Abstract
Background
Oligodendroglioma is a rare primary central nervous system (CNS) tumor with highly variable outcome and for which therapy is usually not curative. At present, little is known regarding the pathways involved with progression of oligodendrogliomas or optimal biomarkers for stratifying risk. Developing new therapies for this rare cancer is especially challenging. To overcome these challenges, the neuro-oncology community must be particularly innovative, seeking multi-institutional and international collaborations, and establishing partnerships with patients and advocacy groups thereby ensuring that each patient enrolled in a study is as informative as possible.
Methods
The mission of the National Cancer Institute’s NCI-CONNECT program is to address the challenges and unmet needs in rare CNS cancer research and treatment by connecting patients, health care providers, researchers, and advocacy organizations to work in partnership. On November 19, 2018, the program convened a workshop on oligodendroglioma, one of the 12 rare CNS cancers included in its initial portfolio. The purpose of this workshop was to discuss scientific progress and regulatory challenges in oligodendroglioma research and develop a call to action to advance research and treatment for this cancer.
Results
The recommendations of the workshop include a multifaceted and interrelated approach covering: biology and preclinical models, data sharing and advanced molecular diagnosis and imaging; clinical trial design; and patient outreach and engagement.
Conclusions
The NCI-CONNECT program is well positioned to address challenges in oligodendroglioma care and research in collaboration with other stakeholders and is developing a list of action items for future initiatives.
Keywords: NCI-CONNECT, oligodendroglioma, rare CNS tumors, workshop
Key Points.
Patients with oligodendroglioma have highly variable outcome and therapy is usually not curative.
Risk stratification biomarkers and pathways involved with resistance to therapy and progression are being investigated.
The NCI-CONNECT Workshop provides a call to action to address challenges in patient care and research.
Introduction and Background
Developing therapies for rare cancers of the central nervous system (CNS) is especially formidable for several reasons. First, unlike some other diseases, including many cancers, there are few good preclinical models on which to build clinical trials. Second, the complexity of the CNS and potential for therapy-induced injury poses strategic, pragmatic, and ethical challenges. Third, there are inherent challenges in both providing care and conducting clinical research. These challenges include the potential for delays in diagnosis, lack of well-defined standards of care, limited social and advocacy support, and difficulties in conducting clinical trials because of low incidence compounded by the limited experience of health care providers, even those in academic centers with specialized neuro-oncology programs. As these cancers are extremely rare, it is difficult to recruit enough patients to statistically power a study. To overcome these multilayered challenges, the neuro-oncology community must be particularly innovative, seeking multi-institutional and international collaboration, and establishing partnerships with patients and advocacy groups thereby ensuring that each patient enrolled in a study is as informative as possible.
Responding to the Challenges
The Beau Biden Cancer Moonshot Program, part of the 21st Century Cures Act, appropriated $1.7 billion toward cancer care in 10 select areas, including patient engagement. The National Cancer Institute (NCI) was granted support from this program to focus specifically on adult rare CNS tumors through the NCI-CONNECT program, housed in the Neuro-Oncology Branch in NCI’s Center for Cancer Research. The program’s mission is to address the challenges and unmet needs in adult rare CNS cancer research and treatment by connecting patients, health care providers, researchers, and advocacy organizations to work in partnership. The program is first focusing on 12 types of rare CNS tumors, each with less than 2,000 people diagnosed a year in the United States. NCI-CONNECT has three program goals: (1) develop an infrastructure across a network of national and international sites to study select adult rare CNS tumors; (2) collect, analyze, and share information to promote discovery and improve understanding of adult rare CNS tumors; and (3) build the network to facilitate the translation of discoveries into new therapies through collaborative efforts to improve patient clinical outcomes.
As part of NCI-CONNECT, the Neuro-Oncology Branch has undertaken a large international study, the “Outcomes and Risk Study for Patients with Rare Brain and Spine Tumors,” which aims to refine the molecular classification of these rare cancers so that physicians will be able to give a more accurate prognosis to patients as well as select more appropriate treatments that have a greater likelihood of being effective in any individual tumor. It also aims to uncover risk factors that might increase a person’s chances of developing brain or spine cancer. Another study, “Evaluation of the Natural History and Specimen Banking for Patients with CNS Cancers,” was developed to better understand brain and spinal cord tumors to uncover areas for future research. NCI-CONNECT also supports several treatment trials, including investigation of the immunotherapy drug nivolumab for treating rare CNS tumors, the multitargeted kinase inhibitor sunitinib for recurrent gliosarcomas and primary CNS sarcomas, the proteasome inhibitor marizomib for recurrent ependymoma, and the antiangiogenic agent bevacizumab in combination with carboplatin for recurrent ependymoma.
Partnering with advocacy groups and patients is critical to NCI-CONNECT’s mission; to advance the understanding of rare adult CNS cancers by establishing and fostering patient–advocacy–provider partnerships and networks to improve approaches to care and treatment. This could lead to increased patient accrual to the program, care, and studies, which take place at the National Institutes of Health (NIH) Clinical Center or one of the more than 30 institutions in the Brain Tumor Trials Collaborative (BTTC) network. An important contribution of NCI-CONNECT is its convening power, that is, the ability to bring experts in rare CNS cancers together to share insights, ideas, and plans for collaboration. On November 19, 2018, NCI-CONNECT convened a workshop on oligodendroglioma, one of the 12 rare CNS cancers included in its initial portfolio. (See https://ccr.cancer.gov/neuro-oncology-branch/connect for the complete list.) This manuscript summarizes the proceedings of this workshop. Worldwide neuro-oncology experts from different disciplines and advocacy partners met to discuss the molecular pathology of oligodendrogliomas, preclinical models, clinical trial efficacy endpoints, and collaborative efforts in data sharing. Presentations by leaders in the field on important areas of scientific progress and associated challenges were followed by in-depth discussions by all participants divided in working groups. Participants recognized the need to combine data, resources, and samples to develop better treatments for patients with oligodendrogliomas. An action plan to address challenges in patient care and research was developed.
About Oligodendroglioma
Oligodendroglioma is a primary CNS tumor believed to originate from oligodendroglial precursor cells. Oligodendrogliomas are diffusely infiltrative gliomas molecularly defined by isocitrate dehydrogenase (IDH) mutation and codeletion of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q), according to the World Health Organization (WHO) 2016 classification.1 Additionally, they are historically classified into two grades, WHO grade II or low grade and grade III or anaplastic, based on their histological characteristics. Other common molecular alterations are described later in this manuscript.2,3 Oligodendrogliomas represent less than 10% of all gliomas.4 Whereas oligodendroglial tumors share common molecular features and typically have a better prognosis than astrocytic tumors, they still have highly variable clinical outcomes even within the same WHO grade (Figures 1 and 2). At present, little is known regarding the pathways involved with resistance to therapy and progression of oligodendrogliomas or optimal biomarkers for stratifying risk.
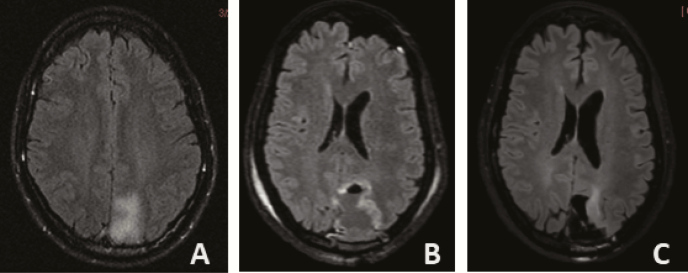
Figure 1.
Axial T2/FLAIR MRI images of patient with left occipitoparietal oligodendroglioma WHO grade 2, IDH1 mutated, 1p19q codeleted with indolent course. (A) Incidental finding at age 39, followed with serial imaging without intervention. (B) Near total resection at age 41 after slight increase in T2/FLAIR hyperintensity and development of headaches, then followed without additional treatment. (C) Slight increase in T2/FLAIR hyperintensity adjacent to the surgical cavity at age 52 (13 years from initial imaging diagnosis; no prior radiation or chemotherapy).
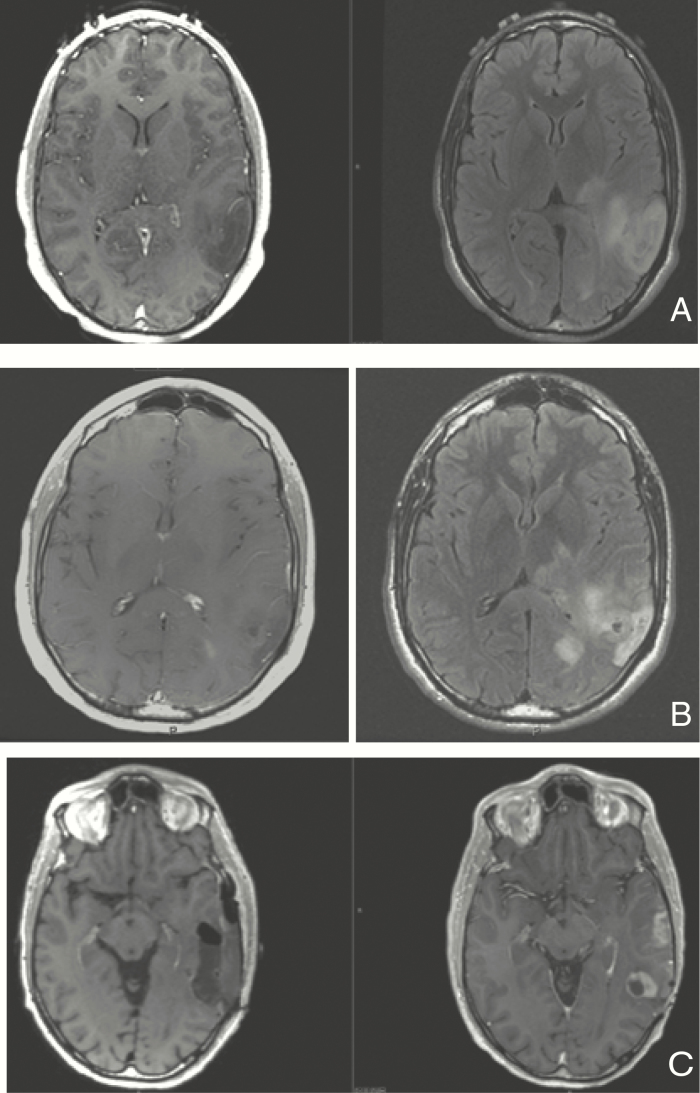
Figure 2.
Axial T1 with contrast and T2/FLAIR MRI images of patient with left posterior temporal oligodendroglioma WHO grade 2, IDH1 mutated, 1p19q codeleted with aggressive course. (A) Imaging at age 28 after presenting with seizures; underwent biopsy without further treatment. (B) Imaging at age 31 after biopsy demonstrating transformation to anaplastic oligodendroglioma. Patient had received 2 years of therapy with temozolomide due to imaging progression detected 6 months after initial diagnosis. (C) Imaging preresection (right) and postresection (left) at the time of 4th progression (age 37), after having received radiation, procarbazine and lomustine; received subsequent treatment with re-irradiation and with a PD-1 inhibitor 3 years later for new recurrence.
Oligodendrogliomas occur most often in young adults between the ages of 35 and 44 but can occur at any age. They occur slightly more often in males and are rare in children, in whom despite presenting classical histological features, the molecular characteristics differ from adults (typically IDH/TERTp/1p19q wild type), with clinical implications that are not fully understood.5,6 According to the Central Brain Tumor Registry of the United States, an estimated 11,757 people are living with this tumor in the United States. The 5-year survival rate is 74.1%.4
Symptoms of oligodendroglioma depend on the size and location of the tumor. The most common presenting symptom is a seizure, which approximately 60% of patients experience before being diagnosed.7 Other commonly occurring symptoms include headaches, memory loss and other cognitive difficulties, focal weakness and/or sensory deficits, or problems with balance and movement. The first intervention is surgery, which allows to make a tissue-based diagnosis including molecular marker assessment; further, surgery is a therapeutic measure and aims to remove as much tumor as possible without causing more neurological deficits. Current treatment guidelines are based on phase 3 clinical trials which were designed separately for grade II and grade III tumors and before the establishment of molecular criteria for the diagnosis of oligodendroglioma. Upfront treatment options for low-grade oligodendrogliomas include observation alone (typically for patients under the age of 40 with gross total resection of tumor) and radiation with adjuvant chemotherapy for patients not meeting these criteria (procarbazine/CCNU/vincristine or PCV, category 1; temozolomide, category 2B according to NCCN guidelines8). Anaplastic oligodendrogliomas are typically treated with radiation and adjuvant PCV chemotherapy (category 1 according to NCCN guidelines8) or adjuvant temozolomide with or without concurrent temozolomide.8–12 An ongoing international phase 3 clinical trial (CODEL, NCT00887146) is comparing the efficacy of radiotherapy with concomitant temozolomide followed by adjuvant temozolomide, versus radiotherapy with adjuvant PCV in patients with newly diagnosed anaplastic gliomas and high-risk low-grade gliomas with 1p/19q codeletion. Whether radiotherapy can be deferred to prevent radiotherapy-induced neurotoxicity in anaplastic oligodendrogliomas is currently being investigated in the POLCA trial, comparing upfront radiotherapy plus PCV versus upfront PCV plus deferred radiotherapy at progression (NCT02444000). Many questions remain regarding treatment of oligodendrogliomas, including the optimal time for initiation of therapy in low-grade tumors, the relative efficacy of PCV versus temozolomide, the identification of favorable molecular markers beyond IDH mutation13 and 1p19q codeletion14 (which are now disease-defining and no longer prognostic within the histology), the potential role of de-escalation of therapy for patients with such favorable molecular markers, and the optimal therapy for recurrent tumors.
Scientific Progress and Regulatory Challenges in Oligodendroglioma Research
To frame the discussion of the working groups, three important and separate areas of scientific progress and challenges were reviewed, as summarized below.
The Molecular Characteristics of Oligodendroglioma: Implications for Diagnosis and Prognosis
Presented by Daniel J. Brat, M.D., Ph.D., Northwestern University Feinberg School of Medicine.
Oligodendrogliomas were first defined histopathologically in the 1920s. The oligodendrocyte had only been defined as a type of glial cell in the 10 years prior. Although treatment strategies have not evolved significantly over recent years, the ability to stratify risk has improved, based increasingly on molecular alterations. Although oligodendrogliomas are defined by the presence of IDH mutations and 1p/19q codeletion, they also frequently harbor mutations in the TERT promoter, CIC, FUBP1,3 and NOTCH1. In addition, these tumors are more chemosensitive than their IDH1/2 mutated astrocytic counterparts, possibly due to the 1p/19q codeletion that accompanies the IDH mutations.15,16
Review of gliomas based on their mutational status shows that oligodendrogliomas have a specific profile, and there is little molecular overlap among oligodendrogliomas, IDH mutant astrocytomas, and IDH wild-type high-grade gliomas. Genes mutated in oligodendrogliomas such as CIC, NOTCH1, FUBP1, are typically not mutated in astrocytomas. These specific molecular fingerprints of glioma subtypes led the WHO in 2016 to distinguish between oligodendrogliomas and astrocytomas based on their molecular characterization. This also resulted in the recognition that the tumor previously called oligoastrocytoma did not have a molecular signature and should not be used as a diagnosis when molecular testing is available.1 This new paradigm has led to an ability to establish reproducible and clinically meaningful diagnoses that stratify risk based on molecular classes rather than on histological findings such as cell morphology, nuclear atypia and vascular proliferation, or necrosis. Leveraging this molecular characterization can point to biomarkers and mechanisms that drive tumor progression so that therapies can be directed against them.
Within each molecular class of diffuse glioma, there is currently a desire to use molecular markers to further stratify risk17 and serve as tools for grading. One study in Japan investigated two cohorts of patients with oligodendroglioma whose tumors had been genetically sequenced.18 Based on multivariate analysis, it was determined that incomplete neurosurgical resection and the presence of NOTCH1 mutations—which are found specifically in oligodendrogliomas but not in IDH mutant astrocytomas or molecularly defined glioblastomas—were two independent predictors of poor overall survival. Patients who had an extensive resection and did not have NOTCH1 mutations had good clinical outcomes long-term. Based on these findings, Brat et al. are developing clinical decision support algorithms to predict patient outcomes and guide treatment decisions. Like the Japanese cohort, Brat et al.’s analyses demonstrated that NOTCH1 pathway inactivation and PI3K pathway activation are strongly associated with poor clinical outcome.19 Further analyses showed that every member of the NOTCH pathway is upregulated dramatically in patients with prolonged survival, suggesting a pathway effect.
In addition to reviewing overall survival and progression-free survival, Brat et al. analyzed endpoints associated with disease progression, such as contrast enhancement on magnetic resonance imaging (MRI), high-cellular density in digitized histopathologic images, and increased cellular proliferation. They searched for enrichment of specific mutations in those tumors with radiologic or pathologic feature of a higher grade. The contrast-enhancing tumors were found to have NOTCH1 mutations, as well as shorter overall survival. Similar correlations were found with regard to cell density and proliferation. In comparison, the nonenhancing tumors are genetically relatively silent. Although NOTCH1 mutations are important in the progression of this disease, but other prognostic markers include PI3K pathway activation and loss of chromosomes 14q and 15q.
Evolution and Progression of Low-Grade Glioma
Presented by Joseph F. Costello, Ph.D., University of California, San Francisco.
Costello et al. have focused on how oligodendrogliomas initiate and progress through malignant transformation (ie, change to a higher histological grade and development of resistance to therapy). Their goal is to understand the mechanism of malignant transformation to ultimately develop strategies to reduce its occurrence.
Using multiple samples from each tumor and accounting for diversity in the genetic makeup in different parts of the tumor, basic techniques of genetic phylogenies can be traced back in time.20 In some cases, the first mutation that occurred earlier in an individual patient’s tumor can be determined. A phylogenetic tree can be constructed that tracks the evolution in the mutations. In the example provided in Figure 3 (tumor evolution in a primary 1p19q codeleted, IDH2 mutant low-grade oligodendroglioma), one can infer that in some cases the recurrence can differ from the primary tumor (shares some mutations but has a greater number of nonshared mutations). Piecing this and other information together, a hypothesis of the steps in the formation of a low-grade glioma can be constructed, including improved understanding of epigenetic and metabolic effects, immune reprogramming, and immunosuppression, all induced by the IDH mutation. The cell cycle is dysregulated through the TP53 mutation and results in reduction in the ability of the cell to undergo apoptosis, making it more resistant to therapy. Regulation of the cell telomeres, and therefore mortality, is influenced by the ATRX mutation in astrocytoma and the TERT promoter mutation in oligodendroglioma.
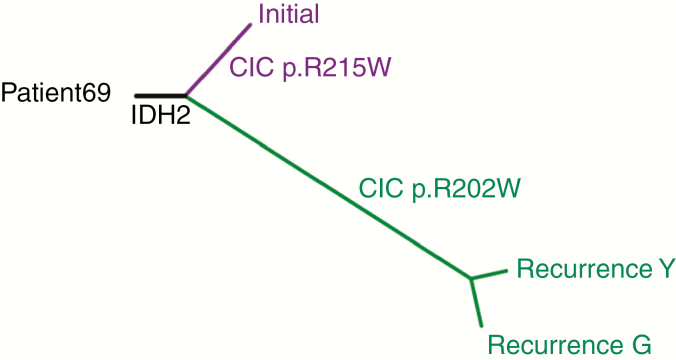
Figure 3.
Early origin of recurrence from a grade II oligodendroglioma. Tumor evolution inferred from mutations in a primary 1p19q codeleted, IDH2 mutant low-grade oligodendroglioma and two tumor samples from a subsequent tumor recurrence from the same patient, resected years later (recurrence Y, recurrence G). The phylogenetic tree is derived from somatic mutations from exome sequencing of the tumor DNA samples. The length of each line in the phylogenetic tree is proportional to the number of mutations. The branch positions illustrate inferred points of evolutionary divergence among the samples. The IDH2 mutation is shared by all three samples whereas a different CIC mutation is found in the initial sample versus in the two tumor samples at recurrence.
Costello et al. have developed a model of low-grade glioma initiation and malignant transformation with disease recurrence. There is great interest in the genetic characterization of cells that are left behind following surgical resection as they can provide clues to the vulnerabilities or resistance of the residual disease to which subsequent treatment is directed. With malignant transformation, tumors can recur as higher grade through spontaneous genetic events or through chemotherapy-associated increases in mutational burden including a profound increase in mutations often referred to as hypermutation.21 Preclinical studies findings suggested that the alkylating chemotherapy agent temozolomide might spur a series of reactions that may lead to resistance to the antitumor effect, consistent with findings from the genomic analysis of patient samples. However, this hypothesis still needs further clinical confirmation and enters in conflict with the known clinical benefit of temozolomide in oligodendroglial tumors. Thus, according to these preliminary findings, this agent could evolve from being cytotoxic to the tumor cells and beneficial to creating more mutations that confer resistance at the time of relapse. Costello et al. have shown that the signature of this chemotherapy can be seen in the exome sequencing of recurrent tumors.22 Hypermutation after temozolomide is statistically associated with an increase in malignancy grade in these studies; that is, the mutations created by chemotherapy are contributing or driving the process of malignant transformation. Further studies have shown that although hypermutation most commonly occurs in astrocytoma it can also be detected readily in oligodendrogliomas. The mutations created by the drug are promoting faster growth, because a critical checkpoint on cell growth is eliminated by mutations occurring following chemotherapy, such as RB and AKT-mTOR pathway mutations. However, the clinical significance of these findings is still unclear. To date, the impact of a hypermutator versus a nonhypermutator oligodendroglioma on patient’s overall survival has not been evaluated prospectively, and clinical trials have shown improved overall survival if chemotherapy is added early on to radiotherapy in oligodendrogliomas, raising the question of to what extent these observations impact clinical outcome.
The Glioma Longitudinal AnalySiS (GLASS) Consortium (https://www.glass-consortium.org/) has shown an increase in the number of CIC mutations after recurrence, suggesting that CIC is a marker of secondary progression. Further, almost all recurrent hypermutated oligodendrogliomas appear to have a defect in the P53 pathway. In fact, analysis of the enrichment of pathway mutations in hypermutated oligodendrogliomas reveals that TP53 is the most enriched gene in these tumors and likely a player in hypermutated oligodendroglioma. This suggests that perhaps the cells that are hypermutated respond differently to therapy because TP53 has such a profound effect on their response.
Additional retrospective data on a small number of cases suggest that the genotype of hypermutation is clinically relevant, although this requires further confirmation in larger and prospective datasets. At recurrence, patients with a hypermutated tumor have a shorter overall survival, suggesting that the hypermutator clone is more aggressive than the standard grade III glioma when data are adjusted for both age and subtype. The time between the point of first chemotherapy and recurrence with a hypermutator varies widely. Understanding the issues of latency and tumor dormancy is the next challenge. Studies with a greater range of patient outcomes are needed to understand the actual rate of hypermutation in the treated population; preliminary data suggest the rate is about 20%, but the number of cases analyzed to date is small.
General Overview of Clinical Trial Efficacy Endpoints
Presented by Paul G. Kluetz, M.D., U.S. Food and Drug Administration.
The Federal Food, Drug, and Cosmetic Act (FD&C Act) provides the framework for approval of new drugs based on substantial evidence of efficacy and acceptable safety for an indicated population. The Public Health Services Act requires evidence that biologic products be safe, pure, and potent for approval. The FDA Modernization Act clarified that the review and approval of drugs and biologics use a similar evidentiary framework, allowing for consistency in trial design and endpoint selection for registration trials to support cancer therapeutics. The traditional approval pathway relies on clinical benefit endpoints or established surrogate endpoints, in other words evidence of a “prolongation of life, a better life or an established surrogate.” Accelerated approval is based on endpoints other than irreversible morbidity or mortality, and can rely on a surrogate endpoint reasonably likely to predict clinical benefit.23 Because there is often residual uncertainty regarding clinical benefit when using surrogate endpoints in the accelerated approval program, post-marketing trials are needed to verify benefit. Direct measures of clinical benefit tend to be clinical outcomes, such as feeling or functioning better, or improvement in survival. In contrast, surrogate endpoints are not directly measuring symptoms, function, or survival, but rather look to predict benefit. In oncology this is most commonly based on control of tumor cell burden, which can be directly measured either through the blood in hematologic malignancy or through imaging of solid tumors. Because tumor measures are monitored and acted upon in clinical practice (eg, changing therapies upon progression), response rate and progression-free survival are considered strong endpoints suitable for either regular or accelerated approval depending on the context and magnitude of effect.24
Clinical outcome assessments are used to quantify symptoms or function. These data can come from several sources, including clinician-reported outcomes, observer-reported outcomes (someone other than the patient or health care provider), patient-reported outcomes, or performance-based outcomes.25 Wearable mobile technology tools may also provide an opportunity to gather data related to activity and other components of function.
When evaluating the strength and limitations of various types of efficacy endpoints, Dr. Kluetz stated that FDA will consider what is being measured (ie, endpoint selection) and whether it directly measures a clinical benefit (eg, survival, symptoms, or function) or whether it is a surrogate endpoint. Another consideration is the accuracy of the measure, its susceptibility to bias, subjectivity, or interpretation, and the accuracy of the timing of the event. For an individual trial, the magnitude of effect on the endpoint is an important consideration. For example, in a single-arm trial of a chemotherapeutic agent treating patients with oligodendroglioma, a high rate of rapid, deep, and durable shrinkage of tumors across a cohort of patients would be compelling evidence of antitumor effect due to the investigational agent, given malignant tumors do not typically regress to a great extent on their own. Thus, treatment effect measured by tumor response can be a surrogate endpoint to support accelerated approval in the appropriate setting (specific disease, large magnitude of the effect, durability of response).
Clinical benefit is determined based on the totality of data within a given context. A critical component of the totality of data is the safety profile of the product. Acute and subacute safety and tolerability must be carefully characterized, including clinician-reported adverse events, dose modifications and, increasingly, patient-reported symptomatic toxicities and their impact on function. The agency will also consider other well-defined functional domains that affect one’s health-related quality of life, such as cognition.
Moving the Field of Oligodendroglioma Research Forward – Reports from Working Groups
Workshop participants broke into four working groups to discuss issues related to advancing the field through collaborative research efforts to establish better preclinical models; data sharing and clinical research approaches, such as advanced imaging; optimization of clinical trial designs; and promoting patient engagement.
Biology of Oligodendrogliomas and Preclinical Models
Session Leads: Daniel P. Cahill, M.D., Ph.D., Harvard Medical School and J. Gregory Cairncross, M.D., University of Calgary.
Molecular diagnosis based on current WHO 2016 criteria allows a more reliable classification of oligodendrogliomas and reflects tumor biology more accurately. This provides an opportunity for further research. First, preclinical models are needed that emulate indolent disease. It is difficult to propagate cell lines either in vitro or these tumors in vivo. The limitations of animal models include the long time to achieve tumor growth and the relatively short lifespan of the animal, whereas some canine models that develop gliomas spontaneously are promising.26 Induced pluripotent stem (iPS) cells provide an opportunity to introduce the various mutations of the disease to generate a faithful model that can be used for in vitro studies. Recent success with creation of IDH mutant mouse models for astrocytoma could be adapted to contain more oligodendroglioma-relevant genes.27–31
A second opportunity involves using clinical trial materials to develop correlative studies to understand the biology of oligodendroglioma, in particular, to collect image-guided tumor samples during longitudinal studies that might provide clues about early progression and spatial heterogeneity. For example, do patients who fail early have particular genetic alterations that could provide a window into what predicts poor prognosis?19,32,33
A third opportunity lies in identifying druggable targets for oligodendrogliomas. This could lead to preventive-type strategies, for example, screening patients for high-risk germline alterations34 and exposing people to a comparably safe drug early in adulthood to decrease their lifetime risk of developing glioma.
These opportunities could be better pursued through access to a central repository containing human and animal tumor samples, prospective genomic analysis conducted in parallel with large clinical trials, and prospective and continuous biospecimen monitoring to search for biomarkers for patients in the indolent phase of the disease.
Collaborative Efforts in Data Sharing and Incorporating Advanced Molecular Diagnosis and Advanced Imaging in Low Grade Glioma (LGG) Research
Session Leads: Terri S. Armstrong, Ph.D., NCI-CONNECT; Mark R. Gilbert, M.D., NCI-CONNECT; Roel G.W. Verhaak, Ph.D., Jackson Laboratory for Genomic Medicine
Successful research networks require strong and well-defined leadership, centralized infrastructure, clearly defined policies (eg, data sharing, authorship), and funding. For example, the Cancer Genome Atlas has been successful because of its infrastructure and large community of scientists. The GLASS consortium35, 44 is emerging as another robust effort in data aggregation and sharing. In each of these cases, champions for this effort and a commitment to inclusion and mutual value have brought success. NCI-CONNECT is well positioned to partner and collaborate with such endeavors.
Future research efforts should consider the use of existing sources of data (alone or expanded) such as The Surveillance, Epidemiology, and End Results Program SEER https://seer.cancer.gov/ (some sites have samples) or case/control (eg, GICC,36 GLIOGENE37). Large cohorts, such as the Nurses’ Health Study (https://www.nurseshealthstudy.org/about-nhs), have stored vast amounts of clinical trial data that can be tapped for comparative controls. The AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE; https://www.aacr.org/Research/Research/pages/aacr-project-genie.aspx) provides a model platform for oligodendrogliomas.
As new data collection goes forward, patient-level information is needed and long-term follow-up with clinical annotation is key. Although studies should always strive to answer specific questions, data collection need not be hypotheses-driven. For example, large companies collect vast amounts of data that can later be evaluated for trends or mined for profit. This is a model that should be emulated in science. Ideally, molecular and imaging data should be included in future data collection, noting that such efforts require additional time and resources.
Collaboration will be essential to acquire large longitudinal sample collections, since historically sample sizes have typically been small and it is difficult to achieve statistical power. Additional efforts are needed to explore the value of serum- and CSF-derived biomarkers. In all cases, annotated tissue sampling will be critical to answering many questions.
A large amount of imaging data already exists and efforts are needed to combine these data. Although pre and postoperative images tend to only include standard sequences that are essential, consideration should be given to adding advanced imaging studies such as perfusion/diffusion, PET (18F-FDG, 11C-MET, others), and 2-HG spectroscopy when available, although many challenges remain regarding their implementation, value, indications and standardization among centers. Workshop participants encouraged the use of the Brain Tumor Imaging Protocol (BTIP) for standardization.38 Existing imaging infrastructure (ie, Quantitative Image Informatics for Cancer Research [QIICR]) can be leveraged. Experience from the French POLA effort to study rare tumors has shown that the biggest challenge has been aggregating MRI images, as some institutions do not save images and there are differences among institutions regarding what is saved and what patient privacy protections are needed. Finally, in cases where no tumor tissue is available, it may be possible to infer classification/genomic properties from radiologic images, which may enable retrospective sample classification, although this remains speculative.
Clinical Trial Designs
Session Leads: Martin van den Bent, M.D., Ph.D., Erasmus MC Cancer Institute, and Marta Penas-Prado, M.D., NCI-CONNECT.
Given the typical long survival of patients with oligodendroglioma, alternative clinical trial endpoints to progression-free survival and overall survival are needed that determine outcome and lead to more efficient trial designs. Examples include imaging endpoints evaluating growth trajectory, and clinical endpoints such as seizure control, objective testing of cognitive function, instrumental activities of daily living (ADLs), and symptom burden.39–43 At present, imaging endpoints evaluating growth trajectory are an active subject of research by different groups but remain poorly defined and further studies are needed to validate them, particularly as related to posttreatment growth rate changes. Use of composite endpoints, including evaluation of imaging trajectory in combination with one or more clinical endpoints, should be considered for the design of future clinical trials. Randomization as opposed to the use of historical controls is also desirable for clinical trials that will be of long duration, as the historical control comparison may be unreliable. Other trial designs to consider include Phase 0/proof-of-concept trials with tissue acquisition and pharmacodynamic endpoints after administration of experimental therapy to compensate for the lack of optimal preclinical models. However, these trials are challenging to implement and coordinate and are typically only feasible in a small number of experienced centers.
Inclusion and exclusion criteria for trials can be better informed through molecular characterization as opposed to classical clinical factors (extent of resection, age), for example, to select patients who should not delay upfront therapy (those at greatest risk of progression due to a high-risk molecular signature). Importantly, the working group emphasized the need for contemporary tissue sampling in trials for recurrent oligodendroglioma to assess molecular changes over time.
The group also recommended that tumor tissue and blood collection be made mandatory for future trials. Having tissue collected upfront would facilitate future analyses as more information becomes available, rather than trying to obtain tumor tissue retrospectively to analyze recently discovered biomarkers once a prospective study is completed. Given the low incidence of oligodendrogliomas, there should be initiatives launched to combine efforts that are not only multi-institutional but also international, recognizing regulatory, and logistical challenges. One potential alternative to conducting collaborative international trials creating an oligodendroglioma consortium similar to the AGILE consortium for glioblastoma (www.gcaresearch.org/gbm-agile/), which has led to the development of an international adaptive platform trial with an external contract research organization (CRO) in charge of supporting the trial (NCT03970447). However, for international studies, it is easier to pool data than to exchange tumor tissue samples or collaborate on trials given the increasingly complex regulatory environment for material transfer agreements and trial sponsorship. Existing longitudinal studies of cancer cohorts have relied on patients prospectively opting into data and specimen collection, which can eliminate some of the regulatory obstacles to seeking permission retrospectively or obtaining institutional permission to do so. Despite these challenges, the NCI-CONNECT program provides an opportunity for conducting either basket or umbrella studies in oligodendroglioma with shared molecular features with other rare CNS tumors.
Patient Outreach and Engagement
Session Leads: Brittany Cordeiro, Terri S. Armstrong, Ph.D., and Mark R. Gilbert, M.D., NCI-CONNECT
Patients need more standardized and reliable information about diagnosis and treatment for their own consumption and for sharing across groups. For example, patients might be misleadingly told that they have the “good tumor” when being diagnosed with a 1p/19q codeletion and not receive the same prognostic information as others with the same diagnosis. Along with discussions about prognosis, guidance regarding impact of the disease on daily life is also critical, including issues with loss of independence (inability to return to previous work, driving restrictions) and family planning for patients in their reproductive years. Contradictory or inaccurate information can not only influence treatment decisions but also deter research participation. For example, patients need to understand the still uncertain clinical implications of the preliminary findings regarding hypermutation and how that might or might not influence treatment decisions. In addition, standardized information and training for providers is a critical need. NCI-CONNECT can be a valuable and trusted resource in communicating accurate information to the patient and provider communities about research, diagnosis, and new treatment options. Finally, patient registries are an important resource for patients to both provide and receive critical clinical and research information.
Conclusions, Recommendations and Action Plan
An important contribution of NCI-CONNECT is its ability to bring experts in rare CNS cancers together to share insights, ideas, and plans for collaboration. Oligodendroglioma is one of the 12 rare CNS cancers included in the initial NCI-CONNECT portfolio. Although oligodendrogliomas typically have better prognoses than other primary brain tumors, they still have highly variable clinical outcomes, and little is known about the pathways involved with progression or the optimal markers for stratifying risk. Current therapies are effective in controlling tumor growth for prolonged periods of time in many instances, but they are usually not curative, and both the tumor itself and the treatments are often linked to long-term sequalae and increased symptom burden with a negative impact on quality of life.
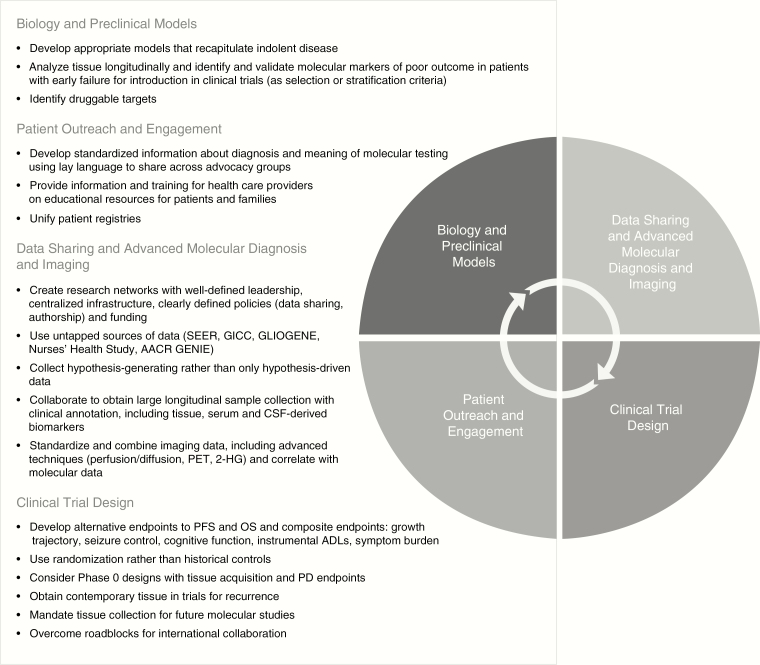
This report summarizes the discussion and recommendations of the Oligodendroglioma Workshop held on November 19, 2018 and provides a call to action to address the challenges noted in oligodendroglioma care and research. The recommendations of the workshop are summarized in Figure 4 and include a multifaceted and interrelated approach covering four distinct areas: biology and preclinical models, data sharing and advanced molecular diagnosis and imaging, clinical trial design, and patient outreach and engagement.
Figure 4.
Workshop recommendations.
The NCI-CONNECT program is well positioned to address many of these needs in collaboration with other stakeholders, and a list of action items emanated from the workshop and is currently being worked on. The Natural History study with its longitudinal collection of clinical data along with tumor tissue and germline samples is serving as a platform for interaction with investigators from other sites to unify patient registries and collaborate on tumor tissue analysis (from both newly diagnosed and recurrent tumors) as well as germline analysis from other large tumor repositories.
Another contribution of the NCI-CONNECT program is a clinical trial infrastructure in which only industry support to provide the experimental agent/s is necessary. Conducting multi-institutional collaborative umbrella or basket trials for oligodendrogliomas together with other rare CNS tumors sharing the same molecular features is possible. Finally, partnering with advocacy groups and patients is critical to NCI-CONNECT, and development of informational resources for patients and health care providers falls within its mission.
Acknowledgments
Brittany Cordeiro, Advocacy Liaison and Navigator; Kristin Odom, Communications Editor; Kathleen Mercure, Program Manager; Alan Dabbiere; Ashley Dabbiere; Kim Brams; Robert Brams; Kathi E. Hanna, Ph.D., M.S., Freelance Writer/Editor.
Contributor Information
NCI-CONNECT Oligodendroglioma Workshop:
David Aarons, Kevin Camphausen, Elizabeth Claus, Brittany Cordeiro, Francois Ducray, Dominique Figarella-Branger, Pim French, Brock Greene, John D Heiss, Robert Jenkins, Amy LeBlanc, Tito Mendoza, Kathy Oliver, Martha Quezado, Margarita Raygada, Carlos Romo, Lawrence Rubinstein, Christine Siegel, Joohee Sul, Keisuke Ueki, Michael Weller, Patrick Y Wen, Nicole Willmarth, Ying Yuan, and Kareem Zaghloul
Funding
The NCI Comprehensive Oncology Network for Evaluating Rare CNS Tumors (NCI-CONNECT) is a program within the Rare Tumor Patient Engagement Network (RTPEN), an initiative supported by the Cancer Moonshot℠ funds and managed at the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Neuro-Oncology Branch.
Conflict of Interest
D.P.C.: Funding support by the MGH Tawingo Chair Fund, NIH R01CA227821, and P50CA165962. D.P.C. has received honoraria/travel reimbursement from Merck and has served as a consultant for Lilly. M.V.D.B.: Agios, Celgene, BMS, Carthera, Boehringer, Bayer. R.G.W.V.: stock in Boundless Bio, Inc. S.M.C.: institutional research support from Agios. P.Y.W.: Agios (ad board and clinical trial support).
Authorship
All listed authors participated in the Oligodendroglioma Workshop and have been involved in the writing of the manuscript and have read and approved the final version.
NCI-CONNECT, Comprehensive Oncology Network Evaluating Rare CNS Tumors, Oligodendroglioma Workshop Participants (alphabetical order)
David Aarons, JD (National Brain Tumor Society), Kenneth Aldape, MD (Laboratory of Pathology, National Institutes of Health, Bethesda, MD), Kevin Camphausen, MD (Radiation Oncology Branch/National Cancer Institute, Bethesda, MD), Elizabeth Claus, MD, PhD (Yale University School of Public Health, Department of Neurosurgery Brigham and Women’s Hospital, New Haven, CT), Brittany Cordeiro (Neuro-Oncology Branch/National Cancer Institute, Bethesda, MD), Francois Ducray, MD (Hospices Civils de Lyon, France), Dominique Figarella-Branger, MD, PhD (APHM, Hôpital de la Timone, Service d’Anatomie Pathologique et de Neuropathologie and Aix-Marseille Univ, CNRS, INP, Inst Neurophysiopathol, Marseille, France), Pim French, PhD (Erasmus MC Cancer Institute, University Medical Center, Rotterdam, The Netherlands), Brock Greene (Oligo Nation), John D. Heiss, MD (Surgical Neurology Branch, NINDS, National Institutes of Health), Robert Jenkins, MD, PhD (Laboratory Medicine, Mayo Clinic, Rochester, MN), Amy LeBlanc, DVM, DACVIM (Comparative Oncology Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD), Tito Mendoza, PhD, MS, MEd (Department of Symptom Research, The UT MD Anderson Cancer Center, Houston, TX), Kathy Oliver (International Brain Tumour Alliance), Martha Quezado, MD (Laboratory of Pathology, National Institutes of Health, Bethesda, MD), Margarita Raygada, PhD, MSC (Neuro-Oncology Branch/National Cancer Institute, Bethesda, MD), Carlos Romo, MD (Johns Hopkins University School of Medicine & National Institutes of Health), Lawrence Rubinstein, PhD (Biometric Research Program, DCTD, NCI, National Institutes of Health, Bethesda, MD), Christine Siegel, NP (Neuro-Oncology Branch/National Cancer Institute, Bethesda, MD), Joohee Sul, MD (Office of Hematology and Oncology Products, CDER, FDA), Keisuke Ueki, MD, PhD (Department of Neurosurgery, Dokkyo Medical University, Japan), Michael Weller, MD (Department of Neurology, University Hospital and University of Zurich, Switzerland), Patrick Y. Wen, MD (Center for Neuro-Oncology, Dana-Farber Cancer Institute, Boston, MA), Nicole Willmarth, PhD (American Brain Tumor Association), Ying Yuan, PhD (Department of Biostatistics, The UT MD Anderson Cancer Center, Houston, TX), Kareem Zaghloul, MD, PhD (Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, MD).
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Cahill DP, Louis DN, Cairncross JG. Molecular background of oligodendroglioma: 1p/19q, IDH, TERT, CIC and FUBP1. CNS Oncol. 2015;4(5):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-oncology. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez FJ, Tihan T, Lin D, et al. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol. 2014;38(8):1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nauen D, Haley L, Lin MT, et al. Molecular analysis of pediatric oligodendrogliomas highlights genetic differences with adult counterparts and other pediatric gliomas. Brain Pathol. 2016;26(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerkhof M, Benit C, Duran-Pena A, Vecht CJ. Seizures in oligodendroglial tumors. CNS Oncol. 2015;4(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Network NCC. Central Nervous System Cancers (Version 3.2019).https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed October 18, 2019.
- 9. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 11. Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Den Bent MJ, Bromberg JE, Buckner J. Low-grade and anaplastic oligodendroglioma. Handb Clin Neurol. 2016;134:361–380. [DOI] [PubMed] [Google Scholar]
- 13. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 15. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu X, Martinez-Ledesma E, Zheng S, et al. Multigene signature for predicting prognosis of patients with 1p19q co-deletion diffuse glioma. Neuro Oncol. 2017;19(6):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoki K, Nakamura H, Suzuki H, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halani SH, Yousefi S, Velazquez Vega J, et al. Multi-faceted computational assessment of risk and progression in oligodendroglioma implicates NOTCH and PI3K pathways. NPJ Precis Oncol. 2018;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazor T, Pankov A, Johnson BE, et al. DNA methylation and somatic mutations converge on the cell cycle and define similar evolutionary histories in brain tumors. Cancer Cell. 2015;28(3):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–856. [DOI] [PubMed] [Google Scholar]
- 24. FDA. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics (December 2018).https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics. Accessed August 20, 2019.
- 25. Group F-NBW. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); 2016-. Glossary 2016 Jan 28 [Updated 2018 May 2]; https://www.ncbi.nlm.nih.gov/books/NBK338448/ Co-published by National Institutes of Health (US), Bethesda (MD). [Google Scholar]
- 26. Koehler JW, Miller AD, Miller CR, et al. A revised diagnostic classification of canine glioma: towards validation of the canine glioma patient as a naturally occurring preclinical model for human glioma. J Neuropathol Exp Neurol. 2018;77(11):1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sasaki M, Knobbe CB, Itsumi M, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev. 2012;26(18):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bardella C, Al-Dalahmah O, Krell D, et al. Expression of Idh1R132H in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell. 2016;30(4):578–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pirozzi CJ, Carpenter AB, Waitkus MS, et al. Mutant IDH1 disrupts the mouse subventricular zone and alters brain tumor progression. Mol Cancer Res. 2017;15(5):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Philip B, Yu DX, Silvis MR, et al. Mutant IDH1 promotes glioma formation in vivo. Cell Rep. 2018;23(5):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nunez FJ, Mendez FM, Kadiyala P, et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 2019;11(479):pii: eaaq1427. doi:10.1126/scitranslmed.aaq1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048–5050. [DOI] [PubMed] [Google Scholar]
- 33. Tateishi K, Nakamura T, Juratli TA, et al. PI3K/AKT/mTOR pathway alterations promote malignant progression and xenograft formation in oligodendroglial tumors. Clin Cancer Res. 2019;25(14):4375–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jenkins RB, Xiao Y, Sicotte H, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012;44(10):1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. GLASS Consortium. Glioma through the looking GLASS: molecular evolution of diffuse gliomas and the Glioma Longitudinal Analysis Consortium. Neuro-oncology. 2018;20(7):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amirian ES, Armstrong GN, Zhou R, et al. The glioma international case-control study: a report from the genetic epidemiology of glioma international consortium. Am J Epidemiol. 2016;183(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malmer B, Adatto P, Armstrong G, et al. GLIOGENE an International Consortium to Understand Familial Glioma. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1730–1734. [DOI] [PubMed] [Google Scholar]
- 38. Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koekkoek JA, Dirven L, Heimans JJ, et al. Seizure reduction is a prognostic marker in low-grade glioma patients treated with temozolomide. J Neurooncol. 2016;126(2):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blakeley JO, Coons SJ, Corboy JR, Kline Leidy N, Mendoza TR, Wefel JS. Clinical outcome assessment in malignant glioma trials: measuring signs, symptoms, and functional limitations. Neuro-oncology. 2016;18(Suppl 2):ii13–ii20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 42. Gabel N, Altshuler DB, Brezzell A, et al. Health related quality of life in adult low and high-grade glioma patients using the national institutes of health patient reported outcomes measurement information system (PROMIS) and neuro-QOL assessments. Front Neurol. 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oort Q, Dirven L, Meijer W, et al. Development of a questionnaire measuring instrumental activities of daily living (IADL) in patients with brain tumors: a pilot study. J Neurooncol. 2017;132(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barthel FP, Johnson KC, Varn FS, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature (2019). doi:10.1038/s41586-019-1775-1 [DOI] [PMC free article] [PubMed] [Google Scholar]