Abstract
Background
lncRNA HCP5 plays a cancer-promoting role in a variety of cancers. This study was the first to explore the mechanism of HCP5 in gastric carcinoma (GC).
Material/Methods
The differences in HCP5 between GC patients and healthy people were revealed in the TCGA database. The expression of HCP5 in GC tissues and adjacent tissues was compared by qRT-PCR. At the same time, the clinic pathological features of the patients were counted. Starbase and luciferase assay predicted and verified that miR-27b-3p is a targeted miRNA for HCP5. The expression of HCP5 and miR-27b-3p in various GC cells was detected by qRT-PCR. Cell viability and metastasis in different treatment groups were assessed by use of Cell Couting Kit-8 assay and clone formation assay, wound-healing assay, and transwell assay. Finally, expression of epithelial-mesenchymal transition (EMT)-associated markers was detected by Western blot.
Results
We found that HCP5 was overexpressed in GC tissues. Patients with higher expression of HCP5 had larger tumors, were more likely to have lymph node metastasis, and had higher TNM stage. HCP5 was overexpressed in GC cells, but this was reversed by miR-27b-3p. Silencing HCP5 inhibited GC cell viability and metastasis by downregulating Vimentin and N-cadherin and up-regulating E-cadherin, but this effect was partially reversed by miR-27b-3p inhibitor.
Conclusions
The effect of silencing HCP5 on repressing GC cells viability and metastasis by regulating EMT-associated markers can be partially reversed by miR-27b-3p inhibitor.
MeSH Keywords: MicroRNAs; RNA, Long Noncoding; Stomach Neoplasms
Background
Gastric carcinoma is a malignant tumor originating from the gastric mucosal epithelium [1]. It is one of the most common malignant tumors in the digestive tract and poses a serious threat to human health. According to the statistics of the International Agency for Research on Cancer (IARC), in 2012 alone there were about 951 000 new cases of gastric carcinoma in the world, of which about 723 000 died of gastric carcinoma, ranking third among global cancer mortality rates [2]. In the statistics released by the Global Cancer Database (GLOBOCAN) in 2018, the number of deaths from gastric carcinoma rose to 786 200 [3]. Epidemiological studies have proved that gastric carcinoma is highly prevalent in developing countries, especially in Asia [4]. Every year there are about 221 000 deaths due to gastric carcinoma in China, nearly half of the world’s gastric cancer deaths [5]. Early diagnosis of gastric carcinoma is difficult due to the lack of obvious symptoms. Diagnosis is performed relying on clinical manifestations combined with endoscopy, B-ultrasound, CT, and other examinations, and exfoliative cytology is another relatively common diagnostic method [6,7]. However, these methods have certain defects, and many patients have missed the best treatment opportunity by the time they are diagnosed. At present, the treatment of gastric carcinoma is mainly by surgical resection and radiotherapy and chemotherapy, but due to late diagnosis, recurrence, and a high metastasis rate, the 5-year survival rate of gastric carcinoma is only 20% [8,9]. Therefore, further research on the pathogenesis of gastric carcinoma is needed to improving the early diagnosis rate, and finding the factors affecting tumor metastasis and recurrence are key to improving early diagnosis and effectively treating gastric carcinoma.
Gastric carcinoma is a complex pathological process in which multiple risk factors induce multiple biological changes in genes and, ultimately, pathogenesis [10]. These include environmental factors such as Helicobacter pylori, EB virus infection, and unhealthy lifestyles, as well as epigenetic changes that have received increasing attention in recent years [11,12]. Epigenetics refers to changes in gene expression levels caused by changes in non-gene sequences, such as DNA methylation, chromatin conformational changes, histone modifications, and transcriptional activities [13]. Non-coding RNA plays a key role in this process. Non-coding RNAs are small RNAs that do not encode proteins, but have transcriptional functions. Long-chain non-coding RNA (lncRNA) is one of these.
lncRNAs are longer than 200 nt and are located in the nucleus or cytoplasm [14]. lncRNA itself has no coding ability on proteins, and the major RNA forms play a role in epigenetic regulation, transcription, and translation. lncRNA is powerful and participates in many important regulatory processes in the human body, such as interfering with the cleavage mode of mRNA, mediating chromatin remodeling, regulating histone modification, and acting as a precursor of small RNA [14,15]. Many experimental studies have shown that lncRNA plays a key role in many tumor diseases such as breast cancer, liver cancer, cervical cancer, and colon cancer. There are more and more studies on lncRNA in gastric carcinoma [16,17]. Wang et al. [18] proved that lncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric carcinoma cells by repressing P15 and P16 expression. Huang et al. [19] found that lncRNA AK023391 expression in gastric carcinoma tissues and cells was significantly upregulated and this was positively correlated with poor survival rates among gastric carcinoma patients. Li reported that MIR22HG can be repressed by attenuating NOTCH2 signaling [20].
lncRNA HCP5 is located between the MICA and MICB genes in the major histocompatibility complex (MHC) class I region [21]. Scientists have discovered that HCP5 is a regulatory lncRNA that is involved in adaptive and innate immune responses in humans and is associated with a variety of autoimmune diseases (e.g., psoriasis) and cancer (e.g., lung adenocarcinoma and cervical cancer) [22–24]. However, there is no published study on the role of lncRNA HCP5 in gastric carcinoma. With the aim of finding a new molecular therapeutic target for gastric carcinoma prevention and treatment, the present study explored the expression of lncRNA HCP5 in gastric carcinoma tissues and assessed its effect on proliferation and metastasis of gastric carcinoma cells.
Material and Methods
Ethics statement
Our clinical research was approved by the Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University (Approval No.: 2016027XHK). All patients signed informed consent and agreed to use of their gastric carcinoma tissue and adjacent tissue samples for clinical studies.
Gastric carcinoma tissue sample collection
We collected gastric carcinoma tissue and adjacent tissues (at least 5 cm away from tumor tissue) from 84 patients from July 2016 to December 2018 at the Affiliated Yantai Yuhuangding Hospital of Qingdao University. All cases were pathologically confirmed and patients did not undergo other treatments such as radiation. The tissue excised during the operation was washed with physiological saline and immediately placed in liquid nitrogen. The tissue was transferred to a −80°C freezer for cryopreservation.
TCGA database
The Cancer Genome Atlas (TCGA) database is a human genome project co-sponsored by the National Cancer Institute and the American Human Genome Research Institute. It contains 34 cancers with multi-omics sequencing data for more than 11 000 samples. The differential expression of lncRNA HCP5 in gastric carcinoma patients and healthy people (375 cases of cancer, 32 cases of normal samples) was assessed using the TCGA database (http://gdc-portal.nci.nih.gov/projects/TCGA-STAD).
Cell culture
Normal gastric mucosal cells GES-1 (CC-Y1572) and gastric carcinoma cells SGC-7901 (CC-Y1456), BGC-823 (CC-Y1071), MKN-45 (CC-Y1358), and HGC27 (CC-Y1228) were purchased from EK-Bioscience (China). All cells were identified by STR. GES-1 and HGC27 needed to be cultured in DMEM (11965118, Life Technologies, USA) containing 10% fetal bovine serum (FBS, C0251, Beyotime, China), while SGC-7901, MKN-45, and BGC-823 cells were cultured in RPMI-1640 medium (SH30809.01, HyClone, USA) containing 10% fetal bovine serum. The above cells were cultured in a Thermo Scientific CO2 cell culture incubator containing 5% CO2 at 37°C.
Starbase database
The Starbase database (http://starbase.sysu.edu.cn/) is a powerful database for studying non-coding RNAs such as lncRNA/circRNA/microRNA. It can find non-coding RNAs based on microRNAs (miRNAs) such as lncRNA, mRNA targets based on miRNAs, ceRNA regulatory molecules, and RNA-binding proteins. We retrieved the targeted miRNAs of lncRNA HCP5 from this database.
RNA interference plasmid construction and cell transfection
We needed to construct the small interfering RNA vector of HCP5 (siHCP5) using shRNA plasmid vector (shrna0001, RIBOBIO, China) after amplification of the target fragment HCP5. The following is the amplification sequence of HCP5: HCP5 forward, 5′-CCGCTGGTCTCTGGACACATACT-3′, and reverse, 5′-CTCACCTGTCGTGGGATTTTGC-3′. After completion of the amplification, the HCP5 fragment was ligated to the shRNA plasmid vector. miR-27b-3p inhibitor (4464084) specifically targets miR-27b-3p and was purchased directly from ThermoFisher (USA). We transfected cells with FuGENE® HD Transfection Reagent (E231A, Promega, USA). BGC-823 cells were digested with trypsin (0.25%, P5255, Abnova, USA) prior to transfection. After 24 h, cancer cells were evenly spread over the bottom of the 6-well plate and the remaining trypsin was aspirated. Next, 5 μl of FuGENE® HD, 2 μg of siHCP5 (or miR-27b-3p inhibitor), and 93 μl of RPMI-1640 medium (without serum) were added to each well. The above mixture was slowly mixed and allowed to stand at room temperature for 15 min. The 6-well plates were then placed in an incubator (37°C, 5% CO2). Transfection efficiency was measured after 24 h.
Luciferase assay
The luciferase assay calculates the ratio of firefly luciferase and Renilla luciferase in different reporter plasmids to determine the ratio of the target gene. We constructed HCP5 wild-type (HCP5-wt) and HCP5 mutant (HCP5-mut) reporter plasmids using the pmirGLO Dual-Luciferase miRNA Target Expression Vector (E1330, Promega, USA). BGC-823 cells were first digested, and RPMI-1640 medium was added to prepare a cell suspension. The cell suspension was added to a 6-well plate at a concentration of 5×105 cells/well and placed in an incubator. After 24 h, 50 ng of the reporter plasmid and 100 pmol of miR-27b-3p inhibitor were added to the cells and transfected for 48 h, after which the cells were washed. We added 500 μl of PLB lysis buffer (1168-019, Invitrogen, USA) to a 6-well plate for 15 min at room temperature. Then, 100 μl of Luciferase Assay Reagent II (LAR II) and 20 μl of sample lysate were added in the plate. We added 100 μl of Stop&Glo® Reagent after detection of firefly luciferase activity using a GloMax® 20/20 Luminometer (E5311, Promega, USA). Finally, we tested the Renilla luciferase activity.
Extraction of total RNA and quantitative real-time-polymerase chain reaction (qRT-PCR)
Total RNA in tissues and cells was extracted using Trizol extraction. First, 100 mg was taken from frozen gastric carcinoma tissue (adjacent tissue). The tissue was minced and 1 ml of Trizol reagent (15596026, Invitrogen, USA) was added. The tissue was then fully ground and the supernatant was aspirated with a pipette. Total RNA in the supernatant was obtained after adding chloroform (40064961, HUSHI, China) and isopropanol (I119459-1L, Aladdin, China). The total RNA extracted from the cells was the same except that the cells did not require grinding. After extracting total RNA, we detected the concentration using NanoDrop2000 (YQ1633128263, ThermoFisher, USA). Next, the total RNA was reverse-transcribed into a complementary Deoxyribonucleic acid (cDNA): 1622 Revert Aid First-Strand cDNA Synthesis Kit (ThermoFisher, USA) reversed transcription of HCP5 and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal reference); miR-27b-3p and U6 (internal reference) required the All-in-One™ miRNA First-Strand cDNA Synthesis Kit (AMRT-0050, GeneCopoeia, USA) and TaqMan® Universal PCR Master Mix (4304440, ABI, USA). The resulting cDNA was diluted 10-fold with DEPC water and used. The purchased primers (Sangon Biotech, China) were thoroughly diluted according to the instructions. The following reaction system was added to a 96-well plate (protected from light): 2 μl cDNA, 6 μl DEPC water, 2 μl primer, and 10 μl SYBR reagent (4913850001, Roche, Switzerland). After the above liquid was thoroughly mixed, the 96-well plate was placed in a Veriti™ 96-Well Fast Thermal Cycler (4375305, ThermoFisher, USA). The amplification conditions of the PCR were set as follows: pre-denaturation at 95°C (10 min), denaturation at 95°C (15 s), and annealing at 61°C (1 min) for 40 cycles. The amount of gene mRNA expression was calculated with the 2−ΔΔCT method [25]. The primer sequences used in this experiment were:
-
HCP5 forward, 5′-CCGCTGGTCTCTGGACACATACT-3′, and
reverse, 5′-CTCACCTGTCGTGGGATTTTGC-3′;
-
GAPDH forward, 5′-TGTGGGCATCAATGGATTTGG-3′, and
reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′;
-
miR-27b-3p forward, 5′-TGCGTCGTATCCAGTGCAAT-3′, and
reverse, 5′-GTCGTATCCAGTGCGTGTCG-3′;
-
U6 forward, 5′-TCTGCTCCTATCCCAATTACCTG-3′, and
reverse, 3′-ACTCCCGGATCTCTTCTAAGTTG-3′.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed by adding CCK-8 reagent to the cells. We first inoculated BGC-823 gastric carcinoma cells cultured in logarithmic growth phase into 96-well plates (100 μl, 5×103 cells/well). After 48 h of incubation, 10 μl of CCK-8 reagent (YZ-CK04-500T, Solarbio, China) was added to 96-well plates and mixed. After incubation for 24 h at 37°C, the optical density (OD) of the cells was assessed using a Multiskan™ FC plate reader (24072800, ThermoFisher, USA). The detection wavelength was set to 450 nm. Absorbance was measured after 48 h of incubation.
Wound-healing assay
The wound-healing assay is currently used to observe cell migration. Compared with the traditional scratch test, it reduces the uncontrollable factors and the consistency is higher. The cells to be tested were first digested. A culture tube caps with insert (SLW4503/20-20EA, Sigma, USA) were placed in the middle of each well of a 6-well plate. The pretreated cells were added to the Culture-Insert. After the cells were observed under the microscope, the culture tube caps with insert were removed with forceps and the medium was added. Cell migration was observed and counted at 0 and 24 h.
Transwell assay
Transwell assay uses the transwell chamber to detect the invasive ability of cancer cells. We selected a transwell chamber (BD Biosciences, USA) with a diameter of 8 μm for detection. The BGC-823 cells were starved for 12 h. The cells were awaiting detection after addition of RPMI-1640 containing 0.1% FBS. After placing an 8-μm transwell chamber in the culture plate, 10% FBS RPMI-1640 was added. The upper and lower chambers were then separated by a polycarbonate membrane (8 μm). Finally, the cells were inoculated in the upper chamber of matrigel mixed gel (356231, BD Biosciences, USA). After 48 h, the cells were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. Finally, the invasion was observed with a microscope and the number of cell transfers was counted.
Colony formation assay
Colony formation assay is a method for determining the ability of cells to proliferate. The rate of clone formation reflects 2 important traits of cell-dependent and proliferation ability. Generally, the formation rate of primary cultured cells is weak, and the passage cell line is strong; the formation rate of normal cell clones is weak, and the tumor cells are strong. Gastric carcinoma cells in logarithmic growth phase were pretreated to prepare a cell suspension. The concentration of the cell suspension was adjusted to 100/ml, then 10 ml of the cell suspension was added to the culture dish, and the cells were gently shaken on the stage to sufficiently disperse the cells. The culture dish was then placed in a cell culture incubator (37°C, 5% CO2). Two weeks later, the supernatant in the petri dish was discarded, then the gastric carcinoma cells were washed. The cells were fixed with 5 ml of methanol for 15 min. After the solution was poured off, Giemsa stain (32884-250ml, Sigma, USA) was added to the petri dish. After 20 min, the staining solution was washed with distilled water and dried at room temperature. Finally, the cells were observed under a microscope and counted.
Western blot analysis
Western blot analysis can be used to analyze protein expression in different treatment groups. Therefore, we first extracted proteins from the various groups of cells, then 100 μl of cell lysate was added to the pretreated cells and mixed well. The supernatant obtained after centrifugation of the cells at 4°C for 10 min at 1600 g was the protein stock we needed. The protein stock concentration was determined using BCA. We transferred 100 μg of the protein separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to a prepared NC membrane (HATF00010, Merck Millipore, Germany). After the NC membrane was washed 3 times with 1×TBST, the corresponding position was cut against the molecular weight of the primary antibody. The NC membrane was then blocked with a 5% blocking solution. After 2 h, the blocking solution was washed off with 1×TBST. The primary antibodies (Vimentin, ab92547, 54 kDa, 1: 1000; N-cadherin, ab18203, 130kD, 1: 1000; E-cadherin, ab40772, 97kD, 1: 10000; GAPDH, ab181602, 36KD, 1: 10000) were then fully covered with the corresponding NC membrane and incubated for 24 h at 4°C. After the incubation, the membrane was washed with 1×TBST. The Anti-Rabbit secondary antibody (ab6721, 1: 10000) and each NC membrane were then incubated for 1 h at room temperature. The membrane was washed again with 1×TBST. Each NC membrane was dropped with 1 ml of ECL luminescent liquid (WBKLS0100, Merck Millipore, Germany) and placed in a GelDoc™ XR+ imaging system (BIO-RAD, USA) for detection. The antibodies used in Western blot analysis were purchased from Abcam, UK. GAPDH was used as an internal reference.
Statistical analysis
The experimental data in this study were all statistically analyzed using Statistical Product and Service Solutions software (SPSS 22.0, USA). In the normally distributed data, the differences between the 2 groups were compared using the two-tailed t test; differences between more than 2 groups were compared using one-way analysis of variance (ANOVA). If the data were not normally distributed, a nonparametric test (Mann-Whitney) was used. P<0.05 was considered to be statistically significant.
Results
Overexpressed lncRNA HCP5 in gastric carcinoma tissues is associated with tumor size, lymph node metastasis, and TNM staging
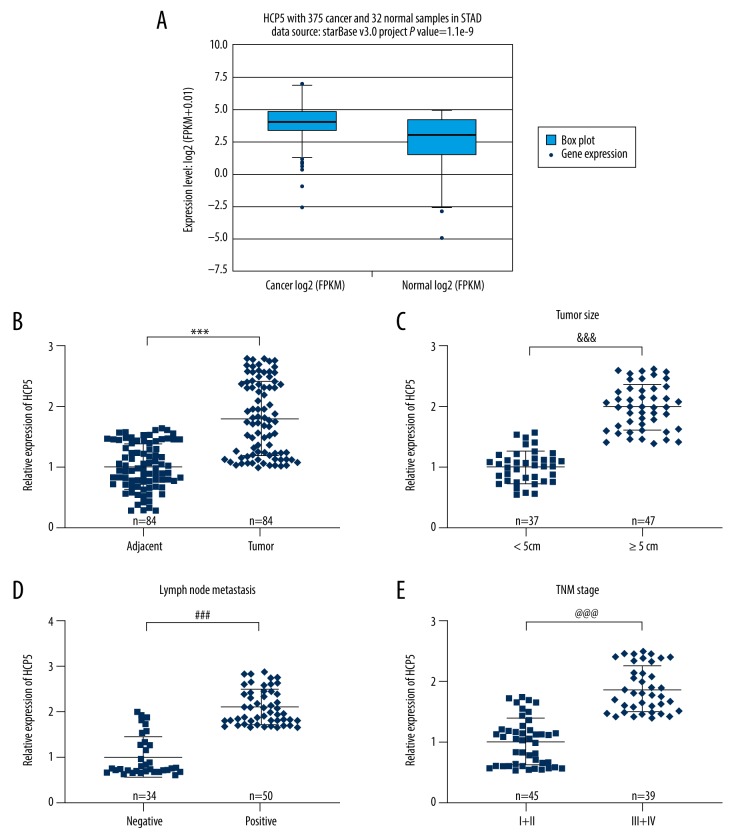
We first compared the expression of lncRNA HCP5 in gastric carcinoma patients and normal people through the TCGA database (Figure 1A). The expression of HCP5 in gastric carcinoma samples was markedly higher (P value=1.1e-9). We then assessed the expression of HCP5 in gastric carcinoma tissues and adjacent tissues by qRT-PCR (Figure 1B). The content of HCP5 in gastric carcinoma tissues was obviously higher than in adjacent tissues (p<0.001). By observing the clinicopathological characteristics of patients (Table 1), we found that HCP5 was highly expressed in patients with large tumors (≥5 cm) and lymphocytic metastasis (Figure 1C, 1D, p<0.001) The expression level in patients with stage III+IV was also higher than in those with stage I+II (Figure 1E, p<0.001). This part of the experimental results revealed that lncRNA HCP5 was overexpressed in gastric carcinoma tissues and was elevated in patients with large tumors, lymph node metastasis, or stage III+IV gastric carcinoma.
Figure 1.
Overexpressed lncRNA HCP5 in gastric carcinoma tissues is associated with tumor size, lymph node metastasis, and TNM staging. (A) TCGA database compared the expression difference of lncRNA HCP5 in gastric carcinoma patients and normal people. (B) QRT-PCR was used to detect the expression of lncRNA HCP5 in gastric carcinoma tissues and adjacent tissues (n=84). (C) Correlation between tumor size and lncRNA HCP5 expression was analyzed. (D) Correlation between lymph node metastasis and lncRNA HCP5 expression was analyzed. (E) Correlation between TNM stage and lncRNA HCP5 expression was analyzed. All experiments were repeated 3 times and results were averaged. *** p<0.001 vs. Adjacent tissue; &&& p<0.001 vs. <5 cm; ### p<0.001 vs. Negative Control; @@@ p<0.001 vs. stage I+II.
Table 1.
Statistical table of clinicopathological characteristics of patients with gastric cancer.
| Clinicopathologic feature | Case number | HCP5 expression | P Value | |
|---|---|---|---|---|
| Low (n=33) | High (n=51) | |||
| Age (year) | ||||
| <60 | 36 | 15 | 21 | 0.699 |
| ≥60 | 48 | 18 | 30 | |
| Sex | ||||
| Male | 49 | 21 | 28 | 0.428 |
| Female | 35 | 12 | 23 | |
| Tumor size (cm) | ||||
| <5 | 37 | 18 | 14 | 0.013 |
| ≥5 | 47 | 15 | 37 | |
| Lymph node metastasis | ||||
| Negative | 34 | 20 | 14 | 0.002 |
| Positive | 50 | 13 | 37 | |
| TNM stage | ||||
| I+II | 45 | 24 | 21 | 0.005 |
| III+IV | 39 | 9 | 30 | |
miR-27b-3p is a targeted miRNA of lncRNA HCP5
The Starbase database helped us to retrieve miR-27b-3p, a targeted miRNA for HCP5 (Figure 2A). Luciferase assay (Figure 2B) showed that the fluorescence activity of the HCP5-wt reporter plasmid transfected with miR-27b-3p mimic was significantly lower than that of the negative control group (p<0.001), demonstrating that miR-27b-3p is a targeted miRNA for lncRNA HCP5.
Figure 2.
miR-27b-3p is the targeted miRNA of lncRNA HCP5. (A) Targeted miRNAs for HCP5 were predicted by Starbase. (B) Luciferase assay was used to validate the targeted miRNAs of HCP5. All experiments were repeated 3 times and results were averaged. *** p<0.001 vs. miR-Negative Control (miR-NC).
lncRNA HCP5 is overexpressed in gastric carcinoma cells, but miR-27b-3p is underexpressed
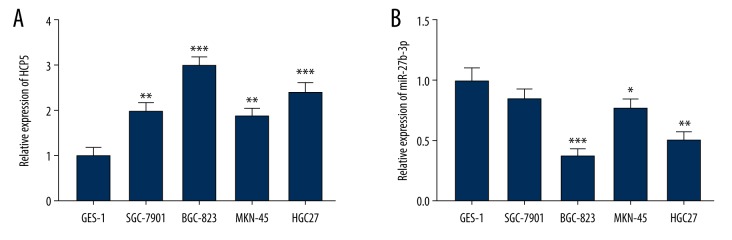
In addition to gastric carcinoma tissues, we also examined mRNA levels of HCP5 and miR-27b-3p in gastric carcinoma cells. As shown in Figure 3, the expression levels of HCP5 in various gastric carcinoma cells were clearly increased; on the contrary, the content of miR-27b-3p in gastric carcinoma cells was downregulated to varying degrees. Based on this set of results, we selected the BGC-823 cells with the highest expression of HCP5 for the next experiment.
Figure 3.
lncRNA HCP5 is overexpressed in gastric carcinoma cells, but miR-27b-3p is underexpressed. (A) QRT-PCR was used to detect the expression of HCP5 in normal gastric mucosal cells (GES-1) and gastric carcinoma cells. GAPDH was used as an internal reference (B) QRT-PCR was used to detect the expression of miR-27b-3p in normal gastric mucosal cells (GES-1) and gastric carcinoma cells. U6 was used as an internal reference. All experiments were repeated 3 times and results were averaged. * p<0.05, ** p<0.01, *** p<0.001 vs. GES-1.
miR-27b-3p inhibitor partially reverses the effect of silencing HCP5 on cell viability and metastasis by regulating epithelial-mesenchymal transition (EMT)-associated markers
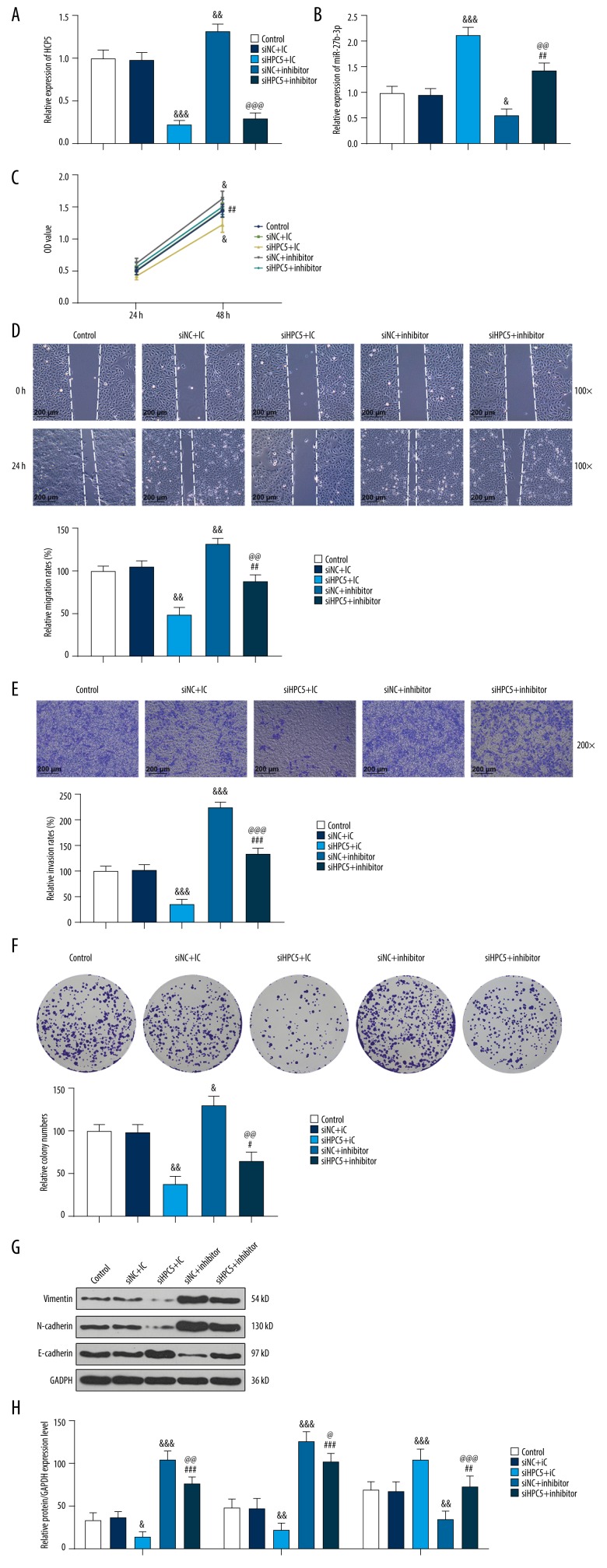
After transfection of BGC-823 cells, expression of HCP5 was obviously inhibited in cells silencing HCP5 (siHCP5, Figure 4A, p<0.001), and silencing HCP5 significantly increased miR-27b-3p expression (Figure 4B, p<0.001). However, miR-27b-3p inhibitor downregulated the expression of miR-27b-3p (Figure 4B, p<0.05). Then, we examined the cell viability, proliferation, migration, and invasion abilities of cells in the various treatment groups. Both the CCK-8 assay (Figure 4C) and the colony-formation assay (Figure 4F) showed that the cell viability and proliferation abilities of gastric carcinoma cells were weakened after silencing HCP5. However, miR-27b-3p inhibitor partially reversed this effect, enhancing cell viability and proliferation. In the assessment of metastatic ability (Figure 4D, 4E), siHCP5 greatly inhibited the migration and invasion abilities of cancer cells. This effect was also partially reversed by the miR-27b-3p inhibitor. In view of the above experimental results, we analyzed EMT-associated markers by Western blot (Figure 4G, 4H), finding that siHCP5 downregulated Vimentin and N-cadherin, and upregulated the E-cadherin gene; and treatment with the miR-27b-3p inhibitor partially reversed this effect. These results indicated that silencing HCP5 inhibited the viability and metastasis of gastric carcinoma cells by regulating EMT-associated markers, and this effect was partially reversed by miR-27b-3p inhibitor.
Figure 4.
miR-27b-3p inhibitor can partially reverse the effect of silencing HCP5 on cell viability and metastasis by modulating epithelial-mesenchymal transition (EMT)-associated markers. (A, B) Cell transfection efficiency was measured by qRT-PCR. GAPDH and U6 were used as internal controls. (C) Cell viability was detected by the Cell Couting Kit-8 assay. (D) Wound-healing assay was used to observe cell migration. (E) Transwell assay was used to observe cell invasion. (F) Colony formation assay was used to observe cell proliferation. (G, H) Western blot analysis was used to assess the expression of EMT-associated markers. GAPDH was used as an internal reference. All experiments were repeated 3 times and results were averaged. & p<0.05, && p<0.01, &&& p<0.001 vs. Silent Negative Control (siNC)+miR-27b-3p inhibitor Control (IC); # p<0.05, ## p<0.01, ### p<0.001 vs. siHCP5+IC; @ p<0.05, @@ p<0.01, @@@ p<0.001 vs. siNC+miR-27b-3p inhibitor (inhibitor).
Discussion
Epithelial-mesenchymal transition (EMT) refers to the process of transforming stable epithelial cells with polarity into embryonic mesenchymal cells in conditions of embryonic development or pathology [26]. In this process, the phenotype and gene expression profile of epithelial cells are changed. Epithelial cells are attached cells that form a tight junction with adjacent cells [27] and thus lack the ability to migrate. Conversely, stromal cells that lose cell polarity lack mutual connectivity, so they can freely shuttle through the cell matrix [27]. The EMT process involves several steps: when induced by EMT-associated markers, the epithelial cell junction is destroyed and the polarity disappears, then the cytoskeleton changes and the basement membrane is digested by proteolytic enzymes, causing the cells to invade the substrate and metastasize [28,29]. EMT not only participates in normal physiological processes such as embryonic growth and development, but also has pivotal effects in the development of tumors. Scientists have found that tumor cells have enhanced ability to move and migrate through the EMT process and promote tumor invasion [30,31]. Therefore, exploring the mechanism of EMT is important for blocking tumors from metastatic pathways.
Through the continuous exploration of scientists, various cytokines have been found to be involved in the EMT process, such as cell surface markers, cytoskeletal markers, extracellular proteins, and transcription factors. Cadherin is a transmembrane glycoprotein whose main function is to mediate cell junctions, participate in cell transformation, and inhibit cell migration [32]. Cadherin is divided into 3 types: E-cadherin, P-cadherin, and N-cadherin. E-cadherin is mainly expressed in epithelial cells and has the ability to maintain intercellular stability, connectivity, and cell polarity and to inhibit tumor cell invasion and migration [33]. Therefore, the expression change of E-cadherin is an important marker of EMT. In contrast to E-cadherin, N-cadherin is expressed in stromal cells and has a significant effect on tumor cell metastasis [34]. Therefore, Catalan et al. proposed that the conversion of E-cadherin to N-cadherin is one of the important mechanisms in the development of EMT [32]. Vimentin is a highly conserved type III intermediate fibrin that is expressed in mesenchymal cells and is an important cytoskeletal protein [35]. It not only maintains cell integrity, but also participates in physiological processes such as cell adhesion, migration, and apoptosis. Studies have shown that TGF-β, Wnt, and other pathways induce EMT by regulating the expression of Vimentin [36]; overexpression of Vimentin can promote the metastasis of tumor cells [37]. Thus, inhibition of Vimentin and N-cadherin expression and upregulation of the E-cadherin gene are important ways to inhibit EMT and reduce cancer cell metastasis. Our study also yielded consistent results: silencing of HCP5 significantly downregulated Vimentin and N-cadherin, while E-cadherin expression was upregulated, and miR-27b-3p can silence HCP5.
It is reported that HCP5 is associated with some types of miRNA in other cancer cells. For example, Zhao reported that HCP5 increased cell invasion and EMT via miR-140-5p/SOX4 in oral squamous cell carcinoma [38]. HCP5 is also involved in the development of gemcitabine-resistance pancreatic cancer cells via the miR-214-3p/HDGF axis [39]. In skin cutaneous melanoma cells, HCP5 can repress cell malignancy via miR-1286/RARRES3 [40]. Wang found that HCP5 can facilitate triple-negative breast cancer progression via miR-219a-5p/BIRC3 [41].
miR-27b-3p is the targeted miRNA of lncRNA HCP5 we found in the Starbase database. We also found a reciprocal association between HCP5 and miR-27b-3p, in which silencing HCP5 promoted miR-27b-3p expression. miR-27b-3p is located on chromosome 9 in humans and belongs to the miR-23b/27b/24-1 cluster [42]. Many studies have confirmed that miR-27b-3p is underexpressed in lung carcinoma cells, endometrial carcinoma cells, and breast carcinoma cells, and can inhibit the proliferation and metastasis of cancer cells [42–44]. The expression level of miR-27b-3p in plasma of patients with oral cancer is also significantly downregulated, which may become a serum test marker for oral cancer [45]. This is evidence to further demonstrate the reliability of this study: miR-27b-3p is underexpressed in gastric carcinoma cells, and can reverse the promotion of gastric carcinoma cell metastasis caused by HCP5, and plays an important role as a tumor-suppressor gene. miRNAs are non-coding single-stranded small molecules that are ubiquitous in animals and plants and are about 19–25 nt in length [46]. They inhibit transcription or translation of the target gene by binding to the 3′ non-coding region of the mRNA. It eventually affects the physiological processes of cell proliferation, differentiation, and apoptosis. Since non-coding RNA was discovered in 2000, miRNAs have been widely used in the prevention and treatment of diseases such as cardiovascular diseases and cancer [47,48]. Many reports indicate that miRNAs are closely involved in the occurrence and metastasis of gastric carcinoma and resistance to chemotherapy drugs [49,50]. miRNA has become one of the specific molecular markers of gastric carcinoma, and it has become a breakthrough in early diagnosis, molecular approach to treatment, and improvement of prognosis.
Conclusions
For the first time, we have demonstrated that the lncRNA HCP5 is highly expressed in gastric carcinoma tissues and cells. The increase in expression is closely related to the size of the tumor, whether the lymph nodes metastasize, and the severity of the disease. lncRNA HCP5 promotes EMT processes by regulating EMT-associated markers and promotes the proliferation and metastasis of cancer cells. However, this effect can be partially reversed by miR-27b-3p inhibitor. Our research provides a new basis for molecular targeted therapy of gastric carcinoma.
Footnotes
Source of support: Departmental sources
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374(9688):477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–90. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112(1):31–37. doi: 10.1002/jso.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20(38):13767–74. doi: 10.3748/wjg.v20.i38.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka M, Oyama Y, Abe A, et al. Clinical significance of an inflammation-based prognostic system for gastric cancer patients with a preoperative normal serum level of carcinoembryonic antigen. Anticancer Res. 2014;34(12):7219–26. [PubMed] [Google Scholar]
- 8.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014;15(1):23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 10.Rocken C. Molecular classification of gastric cancer. Expert Rev Mol Diagn. 2017;17(3):293–301. doi: 10.1080/14737159.2017.1286985. [DOI] [PubMed] [Google Scholar]
- 11.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu DG. Epigenetic alterations in gastric cancer (review) Mol Med Rep. 2015;12(3):3223–30. doi: 10.3892/mmr.2015.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazaly E, Charlesworth J, Dickinson JL, Holloway AF. Genetic determinants of epigenetic patterns: Providing insight into disease. Mol Med. 2015;21:400–9. doi: 10.2119/molmed.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Renganathan A, Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv Exp Med Biol. 2017;1008:199–222. doi: 10.1007/978-981-10-5203-3_7. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Chen T, Li G, et al. LncRNAs: Emerging biomarkers in gastric cancer. Future Oncol. 2015;11(17):2427–41. doi: 10.2217/fon.15.175. [DOI] [PubMed] [Google Scholar]
- 17.Hao S, Lv J, Yang Q, et al. Identification of key genes and circular RNAs in human gastric cancer. Med Sci Monit. 2019;25:2488–504. doi: 10.12659/MSM.915382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang MW, Liu J, Liu Q, et al. LncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric cancer cells by repressing the P15 and P16 expression. Eur Rev Med Pharmacol Sci. 2017;21(20):4613–22. [PubMed] [Google Scholar]
- 19.Huang Y, Zhang J, Hou L, et al. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36(1):194. doi: 10.1186/s13046-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Wang Y. Long noncoding RNA (lncRNA) MIR22HG suppresses gastric cancer progression through attenuating NOTCH2 signaling. Med Sci Monit. 2019;25:656–65. doi: 10.12659/MSM.912813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulski JK. Long noncoding RNA HCP5, a hybrid HLA Class I endogenous retroviral gene: Structure, expression, and disease associations. Cells. 2019;8(5) doi: 10.3390/cells8050480. pii: E480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4(3):e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Wang R, Fang L, et al. HCP5 is a SMAD3-responsive long non-coding RNA that promotes lung adenocarcinoma metastasis via miR-203/SNAI axis. Theranostics. 2019;9(9):2460–74. doi: 10.7150/thno.31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Shen HM, Fang DM, et al. LncRNA HCP5 promotes the development of cervical cancer by regulating MACC1 via suppression of microRNA-15a. Eur Rev Med Pharmacol Sci. 2018;22(15):4812–19. doi: 10.26355/eurrev_201808_15616. [DOI] [PubMed] [Google Scholar]
- 25.Tao J, Zhi X, Zhang X, et al. miR-27b-3p suppresses cell proliferation through targeting receptor tyrosine kinase like orphan receptor 1 in gastric cancer. J Exp Clin Cancer Res. 2015;34:139. doi: 10.1186/s13046-015-0253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 28.Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232(12):3261–72. doi: 10.1002/jcp.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front Med. 2018;12(4):361–73. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 32.Hazan RB, Qiao R, Keren R, et al. Cadherin switch in tumor progression. Ann NY Acad Sci. 2004;1014:155–63. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 33.Onder TT, Gupta PB, Mani SA, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 34.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48(5–6):463–76. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 35.Costigliola N, Ding L, Burckhardt CJ, et al. Vimentin fibers orient traction stress. Proc Natl Acad Sci USA. 2017;114(20):5195–200. doi: 10.1073/pnas.1614610114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S, Yan C, Yang X, et al. Pharmacoproteomic analysis reveals that metapristone (RU486 metabolite) intervenes E-cadherin and vimentin to realize cancer metastasis chemoprevention. Sci Rep. 2016;6:22388. doi: 10.1038/srep22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu R, Zhou Z, Yu W, et al. CPEB4 promotes cell migration and invasion via upregulating Vimentin expression in breast cancer. Biochem Biophys Res Commun. 2017;489(2):135–41. doi: 10.1016/j.bbrc.2017.05.112. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Bai X, Feng C, et al. Long non-coding RNA HCP5 facilitates cell invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by miR-140-5p/SOX4 axis. Cancer Manag Res. 2019;11:10455–62. doi: 10.2147/CMAR.S230324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Wang J, Dong L, et al. Long noncoding RNA HCP5 regulates pancreatic cancer gemcitabine (GEM) resistance by sponging Hsa-miR-214-3p to target HDGF. Onco Targets Ther. 2019;12:8207–16. doi: 10.2147/OTT.S222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Gu X, Ma M, Lou C. Long noncoding RNA HCP5 suppresses skin cutaneous melanoma development by regulating RARRES3 gene expression via sponging miR-12. Onco Targets Ther. 2019;12:6323–35. doi: 10.2147/OTT.S195796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Luan T, Zhou S, et al. LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR-219a-5p. Cancer Med. 2019;8(9):4389–403. doi: 10.1002/cam4.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Hu J, Yu T, et al. miR-27b-3p/MARCH7 regulates invasion and metastasis of endometrial cancer cells through Snail-mediated pathway. Acta Biochim Biophys Sin (Shanghai) 2019;51(5):492–500. doi: 10.1093/abbs/gmz030. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Si W, Shen J, et al. miR-27b-3p inhibits proliferation and potentially reverses multi-chemoresistance by targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis. 2018;9(2):188. doi: 10.1038/s41419-017-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Xu T, Cao YW, Ding XQ. Antitumor effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur Rev Med Pharmacol Sci. 2017;21(18):4113–23. [PubMed] [Google Scholar]
- 45.Lo WY, Wang HJ, Chiu CW, Chen SF. miR-27b-regulated TCTP as a novel plasma biomarker for oral cancer: From quantitative proteomics to post-transcriptional study. J Proteomics. 2012;77:154–66. doi: 10.1016/j.jprot.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 47.Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68(23):2577–84. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 48.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20(30):10432–39. doi: 10.3748/wjg.v20.i30.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W, Ma J, Zhou W, et al. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21(11):1063–75. doi: 10.1080/14728222.2017.1389900. [DOI] [PubMed] [Google Scholar]