Abstract
Rationale: Anxiety is a common comorbidity of chronic obstructive pulmonary disease (COPD) that is associated with higher morbidity and mortality. We evaluated three anxiety screening questionnaires: the Generalized Anxiety Disorder 7-Item Scale (GAD-7), the Hospital Anxiety and Depression Scale Anxiety subscale (HADS-A), and the Anxiety Inventory for Respiratory Disease (AIR).
Objectives: To evaluate and compare the test performance characteristics of three anxiety screening questionnaires, using the Mini-International Neuropsychiatric Interview (MINI), version 7.0, as the “gold standard.”
Methods: Individuals with COPD were recruited at 16 centers. The MINI and questionnaires were administered by trained research coordinators at an in-person visit and readministered by telephone 2–4 weeks later. A composite score for the presence of any Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) anxiety disorder was computed, based on the MINI as the gold standard, compared with a participant screening positive on self-report measures for these analyses.
Results: Two hundred and twenty eligible individuals with COPD were enrolled; 219 completed the study. Eleven percent were identified as having a DSM-V anxiety disorder, based on the MINI. Elevated anxiety symptoms based on questionnaires were 38% for the AIR, 30% for the GAD-7, and 20% for the HADS-A. Area under the receiver operating characteristic curve (AUC) was highest for the GAD-7 (0.78; 95% confidence interval [CI], 0.69–0.87), followed by the HADS-A (0.74; 95% CI, 0.64–0.84) and the AIR (0.66; 95% CI, 0.56–0.76). The AUC for the GAD-7 was significantly greater than for the AIR (P = 0.014). Sensitivity was not statistically different among the questionnaires: 77% for the GAD-7, 63% for the HADS-A, and 66% for the AIR. The HADS-A had the highest specificity, 85%, which was significantly higher than that of the GAD-7 (77%; P < 0.001) and the AIR (65%; P < 0.001); GAD-7 specificity was higher than AIR specificity (P < 0.001).
Conclusions: Symptoms of anxiety among patients with COPD as identified by screening questionnaires were common and significantly higher than the prevalence of anxiety disorder meeting DSM-V criteria. The GAD-7, the HADS-A and the AIR questionnaires had fair to moderate psychometric properties as screening tools for anxiety in individuals with COPD, indicating the need for improved measures for this patient population.
Keywords: test anxiety scale, chronic obstructive pulmonary disease, anxiety, psychometric properties
Chronic obstructive pulmonary disease (COPD) affects 11 to 14 million adults in the United States, and the financial burden of caring for COPD is estimated to cost more than 70 billion dollars annually (1, 2). It is the third leading cause of premature death in the United States (3), a leading cause of disability resulting in poor quality of life (4, 5), and is associated with frequent exacerbations and hospitalizations (6). Comorbidities are common in individuals with COPD. The prevalence of anxiety in individuals with COPD is estimated to be between 16% and 31% (7, 8). Anxiety in patients with COPD is associated with increased mortality and morbidity, including more exacerbations, longer hospital stays, and more functional limitations (7, 9–14). However, anxiety often goes undetected and untreated in individuals with COPD (15) because screening for anxiety is not routinely performed.
Underdiagnosis of anxiety in COPD may also be related to the overlap between somatic symptoms of anxiety with respiratory symptoms of COPD. Generalized anxiety disorder (GAD; chronic worry and physical symptoms) and panic disorder (sudden intense fear with no trigger and physical symptoms) are particularly difficult to identify in this population because of the overlapping symptoms. Moreover, people with COPD often experience unpredictable episodes of breathing distress that may lead to panic and chronic anxiety (14). The overlap of symptoms such as dyspnea makes it difficult to determine how to treat these symptoms, which may lead to a negative cycle between symptoms such as dyspnea resulting in distress and anxiety, and vice versa. A number of anxiety screening tools include questions about somatic symptoms, which complicates their interpretation (16, 17).
Validated screening questionnaires for anxiety that have been used in a numerous studies of various diseases and that do not include somatic symptoms include the Generalized Anxiety Disorder 7-Item Scale (GAD-7 [18]) and the Hospital Anxiety and Depression Scale Anxiety subscale (HADS-A [19]). Although the HADS-A has been validated in patients with COPD (20), to our knowledge the GAD-7 has not been validated in COPD. The AIR is a newer questionnaire developed specifically for assessing nonsomatic anxiety symptoms in patients with COPD (21).The Anxiety Inventory for Respiratory Disease (AIR) places more emphasis on panic-related symptoms than does the GAD-7 or the HADS-A.
We evaluated the GAD-7, HADS-A, and AIR as screening tools for anxiety symptoms in individuals with COPD. The presence of an anxiety disorder was established using the Mini-International Neuropsychiatric Interview (MINI), version 7.0, a structured interview that directly corresponds to Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) diagnoses of anxiety (22).
Methods
Study Design
This prospective, multicenter, cohort study was designed to validate and compare three anxiety screening questionnaires for individuals with COPD, using the MINI version 7.0 diagnostic interview as the “gold standard” for identification of anxiety disorders (i.e., panic disorder, agoraphobia, social anxiety disorder, post-traumatic stress disorder, and generalized anxiety disorder) as defined in the DSM-V. There was an in-person visit for enrollment and baseline assessments; a second visit was conducted by telephone 2–4 weeks later. The study was approved by the institutional review boards for the data coordinating center and clinical centers; all participants provided written informed consent.
Study Participants and Eligibility
Participants were recruited at 16 centers of the American Lung Association Airways Clinical Research Centers Network (ALA-ACRC; a list of participating centers may be found before the beginning of the References). Participants were recruited specifically for this study and were not pooled from other ACRC trials. Enrolled participants were 40 years of age or older with clinically stable COPD, defined as receiving medications for stable maintenance of COPD and the absence of an exacerbation of symptoms requiring treatment with an antibiotic or corticosteroids in the 6 weeks before enrollment. COPD was confirmed by spirometry, that is, post-bronchodilation FEV1 less than 80% of the predicted normal value and an FEV1/FVC ratio less than 0.7 (20). Eligible participants had to score above 18 on the Montreal Cognitive Assessment (MoCA) to rule out significant cognitive impairment (23). Individuals with unstable coronary heart disease or a major psychiatric disorder were excluded because of safety concerns about these individuals performing spirometry and 6-minute walk tests. We also excluded patients with self-reported major psychiatric disorders such as schizophrenia and bipolar disorder as we did not have access to medical records to validate reports of other known psychiatric disorders.
Procedures
Eligible participants underwent a brief physical examination, provided a medical history, performed spirometry testing and a 6-minute walk test, and completed the questionnaires. Questionnaires were administered by coordinators. Coordinators then conducted the structured MINI. At the second visit, 2–4 weeks later, questionnaires were administered by coordinators on the telephone.
Instruments
Coordinators were trained and certified to administer these instruments before the study. They read the questions to participants. If the individual asked for an explanation for a given question, care was taken to avoid any form of guidance that may have biased the patient’s selection of answer to the question. After rigorous training, a psychologist reviewed recorded interviews conducted by coordinators for certification. Coordinators also recorded the first interview conducted with an enrolled participant and every fourth interview thereafter, with review by the same psychologist for quality assurance.
Mini-International Neuropsychiatric Interview, Version 7.0
The MINI, version 7.0 (22) sections on major depression and anxiety disorder including panic disorder, agoraphobia, social anxiety disorder (or social phobia), post-traumatic stress disorder, and generalized anxiety disorder were administered. All disorders were assessed on the basis of current symptoms, with the exception of panic disorder, which included current and lifetime panic disorder and major depression, which included both current and past episodes. Questions elicit a yes/no response and are linked to diagnostic criteria from the DSM-V. A composite score for the presence of any anxiety disorder was computed on the basis of a participant having a diagnosis on at least one of the anxiety scales and used as the gold standard comparator for these analyses.
Anxiety Inventory for Respiratory Disease
The AIR (21) is a 10-item anxiety screening instrument with seven questions about generalized anxiety and three questions about panic. Responses are rated on a 4-point scale ranging from 0 (“not at all”) to 3 (“almost all the time”), with the total score ranging from 0 to 30. In a preliminary study of 22 patients (mean age, 71 yr) with COPD, a score of 8 or greater on the AIR scale indicated clinically significant anxiety symptoms, and a 5-point difference was estimated to be clinically important in the context of a pulmonary rehabilitation program (24).
Generalized Anxiety Disorder 7-Item Scale
The GAD-7 (18) is a component of the Patient Health Questionnaire and is a seven-item measure about generalized anxiety symptoms in the previous 2 weeks. Each of the GAD-7 questions is rated on a 4-point scale from 0 (“not at all”) to 3 (“nearly every day”), and the score is the sum of the seven responses. Scores range from 0 to 21, with a score of 4 or less indicating normal, 5–9 indicating mild, 10–14 indicating moderate, and 15 or greater indicating severe anxiety symptoms. The minimally clinical important difference has not been established. There are no specific questions about panic on the GAD-7.
Hospital Anxiety and Depression Scale
The HADS (19) contains two subscales that screen for anxiety and depression, respectively. Each subscale has seven questions assessing frequency and severity of symptoms over the previous week, rated on a scale from 0 (e.g., not at all, only occasionally, etc.) to 3 (e.g., very much indeed, most of the time, etc.). There are six questions about generalized anxiety and one question about panic on the HADS Anxiety subscale (HADS-A). Scores range from 0 to 21 for each subscale, with a score of 8 or more indicating significant anxiety symptoms. A minimal clinically important difference of 1.5 has been reported for patients with COPD (19).
Modified Medical Research Council Dyspnea Scale
The modified Medical Research Council (mMRC) Dyspnea Scale measures shortness of breath in daily activities (25). The mMRC Dyspnea Scale consists of five statements that describe almost the entire range of dyspnea from none (grade 0) to almost complete incapacity due to severity of dyspnea (grade 4). It is a reliable and responsive scale to pulmonary rehabilitation program (26).
COPD Assessment Test
The COPD Assessment Test (CAT) is a validated measure that quantifies the impact of COPD symptoms on health status (26). The questionnaire has eight items and total scores range from 0 to 40, with higher scores corresponding to greater impact on quality of life.
Data Analysis
The planned sample size of 200 patients with COPD provided greater than 90% power to detect a difference of 0.25 between receiver operating characteristic (ROC) areas under the curve (AUCs) (27, 28) with a two-sided type I error rate of 5%. Descriptive statistics (medians, quartiles, counts, and percentages) were used to summarize the sociodemographic and clinical characteristics of the cohort and the scores for each questionnaire.
Most analyses are based on the scores derived from response to the questionnaires at the first visit. Cronbach’s α (27) was calculated to evaluate the internal consistency of the AIR, GAD-7, and HADS-A for each visit. Test–retest reliability was assessed by calculating intraclass correlation coefficients (ICCs) (28) between scores from questionnaires administered at enrollment and 2–4 weeks later by telephone. The prevalence of participants with elevated anxiety symptoms was determined using established threshold scores for the AIR (≥8) (21), the GAD-7 (≥5) (18), and the HADS-A (≥8) (19). The test performance characteristics of each questionnaire was evaluated using ROC curves, sensitivity and specificity, positive and negative predictive values, and screening accuracy using the MINI anxiety assessment as the gold standard (29, 30). We defined screening accuracy as the number of participants who were correctly identified (e.g., true positives and true negatives) divided by the total number of participants.
Comparisons of the sensitivity, specificity, and test performance characteristics of the three questionnaires were made using McNemar’s test. Linear mixed-effects models were used to compare positive and negative predicted values, and area under the ROC curves (30). P values for the positive and negative likelihood ratios were calculated by bootstrapping. Minimal clinically important differences were estimated on the basis of the distributional properties of the scores, 0.5 SD (30). The same diagnostic parameters were calculated using only a diagnosis of panic disorder on the MINI as the gold standard. Statistically significant differences were defined as comparisons with a P value less than 0.05. No adjustments were made for multiple comparisons. A post-hoc analysis evaluating the diagnostic parameters in the subgroup that reported current treatment with an antidepressant or anxiolytic drugs was performed. Data were analyzed with SAS version 9.4 (SAS Institute).
Results
Two hundred and eighty-two individuals were screened and 223 enrolled in the study between May 2016 and December 2016 at 16 ALA-ACRC clinical centers. Reasons for exclusion included 45 individuals with an FEV1 percent predicted of 80% or greater or an FEV1/FVC ratio of 0.7 or greater, 10 individuals with an MoCA score of 18 or lower, and five individuals for reasons based on both those criteria. One participant was lost to follow-up between visit 1 and visit 2. Two enrolled participants were not included in the analysis because one individual did not have COPD based on lung function tests and one individual’s MINI results were considered unacceptable based on the independent psychologist’s review (see Figure E1 in the online supplement).
Participants were older (median age, 65 yr), 54% were male, and most self-identified as white (76%) as shown in Table 1. Lung function values demonstrated a range of moderate to severe COPD. COPD had a medium to high impact in most participants (86%) in their health-related quality of life, based on a CAT score equal to or exceeding 10.
Table 1.
Characteristics at enrollment
| Characteristic | Total (N = 220) |
|---|---|
| Demographics | |
| Median age at enrollment, median (first–third Q), yr | 65 (59–73) |
| Male, n (%) | 118 (54%) |
| White, n (%) | 168 (76%) |
| Current or former smoker, n (%) | 215 (98%) |
| Pack-years, median (first–third Q) | 42 (28–60) |
| COPD characteristics | |
| Median age at COPD onset, median (first–third Q), yr | 54 (47–62) |
| ≥1 ER visits or hospitalizations for COPD in prior 12 mo, n (%) | 65 (30%) |
| ≥1 course of corticosteroids in prior 12 mo, n (%) | 94 (43%) |
| ≥1 course of antibiotic in prior 12 mo, n (%) | 101 (46%) |
| Use of supplemental oxygen, n (%) | 91 (41%) |
| COPD scores, median (first–third Q) | |
| mMRC Dyspnea Scale score (score range, 1–5) | 3 (2–4) |
| CAT score (score range, 0–40) | 18 (12–24) |
| Pulmonary function measures, median (first–third Q) | |
| Post-bronchodilator FEV1, % predicted | 49.7 (35.8–62.7) |
| Post-bronchodilator FEV1/FVC ratio | 0.51 (0.39–0.60) |
| 6-Min walk test distance, m | 1,165 (905–1,378) |
| Mental health assessments | |
| MoCA score (score range, 0–30), median (first–third Q) | 25 (23–27) |
| PHQ-9 score (score range, 0–27), median (first–third Q) | 4 (2–8) |
| MINI diagnosis of major depression, n (%) | 54 (25%) |
| Use of antidepressants or anxiolytic medications in past 2 wk, n (%) | |
| Benzodiazepines | 20 (9%) |
| Buspirone | 2 (1%) |
| Any antidepressant or anxiolytic medication | 65 (30%) |
| Use of mental health treatments in past 2 wk, n (%) | |
| Mental health therapy or counseling | 22 (10%) |
| Substance abuse treatment (excluding smoking cessation) | 2 (1%) |
| Socioeconomic | |
| Household income, n (%) | |
| <22,0000 | 72 (32%) |
| 22,000–43,999 | 47 (21%) |
| >44,000 | 68 (31%) |
| Don’t know/refused to answer | 33 (15%) |
| Education, n (%) | |
| High school or less | 20 (10%) |
| Some college | 62 (28%) |
| Graduate or post-graduate | 111 (50%) |
| Don’t know/refused to answer | 26 (12%) |
Definition of abbreviations: CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; ER = emergency room; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; MINI = Mini-International Neuropsychiatric Interview, version 7.0; mMRC = Modified Medical Research Council; MoCA = Montreal Cognitive Assessment; PHQ-9 = Patient Health Questionnaire; Q = quartile.
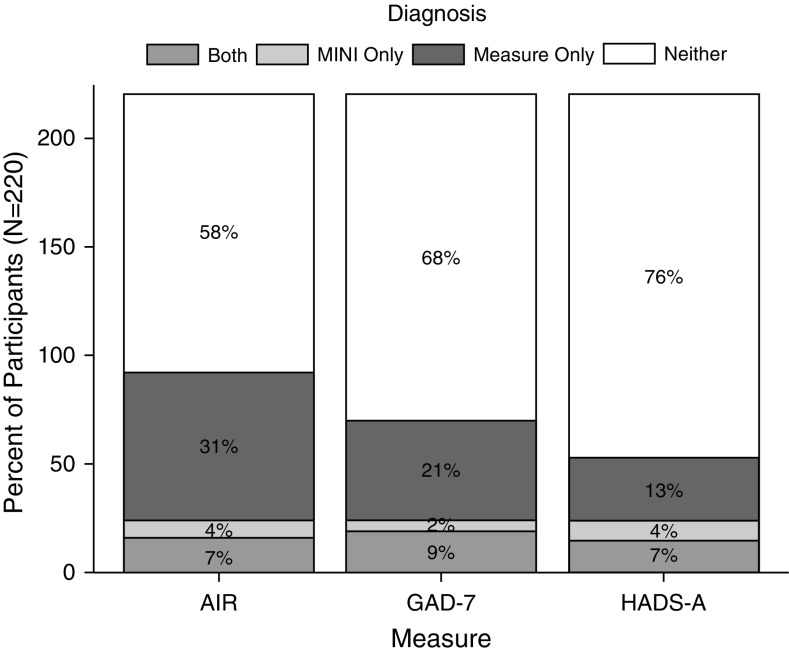
On the basis of the MINIs, the prevalence and 95% confidence intervals (CIs) for any anxiety disorder was 11% (7–16%) and 7% (4–11%) for panic disorder per the DSM-V criteria (Figure 1 and Table 2). A clinical psychologist independently listened to and scored 20% of the MINIs for reliability; the overall κ (95% CI) for agreement on the presence of any anxiety disorder was 0.73 (0.37–1.00) (31); κ values for specific anxiety diagnoses are provided in Table E1. The AIR identified 38% (32–45%) of participants as having elevated anxiety symptoms; the GAD-7 identified 30% (24–36%); and the HADS-A identified 20% (15–26%) (Figure E1). The estimated prevalence of anxiety based on MINIs was significantly lower than the prevalence based on questionnaire scores (P ≤ 0.001 for all pairwise comparisons). Similarly, all the questionnaires had prevalence estimates that were significantly different from each other: AIR versus GAD-7 (P = 0.004), AIR versus HADS-A (P < 0.001), and GAD-7 versus HADS-A (P < 0.001).
Figure 1.
Prevalence of anxiety symptoms by questionnaires. AIR = Anxiety Inventory for Respiratory Disease; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; HADS-A = Hospital Anxiety and Depression Scale Anxiety subscale; MINI = Mini-International Neuropsychiatric Interview, version 7.0.
Table 2.
Summary of Mini-International Neuropsychiatric Interview prevalence of anxiety and individual diagnostic categories
| MINI Diagnoses | N = 220 |
|---|---|
| Diagnosis of any anxiety disorder, n (%) | 24 (11%) |
| Panic disorder, lifetime, n (%)* | 15 (7%) |
| Panic disorder, current, n (%) | 6 (3%) |
| Agoraphobia, n (%) | 10 (5%) |
| Social anxiety disorder, n (%) | 3 (1%) |
| Post-traumatic stress disorder, n (%) | 6 (3%) |
| Generalized anxiety disorder, n (%) | 4 (2%) |
Definition of abbreviation: MINI = Mini-International Neuropsychiatric Interview, version 7.0.
Includes those with current panic disorder.
Cronbach’s α values were similar for all questionnaires. The ICCs for agreements between the in-person and telephone interviews were higher for the GAD-7 and the HADS-A (Table 3). The minimally important differences estimated from distributional analyses (e.g., 0.5 SD) for the AIR, GAD-7, and HADS-A were 3, 2, and 2, respectively. Sensitivity and specificity plots of questionnaire scores showed that the previously defined threshold scores for high risk of anxiety of at least 8 for AIR and at least 5 for GAD-7 were close to optimal for simultaneously maximizing sensitivity and specificity (Figures E2 and E3). For the HADS-A, a lower cutoff score than typically used, at least 7 instead of at least 8, maximized sensitivity and specificity simultaneously (Figure E4).
Table 3.
Summary and comparison of characteristics of AIR, GAD-7, and HADS-A at in-person visit and telephone interview
| Questionnaire (Possible Range of Scores) | |||
|---|---|---|---|
| AIR (0–30)* | GAD-7 (0–21)* | HADS-A (0–21)* | |
| In-person visit (n = 220) | |||
| Median (first–third Q) | 6 (2–10) | 2 (0–5) | 3 (1–7) |
| Cronbach’s α (95% CI) | 0.89 (0.87–0.91) | 0.89 (0.87–0.91) | 0.87 (0.84–0.89) |
| MID† | 3 | 2 | 2 |
| Telephone interview (n = 219) | |||
| Median (first–third Q) | 3 (0–7) | 2 (0–4) | 3 (1–6) |
| Cronbach’s α (95% CI) | 0.90 (0.88–0.92) | 0.89 (0.87–0.91) | 0.86 (0.83–0.89) |
| ICC (95% CI) | 0.60 (0.52–0.68) | 0.70 (0.63–0.77) | 0.68 (0.61–0.75) |
Definition of abbreviations: AIR = Anxiety Inventory for Respiratory Disease; CI = confidence interval; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; HADS-A = Hospital Anxiety and Depression Scale Anxiety subscale; ICC = intraclass correlation coefficient; MID = minimally important difference; Q = quartile.
Higher scores indicate more severe anxiety symptoms.
Minimally important difference defined as 0.5 SD.
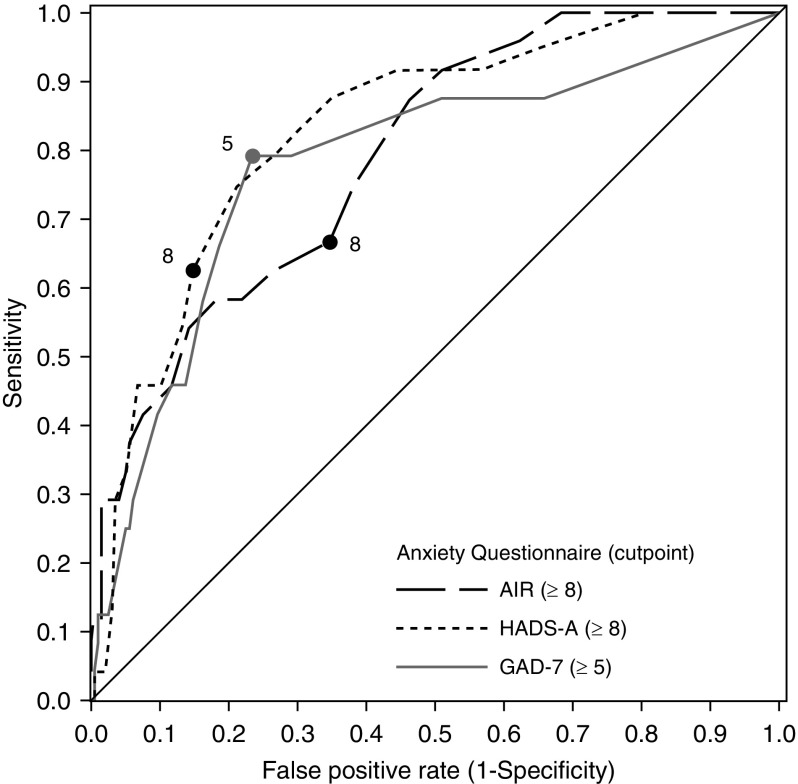
The AUC for the ROC curves was highest for the GAD-7 (Figure 2 and Table 4) and significantly greater than the AUC for AIR (P = 0.014), but not the HADS-A (P = 0.430). AUCs were not statistically different between the HADS-A and AIR (P = 0.116). Using established threshold scores, the sensitivity was 78% for the GAD-7, 66% the for AIR, and 63% for the HADS-A; there were no statistical differences between the questionnaires. Specificity was statistically lower for the AIR (65%) as compared with either the GAD-7 (77%; P < 0.001) or the HADS-A (85%; P < 0.001). Specificity for the GAD-7 was also statistically lower than for the HADS-A (P < 0.001). The HADS-A had significantly higher screening accuracy, 83% compared with 77% for the GAD-7 (P = 0.020) and 66% for the AIR (P < 0.001); the GAD-7 was more accurate than the AIR (P < 0.001).
Figure 2.
Receiver operating characteristic curves for three questionnaires to correctly identify the MINI diagnosis of any anxiety. AIR = Anxiety Inventory for Respiratory Disease; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; HADS-A = Hospital Anxiety and Depression Scale Anxiety subscale; MINI = Mini-International Neuropsychiatric Interview, version 7.0.
Table 4.
Diagnostic properties of AIR, GAD-7, and HADS-A assessments of anxiety and panic compared with gold standard MINI evaluation
| Diagnostic Properties Estimate (95% CI) |
P Value* |
|||||
|---|---|---|---|---|---|---|
| Questionnaire Cutoff Score |
AIR vs. GAD-7 | AIR vs. HADS-A | GAD-7 vs. HADS-A | |||
| AIR ≥ 8 | GAD-7 ≥ 5 | HADS-A ≥ 8 | ||||
| Any MINI anxiety diagnosis | |
|||||
| AUC | 0.66 (0.56–0.76) | 0.78 (0.69–0.87) | 0.74 (0.64–0.84) | 0.014 | 0.116 | 0.430 |
| Sensitivity | 0.67 (0.48–0.86) | 0.79 (0.63–0.95) | 0.63 (0.43–0.82) | 0.180 | 0.655 | 0.102 |
| Specificity | 0.65 (0.59–0.72) | 0.77 (0.71–0.82) | 0.85 (0.8–0.9) | <0.001 | <0.001 | <0.001 |
| Positive predictive value | 0.19 (0.11–0.27) | 0.29 (0.18–0.4) | 0.34 (0.2–0.48) | 0.002 | 0.002 | 0.275 |
| Negative predictive value | 0.94 (0.9–0.98) | 0.97 (0.94–1) | 0.95 (0.92–0.98) | 0.087 | 0.590 | 0.171 |
| Screening accuracy | 0.66 (0.59–0.72) | 0.77 (0.71–0.82) | 0.83 (0.78–0.88) | <0.001 | <0.001 | 0.020 |
| LR+ | 1.92 (1.37–2.7) | 3.37 (2.44–4.67) | 4.22 (2.67–6.67) | <0.001 | <0.001 | 0.091 |
| LR− | 0.51 (0.29–0.91) | 0.27 (0.12–0.6) | 0.44 (0.26–0.74) | 0.048 | 0.655 | 0.140 |
| MINI panic/agoraphobia diagnosis | |
|||||
| AUC | 0.68 (0.57–0.78) | 0.75 (0.65–0.85) | 0.72 (0.61–0.83) | 0.108 | 0.391 | 0.593 |
| Sensitivity | 0.7 (0.5–0.9) | 0.75 (0.56–0.94) | 0.6 (0.39–0.81) | 0.564 | 0.317 | 0.180 |
| Specificity | 0.65 (0.58–0.72) | 0.75 (0.69–0.81) | 0.84 (0.79–0.89) | 0.002 | <0.001 | <0.001 |
| Positive predictive value | 0.17 (0.09–0.25) | 0.23 (0.13–0.33) | 0.27 (0.14–0.4) | 0.021 | 0.015 | 0.309 |
| Negative predictive value | 0.96 (0.92–0.99) | 0.97 (0.94–1) | 0.96 (0.92–0.99) | 0.323 | 0.914 | 0.299 |
| Screening accuracy | 0.66 (0.59–0.72) | 0.75 (0.69–0.81) | 0.82 (0.77–0.87) | 0.001 | <0.001 | 0.007 |
| LR+ | 2 (1.42–2.82) | 3 (2.12–4.25) | 3.75 (2.32–6.05) | 0.002 | <0.001 | 0.205 |
| LR− | 0.46 (0.23–0.91) | 0.33 (0.16–0.72) | 0.48 (0.28–0.82) | 0.165 | 0.890 | 0.228 |
Definition of abbreviations: AIR = Anxiety Inventory for Respiratory Disease; AUC = area under receiver operator curves; CI = confidence interval; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; HADS-A = Hospital Anxiety and Depression Anxiety subscale; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; MINI = Mini-International Neuropsychiatric Interview, version 7.0.
The P values for AUC, and positive and negative predictive values, are based on linear mixed-effects models; P values for sensitivity, specificity, and diagnostic accuracy are based on McNemar’s test.
Includes current and lifetime panic.
The patterns of results were similar for the specific MINI diagnosis of anxiety related to panic (Table 4, bottom) and in the subgroup of 60 individuals reporting current treatment with an antidepressant or anxiolytic medications (Tables E2–E4, FigureE5). Positive and negative predictive values of anxiety and panic for each of the questionnaires over plausible prevalence are shown in Tables E4 and E5, respectively.
Discussion
Anxiety as a comorbidity of COPD has been associated with higher risk of mortality than other common comorbid diseases, for example, heart disease and diabetes (9). Barriers for identifying anxiety in patients with COPD include patient-level factors (e.g., stigma, physical symptoms masking disorder), provider-level factors (e.g., lack of standardized approach to diagnosis, short visit times), and system-level factors (e.g., poor integration with mental health systems) (7, 15). A simple and easily administered assessment of anxiety symptoms that overcomes these barriers to screening is needed in this population as screening tools for further evaluation and clinical trials (32, 33).
The current study is one of the few studies to use a recognized gold standard for the diagnosis of anxiety disorders, the MINI diagnosis of any DSM-V anxiety disorder, and to compare the performance of three questionnaires designed to screen for anxiety disorders. Although a true gold standard would have been the performance of interviews by a psychiatrist or psychologist, that would not have been feasible for this multicenter study, because not every site had a psychologist/psychiatrist available and familiar with COPD. The MINI can be administered by trained non–mental health professionals, is updated for DSM-V criteria, and has been used in multiple other studies for mental health diagnosis. Two of the questionnaires evaluated were designed to be used in a broad range of patients, the GAD-7 (34–36) and the HADS-A (37), whereas the AIR is a newer questionnaire designed specifically for individuals with COPD (21). The three anxiety screening questionnaires identified more people with elevated symptoms of anxiety compared with the proportion of people who met diagnostic criteria on the MINIs. DSM-V diagnostic criteria for all of the anxiety disorders require functional limitations due to anxiety symptoms. The higher prevalence estimates of anxiety symptoms may be due to the study participants being older and less likely to be active because of the severity of respiratory impairment compared with the general population. However, our estimate of the prevalence of anxiety, 11%, is consistent with estimates for anxiety in the past 12 months of 9–19% among U.S. adults in early 2000 and the 1990s (38). In an older U.S. cohort, the prevalence of any anxiety disorder was 14% in women and 8% in men (39).
The three questionnaires used had acceptable values on measures of consistency; Cronbach’s α values were about 0.9 at both visits for all questionnaires (40). On the basis of the ICC values there was moderate reliability for the GAD-7 and HADS-A (40), but not the AIR. Test–retest reliability may have been reduced because the second questionnaire was administered by telephone. The results suggest that telephone interviews for the GAD-7 and HADS-A are an acceptable method for screening for anxiety. Telephone administration could augment screening programs at a relatively low cost. Consistent with the overall evaluation based on AUCs, using established threshold scores the GAD-7 and HADS-A had superior screening accuracy properties as compared with the AIR for any anxiety disorder and for panic-related anxiety disorder. A lower screening threshold value for the HADS-A, at least 7 instead of more than 8, resulted in higher sensitivity and specificity (Figure E4). Others have identified higher (41) and lower (42) cutoff values as being optimal for the HADS-A. Regardless, those studies had similar sensitivity (71%, 79%) and specificity (79%, 71%) for the HADS-A among 56 and 55 patients with COPD, respectively. However, the prevalence of anxiety as measured by MINIs was higher in both those studies, 25%.
Our findings were unexpected because the AIR was developed specifically for screening patients with COPD, includes more items specifically about panic, and performed well in a feasibility study of patients with a mean age of 71 years and severe functional impairment (43). We hypothesized that sudden episodes of breathlessness due to COPD might lead to more panic-related anxiety symptoms, and panic disorder was the most common type of anxiety in this population. Sensitivity was not statistically different among the three questionnaires, and the GAD-7 and HADS-A had higher specificity in identifying individuals without anxiety or panic disorder. Like the AIR, neither the GAD-7 nor HADS-A includes questions about somatic symptoms that can be confused with the physical symptoms of COPD.
The psychometric properties of all three questionnaires were similar to the overall results in the subgroup of individuals reporting current use of an antidepressant or anxiolytic drugs. As expected, positive predictive values were numerically higher in this subgroup COPD population that had a higher prevalence anxiety; however, sensitivity was also numerically higher.
Strengths of the current study include use of the MINI as a gold standard, comparison of three questionnaires, multiple recruitment sites, and administration by trained coordinators.
This study has several limitations that need into consideration. First, neither the GAD-7 nor HADS-A questionnaire emerged as clearly superior across all metrics. The GAD-7 had a larger AUC and higher sensitivity, but neither was statistically different from the HADS-A. Specificity and screening accuracy were statistically higher for the HADS-A compared with the GAD-7. AUCs, which favored the GAD-7, reflect both sensitivity and specificity. Screening accuracy is dependent on prevalence and anxiety, as identified by the MINI, was relatively low in this population. Using a lower threshold score for anxiety symptoms, 7 instead of 8, for the HADS-A would have increased sensitivity and AUC because the lower score simultaneously maximized sensitivity and specificity (Figure E4). However, using variable threshold scores based on specific populations would complicate the use of an instrument, and our results may have been idiosyncratic to our participants.
Second, telephone administration of instruments at the second time point moderate κ agreement for the anxiety diagnosis based on an independent psychologist’s review of 20% of the sample. In addition, there was no evaluation of responsiveness of questionnaires to changes in anxiety level to an intervention. Third, trained interviewers who administered the MINI diagnostic scale for anxiety were not blinded to the results of the screening tools. This may have introduced unwanted bias to the study.
Conclusion
Anxiety in COPD constitutes a significant problem leading to impaired quality of life, functional limitations, and poor outcomes and is associated with higher dropout from pulmonary rehabilitation (17, 18). We found a low prevalence of clinical anxiety as defined by DSM-V criteria in our population: 11% based on MINIs. However, based on questionnaire scores, the prevalence of anxiety symptoms ranges between 20% and 38%. The GAD-7 and HADS-A, which are established anxiety screening instruments, demonstrated only fair to moderate psychometric characteristics for identifying anxiety symptoms in COPD that varied across the range of prevalence of anxiety. The choice of which questionnaire to use depends on the objectives of screening. If the objective is to cast a wide net for more intensive evaluation, the GAD-7 may be marginally preferable. Regular screening with well-validated instruments for anxiety should be instituted in patients with COPD for both early identification and treatment. It is also crucial to consider referral of a patient who screens positive for anxiety to a mental health specialist for appropriate diagnosis (further assessment) and treatment. These results suggest that further work is needed to develop and test anxiety screening measures that perform better across the full range of prevalence of anxiety disorders.
Acknowledgments
American Lung Association Airways Clinical Research Centers
Baylor College of Medicine, Houston, Texas: Nicola Hanania, M.D., M.S., F.C.C.P. (principal investigator), Marianna Sockrider, M.D., Ph.D. (co-principal investigator), Laura Bertrand, R.N., R.P.F.T. (principal clinic coordinator), Mustafa Atik, M.D. (coordinator), Amit Parulekar, M.D., Harold Farber, M.D. (study physicians). Former member: Blanca A. Lopez, L.V.N.
Columbia University–New York University Consortium, New York: Joan Reibman, M.D. (principal investigator), Emily DiMango, M.D., Linda Rogers, M.D. (co-principal investigators), Karen Carapetyan, M.A. (principal clinic coordinator at New York University), Kristina Rivera, M.P.H., Melissa Scheuerman, B.Sc. (clinic coordinators at Columbia University). Former members: Elizabeth Fiorino, M.D., Newel Bryce-Robinson.
Duke University Medical Center, Durham, North Carolina: Loretta G. Que, M.D. (principal investigator), Jason Lang, M.D., Ph.D. (co-principal investigator), Erika M. Coleman, B.S., Eli Morgan, B.A. (coordinators), Heather Kuehn, B.S. (regulatory coordinator), Anne Matthews, M.D., Devon Paul, M.D., M.P.H. (study physicians), Catherine Foss, B.S., R.R.T., C.C.R.C. (principal clinic coordinator), Nicholas Eberlein, B.A.
Illinois Consortium, Chicago, Illinois: Lewis Smith, M.D. (principal investigator), Ravi Kalhan, M.D., James Moy, M.D., Edward Naureckas, M.D. (co-principal investigators), Jenny Hixon, B.S., C.C.R.C. (principal clinic coordinator), Zenobia Gonsalves, Virginia Zagaja, Jennifer Kustwin, Ben Xu, B.S., C.C.R.C., Thomas Matthews, M.P.H., R.R.T., Lucius Robinson III, Noopur Singh (coordinators).
Louisiana State University Health Sciences Center-New Orleans, Section of Pulmonary and Critical Care/Allergy and Immunology: Kyle Happel, M.D. (principal investigator), Marie C. Sandi, F.N.P.-B.C. (principal clinic coordinator), Jennifer M. Graham, Katelyn Sullivan, Elizabeth Poretta (data system operators).
National Jewish Health, Denver, Colorado: Rohit Katial, M.D. (principal investigator), Flavia Hoyte, M.D., Maria Rojas (principal clinic coordinator).
St. Louis Asthma Clinical Research Center: Washington University, St. Louis, Missouri: Mario Castro, M.D., M.P.H. (principal investigator), Leonard B. Bacharier, M.D., Kaharu Sumino, M.D., Roger D. Yusen, M.D., M.P.H.(co-investigators); Jaime J. Tarsi, R.N., M.P.H. (principal clinic coordinator), Brenda Patterson, M.S.N., R.N., F.N.P. (coordinator); Terri Montgomery (data entry operator).
St. Vincent Hospital and Health Care Center, Inc, Indianapolis, Indiana: Michael Busk, M.D., M.P.H. (principal investigator), Debra Weiss (principal clinic coordinator). Former member: Kimberly Sundblad.
Vermont Lung Center at the University of Vermont, Colchester, Vermont: Charles Irvin, Ph.D. (principal investigator), Anne E. Dixon, M.D., David A. Kaminsky, M.D., Charlotte Teneback, M.D., Jothi Kanagalingam, M.D. (co-principal investigators), Stephanie M. Burns (principal clinic coordinator), Kathleen Dwinell (data system operator).
University of Arizona, Tucson, Arizona: Lynn B. Gerald, Ph.D., M.S.P.H. (principal investigator), James L. Goodwin, Ph.D., Mark A. Brown, M.D. (co-investigators), Tara F. Carr, M.D., Cristine E. Berry, M.D., M.H.S., Christian Bime, M.D., Mark A. Goforth, F.N.P.-C. (study physicians), Elizabeth A. Ryan, B.S., R.R.T. (principal clinic coordinator), Jesus A. Wences, B.S., Silvia L. Lopez, R.N., Janette C. Priefert, B.S. (coordinators), Natalie S. Provencio-Dean, B.S., Destinee R. Ogas (data system operator). Former member: Valerie R. Bloss, B.S.
University of California, San Diego: Stephen I. Wasserman, M.D. (principal investigator), Joe W. Ramsdell, M.D., Xavier T. Soler, M.D., Ph.D. (co-principal investigators), Katie H. Kinninger, R.C.P. (principal clinic coordinator), Amber J. Martineau (coordinator). Former members: Tonya Greene, Samang Ung.
University of Miami, Miami, Florida–University of South Florida, Tampa, Florida: Adam Wanner, M.D. (principal investigator, Miami), Richard Lockey, M.D. (principal investigator, Tampa), Thomas B. Casale, M.D. (co-principal investigator, Tampa), Andreas Schmid, M.D., Michael Campos, M.D. (co-principal investigators, Miami), Monroe King, D.O. (co-principal investigator, Tampa), Eliana S. Mendes, M.D. (principal clinic coordinator, Miami), Catherine Renee Smith (principal clinic coordinator, Tampa), Jeaneen Ahmad, Patricia D. Rebolledo, Johana Arana, Lilian Cadet, Shawna Atha, C.R.C., Rebecca McCrery, Sarah M. Croker, B.A. (coordinators).
University of Missouri, Kansas City School of Medicine, Kansas City: Gary Salzman, M.D. (principal investigator), Asem Abdeljalil, M.D., Abid Bhat, M.D., Ashraf Gohar, M.D. (co-principal investigators), Mary Reed, R.N., B.S.N, C.C.R.C. (principal clinic coordinator).
University of Virginia, Charlottesville, Virginia: W. Gerald Teague, M.D. (principal investigator), Larry Borish, M.D. (co-principal investigator), Kristin W Wavell, B.S., C.C.R.C. (principal clinic coordinator), Theresa A. Altherr (study coordinator). Former members: Donna Wolf, Ph.D., Shahleen Ahmed.
Chairman’s Office, University of Alabama, Birmingham, Alabama: William C. Bailey, M.D.
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore, Maryland: Robert Wise, M.D. (center director), Janet Holbrook, Ph.D., M.P.H. (deputy director), Alexis Rea (principal coordinator), Joy Saams, R.N., Anne Casper, Robert Henderson, Andrea Lears, B.S., Deborah Nowakowski, David Shade, J.D., Elizabeth Sugar, Ph.D. Former members: Bethany Grove, Adante Hart, B.S., Lea T. Drye, Ph.D.
Data and Safety Monitoring Board: Vernon M. Chinchilli, M.D. (chair), Paul N. Lanken, M.D., Donald P. Tashkin, M.D.
Project Office, American Lung Association, New York: Alexandra Sierra (project officer), Norman H. Edelman, M.D. (scientific consultant), Susan Rappaport, M.P.H. The sponsor had a role in the management and review of the study. Former member: Elizabeth Lancet, M.P.H.
Footnotes
Supported by the American Lung Association (DC002N).
A complete list of members for the American Lung Association Airway Clinical Research Centers may be found before the beginning of the References.
Author Contributions: All authors met the four following criteria for authorship: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version submitted for publication; accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the American Lung Association Airways Clinical Research Centers, Nicola Hanania, Marianna Sockrider, Laura Bertrand, Mustafa Atik, Blanca A. Lopez, Joan Reibman, Emily DiMango, Linda Rogers, Karen Carapetyan, Kristina Rivera, Melissa Scheuerman, Elizabeth Fiorino, Newel Bryce-Robinson, Loretta G. Que, Deanna Green, Robert Noveck, Catherine Foss, Jessica Ghidorzi, Zongyao Wang, Elise Pangborn, V. Susan Robertson, Nicholas Eberlein, Jane Stiles, Michael Land, Brian Vickery, Eveline Wu, Denise Jaggers, Stephanie Allen, Sabrena Mervin-Blake, Lewis Smith, Ravi Kalhan, James Moy, Edward Naureckas, Jenny Hixon, Zenobia Gonsalves, Virginia Zagaja, Jennifer Kustwin, Ben Xu, Thomas Matthews, Lucius Robinson, III, Noopur Singh, Kyle Happel, Marie C. Sandi, Jennifer M. Graham, Katelyn Sullivan, Elizabeth Poretta, Rohit Katial, Flavia Hoyte, Maria Rojas, Mario Castro, Leonard B. Bacharier, Kaharu Sumino, Roger D. Yusen, Jaime J. Tarsi, Brenda Patterson, Terri Montgomery, Michael Busk, Debra Weiss, Kimberly Sundblad, Charles Irvin, Anne E. Dixon, David A. Kaminsky, Charlotte Teneback, Jothi Kanagalingam, Stephanie M. Burns, Kathleen Dwinell, Lynn B. Gerald, James L. Goodwin, Mark A. Brown, Tara F. Carr, Cristine E. Berry, Christian Bime, Mark A. Goforth, Elizabeth A. Ryan, Jesus A. Wences, Silvia L. Lopez, Janette C. Priefert, Natalie S. Provencio-Dean, Destinee R. Ogas, Valerie R. Bloss, Stephen I. Wasserman, Joe W. Ramsdell, Xavier T. Soler, Katie H. Kinninger, Amber J. Martineau, Tonya Greene, Samang Ung, Adam Wanner, Richard Lockey, Thomas B. Casale, Andreas Schmid, Michael Campos, Monroe King, Eliana S. Mendes, Catherine Renee Smith, Jeaneen Ahmad, Patricia D. Rebolledo, Johana Arana, Lilian Cadet, Shawna Atha, Rebecca McCrery, Sarah M. Croker, Gary Salzman, Asem Abdeljalil, Abid Bhat, Ashraf Gohar, Mary Reed, W. Gerald Teague, Larry Borish, Kristin W Wavell, Theresa A. Altherr, Donna Wolf, Shahleen Ahmed, William C. Bailey, Robert Wise, Janet Holbrook, Alexis Rea, Joy Saams, Bethany Grove, Adante Hart, Andrea Lears, Deborah Nowakowski, David Shade, Elizabeth Sugar, Anne Shanklin Casper, Lea T. Drye, Vernon M. Chinchilli, Paul N. Lanken, Donald P. Tashkin, Alexandra Sierra, Norman H. Edelman, Susan Rappaport, and Elizabeth Lancet
References
- 1.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest. 2013;143:1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics Health, United States, 2015: with special feature on racial and ethnic health disparities. [2016 May; updated 2017 June 22]Available from: https://www.cdc.gov/nchs/data/hus/hus15.pdf [PubMed]
- 3.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance: United States, 1999–2011. Chest. 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper CB. Airflow obstruction and exercise. Respir Med. 2009;103:325–334. doi: 10.1016/j.rmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130:647–656. doi: 10.1378/chest.130.3.647. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147:989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Pifarré J, Alsedà M, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Hai. 2017;195:77–85. doi: 10.1007/s00408-016-9966-0. [DOI] [PubMed] [Google Scholar]
- 8.Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205–1211. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- 9.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 10.Yohannes AM, Baldwin RC, Connolly MJ. Prevalence of depression and anxiety symptoms in elderly patients admitted in post-acute intermediate care. Int J Geriatr Psychiatry. 2008;23:1141–1147. doi: 10.1002/gps.2041. [DOI] [PubMed] [Google Scholar]
- 11.Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15:1090–1096. doi: 10.1002/1099-1166(200012)15:12<1090::aid-gps249>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178:913–920. doi: 10.1164/rccm.200804-619OC. [DOI] [PubMed] [Google Scholar]
- 13.Felker B, Katon W, Hedrick SC, Rasmussen J, McKnight K, McDonnell MB, et al. The association between depressive symptoms and health status in patients with chronic pulmonary disease. Gen Hosp Psychiatry. 2001;23:56–61. doi: 10.1016/s0163-8343(01)00127-x. [DOI] [PubMed] [Google Scholar]
- 14.Cully JA, Graham DP, Stanley MA, Ferguson CJ, Sharafkhaneh A, Souchek J, et al. Quality of life in patients with chronic obstructive pulmonary disease and comorbid anxiety or depression. Psychosomatics. 2006;47:312–319. doi: 10.1176/appi.psy.47.4.312. [DOI] [PubMed] [Google Scholar]
- 15.Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4) Suppl:43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 17.Speilberger CD, Gorsuch R, Lushene R, Vagg PR, Jacobs AG. Palo Alto, CA: Consulting Psycholgists Press; 1983. Manual for the State-Trait Anxiety Inventory (form Y) [Google Scholar]
- 18.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Janssen DJ, Spruit MA, Leue C, Gijsen C, Hameleers H, Schols JM, et al. on behalf of the Ciro network. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chron Respir Dis. 2010;7:147–157. doi: 10.1177/1479972310369285. [DOI] [PubMed] [Google Scholar]
- 21.Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the Anxiety Inventory for Respiratory Disease. Chest. 2013;144:1587–1596. doi: 10.1378/chest.13-0168. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12:232–241. [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Yohannes AM, Dryden S, Hanania NA. The responsiveness of the Anxiety Inventory for Respiratory Disease Scale following pulmonary rehabilitation. Chest. 2016;150:188–195. doi: 10.1016/j.chest.2016.02.658. [DOI] [PubMed] [Google Scholar]
- 25.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) Dyspnoea Scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 27.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 28.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 30.Eusebi P. Diagnostic accuracy measures. Cerebrovasc Dis. 2013;36:267–272. doi: 10.1159/000353863. [DOI] [PubMed] [Google Scholar]
- 31.Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80:99–103. doi: 10.1207/S15327752JPA8001_18. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Donker T, van Straten A, Marks I, Cuijpers P. Quick and easy self-rating of generalized anxiety disorder: validity of the Dutch web-based GAD-7, GAD-2 and GAD-SI. Psychiatry Res. 2011;188:58–64. doi: 10.1016/j.psychres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Kertz S, Bigda-Peyton J, Bjorgvinsson T. Validity of the Generalized Anxiety Disorder-7 Scale in an acute psychiatric sample. Clin Psychol Psychother. 2013;20:456–464. doi: 10.1002/cpp.1802. [DOI] [PubMed] [Google Scholar]
- 35.Wild B, Eckl A, Herzog W, Niehoff D, Lechner S, Maatouk I, et al. Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 Scales: results of a validation study. Am J Geriatr Psychiatry. 2014;22:1029–1038. doi: 10.1016/j.jagp.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 36.Terrill AL, Hartoonian N, Beier M, Salem R, Alschuler K. The 7-item Generalized Anxiety Disorder Scale as a tool for measuring generalized anxiety in multiple sclerosis. Int J MS Care. 2015;17:49–56. doi: 10.7224/1537-2073.2014-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 38.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourdon KH, Rae DS, Locke BZ, Narrow WE, Regier DA. Estimating the prevalence of mental disorders in U.S. adults from the Epidemiologic Catchment Area Survey. Public Health Rep. 1992;107:663–668. [PMC free article] [PubMed] [Google Scholar]
- 40.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan T, Carter O, Adams C, Waterer G, Chung LP, Hawkins M, et al. Discriminant validity of the Hospital Anxiety and Depression Scale, Beck Depression Inventory (II) and Beck Anxiety Inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13:220–228. doi: 10.1177/1479972316634604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung G, Patrick C, Sullivan G, Cooray M, Chang CL. Sensitivity and specificity of the Geriatric Anxiety Inventory and the Hospital Anxiety and Depression Scale in the detection of anxiety disorders in older people with chronic obstructive pulmonary disease. Int Psychogeriatr. 2012;24:128–136. doi: 10.1017/S1041610211001426. [DOI] [PubMed] [Google Scholar]
- 43.Yohannes AM, Willgoss TG. The accuracy of the Anxiety Inventory Respiratory Disease Scale for patients with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2015;30:106–108. doi: 10.1002/gps.4202. [DOI] [PubMed] [Google Scholar]