Abstract
Tumors have long been compared to chronic wounds that do not heal, since they share many of the same molecular and cellular processes. In normal wounds, healing processes lead to restoration of cellular architecture, while in malignant tumors these healing processes become dysregulated and contribute to growth and invasion of neoplastic cells into the surrounding tissues. Fibrocytes are fibroblast-like cells that differentiate from bone marrow-derived CD14+ circulating monocytes and aid wound healing. Although most monocytes will differentiate into macrophages after extravasating into a tissue, signals present in a wound environment can cause some monocytes to differentiate into fibrocytes. The fibrocytes secrete matrix proteins and inflammatory cytokines, activate local fibroblasts to proliferate and increase extracellular matrix production, promote angiogenesis, and because fibrocytes are contractile, they also help wound contraction. There is now emerging evidence that fibrocytes are present in the tumor microenvironment, attracted by the chronic tissue damage and cytokines from both cancer cells and other immune cells. Fibrocytes may aid in the survival and spread of neoplastic cells, so these wound healing cells may be a promising target for anti-cancer research in future studies.
Keywords: Tumor, Microenvironment, Stroma, Cancer, Fibrocyte, Fibroblast, Myofibroblast, Monocyte, Scar, Wound, Fibrosis, Benign, Malignant, Metastasis
Introduction
A major advance in the understanding of tumor biology came in 1986 when Dvorak et al. published an essay detailing the similarities between tumors and chronic wounds that do not heal.1 In the wound environment, repair of tissue damage was originally thought to be dependent upon fibroblasts which were quiescent cells resident in the local tissues that only became activated after tissue damage occurred. These local cells would then migrate to the site of injury, proliferate, and engage in tissue repair. Another school of thought held the possibility that the wound environment is populated in part by cells that originated from distant sites. Sir James Paget in his “Lectures on Surgical Pathology” first published the observation of circular mononuclear cells entering a wound site from the blood and transforming themselves into long spindle-shaped cells in the wound bed.2 These cells were characterized in 1994 when Bucala et al. discovered the presence of large numbers of fibroblast-like cells coinciding with the entry of circulating inflammatory cells in wound chamber experiments. These cells, termed fibrocytes, were found to enter sites of sites of tissue injury and contribute to connective tissue and scar formation.3
Over the past 25 years there has been a significant amount of progress on research of the differentiation and roles of fibrocytes in physiologic and pathologic processes. Fibrocytes differentiate from a CD14+ peripheral blood monocyte precursor population.4 Fibrocytes express both hematopoietic (CD45, MHC II, CD34) and connective tissue markers (Collagen I and III and fibronectin).3,4 Mature fibrocytes secrete inflammatory cytokines and extracellular matrix proteins to promote angiogenesis and wound contraction.5,6 Fibrocytes can be specifically identified in culture by their unique co-expression of CD45RO, 25F9, and S100A8/A9, but not PM-2K. Fibrocytes also change expression of some markers with longer time in culture after differentiation from monocytes, including a loss of CD34, increased expression of Mac-2/galectin 3, and expression of CD49c.7 Fibrocytes can further become activated by local inflammatory signals such as TGFβ and begin expressing α-smooth muscle actin, transitioning into myofibroblasts, as has been shown in bronchial asthma.8 Fibrocytes are antigen presenting cells, participating in parts of the innate response to tissue damage and invasion.9 Since their identification, fibrocytes have been found in a variety of disease processes, including aberrant wound repair such as hypertrophic scarring and keloid formation, fibrosing diseases such as idiopathic pulmonary fibrosis and myelofibrosis, and autoimmunity.10–15 More recently, studies have also identified fibrocytes in neoplastic processes. In this chapter, we briefly review our understanding fibrocytes in the tumor microenvironment.
Fibrocytes in benign tumors
One of the first studies that described CD34 positive spindle shaped cells in benign and malignant tumors was the pathological study of skin lesions by Kirchmann et al. in 1994, the same year as the discovery of fibrocytes by Bucala and colleagues.16 CD34 positive spindle shaped cells, which were likely fibrocytes, were found around trichoepithelioma, a benign skin tumor with follicular differentiation. Basal cell cancers were also surrounded by similar spindle cells, however the spindle-shaped cells did not appear to express CD34, leading the authors to conclude that a loss of CD34 expression was an indicator of malignancy.16 This was a finding that was reproduced in many studies to come. Fibrocytes identified by spindle shaped morphology and CD34 expression were observed surrounding breast ducts containing intraepithelial hyperplasia, ductal carcinoma in situ (DCIS), fibroadenomas, and phyllodes tumors.17 Of note, there was an observed loss of CD34 positivity as DCIS specimens were higher in grade, and a variable expression of αSMA in the different tumors.17 Given these pathologic observations, one hypothesis is that local factors from the tumor influence the further differentiation of a fibrocyte into a myofibroblast, including loss of CD34 and gain of αSMA. As we will discuss more in the next section, the mechanisms and prognostic effects of this transition are not known at this time, but this staining pattern may be a method of differentiating benign from malignant processes. CD34 positive spindle shaped cells have been observed to be interspersed in benign tumors of adipose tissue such as lipomas, angiolipomas, angiomyolipomas, and intramuscular lipomas.18 Although this study did not specify that these cells were fibrocytes, the presence of CD34 staining on a spindle shaped cell strongly suggests that it is a fibrocyte.
Fibrocytes in malignant tumors
In 1997, Suster et al. reported one of the earliest descriptions of what were likely fibrocytes in malignant tumors.18 They described CD34+ spindle shaped cells that were present not only on benign fatty tumors but also on locally aggressive and malignant fatty tumors such as atypical lipomatous tumors, well-differentiated liposarcomas, myxoid liposarcomas, and the sarcomatous component of dedifferentiated liposarcomas.18 Since then, multiple studies that we will describe below found CD34+ fibrocytes in malignancies. These studies also often observed that the spindle-shaped cells show less CD34 expression in proximity to the tumor, and more expression of αSMA.17,19–24 This may in fact reflect the transition of a fibrocyte to a myofibroblast in reaction to cytokines expressed by the tumor. This pattern has been found in pancreatic cancer, ductal carcinoma in situ and invasive ductal carcinoma of the breast, invasive lobular carcinoma of the breast, high grade cervical intraepithelial neoplasia and squamous cell carcinoma of the cervix, squamous cell carcinomas of the oropharynx and larynx, and urothelial carcinoma of the bladder.17,19–23,25,26 Figure 1 shows immunofluorescent staining of fibrocytes in pancreatic adenocarcinoma and invasive ductal carcinoma of the breast. Instead of staining for CD34, we identified fibrocytes by colocalization of Collagen-I and CD45RO. We have found this to be a more accurate method to identify fibrocytes, as we and other groups have found CD34 expression to be lost as fibrocytes become more mature and activated.
Figure 1:
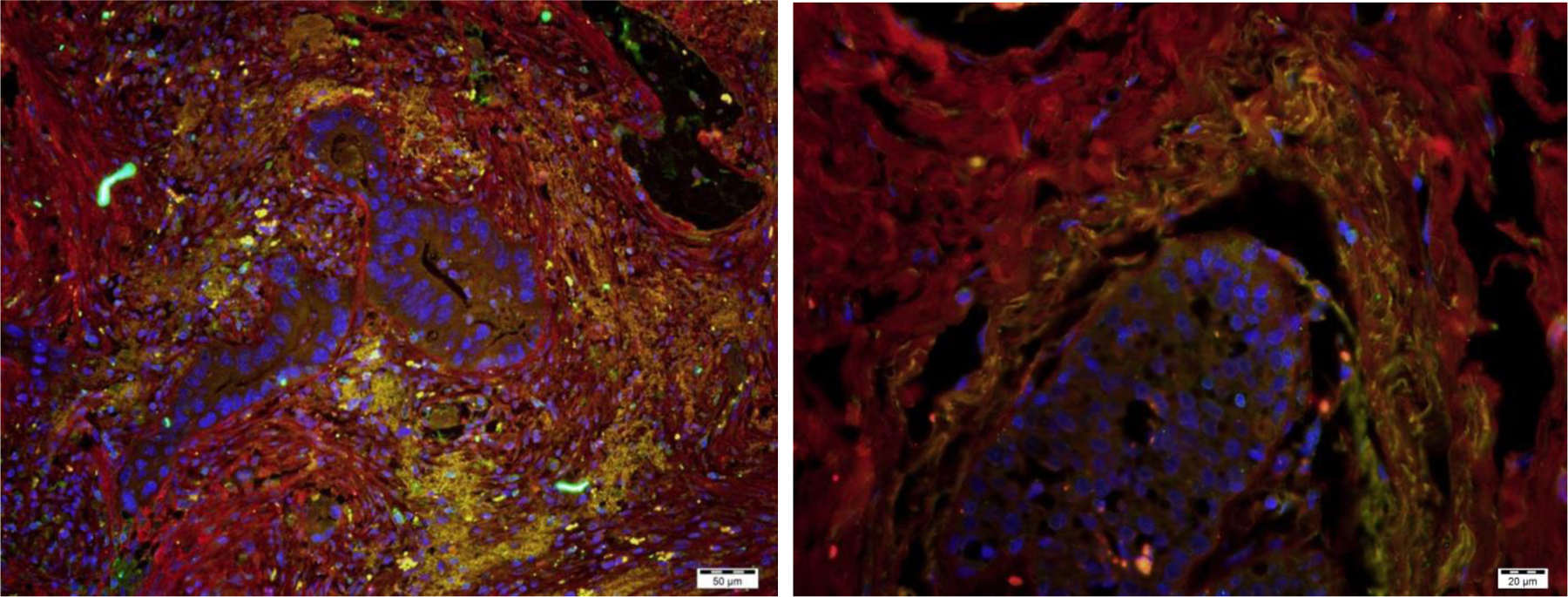
Fibrocytes surrounding neoplastic ducts in pancreatic adenocarcinoma (left) and invasive ductal carcinoma of the breast (right). The fibrocytes are identified by co-expression (yellow) of Collagen-I (red) and CD45RO (green). DAPI staining of nuclei is shown in blue. Bar is 50 μm on left, 20 μm on right.
To understand the possible of contribution of fibrocytes to tumor growth, we will discuss the stroma of pancreatic adenocarcinoma in more detail, as it is known to have a strong desmoplastic component, in that the majority of the tumor volume is fibrotic stroma rather than neoplastic cells.27 The tumor cells of solid tumors such as pancreatic cancer attract fibrocytes through paracrine signaling. These solid tumors produce Th2 cytokines such as interleukin-4 and 13, and high expression of these cytokines have been linked to cancer cell survival, invasion, and metastasis, and poor prognosis.27–32
We previously found that Th2 cytokines such as IL-4 and IL-13 promote fibrocyte differentiation from monocytes.34 This desmoplastic reaction also promotes angiogenesis, resulting in numerous disorganized, small, leaky blood vessels and capillaries that provide the tumor cells with oxygen.35 This environment created by the tumor cells and the tumor stroma drives forward its own progression in a positive feedback cycle by production of factors such as transforming growth factor β (TGF-β), matrix-metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and fibroblast growth factor (FGF).36 In pancreatic ductal adenocarcinoma, α-SMA+ myofibroblasts form thick sheets surrounding the infiltrating tumor cells. In the pancreatic tissue surrounding the tumor, there is a local concentration of CD34+ fibrocytes around the infiltrating border of the carcinoma. The gradient from tumor-free tissue to invasive carcinoma reveals an abrupt loss of the CD34 positivity.19 One explanation for this could be that after CD34+ fibrocytes are recruited to the edge of the expanding tumor, they begin secreting ECM and become trapped by abundant ECM and new fibrocytes, myofibroblasts, and stellate cells being recruited around them. Once they are trapped and come under the influence of paracrine signals such as TGF-β, they lose CD34 expression and increase α-SMA expression, thereby becoming a contractile myofibroblast and secreting even greater amounts of ECM in a futile attempt to seal this ever growing wound environment, unknowingly also contributing to tumor survival by secreting growth factors and promoting angiogenesis. Fibrocytes secrete many of the growth factors found in the tumor microenvironment, such as PDGF, VEGF, TGF-β, MMP-9, and FGF, and these could enhance neoplastic cell proliferation by a paracrine effect.36,37 ECM proteins such as collagen and fibronectin that are produced by fibrocytes have been shown to contribute to proliferation, survival, metastasis, and drug resistance of cancer cells.38–45 Bone marrow derived fibrocytes were also found to be the source of cancer-associated fibroblasts (CAFs) in a mouse model of gastric cancer, and caused significantly larger tumors when inoculated along with the cancer cell line.46
Fibrocytes also appear to contribute to resistance of cancer to therapeutic drugs. In mouse models of malignant pleural mesothelioma and lung cancer, fibrocytes were found to mediate resistance to bevacizumab, an FDA-approved VEGF inhibitor used as a treatment for several cancers, by production of fibroblast growth factor 2 (FGF2).47
Fibrocytes in metastasis
Following in his aforementioned father’s footsteps, the surgeon Stephen Paget contributed a profound theory to cancer research in his “seed and soil” hypothesis. He suggested that the metastasis of cancer is not coincidental and just a matter of random travel through blood vessels of lymphatics, but that a receptive microenvironment must be present for malignant cells to establish a metastatic tumor.48 Recent studies have shown that fibrocytes are one of the cell types that are necessary for tumor metastases. In a mouse model of melanoma, fibrocytes were found to prepare lungs for pulmonary metastases by recruiting Ly-6G monocytes, and the process was dependent on MMP9, CCL2, CCR2 and CCR5.49,50 Fibrocytes have also been observed in liver metastases in a mouse model of colon cancer, and in this study fibrocyte recruitment was dependent both on CCR1+ neutrophils and subsequent expression of MMP2 and MMP9.51 Another study found that myeloid-derived suppressor cells (MDSC) may ultimately be the source of fibrocytes in cancer metastases, and that by inhibiting monocyte differentiation from these MDSCs by knocking out Kruppel-like factor 4 (KLF4) in a mouse model, there were significantly less fibrocytes and myofibroblasts in the lungs and significantly fewer pulmonary metastases of melanoma and breast cancer cell lines52.
In addition to being present in the stroma surrounding solid tumors, one study has identified higher numbers of fibrocytes in the circulating blood of metastatic cancer patients. These fibrocytes were identified as a novel subset of circulating MDSCs which were expanded in metastatic pediatric sarcoma patients but absent from healthy controls.53 These cells expressed previously described fibrocytes markers including CD34, CD45, HLA-DR, αSMA (for activated fibrocytes), Collagen I/V, MMP9, S100A8/A9, fibronectin, and LSP-1, and had the capability to produce ECM and promote angiogenesis, however these cells functioned as immune suppressors rather than antigen presenting cells as in healthy controls. In the same study, fibrocytes from cancer patients inhibited T-cell proliferation in vitro through production of indoleamine oxidase.53 Taken together, this work suggests that fibrocytes may have a role in global immune suppression in addition to their local effects in the tumor microenvironment, and as a result help metastatic tumor cells initiate new tumors.
Conclusion and Future Directions
Fibrocytes appear to play an important role in the tumor microenvironment, likely participating in most if not all of the hallmarks of cancer as described by Hanahan and Weinberg, which include activating invasion and metastasis, inducing angiogenesis, resisting cell death, sustaining proliferative signaling, and evading growth suppressors.54 As demonstrated by the recent successes with immunotherapy, the next frontier of cancer treatment is to continue learning how to harness the normal cells of the body in order to prevent and fight cancer growth. Further research in controlling fibrocytes and their signals for differentiation may become an attractive target for new methods of anti-cancer therapeutics.
Acknowledgements
We thank Darrell Pilling for helpful comments on the manuscript. This work was supported by NIH HL132919.
References
- 1.Flier JS, Underhill LH, Dvorak HF. Tumors: Wounds That Do Not Heal. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 2.Paget J Lectures on Surgical Pathology. Lindsay and Blakiston; 1871. [Google Scholar]
- 3.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71. [PMC free article] [PubMed] [Google Scholar]
- 4.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated Production of Type I Collagen and Inflammatory Cytokines by Peripheral Blood Fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral Blood Fibrocytes: Differentiation Pathway and Migration to Wound Sites. J Immunol. 2001;166(12):7556–7562. [DOI] [PubMed] [Google Scholar]
- 6.Hartlapp I, Abe R, Saeed RW, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15(12):2215–2224. doi: 10.1096/fj.01-0049com [DOI] [PubMed] [Google Scholar]
- 7.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of Markers that Distinguish Monocyte-Derived Fibrocytes from Monocytes, Macrophages, and Fibroblasts. PLoS One. 4(10). doi: 10.1371/journal.pone.0007475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380. [DOI] [PubMed] [Google Scholar]
- 9.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94(12):6307–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal SA, Sidgwick GP, Bayat A. Identification of fibrocytes from mesenchymal stem cells in keloid tissue: a potential source of abnormal fibroblasts in keloid scarring. Arch Dermatol Res. 2012;304(8):665–671. doi: 10.1007/s00403-012-1225-5 [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Scott PG, Dodd C, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2005;13(4):398–404. doi: 10.1111/j.1067-1927.2005.130407.x [DOI] [PubMed] [Google Scholar]
- 12.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149 [DOI] [PubMed] [Google Scholar]
- 13.Verstovsek S, Manshouri T, Pilling D, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016;213(9):1723–1740. doi: 10.1084/jem.20160283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum. 2012;64(11):3583–3593. doi: 10.1002/art.34589 [DOI] [PubMed] [Google Scholar]
- 15.Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–438. doi: 10.1210/jc.2009-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchmann TTT, Prieto VG, Smoller BR. CD34 Staining Pattern Distinguishes Basal Cell Carcinoma From Trichoepithelioma. Arch Dermatol. 1994;130(5):589–592. doi: 10.1001/archderm.1994.01690050057008 [DOI] [PubMed] [Google Scholar]
- 17.Chauhan H, Abraham A, Phillips JRA, Pringle JH, Walker RA, Jones JL. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003;56(4):271–276. doi: 10.1136/jcp.56.4.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suster S, Fisher C. Immunoreactivity for the Human Hematopoietic Progenitor Cell Antigen (CD34) in Lipomatous Tumors. Am J Surg Pathol. 1997;21(2):195. [DOI] [PubMed] [Google Scholar]
- 19.Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch. 2002;440(2):128–133. doi: 10.1007/s00428-001-0551-3 [DOI] [PubMed] [Google Scholar]
- 20.Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch. 2002;440(3):298–303. doi: 10.1007/s004280100530 [DOI] [PubMed] [Google Scholar]
- 21.Barth P, Ramaswamy A, Moll R. CD34 + fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch. 2002;441(6):564–568. doi: 10.1007/s00428-002-0713-y [DOI] [PubMed] [Google Scholar]
- 22.Barth PJ, Schenck zu Schweinsberg T, Ramaswamy A, Moll R. CD34+ fibrocytes, α-smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch. 2004;444(3):231–234. doi: 10.1007/s00428-003-0965-1 [DOI] [PubMed] [Google Scholar]
- 23.Nimphius W, Moll R, Olbert P, Ramaswamy A, Barth PJ. CD34+ fibrocytes in chronic cystitis and noninvasive and invasive urothelial carcinomas of the urinary bladder. Virchows Arch. 2007;450(2):179–185. doi: 10.1007/s00428-006-0347-6 [DOI] [PubMed] [Google Scholar]
- 24.Catteau X, Simon P, Vanhaeverbeek M, Noël J-C. Variable Stromal Periductular Expression of CD34 and Smooth Muscle Actin (SMA) in Intraductal Carcinoma of the Breast. PLOS ONE. 2013;8(3):e57773. doi: 10.1371/journal.pone.0057773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimsade S, Westhoff CC, Barth PJ. CD34+ fibrocytes are preserved in most invasive lobular carcinomas of the breast. Pathol - Res Pract. 2007;203(9):695–698. doi: 10.1016/j.prp.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy A, Moll R, Barth PJ. CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch Int J Pathol. 2003;443(4):536–540. doi: 10.1007/s00428-003-0855-6 [DOI] [PubMed] [Google Scholar]
- 27.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101(4):887–907. doi: 10.1002/jcb.21209 [DOI] [PubMed] [Google Scholar]
- 28.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69(22):8678–8685. doi: 10.1158/0008-5472.CAN-09-2100 [DOI] [PubMed] [Google Scholar]
- 29.Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, Torres S, Casal JI. High Expression of IL-13 Receptor α2 in Colorectal Cancer Is Associated with Invasion, Liver Metastasis, and Poor Prognosis. Cancer Res. 2012;72(11):2780–2790. doi: 10.1158/0008-5472.CAN-11-4090 [DOI] [PubMed] [Google Scholar]
- 30.Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer. 2012;131(2):344–356. doi: 10.1002/ijc.26366 [DOI] [PubMed] [Google Scholar]
- 31.Todaro M, Lombardo Y, Francipane MG, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15(4):762–772. doi: 10.1038/sj.cdd.4402305 [DOI] [PubMed] [Google Scholar]
- 32.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92(5):921. doi: 10.1038/sj.bjc.6602416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi BH, Leland P, Lababidi S, Varrichio F, Puri RK. Interleukin-4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med. 2014;3(6):1615–1628. doi: 10.1002/cam4.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–1333. doi: 10.1189/jlb.1107782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vong S, Kalluri R. The Role of Stromal Myofibroblast and Extracellular Matrix in Tumor Angiogenesis. Genes Cancer. 2011;2(12):1139–1145. doi: 10.1177/1947601911423940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186. [DOI] [PubMed] [Google Scholar]
- 37.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. [DOI] [PubMed] [Google Scholar]
- 38.Sawai H, Okada Y, Funahashi H, et al. Basement membrane proteins play an important role in the invasive processes of human pancreatic cancer cells. J Surg Res. 2008;144(1):117–123. doi: 10.1016/j.jss.2007.03.023 [DOI] [PubMed] [Google Scholar]
- 39.Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH2-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66(24):11745. [DOI] [PubMed] [Google Scholar]
- 40.Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66(9):4662. [DOI] [PubMed] [Google Scholar]
- 41.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2(1):14. [DOI] [PubMed] [Google Scholar]
- 42.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways* 1. Gastroenterology. 2003;125(4):1188–1202. [DOI] [PubMed] [Google Scholar]
- 43.Edderkaoui M, Hong P, Vaquero EC, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol- Gastrointest Liver Physiol. 2005;289(6):G1137. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28(1):38. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(21):7427. [DOI] [PubMed] [Google Scholar]
- 46.Terai S, Fushida S, Tsukada T, et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer. 2015;18(2):306–313. doi: 10.1007/s10120-014-0380-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsuhashi A, Goto H, Saijo A, et al. Fibrocyte-like cells mediate acquired resistance to anti-angiogenic therapy with bevacizumab. Nat Commun. 2015;6:8792. doi: 10.1038/ncomms9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paget S THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. The Lancet. 1889;133(3421):571–573. doi: 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 49.van Deventer HW, Wu QP, Bergstralh DT, et al. C-C Chemokine Receptor 5 on Pulmonary Fibrocytes Facilitates Migration and Promotes Metastasis via Matrix Metalloproteinase 9. Am J Pathol. 2008;173(1):253–264. doi: 10.2353/ajpath.2008.070732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deventer HW van Palmieri DA, Wu QP McCook EC, Serody JS. Circulating Fibrocytes Prepare the Lung for Cancer Metastasis by Recruiting Ly-6C+ Monocytes Via CCL2. J Immunol. 2013;190(9):4861–4867. doi: 10.4049/jimmunol.1202857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirai H, Fujishita T, Kurimoto K, et al. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis. 2014;31(8):977–989. doi: 10.1007/s10585-014-9684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Ou L, Han S, et al. Deficiency of Kruppel-like factor KLF4 in myeloid-derived suppressor cells inhibits tumor pulmonary metastasis in mice accompanied by decreased fibrocytes. Oncogenesis. 2014;3:e129. doi: 10.1038/oncsis.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Maric I, DiPrima MJ, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013;122(7):1105–1113. doi: 10.1182/blood-2012-08-449413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]


