Abstract
Assembled α-synuclein in nerve cells and glial cells is the defining pathological feature of neurodegenerative diseases called synucleinopathies. Seeds of α-synuclein can induce the assembly of monomeric protein. Here, we used sucrose gradient centrifugation and transiently transfected HEK 293T cells to identify the species of α-synuclein from the brains of homozygous, symptomatic mice transgenic for human mutant A53T α-synuclein (line M83) that seed aggregation. The most potent fractions contained Sarkosyl-insoluble assemblies enriched in filaments. We also analyzed six cases of idiopathic Parkinson's disease (PD), one case of familial PD, and six cases of multiple system atrophy (MSA) for their ability to induce α-synuclein aggregation. The MSA samples were more potent than those of idiopathic PD in seeding aggregation. We found that following sucrose gradient centrifugation, the most seed-competent fractions from PD and MSA brains are those that contain Sarkosyl-insoluble α-synuclein. The fractions differed between PD and MSA, consistent with the presence of distinct conformers of assembled α-synuclein in these different samples. We conclude that α-synuclein filaments are the main driving force for amplification and propagation of pathology in synucleinopathies.
Keywords: alpha-synuclein (α-synuclein), oligomer, neurodegeneration, Parkinson disease, fibril, brain disease, Lewy body, multiple system atrophy, seeded aggregation, synucleinopathy
Introduction
Assemblies of filamentous α-synuclein define a group of neurodegenerative diseases called synucleinopathies (1). Parkinson's disease (PD),2 the most common synucleinopathy, is characterized by the presence of Lewy body and Lewy neurite inclusions in a number of brain regions, including the substantia nigra (2, 3). Lewy pathology also defines Lewy body dementia, which encompasses dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD) (2–4). Unlike PD, in Lewy body dementia, α-synuclein pathology is predominant in neocortical and limbic regions, as well as in substantia nigra (5, 6). Multiple system atrophy (MSA) is defined by the presence of abundant filamentous α-synuclein inclusions in oligodendrocytes, also known as glial cytoplasmic inclusions or Papp-Lantos bodies (7–10); smaller numbers of α-synuclein inclusions are present in nerve cells (11, 12). Cases of MSA are classified as MSA-P, with predominant parkinsonism caused by striatonigral degeneration, and MSA-C, with cerebellar ataxia associated with olivopontocerebellar atrophy (13). Autonomic dysfunction is common to both MSA-P and MSA-C.
α-Synuclein is a 140-amino acid protein, which is natively unfolded (1). It interacts with membranes of different lipid composition, and binds preferentially to lipids with high curvature (14, 15). α-Synuclein is believed to facilitate exocytosis of neurotransmitters through dilation of synaptic vesicle pores (16). In synucleinopathies, there is a conversion from monomeric to filamentous α-synuclein. To understand disease pathogenesis, it is essential to work out how α-synuclein assembles into filaments and how these assemblies propagate through the brain.
Six missense mutations in SNCA, the α-synuclein gene, cause familial PD (17–23); duplications and triplications of SNCA also give rise to PD (24–26). The brains of individuals with these mutations are littered with α-synuclein inclusions that are indistinguishable from those in sporadic PD, indicating that dysfunction or overexpression of α-synuclein is sufficient to cause assembly and neurodegeneration.
α-Synuclein inclusions originate in distinct parts of the brain, from where they appear to spread to anatomically connected regions (27, 28). Based on this, a staging system for PD has been proposed (29). Apparent spread of assembled α-synuclein has also been observed in PD patients who received fetal mesencephalic grafts for therapeutic relief (30, 31). Transfer of assembled α-synuclein has been shown experimentally, both in vivo and in vitro. Grafts implanted in transgenic mice developed α-synuclein inclusions, demonstrating inter-neuronal spread (32, 33). Cell-based assays have also demonstrated the transfer of α-synuclein between cells (34) and have identified some pathways for internalization (35).
Noncell-autonomous mechanisms have come to explain how toxic amyloid aggregates can spread through prion-like mechanisms (36). Intracerebral injection of brain homogenates has been shown to induce or promote the assembly of α-synuclein (37–39). Injection of filaments assembled from recombinant α-synuclein also induced aggregation and neurodegeneration (40, 41). Moreover, peripheral injections of assembled α-synuclein seeded α-synuclein aggregation in the brains of transgenic mice (42–45). A recent study reported that injection of α-synuclein filaments assembled from recombinant protein into the muscle layers of pylorus and duodenum of WT mice led to the assembly of α-synuclein and neurodegeneration in brain regions affected in PD (46).
The most seed-competent species of assembled α-synuclein are only poorly understood. We therefore used sucrose gradient centrifugation and transiently transfected human embryonic kidney (HEK 293T) cells to identify α-synuclein seeds from the brains of homozygous, symptomatic mice transgenic for human A53T α-synuclein (line M83) (47), and from PD and MSA brains.
Results
HEK 293T cell-seeding assay
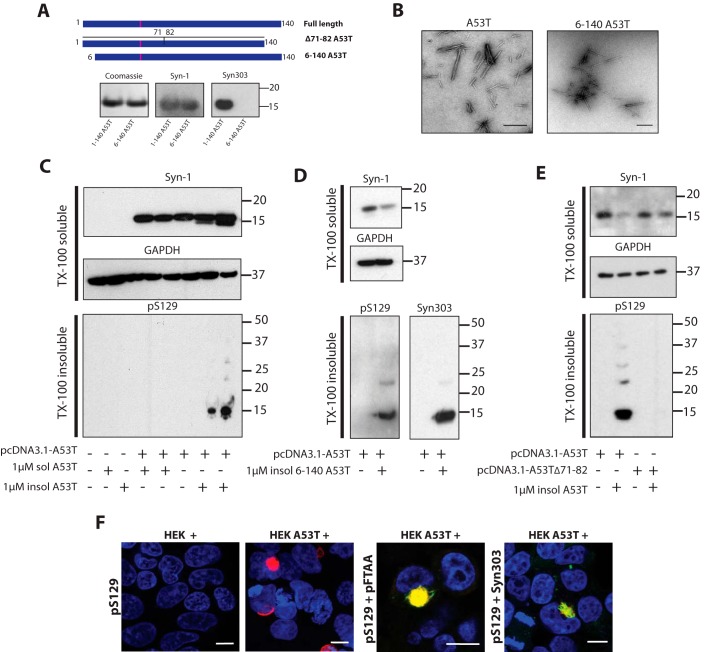
Recombinant full-length (1–140) and N terminally truncated (6–140) human A53T α-synucleins were expressed, purified, and assembled into filaments (Fig. 1, A and B). They were then used as seeds following transient expression of full-length A53T α-synuclein in HEK 293T cells. Addition of seeds induced the formation of Triton X-100–insoluble α-synuclein that was positive with an antibody specific for phosphorylation at serine 129 (pS129) (Fig. 1C). Assembled α-synuclein is phosphorylated at Ser-129 in vivo (48, 49). Nonassembled α-synuclein did not induce aggregation (Fig. 1C). To establish that insoluble α-synuclein was different from the seeds, assembled (6–140) A53T α-synuclein was used. Antibody Syn303 is specific for amino acids (1–5) of α-synuclein (50). Seeds of 6–140 A53T α-synuclein induced the formation of Triton X-100–insoluble α-synuclein that was positive with antibodies pS129 and Syn303 (Fig. 1D), showing that the assemblies were made of full-length α-synuclein. When Δ71–82 A53T α-synuclein, which is unable to assemble (51), was expressed, no seeded aggregation was observed (Fig. 1E). Moreover, immunohistochemistry showed perinuclear and skein-like inclusions, which were positive for antibody pS129 and the luminescent-conjugated oligothiophene pFTAA, in both transfected and seeded and cells (Fig. 1F). pFTAA stains the pS129–positive α-synuclein inclusions of PD and MSA (52).
Figure 1.
Seeding assay for A53T α-synuclein in HEK 293T cells. A, full-length 1–140 and N terminally truncated 6–140 A53T α-synuclein in pRK172 were expressed in E. coli BL21 (DE3) cells. Following purification (Coomassie), immunoblotting showed that Syn-1 labeled both full-length and truncated α-synuclein, whereas Syn303 labeled full-length, but not truncated, α-synuclein. After transient transfection, full-length and Δ71–82 A53T α-synuclein were expressed in HEK 293T cells. B, electron micrographs of assembled full-length and truncated A53T α-synuclein. Scale bar, 500 nm. C, Western blot analysis of untransfected and A53T α-synuclein–transfected HEK 293T cells seeded with unassembled and assembled full-length A53T α-synuclein. Cell lysates were separated into Triton X-100–soluble and -insoluble fractions by ultracentrifugation at 100,000 × g for 1 h at 4 °C. Blots of anti-α-synuclein antibody Syn-1 and anti-GAPDH were processed for the Triton X-100–soluble fractions and anti-α-synuclein antibody pS129 was used for Triton X-100–insoluble fractions. Twenty μg of Triton X-100–soluble and 50 μg of Triton X-100–insoluble proteins were run on 4–12% BisTris SDS-PAGE. D, Western blot analysis of full-length A53T α-synuclein expressing HEK 293T cells seeded with N terminally truncated A53T α-synuclein aggregates. Triton X-100–soluble fractions were blotted with Syn-1 and anti-GAPDH. Triton X100–insoluble fractions were blotted with pS129 and Syn303. E, Western blot analysis of full-length and Δ71–82 A53T α-synuclein expressing HEK 293T cells seeded with 1 μm full-length α-synuclein aggregates. Triton X-100–soluble fractions were blotted with Syn-1 and anti-GAPDH antibodies. Triton X-100–insoluble fractions were blotted with anti-pS129 antibody. F, staining of untransfected (HEK+) and full-length A53T α-synuclein–transfected (HEK A53T+) cells seeded with 1 μm aggregated N terminally truncated 6–140 A53T α-synuclein with pS129 (red), Syn303 (green), and pFTAA (green). Nuclei were visualized with DAPI (blue). Scale bar, 10 μm.
Characterization of α-synuclein from the brains of mice transgenic for human A53T α-synuclein (line M83)
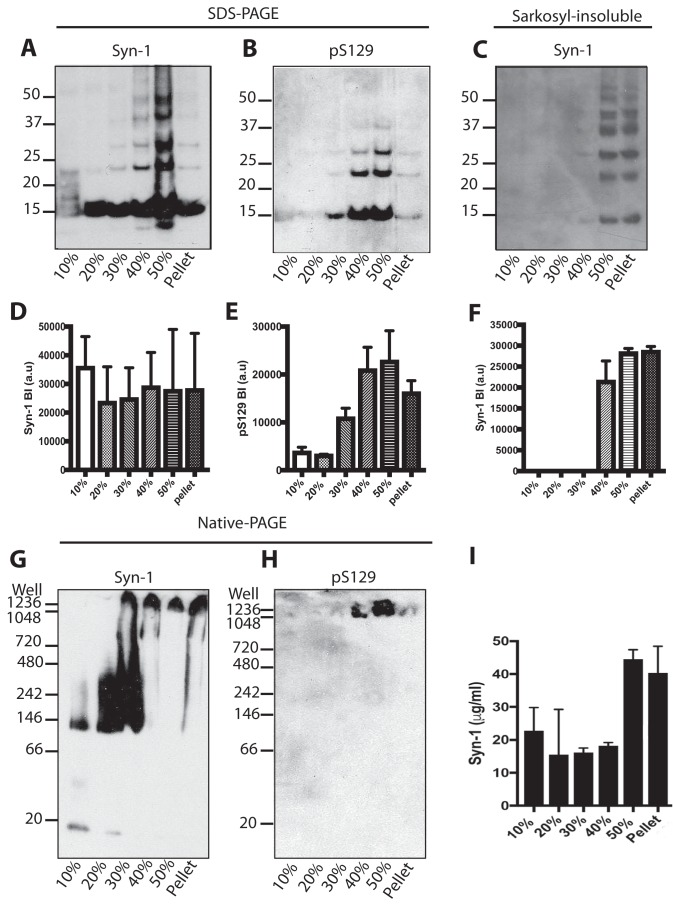
We used sucrose gradient centrifugation to separate α-synuclein assemblies from the brains of homozygous, symptomatic mice transgenic for human A53T α-synuclein (line M83). Sucrose densities were: 50, 40, 30, 20, and 10% in PBS. Brain lysates were spun at 281,000 × g for 4 h at 20 °C. Fractions were analyzed by Western blotting with anti-α-synuclein antibodies Syn-1 and pS129 to detect total and phosphorylated α-synuclein, respectively. By SDS-PAGE, all fractions contained monomeric α-synuclein that ran as a 15-kDa band (Fig. 2, A, C, D, and F). It is well-established that some higher molecular mass bands, such as those at 22 and 29 kDa, correspond to ubiquitinated α-synuclein (49, 53–55) Assembled α-synuclein, which was pS129–positive, was present in the 30, 40, 50%, and pellet fractions, with highest levels in the 40 and 50% fractions (Fig. 2, B and E). Sarkosyl-insoluble α-synuclein was detected in the 40, 50%, and pellet fractions (Fig. 2, C and F). Native-PAGE Western blot analysis with Syn-1 revealed monomeric α-synuclein in the 10% sucrose fraction, which ran at ∼15 kDa and a higher molecular mass species that ran at 140 kDa (Fig. 2G). The 20% fraction contained predominantly higher molecular mass multimeric α-synuclein, which ranged from 140 to 480 kDa. The same was true of the 30% fraction, which contained in addition multimeric α-synuclein between the well and 480 kDa. Moreover, this fraction also contained α-synuclein that did not enter the gel. Material that did not enter the gel was also present in the 40, 50%, and pellet fractions. Antibody pS129 only recognized α-synuclein species that were trapped in the wells (Fig. 2I). The amount of α-synuclein in each fraction was determined by dot-blotting with Syn-1 (Fig. 2F). In contrast to homozygous M83 mice, heterozygous mice did not develop motor dysfunction. Sucrose gradient fractionated brain extracts from heterozygous M83 mice were unable to seed aggregation of human A53T α-synuclein in transfected HEK 293T cells (Fig. S1).
Figure 2.
Characterization of α-synuclein from the brains of homozygous M83 mice following sucrose gradient centrifugation. A and B, Western blot analysis (anti-α-synuclein antibodies Syn-1 and pS129) following SDS-PAGE of brain lysates fractionated by sucrose gradient centrifugation. A representative blot is shown. C, Western blot analysis (antibody Syn-1) of Sarkosyl-insoluble brain lysates. D–F, densitometric analysis of fractionated brain lysates and Sarkosyl-insoluble lysates. The results are the mean ± S.D. (n = 3). G and H, Western blot analysis (anti-α-synuclein antibodies Syn-1 and pS129) following native-PAGE of brain lysates fractionated by sucrose gradient centrifugation. I, to quantify the amount of α-synuclein in the sucrose gradient fractions, dot-blot analysis was performed with α-synuclein protein standards. The results are expressed as the mean ± S.D. (n = 3).
Immunoelectron microscopy
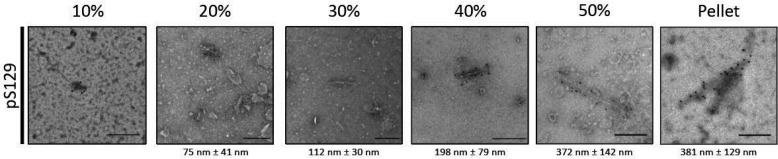
Immunoelectron microscopy using an antibody specific for α-synuclein phosphorylated at Ser-129 was used to study the presence of α-synuclein assemblies in sucrose gradient fractions from the brains of mice transgenic for human A53T α-synuclein (Fig. 3). In the 10% fraction, we did not detect any pS129–positive α-synuclein assemblies. The 20% fraction contained pS129–positive filaments of 75 ± 41 nm. Filaments in the 30% fraction were 112 ± 30 nm, 198 ± 79 nm in the 40% fraction, and 372 ± 142 nm in the 50% fraction (Fig. 3).
Figure 3.
Immunoelectron microscopy of brain lysates from homozygous M83 mice following sucrose gradient fractionation. A, immunoelectron microscopy of fractionated brain lysates from homozygous, symptomatic mice transgenic for human A53T α-synuclein (line M83) with anti-α-synuclein pS129 antibody. The measured filament lengths (20, 30, 40, 50%, and pellet) are shown (n = 15). Scale bar, 200 nm.
Seeded aggregation of α-synuclein with sucrose gradient-fractionated brain lysates from mice transgenic for human A53T α-synuclein
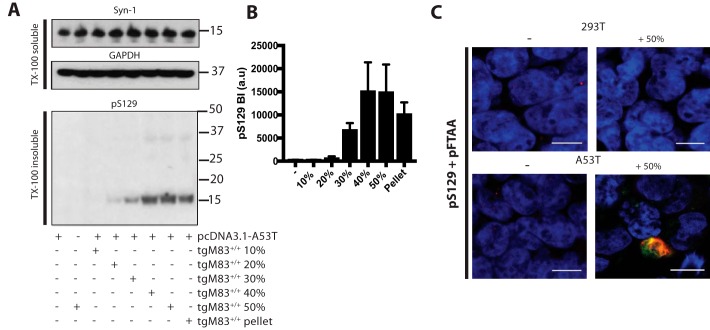
The ability of fractionated assemblies to seed aggregation was investigated in the HEK 293T cell-seeding assay. Dot-blot analysis was used to quantify α-synuclein levels. One μg of α-synuclein from each fraction was then added to HEK 293T cells expressing human A53T α-synuclein and incubated for 72 h. The 30, 40, and 50% sucrose fractions, as well as the pellet, induced seeded aggregation (Fig. 4, A and B). 10 and 20% fractions were inactive. The 40 and 50% fractions were the most potent, followed by pellet and 30% fractions. Immunohistochemistry with anti-α-synuclein antibody pS129 and staining with pFTAA confirmed seeded aggregation (Fig. 4C). When HEK 293T cells that did not express α-synuclein were seeded with the 50% sucrose fraction, no staining was observed with the pS129 antibody.
Figure 4.
Seeding potency of sucrose gradient fractionated brain lysates from homozygous M83 mice. A, Western blot analysis of untransfected and full-length human A53T α-synuclein–transfected HEK 293T cells seeded with sucrose gradient-fractionated homozygous M83 brain lysates. Cells were incubated with sucrose gradient fractions for 72 h and harvested for Western blot analysis. Cell lysates were separated into Triton X-100–soluble and –insoluble fractions by ultracentrifugation at 100,000 × g for 1 h at 4 °C. Twenty μg of protein of the supernatant was loaded onto 4–12% BisTris SDS-PAGE for Triton X-100–soluble fractions and 50 μg of protein from the pellet for Triton X-100–insoluble fractions. A representative Western blotting is shown. B, densitometric analysis of Triton X-100–insoluble pS129 in seeded cells. The results are expressed as the mean ± S.D. (n = 3). C, staining of untransfected (293T) and full-length A53T α-synuclein–transfected (A53T) cells unseeded (−) and seeded with 50% sucrose fraction using antibody pS129 (red) and LCO pFTAA (green). Nuclei were visualized with DAPI (blue). Scale bar, 10 μm.
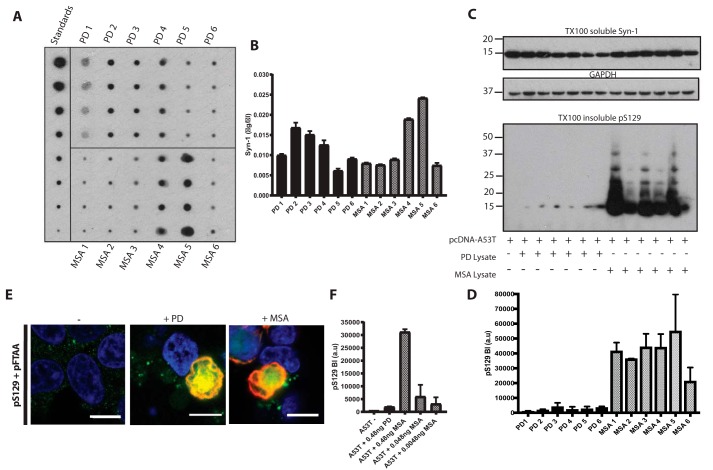
α-Synuclein assemblies from Parkinson's disease and multiple system atrophy brains seed aggregation
α-Synuclein levels were measured by dot blotting of substantia nigra from six cases of neuropathologically confirmed idiopathic PD and cerebellum from six cases of neuropathologically confirmed MSA-C using recombinant α-synuclein standards (Fig. 5, A and B). One ng of α-synuclein from each brain lysate was added to HEK 293T cells expressing human A53T α-synuclein and incubated for 72 h. Western blot analysis showed that MSA brain lysates induced α-synuclein assembly more robustly than PD lysates (Fig. 5, C, D, and F). Staining confirmed the presence of α-synuclein assemblies in seeded cells, which were positive using antibody pS129 and pFTAA (Fig. 5E). In some experiments, superior temporal gyrus from a case of neuropathologically confirmed PD and diffuse DLB with a heterozygous duplication of SNCA was used (56).
Figure 5.
Parkinson's disease and multiple system atrophy brain lysates induce α-synuclein aggregation in transfected HEK 293T cells. A and B, to quantify α-synuclein in six idiopathic PD (PD1–6) and six MSA (MSA1–6) brain lysates, we used dot blotting with anti-α-synuclein antibody Syn-1. Recombinant α-synuclein protein standards were blotted alongside diluted brain lysates (n = 3). C, Western blot analysis of human A53T full-length α-synuclein–transfected cells seeded with 1 ng of α-synuclein from each of the PD and MSA brain lysates. Triton X-100–soluble fractions were blotted with Syn-1 and anti-GAPDH antibodies, and Triton X-100–insoluble fractions were blotted with anti-α-synuclein pS129 antibody. D, densitometric analysis of Triton X-100–insoluble pS129 band intensities from seeded HEK 293T cells. E, staining of human A53T α-synuclein–transfected cells unseeded (−) and seeded with PD (+PD) and MSA (+MSA) brain lysates stained for anti-α-synuclein antibody pS129 (red) and pFTAA (green). Nuclei were visualized with DAPI (blue). Scale bar, 10 μm. F, densitometric analysis of human A53T α-synuclein–transfected cells seeded with 0.48 ng of PD lysate and serially diluted MSA lysate. The results are expressed as the mean ± S.D. (n = 3).
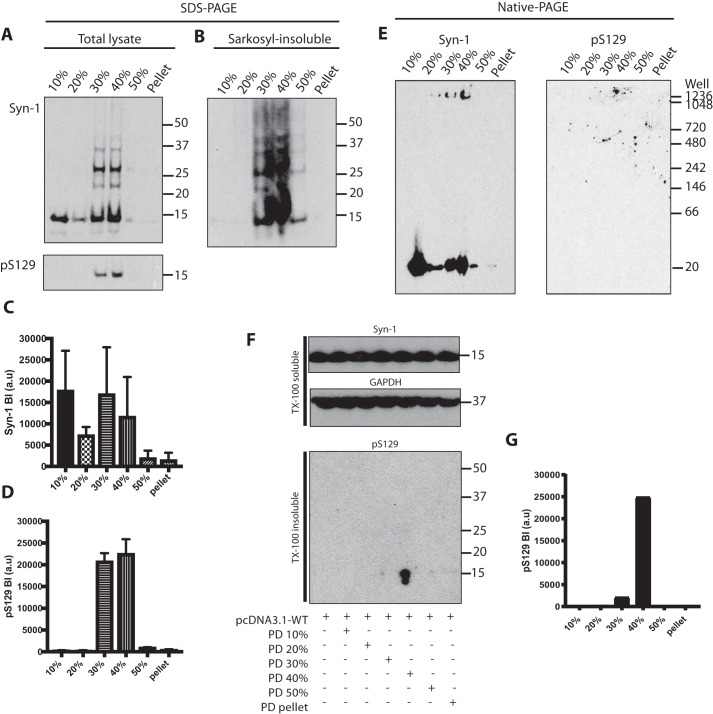
Characterization of α-synuclein from Parkinson's disease brains and identification of seed-competent species
Brain lysates of substantia nigra from two cases of idiopathic PD (PD2 and PD3) and the case of familial PD were fractionated by sucrose gradient centrifugation. Western blot analysis with Syn-1 showed monomeric α-synuclein in all fractions and higher molecular weight α-synuclein in the 30 and 40% fractions (Fig. 6, A–D). Anti-pS129 antibody was only positive with material in the 30 and 40% fractions (Fig. 6A). These fractions contained Sarkosyl-insoluble α-synuclein, as did the 50% fraction (Fig. 6B). Nondenaturing gel electrophoresis showed the presence of high-molecular weight assemblies of α-synuclein that did not migrate into the gel in the 30 and 40% fractions, and confirmed the presence of monomeric α-synuclein in most fractions (Fig. 6E). Brain lysates from PD2 and PD3, as well as from the case of familial PD, were tested in the cell-seeding assay. One ng of α-synuclein from each fraction was added to HEK 293T cells expressing WT α-synuclein. The case of familial PD (Fig. 6, F and G), but not PD2 or PD3, seeded aggregation. Only the 40% sucrose gradient fraction seeded robustly. Occasionally, the 30% fraction also seeded.
Figure 6.
Characterization of α-synuclein from Parkinson's disease brains following sucrose gradient centrifugation and identification of seed-competent species. A, Western blot analysis (anti–α-synuclein antibodies Syn-1 and pS129) following SDS-PAGE of brain lysates fractionated by sucrose gradient centrifugation. A representative blot using PD2 is shown. B, Western blot analysis (antibody Syn-1) of Sarkosyl-insoluble brain lysates. C and D, densitometric analysis of fractionated brain lysates. The results are expressed as the mean ± S.D. (n = 3). E, Western blot analysis (anti-α-synuclein antibodies Syn-1 and pS129) following native-PAGE of brain lysates fractionated by sucrose gradient centrifugation. F, Western blot analysis following SDS-PAGE of HEK 293T cells expressing human WT α-synuclein seeded with fractionated lysates of the superior temporal gyrus from an SNCA duplication case. Triton X-100–soluble cell lysates were blotted with Syn-1 and anti-GAPDH antibodies. Triton X-100 insoluble fractions were blotted with pS129. G, densitometric analysis of Triton X-100–insoluble pS129 bands of seeded cells.
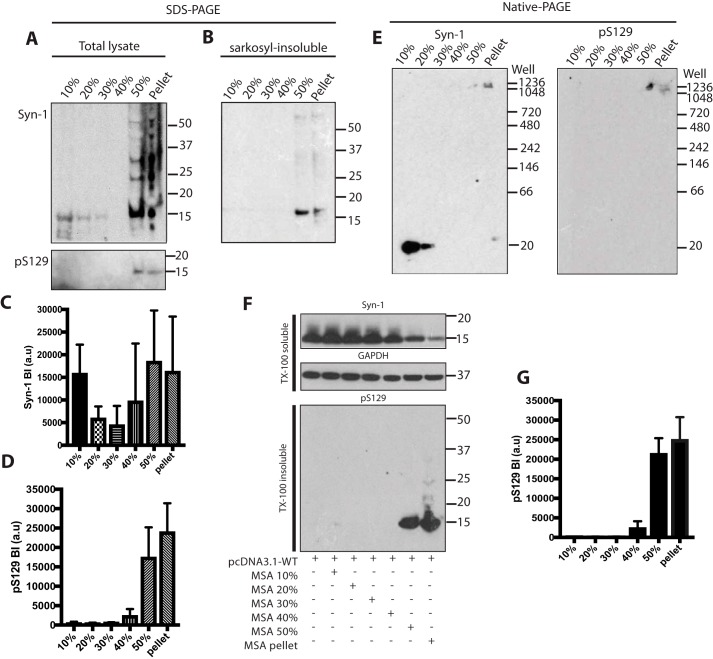
Characterization of α-synuclein from multiple system atrophy brains and identification of seed-competent species
Brain lysates of cerebellum from cases MSA1, MSA4, and MSA5 were fractionated by sucrose gradient centrifugation. Western blot analysis with Syn-1 showed monomeric α-synuclein in all fractions and higher molecular weight α-synuclein in the 50% and pellet fractions (Fig. 7, A–D). Anti-pS129 antibody was only positive with material in the 50% and pellet fractions (Fig. 7A). These fractions contained Sarkosyl-insoluble α-synuclein (Fig. 7B). Nondenaturing gel electrophoresis showed the presence of high-molecular weight assemblies of α-synuclein that did not migrate into the gel in the 50% and pellet fractions, and confirmed the presence of monomeric α-synuclein in the less dense fractions (Fig. 7E). One ng of α-synuclein from each fraction was added to HEK 293T cells expressing WT α-synuclein. Only the 50% and pellet fractions seeded robustly. Occasionally the 40% fraction also seeded (Fig. 7, F and G). All three cases of MSA gave similar results.
Figure 7.
Characterization of α-synuclein from multiple system atrophy brains following sucrose gradient centrifugation and identification of seed-competent species. A, Western blot analysis (anti-α-synuclein antibodies Syn-1 and pS129) following SDS-PAGE of brain lysates fractionated by sucrose gradient centrifugation. A representative blot using MSA5 is shown. B, Western blot analysis (antibody Syn-1) of Sarkosyl-insoluble brain lysates. C and D, densitometric analysis of fractionated brain lysates. E, Western blot analysis (anti-α-synuclein antibodies Syn-1 and pS129) following native-PAGE of brain lysates fractionated by sucrose gradient centrifugation. F, Western blot analysis following SDS-PAGE of HEK cells expressing human WT α-synuclein seeded with fractionated lysates. Triton X-100–soluble cell lysates were blotted with Syn-1 and anti-GAPDH antibodies. Triton X-100–insoluble fractions were blotted with pS129. G, densitometric analysis of Triton X-100–insoluble pS129 bands of seeded cells. Densitometric results are expressed as the mean ± S.D. (n = 3).
Discussion
Assembled α-synuclein can spread between anatomically connected neurons and seed the aggregation of soluble α-synuclein into filaments (57). α-Synuclein species responsible for seeding are not completely known. We therefore used sucrose gradient centrifugation (58) to analyze brain lysates from symptomatic mice transgenic for human mutant A53T α-synuclein (line M83) (47); such lysates support α-synuclein propagation (37, 38). Highly aggregated forms pellet more rapidly than amorphous aggregates, small oligomers, and monomeric α-synuclein.
Analysis of brain lysates by SDS-PAGE following sucrose gradient centrifugation revealed the presence of monomeric and multimeric α-synuclein. Syn-1 identified monomeric α-synuclein in all fractions. Ubiquitinated α-synuclein was mostly present in the 40 and 50% fractions. The latter were also labeled predominantly by an antibody specific for α-synuclein phosphorylated at Ser-129. The 40 and 50% sucrose gradient fractions were the most potent at seeding aggregation of α-synuclein in the HEK 293T cell assay. The pellet fraction was slightly less effective, followed by the 30% sucrose fraction. The 10 and 20% fractions were devoid of significant activity. Sarkosyl-insoluble α-synuclein was present in the 40, 50%, and pellet fractions. Because these fractions are enriched in filaments and because filaments have been shown to seed aggregation (38, 59), it appears likely that α-synuclein filaments of 198–381 nm were the most seed-competent species. Previous work using α-synuclein assembled from recombinant protein and untransfected SH-SY5Y cells also showed that short filaments were the most seed-potent (60). Moreover, sonicated filaments assembled from recombinant human α-synuclein were more potent at seeding assembly than nonsonicated filaments (61).
By native PAGE, several types of α-synuclein assemblies were observed upon fractionation of brain lysates from symptomatic M83 mice. Syn-1–positive α-synuclein resolved as a ladder ranging from 140 to 1,200 kDa in the 10–30% fractions. The 30% fraction also contained high-molecular weight α-synuclein (>1,240 kDa). The latter, which did not enter the gel, was the major species in the 40, 50%, and pellet fractions. The pS129 α-synuclein antibody only labeled material that did not enter the gel.
These findings are reminiscent of those obtained for tau protein from the brains of symptomatic mice transgenic for human mutant P301S tau following sucrose gradient fractionation and seeding in HEK 293T cells (62). Short filaments were the major species of seed-competent tau. It thus appears that filamentous tau and filamentous α-synuclein are the molecular species with the most seeding activity. Monomeric proteins and small oligomers were inactive. These findings are in contrast to those reporting that some species of tau monomers were able to seed aggregation of human mutant, truncated tau in a biosensor cell line (63). Although human A53T α-synuclein was transiently expressed in the present study, previous work (62) used HEK 293T cells stably expressing human 0N4Rtau. It remains to be seen if stable expression of A53T α-synuclein will result in less variability.
Different conformers of assembled α-synuclein may be characteristic of PD and MSA (64–69). Thus, previous studies have demonstrated α-synuclein seeding activity in MSA, but not PD, brains (65, 66). We found that α-synuclein seeds from the cerebellum of MSA patients seeded aggregation more potently than seeds from the substantia nigra of PD patients. However, in contrast to the previous work (65, 66), we found that PD brain extracts were also seed-competent in HEK 293T cells expressing human mutant A53T α-synuclein. When expressed relative to a constant amount of α-synuclein, MSA extracts were ∼100 times more potent at seeding aggregation than PD extracts. It is possible that MSA extracts contained more seed-competent α-synuclein than PD extracts, but such differences are unlikely to have been as high as 100-fold. Recently, a biosensor cell line stably expressing A53T α-synuclein-CFP/YFP fusion proteins was used to detect α-synuclein seeds from PD and MSA brains (70).
When fractionating substantia nigra extracts from PD and cerebellar extracts from MSA, the levels of pS129 α-synuclein and Sarkosyl-insoluble protein were highest in the 30 and 40% fractions for PD, and in the 50% and pellet fractions for MSA. Because sucrose gradient centrifugation separates proteins according to size and density, these differences are consistent with the existence of distinct conformers of assembled α-synuclein. Following sucrose gradient centrifugation, the 50% and pellet fractions from the MSA cases tested readily seeded aggregation of WT α-synuclein. By contrast, the 30 and 40% fractions of only one case of PD (familial disease caused by duplication of SNCA) seeded aggregation. Future experiments will use additional cases of idiopathic PD.
The existence of distinct conformers of assembled α-synuclein is also supported by the morphological differences between isolated, negatively stained α-synuclein filaments from PD and DLB brains when compared with MSA brains (9, 72, 73). To establish the existence of distinct filament conformers unambiguously and to understand their differences, high-resolution structures of α-synuclein filaments from human brain are required.
Experimental procedures
Cloning
α-Synuclein cDNAs were amplified from pRK172 vectors by polymerase chain reaction (PCR) with PFU Turbo polymerase. Thermocycles were performed using a Veriti 96-well-thermal cycler (Applied Biosciences). The primers used for constructs to be cloned into pcDNA3.1 mammalian expression vector (Invitrogen) contained a Kozak consensus sequence (ACCACCAUGG) and restriction endonuclease sites (5′) HindIII and (3′) EcoRV. Primer sequences for WT and mutant full-length α-synuclein were as follows: forward primer: 5′-AAGCTTCCACCATGGATGTATTCATG-3′ and reverse primer: 5′GATATCTTAGGCTTCAGGTTC3′. α-Synuclein constructs 6–140 and Δ71–82 were generated using site-directed mutagenesis (Aligent QuikChange Lightening Kit) to remove the relevant amino acids in the human A53T α-synuclein pRK172 plasmid. The designed primer sequences were 5′-CATATGAAAGGACTTTCAAAGGCC-3′ and 5′-AAAAGCTTTTAGGCTTCAGGTTC-3′ for 6–140 A53T α-synuclein and 5′-ACAAATGTTGGAGGAGAGGGAGCAGGGA-3′ and 5′-TCCCTGCTCCCTCTCCTCCAACATTTGT-3′ for Δ71–82 A53T α-synuclein.
Recombinant α-synuclein filaments
Full-length (1–140) and truncated (6–140) human A53T α-synucleins were expressed using pRK172 vectors in Escherichia coli BL21 (DE3) cells and purified using modifications of a published protocol (74). Briefly, anion-exchange chromatography using a HiTrap Q HP column (GE Life Sciences) was followed by size exclusion chromatography on a HiLoad 16/600 Superdex 200 column (GE Life Sciences). α-Synuclein was concentrated to 400 μm in 3K cut-off ultrafiltration spin columns (Vivaspin), dialyzed against phosphate-buffered saline (PBS) overnight at 4 °C, and assembled using rotational shaking (450 rpm) at 37 °C for 5 days. Assembled protein was spun at 100,000 × g for 1 h at 4 °C and the pellets resuspended in PBS at 400 μm, followed by a 30-s sonication (Misonix XL).
Brain lysates
Transgenic mouse line M83, which expresses human A53T α-synuclein under the control of the mouse prion protein promoter (47), was purchased from the Jackson Laboratory (stock number 004479). Symptomatic, homozygous M83 mice aged 14 (female), 16 (male), and 24 (female) months were used. Heterozygous M83 mice used were aged 6 (female), 10 (male), and 14 (male) months. Experiments were carried out in compliance with the UK Animals (Scientific Procedures) Act of 1986 and were approved by the local Animal Welfare and Ethical Review Board. Mice were euthanized by cervical dislocation, followed by exsanguination. Whole brains were dissected, frozen on dry ice, and stored at −80 °C. They were homogenized at 10% (w/v) in sterile PBS plus Complete protease inhibitor mixture (Roche Applied Science) using a Polytron (PT 2100 Kinematica AG). Homogenates were spun at 5,000 × g for 5 min at 4 °C. Supernatants were stored in aliquots at −80 °C until use. α-Synuclein levels were measured by dot blotting. Fresh-frozen substantia nigra from six cases of neuropathologically confirmed idiopathic PD (1 woman (PD1), age at death, 74 years; and 5 men (PD2-PD6), ages at death, 68, 83, 74, 79, and 75 years), superior temporal gyrus from a man with neuropathologically confirmed familial PD and diffuse DLB (SNCA duplication, age at death, 63 years) and cerebellum from six neuropathologically confirmed cases of MSA-C (2 men (MSA2, MSA5), ages at death, 77 and 52 years; and 4 women (MSA1, MSA3, MSA4, MSA6), ages at death, 81, 65, 71, and 83 years) were homogenized in sterile PBS at 200 mg/ml using a Polytron (PT 2500 E, Kinematica AG). Samples were kept on ice and centrifuged at 5,000 rpm at 4 °C for 5 min. Supernatants were stored in aliquots at −80 °C.
Sucrose gradient centrifugation
Ten, 20, 30, 40, and 50% sucrose fractions were prepared in PBS and layered onto 5-ml centrifuge tubes (BeckmanCoulter Ultra-Clear). Volumes were: 0.5 ml at 50%, 0.5 ml at 40%, 0.5 ml at 30%, 0.5 ml at 20%, and 1 ml at 10% sucrose, layered in decreasing density. One ml of brain lysate (15–20 mg of protein) was added on top of the gradient. The tubes were spun at 250,000 × g in a Beckman Ti 60 swing-out rotor for 4 h at 20 °C (58). Fractions were collected and stored at −80 °C. Pellets were resuspended in 0.5 ml of PBS.
Sarkosyl extraction
One hundred μl of the sucrose gradient fractions were incubated with 1% Sarkosyl for 1 h at 22 °C on a flat rotational shaker (700 rpm). Samples were centrifuged at 100,000 × g for 1 h at 4 °C and pellets were resuspended in 50 mm Tris-HCl, pH 7.4.
Dot blotting
Recombinant α-synuclein proteins of known concentration and brain lysates diluted 1:1,000 were vacuum pulled onto a polyvinylidene fluoride (PVDF) membrane (0.22 μm) using a minifold I spot-blot system (GE Healthcare). Membranes were blocked in 5% milk for 1 h and incubated with anti-α-synuclein antibody Syn-1 (1:1,000, Transduction Laboratories) for 1 h at room temperature, washed three times in PBS-Tween, incubated in horseradish peroxidase secondary antibody in 5% milk for 1 h, and washed again. Chemiluminescence was used to quantify the signal. Serial dilutions (0.5–3.5 ng) of recombinant α-synuclein were used as standard. The linear parts of the standard curves were used to determine the concentrations of α-synuclein. Syn-1 recognizes residues 91–99 of human α-synuclein (71).
EM
Recombinant α-synuclein aggregates and sucrose gradient fractions of M83 brain lysates were placed onto 400-meshed Formvar/carbon film-coated, glow-discharged copper grids (Sigma) for 3 min, blocked with distilled water + 0.1% gelatin for 10 min, and incubated with anti-α-synuclein antibody pS129 (1:50, ab51253, Abcam) for 3 h in a humidity chamber. Grids were washed in distilled water and incubated with 10-nm gold-conjugated anti-rabbit secondary antibody (Sigma, 1:25) for 1 h, washed, and stained with 0.1% uranyl acetate. Images were taken on a Philips Spirit transmission microscope and measurements made in Fiji (ImageJ).
HEK 293T cell-seeding assay
HEK 293T cells were transiently transfected with 1 μg of WT human α-synuclein, A53T α-synuclein, or Δ71–82 A53T α-synuclein cDNAs in pcDNA3.1 using Lipofectamine 2000 (Invitrogen) and 6-well-plates. Following overnight incubation, the medium (DMEM + GlutaMAXTM with 10% fetal calf serum) was replaced. Cells were then incubated with sucrose gradient fractions. After 24 h, fresh medium was added and the cells were incubated for a further 48 h. They were then harvested, lysed by incubation in Tris-HCl, pH 7.4, + 1% Triton X-100 for 10 min on ice, and homogenized with a cell sonicator (Misonix, Microson Ultrasonic Cell Disruptor). Lysates were separated into soluble and insoluble fractions by centrifugation at 100,000 × g for 30 min at 4 °C.
Gel electrophoresis and Western blotting
Samples were boiled in buffer containing 2-mercaptoethanol, loaded onto Novex 4–12% BisTris gels (Life Technologies), and run at 200 V for 40 min. Proteins were transferred onto PVDF membranes in a semi-dry transfer tank (Bio-Rad), blocked with 5% milk, and immunoblotted with Syn-1 (1:1,000), Syn303 (1:1,000, BioLegend), or pS129 (1:1,000). After incubation with horseradish peroxidase secondary antibody (1:5,000), bands were visualized using chemiluminescence. For native-PAGE, samples were added to buffer (Invitrogen), loaded onto 4–16% Native-PAGE BisTris gels, and run for 2 h at 150 V. Proteins were transferred onto PVDF membranes by semi-dry transfer.
Immunofluorescence
Cells were seeded and plated onto poly-l-lysine–coated coverslips (BD Biosciences), fixed with 4% paraformaldehyde for 20 min at 37 °C, and permeabilized with 0.1% Triton X-100, 3% BSA in PBS for 1 h. Primary antibodies were incubated overnight at 4 °C, three PBS washes preceded and followed incubation for 1 h with fluorescent secondary antibodies (1:1,000). Stained cells were mounted in Prolong Gold with DAPI and confocal images were taken on a Zeiss710 microscope. Primary antibodies were: pS129 (ab51253, Abcam) at 1:2,000 and Syn303 (BioLegend) at 1:1,000. pFTAA (0.6 μm in PBS) was added to cells for 3 h at room temperature after primary antibody addition.
Data availability
All data presented are contained within the manuscript.
Author contributions
S. A. M. and M. G. conceptualization; S. A. M. data curation; S. A. M. formal analysis; S. A. M. writing-original draft; S. A. M. and M. G. writing-review and editing; I. L., M. A. D., D. W. D., and B. G. resources; J. F., M. M.-S., and D. P. methodology.
Supplementary Material
Acknowledgments
We thank the patients' families for donating brain tissues and Dr. T. Klingstedt for pFTAA.
This work was supported by the United Kingdom Medical Research Council Grant MC_U105184291 (to M. G.) and National Institutes of Health NIA Grant PHS P30 AG01033 (to B. G.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- PD
- Parkinson's disease
- DLB
- dementia with Lewy bodies
- PDD
- Parkinson's disease dementia
- MSA
- Multiple system atrophy
- HEK
- human embryonic kidney
- PVDF
- polyvinylidene fluoride
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- DAPI
- 4′,6-diamidino-2-phenylindole.
References
- 1. Goedert M., Jakes R., and Spillantini M. G. (2017) The synucleinopathies: twenty years on. J. Parkinsons Dis. 7, 51–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., and Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 3. Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., and Goedert M. (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corriveau R. A., Koroshetz W. J., Gladman J. T., Jeon S., Babcock D., Bennett D. A., Carmichael S. T., Dickinson S. L., Dickson D. W., Emr M., Fillit H., Greenberg S. M., Hutton M. L., Knopman D. S., Manly J. J., et al. (2017) Alzheimer's disease-related dementias summit 2016: National research priorities. Neurology 89, 2381–2391 10.1212/WNL.0000000000004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kosaka K. (1978) Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol. 42, 127–134 10.1007/BF00690978 [DOI] [PubMed] [Google Scholar]
- 6. Jellinger K. A., and Korczyn A. D. (2018) Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC Med. 16, 34 10.1186/s12916-018-1016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papp M. I., Kahn J. E., and Lantos P. L. (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J. Neurol. Sci. 94, 79–100 10.1016/0022-510X(89)90219-0 [DOI] [PubMed] [Google Scholar]
- 8. Wakabayashi K., Yoshimoto M., Tsuji S., and Takahashi H. (1998) α-Synuclein immunoreractivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 249, 180–182 10.1016/S0304-3940(98)00407-8 [DOI] [PubMed] [Google Scholar]
- 9. Spillantini M. G., Crowther R. A., Jakes R., Cairns N. J., Lantos P. L., and Goedert M. (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 251, 205–208 10.1016/S0304-3940(98)00504-7 [DOI] [PubMed] [Google Scholar]
- 10. Tu P. H., Galvin J. E., Baba M., Giasson B., Tomita T., Leight S., Nakajo S., Iwatsubo T., Trojanowski J. Q., and Lee V. M. (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann. Neurol. 44, 415–422 10.1002/ana.410440324 [DOI] [PubMed] [Google Scholar]
- 11. Kato S., and Nakamura H. (1990) Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol. 79, 584–594 10.1007/BF00294235 [DOI] [PubMed] [Google Scholar]
- 12. Cykowski M. D., Coon E. A., Powell S. Z., Jenkins S. M., Benarroch E. E., Low P. A., Schmeichel A. M., and Parisi J. E. (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138, 2293–2309 10.1093/brain/awv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fanciulli A., and Wenning G. K. (2015) Multiple system atrophy. N. Engl. J. Med. 372, 249–263 10.1056/NEJMra1311488 [DOI] [PubMed] [Google Scholar]
- 14. Davidson W. S., Jonas A., Clayton D. F., and George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 10.1074/jbc.273.16.9443 [DOI] [PubMed] [Google Scholar]
- 15. Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R., and Südhof T. C. (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Logan T., Bendor J., Toupin C., Thorn K., and Edwards R. H. (2017) α-Synuclein promotes dilation of the exocytotic fusion pore. Nat. Neurosci. 20, 681–689 10.1038/nn.4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- 18. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., and Riess O. (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 10.1038/ng0298-106 [DOI] [PubMed] [Google Scholar]
- 19. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., and de Yebenes J. G. (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 20. Lesage S., Anheim M., Letournel F., Bousset L., Honoré A., Rozas N., Pieri L., Madiona K., Dürr A., Melki R., Verny C., Brice A., and French Parkinson's Disease Genetics Study Group. (2013) G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471 10.1002/ana.23894 [DOI] [PubMed] [Google Scholar]
- 21. Kiely A. P., Asi Y. T., Kara E., Limousin P., Ling H., Lewis P., Proukakis C., Quinn N., Lees A. J., Hardy J., Revesz T., Houlden H., and Holton J. L. (2013) α-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson's disease and multiple system atrophy? Acta Neuropathol. 125, 753–769 10.1007/s00401-013-1096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., Tienari P. J., and Pöyhönen M. (2014) A novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol. Aging 35, 2180.e1–2180.e5 [DOI] [PubMed] [Google Scholar]
- 23. Yoshino H., Hirano M., Stoessl A. J., Imamichi Y., Ikeda A., Li Y., Funayama M., Yamada I., Nakamura Y., Sossi V., Farrer M. J., Nishioka K., and Hattori N. (2017) Homozygous α-synuclein p.A53V in familial Parkinson's disease. Neurobiol. Aging 57, 248.e7–248.e12 [DOI] [PubMed] [Google Scholar]
- 24. Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., and Destée A. (2004) α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 10.1016/S0140-6736(04)17103-1 [DOI] [PubMed] [Google Scholar]
- 25. Ibáñez P., Bonnet A. M., Débarges B., Lohmann E., Tison F., Pollak P., Agid Y., Dürr A., and Brice A. (2004) Causal relation between α-synuclein gene duplication and familial Parkinson's disease. Lancet 364, 1169–1171 10.1016/S0140-6736(04)17104-3 [DOI] [PubMed] [Google Scholar]
- 26. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., and Gwinn-Hardy K. (2003) α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- 27. Goedert M., Spillantini M. G., Del Tredici K., and Braak H. (2013) 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- 28. Braak H., and Del Tredici K. (2017) Neuropathological staging of brain pathology in sporadic Parkinson's disease: separating the wheat from the chaff. J. Parkinsons Dis. 7, S71–S85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braak H., Del Tredici K., Rüb U., de Vos R. A., Jansen Steur E. N., and Braak E. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 30. Li J. Y., Englund E., Holton J. L., Soulet D., Hagell P., Lees A. J., Lashley T., Quinn N. P., Rehncrona S., Björklund A., Widner H., Revesz T., Lindvall O., and Brundin P. (2008) Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 10.1038/nm1746 [DOI] [PubMed] [Google Scholar]
- 31. Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., and Olanow C. W. (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- 32. Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., and Lee S. J. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hansen C., Angot E., Bergström A. L., Steiner J. A., Pieri L., Paul G., Outeiro T. F., Melki R., Kallunki P., Fog K., Li JY, Brundin P. (2011) α-Synuclein propagates from human mouse brain to grafted dopaminergic neurons and seeds aggregation in cultures of human cells. J. Clin. Invest. 121, 715–725 10.1172/JCI43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee H. J., Suk J. E., Bae E. J., Lee J. H., Paik S. R., and Lee S. J. (2008) Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 10.1016/j.biocel.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 35. Holmes B. B., DeVos S. L., Kfoury N., Li M., Jacks R., Yanamandra K., Ouidja M. O., Brodsky F. M., Marasa J., Bagchi D. P., Kotzbauer P. T., Miller T. M., Papy-Garcia D., and Diamond M. I. (2013) Heparan sulphate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. U.S.A. 110, E3138–E3147 10.1073/pnas.1301440110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goedert M. (2015) Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349, 1255555 10.1126/science.1255555 [DOI] [PubMed] [Google Scholar]
- 37. Mougenot A. L., Nicot S., Bencsik A., Morignat E., Verchère J., Lakhdar L., Legastelois S., and Baron T. (2012) Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging 33, 2225–2228 10.1016/j.neurobiolaging.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 38. Luk K. C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J. Q., and Lee V. M. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D. M., and Hasegawa M. (2013) Prion-like spreading of pathological α-synuclein in brain. Brain 136, 1128–1138 10.1093/brain/awt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luk K. C., Kehm V. M., Zhang B., O'Brien P., Trojanowski J. Q., and Lee V. M. (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 10.1084/jem.20112457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osterberg V. R., Spinelli K. J., Weston L. J., Luk K. C., Woltjer R. L., and Unni V. K. (2015) Progressive aggregation of α-synuclein and selective degeneration of Lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 10, 1252–1260 10.1016/j.celrep.2015.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sacino A. N., Brooks M., Thomas M. A., McKinney A. B., Lee S., Regenhardt R. W., McGarvey N. H., Ayers J. I., Notterpek L., Borchelt D. R., Golde T. E., and Giasson B. I. (2014) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10732–10737 10.1073/pnas.1321785111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Breid S., Bernis M. E., Babila J. T., Garza M. C., Wille H., and Tamgüney G. (2016) Neuroinvasion of α-synuclein prionoids after intraperitoneal and intraglossal inoculation. J. Virol. 90, 9182–9193 10.1128/JVI.01399-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ayers J. I., Brooks M. M., Rutherford N. J., Howard J. K., Sorrentino Z. A., Riffe C. J., and Giasson B. I. (2017) Robust central nervous system pathology in transgenic mice following peripheral injection of α-synuclein fibrils. J. Virol. 91, e02095–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woerman A. L., Kazmi S. A., Patel S., Freyman Y., Oehler A., Aoyagi A., Mordes D. A., Halliday G. M., Middleton L. T., Gentleman S. M., Olson S. H., and Prusiner S. B. (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol. 135, 49–63 10.1007/s00401-017-1762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim S., Kwon S. H., Kam T. I., Panicker N., Karuppagounder S. S., Lee S., Lee J. H., Kim W. R., Kook M., Foss C. A., Shen C., Lee H., Kulkamì S., Pasricha P. J., Lee G., et al. (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron 103, 627–641.e7 10.1016/j.neuron.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giasson B. I., Duda J. E., Quinn S. M., Zhang B., Trojanowski J. Q., and Lee V. M. (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 10.1016/S0896-6273(02)00682-7 [DOI] [PubMed] [Google Scholar]
- 48. Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., and Iwatsubo T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- 49. Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., Seubert P., Schenk D., Sinha S., Gai W. P., and Chilcote T. J. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 10.1074/jbc.M600933200 [DOI] [PubMed] [Google Scholar]
- 50. Giasson B. I., Jakes R., Goedert M., Duda J. E., Leight S., Trojanowski J. Q., and Lee V. M. (2000) A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson's disease. J. Neurosci. Res. 59, 528–533 [DOI] [PubMed] [Google Scholar]
- 51. Giasson B. I., Murray I. V., Trojanowski J. Q., and Lee V. M. (2001) A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386 10.1074/jbc.M008919200 [DOI] [PubMed] [Google Scholar]
- 52. Klingstedt T., Ghetti B., Holton J. L., Ling H., Nilsson K. P. R., and Goedert M. (2019) Luminescent conjugated oligothiophenes distinguish between α-synuclein assemblies of Parkinson's disease and multiple system atrophy. Acta Neuropathol. Commun. 7, 193 10.1186/s40478-019-0840-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hasegawa M., Fujiwara H., Nonaka T., Wakabayasi K., Takahashi H., Lee V. M., Trojanowski J. Q., Mann D., and Iwatsubo T. (2002) Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J. Biol. Chem. 277, 49071–49076 10.1074/jbc.M208046200 [DOI] [PubMed] [Google Scholar]
- 54. Sampathu D. M., Giasson B. I., Pawlyk A. C., Trojanowski J. Q., and Lee V. M. (2003) Ubiquitination of α-synuclein is not required for formation of pathological inclusions in α-synucleinopathies. Am. J. Pathol. 163, 91–100 10.1016/S0002-9440(10)63633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tofaris G. K., Razzaq A., Ghetti B., Lilley K. S., and Spillantini M. G. (2003) Ubiquitination of α-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J. Biol. Chem. 278, 44405–44411 10.1074/jbc.M308041200 [DOI] [PubMed] [Google Scholar]
- 56. Konno T., Ross O. A., Puschmann A., Dickson D. W., and Wszolek Z. W. (2016) Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinson Rel. Disord. 22, S1–S6 10.1016/j.parkreldis.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karpowicz R. J. Jr., Trojanowski J. Q., and Lee V. M. (2019) Transmission of α-synuclein seeds in neurodegenerative disease: recent developments. Lab. Invest. 99, 971–981 10.1038/s41374-019-0195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maeda S., Sahara N., Saito Y., Murayama S., Ikai A., and Takashima A. (2006) Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease. Neurosci. Res. 54, 197–201 10.1016/j.neures.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 59. Froula J. M., Castellana-Cruz M., Anabtawi N. M., Camino J. D., Chen S. W., Thrasher D. R., Freire J., Yazdi A. A., Fleming S., Dobson C. M., Kumita J. R., Cremades N., and Volpicelli-Daley L. A. (2019) Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice. J. Biol. Chem. 294, 10392–10406 10.1074/jbc.RA119.007743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aulić S., Le T. T., Moda F., Abounit S., Corvaglia S., Casalis L., Gustincich S., Zurzolo C., Tagliavini F., and Legname G. (2014) Defined α-synuclein prion-like molecular assemblies spreading in cell culture. BMC Neurosci. 15, 69 10.1186/1471-2202-15-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tarutani A., Suzuki G., Shimozawa A., Nonaka T., Akiyama H., Hisanaga S., and Hasegawa M. (2016) The effect of fragmented pathogenic α-synuclein seeds on prion-like propagation. J. Biol. Chem. 291, 18675–18688 10.1074/jbc.M116.734707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jackson S. J., Kerridge C., Cooper J., Cavallini A., Falcon B., Cellas C. V., Landi A., Szekeres P. G., Murray T. K., Ahmed Z., Goedert M., Hutton M., O'Neill M. J., and Bose S. (2016) Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. J. Neurosci. 36, 762–772 10.1523/JNEUROSCI.3542-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mirbaha H., Chen D., Morazova O. A., Ruff K. M., Sharma A. M., Liu X., Goodarzi M., Pappu R. V., Colby D. W., Mirzaei H., Joachimiak L. A., and Diamond M. I. (2018) Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife 7, e36584 10.7554/eLife.36584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Watts J. C., Giles K., Oehler A., Middleton L., Dexter D. T., Gentleman S. M., DeArmond S. J., and Prusiner S. B. (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 110, 19555–19560 10.1073/pnas.1318268110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woerman A. L., Stöhr J., Ayoagi A., Rampersaud R., Krejciova Z., Watts J. C., Ohyama T., Patel S., Widjaja K., Oehler A., Sanders D. W., Diamond M. I., Seeley W. W., Middleton L. T., Gentleman S. M., Mordes D. A., Südhof T. C., Giles K., and Prusiner S. B. (2015) Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 112, E4949–E4958 10.1073/pnas.1513426112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Prusiner S. B., Woerman A. L., Mordes D. A., Watts J. C., Rampersaud R., Berry D. B., Patel S., Oehler A., Lowe J. K., Kravitz S. N., Geschwind D. H., Glidden D. V., Halliday G. M., Middleton L. T., Gentleman S. M., Grinberg L. T., and Giles K. (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 112, E5308–E5317 10.1073/pnas.1514475112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peng C., Gathagan R. J., Covell D. J., Medellin C., Stieber A., Robinson J. L., Zhang B., Pitkin R. M., Olufemi M. F., Luk K. C., Trojanowski J. Q., and Lee V. M. (2018) Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557, 558–563 10.1038/s41586-018-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peelaerts W. M., Bousset L., Baekelandt V., and Melki R. (2018) α-Synuclein strains and seeding in Parkinson's disease, incidental Lewy body disease, dementia with Lewy bodies and multiple system atrophy: similarities and differences. Cell Tissue Res. 373, 195–212 10.1007/s00441-018-2839-5 [DOI] [PubMed] [Google Scholar]
- 69. Lavenir I., Passarella D., Masuda-Suzukake M., Curry A., Holton J. L., Ghetti B., and Goedert M. (2019) Silver staining (Campbell-Switzer) of neuronal α-synuclein assemblies induced by multiple system atrophy and Parkinson's disease brain extracts in transgenic mice. Acta Neuropathol. Commun. 7, 148 10.1186/s40478-019-0804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamasaki T. R., Holmes B. B., Furman J. L., Dhavale D. D., Su B. W., Song E. S., Cairns N. J., Kotzbauer P. T., and Diamond M. I. (2019) Parkinson's disease and multiple system atrophy have distinct α-synuclein seed characteristics. J. Biol. Chem. 294, 1045–1058 10.1074/jbc.RA118.004471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perrin R. J., Payton J. E., Barnett D. H., Wraight C. L., Woods W. S., Ye L., and George J. M. (2003) Epitope mapping and specificity of the anti-α-synuclein monoclonal antibody Syn-1 in mouse brain and cultured cell lines. Neurosci. Lett. 349, 133–135 10.1016/S0304-3940(03)00781-X [DOI] [PubMed] [Google Scholar]
- 72. Crowther R. A., Daniel S. E., and Goedert M. (2000) Characterisation of isolated α-synuclein filaments from substantia nigra of Parkinson's disease brain. Neurosci. Lett. 292, 128–130 10.1016/S0304-3940(00)01440-3 [DOI] [PubMed] [Google Scholar]
- 73. Tarutani A., Arai T., Murayama S., Hisanaga S. I., and Hasegawa M. (2018) Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 6, 29 10.1186/s40478-018-0532-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Crowther R. A., Jakes R., Spillantini M. G., and Goedert M. (1998) Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett. 436, 309–312 10.1016/S0014-5793(98)01146-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented are contained within the manuscript.