Abstract
Background: To investigate the risk factors related to aggravation and clinical outcomes in coronavirus disease 2019 (COVID-19) patients.
Methods: We performed a retrospective study on the risk factors for disease progression of cases with COVID-19. Based on the clinical types, the patients were divided into a progression group and an improvement group. Multivariable logistic regression and ROC curve analysis were performed to explore the risk factors for disease progression.
Results: A total of 101 patients were included in this study; diseases progression occurred in 17 patients, 84 patients improved, 6 were transferred to intensive care unit (ICU), and 5 died. The mean time to obtain negative nucleic acid results was 12.5 ± 5.0 days. Multivariate logistic analysis indicated that age (OR, 0.104; p = .002), C-reactive protein (CRP) (OR, 0.093; p < .001) and lymphocyte count (OR, 3.397; p = .022) were risk factors for disease progression. ROC curve analysis revealed that the AUC of age, CRP and lymphocyte count for disease progression were 0.873, 0.911 and 0.817, respectively.
Conclusions: Older age increased CRP and decreased lymphocyte count resulted in potential risk factors for COVID-19 progression. This may be helpful in identifying patients whose condition worsens at an early stage.
Keywords: COVID-19, risk factors outcome
Introduction
Since December 2019, unexplained pneumonia, today known as a novel coronavirus (2019-nCoV), has been identified in several patients across multiple Wuhan city hospitals (Hubei Province, China). Within 2 months, the infection spread rapidly to other Countries and regions [1,2]. Today (11 April 2020), the virus has spread to 212 countries around the world, infecting more than 1,607,467 people and causing more than 98,866 deaths [3].
Coronavirus is a large family of viruses that cause illness ranging from the common cold to more severe diseases, such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The novel coronavirus (nCoV) is a new strain that has not been previously identified in humans. On 11 February 2020, the International Committee on Taxonomy of viruses issued a statement stating an official designation for the novel virus: “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) [3].
Fever, dry cough, and fatigue are the main clinical manifestations of the disease. Many infected patients present with mild flu-like symptoms and recover quickly. Yet, in certain populations, especially the elderly, the virus may cause respiratory failure and even result in death. Therefore, early identification of risk factors for disease progression is helpful for early intervention in severe patients. Previous studies have suggested that the highest temperature, dyspnoea, respiratory rate, white blood cell count, neutrophil count, lymphocyte count, d-dimer, albumin, and procalcitonin are risk factors for ICU care in patients with coronavirus disease 2019 (COVID-19) [4,5]. In this study, we retrospectively examined 101 laboratory-confirmed cases of COVID-19 admitted to our hospital between 21 January 2020 and 9 March 2020, so as to investigate the factors affecting the outcomes with the hope of improving the treatment and reducing mortality.
Methods
Subjects
We performed a retrospective study of clinical characteristics and risk factors for disease progression in laboratory-confirmed cases with COVID-19 hospitalized at Beijing YouAn hospitals (Capital Medical University, Beijing) between 21 January 2020 and 9 March 2020. All patients were evaluated and clinically typed upon admission, according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia version 7 (trial)” [6]. Specific clinical types included: (1) mild: mild clinical manifestation with no unusual imaging data; (2) common: fever, respiratory symptoms, pneumonia performance on X-ray or CT; (3) severe (meet any of the followings): (i) respiratory distress, RR ≥ 30/min; (ii) oxygen saturation ≤ 93% at rest state; (iii) arterial partial pressure of oxygen (PaO2)/fraction of inspiration O2 (FiO2) ≤ 300 mmHg, 1 mmHg = 0.133 kPa; (4) critically ill (meet any of the followings): (i) respiratory failure needs mechanical ventilation; (ii) shock; (iii) combined with other organ failure, patients need ICU monitoring and treatment.
All patients were divided into two groups: progression group and improvement group. The definition of the progression group: clinically advanced types; patients admitted to ICU; death during hospitalization. The improvement group: the clinical types remained unchanged or changed to a lighter type, and patients were discharged from the hospital.
Laboratory testing
Patients’ pharyngeal swab specimens were collected for the SARS-CoV-2 viral nucleic acid detection using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay. Influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus, parainfluenza virus, enterovirus, human metapneumovirus, coxsackievirus, chlamydia, and mycoplasma were detected by collecting body fluid (nasopharyngeal swabs and sputum) samples. Laboratory variables were tested with conventional methods, including (i) routine blood tests: the numbers of leukocytes, lymphocytes, and neutrophil, haemoglobin, platelet; (ii) blood biochemistry: alanine transaminase, aspartate aminotransferase, total bilirubin, albumin, creatinine, creatine kinase, myoglobin, troponin; (iii) partial pressure of oxygen and oxygen saturation of blood; (iv) infection indices: procalcitonin and CRP.
Data collection
We reviewed the electronic medical records, laboratory findings, and chest X-ray data for all patients. Personal data, epidemiological data, comorbidities, clinical data, laboratory data, radiological data, treatment and outcomes data were obtained with standardized data collection forms from electronic medical records. Two researchers also independently reviewed the data collection forms to double-check the collected data.
Statistical analysis
Categorical variables were described as frequency rates and percentages, analyzed using the Chi-squared test or Fisher’s exact test. Continuous variables were described using mean, median, and interquartile range (IQR) values. Means for continuous variables were compared using independent group t-tests when the data were normally distributed; otherwise, the Mann–Whitney U test was used. Univariate and multivariate analyses of prognostic factors were performed using the logistics regression. SPSS software version 20.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. The area under curve (AUC), cut-off value, sensitivity, and specificity of ROC were analyzed by the receiver operator characteristic curve (ROC). p < .05 was considered statistically significant, and all p values were two-tailed.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived, given the context of emerging infectious diseases.
Results
Demographics and case grouping
The study population included 101 hospitalized patients with laboratory-confirmed COVID-19, and 44 (43.6%) were men. The median age was 50.9 ± 20.1 years, and 27 patients were aged ≥65 years (26.7%). Among the 101 patients, there were 4 patients with the mild type (4.0%), 75 patients with the common type (74.3%), 13 patients with the severe type (12.9%), and nine patients with the critically ill type (8.9%) at admission.
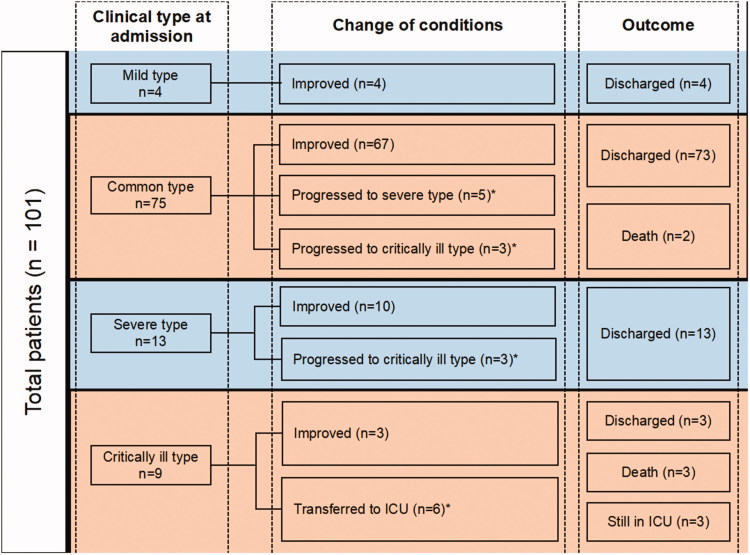
Among the 101 patients, 8 cases with common type showed progression of the disease, 5 of them developed a severe type, while 3 progressed to critically ill type. Three cases with severe type progressed to critically ill type, and 6 cases with the critically ill type required ICU treatment after admission during hospitalization. A total of 17 patients (16.8%) were included in the progression group and 84 patients (83.2%) in the improvement group.
In the improvement group, 4 cases were with mild type, 67 cases with common type, 10 cases with severe type, and 3 cases with critically ill type. All of them were successfully discharged. Changes in conditions and outcomes of 101 patients after admission are shown in Figure 1.
Figure 1.
The change in condition and outcomes of 101 patients after admission. Among the 101 patients, 8 cases with common type showed progression of the disease, 5 of them developed a severe type, while 3 progressed to critically ill type. Three cases with severe type progressed to critically ill type, and 6 cases with the critically ill type required ICU treatment after admission during hospitalization. A total of 17 patients (16.8%) were included in the progression group and 84 patients (83.2%) in the improvement group. ICU: intensive care unit; *included in the progression group.
Clinical characteristics
Among these patients, 43 (42.6%) were in Hubei province during the virus outbreak, 78 (77.2%) clustering onset. The median duration from the first symptoms to diagnosis was 4.8 ± 2.9 days. Of the 101 patients, 30 (29.7%) had one or more comorbidities. Hypertension [21(20.8%)], cardiovascular disease [11(10.9%)], diabetes [6(5.9%)], and malignancy [5 (5.0%)] were the most common comorbidities. The most common symptoms were fever [74 (73.3%)] and dry cough [63 (62.4%)]. The median Sequential Organ Failure Assessment (SOFA) score was 1 (IQR, 0–1).
The patients in the progression group were significantly older than those in the improvement group (72.4 ± 13.9 versus 46.6 ± 18.3). There was no significant difference in sex between the two groups (p > .05). Compared with the improvement group, the patients in the progression group were more likely to have underlying comorbidities (58.8% versus 23.8%, χ2 = 8.301, p = .004). Besides, the progression group had a significantly higher proportion of patients with hypertension than the improvement group (47.1% versus 15.5%, χ2 = 6.753, p = .009). There was no significant difference in other comorbidities.
The maximum body temperature, heart rate, mean arterial pressure, and blood oxygen saturation did not differ between patients in the progression and improvement group. The respiratory rate in the progression group was significantly higher than in the improvement group (21.0 ± 2.0 breaths/min versus 20.0 ± 1.7 breaths/min, t = –2.072, p = .041). The median SOFA scores in the progression group were significantly higher than those in the improvement group [3(1.5, 4) versus 3(0, 1), U = 6.175, p < .001]. Demographic data and clinical characteristics of 101 patients with COVID-19 are shown in Table 1.
Table 1.
Demographic data and clinical characteristics of 101 patients with COVID-19.
| Total n = 101 |
Improvement n = 84 |
Progression n = 17 |
t/U/χ2 value | p Value | |
|---|---|---|---|---|---|
| Age (years, mean ± S.D.) | 50.9 ± 20.1 | 46.6 ± 18.3 | 72.4 ± 13.9 | −5.496 | <.001 |
| Gender (male/female) | 44/57 | 34/50 | 10/7 | 1.936 | .164 |
| History of exposure to Hubei province (n, %) | 43 (42.6) | 37 (44.0) | 6 (35.3) | 0.443 | .506 |
| Cluster (n, %) | 78 (77.2) | 63 (75.0) | 15 (88.2) | 0.756 | .385 |
| Duration from first symptoms to diagnosis (d, mean ± S.D.) | 4.8 ± 2.9 | 4.7 ± 2.7 | 5.2 ± 3.6 | −0.600 | .550 |
| Comorbidities (n, %) | 30 (29.7) | 20 (23.8) | 10 (58.8) | 8.301 | .004 |
| Hypertension | 21 (20.8) | 13 (15.5) | 8 (47.1) | 6.753 | .009 |
| Coronary artery disease | 11 (10.9) | 7 (8.3) | 4 (23.5) | 1.981 | .159 |
| Diabetes mellitus | 6 (5.9) | 4 (4.8) | 2 (11.8) | 0.304 | .581 |
| Cerebrovascular | 3 (3.0) | 2 (2.4) | 1 (5.9) | 0.595 | .428 |
| Chronic obstructive pulmonary disease | 4 (4.0) | 2 (2.4) | 2 (11.8) | 3.241 | .131 |
| Malignancy | 5 (5.0) | 4 (4.8) | 1 (5.9) | 0.038 | .846 |
| Symptoms (n, %) | |||||
| Fever | 74 (73.3) | 59 (70.2) | 15 (88.2) | 1.510 | .219 |
| Dry cough | 63 (62.4) | 53 (63.1) | 10 (58.8) | 0.110 | .740 |
| Temperature max (°C, mean ± S.D.) | 38.5 ± 0.6 | 38.5 ± 0.6 | 38.6 ± 0.5 | –0.849 | .399 |
| Respiratory rate (breaths/min, mean ± S.D.) | 20.2 ± 1.8 | 20.0 ± 1.7 | 21.0 ± 2.0 | −2.072 | .041 |
| Heart rate (beats/min, mean ± S.D.) | 85.5 ± 12.8 | 85.6 ± 12.5 | 84.9 ± 14.5 | 0.188 | .851 |
| Mean arterial pressure (mmHg, mean ± S.D.) | 89.7 ± 13.8 | 90.8 ± 10.4 | 84.7 ± 24.4 | 1.678 | .097 |
| Blood oxygen saturation (%, mean ± S.D.) | 96.5 ± 4.0 | 96.8 ± 3.0 | 95.3 ± 7.1 | 1.376 | .172 |
| SOFA (median, IQR) | 1.0 (0.0,1.0) | 0.0 (0.0,1.0) | 3.0 (1.5,4.0) | 6.157 | <.001 |
SOFA: Sequential Organ Failure Assessment.
Laboratory findings and imaging characteristics
Laboratory indices of the 101 patients with COVID-19 were determined at the time of admission. There were numerous differences in laboratory data between patients in the progression group and the improvement group (Table 2). White blood cell count and neutrophil count were significantly elevated, and lymphocyte count significantly decreased in the progression group compared to the improvement group. Albumin was significantly decreased in the progression group compared to the improvement group. In addition, the levels of estimated glomerular filtration rate, creatine kinase isoenzymes, myoglobin, troponin, C-reactive protein, procalcitonin, and lactic acid were significantly increased in the progression group compared to the improvement group. Eighty-seven out of 101 enrolled patients showed bilateral involvement of chest CT scan, and 10 patients showed unilateral involvement. There were no significant differences in imaging findings between the two groups (Table 2).
Table 2.
Laboratory results and imaging findings of 101 patients with COVID-19.
| Total n = 101 |
Improvement n = 84 |
Progression n = 17 |
t/U/χ2 value | p Value | |
|---|---|---|---|---|---|
| Laboratory results (mean ± S.D. or median, IQR) | |||||
| WBC (× 109/L) | 4.3 (3.5,5.8) | 4.1 (3.5,5.6) | 6.4 (3.7,8.2) | 2.360 | .018 |
| N (× 109/L) | 2.6 (1.8,3.8) | 2.3 (1.8,3.3) | 4.9 (2.7,7.3) | 3.556 | <.001 |
| L (× 109/L) | 1.2 ± 0.8 | 1.3 ± 0.8 | 0.7 ± 0.3 | 3.205 | .002 |
| PLT (× 109/L) | 192.1 (156.2,245.8) | 192.2 (158.8,248.8) | 193.5 (111.4,218.5) | −1.002 | .316 |
| ALT (U/L) | 28.3 (20.0,46.70) | 28.6 (20.5,45.3) | 36.2 (18.4,63.1) | 0.743 | .458 |
| AST (U/L) | 30.3 (22.5,45.6) | 30.5 (22.6,42.8) | 37.8 (22.5,76.4) | 1.569 | .117 |
| TBIL (μmol/L) | 9.2 (6.6,12.4) | 8.9 (6.4,12.0) | 12.1 (7.6,15.6) | 1.633 | .102 |
| ALB (g/L) | 35.8 ± 5.8 | 36.5 ± 5.2 | 32.3 ± 7.2 | 2.793 | .006 |
| Cr (umol/L) | 64.3 (53.5,77.8) | 62.9 (53.1,74.8) | 77.3 (54.5,88.1) | 1.680 | .093 |
| eGFR | 99.5 (89.0,114.7) | 105.3 (94.0,118.8) | 91.2 (66.7,96.9) | −3.512 | <.001 |
| CK (U/L) | 80.5 (46.0,130.3) | 74.5 (45.8,122.3) | 96.0 (59.7,161.3) | 1.105 | .269 |
| CKMB (U/L) | 0.3 (.02,0.9) | 0.3 (0.2,0.6) | 1.1 (0.3,2.5) | 2.919 | .004 |
| MYO (ng/ml) | 44.5 (30.0,67.5) | 36.5 (28.2,59.4) | 97.4 (52.3,198.5) | 3.755 | <.001 |
| TNI (ng/ml) | 0.01 (0.01,0.02) | 0.01 (0.01,0.02) | 0.04 (0.01,0.08) | 3.306 | .001 |
| PT (s) | 12.6 (11.9,13.1) | 12.6 (12.0,13.1) | 12.8 (11.6,13.6) | 0.164 | .869 |
| INR | 1.1 (1.1,1.2) | 1.1 (1.1,1.2) | 1.1 (1.0,1.2) | 0.052 | .959 |
| CRP (mg/L) | 19.3 (3.6,59.1) | 13.9 (2.6,26.9) | 86.0 (66.7,122.5) | 5.312 | <.001 |
| PCT (ng/ml) | 0.1 (0.1,0.1) | 0.1 (0.1,0.1) | 0.1 (0.1,0.2) | 2.944 | .003 |
| LAC (mmol/L) | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.7 ± 0.8 | −2.946 | .004 |
| Imaging findings (n, %) | |||||
| Normal | 4 (4.0) | 4 (4.8) | 0 (0) | 0.056 | .813 |
| Unilateral involvement | 10 (10.0) | 10 (11.9) | 0 (0) | 1.110 | .292 |
| Bilateral involvement | 87 (86.1) | 70 (83.3) | 17 (100.0) | 2.041 | .153 |
WBC: white blood cell count; N: neutrophil count; L: lymphocyte count; PLT: platelet count; ALT: alanine transaminase; AST: aspartate transaminase; TBIL: total bilirubin; ALB: albumin; Cr: creatinine; CK: creatinine kinase; CKMB: creatine kinase isoenzymes; MYO: myoglobin; TNI: troponin; PT: prothrombin time; INR: international normalized ratio; CRP: C-reactive protein; PCT: procalcitonin; LAC: lactic acid.
Treatments, complications and outcomes
The main treatments, complications, and outcomes are shown in Table 3. Of the 101 patients, 35 (34.7) patients received antiviral therapy. The proportion of systemic corticosteroid treatment and human-immunoglobulin treatment in the progression group was significantly higher than that in the improvement group (82.3% versus 21.4%; 35.3% versus 1.2%). The proportion of patients in the progression group who required ICU care, continuous renal replacement therapy (CRRT), invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) treatment were 58.8%, 29.4%, 41.2%, and 23.5%, respectively.
Table 3.
Treatments, complications and outcomes of 101 patients with COVID-19.
| Total n = 101 |
Improvement n = 84 |
Progression n = 17 |
t/U/χ2 value | p Value | |
|---|---|---|---|---|---|
| Treatments (n, %) | |||||
| Antiviral treatment | 35 (34.7) | 29 (34.5) | 6 (35.3) | 0.004 | .951 |
| Systemic corticosteroid treatment | 32 (31.7) | 18 (21.4) | 14 (82.3) | 24.246 | <.001 |
| Human-immunoglobulin | 7 (6.9) | 1 (1.2) | 6 (35.3) | 20.480 | <.001 |
| ICU care | 10 (10.0) | 0 (0) | 10 (58.8) | 48.446 | <.001 |
| CRRT | 5 (5.0) | 0 (0) | 5 (29.4) | 20.118 | <.001 |
| Oxygen support | |||||
| Nasal cannula | 63 (62.4) | 46 (54.8) | 17 (100.0) | 12.329 | <.001 |
| Non-invasive ventilation or high-flow nasal cannula | 3 (3.0) | 0 (0) | 3 (17.6) | 9.768 | .002 |
| Invasive mechanical ventilation |
7 (6.9) | 0 (0) | 7 (41.2) | 31.054 | <.001 |
| Invasive mechanical ventilation and ECMO |
4 (4.0) | 0 (0) | 4 (23.5) | 14.858 | .001 |
| Complications (n, %) | |||||
| Acute Respiratory Distress Syndrome | 7 (6.9) | 0 (0) | 7 (41.2) | 31.054 | <.001 |
| Sepsis | 7 (6.9) | 1 (1.2) | 6 (35.3) | 20.480 | <.001 |
| Septic shock | 6 (5.9) | 0 (0) | 6 (35.3) | 25.520 | <.001 |
| Cardiogenic shock | 3 (3.0) | 0 (0) | 3 (17.6) | 9.768 | .002 |
| Arrhythmia | 7 (6.9) | 0 (0) | 7 (41.2) | 31.054 | <.001 |
| Myocardial injury | 24 (23.8) | 10 (11.9) | 14 (82.3) | 34.942 | <.001 |
| Acute kidney injury | 12 (11.9) | 6 (7.1) | 6 (35.3) | 8.182 | .004 |
| Liver injury | 38 (37.6) | 28 (33.3) | 10 (58.8) | 3.914 | .048 |
| Secondary infection | 9 (8.9) | 1 (1.2) | 8 (47.1) | 31.242 | <.001 |
| Outcomes (n, % or mean ± S.D.) | |||||
| Time taken for temperature normalization (d) | 8.6 ± 4.3 | 8.0 ± 3.8 | 11.3 ± 5.4 | −2.798 | .006 |
| Time required to obtain negative nucleic acid results (d) | 12.5 ± 5.0 | 11.7 ± 4.4 | 17.2 ± 5.9 | −4.246 | <.001 |
| Time taken for imaging improvement (d) | 14.9 ± 5.4 | 14.0 ± 3.2 | 22.8 ± 11.3 | −2.319 | .048 |
| Improved and discharged (n) | 93 (92.1) | 84 (100.0) | 9 (52.9) | 26.827 | <.001 |
| Death (n) | 5 (5.0) | 0 (0) | 5 (29.4) | 20.118 | <.001 |
| Length of hospital stay [d, (median, IQR)] | 13.0 (10.0, 17.0) | 13.0 (10.0, 16.0) | 20.5 (15.3, 22.3) | 3.065 | .002 |
ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; CRRT: continuous renal replacement therapy.
Seven out of 101 patients (6.9%) developed acute respiratory distress syndrome (ARDS). Seven patients were complicated with sepsis; six of them developed into septic shock. Cardiogenic shock and arrhythmia were found in 3 patients and seven patients, respectively. The proportion of myocardial injury, acute renal injury, and liver injury was 23.8%, 11.9%, and 37.6%, respectively. Nine patients developed a secondary bacterial infection. The rate of secondary bacterial infection in the progression group was 47.1%, which was significantly higher than that in the improvement group (1.2%).
The mean time for the body temperature to decrease was 8.6 ± 4.3 days, the mean time to obtain negative nucleic acid result was 12.5 ± 5.0 days, and the mean time for imaging improvement was 14.9 ± 5.4 days. Compared with the improvement group, the body temperature decreased more slowly, and negative nucleic acid results and imaging improvements were achieved later in patients from progression group (11.3 ± 5.4 versus 8.0 ± 3.8; 17.2 ± 5.9 versus 11.7 ± 4.4; 22.8 ± 11.3 versus 14.0 ± 3.2 days). The mortality in these patients was 5%, and the median hospital stay was 13 days (IQR, 10.0–17.0).
Risk factors for disease progression
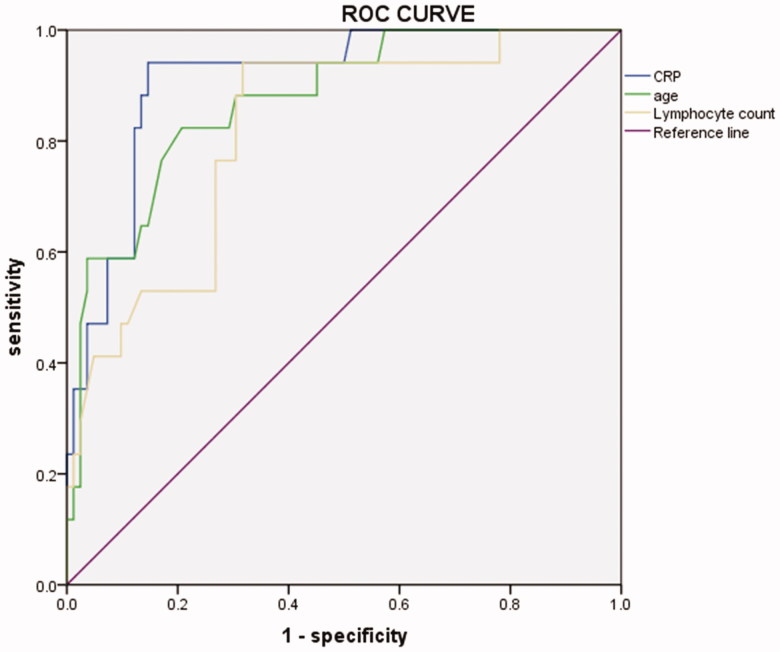
The results of the univariate logistic analysis found that age, comorbidities, neutrophil count, lymphocyte count, albumin, myoglobin, and CRP were significantly associated with the disease progression. Furthermore, the multivariate logistic analysis indicated that age (OR, 0.104; 95% CI: 0.024–0.449; p = .002), CRP (OR, 0.093; 95% CI: 0.025–0.314; p < .001) and lymphocyte count (OR, 3.397; 95% CI: 1.195–9.565; p = .022) were risk factors for disease progression (Table 4). ROC curve analysis revealed that the AUC of age, CRP and lymphocyte count for disease progression were 0.873 (95% CI: 0.786–0.961, p < .001), 0.911 (95% CI: 0.843–0.978, p < .001), 0.817 (95% CI: 0.711–0.923, p < .001), respectively (Figure 2). Age > 62.5 years, CRP > 51.4 mg/L and lymphocyte count < 0.945 × 109/L indicated the progression of the disease, where the sensitivity was 82.4%, 94.1.2% and 94.1%, and the specificity was 79.3%, 85.4% and 68.3%.
Table 4.
Logistic analysis results of risk factors for disease progression (n = 101).
| B | OR | p Value | 95% CI | |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 1 (ref) | |||
| ≥60 | −2.267 | 0.104 | .002 | 0.024–0.449 |
| CRP (mg/L) | ||||
| <60 | 1 (ref) | |||
| ≥60 | −2.373 | 0.093 | <.001 | 0.025–0.341 |
| Lymphocyte count (× 109/L) | ||||
| <1.1 | 1 (ref) | |||
| ≥1.1 | 1.223 | 3.397 | .022 | 1.195–9.565 |
OR: odds ratio; CRP: C-reactive protein.
Figure 2.
ROC curve of independent risk factors for disease progression in patients with COVID-19.
Discussion
In this retrospective cohort study, 17 patients (16.8%) developed disease progression after admission; 6 were transferred to ICU, and five (4.9%) patients died. Similar data were reported in the recent meta-analysis that examined 50466 cases with SARS-CoV-2 [7].
This study identified several risk factors for disease progression in patients who were hospitalized with COVID-19 in Beijing. In particular, older age, higher CRP, and lower lymphocyte count on admission were associated with higher odds of in-hospital disease progression. Additionally, more comorbidities, elevated levels of white blood cell count, myoglobin, troponin, and decreased levels of albumin were more commonly seen in patients with disease progression. In addition, the time to obtain negative nucleic acid results was significantly longer in patients with disease progression compared to those who showed improvement.
Coronaviruses are a large family of viruses found in several domestic animals, pets, and humans, causing a variety of acute and chronic diseases [8]. Currently, the pathogenesis of the COVID-19 still remains unclear, and there is no precise and effective treatment. Recent clinical research suggests that some patients have mild symptoms and can recover quickly, while others may develop rapid disease progression, which often leads to respiratory failure and even death. Besides, advanced age at presentation has shown to be very useful in predicting the mortality in SARS [9] and MERS [10] cases. Elderly patients tend to develop a greater number of comorbidities, potentially leading to poor outcomes [11]. Besides, studies have shown that older macaques have stronger host innate responses to virus infection than younger adults, which promotes inflammation [12]. The current study confirmed that increased age was associated with disease progression in patients with COVID-19.
CRP is an important inflammatory index and a significant predictor of disease progression. Previous studies on SARS have suggested the initial CRP level as a predictive factor of death [13]. Rapid elevation of inflammatory cytokines-IL-6, IL-8, and TNF-alpha might have a role in the development of SARS-related ARDS. The timing of elevations in inflammatory cytokines and CRP is correlated with the progression of pulmonary infiltrates of SARS patients [14]. In addition, studies have found that CRP is one of the risk factors for disease progression in Wuhan patients with COVID-19 [15]. Moreover, lymphocyte count is another predictor of disease progression. The most common laboratory abnormalities observed across several studies were depressed total lymphocytes [16]. Recently, it has been found that the number of CD4 and CD8 T cells in the peripheral blood of patients with COVID-19 is significantly reduced, but the state is over-activated, which shows the increase of Th17 and the high cytotoxicity of CD8 T cells thus suggesting serious damage of immune system in these patients [16]. Our study confirmed that CRP and lymphocytopenia were independent risk factors for disease progression.
Our study also found that patients with disease progression were more likely to have various complications, especially cardiac complications. The incidence of cardiogenic shock and arrhythmia in patients with disease progression was significantly higher than that in the improvement group. Moreover, the incidence of myocardial injury, including an abnormal increase of creatine kinase isoenzymes (CKMB) and/or troponin (TnI) was significantly higher than that of the improvement group. The previous study reported an incidence of acute cardiac injury of 7.2–12% in Wuhan patients with COVID-19 [17,18], which was lower than our results (23.8%). In addition, ARDS, secondary infection, sepsis and septic shock, kidney injury, and liver injury also resulted as related to disease progression.
Compared with the improvement group, the body temperature decreased more slowly in patients from the progression group (11.3 ± 5.4 versus 8.0 ± 3.8). Besides, detectable SARS-CoV-2 RNA persisted for a mean of 17.2 ± 5.9 days in the progression group, which was significantly longer compared to the disease improvement group (11.7 ± 4.4 days). Therefore, it is critical for these patients to receive effective antiviral treatment as early as possible [19]. Currently, there are no FDA-approved treatments for human CoV infection; however, there are agents that have shown to be effective for SARS and MERS epidemics, such as Lopinavir and Ritonavir [20,21].
Our study has few limitations. First, our sample size was relatively small, which may lead to biased results. Consequently, a multi-center large-scale study is required. Second, not all patients underwent laboratory testing, such as detecting lactate dehydrogenase and cytokine levels, so they were not included in the risk factor analysis.
In conclusion, patients with COVID-19 showed similar clinical manifestations to those with MERS or SARS. Older age, CRP, and lymphocyte count at admission were identified as risk factors for disease progression in patients with COVID-19 and may be useful for identifying an early-stage disease. Effective antiviral therapy may improve the patients’ outcomes.
Author contributions
Wei Hou wrote the article; participated in designing the concept of the study, the definition of intellectual content, and data acquisition. Wei Zhang, Lianchun Liang and Bin Xu contributed to data acquisition. Ronghua Jin and Zhongjie Hu designed and reviewed the manuscript.
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Hui DS, E IA, Madani TA, et al. . The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Wang L, Deng X, et al. . Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO main website https://www.who.int [accessed 11 April 2020].
- 4.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Koh V, Marimuthu K, et al. . Epidemiological and clinical predictors of COVID-19. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia version 7 (trial). National Health and Health Commission of China Available from : http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf [Accessed on 9 March 2020]
- 7.Sun P, Qie S, Liu Z, et al. . Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. J Med Virol. 2020;92(6):612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Liu Q, Guo D. Coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JCK, Tsui ELH, Wong V. The Hospital Authority SARS Collaborative Group. Prognostication in severe acute respiratory syndrome: a retrospective time-course analysis of 1312 laboratory-confirmed patients in Hong Kong. Respirology. 2007;12(4):531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong KH, Choi JP, Hong SH, et al. . Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax. 2018;73(3):286–289. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, He W, Yu XM, et al. . Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020. DOI: 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits SL, de Lang A, van den Brand JMA, et al. . Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2):e1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng WH, Chiang BL, Chang SC, et al. . Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. J Formos Med Assoc. 2005;104(10):715–723. [PubMed] [Google Scholar]
- 14.Wang JT, Sheng WH, Fang CT, et al. . Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10(5):818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Tao ZW, Lei W, et al. . Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020. DOI: 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Shi L, Wang YJ, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CL, Wang YM, Li XW, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang DW, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian GQ, Chen XQ, Lv DF, et al. . Duration of SARS-CoV-2 viral shedding during COVID-19 infection. Infect Dis. 2020. DOI: 10.1080/23744235.2020.1748705 [DOI] [PubMed] [Google Scholar]
- 20.Yao TT, Qian JD, Zhu WY, et al. . A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus – a possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020;92(6):556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Du RH, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]