The prospective REGOMA phase 2 trial showed an encouraging and significant median progression-free and overall survival (PFS, OS) benefit for glioblastoma patients at first progression treated with the oral multikinase inhibitor regorafenib compared to lomustine monotherapy.1 In particular, the OS benefit was 1.8 months compared to lomustine (7.4 vs. 5.6 months; P = .0009), and the hazard ratio was 0.5 (95% confidence interval, 0.33–0.75). Interestingly, despite unchanged MRI findings at first follow-up (“Stable Disease,” 39%) following regorafenib, complete or partial radiological responses according to the RANO criteria2 were only seen in 5% of cases treated with regorafenib. On the other hand, 59% of patients in the regorafenib arm had grade 3–4 adverse events.
In contrast, a subsequent retrospective case series with predominantly glioblastomas (19 of 24 patients) treated with regorafenib at a later stage of disease (88% of cases had two or more relapses; range, 2–7) showed a clearly lower efficacy in terms of OS (4.1 months).3 Furthermore, radiologically only either a “Stable Disease” or a “Partial Response” according to the RANO criteria after 6–12 weeks after starting regorafenib were observed (13% each).
Moreover, another retrospective case series with six progressive high-grade astrocytomas (range of relapses, 2–6) undergoing regorafenib treatment reported both a high rate of regorafenib-related adverse advents (all but one patient had grade 3 laboratory abnormalities or clinical adverse advents) and no radiological responses at follow-up (all “Progressive Disease”) according to the RANO criteria.4 In contrast, the OS in that series was 5.3 months (range, 2.4–6.7 months).
Taken together, a more objective surrogate imaging parameter for an earlier assessment of both response and nonresponse seems mandatory since regorafenib is associated with considerable treatment-related adverse advents.1,4 Additionally, monitoring of regorafenib treatment imposes specific requirements on neuroimaging which are not met by anatomical MRI, for example, the identification of pseudoresponse.
To overcome these limitations, other imaging techniques such as perfusion MRI or efforts of the immunotherapy Response Assessment in Neuro-Oncology (iRANO) Working Group may be of value. Furthermore, the PET/RANO group and others highlighted the additional clinical value of amino acid PET for response assessment of chemotherapy in patients with malignant glioma, especially when drugs with antiangiogenic properties are used.5,6 To assess whether amino acid PET using O-(2-[18F]-fluoroethyl)-L-tyrosine (FET) might be valuable for the assessment of regorafenib treatment effects, we conducted a small pilot study. A total of five patients with progressive IDH-wildtype malignant glioma (mean age, 44 ± 8 years) treated with regorafenib at an early stage of disease (median number of relapses, 1; range, 1–4) were examined using FET PET at baseline (ie, before regorafenib initiation) and 8 weeks later (ie, after two regorafenib cycles; 120–160 mg daily, 21 days on, 7 days off). Further clinical information is provided in Supplementary Table 1.
In the majority of patients (in four of five patients), FET PET provided valuable additional clinical information. Unlike contrast-enhanced MRI, FET PET was able to detect both pseudoresponse (n = 2 patients) and pseudoprogression (n = 1 patient) following regorafenib treatment (Fig. 1). Furthermore, in another patient, FET PET allowed an earlier diagnosis of tumor progression although MRI findings were unchanged during follow-up (“Stable Disease” according to RANO criteria). The therapeutic efficacy of regorafenib in our series (OS, 4.8 months) was similar to previously reported results,3,4 and three of five patients had grade 3 adverse events.
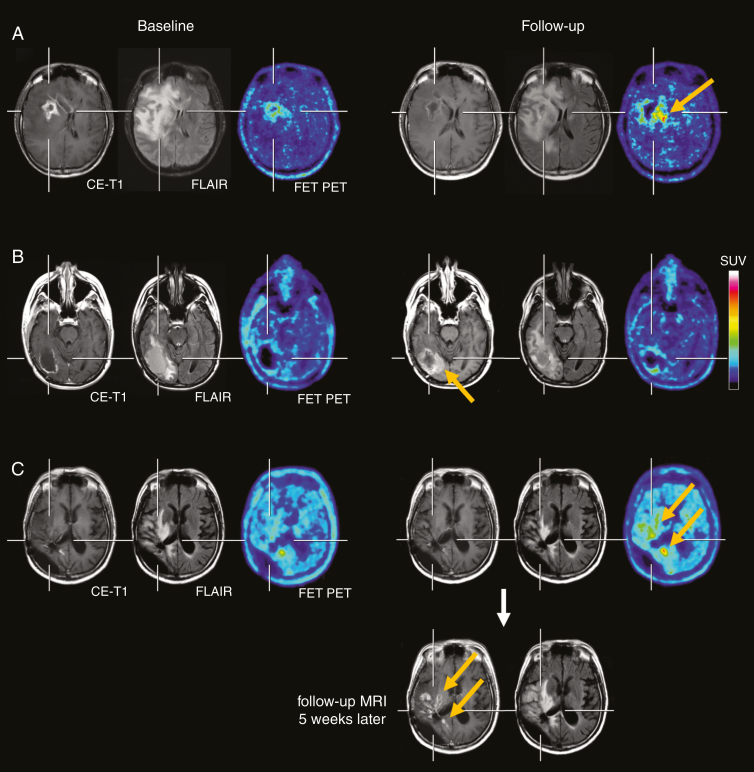
Figure 1.
(A, top row) The contrast-enhanced MRI, the FLAIR-weighted MR image and FET PET at baseline (left three images) and after two cycles of regorafenib (right three images) of a 35-year-old glioblastoma patient (IDH-wildtype). Following regorafenib, the MRI showed a “Partial Response” according to RANO criteria, whereas the FET PET depicted increased metabolic activity spatially located medial to the area with the necrotic core on MRI (arrow), indicating pseudoresponse on MRI. The patient deceased 3 months later. (B, second row) Discrepant MRI and FET PET findings in a 39-year-old glioblastoma patient (IDH-wildtype), which indicate pseudoprogression on MRI. Due to a distant tumor recurrence (right parietal, images not shown), the patient was treated with regorafenib. After two cycles of regorafenib, the MRI showed an increase of both the FLAIR signal alteration and contrast enhancement at the medial edge of the initial resection cavity (arrow, second row), whereas the FET PET revealed no pathologically increased tracer uptake. Despite a clinical stable course for the next three months, the patient died unexpectedly most probably due to a progression of the distant tumor. (C) Pathological increase of FET uptake after two cycles of regorafenib (arrows, third row), whereas the corresponding MRI is consistent with “Stable Disease” according to RANO criteria. Five weeks later, the 43-year-old patient deteriorated clinically, and the MRI showed a progression of the contrast-enhancing lesions and of the FLAIR signal (arrows, bottom row).
These initial findings suggest that FET PET allows an early diagnosis of both pseudoresponse and pseudoprogression already 8 weeks after initiation of regorafenib treatment initiation. Thus, FET PET offers great potential for clinical decision-making and justifies a more widespread use, especially when newer treatment options with potential efficacy such as regorafenib and without well-defined parameters for a response or treatment failure are being used. Further investigations assessing the value of FET PET in a more significant number of patients undergoing regorafenib treatment are warranted.
Funding
The Wilhelm-Sander Stiftung, Germany, supported this work.
Conflict of interest statement. Related to the present work, the authors disclosed no potential conflicts of interest.
Supplementary Material
References
- 1. Lombardi G, De Salvo GL, Brandes AA, et al. . Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–119. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 3. Tzaridis T, Gepfner-Tuma I, Hirsch S, et al. . Regorafenib in advanced high-grade glioma: a retrospective bicentric analysis. Neuro Oncol. 2019. doi:10.1093/neuonc/noz071 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kebir S, Rauschenbach L, Radbruch A, et al. . Regorafenib in patients with recurrent high-grade astrocytoma. J Cancer Res Clin Oncol. 2019;145(4):1037–1042. [DOI] [PubMed] [Google Scholar]
- 5. Albert NL, Weller M, Suchorska B, et al. . Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.