Abstract
Objective:
The aim of this study was to grade cartilage damage in a swine model of osteoarthritis using a whole-body photon-counting detector (PCD) CT.
Materials and Methods:
A multienergy phantom containing gadolinium (Gd) (2, 4, 8, and 16 mg/mL) and hydroxyapatite (200 and 400 mg/cc) was scanned using a PCD-CT system (48 [notdef] 0.25 mm collimation, 80 kV, 800 mAs, D50 reconstruction kernel) to serve as calibration for material decomposition and to assess quantification accuracy. Osteoarthritis was induced in Yucatan miniswine (n = 8) using 1.2 mg monoiodoacetate (MIA) injected into a randomized knee, whereas the contralateral control knee received saline. Twenty-one days later, a contrast bolus (gadoterate meglumine, 4 mL/knee) was intra-articularly administered into both knees. The knees were simultaneously scanned on the PCD-CT system (48 [notdef] 0.25 mm collimation, 80 kV, 800 mAs). Multienergy images were reconstructed with a sharp “V71” kernel and a quantitative “D50” kernel. Image denoising was applied to the V71 images before grading cartilage damage, and an iterative material decomposition technique was applied to D50 images to generate the Gd maps. Two radiologists blinded to the knee injection status graded the cartilage integrity based on a modified International Cartilage Repair Society scoring system. Histology was performed on excised cartilage using methylene blue/basic fuchsin. Statistical analysis of grade distribution was performed using an exact test of omnibus symmetry with P < 0.05 considered significant.
Results:
Material decomposed images from the multienergy phantom scan showed delineation and quantification of Gd and hydroxyapatite with a rootmean-squared error of 0.3 mg/mL and 18.4 mg/cc, respectively. In the animal cohort, the radiologists reported chondromalacia in the MIA knees with International Cartilage Repair Society scores ranging from grade 1 (cartilage heterogeneity, n = 4 knees) to grade 3 (up to 100% cartilage loss, n = 4 knees). Grade 1 was characterized by cartilage heterogeneity and increased joint space in the patellofemoral compartment, whereas grade 3 was characterized by cartilage erosion and bone-on-bone articulation in the patellofemoral compartment. All control knees were scored as grade 0 (normal cartilage). Significant difference (P = 0.004) was observed in the grade distribution between the MIA and control knees. Gross examination of the excised knees showed cartilage lesions in the grade 3 MIA knees. The Gd maps from material decomposition showed lower contrast levels in the joint space of the MIA knee compared with the contralateral control knee due to joint effusion. Histology revealed chondrocyte loss in the MIA knee cartilage confirming the chondrotoxic effects of MIA on cartilage matrix.
Conclusions:
We demonstrated a high-resolution and quantitative PCD-CT arthrography technique for grading cartilage damage in a large animal model of osteoarthritis. Photon-counting detector CT offers simultaneous high-resolution and multienergy imaging capabilities that allowed morphological assessment of cartilage loss and quantification of contrast levels in the joint as a marker of joint disease. Cartilage damage in the MIA knees was graded using PCD-CT images, and the image-based findings were further confirmed using histology and gross examination of the excised knees.
Keywords: osteoarthritis, arthrography, x-ray computed tomography, contrast media, monoiodoacetate, experimental animal models, radiologic phantoms
Osteoarthritis (OA) is a progressive joint disease that causes cartilage degeneration and immobility. Knee OA is the most common type of OA, with 1 in 2 adults developing symptoms during their lives.1 Early detection of OA using diagnostic imaging techniques may facilitate individualized treatment regimens to minimize the disease progression. Recent developments in high-resolution CT technology using photon-counting detectors (PCDs) may facilitate early detection of cartilage damage as a marker of OA. However, the benefits of new imaging techniques need to be demonstrated in appropriate animal models before use in clinical practice.
Osteoarthritis is typically modeled in animals through surgical induction or intra-articular administration of chondrotoxic agents.2 Surgical induction often involves creating focal osteochondral defects that progress into OA,3 whereas chemical induction causes direct matrix damage, chondrocyte death, or inflammation using chondrotoxic agents such as monoiodoacetate (MIA).2,4 Chemical induction is suitable for OA imaging models since the induced damage is usually nonfocal and controlled disease progression can be achieved by altering the dose-time response.
Magnetic resonance imaging (MRI) is clinically used for noncontrast or contrast-enhanced cartilage imaging but has limited spatial resolution,5,6 which often requires longer acquisition times. Computed tomography (CT) offers superior spatial resolution compared with MRI and is often preferred for detecting meniscal tear7 that are not visible with MRI, or in patients with contraindications for MRI.6,8 Multidetector CT acquisitions feature isotropic volumes, consequently allowing retrospective reconstruction in different spatial planes9 as a valuable feature for visualizing joint structures. Computed tomography offers dual-energy capabilities for material discrimination and ultra-highresolution (UHR) acquisition for imaging of the extremities. Ultrahigh-resolution is typically enabled through the use of comb filters10 placed near the detector array, to reduce the pixel aperture and, consequently, yield higher imaging resolution. Alternatively, dual-energy protocols allow material classification to yield volumetric bone density maps or virtual noncalcium images for visualization of bone edema.11–13 A current limitation of conventional CT arthrography techniques is that dual-energy and UHR capabilities are not simultaneously available from the same acquisition. Recent advances in CT technology involving PCDs14–16 have demonstrated simultaneous high-resolution and multienergy imaging for a range of preclinical and clinical imaging tasks. Photon-counting detector CT offers UHR imaging (150 micron in-plane resolution17,18) using smaller detector pixels, and multienergy imaging capabilities15,16,19 using thresholding and energy binning of x-ray photons. Although several studies have demonstrated the benefits of PCD-CT for vascular imaging,22–25 neuroimaging,20,21 and chest imaging,26,27 the application of PCD-CT for in vivo musculoskeletal imaging (such as cartilage damage occurring from OA) has not been reported. The multienergy acquisitions from PCDs capture the spectral signature of different material types; this facilitates discrimination and quantification of contrast media. The use of smaller detector pixels in PCD-CT without additional UHR comb filters improves the imaging spatial resolution and radiation dose efficiency that could be beneficial for assessing cartilage damage. In this study, we aimed to detect and score cartilage damage in the knee joint of a large animal OA model using high-resolution PCD-CT and assess changes in anionic gadoterate meglumine (Gd-DOTA) contrast levels in the knee joint using material decomposition. Ionic contrast agents such as Gd-DOTA undergo preferential uptake in the cartilage based on electrostatic interaction with the negatively charged glycosaminoglycans (GAGs) in the cartilage matrix, a principle described as equilibrium-partitioned imaging of cartilage.28
MATERIALS AND METHODS
Multienergy Phantom
A 20-cm multienergy phantom containing Gd (2, 4, 8, and 16 mg/mL) and hydroxyapatite (HA) (200 and 400 mg/cc) was scanned on the PCD-CT system (see Table 1 for scan parameters). The phantom experiment was designed to assess the accuracy of material decomposition (root-mean-squared error in the image-based measurements relative to ground truth) and serve as a calibration for material decomposition of the pig knees.
TABLE 1.
PCD-CT Acquisition and Image Reconstruction Parameters
| Animal Scans | Phantom Scan | ||
|---|---|---|---|
| Acquisition mode | Sharp (48 × 0.25 mm collimation) | ||
| Tube potential | 80 kV | ||
| Tube-current-time product | 800 mAs (effecttive) | ||
| CTDIvol (32 cm) | 19 mGy | ||
| Pitch | 0.6 | ||
| Scan FOV | 275 mm | ||
| Energy thresholds | 25, 50 keV | ||
| Image matrix size | 512 × 512 | ||
| Reconstruction technique | Iterative* | wFBP† | wFBP |
| Kernel | V71 | D50 | D50 |
| Iterative strength | 5 | — | — |
| Image thickness | 0.5mm | 2.0 mm | |
| Reconstruction FOV | 80 mm | 275 mm | |
SAFIRE
weighted-filtered-back projection (Siemens Healthcare, Forchheim, Germany).
FOV indicates field of view.
Imaging Swine Model of Osteoarthritis
After approval from our institutional animal care and use committee, 1-year-old Yucatan swine (n = 8, mixed sex) were employed in our study. Chemical induction of OA was performed by administering chondrotoxic MIA in randomized stifle joints. The stifle joint was chosen due to its similarity to the human knee. Approximately 2 hours before the intra-articular injections, MIA was dissolved in sterile phosphate-buffered saline (PBS, pH = 7.2, Gibco Biosciences, Dublin, Ireland) in a laminar flow hood to yield a MIA concentration of0.6 mg/mL (1.2 mg/knee). The MIA solution was vortexed, sterile filtered, and drawn into syringes in 2-mL volumes. The 1.2-mg MIA dose per knee was determined based on a previous study by Unger et al29 involving Yucatan OA model. Sham injections (2-mL sterile PBS) were also drawn for the control knees. A blinding color code was assigned to the MIA and control syringes by a member of another laboratory who did not participate in this study.
The pigs were anesthetized using telazol/xylaxine and maintained using isofluorane (1.5% to 3%) through tracheal intubation. Physiological parameters (heart rate, respiration, blood pressure, body temperature, and anesthesia) were constantly monitored and documented. The animals were placed on their back on the surgery table, and the skin over the knee joints was scrubbed using 1% chlorhexidine. The injections were performed under strict aseptic conditions by a board-certified musculoskeletal radiologist (N.S.M.; 13 years of experience) blinded to the injectate. A 22-gauge sterile needle was inserted from the lateral side of the knee toward the synovial space between patella and trochlea using ultrasound (US) guidance. A test injection using normal saline (~200 μL) was initially performed to confirm desired needle placement and dispersion into the joint space under US, followed by administration of MIA or PBS. After delivering the injectate, the needle was withdrawn and the knee was flexed and extended for about a minute to ensure uniform distribution of the injectate in the synovial space before proceeding to the contralateral knee. The animals were allowed to recover after the intra-articular injections and were fed a normal diet thereafter until PCD-CT scanning. Potential joint pain arising from MIAwas managed using analgesics: buprenorphine 0.18 mg/kg subcutaneously once pre-MIA, and 4 mg/kg carprofen subcutaneously once pre-MIA, and then orally for 3 to 4 days post-MIA.
PCD-CT Data Acquisition and Image Reconstruction
A research PCD-CT scanner (Somatom CounT; Siemens Health care, Forchheim, Germany) was employed in this study. The scanner is based on a second-generation clinical dual-source scanner (Definition Flash; Siemens Healthcare), with the A-subsystem equipped with a conventional energy-integrating detector, and the B-subsystem equipped with a PCD array. The PCD subsystem allows a 27.5-cm scan field of view (FOV). Multienergy CT on the PCD system is enabled using energy-thresholds, and the sharp mode (0.25-mm detector pixel size) offers 2 energy thresholds per detector pixel for simultaneous multienergy high-resolution acquisitions. Additional details about the scanner and the acquisition modes can be found elsewhere.17,30
Three weeks after the MIA injections, the pigs were anesthetized and scanned on the PCD-CT system. Before CT scanning, a 4-mL contrast bolus of anionic Gd-DOTA (Dotarem, Guerbet, France) at stock concentration (376.9 mg/mL gadoterate meglumine) was injected under US guidance into the intra-articular space similar to the MIA injections. For each pig, both knees received the same amount of contrast, and the injections were performed within 5 minutes between knees. The knees were flexed and extended for about 1 minute to ensure uniform distribution of the imaging contrast agent in the synovial space. The pigs were then placed laterally on the scan table in feet-first position, and the knees were extended and held in position as shown in Figure 1. Both knees were accommodated within the PCD FOV (27.5 cm), therefore scanned together in a single scan preventing the need for separate arthrograms for each knee. The knees were scanned approximately 30 minutes after intra-articular contrast administration. For the swine study and the multienergy phantom experiment, spiral PCD-CT scans and image reconstructions were performed using the settings tabulated in Table 1.
FIGURE 1.
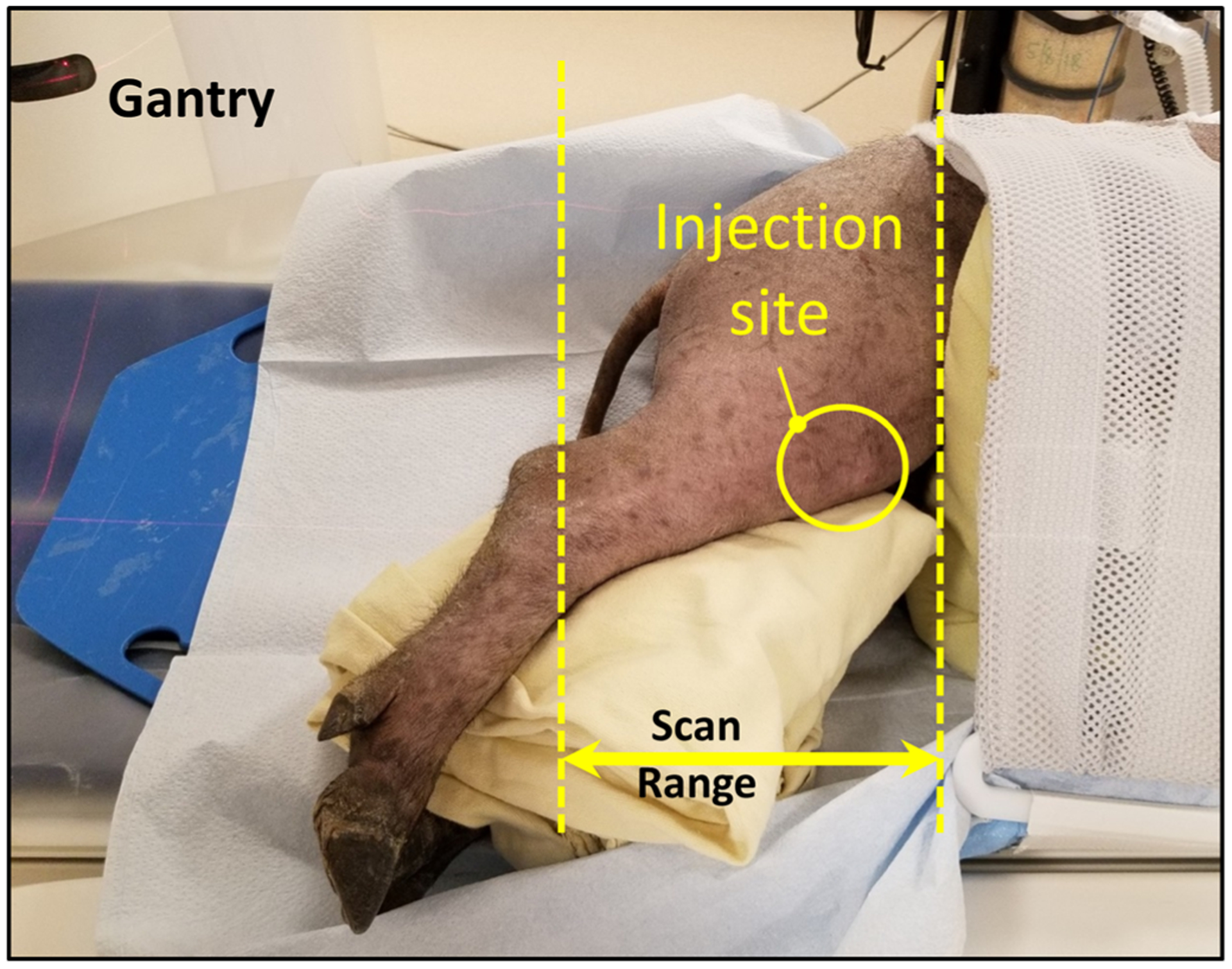
Swine scan orientation and location of scan. Bothhind knees received 4 mL of Gd-DOTA contrast at the injection site before scanning.
Although both knees were scanned simultaneously using PCD-CT, the images for each knee were reconstructed separately (at 80-mm FOV) for blinded image review. Also, sagittal reformats (0.5-mm thickness) were generated from the axial images for each knee. The full spectrum image (25–80 keV) obtained using the dedicated sharp kernel (V71, 10% MTF = 19 cm−1) was used in the CT image analysis, whereas multienergy bin images (25–50 keVand 50–80 keV) obtained using the quantitative D50 kernel were used for material decomposition (Gd quantification). For the high-resolution images obtained using V71, an additional image denoising technique19,31 was applied to reduce the image noise. An image-domain material decomposition algorithm known as Prior-Knowledge Aware Iterative Denoising19 (MD-PKAID) was employed to generate the Gd map. Briefly, the material decomposition algorithm utilizes the noise properties of the full-spectrum image (25–80 keV) as prior knowledge to reduce noise in the material maps using a constrained regularization. The full-spectrum image exhibits the lowest noise among the multienergy data and therefore was used as the prior image. Material decomposition was applied to D50 images for both swine and phantom experiments.
Grading Cartilage Integrity Using PCD-CT Images
A consensus review panel graded the PCD-CT V71 images (25–80 keV, axial and saggital images) from the swine knee arthrograms. The panel consisted of 2 board-certified musculoskeletal radiologists (N.S.M. and M.A.F.; with combined 28 years of experience) who were blinded to the injection status of each knee for all the pigs in the cohort. A modified clinical grading system for the images based on the International Cartilage Repair Society system29 was adopted for scoring the cartilage integrity as shown in Table 2. The patellofemoral compartment from each knee joint was partitioned into 4 regions for radiologic assessment (trochlea and patella, medial and lateral), and the cartilage integrity was scored.
TABLE 2.
Modified-Clinical Grading System for Scoring Cartilage Integrity Using CT Images
| Grade 0 | Normal cartilage thickness and appearance |
| Grade 1 | Minor superficial indentation and cartilage heterogeneity |
| Grade 2 | Cartilage thinning up to 50% |
| Grade 3 | Cartilage thinning from 50% to 100%, no subchondral bone involvement |
| Grade 4 | Complete cartilage loss and erosion of underlying subchondral bone |
CT indicates computed tomography
Gross Evaluation of Harvested Knees
The swine were euthanized immediately after CT scanning using an intravenous injection of pentobarbital (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI), and their knees were harvested the same day. Skin was removed, and the tibia, femur, and patella were separated. The knees were qualitatively examined for gross morphological changes and compromised cartilage integrity before storing in 10% neutral-buffered formalin for histological analysis. Patella, trochlea, femoral condyles, and tibial plateau from each knee were digitally photographed for reference.
Cartilage Histology
The formalin-fixed excised knees were transferred to 70% ethyl alcohol (ETOH) and vacuumed at 15 psi for 1 hour at room temperature, then placed in 95% ETOH and stored in 4°C overnight. The specimens were then transferred to 100% ETOH (changed each day) and stored in 4°C for a period of 2 weeks. The samples were transferred to a methylmethacrylate (MMA; vacuumed base media, changed each day) and 2 weeks later placed in a 30°C water bath for 2 days for the plastic to firm up. Excess MMA was removed, and 200-μm-thick sections were obtained using a band saw equipped with diamond blade (E310; Exakt Technologies, Oklahoma City, OK). Final 20-μm-thick sections were obtained using a microgrinder (E400; Exakt Technologies, Oklahoma City, OK) and stained using cationic methylene blue with basic fuchsin as a counterstain.
Statistical Analysis
All statistical analyses were performed using the R v3.5.3 statistical software (R Core Team, Vienna). After grading the cartilage loss using high-resolution PCD-CT images, to assess whether there was a significant difference in grade distribution by MIA injection status, we tested the hypothesis of global symmetry via an exact multinomial goodness-of-fit test using the rcompanion R package. This is similar to a McNemar-Bowker test for paired observations and appropriate for small discordant cell frequencies. Statistical significance was declared at alpha level of 0.05.
RESULTS
Material decomposition results from the multienergy phantom experiment are shown in Figure 2. The Gd and HA inserts were delineated, and their concentrations were quantified. The root-meansquared error between measured concentrations and ground truth was0.3 mg/mL for Gd and 18.4 mg/cc for HA.
FIGURE 2.
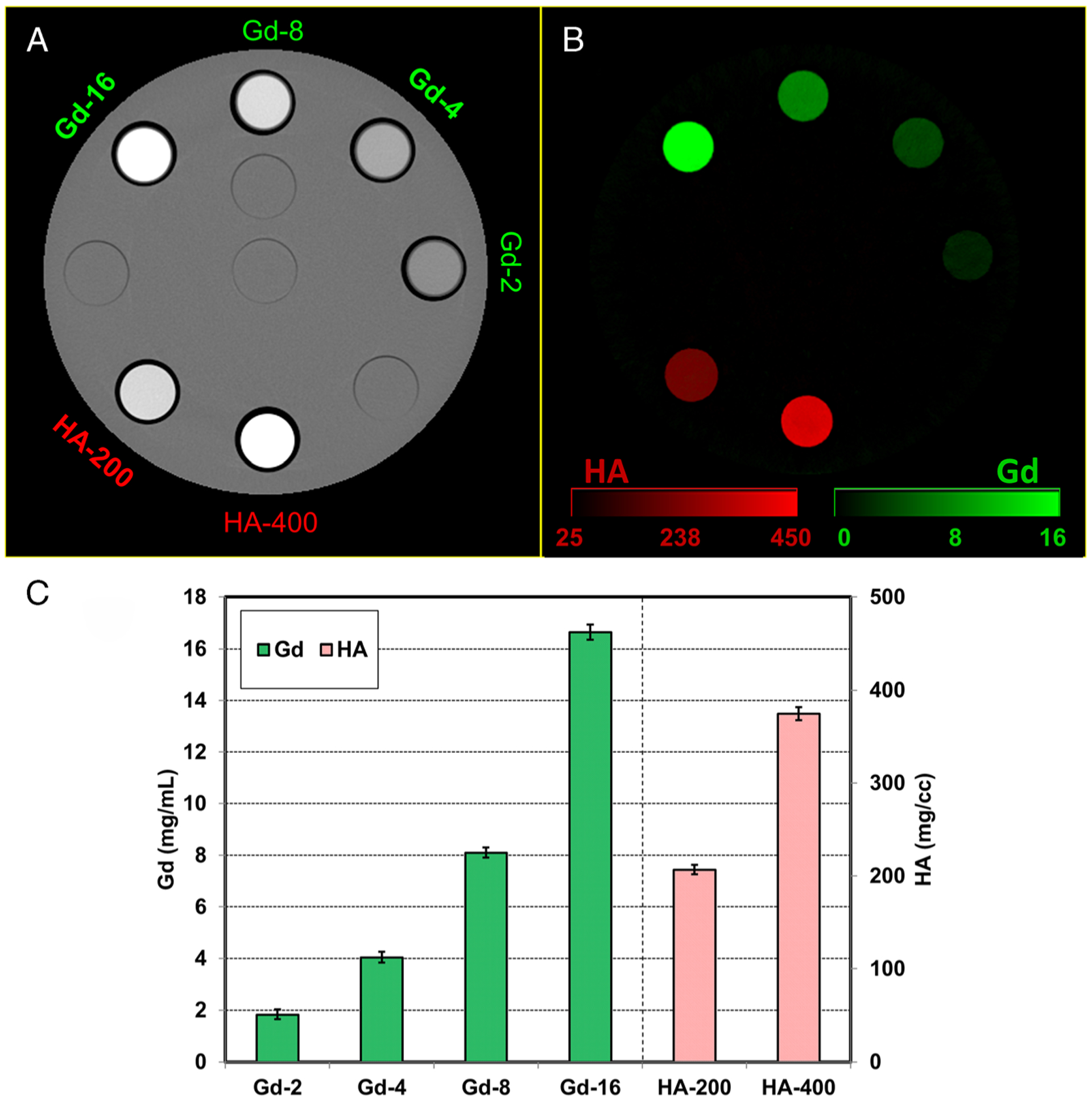
Photon-counting detector computed tomography (PCD-CT) images from the multienergy phantom scan. A, PCD-CT (25–80) keV image with gadolinium (Gd 2, 4, 8, and 16 mg/mL) and hydroxyapatite (HA, 200 and 400 mg/cc) inserts. B, Material decomposition results showing delineation and quantification of Gd and HA inserts. C, Estimated mass densities compared with ground truth for Gd and HA (root-mean-squared error = 0.3 mg/mL and 18.4 mg/cc for Gd and HA, respectively). Horizontal axis entries represent material-concentration (eg, Gd-2 represents Gd at 2 mg/mL).
Axial and sagittal knee images (MIA and control knees) from a miniswine PCD-CT scan are shown in Figure 3. Chondromalacia was detected in the MIA knee, which was scored by the blinded panel as grade 1 (cartilage heterogeneity), whereas the control knee was scored as grade 0 (normal cartilage thickness and appearance). In addition, the patellofemoral joint space in the MIA knee was found to be larger compared with the control knee (Fig. 3B). The contrast enhancement in the joint space was noticeably lower in the MIA knee, which was further confirmed by the Gd material maps obtained from MD-PKAID (Fig. 4), where the Gd levels in the MIA knee was lower compared with that of the control knee despite both knees receiving the same volume and concentration of the Gd agent. This is indicative of increased contrast dilution from joint effusion in the MIA-injected knee. Harvested knees did not show any global or focal damage to the cartilage in the MIA and control knees; however, the MIA knee showed excessive fluid leakage during the excision of joint capsule, further confirming joint effusion in the MIA knees. Histology sections stained using methylene blue/basic fuchsin stains revealed chondrocyte loss, clustering, and degeneration in the MIA knee, whereas the control knee cartilage showed normal chondrocyte distribution and appearance as shown in Figure 5. Matrix and cell loss has been associated with MIA-induced inflammation and cartilage destruction, and has been previously reported in an MIA rat OA model.32
FIGURE 3.
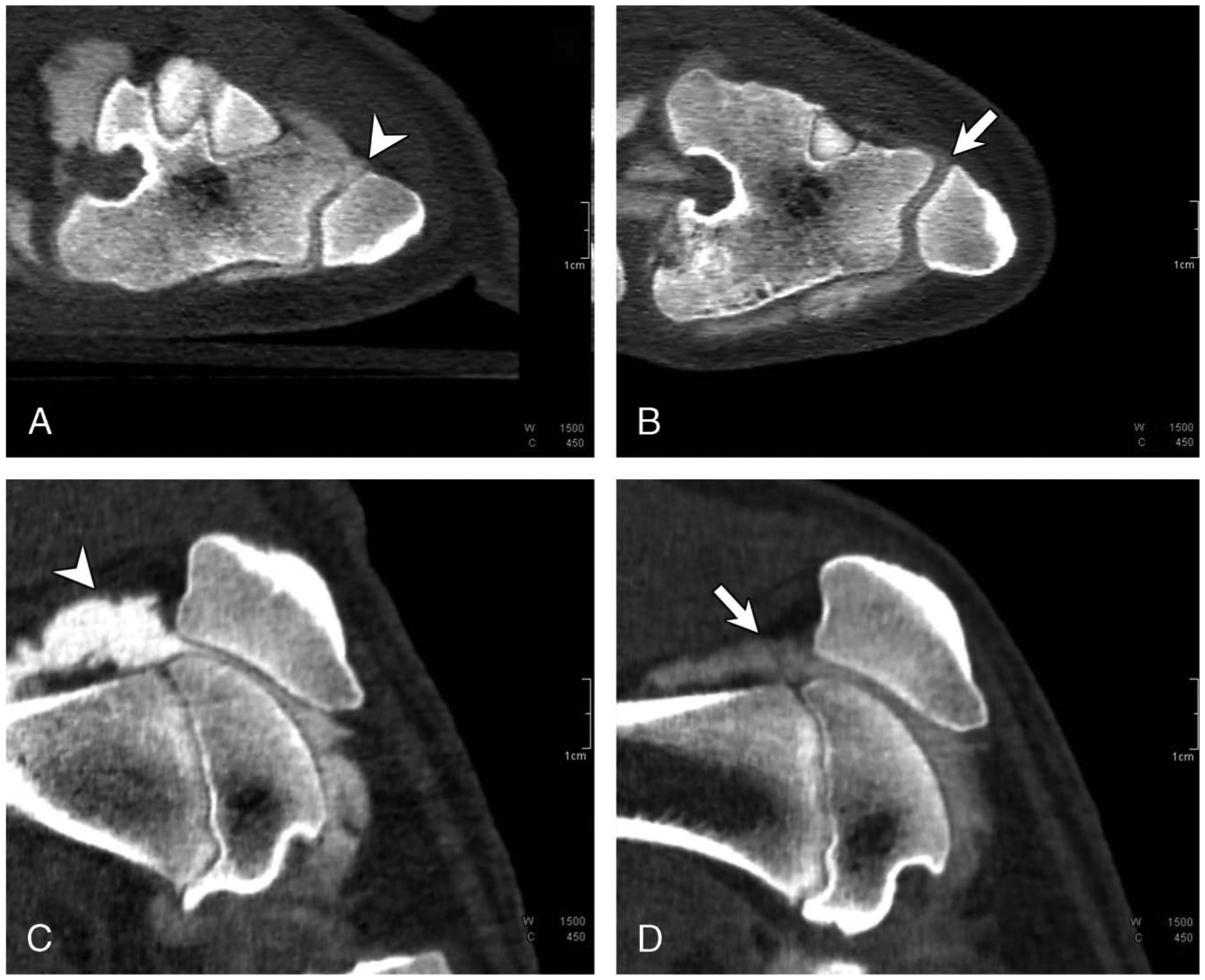
Axial and sagittal PCD-CT images of a control knee (A and C) and the contralateral MIA knee (B and D) that was scored as grade 1 (cartilage heterogeneity) from a miniswine. Cartilage heterogeneity and increased intra-articular space (arrow line in B) in the patellofemoral compartment was noticed in the MIA knee. Lower CT enhancement (arrow line in D) was observed in the joint space of the MIA knee compared with the control knee signifying increased contrast dilution from joint effusion in the MIA knee.
FIGURE 4.
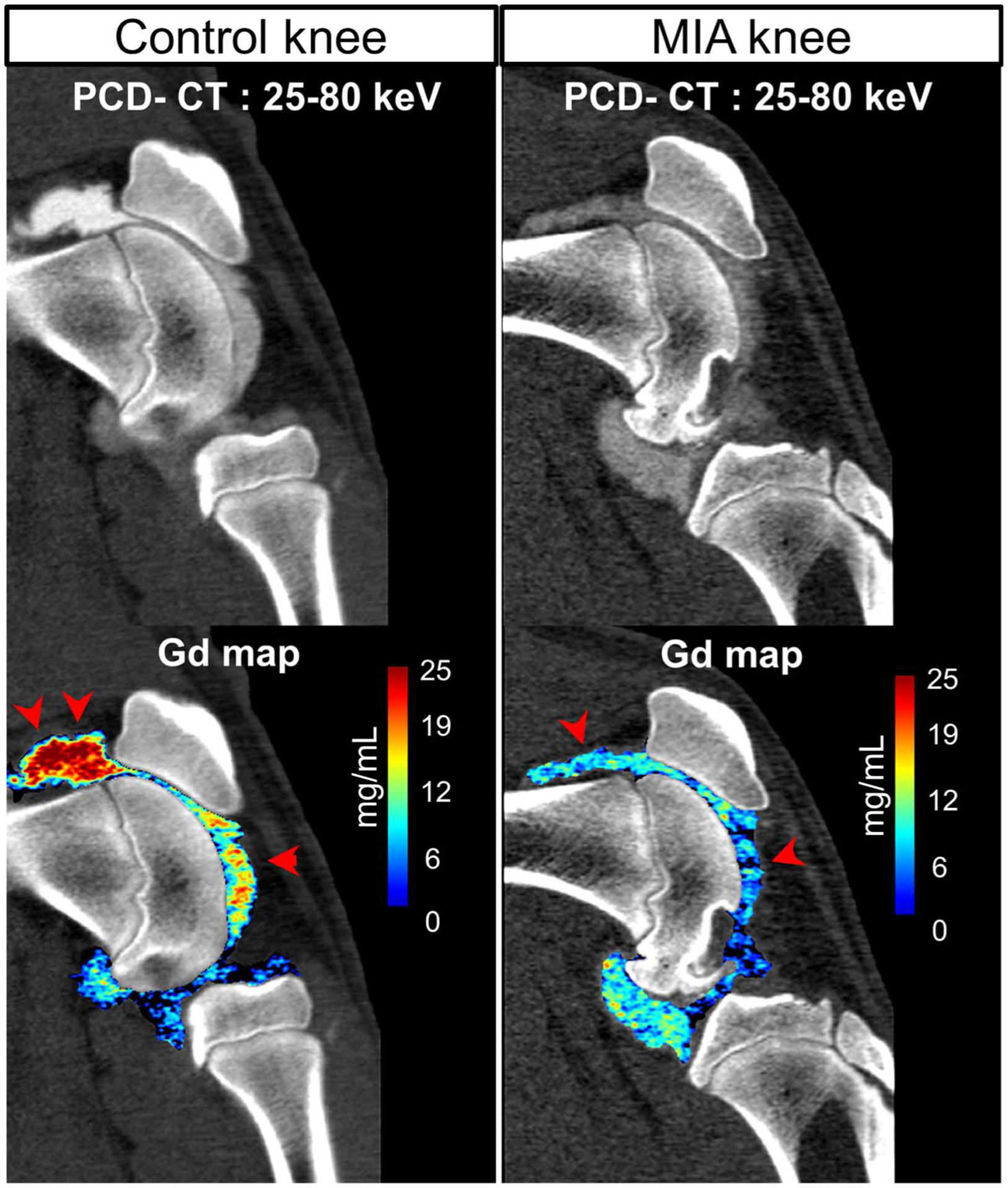
Gadolinium maps from material decomposition applied to sagittal PCD-CT images from the same miniswine shown in Figure 3. The MIA knee showed decreased levels of Gd contrast (marked using arrow heads, displayed in units of mass density mg/mL) in the joint space compared with the contralateral control knee.
FIGURE 5.
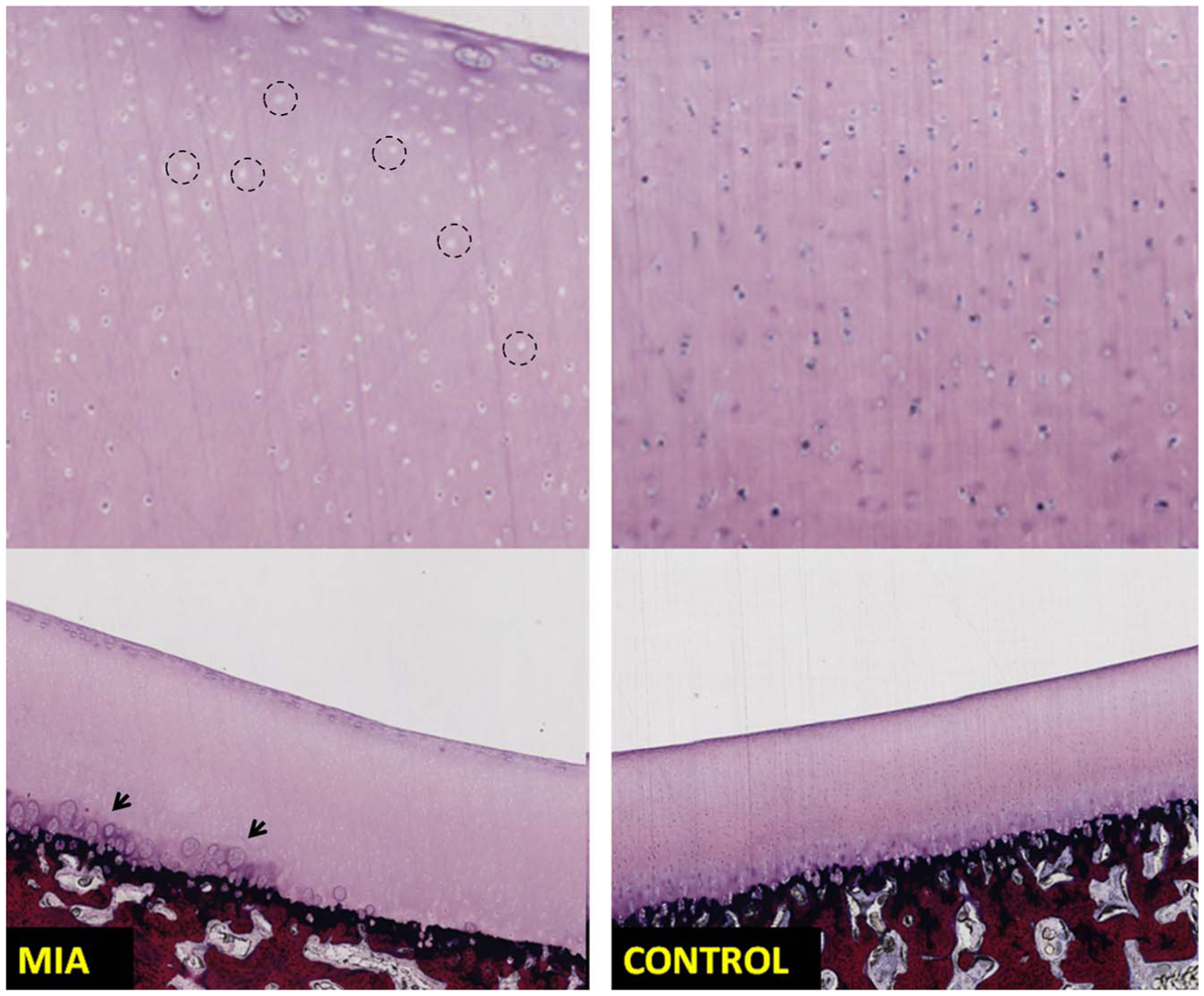
Sections of patella stained using methylene blue/basic fuchsin show chondrocytes loss (left top image, example regions are marked using circles) in the MIA injected knee (left column) compared with the control knee (right column) exhibiting normal chondrocyte appearance. Chondrocyte clustering and degeneration (marked using arrows) was noticed in the MIA knee. The MIA knee from the same swine was marked as grade 1 (cartilage heterogeneity) on the PCD-CT images.
Bilateral knee images from a different swine where the left knee (MIA knee) was scored by the radiologists as grade 3 (up to 100% cartilage loss) are shown in Figure 6. The PCD-CT images from the MIA knee showed bone-on-bone articulation in the lateral patellofemoral compartment due to complete cartilage loss. No damage to the subchondral bone at these sites was reported. The control knee (right knee) showed normal articulation and cartilage appearance (Fig. 6B, D) and was marked as grade 0. In addition, similar to the previous swine example, a lower CT number enhancement was noticed in the joint space of the MIA-injected knee. Harvested MIA knee showed severe cartilage lesions in the trochlear and patellar surfaces, which correlates with the PCD-CT images, whereas the control knee showed normal cartilage appearance.
FIGURE 6.
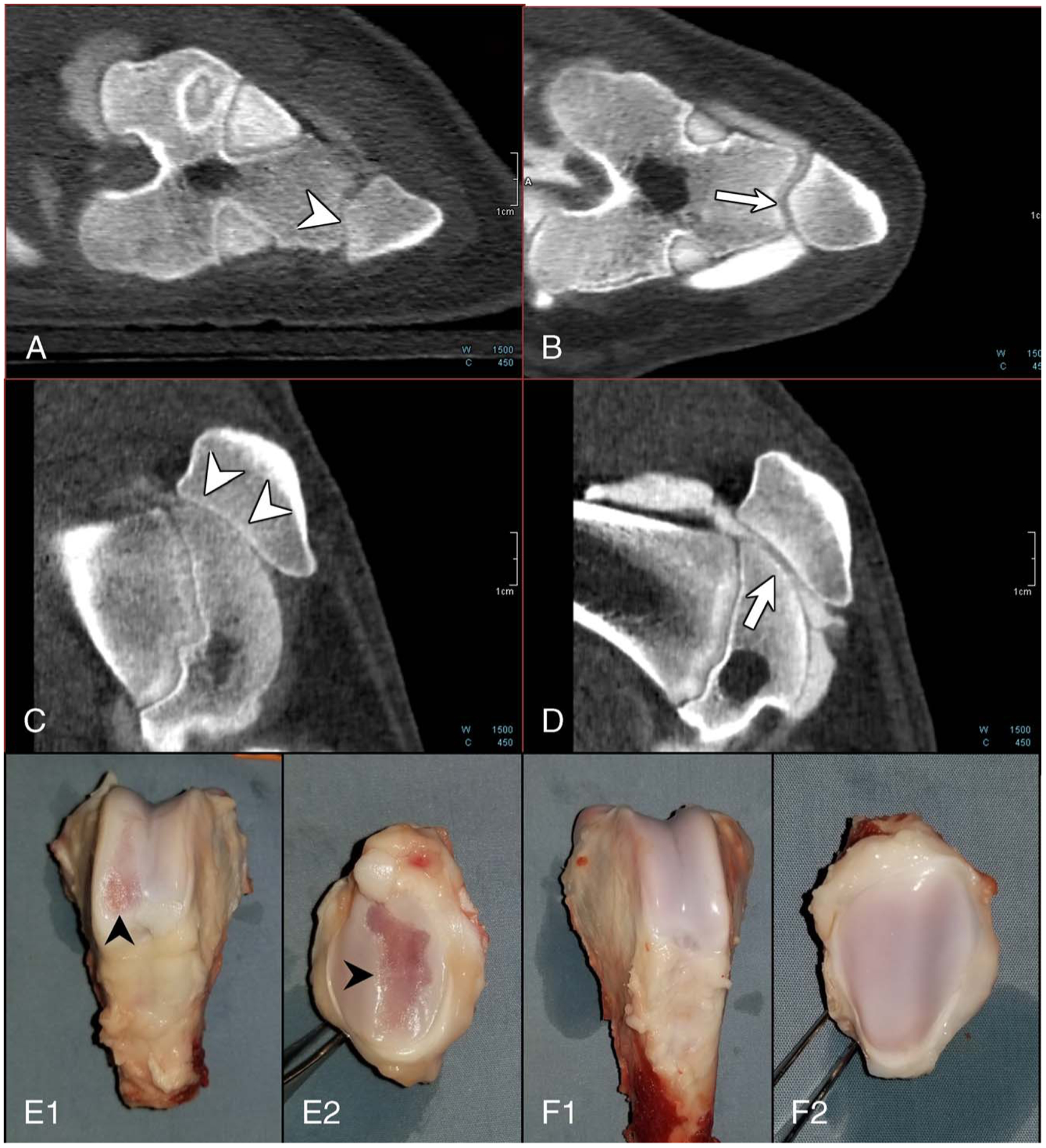
Photon-counting detector computed tomography (PCD-CT) images and photographs of harvested knees showing substantial cartilage loss from the articular surface. PCD-CT images from the MIA knee (A and C) show bone-on-bone articulation (arrowheads in A, C) due to complete cartilage loss (grade 3), whereas the control knee showed normal cartilage thickness and appearance (grade 0). Excised femur (E1, F1) and patella (E2, F2) specimens from the same pig showed substantial cartilage damage (black arrowheads) in the MIA knee (E1, E2) compared with the control knee, which exhibited normal cartilage appearance (F1, F2).
Monoiodoacetate-injected knees from all animals in the cohort were compared against the contralateral control knees (Table 3), and an exact test of omnibus symmetry applied to the cohort showed compromised cartilage integrity in the MIA-injected knees (P = 0.004), whereas all the control knees showed normal cartilage thickness and appearance.
TABLE 3.
Cartilage Integrity Scores for the Yucatan Cohort Assigned Using PCD-CT Images
| Sex Index, Male/Female-Number | Control Knee | MIA Knee |
|---|---|---|
| F-1 | Grade 0 | Grade 1 |
| F-2 | Grade 1 | |
| F-3 | Grade 3 | |
| F-4 | *Grade 1/grade 2 | |
| M-1 | Grade 0 | Grade 3 |
| M-2 | Grade 3 | |
| M-3 | Grade 3 | |
| M-4 | Grade 3 |
Cartilage heterogeneity observed with potential thinning <50%. Exact test of omnibus symmetry between MIA and control knee scores was applied, P = 0.004, statistically significant.
PCD-CT indicates photon-counting detector computed tomography; MIA, monoiodoacetate.
Photon-counting detector CT images from all pigs in the cohort showed osteochondritis dissecans (OCD) in the femoral condyles irrespective of the knee treatment status (MIA-injected or control). These findings could be attributed to factors pertinent to skeletal maturity in the miniswine and not related to the MIA injection.
DISCUSSION
In this study, dedicated sharp kernel (V71) images for PCD-CT scans of swine knees facilitated grading of cartilage loss caused by MIA. Photon-counting detector CTarthrography maintains the benefits of traditional CTarthrography (fast scan time and better intrinsic spatial resolution than MR) and provides additional quantitative capabilities for material discrimination and mass density quantification,19 which collectively facilitates assessment and grading of cartilage heterogeneity, joint-space narrowing, and joint effusion associated with OA as demonstrated in our study. Grade 1 cartilage damage was associated with cartilage heterogeneity in the patellofemoral compartment, and increased joint space and contrast dilution in the joint. Grade 3 damage was characterized by severe cartilage lesions resulting in bone-on bone articulation in the MIA knees. Quantification of Gd contrast levels in the joints was demonstrated using an image-based iterative material decomposition. The quantitative accuracy of material decomposition for Gd and HA was assessed using a multienergy phantom. The Gd maps, along with the high-resolution PCD-CT knee images, confirmed lower contrast levels in the MIA knee signifying joint effusion, which was further confirmed during excision of the joint capsule revealing excess fluid in the MIA knees. High-resolution multienergy CT imaging is achieved using smaller detector pixels (0.25 mm at scan isocenter) thereby eliminating the need for comb filter typically used in conventional CT for UHR imaging. This could be beneficial for imaging of joints affected by chronic conditions where repeat scans are often warranted for longitudinal evaluation. Also, due to the uniform photon weighting, PCDs improve the image contrast-to-noise ratio in contrast enhanced CT,33 which could reduce the contrast dosage required for CT arthrography.
Our animal imaging experiments utilized ionic Gd-DOTA agent for PCD-CTof cartilage; however, our method does not entirely rely on the use of Gd agent(s). For instance, clinically approved ionic iodine agents (such as iothalamate meglumine) could be used as an alternative to Gd for PCD-CT arthrography. When required, the multienergy PCD-CT data could be used to generate volumetric bone density maps for further assessment of subchondral bone in the joints. In terms of cartilage damage, grade 3 (up to 100% cartilage loss) was more prevalent in male Yucatans than females of the same weight. However, due to the limited number of animals in the cohort, further studies are required to establish a statistical significance for the sex disparity.
A key limitation of this study is that the imaging of GAGs distribution in the articular cartilage was not pursued despite using an ionic contrast agent. Glycosaminoglycans are negatively charged, and an ionic contrast agent (cationic or anionic) targets GAG in the cartilage matrix34 through electrostatic interaction. Quantifying intra-articular GAG distribution was not pursued due to the small thickness (<0.5 mm) of the knee cartilage in Yucatan miniswine. However, quantifying ionic contrast in articular cartilage to visualize GAG distribution has been previously demonstrated ex vivo in cadaveric34 and domestic swine cartilage35 specimens using PCD-CT. In our study, early OA-like changes (grade 1) were characterized in the PCD-CT images by increased patellofemoral joint space and joint effusion (causing increased contrast dilution). Detection and grading of MIA-induced OCD was not pursued due to the presence of naturally occurring OCD lesions in the Yucatan cohort. Since we used a whole-body PCD-CT scanner, the imaging methodology could be translated to humans with minimal changes to the CT acquisition settings. However, additional studies in humans with independent readers are warranted to compare the performance of PCD-CT and conventional CT arthrography techniques.
In conclusion, we demonstrated a high-resolution, quantitative PCD-CTarthrography technique for grading cartilage damage in a large animal model of OA. Osteoarthritis markers ranging from cartilage heterogeneity (grade 1) to complete cartilage loss (grade 3) were detected by radiologists using high-resolution PCD-CT images obtained using a dedicated sharp reconstruction kernel. Material decomposition of multienergy PCD-CT images allowed the quantification of contrast levels in the joint as an indicator of joint disease. Cartilage loss in the MIA-injected knees was evaluated using PCD-CT, and the image based findings were further confirmed using histology and gross examination of the excised knees.
ACKNOWLEDGMENTS
The authors thank Jill Anderson and Amy Benike for their support in the animal study, and Robert Brown and James Herrick for specimen histology. The authors acknowledge Mayo Clinic’s X-ray Imaging Core, and Biomaterials and Histomorphometry Core for supplies and services for this study.
Conflicts of interest and source of funding: This study was funded by the Imaging Biomarker Discovery Program, Mayo Clinic-Center for Individualized Medicine, and partially supported by the National Institutes of Health under award numbers R01 EB016966 and C06 RR018898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The device described is a research scanner and not commercially available. Dr McCollough receives industry funding from Siemens Healthcare. For the remaining authors, none were declared.
REFERENCES
- 1.Arthritis Foundation. Arthritis by the Numbers. Atlanta, GA: Arthritis Foundation; 2019. [Google Scholar]
- 2.Teeple E, Jay GD, Elsaid KA, et al. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Art Ther. 2012;2012:764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon JS, Won SJ, Kim SJ, et al. Phase-contrast radiography enables detection of early changes in articular cartilage in a mouse model of osteoarthritis. Am J Phys Med Rehabil. 2015;94:644–648. [DOI] [PubMed] [Google Scholar]
- 5.Hirvasniemi J, Kulmala KA, Lammentausta E, et al. In vivo comparison of delayed gadolinium-enhanced MRI of cartilage and delayed quantitative CT arthrography in imaging of articular cartilage. Osteoarthr Cartil. 2013;21: 434–442. [DOI] [PubMed] [Google Scholar]
- 6.Kalke RJ, Di Primio GA, Schweitzer ME. MR and CT arthrography of the knee. Semin Musculoskelet Radiol. 2012;16:57–68. [DOI] [PubMed] [Google Scholar]
- 7.Lefevre N, Naouri JF, Herman S, et al. A current review of the meniscus imaging: proposition of a useful tool for its radiologic analysis. Radiol Res Pract. 2016; 2016:8329296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guggenberger R, Winklhofer S, Spiczak JV, et al. In vitro high-resolution flat panel computed tomographic arthrography for artificial cartilage defect detection: comparison with multidetector computed tomography. Invest Radiol. 2013;48: 614–621. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo M, Bertellini A, Pogliacomi F, et al. Multidetector computed tomography arthrography of the knee: diagnostic accuracy and indications. Eur J Radiol. 2009;70:342–351. [DOI] [PubMed] [Google Scholar]
- 10.Flohr TG, Stierstorfer K, Suss C, et al. Novel ultrahigh resolution data acquisition and image reconstruction for multi-detector row CT. Med Phys. 2007;34:1712–1723. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman AS, Koskinen SK, Lindblom M, et al. Diagnostic accuracy of dual-energy CT for detection of bone marrow lesions in the subacutely injured knee with MRI as reference method. Acta Radiol. 2019;284185119877343. [DOI] [PubMed] [Google Scholar]
- 12.Frellesen C, Azadegan M, Martin SS, et al. Dual-energy computed tomography based display of bone marrow edema in incidental vertebral compression fractures: diagnostic accuracy and characterization in oncological patients undergoing routine staging computed tomography. Invest Radiol. 2018;53:409–416. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Zhang G, Shi L, et al. Axial spondyloarthritis: dual-energy virtual noncalcium CT in the detection of bone marrow edema in the sacroiliac joints. Radiology. 2019;290:157–164. [DOI] [PubMed] [Google Scholar]
- 14.Lell MM, Kachelrieß M. Recent and upcoming technological developments in computed tomography: high speed, low dose, deep learning, multienergy. Invest Radiol. 2020;55:8–19. [DOI] [PubMed] [Google Scholar]
- 15.Leng S, Bruesewitz M, Tao S, et al. Photon-counting detector CT: system design and clinical applications of an emerging technology. Radiographics. 2019;39:729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willemink MJ, Persson M, Pourmorteza A, et al. Photon-counting CT: technical principles and clinical prospects. Radiology. 2018;289:293–312. [DOI] [PubMed] [Google Scholar]
- 17.Leng S, Rajendran K, Gong H, et al. 150-mum spatial resolution using photon counting detector computed tomography technology: technical performance and first patient images. Invest Radiol. 2018;53:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng S, Yu Z, Halaweish A, et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J Med Imaging (Bellingham). 2016;3:043504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao S, Rajendran K, McCollough CH, et al. Material decomposition with prior knowledge aware iterative denoising (MD-PKAID). Phys Med Biol. 2018;63:195003. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Bartlett DJ, Diehn FE, et al. Reduction of metal artifacts and improvement in dose efficiency using photon-counting detector computed tomography and tin filtration. Invest Radiol. 2019;54:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendran K, Voss BA, Zhou W, et al. Dose reduction for sinus and temporal bone imaging using photon-counting detector CT with an additional tin filter. Invest Radiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannil M, Hickethier T, von Spiczak J, et al. Photon-counting CT: high-resolution imaging of coronary stents. Invest Radiol. 2018;53:143–149. [DOI] [PubMed] [Google Scholar]
- 23.Pourmorteza A, Symons R, Sandfort V, et al. Abdominal imaging with contrastenhanced photon-counting CT: first human experience. Radiology. 2016;279:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symons R, Reich DS, Bagheri M, et al. Photon-counting computed tomographyfor vascular imaging of the head and neck: first in vivo human results. Invest Radiol. 2018;23:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratke G, Hickethier T, Bar-Ness D, et al. Spectral photon-counting computed tomography for coronary stent imaging: evaluation of the potential clinical impact for the delineation of in-stent restenosis. Invest Radiol. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett DJ, Koo CW, Bartholmai BJ, et al. High-resolution chest computed tomography imaging of the lungs: impact of 1024 matrix reconstruction and photon-counting detector computed tomography. Invest Radiol. 2019;54:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symons R, Cork TE, Sahbaee P, et al. Low-dose lung cancer screening with photon-counting CT: a feasibility study. Phys Med Biol. 2016;62:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci. 2006;103:19255–19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger MD, Murthy NS, Kanwar R, et al. Clinical magnetic resonance-enabled characterization of mono-iodoacetate-induced osteoarthritis in a large animal species. PLoS One. 2018;13:e0201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Leng S, Jorgensen SM, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol. 2016;61:1572–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao S, Rajendran K, Zhou W, et al. Noise reduction in CT image using prior knowledge aware iterative denoising. AAPM: Medical Physics (Wiley). 2019;E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam J, Perera P, Liu J, et al. Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate induced arthritis. PLoS One. 2011;6:e24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutjahr R, Halaweish AF, Yu Z, et al. Human imaging with photon counting based computed tomography at clinical dose levels: contrast-to-noise ratio and cadaver studies. Invest Radiol. 2016;51:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajendran K, Lobker C, Schon BS, et al. Quantitative imaging of excised osteoarthritic cartilage using spectral CT. Eur Radiol. 2017;27:384–392. [DOI] [PubMed] [Google Scholar]
- 35.Rajendran K, Tao S, Benike A, et al. Quantitative cartilage imaging using spectral photon-counting detector based computed tomography: SPIE; 2019.


