Abstract
Carbon monoxide (CO) remains the most common cause of human poisoning. The consequences of CO poisoning include cardiac dysfunction, brain injury, and death. CO causes toxicity by binding to hemoglobin and by inhibiting mitochondrial cytochrome c oxidase (CcO), thereby decreasing oxygen delivery and inhibiting oxidative phosphorylation. We have recently developed a CO antidote based on human neuroglobin (Ngb-H64Q-CCC). This molecule enhances clearance of CO from red blood cells in vitro and in vivo. Herein, we tested whether Ngb-H64Q-CCC can also scavenge CO from CcO and attenuate CO-induced inhibition of mitochondrial respiration. Heart tissue from mice exposed to 3% CO exhibited a 42 ± 19% reduction in tissue respiration rate and a 33 ± 38% reduction in CcO activity compared with unexposed mice. Intravenous infusion of Ngb-H64Q-CCC restored respiration rates to that of control mice correlating with higher electron transport chain CcO activity in Ngb-H64Q-CCC–treated compared with PBS-treated, CO-poisoned mice. Further, using a Clark-type oxygen electrode, we measured isolated rat liver mitochondrial respiration in the presence and absence of saturating solutions of CO (160 μm) and nitric oxide (100 μm). Both CO and NO inhibited respiration, and treatment with Ngb-H64Q-CCC (100 and 50 μm, respectively) significantly reversed this inhibition. These results suggest that Ngb-H64Q-CCC mitigates CO toxicity by scavenging CO from carboxyhemoglobin, improving systemic oxygen delivery and reversing the inhibitory effects of CO on mitochondria. We conclude that Ngb-H64Q-CCC or other CO scavengers demonstrate potential as antidotes that reverse the clinical and molecular effects of CO poisoning.
Keywords: carbon monoxide, nitric oxide, mitochondria, mitochondrial disease, mitochondrial respiratory chain complex, hypoxia, hypoxia-inducible factor (HIF), antidotes, CO poisoning, hemoglobin, medical toxicology, neuroglobin
Introduction
Carbon monoxide (CO)3 poisoning is estimated to affect 50,000 individuals in the United States every year (1), causing mortality in 1–3% (1, 2). Long-term mortality in survivors of CO poisoning is double compared with age-matched controls and even higher in those patients who suffer a cardiac complication (3–5). Up to 40% of survivors will suffer permanent neurological or cognitive deficits (6–10). The cardiovascular effects of CO poisoning are significant. In patients hospitalized for CO poisoning, one-third to one-half have been reported to develop myocardial injury or left ventricular dysfunction (3, 11, 12). Physiological studies in animals show that CO poisoning decreases systemic oxygen delivery, which is compensated for by increased cardiac output and oxygen extraction (13). With continued poisoning, these compensatory mechanisms are overwhelmed, and the cardiomyocytes can be directly poisoned, leading to cardiovascular collapse (13).
CO toxicity is induced, in part, by the binding of CO to hemoglobin (Hb), to form carboxyhemoglobin (HbCO). Hb has an approximately 250-fold greater affinity for CO than for oxygen (14). Upon binding, CO displaces oxygen from Hb, which decreases the oxygen carrying capacity of the protein. CO binding also stabilizes the “relaxed” or R-state high-ligand-affinity conformation of Hb, which reduces oxygen unloading, resulting in decreased oxygen delivery throughout the body (1, 15). Notably, although the formation of HbCO had long been considered the major mechanism of CO toxicity (16), the clinical severity of the CO-poisoned patient does not directly correlate with the blood HbCO level (6, 17). It is now recognized that in addition to binding Hb, CO toxicity is potentiated by the binding of CO to other heme-containing proteins, including myoglobin in heart and muscle tissue, and cytochrome c oxidase (CcO; complex IV), the terminal respiratory complex in the mitochondrial electron transport chain (1, 18–23).
Cytochrome c oxidase is a well-characterized target of CO. CO directly inhibits the enzyme by binding the heme a3 site of CcO, resulting in the inhibition of respiration and oxidative phosphorylation (22, 24, 26–30). Because of the competitive binding of oxygen with CO to CcO, CO-dependent inhibition of respiration is potentiated by hypoxia; the affinity of CcO for CO being similar to oxygen (31, 32, 69–71). Further, hypoxia favors the reduced state of the heme a3 of CcO, which promotes CO binding to the reduced, ferrous heme (33, 34). In addition to the loss of tissue ATP production caused by CO-dependent inhibition of CcO, this inhibition is suggested to potentiate superoxide production by the mitochondrion resulting in oxidative damage to cells and tissues (35). It has also been proposed that excess CO can displace NO from heme-containing proteins and activate nitric-oxide synthase enzymes, causing a rapid rise in free NO (36). The increased NO can react with superoxide to form peroxynitrite, leading to additional nitrosative damage and further NO-dependent inhibition of CcO (36–42).
Currently, the only therapeutic options available for CO-poisoned patients are normobaric oxygen and hyperbaric oxygen (HBO2). Supplemental normobaric oxygen therapy and HBO2 significantly decrease the carboxyhemoglobin half-life from 270 min to 90 and 20–40 min, respectively (1, 15, 43–45). Some clinical trials suggest that HBO2 reduces long-term neurocognitive morbidity after CO poisoning; however, it remains unknown whether HBO2 has mortality or cardiovascular benefits (1, 43). A deliverable antidotal therapy is greatly needed, especially for the most severely poisoned patients.
We have established a recombinant neuroglobin with four point mutations: H64Q and three surface thiol substitutions (C46G, C55S, and C120S): Ngb-H64Q-CCC. The H64Q mutation increases CO affinity to a value 500-fold higher than Hb (46) and thus renders Ngb-H64Q-CCC a potential antidote for CO poisoning. The three surface thiol substitutions increase protein solubility and limit oligomerization at high protein concentrations (46). The concept of CO scavenging revolves around a molecule that can reverse the binding of CO to Hb and CcO.
We have shown that Ngb-H64Q-CCC can scavenge CO from molecular Hb and packed red blood cell encapsulated Hb (46). In a model of severe, lethal CO poisoning in ventilated mice, the animals developed hypotension and cardiovascular collapse, and only 10% of animals survived. Treatment with Ngb-H64Q-CCC in this model increased the survival rate to 87.5% at 40 min (46). The CO molecules were directly removed via urinary excretion of CO-bound Ngb-H64Q-CCC (46). Although the efficacy in removing CO from Hb in red cells and in reversing cardiovascular collapse was demonstrated, the effects of Ngb-H64Q-CCC on oxidative phosphorylation were not tested (46). Herein we use both an in vivo CO exposure with ex vivo biochemical measurement and an in vitro model of CO poisoning to test the hypothesis that Ngb-H64Q-CCC attenuates CO-dependent inhibition of mitochondrial respiration.
Results
Ngb-H64Q-CCC restores tissue respiration in hearts from CO-poisoned mice
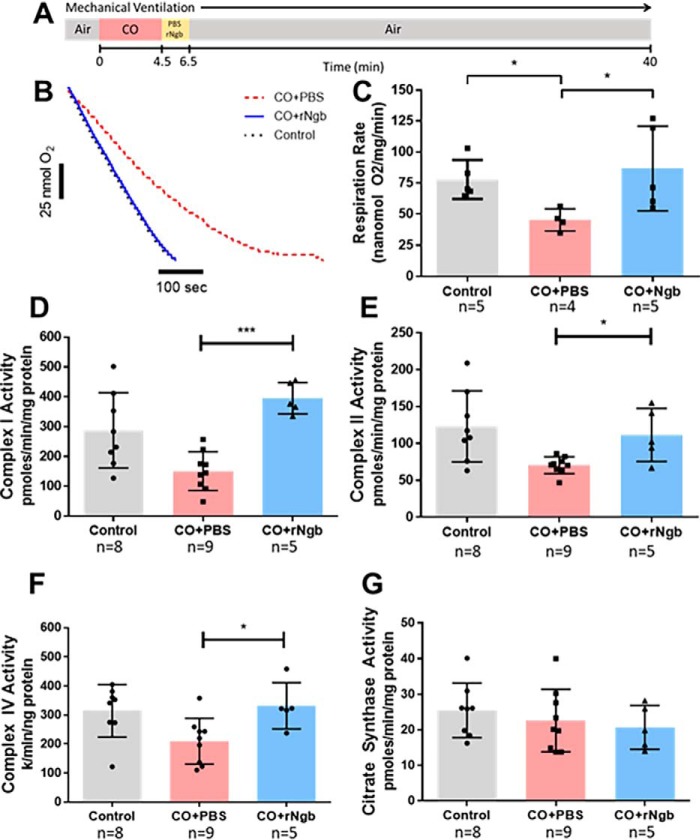
Using a murine model of lethal CO poisoning, we previously reported that Ngb-H64Q-CCC increases survival after exposure to CO Ngb-H64Q-CCC (46). In the current study, the mice were exposed to CO (30,000 ppm; 4.5 min) and then treated with either PBS (as a control) or Ngb-H64Q-CCC (11 ± 0.2 μm) (Fig. 1A). Treatment with Ngb-H64Q-CCC increased survival rate of the mice to 88% as compared with only 10% with control PBS infusions (46). To determine whether Ngb-H64Q-CCC reversed CO-dependent inhibition of respiration in this in vivo CO exposure with ex vivo biochemical measurement model, we next assessed respiration in the cardiac tissue recovered from mice in this model (Fig. 1B). Heart tissue from mice exposed to CO and treated with PBS showed significant inhibition of state 3 respiration (45 nmol oxygen/mg/min) compared with heart tissue from mice not treated with CO (78 ± 15 nmol/mg/min) (58 ± 19%; Mann–Whitney test, p = 0.032; Fig. 1C). Treatment of mice with Ngb-H64Q-CCC after CO exposure restored tissue respiration to 87 ± 34 nmol/mg/min, similar to control tissue, and to rates significantly higher than CO-poisoned mice treated with PBS (p = 0.016; Fig. 1C). These data demonstrate that CO-dependent inhibition of respiration occurs in an in vivo CO exposure with ex vivo biochemical measurement and that Ngb-H64Q-CCC is able to reverse this inhibition.
Figure 1.
A, severe CO-poisoning experiment design as described by Azarov et al. (46). Ventilated mice, sedated with 1.5% isofluorane, were exposed for 4.5 min of 30,000 parts per million CO gas. After exposure, Ngb-H64Q-CCC or PBS were infused for 2 min. Mouse arterial blood pressure and heart rate were measured for 40 min or until death. Using hearts recovered from animals in these experiments, we evaluated whether Ngb-H64Q-CCC reversed CO-dependent inhibition of respiration in an in vivo CO exposure with ex vivo biochemical measurement. Control animals were sedated with 1.5% isofluorane but not exposed to CO gas. The hearts were immediately removed upon death of the animal or sacrificed after 40 min of exposure at the cessation of the experiment (or 20 min in sedated control mice). The mouse heart was homogenized, and tissue respiration was measured after the addition of pyruvate, malate and ADP with a Clark-like electrode respiration system. B, rates were adjusted for protein level confirmed with BCA (representative raw traces). C, in CO-treated mice infused with PBS, respiration of heart tissue was significantly inhibited at 58 ± 19% of the rate of control (Mann–Whitney test; *, p = 0.032). Treatment with Ngb-H64Q-CCC restored tissue respiration to the level of control (Mann–Whitney test; p = 0.55) and to rates significantly higher than CO-poisoned mice treated with PBS (p = 0.032, *). D, to evaluate the effects of severe CO poisoning on the components of the electron transport chain, the same heart homogenate tissue underwent spectrophotometric kinetic assays. Complex I activity in animals treated with Ngb-H64Q-CCC was significantly higher than those treated with PBS (400 ± 52 versus 150 ± 64 pmol/min/mg protein; Mann–Whitney test; ***, p = 0.001) and similar to control (290 ± 130; p = 0.13). E, CO-poisoned animals treated with neuroglobin showed complex II activity levels higher than those treated with PBS (110 ± 35 versus 71 ± 11 pmol/min/mg protein; *, p = 0.029) and similar to control (120 ± 48; p = 0.72). F, animals treated with Ngb-H64Q-CCC after CO exposure showed significantly higher complex IV activity compared with those treated with PBS (330 ± 80 versus 210 ± 79 k/min/ng protein; *, p = 0.042) and similar to sedated controls (310 ± 90; p = 0.83). G, citrase synthase activity, was similar between all groups. All experiments were performed with at least n = 4 animals in each group. All statistical analyses were using the Mann–Whitney test for these in vivo CO exposure with ex vivo biochemical measurement studies. rNgb, Ngb-H64Q-CCC.
Treatment with Ngb-H64Q-CCC restores the activity of complexes I, II, and IV of the electron transport chain after CO poisoning
To determine whether the protective effect of Ngb-H64Q-CCC in the in vivo CO exposure model altered mitochondrial electron transport chain enzymatic activity, we next performed spectrophotometric assays to measure the activities of the complexes in heart tissues isolated immediately after CO poisoning in this in vivo model. Consistent with CO-mediated inhibition of CcO, CcO activity was decreased in mice exposed to CO and treated with PBS compared with sedated control mice (210 ± 79 versus 310 ± 91 k/min/ng protein, Mann–Whitney test, p = 0.021; Fig. 1F). Animals treated with Ngb-H64Q-CCC after CO exposure showed significantly higher CcO activity compared with those treated with PBS (330 ± 80 versus 210 ± 79, p = 0.042) and similar to control mice (p = 0.83). Interestingly, in addition to inhibition of CcO activity, the activities of complex I and complex II were also significantly decreased with CO exposure (53 ± 42% for complex I and 57 ± 16% for complex II). However, the activity of these enzymes was restored to the levels of control when exposed to CO and then treated with Ngb-H64Q-CCC (Fig. 1, D and E). The activity of citrate synthase, a matrix TCA cycle enzyme used as control, was similar between all groups (Fig. 1G).
NO inhibits mitochondrial respiration and Ngb-H64Q-CCC reverses NO-induced inhibition of mitochondrial respiration
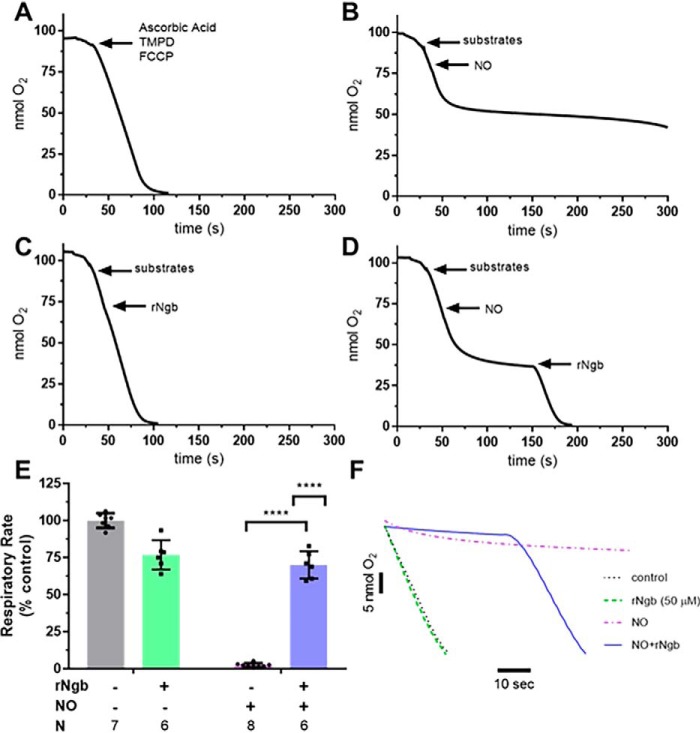
NO inhibits mitochondrial respiration by binding the binuclear center of CcO, similar to CO (29, 48–51). NO-dependent inhibition can be reversed in vitro by NO scavengers such as Hb and myoglobin (52–56). Ngb-H64Q-CCC is known to bind the gaseous ligands CO and NO with high-affinity constants (46, 47, 57). Thus, we first tested whether Ngb-H64Q-CCC could reverse the well-established reversible, NO-dependent inhibition of respiration (52–56) utilizing isolated rat liver mitochondria as a proof of concept to the mitochondrial-specific effects of Ngb-H64Q-CCC.
Because NO and CO more potently inhibit respiration at lower oxygen tensions, we first developed an experimental system to measure mitochondrial respiration using a Clark-type oxygen electrode at low oxygen tensions. This system allowed for repeated measures of respiration in the same mitochondrial sample. Isolated rat liver mitochondria were suspended in a Clark-type oxygen electrode chamber and stimulated to respire with the addition of succinate (7.5 mm) and ADP (7.5 mm). Once the mitochondria consumed all the initial oxygen in the chamber, 50 μl of aerobic PBS was injected to partially reoxygenate the chamber, and the new rate of respiration was measured as the injected oxygen was consumed. The process was performed three consecutive times in the control condition (no addition of NO or Ngb-H64Q-CCC). There was no significant difference between the three measured rates of respiration (Fig. 2A).
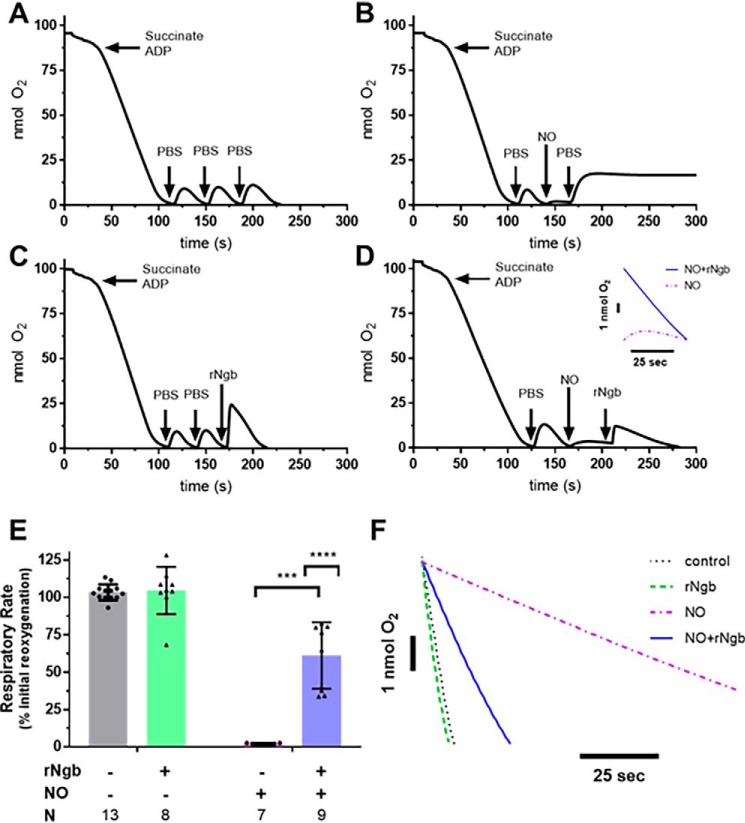
Figure 2.
In vitro effects of NO on mitochondrial respiration and reversal by Ngb-H64Q-CCC treatment. Four experimental arms were used. For control, isolated liver mitochondria was respired to hypoxia after addition of succinate and ADP. A, after hypoxia, 50 μl of aerobic PBS was added and allowed to respire to hypoxia three times (labeled raw data). B, for NO only, initially 50 μl of aerobic PBS was added and respired to hypoxia, and then aerobic PROLI-NONOate (50 μm) was added into the chamber to inhibit respiration. The small amount of oxygen dissolved in the PROLI-NONOate solution increased the oxygen but demonstrated inhibition of respiration. Further injection of 50 μl of aerobic PBS into the chamber increased oxygen concentration; however, respiration remained inhibited until the NO released from PROLI-NONOate began diffusing from the chamber (not pictured). C, for Ngb-H64Q-CCC only, 50 μl of aerobic PBS was added and respired to hypoxia twice. Oxygen-bound Ngb-H64Q-CCC was added to a concentration of 50 μm to the chamber and respired again to hypoxia. D, for NO + Ngb-H64Q-CCC, after the initial aerobic PBS (step 1), PROLI-NONOate (step 2) was added, followed by oxygen-bound Ngb-H64Q-CCC to a concentration of 50 μm (step 3) (labeled raw data). Inset, comparison of NO-inhibited respiration versus Ngb-H64Q-CCC–treated post-NO exposure respiration). The rate of the final respiration rate was compared with the initial reoxygenation respiration rate for each arm (rate respiration final step/rate of respiration initial step). E, the ratios were compared between groups. Control rates had no effect of respiration versus initial reoxygenation. Exposure to PROLI-NONOate with reoxygenation by aerobic PBS decreased respiration to 2 ± 1% of the initial rate. In mitochondria that were exposed to PROLI-NONOate and then treated with Ngb-H64Q-CCC, respiration was 61 ± 22% of the initial rate (unpaired Welch's t test NO-exposed and NO-exposed, Ngb-H64Q-CCC–treated mitochondria; ***, p = 0.0001). In an unmatched regular two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to NO (interaction term; ****, p < 0.0001; single line) (F, representative raw traces compared). rNgb, Ngb-H64Q-CCC.
To test the effect of NO on mitochondrial respiration, after adding 50 μl of aerobic PBS to the hypoxic chamber to measure the initial respiration rate for comparison, 50 μm of the NO donor PROLI-NONOate (t½ = 1.8 s at 37 °C, pH 7.4) was added. A small amount of oxygen dissolved in NO donor solution increased the oxygen concentration of the chamber, but no respiration was observed, indicating complete mitochondrial inhibition. Even with an additional injection of aerobic PBS, respiration remained inhibited to 2 ± 1% of the initial rate (Fig. 2B). To test whether Ngb-H64Q-CCC alone had an effect on respiration, oxygen-bound Ngb-H64Q-CCC (50 μm) was added to the chamber after two aerobic PBS additions and ensuing respiration to 0% oxygen (Fig. 2C). To test whether Ngb-H64Q-CCC could reverse NO-dependent mitochondrial inhibition, after aerobic PBS addition to establish an initial respiration rate, PROLI-NONOate was added, inhibiting mitochondrial respiration, followed by the addition of oxygen-bound Ngb-H64Q-CCC (Fig. 2D).
To analyze these data, the final respiration rate after the third addition to the chamber was calculated as a percentage of the initial respiratory rate (after the first addition of PBS) (Fig. 2E). Exposure to PROLI-NONOate significantly inhibited respiration to 2 ± 1% of the initial rate. Although Ngb-H64Q-CCC alone had no significant effect on respiration rate, Ngb-H64Q-CCC increased the respiration rate (61 ± 22% of the initial rate) of mitochondria that were inhibited by PROLI-NONOate, indicative of a reversal of inhibition (unpaired Welch's t test NO-exposed and NO-exposed, Ngb-H64Q-CCC–treated mitochondria; p = 0.0001). In an unmatched, two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to NO (interaction term, p < 0.0001; representative raw traces compared in Fig. 2F).
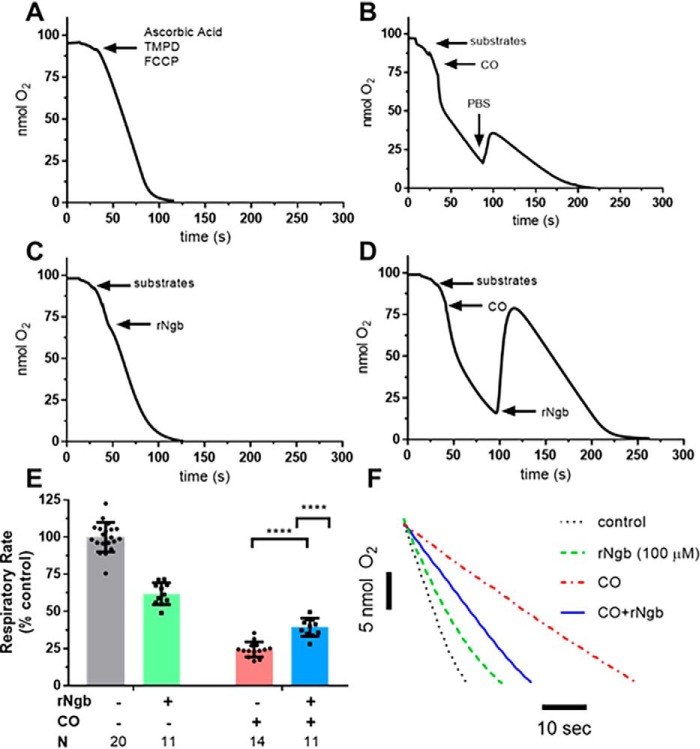
Ngb-H64Q-CCC reverses CO-dependent inhibition of mitochondrial respiration
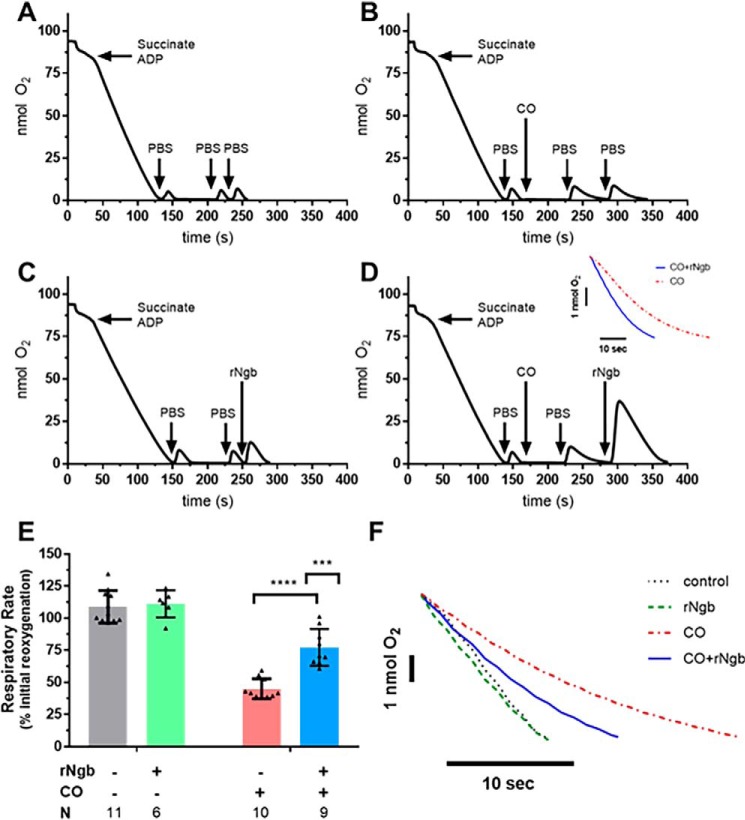
Given the validation of the respirometry model of serial reoxygenation with NO-induced mitochondrial inhibition and subsequent restoration of respiration with Ngb-H64Q-CCC, we sought to determine the whether Ngb-H64Q-CCC could reverse CO-induced respiratory inhibition. Controls and experiments were performed in a similar manner to those with NO (Fig. 2), except that upon addition of CO to the chamber, the solution was allowed to remain at low oxygen tension for 60 s to facilitate CO binding to CcO because of the higher affinity of CO for the complex in hypoxia (35, 58). Additionally, CO was delivered using 100 μl of CO-saturated PBS to a concentration of ∼160 μm (confirmed by measuring spectroscopic change of solution combined with a known concentration of deoxy-hemoglobin). The CO solution was consequently anaerobic; to measure a CO-inhibited respiration rate, aerobic PBS was added to the system. An additional bolus of aerobic PBS demonstrated persistent inhibition of respiration (Fig. 3B) to 45 ± 8% the initial rate. The addition of oxy-Ngb-H64Q-CCC (which contains oxygen-bound Ngb-H64Q-CCC and aerobic buffer) to CO-treated mitochondria restored mitochondrial respiration (Fig. 3D) to 77 ± 14% of the initial rate (unpaired t test CO-exposed and CO-exposed/Ngb-H64Q-CCC–treated mitochondria; p < 0.0001; Fig. 3E). Oxygen concentration in the chamber increased because of its displacement from oxygen-bound Ngb-H64Q-CCC (Fig. 3D). In an unmatched regular two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to CO (interaction term, p = 0.0007; representative raw traces compared; Fig. 3F). As with the NO experiments, it should be noted that oxygen-bound Ngb-H64Q-CCC had no effect on respiration alone (Fig. 3, C and F).
Figure 3.
In vitro effects of CO on mitochondrial respiration and reversal by Ngb-H64Q-CCC treatment. Experiments were performed in a similar manner to the NO testing; however, instead of PROLI-NONOate, 100 μl of CO-saturated PBS (to a concentration of ∼160 μm) was added to the chamber. Additionally, because CO has a higher affinity for cytochrome c oxidase in hypoxia, the chamber was left in hypoxia for 60 s before adding additional aerobic PBS. The CO-saturated PBS did not contain oxygen; therefore 50 μl of PBS was added to demonstrate CO-slowed mitochondrial respiration. B, an additional 50 μl of aerobic PBS was used to show persistent inhibition of respiration (labeled raw data). A and C, controls were similar to the NO experiments (Fig. 2): no exposure to CO or Ngb-H64Q-CCC and addition of 100 μm Ngb-H64Q-CCC without the presence of CO. D, to demonstrate the effect of Ngb-H64Q-CCC, in the final step, instead of PBS, aerobic, oxygen-bound Ngb-H64Q-CCC was added to a concentration of 100 μm to the chamber. This increased the oxygen concentration in the chamber, and the mitochondria respired at an increased rate (labeled raw data). Inset, comparison of CO-inhibited respiration versus Ngb-H64Q-CCC–treated post-CO exposure respiration). E, Control rates had little effect of respiration versus initial reoxygenation. Exposure to CO with reoxygenation by aerobic PBS decreased respiration to 45 ± 8% of the initial rate. In mitochondria that were exposed to CO but treated with Ngb-H64Q-CCC, respiration was 77 ± 14% of the initial rate (unpaired Student's t test CO-exposed and CO-exposed, Ngb-H64Q-CCC–treated mitochondria; p < 0.0001, ****). In an unmatched regular two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to CO (interaction term; ***, p = 0.0007; single line) (F, representative raw traces compared). rNgb, Ngb-H64Q-CCC.
Further, to assess for a potential dose effect of Ngb-H64Q-CCC, both 10 and 150 μm of Ngb-H64Q-CCC were also tested in this model (Fig. S1). There was a dose-dependent effect between 10 and 100 μm of Ngb-H64Q-CCC (10 μm had no effect on respiration compared with 100 μm, Student's t test, p = 0.0002). There was no significant difference in the level of respiration between 100 and 150 μm Ngb-H64Q-CCC, indicating a maximized therapeutic effect observed at the 100 μm level.
CO inhibition of CcO activity is the main cause of CO inhibition of mitochondrial respiration in vitro
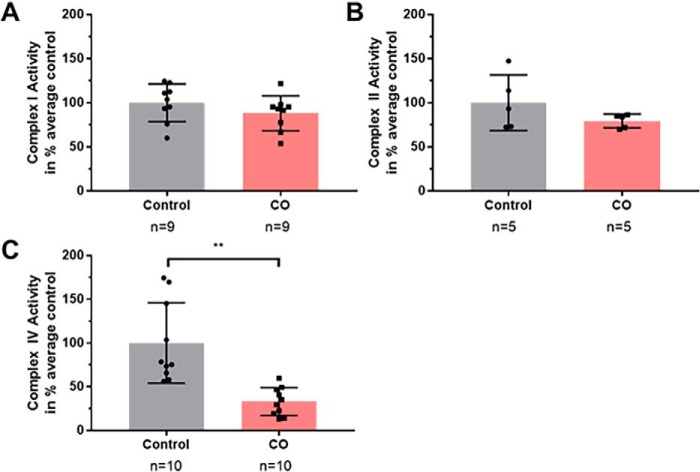
Because we observed inhibition of mitochondrial respiration with CO exposure and partial restoration of mitochondrial respiration with Ngb-H64Q-CCC treatment, we next sought to determine which electron transport complexes CO was inhibiting. We directly measured the effects of CO on complex I, II, and IV activities using spectrophotometry. Activity levels were determined in hypoxic conditions (2% oxygen) with and without the presence of CO-saturated buffer solution. Complex I activity was not significantly decreased by CO in isolation (79 ± 4% of control, Welch's t test, p = 0.22) (Fig. 4A). Complex II activity was not significantly decreased by CO in isolation (88 ± 7% of control, Student's t test, p = 0.24) (Fig. 4B). CO exposure significantly inhibited complex IV activity, with the CO-exposed activity level at 33 ± 5% of control (Welch's t test p = 0.0011) (Fig. 4C). These data demonstrate that in isolated mitochondria, CO-induced inhibition of mitochondrial respiration is due to the inhibition of CcO.
Figure 4.
In vitro effects of CO on complex I, II, and IV activity. Activity levels were determined at hypoxic conditions (2% oxygen) with and without the presence of CO-saturated buffer solution. A, complex I was not significantly reduced by CO in isolation (79 ± 4% of control; Welch's t test; p = 0.22). B, complex II was not significantly reduced by CO in isolation (88 ± 7% of control; Student's t test; p = 0.24). C, complex IV activity after CO exposure was significantly reduced, with the CO-exposed activity level 33 ± 5% of control (Welch's t test; **, p = 0.0011).
Ngb-H64Q-CCC reverses NO-dependent inhibition of CcO activity
To confirm that NO-dependent inhibition of respiration was due to inhibition of CcO in our model, we directly measured the effect of NO and Ngb-H64Q-CCC on CcO activity. N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) (0.3 mm) and ascorbic acid (3.6 mm) were added to isolated mitochondria to directly donate electrons to cytochrome c, bypassing complexes I–III, such that all oxygen consumption measured was due to CcO activity (59–61). Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) (0.015 mm) was then used to uncouple oxidative phosphorylation, maximizing oxygen consumption rates by CcO (59–61). Oxygen was consumed at a steady rate in the absence of any treatment (Figs. 5A and 6A). To establish the model, PROLI-NONOate (50 μm) was again used to inhibit CcO activity (2 ± 1% of baseline) (Fig. 5B). The addition of an equivalent of oxyNgb-H64Q-CCC rescued activity to 70 ± 9% of baseline (Fig. 5, D–F). Notably, addition of oxyNgb-H64Q-CCC to the maximally respiring CcO alone significantly decreased oxygen consumption (77 ± 10% of baseline; Fig. 5, C and E). This decrease could be due to heme-mediated lipid peroxidation or free heme-induced mitochondrial dysfunction without the presence of excess CO or NO, which has previously been reported in the literature (62–64).
Figure 5.
In vitro effects of NO on complex IV activity and reversal by Ngb-H64Q-CCC treatment. To demonstrate the specific effect of NO on complex IV activity in isolated rate mitochondria, a closed chamber with a Clark-like oxygen electrode was utilized. Here, instead of succinate and ADP as substrates for oxidative phosphorylation, a combination of FCCP, TMPD, and ascorbic acid was added. These substrates facilitate the direct transfer of electrons to cytochrome c and vis-à-vis reflect cytochrome c oxidase activity. A–D, four conditions were measured: baseline level of TMPD-ascorbate–driven respiration (labeled raw data, A), respiration after the addition of 50 μm of PROLI-NONOate (B), respiration after the addition of 50 μm chamber concentration of Ngb-H64Q-CCC (C), and finally, respiration after the addition of 50 μm of PROLI-NONOate, followed by the addition of 50 μm of Ngb-H64Q-CCC (D). These rates were compared with the average rate of baseline TMPD-ascorbate–driven respiration for a given day of experiments and the same animal (measured TMPD-ascorbate–driven respiration rate/average baseline TMPD-ascorbate–driven respiration rate). E, these ratios were compared between experimental arms. Here the addition of Ngb-H64Q-CCC itself slowed complex IV activity to 77 ± 10% of baseline. The addition of NO slowed complex IV activity to 2 ± 1% of baseline. The addition of Ngb-H64Q-CCC after NO lead to a respiration rate of 70 ± 9% of baseline, markedly higher than NO untreated complex IV activity (unpaired Welch's t test; ****, p < 0.0001). In an unmatched regular two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to NO (interaction term; ****, p < 0.0001; single line) (F, representative raw traces compared). rNgb, Ngb-H64Q-CCC.
Figure 6.
In vitro effects of CO on complex IV activity and reversal by Ngb-H64Q-CCC treatment. To demonstrate the specific effect of CO on complex IV activity in isolated rate mitochondria, a closed chamber with a Clark-like oxygen electrode was utilized. A combination of FCCP, TMPD, and ascorbate were added to demonstrate cytochrome c oxidase activity. A–D, four conditions were measured: baseline level of TMPD-ascorbate–driven respiration (labeled raw data, A), respiration after the addition of 200 μm CO in CO-saturated PBS followed by 100 μl of aerobic PBS (B), respiration after the addition of 100 μm chamber concentration of oxygenated-Ngb-H64Q-CCC (C), and finally, respiration after the addition of 200 μm CO in CO-saturated PBS followed by 100 μm of oxygenated-Ngb-H64Q-CCC (D). These rates were compared with the average rate of baseline TMPD-ascorbate–driven respiration for a given day of experiments and the same animal. E, the ratios between experimental arms were compared. In these experiments, the addition of Ngb-H64Q-CCC itself slowed complex IV activity to 62 ± 7% of baseline. The addition of CO slowed complex IV activity to 24 ± 5% of baseline. The addition of Ngb-H64Q-CCC after CO lead to a respiration rate of 39 ± 6% of baseline, markedly higher than CO-exposed, untreated complex IV activity (unpaired Student's t test; ****, p < 0.0001). In an unmatched regular two-way ANOVA, we determined that there was a significant interaction between Ngb-H64Q-CCC and exposure to CO (interaction term; ****, p < 0.0001; single line) (F, representative raw traces compared). rNgb, Ngb-H64Q-CCC. #, Figs. 5A and 6A show the same control conditions, represented for clarity.
Ngb-H64Q-CCC reverses CO-dependent inhibition of CcO activity
After establishing CcO inhibition with NO and subsequent rescue with oxyNgb-H64Q-CCC, we demonstrated the effect of CO on CcO. In the presence of CO (and added aerobic PBS), oxygen consumption significantly decreased to 24 ± 5% of baseline activity (Fig. 6B). OxyNgb-H64Q-CCC was able to partially rescue CcO activity in the CO-exposed mitochondria to 39 ± 6% of baseline activity (p < 0.0001; Fig. 6D). These data (Fig. 6, E and F), although confounded by the lower respiration rate caused by rNgb itself in the isolated CcO model (Figs. 5C and 6C), indicate that CO inhibits CcO activity and that Ngb-H64Q-CCC reverses this inhibition.
Discussion
It has been established that Ngb-H64Q-CCC rapidly binds CO in vitro and in vivo and decreases the half-life of the HbCO species in red blood cells from >200 min to less than 25 s (46). Although Ngb-H64Q-CCC causes a dramatic therapeutic effect in terms of increasing the survival of mice severely poisoned by CO, the effects of Ngb-H64Q-CCC on mitochondrial respiration have not been directly examined. In the heart tissue of mice subjected to severe CO poisoning, intravenous treatment with Ngb-H64Q-CCC restored inhibited heart tissue respiration and restored the activity of electron transport enzyme complexes I and II and CcO. Although the severity of the poisoning model could lead to hypoxic and reactive oxygen species related damage to tissue function, there is a clear restoration of tissue respiration with Ngb-H64Q-CCC treatment despite treated mice initially suffering severe poisoning and hypotension. These effects were also observed in isolated mitochondria exposed to CO and NO, clearly demonstrating that Ngb-H64Q-CCC reverses CO- and NO-dependent mitochondrial inhibition in vitro as well. Combined, these results suggest that Ngb-H64Q-CCC scavenges CO from not just carboxyhemoglobin, as demonstrated previously (46), but also scavenges CO directly from the CO-inhibited mitochondrial CcO. Through CO scavenging, Ngb-H64Q-CCC reverses one of the key mechanisms of CO toxicity.
Neuroglobin is a cellular hemoprotein expressed in brain and retina that protects cells from death after ischemia and reperfusion injury (46, 65–68). A mutation of the distal histidine to glutamine (H64Q) dramatically increases the affinity for CO (46). The comparison of the binding properties of Ngb-H64Q-CCC with Hb and CcO (Table 1) indicates that Ngb-H64Q-CCC has almost 500-fold greater affinity for CO than Hb and more than 80,000-fold greater affinity for CO than CcO (46, 69–73). The measured M value (KA CO/KA O2) for Ngb-H64Q-CCC is 9.7 × 103, suggesting preferential binding of CO even in the presence of oxygen (Table 1) (46). The low M value of CcO, calculated as 1.3 × 10−1, indicates a moderate affinity toward CO in the presence of oxygen and can explain the stronger inhibitory effects of CO in hypoxia for CO-induced mitochondrial respiration inhibition observed (69–72). The binding properties of Ngb-H64Q-CCC indicate that Ngb-H64Q-CCC, and in general a molecule capable of scavenging CO from Hb, is also a feasible scavenger of CO from CcO.
Table 1.
Binding parameters for oxygen and CO of Hb, hNgb, Ngb-H64Q-CCC, and CcO
R, R state; T, T state; hNgb, human neuroglobin; Ngb-H64Q-CCC, recombinant neuroglobin with H64Q and three surface thiol substitutions (C46G, C55S, and C120S) (46, 69–73).
| Molecule | O2 |
CO |
M (KA CO/KA O2) | ||||
|---|---|---|---|---|---|---|---|
| kon | koff | KA | kon | koff | KA | ||
| m−1 s−1 | s−1 | m−1 | m−1 s−1 | s−1 | m−1 | ||
| Hb Ra | 5.0 × 107 | 1.5 × 101 | 3.3 × 106 | 6.0 × 106 | 1.0 × 10−2 | 6.0 × 108 | 1.8 × 102 |
| Hb Ta | 4.5 × 106 | 1.9 × 103 | 2.4 × 103 | 8.3 × 104 | 9.0 × 10−2 | 9.2 × 105 | 3.9 × 102 |
| hNgba | 2.5 × 108 | 0.8 | 3.1 × 108 | 6.5 × 107 | 1.4 × 10−2 | 4.6 × 109 | 1.5 × 101 |
| Ngb-H64Q-CCCa | 7.2 × 108 | 18 | 4.0 × 107 | 1.6 × 108 | 4.2 × 10−4 | 3.8 × 1011 | 9.5 × 103 |
| CcOb | 1.0 × 108 to 6.0 × 108 | 10 | 1.0 × 107 to 6.0 × 107c | 7.0 × 104 to 1.2 × 105 | 2.2 × 10−2 | 3.0 × 106 to 6.0 × 106c | 1.0 × 10−1 to 6.0 × 10−1c |
When tested in a severe mouse model of CO poisoning that causes bradycardia, hypotension, and death, therapy with Ngb-H64Q-CCC reverses the deleterious effects of CO compared with PBS control–treated animals (46). Ngb-H64Q-CCC scavenged CO from carboxyhemoglobin and improved lactate clearance, suggesting improved oxygen delivery (46). In the current study, we demonstrate the reversal of CO-induced tissue respiration and CcO inhibition in heart tissue from these same mice. Treatment with Ngb-H64Q-CCC normalized heart tissue homogenate oxygen respiration in the CO-poisoned animals (Fig. 1B) as well as CcO activity levels (Fig. 1D). These data suggest removal of CO bound to a ferrous heme center of CcO by Ngb-H64Q-CCC, attributed to neuroglobin's higher affinity for CO (KA = 3.8 × 1011 m−1) versus CcO (KA = 4.4 × 106 m−1) (Table 1) (46, 69–72). Because CO and NO are freely diffusible ligands, Ngb-H64Q-CCC does not need to be taken up by cells to work: it traps the CO outside the cells, shifting the equilibrium and favoring diffusion of CO out of mitochondria and cells, leading to further scavenging. Further, because of the preferential scavenging for CO by Ngb-H64Q-CCC and the higher affinity of CO for oxygen bound to Ngb-H64Q-CCC, the antidotal oxy-form of Ngb-H64Q-CCC releases its bound oxygen, delivering oxygen to the hypoxic tissue. This phenomenon is best observed in the in vitro studies, where an increase of oxygen is observed in the sealed reaction chamber after oxy-Ngb-H64Q-CCC delivery only in the presence of CO, but not when the oxygen-bound protein was injected to non–CO-exposed respiring mitochondria (Figs. 3, C and D, and 6, C and D). Moreover, this effect of increased oxygen release after oxy-Ngb-H64Q-CCC delivery was not observed in our NO experiments in vitro (Fig. 5D), presumably because of the NO dioxygenation reaction, in which oxygen-bound globin proteins in the presence of NO yield nitrate and the corresponding met-form of globin protein at near diffusion limited rates (74). These results suggest Ngb-H64Q-CCC acts through three mechanisms to mitigate the toxicity of CO poisoning: 1) scavenging of CO from carboxyhemoglobin allowing for improving systemic oxygen delivery, 2) reversing the inhibitory effects of CO on mitochondria and specifically cytochrome c oxidase allowing for improved oxygen utilization, and 3) directly delivering oxygen to hypoxic tissue itself by releasing an oxygen molecule upon scavenging CO molecules from inhibited mitochondria and Hb.
In addition to inhibiting CcO, severe CO poisoning decreased complex I and II activity in mice in heart tissue extracted from CO-poisoned mice. Treatment with Ngb-H64Q-CCC restored complex I and II activity levels to control levels. In our in vitro studies directly exposing isolated mitochondria to CO, we showed that complex IV is inhibited significantly by CO, whereas complexes I and II are not (Fig. 4). We then showed that CO-induced CcO inhibition is partially reversed by treatment Ngb-H64Q-CCC (Fig. 6). Although alterations in complexes I and II have not classically been associated with CO poisoning, decreases in complex I and II activity were recently reported in the peripheral blood mononuclear cells of acutely CO-poisoned patients (75–77). This was similar to our observation of complex activities in heart homogenate from our in vivo CO exposure model. Cytochrome b560 in complex II of bovine heart was reported to react with carbon monoxide when isolated from the membrane-anchoring protein fraction of bovine heart mitochondrial succinate-ubiquinone reductase; however, no reactivity with CO was observed when cytochrome b560 was isolated intact (78). It is possible that homogenization of the heart tissue from the in vivo model altered complex II structure in a similar manner.
Although some possible molecular effects of CO have been noted in complex II, more likely in the setting of in vivo CO exposure, complexes I and II are affected more from the secondary clinical effects of severe CO poisoning, such as hypotension, systemic hypoxia, and ensuing ischemia-reperfusion injury. Those animals treated with PBS were unable to recover from cardiovascular shock in contrast to the recovery seen in Ngb-H64Q-CCC–treated mice. Both complex I and II activities are known to be decreased in septic shock, cardiogenic shock, and ischemia-reperfusion injury (79–81). Hypoxia alone can decrease complex I activity (82). Although inhibition of CcO by CO could cause upstream mitochondrial ROS generation, leading to oxidation of complexes I and II, decreasing activity levels, we did not observe direct inhibition of complex I and II activity by CO in our in vitro studies (82–86).
There was incomplete recovery of CO-induced mitochondrial respiration inhibition and CcO inhibition with the treatment of Ngb-H64Q-CCC in the in vitro system. We did not observe full reversal with increased concentration of Ngb-H64Q-CCC (Fig. S1). We did not observe CO-induced effects on complex I or complex II that could have decreased total mitochondrial respiration. The incomplete recovery of respiration may be due to a component of nonreversible, free radical–induced damage to the mitochondria electron transport chain from the initial complex IV CO-induced inhibition (83) and prolonged, recurrent hypoxia in this in vitro respirometry system (87).
In juxtaposition to the in vitro findings, complete reversal of heart tissue respiration and complex activity was observed in the heart tissue isolated after in vivo CO exposure. The complete restoration of tissue respiration in this in vivo CO-poisoning model versus only partial reversal of CO-induced inhibition of mitochondrial respiration in the isolated mitochondrial model is possibly due to the adaptive systems available in vivo to respond to acute hypoxia, such as intracellular anti-oxidant enzymes (86, 92). These protective systems would not be available in isolated mitochondria. Further, in this model, severe CO poisoning produced not only molecular binding of CO to hemoglobin and CcO; acute CO poisoning caused systemic hypoxia and hypotension. Because Ngb-H64Q-CCC therapy reversed hypoxia and hypotension, resolving the physiologic conditions themselves could have contributed to the beneficial effect on tissue respiration and complex activity. More studies are needed to examine the effect of Ngb-H64Q-CCC on CO-induced neurocognitive dysfunction, which is thought to be directly related to CO-induced mitochondrial effects and cellular injury.
CO poisoning is the most common cause of human poisoning; however, there is still no antidotal therapy available (1, 2). Up to 40% of survivors suffer long-term neurocognitive deficits, and up to 50% of moderately poisoned, hospitalized patients suffer cardiovascular dysfunction or injury (3–5, 11, 12). The standard of care in the management of CO-poisoning patients is limited to normobaric oxygen and hyperbaric oxygen therapy, both of which rely on increased oxygen levels to improve respiratory clearance of CO from the HbCO species (1, 15). An antidotal therapy would allow for more rapid point-of-care treatment in the field or in the emergency department. The current studies support the further development of Ngb-H64Q-CCC as a biological therapeutic for CO poisoning. We have shown previously that Ngb-H64Q-CCC decreases HbCO levels, restores blood pressure, and increases survival in a severe CO-poisoning mouse model. Further studies are planned on the specific cardiovascular hemodynamics involved in CO-induced cardiogenic shock. Our new data demonstrate that Ngb-H64Q-CCC can also help to reverse the toxic effects of CO through the scavenging of CO directly from CO-bound CcO in the mitochondria as shown in both isolated mitochondria and cardiac tissue. These data show that the resolution of hypotension and of bradycardia and, ultimately, the improved survival observed in severely CO-poisoned mice treated with Ngb-H64Q-CCC versus PBS are associated with an improvement in tissue respiration and CcO activity. If shown to be safe and efficacious in humans, Ngb-H64Q-CCC could be used as an antidote for clinical CO poisoning.
Experimental procedures
Neuroglobin expression and purification
Site-directed mutagenesis of Ngb to H64Q combined with three surface thiol substitutions (C46G, C55S, and C120S mutations), referred to as Ngb-H64Q-CCC, was obtained as described previously (46). After endotoxin removal, an excess amount of ferricyanide was added to oxidize the protein and removed via gravity Sephadex G25 size-exclusion columns (PD-10, GE Healthcare). The oxidized protein was concentrated and frozen at −80 °C. The protein was thawed at the time of use and reduced by an excess amount of sodium dithionite (50 mm). The sodium dithionite was removed by a gravity G25 size-exclusion column yielding oxygen-bound Ngb-H64Q-CCC. For the CO-poisoning mouse model studies, the G25 size-exclusion column step was performed in an anaerobic glove box. Afterward, 2.5 mm of sodium dithionite was added to keep the protein in the reduced state during infusion in animal studies. The concentration of total Ngb-H64Q-CCC and of the amount of oxygen-bound, deoxy-, and met-species were confirmed by absorbance spectra collected on a Cary 50 spectrophotometer.
Severe lethal CO-poisoning mouse model
The lethal CO-poisoning murine model was performed as previously described (46). Briefly, male C57BL/6 WT mice were used at a mean age of 10–13 weeks (weight, 23–27 g). The mice were anesthetized by isoflurane, and then a tracheal tube was placed followed by cannulation of right jugular vein and left carotid artery. Arterial blood pressure was monitored and recorded (DATAQ instruments, Akron, OH) through a catheter in the carotid artery. An intravenous catheter was placed and used for Ngb-H64Q-CCC or control PBS infusions using a syringe pump. Infusion volume was calculated according to the mouse weight (10 μl/g) and dead volume of the catheter.
Ventilation was initiated after the surgery with a volume controlled ventilator (MiniVent, type 845; Hugo Sachs, March-Hugstetten, Germany). Air was administrated (21% oxygen) with 1.5% isoflurane at tidal volumes of 230–270 μl (8.8 μl/g weight) and respiratory frequencies of 175 breaths/min. CO (3%) was delivered in air for 4.5 min via the ventilator. After CO delivery, the ventilator delivered air without CO and the Ngb-H64Q-CCC or PBS control was infused for 2 min. Ngb-H64Q-CCC solutions of 11.6 ± 0.6 mm (11∼12 mm) or solutions of PBS in a volume of 10 μl/g mouse weight were infused. A schematic of the severe CO-poisoning model is shown in Fig. 1A. Arterial blood pressure and heart rate signals were processed using Labchart software (ADInstruments Ltd.). Mortality was established based on an unmeasurable heart rate and blood pressure for 5 min, and all mice were followed for a predefined experimental observation period of 40 min from CO exposure, followed by sacrifice. Hearts were removed from the mice immediately following death or at sacrifice upon the end of the observation period.
For control mice, animals underwent sedation with 1.5% isofluorane via a face mask for at least 20 min. The hearts were recovered after euthanasia for further analysis.
Tissue respiration of mouse hearts from severe CO-poisoning model
To measure cardiac tissue respiration in our lethal CO-poisoned mouse model, the hearts of mice immediately after death or euthanasia by cervical neck dislocation and exsanguination (in mice surviving 40 min) were collected. Control mice were sedated with 1.5% isofluorane gas for 20 min to control for the effects of anesthesia and then euthanized. Heart tissue was suspended in a buffer composed of KCl (120 mm), HEPES (10 mm), EGTA (1 mm), KH2PO4 (5 mm), and sucrose (25 mm) (pH 7.4) and electronically homogenized. Homogenate was placed into the respirometry system (YSI 5300 A-1; Instech Laboratories, Plymouth Meeting, PA). The tissue was stimulated to respire with addition of pyruvate (1 mm), malate (1 mm), and ADP (0.5 mm) until the chamber oxygen reached 0%. Rates of oxygen consumption were measured with a Clark-type oxygen electrode. Respiration rates were adjusted for protein concentration. Oxygen concentrations were recorded using a digital recording device (Dataq, Akron, OH). All heart tissue respiration was established with at least four separate experiments each.
Electron transport chain complex activity measurements
Spectrophotometric kinetic enzymatic activity assays of complexes I, II, and IV and citrate synthase were performed on mouse heart homogenate as previously described (88–90). Heart tissue was extracted from the same mice undergoing CO poisoning treated with PBS or Ngb-H64Q-CCC or with isofluorane sedation without CO exposure used in the tissue respiration experiments. Homogenized heart tissues from mice poisoned with CO treated with PBS (n = 9), Ngb-H64Q-CCC (n = 5) or treated only with 1.5% isofluorane as a control (n = 8) were used for spectrophotometric studies. For complex I, NADH oxidation by complex I vis-à-vis oxidation of co-enzyme Q2 after the addition of NADH was measured in the presence of potassium cyanide to inhibit complex IV. For complex II, reduction of co-enzyme Q2 by complex II after the addition of succinate was measured in the presence of rotenone and potassium cyanide to inhibit complexes I and IV. For CcO, the oxidation of reduced cytochrome c by complex IV was measured. For citrate synthase, the rate of production of CoA from oxaloacetate was measured by measuring the production of free 5-thio-2-nitrobenzoate anions from the reaction between the thiol reagent 5,5-dithio-bis-2-nitrobenzoate and CoA.
Isolated liver mitochondrial respiration measurements
All experimental protocols using mice and rats were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and in accordance with National Institutes of Health guidelines.
Liver mitochondria were isolated from livers extracted from healthy, male Sprague–Dawley rats by differential centrifugation (29) and suspended in respiration buffer composed of KCl (120 mm), HEPES (10 mm), EGTA (1 mm), KH2PO4 (5 mm), and sucrose (25 mm) (pH 7.4). The animals were euthanized with carbon dioxide asphyxiation, cervical dislocation, and heart puncture. The mitochondria were placed into a sealed, magnetically stirred Clark-like oxygen electrode respirometry system at 37 °C (Mitocell S200 micro respirometry system; Strathkelvin Instruments Limited). Total volume of the solution in the chamber was initially 400 μl (0.9–1.5 mg/ml protein concentration or 7.8 × 109–1.3 × 1010 total mitochondria) (91). The mitochondria were stimulated to respire with addition of succinate (7.5 mm) and ADP (7.5 mm) until the chamber oxygen level reached 0. The rates of oxygen consumption were measured with a Clark-type oxygen electrode. Following full deoxygenation caused by state 3 respiration, the chamber was reoxygenated by the addition of 50 μl of aerated PBS, and the respiratory rate was measured to establish a base respiration rate for each experimental arm. This was repeated three consecutive times for each study (Figs. 2A and 3A).
For control experiments, the chamber was allowed to reach hypoxia (caused by respiration) and reoxygenated with 50 μl of aerobic PBS three times (Figs. 2A and 3A). The third respiratory rate was compared with initial respiration rate (third respiration rate/initial respiration rate). To assess the effect of Ngb-H64Q-CCC alone, after the initial respiration rate was established with aerobic PBS, a second 50 μl of PBS was added to the chamber. Ngb-H64Q-CCC (50 or 100 μm) was added to the chamber after the second reoxygenation (Figs. 2C and 3C). The rate of respiration was calculated and compared with the initial rate.
For NO or CO exposure experiments, the chamber was reoxygenated with 50 μl of PBS to measure the initial rate. Then NO (through PROLI-NONOate) or CO was introduced into the chamber. An additional bolus (50 μl) of aerobic PBS was added to the chamber to demonstrate the persistence of inhibition. This inhibited rate is compared with the initial rate (Figs. 2B and 3B).
To test the effect of Ngb-H64Q-CCC on NO- and CO-induced inhibition, the chamber was reoxygenated with 50 μl of aerobic PBS to establish a baseline rate. NO and CO exposure occurred in the second step, as described above. On the third and final step, instead of 50 μl of aerobic PBS, oxygen-bound Ngb-H64Q-CCC was added. The third respiration rate, after NO or CO exposure followed by Ngb-H64Q-CCC, was measured and compared with the baseline respiration rate (Figs. 2D and 3D). In the case of the CO experiments, two additional concentrations of oxygen-bound Ngb-H64Q-CCC were added: 10 and 150 μm. The third respiration rate compared with the baseline respiration rate was evaluated among the three doses of Ngb-H64Q-CCC used (10, 100, and 150 μm versus 160 μm of CO) (Fig. S1).
Rates were measured from the highest comparable level of oxygen (∼10–20 nmol oxygen) for each reoxygenation step to the point at which the baseline rate became nonlinear (∼0–2 nmol oxygen) (Fig. S2). A minimum of five experiments were performed for each experimental arm for the main study.
NO and CO solutions
PROLI-NONOate solution was made in 0.01 m NaOH. NO concentration was confirmed by absorbance spectroscopy by measuring the stock solution at 252 nm (ε = 8,400 m cm−1) (25) (Cary 50 UV-visible spectrophotometer; Agilent Technologies).
CO was introduced by aerating PBS with >99.0% CO gas (Matheson) to saturate the solution in a sealed glass vial. Concentrations of CO in solution were 792 ± 13.5 μm confirmed with absorbance spectroscopy when combined with isolated deoxy-hemoglobin.
Individual complex activity measurements
The mitochondria were isolated from rat liver mitochondria by differential centrifugation. The mitochondria under went three freeze/thaw cycles in ethanol/dry ice and 37 °C water bath. All experiments were performed in an anaerobic glove box with controlled oxygen levels (2%) through aerobic buffer titration at 37 °C.
Complex I
Anaerobic complex I buffer (25 mm K2PO4 and 10 mm MgCl2), 2% oxygen via 40 μl of aerated complex I buffer, 50 μg/μl isolated mitochondria, 2.5 mg/ml BSA (fat free), 1 mm KCN, and 100 μm NADH were added to a 2-mm sealed cuvette to a final volume of 400 μl. Absorbance spectroscopy was performed in the 250–700-nm wavelength range (Agilent HP8453; Agilent Technologies). To begin the reaction, 250 μm co-enzyme Q2 was added (88, 89). The experiments were performed for 10 min; 3 μm rotenone was then added to obtain a background rate. To obtain a CO-inhibited activity rate, 100 μl of CO-saturated anaerobic buffer was used at the beginning of the experiment instead of 100 μl of anaerobic buffer.
Complex II
Anaerobic complex II buffer (100 mm K2PO4), 2% oxygen via 40 μl of aerated complex II buffer, 9.5 μg/μl isolated mitochondria, 20 mm succinate, 100 μm EDTA, 120 μm DCPIP, 1 mm KCN, and 10 μm rotenone were added to a 1-cm sealed cuvette to a final volume of 400 μl. Absorbance spectroscopy was performed in the 250–700-nm wavelength range. To begin the reaction, 100 μm co-enzyme Q2 was added (88, 89). The experiments were performed for 10 min; 1.5 mm 2-Thenoyltrifluoroacetone was then added to obtain a background rate. To obtain a CO-inhibited activity rate, 100 μl of CO-saturated anaerobic buffer was used at the beginning of the experiment instead of 100 μl of anaerobic buffer.
Complex IV
Anaerobic complex IV buffer (10 mm K2PO4), 2% oxygen via 40 μl of aerated complex IV buffer, and 2.5 μg/μl isolated mitochondria were added to a 1-cm sealed cuvette to a final volume of 400 μl. Absorbance spectroscopy was performed in the 250–700-nm wavelength range. To begin the reaction, 50 μm reduced cytochrome c was added (88, 89). Experiments were performed for 10 min; 1 mm KCN was added to verify that CcO activity was being observed. To obtain a CO-inhibited activity rate, 100 μl of CO-saturated anaerobic buffer was used at the beginning of the experiment instead of 100 μl of anaerobic buffer.
Cytochrome c oxidase activity measurements
The mitochondria were placed into a sealed, magnetically stirred Clark-like oxygen electrode respirometry system at 37 °C (Mitocell S200 micro respirometry system; Strathkelvin Instruments Limited). Total volume of the solution in the chamber was initially 400 μl (0.9–1.6 mg/ml protein concentration). Electron transfer to complex IV was stimulated by the addition of TMPD (0.3 mm), ascorbic acid (3.6 mm) and FCCP (0.015 mm) (59–61). Oxygen consumption was measured versus time to determine CcO activity levels.
Four experimental arms were used for NO studies (Fig. 5, A–D), and four experimental arms were used for CO studies (Fig. 6, A–D). For NO studies, 1) control rate was established with mitochondria only; 2) NO only rate was determined with mitochondria and PROLI-NONOate (50 μm); 3) Ngb-H64Q-CCC only rate was determined with mitochondria and 50 μm Ngb-H64Q-CCC; and 4) NO+Ngb-H64Q-CCC, PROLI-NONOate was added to the mitochondria to an end concentration of 50 μm, then a small delay followed to confirm inhibition, then oxy-Ngb-H64Q-CCC was added to an end concentration of 50 μm (therefore a 1:2 ratio to NO molecules as PROLI-NONOate releases two molecules of NO).
For the CO studies, 1) control rate was established with mitochondria only; 2) for CO only, 100 μl of CO-saturated PBS (792 ± 13.5 μm) was added to a final concentration of ∼160 μm CO to the chamber, followed shortly after by 100 μl of aerobic PBS to mimic the addition of aerobic oxy-Ngb-H64Q-CCC (with additional oxygen added to chamber dissolved in solution and bound to Ngb-H64Q-CCC); 3) for Ngb-H64Q-CCC only, more Ngb-H64Q-CCC (100 μm) was added because of higher amounts of CO versus NO studies; and 4) for CO+Ngb-H64Q-CCC, 100 μl of CO-saturated PBS was added to a concentration of 160 μm CO to the reaction chamber, and then oxy-Ngb-H64Q-CCC was added to an end concentration of 100 μm.
The rate of activity for each arm was compared with the average baseline (nonpoisoned, nontreated mitochondria) for each experimental day (to control for variances between animals). For NO exposure studies, the rate was measured from ∼30 nmol of oxygen to the point at which the nonpoisoned, nontreated baseline became nonlinear (between 0 and 5 nmol of oxygen) (Fig. S3). For the NO-only group, where complete cessation of respiration occurred for a long duration of time after exposure, the rate was calculated from 45 nmol of oxygen to capture prolonged near total cessation of CcO activity. For CO exposure studies, the rate was measured from 30 nmol of oxygen to the point at which the nonpoisoned, nontreated baseline became nonlinear (between 0 and 5 nmol oxygen) (Fig. S4). A minimum of five experiments were performed for each experimental arm.
Statistical analyses
The data are means ± S.E. and were analyzed by unpaired Student's t test. Welch's correction was used when an f test demonstrated a significant difference in variance between groups. Mann–Whitney test was used to evaluate the in vivo CO exposure with ex vivo biochemical measurement studies because of lower sample sizes and greater biological variance. The statistical tests used are noted in the figure legends. Additionally, we used an unmatched regular two-way ANOVA to demonstrate the interaction effect of exposure to CO or NO inhibition and treatment with Ngb-H64Q-CCC. These statistical analyses were performed using GraphPad Prism software version 7.0. Investigators were not blinded to experiments. p values of <0.05 were considered significant.
Data availability
All data are contained within the article and supporting information.
Author contributions
J. J. R., K. A. B., Q. X., L. W., A. W. D., X. C., C. G. C., D. A. G., I. A., X. N. H., L. G., M. N., C. F. M., C. P. O., J. T., S. S., and M. T. G. conceptualization; J. J. R., M. N., C. P. O., J. T., S. S., and M. T. G. resources; J. J. R., K. A. B., Q. X., L. W., X. C., C. G. C., D. A. G., I. A., X. N. H., Q. T., L. G., M. N., C. F. M., C. P. O., J. T., S. S., and M. T. G. data curation; J. J. R., M. N., C. P. O., J. T., S. S., and M. T. G. software; J. J. R., K. A. B., Q. X., L. W., M. N., C. F. M., J. T., S. S., and M. T. G. formal analysis; J. J. R., Q. X., J. T., S. S., and M. T. G. supervision; J. J. R., J. T., S. S., and M. T. G. funding acquisition; J. J. R., K. A. B., Q. X., A. W. D., J. T., S. S., and M. T. G. validation; J. J. R., Q. X., L. W., A. W. D., X. C., C. G. C., D. A. G., I. A., X. N. H., Q. T., M. N., C. F. M., C. P. O., J. T., S. S., and M. T. G. investigation; J. J. R., K. A. B., Q. X., L. W., A. W. D., J. T., S. S., and M. T. G. visualization; J. J. R., K. A. B., Q. X., L. W., X. C., C. G. C., D. A. G., I. A., X. N. H., Q. T., L. G., M. N., C. F. M., C. P. O., J. T., S. S., and M. T. G. methodology; J. J. R., K. A. B., Q. X., L. W., A. W. D., M. N., C. F. M., C. P. O., J. T., S. S., and M. T. G. writing-original draft; J. J. R., K. A. B., Q. X., J. T., S. S., and M. T. G. project administration; J. J. R., K. A. B., Q. X., L. W., A. W. D., M. N., J. T., S. S., and M. T. G. writing-review and editing.
Supplementary Material
Acknowledgments
We thank S. Tiwari and B. Lemster for help with protein production; D. Osei-Hwedieh for assistance with mice injections; and M. Reynolds and Q. Nguyen for helpful discussion on mitochondrial studies.
This work was supported in part by National Institutes of Health Grants F32 HL132418 and K08 HL136857 (to J. J. R.), R01 HL111706 (to C. P. O.), R01 HL125886 (to J. T. and M. T. G.), R01 HL098032, P01 HL103455, T32 HL110849, and T32 HL007563 (to M. T. G.), R01 GM113816 (to S. S.) and R21 ES027390 (to J. T.). This work was also supported by the National Institutes of Health, NHLBI SMARTT (Science Moving towArd Research Translation and Therapy) Program, the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania (to M. T. G.), and Parker B. Francis Foundation (to J. J. R.). J. J. R., L. W., C. F. M., J. T., and M. T. G. are shareholders in Globin Solutions. J. J. R., Q. X., A. W. D., J. T., and M. T. G. are coinventors of provisional, pending, and granted patents for the use of recombinant neuroglobin and other heme-based molecules as antidotes for carbon monoxide poisoning. J. J. R. and J. T. are officers and directors of Globin Solutions, Inc. M. T. G. is a director and advisor of Globin Solutions, Inc., which has licensed this technology and had an option to technology directed at using hydroxycobalamin for carbon monoxide poisoning from Virginia Commonwealth University that has expired. M. T. G. is a coinventor on patents directed to the use of nitrite salts in cardiovascular diseases licensed and exclusively optioned to Globin Solutions, Inc., which has a sponsored research agreement with the University of Pittsburgh aimed at developing carbon monoxide poisoning antidotes into therapeutics that did not support the research contained in this grant that partially supports the efforts of M. T. G., J. T., Q. X., X. C., Q. T., and X. N. H., M. T. G. is a coinvestigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguate as a treatment for patients with sickle cell disease. The financial conflicts of interest of J. J. R., J. T., Q. X., L. W., A. W. D., C. F. M., and M. T. G. were managed by the University of Pittsburgh Conflict of Interest Committee and a data stewardship committee. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4.
- CO
- carbon monoxide
- Hb
- hemoglobin
- HbCO
- carboxyhemoglobin
- CcO
- cytochrome c oxidase
- HBO2
- hyperbaric oxygen
- ANOVA
- analysis of variance
- TMPD
- N,N,N′,N′-tetramethyl-p-phenylenediamine
- FCCP
- carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone.
References
- 1. Rose J. J., Wang L., Xu Q., McTiernan C. F., Shiva S., Tejero J., and Gladwin M. T. (2017) Carbon monoxide poisoning: pathogenesis, management and future directions of therapy. Am. J. Respir. Crit. Care Med. 195, 596–606 10.1164/rccm.201606-1275CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hampson N. B. (2016) U.S. mortality due to carbon monoxide poisoning, 1999–2014: accidental and intentional deaths. Ann. Am. Thorac. Soc. 13, 1768–1774 [DOI] [PubMed] [Google Scholar]
- 3. Henry C. R., Satran D., Lindgren B., Adkinson C., Nicholson C. I., and Henry T. D. (2006) Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 295, 398–402 10.1001/jama.295.4.398 [DOI] [PubMed] [Google Scholar]
- 4. Hampson N. B., Rudd R. A., and Hauff N. M. (2009) Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit. Care Med. 37, 1941–1947 10.1097/CCM.0b013e3181a0064f [DOI] [PubMed] [Google Scholar]
- 5. Huang C. C., Chung M. H., Weng S. F., Chien C. C., Lin S. J., Lin H. J., Guo H. R., Su S. B., Hsu C. C., and Juan C. (2014) Long-term prognosis of patients with carbon monoxide poisoning: a nationwide cohort study. PLoS One 9, e105503 10.1371/journal.pone.0105503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weaver L. K., Hopkins R. O., Churchill S. K., and Deru K. (2008) Neurological outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb. Med. 35, 258–259 [Google Scholar]
- 7. Hopkins R., and Weaver L. K. (2008) Cognitive outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb. Med. 35, 258 [Google Scholar]
- 8. Mimura K., Harada M., Sumiyoshi S., Tohya G., Takagi M., Fujita E., Takata A., and Tatetsu S. (1999) Long-term follow-up study on sequelae of carbon monoxide poisoning; serial investigation 33 years after poisoning. Seishin Shinkeigaku Zasshi 101, 592–618 [PubMed] [Google Scholar]
- 9. Hsaio C. L., Kuo H. C., and Huang C. C. (2004) Delayed encephalopathy after carbon monoxide intoxication: long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurol. Taiwan 13, 64–70 [PubMed] [Google Scholar]
- 10. Chambers C. A., Hopkins R. O., Weaver L. K., and Key C. (2008) Cognitive and affective outcomes of more severe compared to less severe carbon monoxide poisoning. Brain Inj. 22, 387–395 10.1080/02699050802008075 [DOI] [PubMed] [Google Scholar]
- 11. Dziewierz A., Ciszowski K., Gawlikowski T., Rakowski T., Kleczynski P., Surdacki A., and Dudek D. (2013) Primary angioplasty in patient with ST-segment elevation myocardial infarction in the setting of intentional carbon monoxide poisoning. J. Emerg. Med. 45, 831–834 10.1016/j.jemermed.2013.05.061 [DOI] [PubMed] [Google Scholar]
- 12. Lippi G., Rastelli G., Meschi T., Borghi L., and Cervellin G. (2012) Pathophysiology, clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin. Biochem. 45, 1278–1285 10.1016/j.clinbiochem.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 13. Smithline H. A., Ward K. R., Chiulli D. A., Blake H. C., and Rivers E. P. (2003) Whole body oxygen consumption and critical oxygen delivery in response to prolonged and severe carbon monoxide poisoning. Resuscitation 56, 97–104 10.1016/S0300-9572(02)00272-1 [DOI] [PubMed] [Google Scholar]
- 14. Hall J. (2010) Guyton and Hall Textbook of Medical Physiology, Saunders/Elsevier, Philadelphia, PA [Google Scholar]
- 15. Hampson N. B., Piantadosi C. A., Thom S. R., and Weaver L. K. (2012) Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 186, 1095–1101 10.1164/rccm.201207-1284CI [DOI] [PubMed] [Google Scholar]
- 16. Bernard C. (1857) Leçons sur les Effets des Substances Toxiques et Médicamenteuses, J.-B. Bailliere et Fils, Paris, France: [PubMed] [Google Scholar]
- 17. Hampson N. B., and Hauff N. (2008) Risk factors for short-term mortality from carbon monoxide poisoning treated with hyperbaric oxygen. Crit. Care Med. 36, 2523–2527 10.1097/CCM.0b013e31818419d8 [DOI] [PubMed] [Google Scholar]
- 18. Weaver L. (2009) Clinical practice: carbon monoxide poisoning. N. Engl. J. Med. 360, 1217–1225 10.1056/NEJMcp0808891 [DOI] [PubMed] [Google Scholar]
- 19. Hampson N. B., Dunn S. L., and UHMCS/CDC CO Poisoning Surveillance Group (2012) Symptoms of acute carbon monoxide poisoning do not correlate with the initial carboxyhemoglobin level. Undersea Hyperb. Med. 39, 657–665 [PubMed] [Google Scholar]
- 20. Coburn R. (1973) Endogenous carbon monoxide metabolism. Annu. Rev. Med. 24, 241–250 10.1146/annurev.me.24.020173.001325 [DOI] [PubMed] [Google Scholar]
- 21. Motterlini R., and Otterbein L. E. (2010) The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 9, 728–743 10.1038/nrd3228 [DOI] [PubMed] [Google Scholar]
- 22. Brown S. D., and Piantadosi C. A. (1990) In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J. Appl. Physiol. 68, 604–610 10.1152/jappl.1990.68.2.604 [DOI] [PubMed] [Google Scholar]
- 23. Brown S. D., and Piantadosi C. (1989) Reversal of carbon monoxide-cytochrome c oxidase binding by hyperbaric oxygen in vivo. Adv. Exp. Med. Biol. 248, 747–754 10.1007/978-1-4684-5643-1_84 [DOI] [PubMed] [Google Scholar]
- 24. Iheagwara K. N., Thom S. R., Deutschman C. S., and Levy R. J. (2007) Myocardial cytochrome oxidase activity is decreased following carbon monoxide exposure. Biochim. Biophys. Acta 1772, 1112–1116 10.1016/j.bbadis.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., and Keefer L. K. (1991) Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide: vasorelaxant effects. J. Med. Chem. 34, 3242–3247 10.1021/jm00115a013 [DOI] [PubMed] [Google Scholar]
- 26. Brown S. D., and Piantadosi C. A. (1992) Recovery of energy metabolism in rat brain after carbon monoxide hypoxia. J. Clin. Invest. 89, 666–672 10.1172/JCI115633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner M., Hamilton-Farrell M. R., and Clark R. J. (1999) Carbon monoxide poisoning: an update. J. Accid. Emerg. Med. 16, 92–96 10.1136/emj.16.2.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldbaum L. R., Orellano T., and Dergal E. (1976) Mechanism of the toxic action of carbon monoxide. Ann. Clin. Lab. Sci. 6, 372–376 [PubMed] [Google Scholar]
- 29. Shiva S., Brookes P. S., Patel R. P., Anderson P. G., and Darley-Usmar V. M. (2001) Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. U.S.A. 98, 7212–7217 10.1073/pnas.131128898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiva S., Huang Z., Grubina R., Sun J., Ringwood L. A., MacArthur P. H., Xu X., Murphy E., Darley-Usmar V. M., and Gladwin M. T. (2007) Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100, 654–661 10.1161/01.RES.0000260171.52224.6b [DOI] [PubMed] [Google Scholar]
- 31. Gnaiger E., Lassnig B., Kuznetsov A., Rieger G., and Margreiter R. (1998) Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 201, 1129–1139 [DOI] [PubMed] [Google Scholar]
- 32. Wald G., and Allen D. W. (1957) The equilibrium between cytochrome oxidase and carbon monoxide. J. Gen. Physiol. 40, 593–608 10.1085/jgp.40.4.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piantadosi C. A., Carraway M. S., and Suliman H. B. (2006) Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic. Biol. Med. 40, 1332–1339 10.1016/j.freeradbiomed.2005.11.020 [DOI] [PubMed] [Google Scholar]
- 34. Piantadosi C. A. (2008) Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 45, 562–569 10.1016/j.freeradbiomed.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo Iacono L., Boczkowski J., Zini R., Salouage I., Berdeaux A., Motterlini R., and Morin D. (2011) A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Radic. Biol. Med. 50, 1556–1564 10.1016/j.freeradbiomed.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 36. Thom S. R., Ohnishi S. T., and Ischiropoulos H. (1994) Nitric oxide released by platelets inhibits neutrophil integrin function following acute carbon monoxide poisoning. Toxicol. Appl. Pharmacol. 128, 105–110 10.1006/taap.1994.1186 [DOI] [PubMed] [Google Scholar]
- 37. Thom S. R., Xu Y. A., and Ischiropoulos H. (1997) Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem. Res. Toxicol. 10, 1023–1031 10.1021/tx970041h [DOI] [PubMed] [Google Scholar]
- 38. Thom S. R., Bhopale V. M., Han S. T., Clark J. M., and Hardy K. R. (2006) Intravascular neutrophil activation due to carbon monoxide poisoning. Am. J. Respir. Crit Care Med. 174, 1239–1248 10.1164/rccm.200604-557OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thom S. (2008) Carbon monoxide pathophysiology and treatment. In Physiology and Medicine of Hyperbaric Oxygen Therapy (Neuman T., and Thom S. R., eds) pp. 321–347, Saunders Elsevier, Philadelphia, PA [Google Scholar]
- 40. Roderique J. D., Josef C. S., Feldman M. J., and Spiess B. D. (2015) A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 334, 45–58 10.1016/j.tox.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 41. Fischer G. W., and Levin M. A. (2010) Vasoplegia during cardiac surgery: current concepts and management. Semin. Thorac. Cardiovasc. Surg. 22, 140–144 10.1053/j.semtcvs.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 42. Ketteler M., Cetto C., Kirdorf M., Jeschke G. S., Schäfer J. H., and Distler A. (1998) Nitric oxide in sepsis-syndrome: potential treatment of septic shock by nitric oxide synthase antagonists. Kidney Int. Supp. 64, S27–S30 [PubMed] [Google Scholar]
- 43. Weaver L. K., Hopkins R. O., Chan K. J., Churchill S., Elliott C. G., Clemmer T. P., Orme J. F. Jr., Thomas F. O., and Morris A. H. (2002) Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 347, 1057–1067 10.1056/NEJMoa013121 [DOI] [PubMed] [Google Scholar]
- 44. Ernst A., and Zibrak J. (1998) Carbon monoxide poisoning. N. Engl. J. Med. 339, 1603–1608 10.1056/NEJM199811263392206 [DOI] [PubMed] [Google Scholar]
- 45. Winter P. M., and Miller J. (1976) Carbon monoxide poisoning. JAMA 236, 1502–1504 10.1001/jama.1976.03270140054029 [DOI] [PubMed] [Google Scholar]
- 46. Azarov I., Wang L., Rose J. J., Xu Q., Huang X. N., Belanger A., Wang Y., Guo L., Liu C., Ucer K. B., McTiernan C. F., O'Donnell C. P., Shiva S., Tejero J., Kim-Shapiro D. B., et al. (2016) Five-coordinate H64Q neuroglobin as a ligand-trap antidote for carbon monoxide poisoning. Sci. Transl. Med. 8, 368ra173 10.1126/scitranslmed.aah6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tiso M., Tejero J., Basu S., Azarov I., Wang X., Simplaceanu V., Frizzell S., Jayaraman T., Geary L., Shapiro C., Ho C., Shiva S., Kim-Shapiro D. B., and Gladwin M. T. (2011) Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 286, 18277–18289 10.1074/jbc.M110.159541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., and Schapira A. H. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54 10.1016/0014-5793(94)00424-2 [DOI] [PubMed] [Google Scholar]
- 49. Brookes P. S., Kraus D. W., Shiva S., Doeller J. E., Barone M. C., Patel R. P., Lancaster J. R. Jr., and Darley-Usmar V. (2003) Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. J. Biol. Chem. 278, 31603–31609 10.1074/jbc.M211784200 [DOI] [PubMed] [Google Scholar]
- 50. Brookes P. S., Shiva S., Patel R. P., and Darley-Usmar V. M. (2002) Measurement of mitochondrial respiratory thresholds and the control of respiration by nitric oxide. Methods Enzymol. 359, 305–319 10.1016/S0076-6879(02)59194-1 [DOI] [PubMed] [Google Scholar]
- 51. Brown G. C., and Borutaite V. (2002) Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 33, 1440–1450 10.1016/S0891-5849(02)01112-7 [DOI] [PubMed] [Google Scholar]
- 52. Kamga C., Krishnamurthy S., and Shiva S. (2012) Myoglobin and mitochondria: a relationship bound by oxygen and nitric oxide. Nitric Oxide 26, 251–258 10.1016/j.niox.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brunori M. (2001) Nitric oxide, cytochrome c oxidase and myoglobin. Trends Biochem. Sci. 26, 21–23 10.1016/S0968-0004(00)01698-4 [DOI] [PubMed] [Google Scholar]
- 54. Flögel U., Merx M. W., Godecke A., Decking U. K., and Schrader J. (2001) Myoglobin: a scavenger of bioactive NO. Proc. Natl. Acad. Sci. U.S.A. 98, 735–740 10.1073/pnas.98.2.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarti P., Arese M., Bacchi A., Barone M. C., Forte E., Mastronicola D., Brunori M., and Giuffrè A. (2003) Nitric oxide and mitochondrial complex IV. IUBMB Life 55, 605–611 [DOI] [PubMed] [Google Scholar]
- 56. Sarti P., Forte E., Mastronicola D., Giuffrè A., and Arese M. (2012) Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim. Biophys. Acta 1817, 610–619 10.1016/j.bbabio.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 57. Tsai A. L., Berka V., Martin E., and Olson J. S. (2012) A “sliding scale rule” for selectivity among NO, CO, and O2 by heme protein sensors. Biochemistry 51, 172–186 10.1021/bi2015629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D'Amico G., Lam F., Hagen T., and Moncada S. (2006) Inhibition of cellular respiration by endogenously produced carbon monoxide. J. Cell Sci. 119, 2291–2298 10.1242/jcs.02914 [DOI] [PubMed] [Google Scholar]
- 59. Bai Y., Hu P., Park J. S., Deng J. H., Song X., Chomyn A., Yagi T., and Attardi G. (2004) Genetic and functional analysis of mitochondrial DNA-encoded complex I genes. Ann. N.Y. Acad. Sci. 1011, 272–283 10.1196/annals.1293.026 [DOI] [PubMed] [Google Scholar]
- 60. Li Y., Park J. S., Deng J. H., and Bai Y. (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 38, 283–291 10.1007/s10863-006-9052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wen J. J., and Garg N. J. (2010) Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi–infected mice. Antioxid. Redox Signal. 12, 27–37 10.1089/ars.2008.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schewe T., Hiebsch C., Ludwig P., and Rapoport S. M. (1983) Haemoglobin potentiates the respiration-inhibitory action of lipoxygenases via its pseudolipohydroperoxidase activity. Biomed. Biochim. Acta 42, 789–803 [PubMed] [Google Scholar]
- 63. Plotnikov E. Y., Chupyrkina A. A., Pevzner I. B., Isaev N. K., and Zorov D. B. (2009) Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney's mitochondria. Biochim. Biophys. Acta 1792, 796–803 10.1016/j.bbadis.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 64. Kumar S., and Bandyopadhyay U. (2005) Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 157, 175–188 10.1016/j.toxlet.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 65. Cai B., Lin Y., Xue X. H., Fang L., Wang N., and Wu Z. Y. (2011) TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp. Neurol. 227, 224–231 10.1016/j.expneurol.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 66. Fordel E., Thijs L., Martinet W., Schrijvers D., Moens L., and Dewilde S. (2007) Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene 398, 114–122 10.1016/j.gene.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 67. Greenberg D. A., Jin K., and Khan A. A. (2008) Neuroglobin: an endogenous neuroprotectant. Curr. Opin. Pharmacol. 8, 20–24 10.1016/j.coph.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu Z., Liu J., Guo S., Xing C., Fan X., Ning M., Yuan J. C., Lo E. H., and Wang X. (2009) Neuroglobin-overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation. Neuroscience 162, 396–403 10.1016/j.neuroscience.2009.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cooper C. E. (1999) Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 10.1016/S0005-2728(99)00021-3 [DOI] [PubMed] [Google Scholar]
- 70. Pannala V. R., Camara A. K., and Dash R. K. (2016) Modeling the detailed kinetics of mitochondrial cytochrome c oxidase: catalytic mechanism and nitric oxide inhibition. J. Appl. Physiol. (1985) 121, 1196–1207 10.1152/japplphysiol.00524.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cooper C. E., and Brown G. C. (2008) The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 40, 533–539 10.1007/s10863-008-9166-6 [DOI] [PubMed] [Google Scholar]
- 72. Wilson D. F., and Vinogradov S. A. (2014) Mitochondrial cytochrome c oxidase: mechanism of action and role in regulating oxidative phosphorylation. J. Appl. Physiol. (1985) 117, 1431–1439 10.1152/japplphysiol.00737.2014 [DOI] [PubMed] [Google Scholar]
- 73. Dewilde S., Kiger L., Burmester T., Hankeln T., Baudin-Creuza V., Aerts T., Marden M. C., Caubergs R., and Moens L. (2001) Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 276, 38949–38955 10.1074/jbc.M106438200 [DOI] [PubMed] [Google Scholar]
- 74. Tejero J., and Gladwin M. T. (2014) The globin superfamily: functions in nitric oxide formation and decay. Biol. Chem. 395, 631–639 10.1515/hsz-2013-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jang D. H., Khatri U. G., Shortal B. P., Kelly M., Hardy K., Lambert D. S., and Eckmann D. M. (2018) Alterations in mitochondrial respiration and reactive oxygen species in patients poisoned with carbon monoxide treated with hyperbaric oxygen. Intensive Care Med. Exp. 6, 4 10.1186/s40635-018-0169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miró O., Casademont J., Barrientos A., Urbano-Márquez A., and Cardellach F. (1998) Mitochondrial cytochrome c oxidase inhibition during acute carbon monoxide poisoning. Pharmacol. Toxicol. 82, 199–202 10.1111/j.1600-0773.1998.tb01425.x [DOI] [PubMed] [Google Scholar]
- 77. Alonso J. R., Cardellach F., López S., Casademont J., and Miró O. (2003) Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol. 93, 142–146 10.1034/j.1600-0773.2003.930306.x [DOI] [PubMed] [Google Scholar]
- 78. Yang X., Yu L., and Yu C. A. (1997) Resolution and reconstitution of succinate-ubiquinone reductase from Escherichia coli. J. Biol. Chem. 272, 9683–9689 10.1074/jbc.272.15.9683 [DOI] [PubMed] [Google Scholar]
- 79. Gellerich F. N., Trumbeckaite S., Hertel K., Zierz S., Müller-Werdan U., Werdan K., Redl H., and Schlag G. (1999) Impaired energy metabolism in hearts of septic baboons: diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock 11, 336–341 10.1097/00024382-199905000-00006 [DOI] [PubMed] [Google Scholar]
- 80. Protti A., Fortunato F., Artoni A., Lecchi A., Motta G., Mistraletti G., Novembrino C., Comi G. P., and Gattinoni L. (2015) Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Crit. Care 19, 39 10.1186/s13054-015-0762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Powell C. S., and Jackson R. M. (2003) Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L189–L198 10.1152/ajplung.00253.2002 [DOI] [PubMed] [Google Scholar]
- 82. Hernansanz-Agustín P., Ramos E., Navarro E., Parada E., Sánchez-López N., Peláez-Aguado L., Cabrera-García J. D., Tello D., Buendia I., Marina A., Egea J., López M. G., Bogdanova A., and Martínez-Ruiz A. (2017) Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 12, 1040–1051 10.1016/j.redox.2017.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang J., and Piantadosi C. A. (1992) Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J. Clin. Invest. 90, 1193–1199 10.1172/JCI115980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Paradies G., Petrosillo G., Pistolese M., and Ruggiero F. M. (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286, 135–141 10.1016/S0378-1119(01)00814-9 [DOI] [PubMed] [Google Scholar]
- 85. Gao L., Laude K., and Cai H. (2008) Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet. Clin. North Am. Small Anim. Pract. 38, 137–155 10.1016/j.cvsm.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Handy D. E., and Loscalzo J. (2012) Redox regulation of mitochondrial function. Antioxid. Redox Signal. 16, 1323–1367 10.1089/ars.2011.4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Al-Hasan Y. M., Evans L. C., Pinkas G. A., Dabkowski E. R., Stanley W. C., and Thompson L. P. (2013) Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal Guinea pig organs. Reprod. Sci. 20, 299–307 10.1177/1933719112453509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kirby D. M., Thorburn D. R., Turnbill D. M., and Taylor R. W. (2007) Biochemical assays of respiratory chain complex activity. In Methods in Cell Biology: Mitochondria, 2nd Ed. (Pon L. A., and Schon E. A., eds) pp. 93–119, Elsevier, Inc., Amsterdam: [DOI] [PubMed] [Google Scholar]
- 89. Spinazzi M., Casarin A., Pertegato V., Salviati L., and Angelini C. (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7, 1235–1246 10.1038/nprot.2012.058 [DOI] [PubMed] [Google Scholar]
- 90. Shiva S., Sack M. N., Greer J. J., Duranski M., Ringwood L. A., Burwell L., Wang X., MacArthur P. H., Shoja A., Raghavachari N., Calvert J. W., Brookes P. S., Lefer D. J., and Gladwin M. T. (2007) Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 204, 2089–2102 10.1084/jem.20070198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schwerzmann K., Cruz-Orive L. M., Eggman R., Sänger A., and Weibel E. R. (1986) Molecular architecture of the inner membrane of mitochondria from rat liver: a combined biochemical and stereological study. J. Cell Biol. 102, 97–103 10.1083/jcb.102.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Merino J. J., Roncero C., Oset-Gasque M. J., Naddaf A., and González M. P. (2014). Antioxidant and protective mechanisms against hypoxia and hypoglycaemia in cortical neurons in vitro. Int J Mol Sci. 15, 2475–2493 10.3390/ijms15022475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article and supporting information.