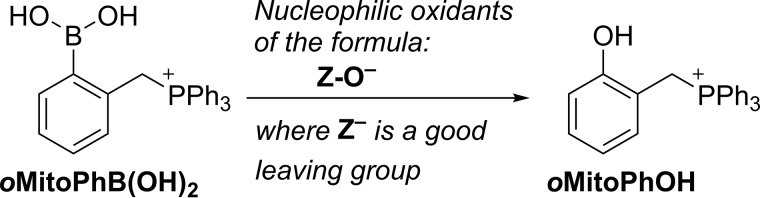Abstract
Reactive oxygen and nitrogen species have been implicated in many biological processes and diseases, including immune responses, cardiovascular dysfunction, neurodegeneration, and cancer. These chemical species are short-lived in biological settings, and detecting them in these conditions and diseases requires the use of molecular probes that form stable, easily detectable, products. The chemical mechanisms and limitations of many of the currently used probes are not well-understood, hampering their effective applications. Boronates have emerged as a class of probes for the detection of nucleophilic two-electron oxidants. Here, we report the results of an oxygen-18–labeling MS study to identify the origin of oxygen atoms in the oxidation products of phenylboronate targeted to mitochondria. We demonstrate that boronate oxidation by hydrogen peroxide, peroxymonocarbonate, hypochlorite, or peroxynitrite involves the incorporation of oxygen atoms from these oxidants. We therefore conclude that boronates can be used as probes to track isotopically labeled oxidants. This suggests that the detection of specific products formed from these redox probes could enable precise identification of oxidants formed in biological systems. We discuss the implications of these results for understanding the mechanism of conversion of the boronate-based redox probes to oxidant-specific products.
Keywords: hydrogen peroxide, superoxide ion, reactive oxygen species (ROS), reactive nitrogen species (RNS), oxygen radicals, isotopic tracer, mass spectrometry (MS), oxygen-18, peroxymonocarbonate, peroxynitrite, reaction mechanism, redox probes, hypochlorous acid, superoxide radical anion, isotope tracing
Introduction
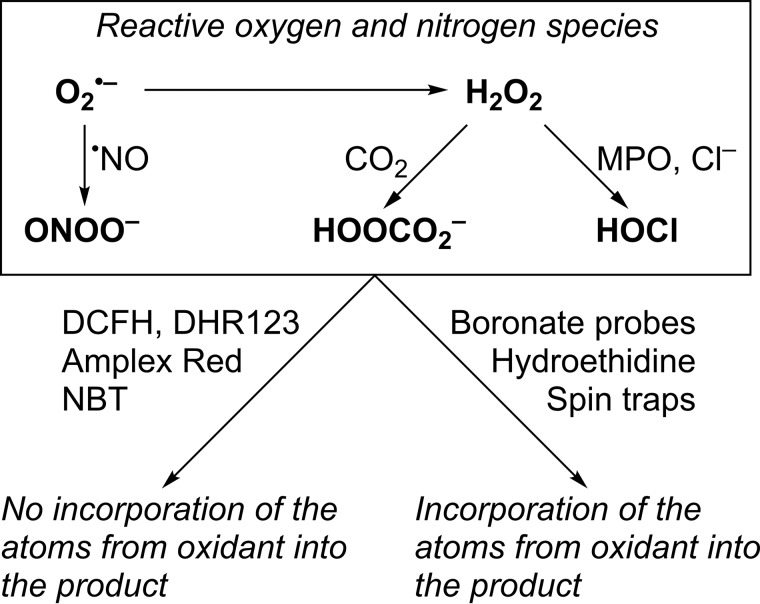
Reactive oxygen species (ROS),2 including superoxide (O2˙̄/HO2•), hydrogen peroxide (H2O2), and peroxynitrite (ONOO−/ONOOH), have been implicated in (patho)physiological mechanisms in redox biology and medicine (1–4). Both superoxide and H2O2 are relatively slow reacting and/or weak oxidants (4–6) but in biological systems can be converted to more reactive species (see Fig. 1), including peroxynitrite (7, 8), peroxymonocarbonate (HCO4−) (9, 10), or hypochlorous acid (HOCl) (11), resulting in enhanced redox signaling and/or damage to cell components (5, 12, 13). Because of the short lifetime of most ROS in biological settings, detection and quantitative analyses of those species have remained a challenge, and development of new probes for redox biology is an active area of research. Most chemical probes used for the detection of cellular oxidants lack selectivity toward a single species. For example, dichlorodihydrofluorescein (DCFH), dihydrorhodamine-123 (DHR123), and Amplex Red undergo two-electron oxidation to fluorescent dichlorofluorescein, rhodamine, and resorufin, respectively, and nitro blue tetrazolium undergoes four-electron reduction to diformazan, without incorporation of the reactive species detected into the product formed (Fig. 1). This often leads to ambiguity regarding the identity of the species detected and prevents tracking of the oxidants using isotope-labeling approach. ROS detection and their unambiguous identification in biological systems requires the use of chemical probes, which upon reaction form species-specific product(s) (14–19). As an example, spin traps react with most radicals by the formation of a covalent bond between the probe and the radical trapped, and the product formed is typically highly specific for the trapped species. Also, the conversion of hydroethidine (HE) into 2-hydroxyethidium (2-OH-E+) has been used to detect O2˙̄ in cultured cells in vitro and in animal models in vivo (20–27). Other products formed from the HE probe, including diethidium and 2-chloroethidium (2-Cl-E+), have been proposed as specific marker products of one-electron oxidants and hypochlorous acid, respectively (28, 29).
Figure 1.
In contrast to commonly used redox probes DCFH, DHR123, Amplex Red, and NBT, spin traps, boronate-based probes, and HE incorporate atoms from the oxidants into the products formed.
Oxidation of boronate-based probes into phenolic products has been utilized for the detection of H2O2 (30–32). An array of boronate probes, with similar chemical reactivities and a similar mechanism of response to H2O2 but with different modes of detection, has been reported (33–37). Also, fluorogenic boronate probes targeted to various subcellular compartments have been described (31, 38–40). Triphenylphosphonium (TPP+)-conjugated phenylboronic acid (called MitoB) was designed for MS-based detection of mitochondrial H2O2 (41–43). Resistance of boronates to heme-catalyzed oxidation makes them good candidates for the detection of oxidants in the in vivo settings. Boronate-based probes are oxidized more than a thousand times faster by HOCl and nearly a million times faster by ONOO− than by H2O2 (kH2O2 ∼ 1 m−1s−1; kHOCl ∼ 104 m−1s−1; kONOO− ∼ 106 m−1s−1), and the reaction typically involves a minor pathway, with the formation of ONOO−-specific product(s) (7, 44–48). Recently, it has been reported that peroxymonocarbonate, the product of the reaction of H2O2 with CO2, reacts with coumarin boronic acid nearly 50 times faster than H2O2 (kHCO4− ∼ 102 m−1s−1) (10).
Although the identities of the oxidation, chlorination, and nitration products of boronate probes have been established in many cases, and the reaction mechanisms have been proposed, the origin of oxygen atoms in the oxidation and nitration products of boronate probes has not been experimentally determined. Understanding the mechanisms of formation of the oxidation products is required for their rigorous use as specific ROS markers in the in vitro and in vivo settings. Also, the potential for selective monitoring of the specific oxidizing species, through use of isotopically labeled oxidant and monitoring isotopic labeling of the specific products, remains to be explored.
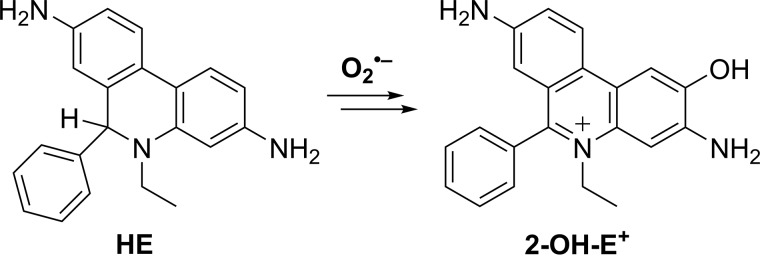
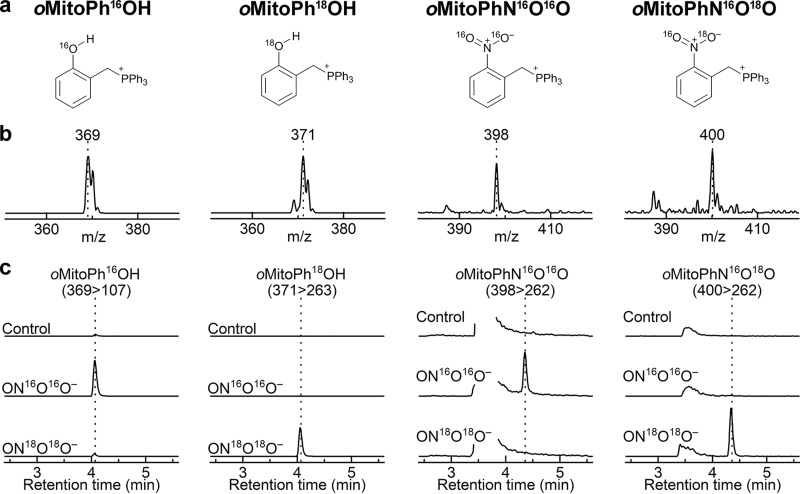
Here, we report on the incorporation of an oxygen atom from the biologically relevant two-electron oxidants, including H2O2, HCO4−, HOCl, and ONOO− in the oxidation and nitration products of the mitochondria-targeted phenyl boronate probe (oMitoPhB(OH)2) (Fig. 2). In addition, we demonstrate the involvement of oxygen atoms from superoxide in the formation of the hydroxylated product, 2-OH-E+, during oxidation of hydroethidine by O2˙̄ (Fig. 3), corroborating the proposed mechanism of the conversion of HE into 2-OH-E+.
Figure 2.
Conversion of oMitoPhB(OH)2 boronate probe in the phenolic product, oMitoPhOH, in the presence of nucleophilic two-electron oxidants.
Figure 3.
Conversion of HE into 2-OH-E+ by O2˙̄.
Results
We have investigated the incorporation of oxygen atoms from different biologically relevant nucleophilic oxidants (Fig. 1) capable of oxidizing boronate probes into the products formed. We chose oMitoPhB(OH)2 as a model boronate probe (Fig. 2) because its reactivity toward H2O2, HOCl, and ONOO− has been studied previously in detail and the products characterized (49–51). To demonstrate the formation of 18O-labeled superoxide, we have also tracked the incorporation of the 18O atom into the hydroxylation product of hydroethidine.
Hydrogen peroxide
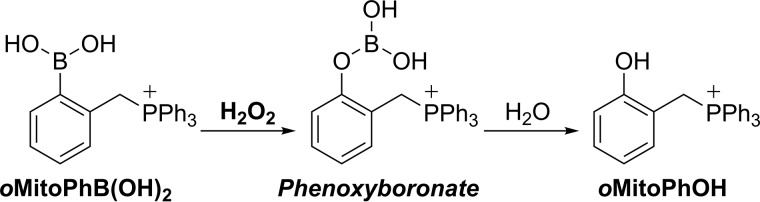
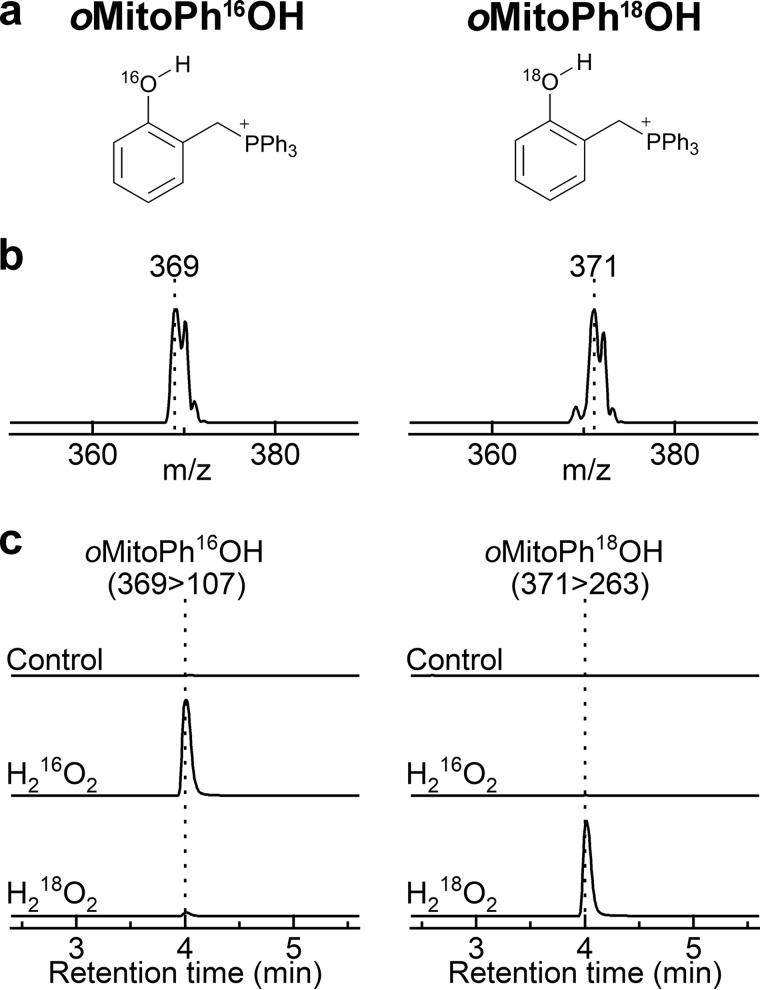
Upon oxidation by H2O2, a conversion of oMitoPhB(OH)2 into the phenolic product (oMitoPhOH) occurs (Fig. 4). H2O2 oxidizes the phenylboronate substrate into a phenoxyboronate intermediate that, upon hydrolysis, yields the phenolic product and boric acid. To determine whether the phenolic oxygen atom derives from H2O2 or water, we performed the oxidation of oMitoPhB(OH)2 by H216O2 in H218O and by H218O2 in H216O (Fig. 5). The product detected in the presence of H216O2 showed the molecular mass of oMitoPh16OH (m/z = 369); in the presence of H218O2, the product had a molecular mass of 371 (Fig. 5, a and b), attributed to oMitoPh18OH. Liquid chromatography with tandem MS (LC-MS/MS) analyses indicated no formation of oMitoPh18OH during the oxidation of the probe by H216O2 in H218O, whereas it was the predominant product in the presence of H218O2 (Fig. 5c). We conclude that during oxidation of boronates by H2O2, the oxygen atom in the phenolic product derives exclusively from H2O2 and not from water.
Figure 4.
Oxidation of the oMitoPhB(OH)2 probe by H2O2.
Figure 5.
Incorporation of an oxygen atom into the phenolic product during the oxidation of oMitoPhB(OH)2 by H2O2. a, chemical structures of the products. b, online mass spectra of the products. c, LC-MS/MS traces of the phenolic products containing 16O (left panel) or 18O (right panel). LC-MS/MS analyses were performed after incubation (20 min) of oMitoPhB(OH)2 (20 μm) alone (control), with H216O2 (10 mm) in H218O (90%), or with H218O2 (10 mm) in H216O.
Peroxymonocarbonate
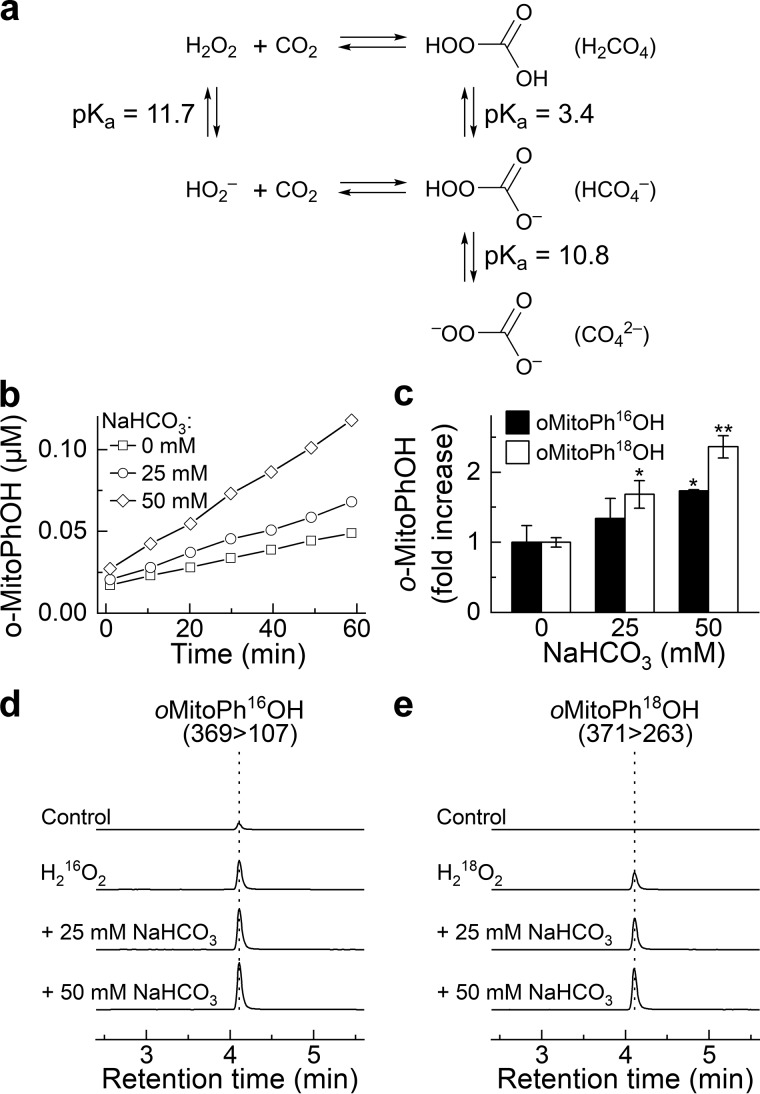
In the presence of CO2, H2O2 is in equilibrium with a more potent oxidant, peroxymonocarbonate (HOOCO2−) (Fig. 6a) (9, 52, 53). Formation of this species has been implicated, for example, in the enhanced hyperoxidation of cellular peroxiredoxins and protein tyrosine phosphatase 1B–mediated signaling cascade observed in the presence of bicarbonate (12, 54–56). Recently, it was shown that the rate of oxidation of the coumarin boronate probe in the presence of H2O2 is increased after the addition of bicarbonate (10). Therefore, we tested if the H2O2-derived HCO4− incorporates the oxygen atom into oMitoPhB(OH)2 probe.
Figure 6.
NaHCO3-enhanced oxidation of oMitoPhB(OH)2 by H2O2 and incorporation of an oxygen atom from HCO4− into the phenolic product. a, chemical scheme of the formation of HCO4− and acid-base equilibria involved. b, dynamics of the formation of oMitoPhOH in the absence and presence of NaHCO3. c, relative increase in the yield of oMitoPhOH after 1-h incubation of the probe with H216O2 or H218O2 in the absence and presence of NaHCO3. d and e, LC-MS/MS traces of the phenolic products containing 16O (d) or 18O (e) atoms. LC-MS/MS analyses were performed after incubation (1 h) of oMitoPhB(OH)2 (1 μm) alone (control), with H216O2 (50 μm, d), or with H218O2 (50 μm, e). All solutions contained 0.1 m phosphate buffer and 0.1 mm dtpa, and the pH of the solutions was adjusted to 7.0.
First, we confirmed that the experimental conditions we used would allow us to detect increased formation of the phenolic product during the reaction of the probe with H2O2 upon addition of NaHCO3. In fact, with increased concentration of NaHCO3, the rate of product formation increased, as determined by LC-MS–based monitoring of the accumulation of oMitoPhOH over the incubation time (Fig. 6, b and c). This effect was observed for both H216O2 and H218O2, when monitoring the 16O- or 18O-phenolic products, respectively (Fig. 6c). The relative increase in the yield of the phenolic product in the case of oMitoPh18OH was higher than in case of oMitoPh16OH, which we attribute to the presence of small amounts of oMitoPh16OH but not oMitoPh18OH in the probe stock solution. The representative LC-MS chromatograms for both H216O2 and H218O2, with increased concentrations of NaHCO3 are shown in Fig. 6, d and e. Under those conditions, HC16O4− and HC18O216O2− were formed, respectively. Incorporation of an 18O atom into the phenolic product indicates the involvement of the peroxyl moiety of HCO4− in the oxidation reaction. Obtained data are consistent with the addition of the deprotonated form of peroxymonocarbonate (CO42−) to the boronate moiety, with elimination of the carbonate anion and incorporation of an oxygen atom from the peroxyl part of the oxidant.
Hypochlorite
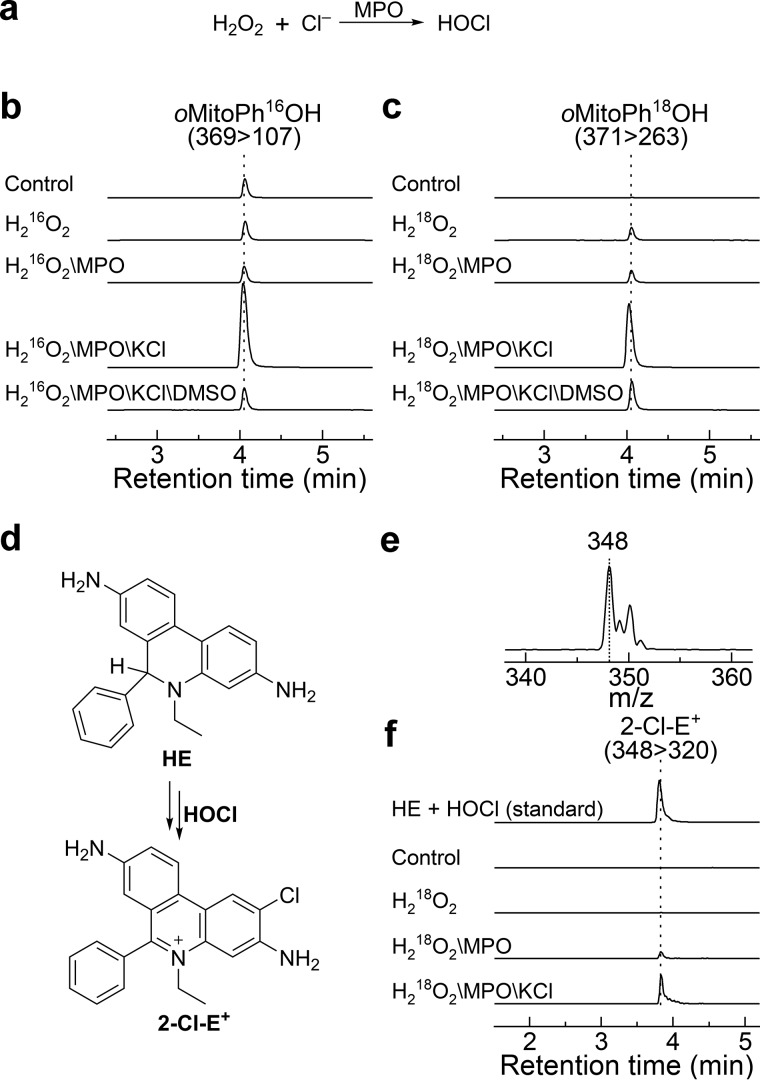
Boronates are oxidized more than a thousand times faster by HOCl than by H2O2 at neutral pH (44). The product of the reaction is a phenol (or alcohol), which may undergo chlorination in the presence of excess HOCl (47, 51, 57). To determine the source of the oxygen atom during the conversion of oMitoPhB(OH)2 into oMitoPhOH, we generated H16OCl and H18OCl in situ from myeloperoxidase (MPO)-catalyzed oxidation of chloride anions by H216O2 and H218O2, respectively (Fig. 7a). To confirm the formation of HOCl in the investigated system, we also performed similar incubations using H218O2 in the presence of the HE probe, and monitored the chlorination product, 2-Cl-E+ (29).
Figure 7.
Incorporation of an oxygen atom into the phenolic product during oxidation of oMitoPhB(OH)2 by HOCl. a, method generating HOCl. b and c, LC-MS/MS traces of the phenolic products containing 16O (b) or 18O (c). LC-MS/MS analyses were performed after incubation (15 min) of oMitoPhB(OH)2 (50 μm) alone (control), with H216O2 (0.1 mm, b), or with H218O2 (0.1 mm, c) in the presence or absence of MPO (20 nm) and KCl (0.1 m). All solutions contained 0.1 m phosphate buffer, pH 7.4. d, scheme of the conversion of HE into 2-Cl-E+. e, online mass spectrum of 2-Cl-E+ detected from reaction of HE with bolus HOCl. f, confirmation of HOCl generation using 2-Cl-E+ marker product. All experimental conditions were the same as described above, but the oMitoPhB(OH)2 probe was replaced by HE (50 μm).
Incubation of oMitoPhB(OH)2 with H2O2, MPO, and potassium chloride (KCl) led to a significant increase in the production of the phenolic product, confirming that HOCl was the major species responsible for oxidation under the conditions used (Fig. 7b). The omission of KCl or MPO resulted in a significantly lower yield of the product. Also, addition of small amounts of dimethyl sulfoxide (DMSO), known to rapidly scavenge HOCl (47, 58), led to a significant attenuation of the formation of the phenolic product. Formation of HOCl was further confirmed by the detection of 2-Cl-E+ in analogous systems, using the HE probe instead of the boronate (Fig. 7, d–f). It previously was shown that HOCl and taurine chloramine are able convert HE into 2-Cl-E+ (29).
Replacement of H216O2 with H218O2 in the incubation mixture containing oMitoPhB(OH)2, MPO, and KCl resulted in a switch from oMitoPh16OH to oMitoPh18OH (Fig. 7c). In the case of both isotopologs, the signal was maximal in a mixture containing H2O2, MPO, and KCl and decreased upon the addition of DMSO. We conclude that the oxygen atom in the product of oMitoPhB(OH)2 oxidation by HOCl derives from the oxidant.
Peroxynitrite
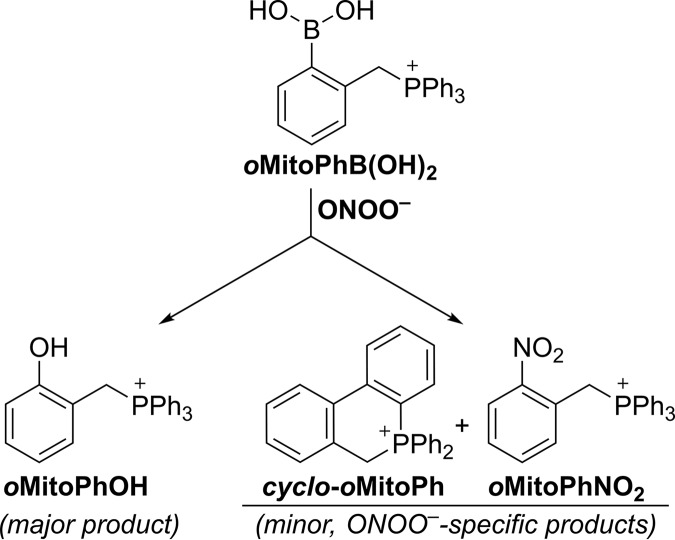
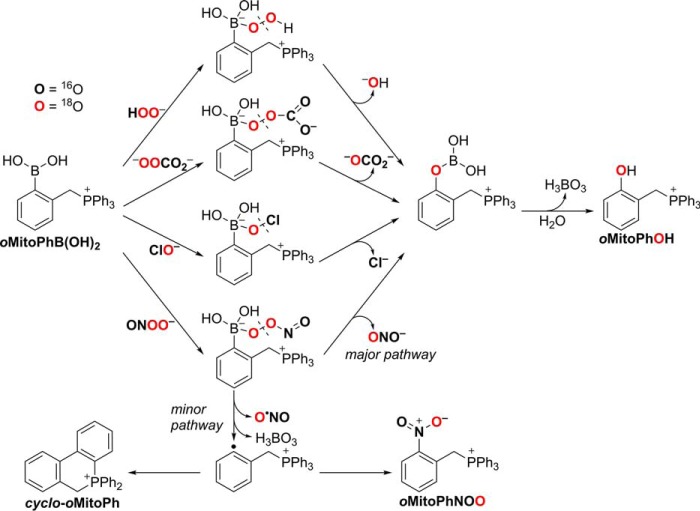
Similar to H2O2, ONOO− reacts with boronates to form a corresponding phenol as the major product. The rate constant of the reaction, however, is significantly higher (∼106 m−1s−1 for ONOO− and ∼1 m−1s−1 for H2O2), and the reaction typically involves a minor pathway, leading to ONOO−-specific minor products (59). The high rate constant provides an opportunity to estimate the absolute flux of ONOO− in cultured cells (48, 60, 61). Formation of ONOO−-specific products provides an opportunity to selectively monitor ONOO− formation in chemical and biological systems (14). We have previously applied this approach to demonstrate the formation of ONOO− during the reaction of nitroxyl with oxygen (62). In the case of oxidation of oMitoPhB(OH)2 by ONOO−, the minor products include cyclo-oMitoPh and oMitoPhNO2 (Fig. 8), formed in 10 and 0.5% yields, respectively (51). Although the mechanism of the oxidation of boronates by ONOO− has been extensively studied, both experimentally and using theoretical calculations (44, 45, 47, 49), the isotope-labeling studies have not been performed. We decided to test the proposed reaction mechanism by reacting oMitoPhB(OH)2 with 18O-labeled ONOO−, produced in situ from co-generated fluxes of nitric oxide (•NO) and 18O2˙̄ (7, 8). •NO flux was generated from the decomposition of spermine NONOate, whereas 18O2˙̄ flux was produced during xanthine oxidase (XO)-catalyzed oxidation of hypoxanthine (HX) in the presence of 18O2. The identity of 18O2˙̄ has been confirmed by using the HE probe and monitoring the incorporation of 18O atoms into the superoxide-specific product 2-OH-E+ (see below). Co-generation of •N16O and 18O2˙̄ leads to the formation of 16ON18O18O−, providing an opportunity to track different oxygen atoms from ONOO− during the conversion of oMitoPhB(OH)2 into oMitoPhOH and oMitoPhNO2. Incubation of oMitoPhB(OH)2 with 16ON 18O18O− led to the formation of the major phenolic product, which showed the mass (m/z = 371) to be two units higher than when using 16ON16O16O− (m/z = 369) (Fig. 9, a and b). LC-MS/MS traces of the phenolic products showed no formation of the oMitoPh18OH in the presence of 16ON16O16O−, although it was a predominant product when 16ON18O18O− was generated (Fig. 9c). In addition, changing the solvent to H218O failed to produce oMitoPh18OH (Fig. S1). These data indicate that the formation of the phenolic product during the reaction of boronates with ONOO− is associated with the incorporation of the oxygen atom from the peroxyl part of the oxidant. Among the minor, ONOO−-specific, products formed, cyclo-oMitoPh did not change its mass when switching from 16ON16O16O− to 16ON18O18O− (Fig. S1) as no oxygen atom is incorporated. Cyclo-oMitoPh was formed in maximal yields when •NO and O2˙̄ were co-generated, and its peak intensity was similar for both 16ON16O16O− and 16ON18O18O− (Fig. S1).
Figure 8.
Oxidation of the oMitoPhB(OH)2 probe by ONOO−.
Figure 9.
Incorporation of an oxygen atom into the phenolic and nitrated products during oxidation of oMitoPhB(OH)2 by ONOO−. a, chemical structures of the products. b, online mass spectra of the detected products. c, LC-MS/MS traces of the phenolic and nitrated products containing 16O (left panels) or 18O (right panels). LC-MS/MS analyses were performed after incubation (30 min) of oMitoPhB(OH)2 (20 μm) alone (control) or with in situ–generated ON16O16O− or ON18O18O−. ON16O16O− and ON18O18O− were produced by cogenerated fluxes of •NO (0.2 μm/min) and 16O2˙̄ or 18O2˙̄ (0.2 μm/min), respectively.
Formation of the other minor product, oMitoPhNO2, was associated with an increase in the mass of this product by two units (m/z = 400) in the presence of 16ON18O18O− as compared with the product formed by 16ON16O16O− (m/z = 398) (Fig. 9, a and b). This indicates that only one oxygen atom originated from the peroxyl (18O-labeled) part of ONOO−. Analyses of the LC-MS/MS traces indicate that oMitoPhN16O18O was formed only when 16ON18O18O− was produced (Fig. 9c), and oMitoPhN16O2 was the product of the reaction with 16ON16O16O−, even when the reaction was carried out in H218O (Fig. S1). The data on the oxidation of oMitoPhB(OH)2 by ONOO− indicate that the oxygen atoms introduced into the products originate from the oxidant and not from the solvent. These data are consistent with the occurrence of two reaction pathways, including heterolytic and homolytic cleavage of the peroxyl bond in the adduct of ONOO− to the boronate probe (Fig. 10). The major pathway, involving a heterolytic cleavage, leads to the formation of the phenolic product, with the oxygen atom incorporated from the peroxyl moiety of the oxidant, similar to the reaction with other tested oxidants, H2O2, HCO4−, and HOCl. The minor pathway, involving the homolytic cleavage of the peroxyl bond, leads to the formation of •NO2 and a phenyl-type radical, which recombine within the solvent cage to form a nitrobenzene-type product (oMitoPhNO2) (Fig. 10). The intramolecular addition of the phenyl radical to the phenyl ring of the TPP+ moiety yields the cyclic product (cyclo-oMitoPh) without incorporating any atom from the oxidant.
Figure 10.
Proposed mechanism of incorporation of oxygen atoms into the oxidation and nitration products of oMitoPhB(OH)2 from H2O2, HCO4−, HOCl, and ONOO−.
Superoxide
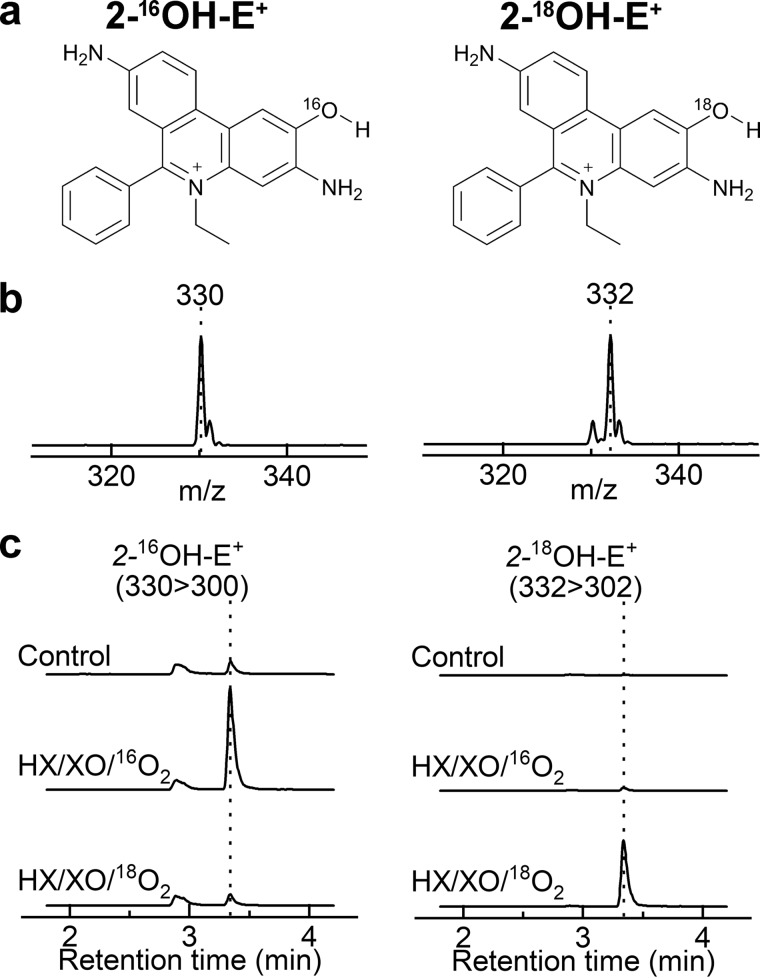
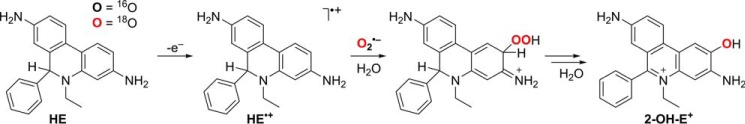
The production of 16ON18O18O− for the study of the oxidation of boronates by ONOO− involved co-generation of •NO and 18O2˙̄. To confirm the formation of 18O2˙̄ in the incubation mixture containing HX, XO, and 18O2, we performed the incubation in the presence of the HE probe and monitored the incorporation of 18O atoms into the 2-OH-E+ product. HE is the most widely used probe for the detection of O2˙̄ in biological systems ranging from cultured cells to animals (21, 63). In the presence of O2˙̄, HE is oxidized to 2-OH-E+, a specific marker product for O2˙̄ (Fig. 3) (20–24). Derivatives of HE for site-specific detection of O2˙̄ have been reported (64–66). Those probes share the same oxidative chemistry with HE (65). A multistep mechanism of the conversion of HE to 2-OH-E+ has been proposed that involves the oxidation of HE to the HE radical cation (HE•+), followed by the reaction of HE•+ with O2˙̄ to form 2-OH-E+ (21, 67). This has been supported by pulse radiolysis data, showing the formation and rapid decay of HE•+ in the presence of pulse-generated O2˙̄ (68) and an increase in the yield of 2-OH-E+ by the addition of peroxidase in the presence of a steady flux of O2˙̄ (67). Here, we provide direct proof of the production of 18O2˙̄ in the HX/XO/18O2 system and the incorporation of the oxygen atom from O2˙̄ into the product during oxidation of HE to 2-OH-E+.
To follow the oxygen atoms, we incubated HE with 16O2˙̄ or 18O2˙̄, produced during enzymatic oxidation of HX by XO in the presence of 16O2 or 18O2, and monitored the formation of 2-16OH-E+ and 2-18OH-E+ (Fig. 11a). The mass spectra of the products showed m/z values of 330 and 332 when the probe was incubated with 16O2˙̄ or 18O2˙̄, respectively (Fig. 11b). The increase in the mass of the product from 18O2˙̄ is consistent with incorporation of 18O into the molecule. The LC-MS/MS traces (Fig. 11c) indicate significant formation of 2-16OH-E+ and negligible formation of 2-18OH-E+ in the presence of 16O2˙̄ (HX/XO/16O2). In the presence of 18O2˙̄ (HX/XO/18O2), only a small peak of 2-16OH-E+ and an intense peak because of 2-18OH-E+ were observed. Furthermore, incubation of HE with 16O2˙̄ in a solvent containing 90% of H218O led to the formation of 2-16OH-E+ but not 2-18OH-E+ (not shown). These data confirm the formation of 18O2˙̄ in the HX/XO/18O2 system and indicate that during the oxidation and hydroxylation of HE, the oxygen atom in the product originates from O2˙̄, consistent with a mechanism involving the reaction of HE•+ with O2˙̄ and forming the hydroperoxyl intermediate (Fig. 12).
Figure 11.
Incorporation of an oxygen atom into the hydroxylated product during oxidation of HE in the presence of O2˙̄. a, chemical structures of the products. b, online mass spectra of the products. c, LC-MS/MS traces of 2-OH-E+ containing 16O (left panel) or 18O (right panel). LC-MS/MS analyses were performed after incubation (30 min) of HE (20 μm) alone (control) or with 16O2˙̄ or 18O2˙̄ (0.2 μm O2˙̄/min, generated from HX/XO and 16O2 or 18O2, respectively).
Figure 12.
Proposed mechanism of incorporation of oxygen atom from O2˙̄ into 2-OH-E+ during oxidation of the HE probe.
Discussion
Isotope tracing is a powerful technique in the study of the mechanism of chemical and enzymatic reactions as well as cellular metabolism (69–71). Isotopically labeled oxidants have been used to identify the spin adducts of O2˙̄ and other oxygen-centered radicals using an EPR spin trapping technique (72). EPR spin trapping, however, is only useful for the detection of radical species and has only limited applicability to detect intracellular ROS. Oxygen tracing in other probes used for cellular oxidants has not been reported.
In this study, we have investigated the origin of the oxygen atom in the products of the reaction of mitochondria-targeted boronate probe with four biologically relevant, two-electron oxidants: hydrogen peroxide, peroxymonocarbonate, hypochlorite, and peroxynitrite. The results support the previously proposed mechanisms of the probes' oxidation and formation of the specific products and provide a solid foundation for the use of those products for identification and tracking isotopically labeled oxidants.
New insights into the selective detection of peroxynitrite
Although initially assumed to be completely selective (specific) for H2O2, boronate-based probes also respond to other biologically relevant nucleophilic oxidants, including HCO4−, HOCl, ONOO−, and amino acid hydroperoxides (44, 57, 73). The main oxidation product in case of all the listed oxidants is the corresponding phenol. In the presence of excess HOCl or ONOO−, the phenolic product may undergo chlorination or nitration, respectively, providing an opportunity to identify the oxidant by profiling the products formed (14, 44). As an example, in the presence of HOCl, the peroxy-caged luciferin probe is converted not only to luciferin but also to chloroluciferin (47). The reaction of boronate probes with ONOO− is of special interest, as this reaction typically proceeds via two pathways of the decomposition of peroxynitrite adduct to the boronate: (i) major pathway (∼85–90%) involving heterolytic cleavage of the peroxyl bond, leading to the formation the phenolic product and (ii) minor pathway (10–15%), involving a homolytic cleavage of the peroxyl bond, with the formation of phenyl-type radical and •NO2, which upon recombination form nitrobenzene-type product (Fig. 10) (45). We have proposed using that product as a specific marker for ONOO− (50), and with such an approach, we demonstrated the formation of ONOO− during the reaction of nitroxyl with oxygen (O2) (62). In the case of the oMitoPhB(OH)2 probe, the nitrated product, oMitoPhNO2, accounts for only 0.5% of ONOO− consumed. The other minor product, cyclo-oMitoPh, is formed at 10% yield, via a rapid intramolecular addition of the phenyl-type radical to one of the phenyl rings of the TPP+ moiety (Fig. 10) (51). Both minor products have been detected in macrophages stimulated to produce ONOO− (51) and can be used as specific marker products for intracellular ONOO−.
Detection of ONOO− in cells has remained a challenge, as most methods were based on the nitrative and/or oxidative properties of ONOO−-derived radicals (e.g. •OH, •NO2, CO3˙̄) (74). However, the same radical species can be formed in biological systems in ONOO−-independent reactions. For example, although nitrated tyrosine residues are commonly used as an endogenous marker of ONOO−, the same product is formed by •NO2 from the MPO-catalyzed oxidation of nitrite by H2O2. Dihydrorhodamine, a fluorogenic probe used for ONOO− detection, cannot distinguish the two pathways of •NO2 formation either. Boronate probes, including oMitoPhB(OH)2 provide the first chemical tool to distinguish these two nitration pathways (51). Formation of the cyclic and nitrobenzene-type products from oMitoPhB(OH)2 occurs in the presence of ONOO− but not in the presence of MPO/H2O2/NO2− (51). This shows that monitoring the conversion of oMitoPhB(OH)2 into cyclo-oMitoPh and oMitoPhNO2 products can be used to selectively detect ONOO− formed in cell-free and cellular systems. Although other boronate probes may not form the cyclic product during the reaction with ONOO−, in most cases they produce nitrobenzene-type minor products. These products may be used to confirm the identity of the oxidant detected. For example, a new boronate probe recently was developed to detect ONOO− in β-amyloid aggregates (76). The minor product(s) formed during the reaction of the probe with ONOO− should be characterized and high-performance LC (HPLC)– or LC-MS–based profiling should accompany fluorescence measurements, which report the yield of the phenolic product. This product is common for various nucleophilic oxidants, as exemplified here by H2O2, HCO4−, HOCl, and ONOO−. Amino acid– and protein-based hydroperoxides also oxidize boronate probes to the phenolic products (73).
Oxidation of aromatic boronates involves initial formation of phenoxyboronic acid, followed by its hydrolysis into phenolic product (Fig. 4). The results obtained in this study demonstrate that during the oxidation of boronates by H2O2, HCO4−, HOCl, or ONOO−, the oxygen atom in the phenolic product derives from those oxidants, not from water. In the case of HCO4− and ONOO−, the oxygen atoms in these oxidants are not equivalent, and the data obtained support the mechanism involving the nucleophilic addition of CO42− or ONOO− to the boron atom, via their peroxyl moieties, followed by elimination of a carbonate or nitrite anion, respectively (Fig. 10). Also, in the case of the formation of nitrobenzene-type product, the pattern of labeling of oMitoPhNO2 during the reaction of oMitoPhB(OH)2 with ONOO− provides insight into the mechanism of the minor pathway of the reaction. Incorporation of only one oxygen-18 atom into the nitrated product from 16ON18O18O− is consistent with the initial homolytic cleavage of the peroxyl bond in the adduct, formation of phenyl-type radical and •NO2, and recombination of both radicals (Fig. 10).
2-Hydroxyethidium as a specific marker for O2˙̄
The HPLC or LC-MS-based analysis of 2-OH-E+ is regarded as a “gold standard” of the detection of O2˙̄ in biological systems (77). However, the utility of 2-OH-E+ as the marker of cellular O2˙̄ recently has been questioned, based on the lack of increase of its amount in HepG2 cells treated with H2O2 or rotenone (78). However, the ability of those treatments to induce O2˙̄ generation in the used cell model has not been shown. Numerous reports demonstrate the utility of HE, MitoSOX Red, and hydropropidine, when coupled with HPLC-based analyses, to detect O2˙̄ in different cellular models, as reviewed elsewhere (20, 21, 63). In those reports, 2-OH-E+, 2-OH-Mito-E+, and 2-OH-Pr2+ were used as specific marker products for O2˙̄. Hydroxylation of ethidium-based probes remains a method of choice for the detection of O2˙̄ in cell-free and cellular systems (17, 22, 64–66, 79).
The presented results demonstrate that the specificity of 2-OH-E+ for O2˙̄ derives from incorporation of an oxygen atom from this species. Together with the pulse radiolysis data on the oxidation of HE by pulse-generated O2˙̄ (68), the 2:1 stoichiometry of the reaction (67), and the lack of incorporation of oxygen from water, observed in this study and during oxidation of HE by Fremy's salt (80), the obtained data are consistent with the mechanism shown in Fig. 12. Initial oxidation of HE by the protonated form of O2˙̄ (hydroperoxyl radical, HO2•) produces a radical cation of HE, which rapidly reacts with O2˙̄ to form a hydroperoxide, containing oxygen atoms from O2˙̄ (Fig. 12). This product must undergo rapid transformation in aqueous solutions to 2-OH-E+ as no intermediates have been detected by HPLC analyses.
Concluding remarks
In summary, we demonstrated that the oxygen atoms in the oxidation and nitration products of the boronate probe, oMitoPhB(OH)2, originate from the corresponding oxidants, H2O2, HCO4−, HOCl, or ONOO−. Also, in the case the conversion of the HE probe into 2-OH-E+, oxygen comes from O2˙̄. The presented data indicate that it is possible to track isotopically labeled oxidants by monitoring the incorporation of the isotopes into the oxidation/nitration products using the boronate and hydroethidine probes. Because no incorporation of the atoms from ROS/reactive nitrogen species occurs in most other commonly used probes, including DCFH, DHR123, Amplex Red, and NBT, they cannot be used for such purposes. The results obtained also corroborate the mechanisms of the conversion of HE into 2-OH-E+ by O2˙̄ and of oxidation and nitration of boronate-based probes, proposed previously based on product analyses, EPR experiments, and density functional theory calculation. We expect that oxygen-18 labeling studies using 18O2, H218O, and H218O2, may also be used in cell-free or cellular systems to reveal whether metal-induced hydrolysis and/or high-valent iron-oxo species may contribute to the hydroxylation of the probes (81). The reaction of the probes with 18O-labeled oxidants also can be used to prepare isotopically labeled standards of the oxidation products. Furthermore, H218O2- or H18O2C16O2−-mediated oxidation of boronates represents a convenient route for the synthesis of 18O-labeled alcohols and phenols.
Experimental procedures
Materials
Ortho-MitoPhB(OH)2 and its oxidation and nitration products were synthesized, as described previously (49–51). The stock solution of oMitoPhB(OH)2 (0.1 m) was prepared in DMSO and stored at −20 °C. The HE probe was obtained from Invitrogen (Carlsbad, CA). The stock solution of HE (20 mm) was prepared in deoxygenated DMSO under argon atmosphere and stored at −80 °C. The standards of the oxidation products were synthesized, as described previously (22, 82). For experiments involving HOCl, both probes were dissolved in ethanol (EtOH) to avoid the scavenging effect of DMSO on HOCl (47). Water-18O (97% oxygen-18), H218O2 (90% oxygen-18), 18O2 (97% oxygen-18), HX, XO, superoxide dismutase (SOD), and catalase were obtained from Sigma-Aldrich. MPO was from Calbiochem.
Determination of the flux of O2˙̄
O2˙̄ was generated from the XO-catalyzed oxidation of HX in a phosphate buffer solution (25 mm, pH = 7.4) containing 0.1 mm diethylenetriamine pentaacetic acid (dtpa). The solution was continuously purged with O2. The flux of O2˙̄ was determined, as described previously (7, 83, 84), by performing the incubation in the presence of ferricytochrome c (50 μm) and monitoring the rate of its reduction following an increase in absorbance at 550 nm (Δϵ = 2.1 × 104 m−1 cm−1) (85). Superoxide dismutase completely blocked the reduction of ferricytochrome c under the conditions used.
Determination of the flux of •NO
•NO was generated from the thermal decomposition of spermine NONOate in a phosphate buffer (25 mm, pH = 7.4) containing 0.1 mm dtpa. The flux of •NO was determined, as described previously (7, 84), by monitoring the rate of decay of spermine NONOate following a decrease in absorbance at 252 nm (ϵ = 8 × 103 m−1cm−1). The release of two molecules of •NO per one molecule of spermine NONOate consumed was assumed in the calculations (86).
Oxidation of oMitoPhB(OH)2 by H2O2
To analyze the product of oxidation of oMitoPhB(OH)2 by H2O2, oMitoPhB(OH)2 (20 μm) was incubated at room temperature with H2O2 (10 mm) for 20 min in a phosphate buffer (25 mm, pH = 7.4) containing 0.1 mm dtpa. When performing the reaction in water-18O, the final concentration of H218O was 90% (by volume).
Oxidation of oMitoPhB(OH)2 by HCO4−
Oxidation oMitoPhB(OH)2 by HCO4− was studied by incubation of the probe with H2O2 in phosphate-buffered (0.1 m) aqueous solution containing dtpa (0.1 mm) in the presence of NaHCO3 (25 and 50 mm). To maximize the involvement of HCO4− in probe oxidation, the probe concentration was lowered to 1 μm, the H2O2 concentration was lowered to 50 μm, and the pH was adjusted to 7.0.
Oxidation of oMitoPhB(OH)2 by HOCl
To study oxidation of oMitoPhB(OH)2 by HOCl, the probe (50 μm, from a stock solution in EtOH) was incubated with H2O2 (0.1 mm), KCl (0.1 m), and MPO (20 nm) for 15 min at 25 °C in a phosphate-buffered (0.1 m) aqueous solution. Where indicated, DMSO was added (final concentration of 0.2% v/v) to scavenge HOCl.
Oxidation of oMitoPhB(OH)2 by ONOO−
To react oMitoPhB(OH)2 with ONOO−, oMitoPhB(OH)2 (20 μm) was incubated with spermine NONOate (200 μm, generating 0.2 μm/min •NO), HX (200 μm), and XO (0.1 milliunit/milliliter, 0.2 μm O2˙̄/min) in an O2-saturated phosphate buffer (25 mm, pH = 7.4) containing 0.1 mm dtpa and 5 kilounits/milliliter catalase. The deoxygenated stock solutions of all components were mixed under argon atmosphere in a hypoxic chamber (final reaction volume: 200 μl). The incubation was started immediately after mixing by passing oxygen gas (16O2 or 18O2) through the solution for 10 min, followed by 20 min further incubation at room temperature. Incubation in water-18O was performed in the presence of 90% (by volume) of H218O.
Chlorination of HE by HOCl
The reaction of HE with HOCl and the formation of 2-Cl-E+ was investigated in the presence of H2O2, MPO, and KCl under conditions identical to those described above for the oMitoPhB(OH)2 probe but using HE (50 μm, from a stock solution in EtOH).
Oxidation of HE by O2˙̄
Conversion of HE into 2-OH-E+ was studied by incubation of HE (20 μm) with HX (200 μm) and XO (0.1 milliunit/milliliter, 0.2 μm O2˙̄/min) in an oxygen-saturated phosphate buffer (25 mm, pH = 7.4) containing 0.1 mm dtpa and 5 kilounits/milliliter catalase. To better control the type of O2 isotopolog present in the solutions, samples were first deoxygenated to remove 16O2 and then reoxygenated using 16O2 or 18O2. The deoxygenated stock solutions of all components were mixed under argon atmosphere in a hypoxic chamber (final reaction volume 200 μl). The incubation was started immediately after mixing by passing O2 gas (16O2 or 18O2) through the solution for 10 min, followed by 20 min further incubation at room temperature. To stop the incubation, SOD was added (final concentration: 0.1 mg/ml) and the sample was taken for LC-MS/MS analysis. The addition of SOD at the beginning of incubation resulted in complete inhibition of 2-OH-E+ formation. When using water-18O as a solvent, the final concentration of H218O was 90% (by volume).
LC-MS/MS analysis of oMitoPhB(OH)2 oxidation products
The oxidation products of oMitoPhB(OH)2 were analyzed using a Shimadzu Nexera2 ultra-HPLC system equipped with UV-visible absorption and LC-MS8030 MS detectors (Columbia, MD). The presence of a positive charge (because of the presence of the TPP+ moiety) allows a sensitive detection by MS, as reported previously for the MitoB probe (41–43). The incubation mixture was injected into a Raptor Biphenyl column (Restek, Bellefonte, PA; 100 mm × 2.1 mm, 2.7 μm) equilibrated with a mobile phase containing 80% water, 20% MeCN, and 0.1% formic acid. The products were eluted by increasing the content of MeCN (containing 0.1% formic acid) from 20% to 60% over 5.5 min. The mobile phase flow rate was 0.5 ml/min. Detection events included continuous scanning of the spectra of the eluate, as well as detection of the specific oxidation products in an MRM mode. MRM transitions were as follows: 397 > 135 for oMitoPhB(OH)2, 369 > 107 for oMitoPh16OH, 371 > 263 for oMitoPh18OH, 398 > 262 for oMitoPhN16O2, 400 > 262 for oMitoPhN16O18O, and 351 > 183 for cyclo-oMitoPh. The MRM transitions of other oxidation products have been reported elsewhere (50, 51, 79).
LC-MS/MS analysis of HE oxidation products
Detection of HE oxidation products, including 2-Cl-E+ and 2-OH-E+, was performed using a Shimadzu Nexera2 ultra-HPLC system equipped with UV-visible absorption and LC-MS8030 MS detectors. The reaction mixture was injected into a Raptor Biphenyl column (Restek, Bellefonte, PA; 100 mm × 2.1 mm, 2.7 μm) equilibrated with the mobile phase containing 90% water, 10% acetonitrile (MeCN), and 0.1% formic acid. The products were eluted by increasing the content of the organic mobile phase (MeCN, 0.1% formic acid) from 10% to 65% over 4.5 min at the flow rate of 0.4 ml/min. Detection events included continuous scanning of the spectra of the eluate, as well as detection of the specific oxidation products in an MRM mode. MRM transitions for 2-Cl-E+, 2-16OH-E+, and 2-18OH-E+ were 348 > 320, 330 > 300, and 332 > 302, respectively. The MRM transitions of other oxidation products were as reported previously (19, 29, 75, 79).
Data availability
All data presented and discussed are contained within the manuscript or in the supporting information.
Author contributions
N. R. and J. Z. data curation; N. R. and J. Z. formal analysis; N. R., R. R., B. K., and J. Z. funding acquisition; N. R. validation; N. R. and J. Z. investigation; N. R. and J. Z. methodology; N. R., R. R., B. K., and J. Z. writing-review and editing; R. R., B. K., and J. Z. conceptualization; R. R., B. K., and J. Z. supervision; R. R. and B. K. project administration; B. K. and J. Z. resources; J. Z. writing-original draft.
Supplementary Material
This work was supported by NCI, National Institutes of Health Grants U01CA178960 and R01CA208648 (to B. K.) and by Espacio Interdisciplinario 2015 and CSIC Grupos 2018, Universidad de la República, Uruguay (to R. R.). This work was also supported by American Cancer Society Institutional Research Grant IRG 16-183-31 and the MCW Cancer Center (to J. Z.); by Agencia Nacional de Investigación e Innovación (ANII) and Universidad de la República fellowships (to N. R.); and by Programa de Desarrollo de Ciencias Básicas (PEDECIBA, Uruguay) (to R. R. and N. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- ROS
- reactive oxygen species
- 2-Cl-E+
- 2-chloroethidium
- DCFH
- dichlorodihydrofluorescein
- DHR123
- dihydrorhodamine-123
- dtpa
- diethylenetriamine pentaacetic acid
- HE
- hydroethidine
- HPLC
- high-performance LC
- HX
- hypoxanthine
- MeCN
- acetonitrile
- MPO
- myeloperoxidase
- MRM
- multiple reaction monitoring
- 2-OH-E+
- 2-hydroxyethidium
- oMitoPhB(OH)2
- mitochondria-targeted phenyl boronate probe
- SOD
- superoxide dismutase
- TPP+
- triphenylphosphonium cationic moiety
- XO
- xanthine oxidase
References
- 1. Winterbourn C. C. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 10.1038/nchembio.85 [DOI] [PubMed] [Google Scholar]
- 2. Forman H. J., Ursini F., and Maiorino M. (2014) An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 73, 2–9 10.1016/j.yjmcc.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reczek C. R., and Chandel N. S. (2015) ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13 10.1016/j.ceb.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winterbourn C. C. (2018) Biological production, detection, and fate of hydrogen peroxide. Antioxid. Redox Signal. 29, 541–551 10.1089/ars.2017.7425 [DOI] [PubMed] [Google Scholar]
- 5. Vissers M. C., Hampton M., and Kettle A. J. (2017) Hydrogen Peroxide Metabolism in Health and Disease. CRC Press, Boca Raton, FL [Google Scholar]
- 6. Winterbourn C. C. (2020) Biological chemistry of superoxide radicals. ChemTexts 6, 7 10.1007/s40828-019-0101-8 [DOI] [Google Scholar]
- 7. Zielonka J., Sikora A., Joseph J., and Kalyanaraman B. (2010) Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 285, 14210–14216 10.1074/jbc.M110.110080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrer-Sueta G., Campolo N., Trujillo M., Bartesaghi S., Carballal S., Romero N., Alvarez B., and Radi R. (2018) Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev. 118, 1338–1408 10.1021/acs.chemrev.7b00568 [DOI] [PubMed] [Google Scholar]
- 9. Bakhmutova-Albert E. V., Yao H., Denevan D. E., and Richardson D. E. (2010) Kinetics and mechanism of peroxymonocarbonate formation. Inorg. Chem. 49, 11287–11296 10.1021/ic1007389 [DOI] [PubMed] [Google Scholar]
- 10. Truzzi D. R., and Augusto O. (2017) Influence of CO2 on hydroperoxide metabolism. in Hydrogen Peroxide Metabolism in Health and Disease (Vissers M. C., Hampton M., and Kettle A. J., eds), pp. 81–99, CRC Press, Boca Raton, FL [Google Scholar]
- 11. Pattison D. I., Davies M. J., and Hawkins C. L. (2012) Reactions and reactivity of myeloperoxidase-derived oxidants: Differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 46, 975–995 10.3109/10715762.2012.667566 [DOI] [PubMed] [Google Scholar]
- 12. Dagnell M., Cheng Q., Rizvi S. H. M., Pace P. E., Boivin B., Winterbourn C. C., and Arnér E. S. J. (2019) Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades. J. Biol. Chem. 294, 12330–12338 10.1074/jbc.RA119.009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins C. L., and Davies M. J. (2019) Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 294, 19683–19708 10.1074/jbc.REV119.006217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardy M., Zielonka J., Karoui H., Sikora A., Michalski R., Podsiadly R., Lopez M., Vasquez-Vivar J., Kalyanaraman B., and Ouari O. (2018) Detection and characterization of reactive oxygen and nitrogen species in biological systems by monitoring species-specific products. Antioxid. Redox Signal. 28, 1416–1432 10.1089/ars.2017.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalyanaraman B., Hardy M., Podsiadly R., Cheng G., and Zielonka J. (2017) Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys. 617, 38–47 10.1016/j.abb.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zielonka J., and Kalyanaraman B. (2018) Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic. Biol. Med. 128, 3–22 10.1016/j.freeradbiomed.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dikalov S. I., and Harrison D. G. (2014) Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 20, 372–382 10.1089/ars.2012.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Debowska K., Debski D., Hardy M., Jakubowska M., Kalyanaraman B., Marcinek A., Michalski R., Michalowski B., Ouari O., Sikora A., Smulik R., and Zielonka J. (2015) Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes—limitations, progress, and perspectives. Pharmacol. Rep. 67, 756–764 10.1016/j.pharep.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koto T., Michalski R., Zielonka J., Joseph J., and Kalyanaraman B. (2014) Detection and identification of oxidants formed during *NO/O2*(−) reaction: A multi-well plate CW-EPR spectroscopy combined with HPLC analyses. Free Radic. Res. 48, 478–486 10.3109/10715762.2014.886774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalyanaraman B., Dranka B. P., Hardy M., Michalski R., and Zielonka J. (2014) HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes—the ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta 1840, 739–744 10.1016/j.bbagen.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zielonka J., and Kalyanaraman B. (2010) Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 48, 983–1001 10.1016/j.freeradbiomed.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zielonka J., Vasquez-Vivar J., and Kalyanaraman B. (2008) Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nat. Protoc. 3, 8–21 10.1038/nprot.2007.473 [DOI] [PubMed] [Google Scholar]
- 23. Zhao H., Joseph J., Fales H. M., Sokoloski E. A., Levine R. L., Vasquez-Vivar J., and Kalyanaraman B. (2005) Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A. 102, 5727–5732 10.1073/pnas.0501719102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vásquez-Vivar J., and Kalyanaraman B. (2003) Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34, 1359–1368 10.1016/S0891-5849(03)00142-4 [DOI] [PubMed] [Google Scholar]
- 25. Proniewski B., Kij A., Sitek B., Kelley E. E., and Chlopicki S. (2019) Multiorgan development of oxidative and nitrosative stress in LPS-induced endotoxemia in C57Bl/6 mice: DHE-based in vivo approach. Oxid. Med. Cell. Longev. 2019, 7838406 10.1155/2019/7838406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seredenina T., Nayernia Z., Sorce S., Maghzal G. J., Filippova A., Ling S. C., Basset O., Plastre O., Daali Y., Rushing E. J., Giordana M. T., Cleveland D. W., Aguzzi A., Stocker R., Krause K. H., and Jaquet V. (2016) Evaluation of NADPH oxidases as drug targets in a mouse model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 97, 95–108 10.1016/j.freeradbiomed.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 27. Fernandes D. C., Wosniak J. Jr., Pescatore L. A., Bertoline M. A., Liberman M., Laurindo F. R., and Santos C. X. (2007) Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am. J. Physiol. Cell Physiol. 292, C413–C422 10.1152/ajpcell.00188.2006 [DOI] [PubMed] [Google Scholar]
- 28. Zielonka J., Srinivasan S., Hardy M., Ouari O., Lopez M., Vasquez-Vivar J., Avadhani N. G., and Kalyanaraman B. (2008) Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radic. Biol. Med. 44, 835–846 10.1016/j.freeradbiomed.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maghzal G. J., Cergol K. M., Shengule S. R., Suarna C., Newington D., Kettle A. J., Payne R. J., and Stocker R. (2014) Assessment of myeloperoxidase activity by the conversion of hydroethidine to 2-chloroethidium. J. Biol. Chem. 289, 5580–5595 10.1074/jbc.M113.539486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lippert A. R., Van de Bittner G. C., and Chang C. J. (2011) Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 44, 793–804 10.1021/ar200126t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin V. S., Dickinson B. C., and Chang C. J. (2013) Boronate-based fluorescent probes: Imaging hydrogen peroxide in living systems. Methods Enzymol. 526, 19–43 10.1016/B978-0-12-405883-5.00002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zielonka J., Cheng G., Zielonka M., Ganesh T., Sun A., Joseph J., Michalski R., O'Brien W. J., Lambeth J. D., and Kalyanaraman B. (2014) High-throughput assays for superoxide and hydrogen peroxide: Design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 289, 16176–16189 10.1074/jbc.M114.548693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carroll V., Michel B. W., Blecha J., VanBrocklin H., Keshari K., Wilson D., and Chang C. J. (2014) A boronate-caged [18F]FLT probe for hydrogen peroxide detection using positron emission tomography. J. Am. Chem. Soc. 136, 14742–14745 10.1021/ja509198w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van de Bittner G. C., Dubikovskaya E. A., Bertozzi C. R., and Chang C. J. (2010) In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. U.S.A. 107, 21316–21321 10.1073/pnas.1012864107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lippert A. R., Gschneidtner T., and Chang C. J. (2010) Lanthanide-based luminescent probes for selective time-gated detection of hydrogen peroxide in water and in living cells. Chem. Commun. 46, 7510–7512 10.1039/c0cc01560a [DOI] [PubMed] [Google Scholar]
- 36. Seven O., Sozmen F., and Turan I. S. (2017) Self immolative dioxetane based chemiluminescent probe for H2O2 detection. Sensors and Actuators B: Chemical 239, 1318–1324 10.1016/j.snb.2016.09.120 [DOI] [Google Scholar]
- 37. Dickinson B. C., Huynh C., and Chang C. J. (2010) A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 132, 5906–5915 10.1021/ja1014103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srikun D., Albers A. E., Nam C. I., Iavarone A. T., and Chang C. J. (2010) Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-Tag protein labeling. J. Am. Chem. Soc. 132, 4455–4465 10.1021/ja100117u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiao H., Li P., Hu X., Shi X., Zhang W., and Tang B. (2016) Simultaneous fluorescence imaging of hydrogen peroxide in mitochondria and endoplasmic reticulum during apoptosis. Chem. Sci. 7, 6153–6159 10.1039/C6SC01793B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickinson B. C., Lin V. S., and Chang C. J. (2013) Preparation and use of MitoPY1 for imaging hydrogen peroxide in mitochondria of live cells. Nat. Protoc. 8, 1249–1259 10.1038/nprot.2013.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cairns A. G., McQuaker S. J., Murphy M. P., and Hartley R. C. (2015) Targeting mitochondria with small molecules: The preparation of MitoB and MitoP as exomarkers of mitochondrial hydrogen peroxide. Methods Mol. Biol. 1265, 25–50 10.1007/978-1-4939-2288-8_3 [DOI] [PubMed] [Google Scholar]
- 42. Cochemé H. M., Quin C., McQuaker S. J., Cabreiro F., Logan A., Prime T. A., Abakumova I., Patel J. V., Fearnley I. M., James A. M., Porteous C. M., Smith R. A., Saeed S., Carré J. E., Singer M., Gems D., Hartley R. C., Partridge L., and Murphy M. P. (2011) Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 10.1016/j.cmet.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cochemé H. M., Logan A., Prime T. A., Abakumova I., Quin C., McQuaker S. J., Patel J. V., Fearnley I. M., James A. M., Porteous C. M., Smith R. A., Hartley R. C., Partridge L., and Murphy M. P. (2012) Using the mitochondria-targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila. Nat. Protoc. 7, 946–958 10.1038/nprot.2012.035 [DOI] [PubMed] [Google Scholar]
- 44. Sikora A., Zielonka J., Lopez M., Joseph J., and Kalyanaraman B. (2009) Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 47, 1401–1407 10.1016/j.freeradbiomed.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sikora A., Zielonka J., Lopez M., Dybala-Defratyka A., Joseph J., Marcinek A., and Kalyanaraman B. (2011) Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC analyses, and quantum mechanical study of the free radical pathway. Chem. Res. Toxicol. 24, 687–697 10.1021/tx100439a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zielonka J., Zielonka M., Sikora A., Adamus J., Joseph J., Hardy M., Ouari O., Dranka B. P., and Kalyanaraman B. (2012) Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J. Biol. Chem. 287, 2984–2995 10.1074/jbc.M111.309062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zielonka J., Podsiadły R., Zielonka M., Hardy M., and Kalyanaraman B. (2016) On the use of peroxy-caged luciferin (PCL-1) probe for bioluminescent detection of inflammatory oxidants in vitro and in vivo. Identification of reaction intermediates and oxidant-specific minor products. Free Radic. Biol. Med. 99, 32–42 10.1016/j.freeradbiomed.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prolo C., Rios N., Piacenza L., Álvarez M. N., and Radi R. (2018) Fluorescence and chemiluminescence approaches for peroxynitrite detection. Free Radic. Biol. Med. 128, 59–68 10.1016/j.freeradbiomed.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 49. Zielonka J., Joseph J., Sikora A., and Kalyanaraman B. (2013) Real-time monitoring of reactive oxygen and nitrogen species in a multiwell plate using the diagnostic marker products of specific probes. Methods Enzymol. 526, 145–157 10.1016/B978-0-12-405883-5.00009-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zielonka J., Sikora A., Adamus J., and Kalyanaraman B. (2015) Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods Mol. Biol. 1264, 171–181 10.1007/978-1-4939-2257-4_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zielonka J., Zielonka M., VerPlank L., Cheng G., Hardy M., Ouari O., Ayhan M. M., Podsiadły R., Sikora A., Lambeth J. D., and Kalyanaraman B. (2016) Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J. Biol. Chem. 291, 7029–7044 10.1074/jbc.M115.702787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richardson D. E., Yao H., Frank K. M., and Bennett D. A. (2000) Equilibria, kinetics, and mechanism in the bicarbonate activation of hydrogen peroxide: Oxidation of sulfides by peroxymonocarbonate. J. Am. Chem. Soc. 122, 1729–1739 10.1021/ja9927467 [DOI] [Google Scholar]
- 53. Bennett D. A., Yao H., and Richardson D. E. (2001) Mechanism of sulfide oxidations by peroxymonocarbonate. Inorg. Chem. 40, 2996–3001 10.1021/ic000910h [DOI] [PubMed] [Google Scholar]
- 54. Peskin A. V., Pace P. E., and Winterbourn C. C. (2019) Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2. Free Radic. Biol. Med. 145, 1–7 10.1016/j.freeradbiomed.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 55. Zhou H., Singh H., Parsons Z. D., Lewis S. M., Bhattacharya S., Seiner D. R., LaButti J. N., Reilly T. J., Tanner J. J., and Gates K. S. (2011) The biological buffer bicarbonate/CO2 potentiates H2O2-mediated inactivation of protein tyrosine phosphatases. J. Am. Chem. Soc. 133, 15803–15805 10.1021/ja2077137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Truzzi D. R., Coelho F. R., Paviani V., Alves S. V., Netto L. E. S., and Augusto O. (2019) The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1. J. Biol. Chem. 294, 14055–14067 10.1074/jbc.RA119.008825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zielonka J., Sikora A., Hardy M., Joseph J., Dranka B. P., and Kalyanaraman B. (2012) Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 25, 1793–1799 10.1021/tx300164j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kabeya L. M., Andrade M. F., Piatesi F., Azzolini A. E., Polizello A. C., and Lucisano-Valim Y. M. (2013) 3,3′,5,5′-tetramethylbenzidine in hypochlorous acid and taurine chloramine scavenging assays: interference of dimethyl sulfoxide and other vehicles. Anal. Biochem. 437, 130–132 10.1016/j.ab.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 59. Cheng G., Zielonka J., Dranka B. P., McAllister D., Mackinnon A. C. Jr., Joseph J., and Kalyanaraman B. (2012) Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 72, 2634–2644 10.1158/0008-5472.CAN-11-3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prolo C., Álvarez M. N., Ríos N., Peluffo G., Radi R., and Romero N. (2015) Nitric oxide diffusion to red blood cells limits extracellular, but not intraphagosomal, peroxynitrite formation by macrophages. Free Radic. Biol. Med. 87, 346–355 10.1016/j.freeradbiomed.2015.06.027 [DOI] [PubMed] [Google Scholar]
- 61. Ríos N., Piacenza L., Trujillo M., Martínez A., Demicheli V., Prolo C., Álvarez M. N., López G. V., and Radi R. (2016) Sensitive detection and estimation of cell-derived peroxynitrite fluxes using fluorescein-boronate. Free Radic. Biol. Med. 101, 284–295 10.1016/j.freeradbiomed.2016.08.033 [DOI] [PubMed] [Google Scholar]
- 62. Smulik R., Dȩbski D., Zielonka J., Michałowski B., Adamus J., Marcinek A., Kalyanaraman B., and Sikora A. (2014) Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological implications. J. Biol. Chem. 289, 35570–35581 10.1074/jbc.M114.597740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zielonka J., Hardy M., Michalski R., Sikora A., Zielonka M., Cheng G., Ouari O., Podsiadły R., and Kalyanaraman B. (2017) Recent developments in the probes and assays for measurement of the activity of NADPH oxidases. Cell Biochem. Biophys. 75, 335–349 10.1007/s12013-017-0813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robinson K. M., Janes M. S., Pehar M., Monette J. S., Ross M. F., Hagen T. M., Murphy M. P., and Beckman J. S. (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 103, 15038–15043 10.1073/pnas.0601945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Michalski R., Zielonka J., Hardy M., Joseph J., and Kalyanaraman B. (2013) Hydropropidine: A novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic. Biol. Med. 54, 135–147 10.1016/j.freeradbiomed.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shchepinova M. M., Cairns A. G., Prime T. A., Logan A., James A. M., Hall A. R., Vidoni S., Arndt S., Caldwell S. T., Prag H. A., Pell V. R., Krieg T., Mulvey J. F., Yadav P., Cobley J. N., et al. (2017) MitoNeoD: A mitochondria-targeted superoxide probe. Cell Chem. Biol. 24, 1285–1298.e1212 10.1016/j.chembiol.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michalski R., Michalowski B., Sikora A., Zielonka J., and Kalyanaraman B. (2014) On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: A reassessment. Free Radic. Biol. Med. 67, 278–284 10.1016/j.freeradbiomed.2013.10.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zielonka J., Sarna T., Roberts J. E., Wishart J. F., and Kalyanaraman B. (2006) Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch. Biochem. Biophys. 456, 39–47 10.1016/j.abb.2006.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rinkel J., and Dickschat J. S. (2015) Recent highlights in biosynthesis research using stable isotopes. Beilstein J. Org. Chem. 11, 2493–2508 10.3762/bjoc.11.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lehmann W. D. (2017) A timeline of stable isotopes and mass spectrometry in the life sciences. Mass Spectrom. Rev. 36, 58–85 10.1002/mas.21497 [DOI] [PubMed] [Google Scholar]
- 71. Jang C., Chen L., and Rabinowitz J. D. (2018) Metabolomics and isotope tracing. Cell 173, 822–837 10.1016/j.cell.2018.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mottley C., Connor H. D., and Mason R. P. (1986) [17O]oxygen hyperfine structure for the hydroxyl and superoxide radical adducts of the spin traps DMPO, PBN and 4-POBN. Biochem. Biophys. Res. Commun. 141, 622–628 10.1016/S0006-291X(86)80218-2 [DOI] [PubMed] [Google Scholar]
- 73. Michalski R., Zielonka J., Gapys E., Marcinek A., Joseph J., and Kalyanaraman B. (2014) Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J. Biol. Chem. 289, 22536–22553 10.1074/jbc.M114.553727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wardman P. (2008) Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol. 441, 261–282 10.1016/S0076-6879(08)01214-7 [DOI] [PubMed] [Google Scholar]
- 75. Talib J., Maghzal G. J., Cheng D., and Stocker R. (2016) Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic. Biol. Med. 97, 124–135 10.1016/j.freeradbiomed.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 76. Sedgwick A. C., Dou W. T., Jiao J. B., Wu L., Williams G. T., Jenkins A. T. A., Bull S. D., Sessler J. L., He X. P., and James T. D. (2018) An ESIPT probe for the ratiometric imaging of peroxynitrite facilitated by binding to Aβ-aggregates. J. Am. Chem. Soc. 140, 14267–14271 10.1021/jacs.8b08457 [DOI] [PubMed] [Google Scholar]
- 77. Dikalov S., Griendling K. K., and Harrison D. G. (2007) Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49, 717–727 10.1161/01.HYP.0000258594.87211.6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xiao Y., and Meierhofer D. (2019) Are hydroethidine-based probes reliable for reactive oxygen species detection? Antioxid. Redox Signal. 31, 359–367 10.1089/ars.2018.7535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng G., Zielonka M., Dranka B., Kumar S. N., Myers C. R., Bennett B., Garces A. M., Dias Duarte Machado L. G., Thiebaut D., Ouari O., Hardy M., Zielonka J., and Kalyanaraman B. (2018) Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future. J. Biol. Chem. 293, 10363–10380 10.1074/jbc.RA118.003044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zielonka J., Zhao H., Xu Y., and Kalyanaraman B. (2005) Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: Stopped-flow optical and EPR studies. Free Radic. Biol. Med. 39, 853–863 10.1016/j.freeradbiomed.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 81. Shan X., and Que L. (2006) High-valent nonheme iron-oxo species in biomimetic oxidations. J. Inorg. Biochem. 100, 421–433 10.1016/j.jinorgbio.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 82. Zielonka J., Hardy M., and Kalyanaraman B. (2009) HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic. Biol. Med. 46, 329–338 10.1016/j.freeradbiomed.2008.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., Kirk M., and Freeman B. A. (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 269, 26066–26075 [PubMed] [Google Scholar]
- 84. Alvarez M. N., Trujillo M., and Radi R. (2002) Peroxynitrite formation from biochemical and cellular fluxes of nitric oxide and superoxide. Methods Enzymol. 359, 353–366 10.1016/S0076-6879(02)59198-9 [DOI] [PubMed] [Google Scholar]
- 85. Massey V. (1959) The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim. Biophys. Acta 34, 255–256 10.1016/0006-3002(59)90259-8 [DOI] [PubMed] [Google Scholar]
- 86. Thomas D. D., Miranda K. M., Espey M. G., Citrin D., Jourd'heuil D., Paolocci N., Hewett S. J., Colton C. A., Grisham M. B., Feelisch M., and Wink D. A. (2002) Guide for the use of nitric oxide (NO) donors as probes of the chemistry of NO and related redox species in biological systems. Methods Enzymol. 359, 84–105 10.1016/S0076-6879(02)59174-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented and discussed are contained within the manuscript or in the supporting information.