour–stromal interface, TIL signature and CTC-based evaluations is difficult to assess because the patient numbers included in studies thus far have been very small and further validation is needed.
Tumour mutational burden.
TMB has also been evaluated as a potential predictive biomarker for patients with SCLC receiving ICIs (FIG. 1; TABLE 2). In CheckMate 032, investigators evaluated TMB using paired blood and pretreatment tumour specimens. Whole-exome sequencing (WES) was used to quantify TMB, and paired blood assessment enabled filtering to remove germline variants. Somatic missense mutations in the tumour were used to define TMB, and the tertiles were defined as <143 mutations (low), 143–247 mutations (intermediate) and ≥248 mutations (high)7. When comparing outcomes among TMB tertiles of patients receiving nivolumab monotherapy, ORRs were 5%, 7% and 21% in the low (n = 42), intermediate (n = 44) and high (n = 47) TMB tertiles, respectively; median PFS durations were 1.3 months, 1.3 months and 1.4 months; median OS durations were 3.1 months, 3.9 months and 5.4 months; 1-year PFS was not evaluable, 3% and 21%; and 1-year OS was 22%, 26% and 35%, respectively. An analysis of data from the nivolumab 1 mg/kg and ipilimumab 3 mg/kg arm of CheckMate 032 reveals similar findings. Divided among the same TMB tertiles of low (n = 27), intermediate (n = 25) and high (n = 26), ORRs were 22%, 16% and 46%, respectively; median PFS duration was 1.5 months, 1.3 months and 7.8 months; median OS was 3.4 months, 3.6 months and 22 months; 1-year PFS was 6%, 8% and 30%; and 1-year OS was 23%, 20% and 62%, respectively7. Although these findings have not been prospectively validated, the WES-based TMB measurements from CheckMate 032 correlated well with the findings of an in silico analysis of TMB determined using the Foundation One CDx assay that included a limited gene set8,51. Additionally, the findings of CheckMate 032 revealed no statistically significant associations between tumour PD-L1 expression determined using the 28-8 pharmDx antibody and TMB7.
TMB determined using WES was also evaluated as a predictive biomarker in an analysis of formalin-fixed paraffin-embedded tumour and matched non-malignant tissue specimens from patients who received pembrolizumab in KEYNOTE-028. In this evaluation, all somatic nonsynonymous mutations (a slightly broader definition than missense mutations, which also includes nonsense mutations) were included48. Similar to Checkmate 032, limited correlations were observed between TMB and PD-L1, although a statistically significant correlation between TMB, ORR and median PFS was reported48. Drawing reliable conclusions about TMB as a predictive biomarker from this analysis is difficult owing to the small number of patients with SCLC in this cohort.
Blood-based tumour mutational burden.
bTMB has only been evaluated as a predictive biomarker of responsiveness to ICIs in patients with treatment-naive SCLC (TABLE 2). In IMPower 133, investigators used a bTMB quantification technique identical to that used to demonstrate the predictive value of bTMB for PFS in patients receiving atezolizumab for the treatment of relapsed NSCLC3,52. This approach involved next-generation sequencing-based assessments of 394 cancer-associated genes52,53. Germline variants were filtered using the dbSNP and ExAC genomic databases, and somatic single-nucleotide variants in these cancer-associated genes were tallied to calculate a bTMB score in terms of mutations per megabase52. Two cut-offs of bTMB were used, 10 mut/Mb and 16 mut/Mb, with conflicting results. Among the 139 patients with TMBs of <10 mut/Mb, a trend towards improved median OS was observed in patients receiving chemotherapy plus atezolizumab compared with chemotherapy alone (11.8 months versus 9.2 months; HR 0.70, 95% CI 0.45–1.07). Among the 212 patients with bTMBs of ≥10 mut/Mb, a substantial improvement in median OS was observed in those receiving chemotherapy plus atezolizumab compared with chemotherapy alone (14.6 months versus 11.2 months; HR 0.68, 95% CI 0.47–0.97). Using a higher bTMB cut-off, among 271 patients with a bTMB of <16 mut/Mb, a significant improvement in median OS was also observed with chemotherapy plus atezolizumab (12.5 months versus 9.9 months; HR 0.71, 95% CI 0.52–0.98). A nonsignificant trend towards improved OS in patients receiving chemotherapy plus atezolizumab was also observed among the 80 patients with a bTMB of ≥16 mut/Mb (17.8 months versus 11.9 months; HR 0.63, 95% CI 0.35–1.15). Given the similar improvements in OS across bTMB subgroups, bTMB is not thought to be predictive of clinical benefit from atezolizumab plus chemotherapy. Caution should be applied in interpreting the significance of these data owing to limited patient numbers, particularly in those with bTMB of ≥16 mut/Mb3 (TABLE 2).
Collectively, data from these studies3,5,48 suggest that tumour-assessed TMB might have some predictive value in patients with relapsed SCLC, although bTMB is not consistently predictive of OS in treatment-naive patients receiving ICIs in combination with chemotherapy. This observation regarding the predictive value of TMB in the treatment-naive setting for patients receiving chemotherapy plus an ICI seems to hold true for both patients with SCLC and those with NSCLC. Data from a retrospective analysis of samples obtained from patients with nonsquamous NSCLC enrolled on KEYNOTE-189 and KEYNOTE-021 indicate a lack of predictive value of TMB for both response and OS in those receiving a platinum-containing agent, pemetrexed and pembrolizumab54,55.
Clinical parameters as biomarkers
Data regarding the validity of clinical predictors of OS benefit from ICIs in patients with SCLC are currently limited to subgroup analyses. The only replicated finding from these analyses has been that patients with liver metastases, regardless of tumour histology, seem not to derive the same level of improvement in OS from ICIs as those without liver metastasis.
Liver metastasis.
In the treatment-naive setting, the presence or absence of liver metastasis has been evaluated as a predictive biomarker in patients with ES-SCLC receiving ICIs. In IMPower 133, a statistically significant OS benefit was observed in 254 patients without liver metastasis who received chemotherapy plus atezolizumab compared with those who received chemotherapy alone (median OS 16.8 months versus 11.2 months; HR 0.64, 95% CI 0.45–0.90). The same difference in the level of benefit was not observed among 145 patients with liver metastases (median OS 9.3 months versus 7.8 months; HR 0.81, 95% CI 0.55–1.20)3. In the phase III RCT evaluating the efficacy of chemotherapy plus ipilimumab versus that of chemotherapy alone in patients with treatment-naive ES-SCLC, no significant differences in OS were detected based on either the presence or absence of liver metastases33.
The presence or absence of liver metastasis has not been identified as a predictor of OS among patients receiving ICI in the maintenance setting41. Although, in CheckMate 331, the subgroup of 364 patients with relapsed SCLC without liver metastasis were again observed to have a significant improvement in OS from nivolumab compared with chemotherapy (median OS 11.2 months versus 10.5 months; HR 0.75, 95% CI 0.59–0.95)43, the same level of OS benefit from nivolumab was not observed among 205 patients with liver metastases (median OS 3.9 months versus 5.9 months; HR 1.34. 95% CI 0.99–1.80)43.
Various other clinical predictors, including age3,33,41,43, gender3,33,41,43,45, ethnicity (white versus Asian)43, performance status (1 versus 0)3,33,41,43,45, platinum sensitivity5,43,45, elevated serum levels of lactate dehydrogenase33,41,43, best response to induction chemotherapy (partial or complete response versus stable disease)41, disease stage at diagnosis (limited versus extensive stage)43,45, presence of central nervous system metastases3,33, number of previous lines of therapy5, previous prophylactic cranial irradiation41 and time from completion of induction chemotherapy41, do not consistently predict either response or OS duration in patients with SCLC receiving ICIs (TABLE 3).
Table 3 |.
Clinical features predictive of overall survival benefit
| Feature | Median OS |
|---|---|
| Upfront | |
| Age | Inconsistent predictive value3,33 |
| Gender | Not predictive3,33 |
| Performance status | Inconsistent predictive value3,33 |
| LDH | Not predictive33 |
| CNS metastasis | Inconsistent predictive value3,33 |
| Liver metastasis | Possibly predictive3,33 |
| First-line maintenance | |
| Best response to induction | Not predictive41 |
| Age | Inconsistent predictive value41 |
| Gender | Not predictive41 |
| Performance status | Not predictive41 |
| LDH | Not predictive41 |
| Liver metastasis | Not predictive41 |
| Previous PCI | Not predictive41 |
| Time from induction chemotherapy | Inconsistent predictive value41 |
| Second line or later | |
| Platinum sensitivity | Inconsistent predictive value43,45 |
| Previous lines of therapy | Not predictive5 |
| Gender | Not predictive43,45 |
| Performance status | Not predictive43,45 |
| LDH | Inconsistent predictive value43 |
| CNS metastasis | Not predictive43 |
| Liver metastasis | Possibly predictive43 |
| Ethnicity | Not predictive43 |
| Stage at diagnosis | Not predictive43,45 |
CNS, central nervous system; LDH, lactate dehydrogenase; OS, overall survival; PCI, prophylactic cranial irradiation.
Future directions
Upfront therapy.
Two trials designed to provide data on the safety and/or efficacy of upfront ICIs are currently ongoing: a phase I, single-arm trial designed to evaluate the effects of pembrolizumab plus standard-of-care chemoradiotherapy followed by pembrolizumab maintenance therapy for 48 weeks in patients with LS-SCLC or ES-SCLC (NCT02402920); and a phase II/III NRG Oncology (NRG-LU005) RCT designed to evaluate the efficacy of standard-of-care concurrent chemoradiotherapy versus that of concurrent chemoradiotherapy plus atezolizumab followed by atezolizumab maintenance therapy for 1 year in patients with LS-SCLC (NCT03811002). In patients with ES-SCLC, in a similar manner to IMPower 133, three RCTs evaluating the efficacy of adding ICI monotherapy in the upfront setting are currently ongoing (nivolumab in ECOG-ACRIN 5161 (NCT03382561); pembrolizumab in EORTC REACTION (NCT02580994); and pembrolizumab in KEYNOTE-604 (NCT03066778)).
The safety and/or efficacy of front-line standard-of-care chemotherapy plus combination ICI therapy with durvalumab and the anti-CTLA-4 antibody tremelimumab is being evaluated in a single-arm, multihistology, phase Ib study that includes patients with ES-SCLC (NCT02658214) and in patients with ES-SCLC in a three-arm, phase III RCT (CASPIAN (NCT03043872)).
First-line maintenance monotherapy or combination therapy.
Two trials designed to evaluate the efficacy of only maintenance use of ICIs in patients with LS-SCLC without disease progression during induction chemoradiotherapy (nivolumab plus ipilimumab in STIMULI (NCT02046733); durvalumab and/or tremelimumab in ADRIATIC (NCT03703297)) are currently ongoing (Supplementary Table 1). Investigators in these trials are seeking to replicate the positive results of the PACIFIC trial involving patients with stage III NSCLC, in whom improvements in microscopic residual disease control resulted in statistically significant improvements in OS in a similar patient population who have a high risk of disease recurrence.
Second-line monotherapy.
One RCT designed to evaluate the efficacy of pembrolizumab versus topotecan as second-line therapy in unselected patients with relapsed SCLC with either platinum-sensitive or platinum-resistant disease, with no requirement of PD-L1 expression, is currently ongoing (AFT-17 (NCT02963090)) (Supplementary Table 1). Of note, previous RCTs comparing the efficacy of ICIs, such as nivolumab43 and atezolizumab45, with that of chemotherapy in this setting have failed to demonstrate improvements in OS compared with chemotherapy.
Second-line or later combination therapy.
In one RCT, patients with relapsed SCLC (either platinum-sensitive or platinum-resistant disease) receiving combination durvalumab and tremelimumab are being randomized to receive stereotactic body radiotherapy or no radiotherapy (NCT02701400). A phase I, open-label, multiarm trial exploring the combination of the anti-CTLA-4 antibody MK-1308 with pembrolizumab in patients with advanced-stage solid tumours, including relapsed SCLC, is currently recruiting patients (NCT03179436) (Supplementary Table 1).
Second-line or later novel agents plus ICIs.
Few therapeutic options have been able to provide a >20–30% ORR in patients with relapsed SCLC, and clinical benefits are often short-lived. Thus, owing to these major unmet needs, the efficacy of ICIs in combination with a variety of novel agents is currently being investigated in this setting (FIG. 2). In a phase II, three-arm trial comparing ICIs to novel agents, patients with ES-SCLC who either had disease progression during induction therapy or relapse within 90 days are being assigned to either four cycles of durvalumab plus tremelimumab, followed by durvalumab maintenance therapy until relapse if they have not previously received an ICI, a WEE1 tyrosine kinase inhibitor (adavosertib) in combination with carboplatin, or an ATR inhibitor (ceralasertib) in combination with the poly(ADP-ribose) polymerase inhibitor olaparib, based on specific contraindications rather than molecular features (NCT02937818). KEYNOTE-603 is a phase I trial designed to evaluate the combination of pembrolizumab with a tumour neoantigen-based cancer vaccine (mRNA-4157; in which exogenous mRNAs are administered with the aim of translation and subsequent presentation of peptide epitopes by antigen-presenting cells), and is currently recruiting patients with resectable or unresectable solid tumours, including SCLC (NCT03313778). Durvalumab is being combined with a variety of novel agents in the relapsed setting. The safety and efficacy of durvalumab are being investigated in combination with olaparib in patients with relapsed, platinum-sensitive SCLC in a phase I/II trial (NCT02734004).
Fig. 2 |. Mechanisms of action of immunotherapies and other novel agents being tested in combination with immunotherapies in patients with small-cell lung cancer.
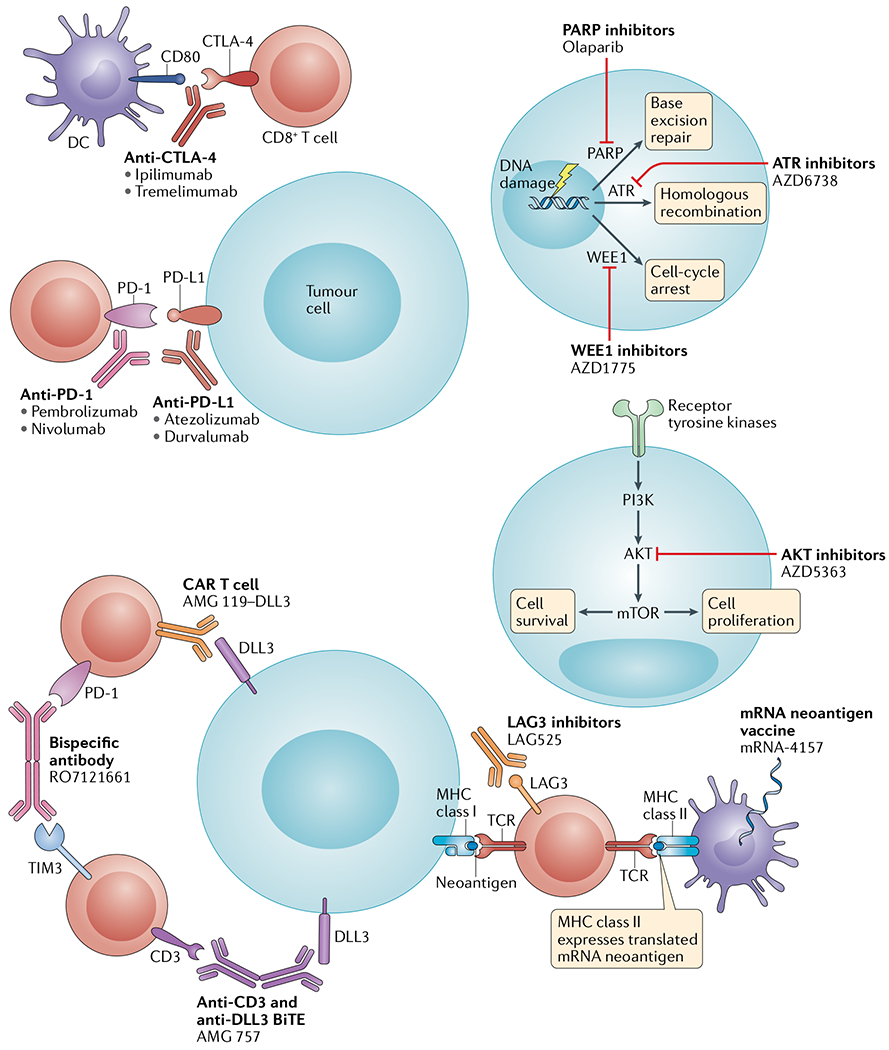
Immunotherapies and other novel agents currently being evaluated in combination with immunotherapies in patients with small-cell lung cancer include immune-checkpoint inhibitors (anti-programmed cell death protein 1 (PD-1), anti-programmed cell death 1 ligand 1 (PD-L1), anti-cytotoxic T lymphocyte protein 4 (CTL A-4), anti-L AG3 and anti-TIM3 antibodies), bispecific antibodies (such as those targeting CD3 plus DLL3 or PD-1 plus TIM3), engineered T cell therapies (such as anti-DLL3 chimeric antigen receptor (CAR) T cells), neoantigen vaccines, antiproliferative agents (AKT inhibitors) and DNA damage repair-directed therapies (poly(ADP-ribose) polymerase (PARP) inhibitors, serine/threonine-protein kinase ATR (ATR) inhibitors and Wee1-like protein kinase (WEE1) inhibitors). BiTE, bispecific T cell engager ; DC, dendritic cell; TCR , T cell receptor.
Novel immunotherapies.
CAR T cells are T cells transduced with a specific, often tumour-associated cell-surface antigen-directed recombinant receptor, containing costimulatory transmembrane domains that promote replication and antitumour activity. In contrast to cellular immunotherapies with transduced T cell receptors, which require host cell antigen processing and MHC presentation and matching for recognition of both cell-surface and intracellular antigens56, CAR T cells are able to engage the target cell-surface antigen independent of MHC expression. CAR T cells targeting the SCLC antigen delta-like protein 3 (DLL3) have entered a phase I clinical trial (AMG 119 (NCT03392064)). Thus far, CAR T cells have demonstrated efficacy in patients with several forms of haematological cancer, although this approach has not achieved promising results in early trials in patients with solid tumours. This early lack of efficacy of CAR T cells in patients with solid tumours most likely reflects the difficulties in assuring that CAR T cells are able to come into contact with solid tumour cells within their respective organs and/or immunosuppressive microenvironments57.
BiTEs are recombinant proteins that contain antibody variable fragments directed at both a T cell surface protein (often CD3) and a tumour-associated cell-surface protein, thereby colocalizing host T cells and tumour cells rather than relying on clearance by the host immune system or direct antitumour effects, similar to monoclonal antibodies58. AMG 757, a BiTE consisting of both anti-CD3 and anti-DLL3 antibodies, is being evaluated in a phase I trial that includes patients with ES-SCLC requiring first-line maintenance therapy and those with recurrent SCLC (NCT03319940). ICIs targeting alternative immune checkpoints to PD-1 or CTLA-4, such as TIM3 and LAG3, have entered clinical trials in combination with anti-PD-1 or anti-PD-L1 antibodies in patients with advanced-stage and/or metastatic solid tumours, including relapsed SCLC (RO7121661 (NCT03708328) and LAG525 (NCT03365791), respectively) (Supplementary Table 1).
Conclusions
Progress has finally been made in the treatment of patients with SCLC with the FDA approval of atezolizumab in combination with chemotherapy in the front-line setting for patients with ES-SCLC based on data from IMPower 133 (REFS3,36) and in relapsed SCLC based on data from CheckMate 032 (REF.5), KEYNOTE-028 (REF.11) and KEYNOTE-158 (REF.44). In the first-line setting, the approval is based on data from an RCT showing survival benefit compared with patients treated with chemotherapy alone, and, in the third-line setting, the approvals are based on promising response rates in a setting in which, despite extensive research efforts, limited treatment options exist. On the basis of data from CheckMate 032 (REF.5), CheckMate 331 (REF.43) and recommendations from the National Comprehensive Cancer Network guidelines59, we advise consideration of the use of ICIs as second-line therapies in patients with platinum-resistant, relapsed SCLC who did not receive an ICI in the first line, because evidence indicates that these patients might derive benefit from nivolumab monotherapy43. In patients with initially platinum-sensitive LS-SCLC, we recommend that ICIs are reserved for third-line use after rechallenge with a platinum-containing agent or topotecan.
The clinical data generated so far have been accompanied by an interest in identifying biomarkers that are predictive of benefit from ICIs in patients with SCLC. Thus far, the limited expression (tumour PD-L1 expression in <20% of patients5–8,34) and inconsistent predictive value5,7,9 of PD-L1 have precluded its adoption as a widely used biomarker of response. TMB has shown some value as a predictor of response and OS in the relapsed setting5,48, although bTMB was not predictive of OS benefit in IMPower 133 with statistically similar OS benefits observed on both sides of two bTMB cut-off values of 10 mut/Mb and 16 mut/Mb3. Differences in the primary source of material for analysis (tumour versus blood) and analysis technique (WES versus targeted gene panels) are important; therefore, TMB remains a potential predictive marker for further investigation. Nonetheless, the challenge with SCLC remains obtaining sufficient tissue to perform analyses involving solid tumour material. At present, other potentially predictive biomarkers, such as tumour–stromal PD-L1 expression, CTCs and TIL signatures, have only been evaluated in small cohorts of patients, thus precluding the development of robust conclusions regarding their predictive value9,48. Similarly, caution should be used in interpreting the presence or absence of liver metastases in guiding the use of ICIs, as the validity of this predictor has only been evaluated in subset analyses3,33,41,43. Ultimately, evaluating potential biomarkers that use multifaceted scores, such as a combination of data on TILs, PD-L1 and TMB plus other yet-to-be-defined factors integrated across multiple tumour histologies, might provide the best way forward given that the breadth of benefits provided by anti-PD-1 or anti-PD-L1 antibodies, in terms of tumour histologies that respond to these agents, is unprecedented48.
Ongoing and future clinical trials for patients with SCLC, involving ICIs and/or other immunotherapies, will evaluate approaches similar to IMPower 133, add treatment scenarios that have not been evaluated using ICIs (such as the maintenance setting in patients with limited-stage disease), further evaluate combination ICIs, evaluate combinations of ICIs with novel targeted therapies (such as poly(ADP-ribose) polymerase inhibitors, AKT1 inhibitors and ATR inhibitors) and test novel immune-based treatment strategies (such as CAR T cells and BiTEs). In order to fully understand the optimal role of immunotherapy in patients with SCLC, these clinical trial results must be followed closely. While progress is being made in using immunotherapies to treat patients with SCLC, a substantial amount of research remains to be done in identifying the optimal therapeutic strategies and predictive biomarkers, as well as developing effective treatment strategies for patients who have disease progression on ICIs.
Supplementary Material
Key points.
Immune-checkpoint inhibitors (ICIs) are approved as first-line and third-line therapies for patients with advanced-stage small-cell lung cancer (SCLC).
In the first-line setting, the anti-programmed cell death 1 ligand 1 ICI atezolizumab plus chemotherapy has been shown to improve overall survival, relative to chemotherapy alone.
In the relapsed setting, nonrandomized data reveal promising responses to several ICIs that have not been corroborated in randomized trials.
No broadly accepted biomarkers that predict benefit from ICI have been identified to date.
Many ongoing trials are evaluating the performance of immune-based treatment strategies in patients with SCLC; these will hopefully enable the optimization of immune-based treatment strategies in this patient population.
Acknowledgements
W.T.I. acknowledges support from the National Institutes of Health and National Cancer Institute Vanderbilt Clinical Oncology Research Career Development Award (VCORCDP) 2K12CA090625-17 and an American Society of Clinical Oncology/Conquer Cancer Foundation Young Investigator Award.
Footnotes
Competing interests
W.T.I. has acted as a consultant for Defined Health, Genentech and Outcomes Insights. L.H. reports clinical trial funding from BMS, Boehringer Ingelheim and Xcovery; and has acted as a consultant for AbbVie, AstraZeneca, EMD Serono, Incyte, Merck, Pfizer, Roche-Genentech, Tesaro and Xcovery. J.P declares no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-019-0316-z.
References
- 1.Bernhardt EB & Jalal SI Small cell lung cancer. Cancer Treat. Res 170, 301–322 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF & Minna JD Developing new, rational therapies for recalcitrant small cell lung cancer. J. Natl Cancer Inst 108, djw119 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Horn L et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med 379, 2220–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17, 883–895 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Schultheis AM et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur. J. Cancer 51, 421–426 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33, 853–861.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boumber Y Tumor mutational burden (TMB) as a biomarker of response to immunotherapy in small cell lung cancer. J. Thorac. Dis. China 10, 4689–4693 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadgeel SM et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J. Thorac. Oncol 13, 1393–1399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarchoan M, Hopkins A & Jaffee EM Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med 377, 2500–2501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott PA et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol 35, 3823–3829 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Carter L et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med 23, 114–119 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Rizvi H et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol 36, 633–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak AK et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol 170, 4905–4913 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Nowak AK, Robinson BW & Lake RA Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemoimmunotherapy. Cancer Res. 62, 2353–2358 (2002). [PubMed] [Google Scholar]
- 16.van der Most RG et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol. Immunother 58, 1219–1228 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonanno L et al. The role of immune microenvironment in small-cell lung cancer: distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur. J. Cancer 101, 191–200 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Wang H et al. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn. Pathol 13, 30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghoff AS et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J. Neurooncol 130, 19–29 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Iams WT et al. Improved prognosis and increased tumor-infiltrating lymphocytes in patients who haveSCLC with neurologic paraneoplastic syndromes. J. Thorac. Oncol 14, 1970–1981 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii H et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J. Thorac. Oncol 10, 426–430 (2015). [DOI] [PubMed] [Google Scholar]
- 22.George J et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirier JT et al. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 34, 5869–5878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YH et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 32, 915–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney DN et al. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 45, 2913–2923 (1985). [PubMed] [Google Scholar]
- 26.Wooten DJ et al. Systems-level network modeling of small cell lung cancer subtypes identifies master regulators and destabilizers. Preprint at bioRxiv 10.1101/506402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McColl K et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 8, 73745–73756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirier JT et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J. Natl Cancer Inst 105, 1059–1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borromeo MD et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 16, 1259–1272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mollaoglu G et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 31, 270–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudin CM et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer 19, 289–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wargo JA, Reuben A, Cooper ZA, Oh KS & Sullivan RJ Immune effects of chemotherapy, radiation, and targeted therapy and opportunities for combination with immunotherapy. Semin. Oncol 42, 601–616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck M et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol 34, 3740–3748 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Reck MLS et al. IMPower133: updated overall survival (OS) analysis of first-line (1L) atezolizumab (atezo) + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann. Oncol 30, mdz264 (2019). [Google Scholar]
- 35.Paz-Ares L et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394, 1929–1939 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Paz-Ares L et al. Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. J. Thorac. Oncol 14, S7–S8 (2019). [Google Scholar]
- 37.Rossi A et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J.Clin. Oncol 30, 1692–1698 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Darnell RB & Posner JB Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med 349, 1543–1554 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Gandhi L et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med 378, 2078–2092 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Paz-Ares L et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med 379, 2040–2051 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Owonikoko TK et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study (LBA1_PR). Ann. Oncol 30, mdz094 (2019). [Google Scholar]
- 42.Ready N et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J. Thorac. Oncol 14, 237–244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reck MVD et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann. Oncol 29 (suppl. 10), x39–x43 (2018). [Google Scholar]
- 44.Chung et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol 36, 8506 (2018). [Google Scholar]
- 45.Pujol JL et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J. Thorac. Oncol 14, 903–913 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Ready NE et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J. Thorac. Oncol 10.1016/j.jtho.2019.10.004 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Tsao MS et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J. Thorac. Oncol 13, 1302–1311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ott PA et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol 37, 318–327 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Ayers M et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade J. Clin. Invest 127, 2930–2940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danaher P et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from The Cancer Genome Atlas (TCGA). J. Immunother. Cancer 6, 63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frampton GM et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol 31, 1023–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandara DR et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med 24, 1441–1448 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Chalmers ZR et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garassino M et al. KEYNOTE 189: tumor mutation burden not significantly associated with efficacy of pembrolizumab. Presented at IASLC World Conference on Lung Cancer 2019. [Google Scholar]
- 55.Langer C et al. KEYNOTE 021: tumor mutation burden not significantly associated with efficacy of pembrolizumab. Presented at IASLC World Conference on Lung Cancer 2019. [Google Scholar]
- 56.Sadelain M, Brentjens R & Riviere I The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Springuel L et al. Chimeric antigen receptor-T cells for targeting solid tumors: current challenges and existing strategies. BioDrugs 33, 515–537 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slaney CY, Wang P, Darcy PK & Kershaw MH CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 8, 924–934 (2018). [DOI] [PubMed] [Google Scholar]
- 59.NCCN Guidelines: Small Cell Lung Cancer Version 1 (National Comprehensive Cancer Network, 2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


