Abstract
Background
Mounting evidence supports the presence of heterogeneity in the presentation of ependymoma patients with respect to location, histopathology, and behavior between pediatric and adult patients. However, the influence of age on treatment outcomes in ependymoma remains obscure.
Methods
The SEER database years 1975–2016 were queried. Patients with a diagnosis of ependymoma were identified using the International Classification of Diseases for Oncology, Third Edition, coding system. Patients were classified into one of 4 age groups: children (age 0–12 years), adolescents (age 13–21 years), young adults (age 22–45 years), and older adults (age >45 years). The weighed multivariate analysis assessed the impact of age on survival outcomes following surgical treatment.
Results
There were a total of 6076 patients identified with ependymoma, of which 1111 (18%) were children, 529 (9%) were adolescents, 2039 (34%) were young adults, and 2397 (40%) were older adults. There were statistically significant differences between cohorts with respect to race (P < .001), anatomical location (P < .001), extent of resection (P < .001), radiation use (P < .001), tumor grade (P < .001), histological classification (P < .001), and all-cause mortality (P < .001). There was no significant difference between cohorts with respect to gender (P = .103). On multivariate logistic regression, factors associated with all-cause mortality rates included males (vs females), supratentorial location (vs spinal cord tumors), and radiation treatment (vs no radiation).
Conclusions
Our study using the SEER database demonstrates the various demographic and treatment risk factors that are associated with increased rates of all-cause mortality between the pediatric and adult populations following a diagnosis of ependymoma.
Keywords: adults, ependymoma, mortality, pediatrics, SEER
Key Points.
The age of ependymoma patients influences tumor grade and histological classification.
Gender, age, radiation, and tumor location affect survival in ependymoma patients.
Importance of the Study.
Prior studies have demonstrated heterogeneity in the presentation of ependymoma with respect to location, histopathology, and behavior observed among different age cohorts. However, there is a paucity of data investigating the influence of age on treatment outcomes in ependymoma. We found significant differences between the cohorts with respect to race, anatomical location, type of resection, use of radiation, tumor grade, histological classification, and all-cause mortality. Additionally, in a multivariable regression analysis, factors associated with all-cause mortality rates included males, supratentorial tumors, and radiation treatment and age older than 45 years. Overall, our study sheds light on the need for identifying risk factors associated with mortality in pediatric and adult patients diagnosed with an ependymoma. Furthermore, standardizing evidence-based treatment regiments focused on pediatrics and adults separately may provide avenues to reducing overall mortality rates and bettering outcomes.
Ependymomas are rare tumors of the central nervous system, traditionally believed to arise from ependymal cells of the ventricular lining of the brain and central canal of the spinal cord.1,2 These tumors have an incident rate of 0.43 patients per 100 000 population in the United States, accounting for 1.8% of all primary brain tumors.3 Ependymomas are subdivided according to the World Health Organization (WHO) classification into 3 grades of malignancy: WHO grades I, II, and III.4–7 A recent molecular classification system has come into use that subdivides ependymal tumors into 9 groups that more completely describe the biological, clinical, and histopathological features of these tumors.8 Furthermore, ependymomas have varying prognosis based on their age of onset, location, and histopathologic appearance.1
A mounting body of evidence shows heterogeneity in the presentation of ependymoma with respect to location, histopathology, and behavior observed among different age cohorts. It has been widely established that ependymoma tumor locations commonly differ between children and adults, with the majority occurring in the posterior fossa for children and in the spinal cord for adults.1–4,9–11 There has also been increasing evidence that pediatric ependymomas tend to present at higher grades.1,8 At the molecular level, investigations have shed light on histopathological discrepancies in pediatric versus adult ependymoma tumors, and different mutations have been partially attributed to these differences in tumor behavior.12 While previous investigations have studied differences in ependymoma tumor characteristics by age, the influence of age on treatment outcomes in ependymoma remains understudied.
Thus, the aim of this study was to investigate differences in management and survival outcomes between different age-stratified cohorts in ependymoma patients.
Methods
Data Source and Patient Population
The sample frame was identified from the most recent SEER datasets (Incidence—SEER 18 Regs) Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying). We queried all patients from all central nervous system sites for International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes: “9391/3: Ependymoma, NOS,” “9392/3: Ependymoma, anaplastic,” “9393/3: Papillary ependymoma,” and “9394/1: Myxopapillary ependymoma” diagnosed from 1975 to 2016. Patients with primary sites that were not included in meninges (C70.0–C70.9), brain (C71.0–C71.9), spinal cord, cranial nerves, and other parts of the central nervous system (C72.0–C72.9) were excluded.
Data Collection
Data collected included gender, race, age at diagnosis, anatomical location of tumor, extent of surgical resection, treatment with radiation therapy, histological classification, tumor grade by SEER ICD-O-3 coding for Solid Tumors, and all-cause mortality. Patients were divided into 4 age group cohorts: 0–12 years (children), 13–21 years (adolescents), 22–45 years (young adults), and older than 45 years (older adults). We divided adults into these groups to correct for comorbidities associated with older adults, as young adults (22–45 years) typically do not have a high prevalence of significant life-threatening comorbidities. Race was dichotomized as “white” and “non-white.” Tumor location was recorded in SEER as 17 categories corresponding to the lobes of the brain, as well as cerebrum, cerebellum, brainstem, spinal cord, spinal meninges, cauda equina, cerebral meninges, ventricles, cranial nerves, brain-not otherwise specified (NOS), and overlapping lesion of the brain. However, due to small numbers in many of these groups, for most analyses anatomical locations were divided into 5 groups: (1) supratentorial brain, overlapping lesion of brain, and brain-NOS; (2) spinal cord, spinal meninges, cerebral meninges, and cauda equina; (3) posterior fossa, cerebellum, and brainstem; (4) ventricles; and (5) cranial nerves and other structures. The extent of surgical resection was divided into no surgery, biopsy, subtotal resection (STR), and gross total resection (GTR) and unknown, based on SEER site-specific coding guidelines.
Statistical Analysis
SEER*STAT v8.3.5 was used to extract case-level data and Stata/IC v13.1 was used for data analyses. Frequencies, proportions, and measures of central tendency were used to describe the data. For bivariate analyses, categorical data were analyzed using the Chi-square test and Fisher’s exact test. Factors associated with all-cause mortality were estimated using multivariate regression. We also included in the model gender, tumor location, radiation, SEER registry, and year of diagnosis. Statistical tests were two-tailed, and P < .05 was considered statistically significant.
Results
Overall Patient Demographic Characteristics
Between 1975 and 2016 the SEER datasets contained 6076 ependymoma patients. The mean age of the patients was 37.1 years (Table 1). All-age demographics included 3276 (53.9%) females and 2800 (46.1%) males (Table 1). The racial composition for the overall patient pool was as follows: 5121 (84.3%) white and 955 (15.7%) non-white or unknown (Table 1). Of all patients, 1116 (18.4%) had supratentorial tumor location, 3323 (54.7%) were spinal, 1083 (17.8%) were in the posterior fossa, cerebellum, or brainstem, 540 (8.9%) were intraventricular, and 14 (0.2%) involved the cranial nerves (Table 1). The extent of surgery receipt for all patients was as follows: 402 (6.6%) received No Surgery, 2031 (33.4%) underwent only Biopsy, 533 (8.8%) underwent STR, 1377 (22.7%) received GTR, 64 (1.1%) underwent NOS Surgery, and 19 (0.3%) had unknown surgical status (Table 1). Of all age patients, 3816 (62.8%) received No radiation and 2260 (37.2%) received Radiation (Table 1). At a 35-month median follow-up, all-cause mortality was 1614 (26.6%) (Table 1). Of all patients, 326 (5.4%) had well-differentiated tumors, 656 (10.8%) had moderately differentiated tumors, 120 (2.0%) had poorly differentiated tumors, 579 (9.5%) had undifferentiated tumors, and 4395 (72.3%) had unknown grade (Table 1). The histological classification of the overall patient pool was as follows: 4126 (67.9%) had NOS histology, 766 (12.6%) had anaplastic histology, 60 (1.0%) had papillary histology, and 1124 (18.5%) had myxopapillary histology (Table 1).
Table 1.
Demographic Characteristics of All Patients With Ependymoma, 1975–2016 (n = 6076)
| Frequency (n) | Percent (%) | |
|---|---|---|
| Mean age at diagnosis in years (standard deviation) | 37.1 (21.87) | |
| Gender | ||
| Male | 2800 | 46.1 |
| Female | 3276 | 53.9 |
| Race | ||
| White | 5121 | 84.3 |
| Not white or unknown | 955 | 15.7 |
| Anatomical location of tumor | ||
| All supratentorial brain, overlapping lesion of brain, and brain, not otherwise specified (NOS) | 1116 | 18.4 |
| Spinal cord, spinal meninges, cerebral meninges, and cauda equina | 3323 | 54.7 |
| Posterior fossa, cerebellum, and brainstem | 1083 | 17.8 |
| Ventricles | 540 | 8.9 |
| Cranial nerves and other | 14 | 0.2 |
| Type of surgery | ||
| Received surgery or unknown | 5674 | 93.4 |
| Did not receive surgery | 402 | 6.6 |
| Biopsy | ||
| No biopsy | 4045 | 66.6 |
| Biopsy | 2031 | 33.4 |
| STR | ||
| No STR | 5543 | 91.2 |
| STR | 533 | 8.8 |
| GTR | ||
| No GTR | 4699 | 77.3 |
| GTR | 1377 | 22.7 |
| Radiation | ||
| No radiation | 3816 | 62.8 |
| Radiation | 2260 | 37.2 |
| All-cause mortality | ||
| Alive | 4462 | 73.4 |
| Died | 1614 | 26.6 |
| Grade | ||
| Well differentiated | 326 | 5.4 |
| Moderately differentiated | 656 | 10.8 |
| Poorly differentiated | 120 | 2.0 |
| Undifferentiated | 579 | 9.5 |
| Unknown | 4395 | 72.3 |
| Histology | ||
| Ependymoma, NOS | 4126 | 67.9 |
| Ependymoma, anaplastic | 766 | 12.6 |
| Papillary ependymoma | 60 | 1.0 |
| Myxopapillary ependymoma | 1124 | 18.5 |
Age-Stratified Demographic Characteristics
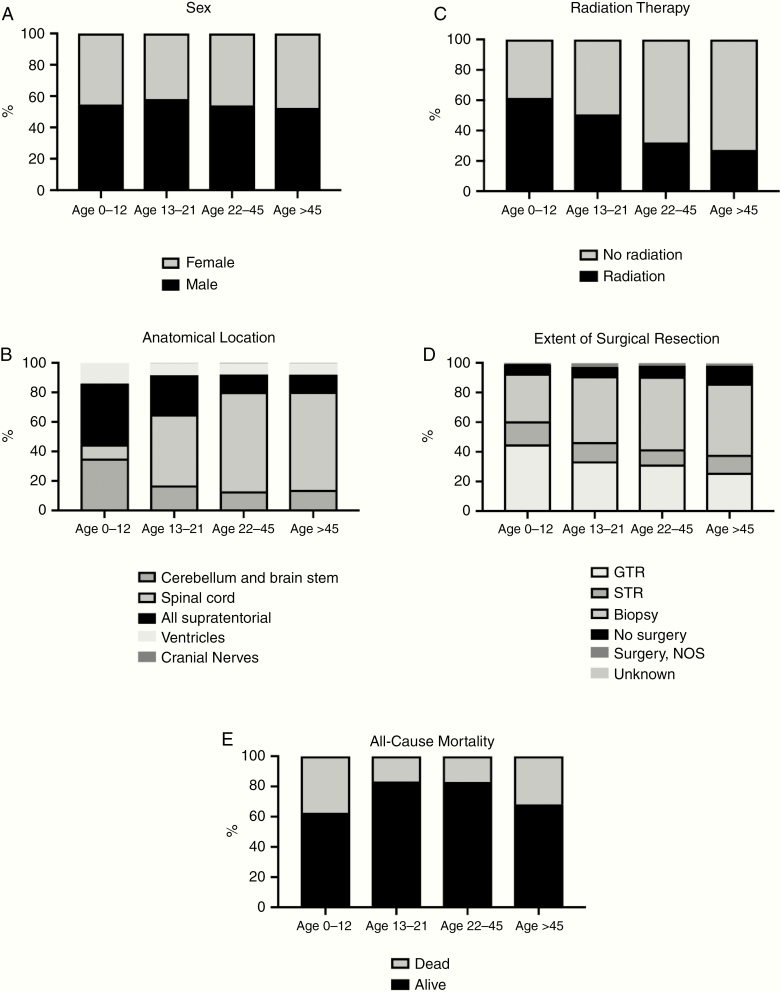
Children (0–12 years) accounted for 1111 (18.3%) patients, Adolescents (13–21 years) accounted for 529 (8.7%) patients, Young adults (22–45 years) accounted for 2039 (33.6%) patients, and Older adults (>45 years) accounted for 2397 (39.5%) patients (Table 2). The median ages of children, adolescents, young adults, and older adults were 4.5, 16.9, 34.2, and 59.0 years, respectively (Table 2). Although ependymomas were more common in males across all age groups, there were no statistically different gender distributions observed between the age cohorts (P = .103) (Figure 1, Table 2). Comparative analyses between children, adolescents, young adults, and older adults revealed statistically significant differences between the 4 cohorts with proportionately more non-whites in the Children cohort (Children: 21.5% vs Adolescents: 17.4% vs Young Adults: 15.9% vs Older Adults: 12.5%, P≤ .001), lower rates of spinal cord tumors in the Children cohort (Children: 9.5% vs Adolescents: 48.0% vs Young Adults: 67.2% vs Older Adults: 66.4%, P≤ .001), and higher rates of GTR in the Children cohort (Children: 24.8% vs Adolescents: 23.4% vs Young Adults: 23.7% vs Older Adults: 20.6%, P≤ .001) (Figure 1, Table 2). Furthermore, there were found to be higher rates of radiation therapy in the Children cohort (Children: 61.5% vs Adolescents: 50.7% vs Young Adults: 32.2% vs Older Adults: 27.2%, P≤ .001), while all-cause mortality was lowest for adolescents (Children: 37.4% vs Adolescents: 16.8% vs Young Adults: 17.0% vs Older Adults: 31.8%, P≤ .001) (Figure 1, Table 2). The Children cohort had significantly lower rates of well-differentiated tumors (Children: 3.4% vs Adolescents: 5.3% vs Young Adults: 6.6% vs Older Adults: 5.3%, P < .001) and higher rates of undifferentiated tumors (Children: 27.8% vs Adolescents: 12.3% vs Young Adults: 5.1% vs Older Adults: 4.2%, P < .001) compared to the adult cohorts (Table 2). Furthermore, the Children cohort had much higher rates of anaplastic histology (Children: 35.6% vs Adolescents: 16.6% vs Young Adults: 6.9% vs Older Adults: 5.9%, P < .001) and lower rates of myxopapillary histology (Children: 3.6% vs Adolescents: 25.3% vs Young Adults: 23.4% vs Older Adults: 19.7%, P < .001) (Table 2).
Table 2.
Demographic Characteristics of Patients With Ependymoma Categorized by Children, Adolescents, Young Adults, and Older Adults, 1975–2016 (n = 6076)
| Total | Children (0–12), n (%) | Adolescents (13–21), n (%) | Young adults (22–45), n (%) | Older adults (>45), n (%) | P | |
|---|---|---|---|---|---|---|
| N = 6076 | N = 1111 (18.3) | N = 529 (8.7) | N = 2039 (33.6) | N = 2397 (39.5) | ||
| Mean age in years (standard deviation) | 4.5 (3.7) | 16.9 (2.7) | 34.2 (6.8) | 59.0 (9.8) | ||
| Gender | .103 | |||||
| Female | 2800 | 504 (45.4) | 221 (41.8) | 936 (45.9) | 1139 (47.5) | |
| Male | 3276 | 607 (54.6) | 308 (58.2) | 1103 (54.1) | 1258 (52.5) | |
| Race | <.001 | |||||
| White | 5121 | 872 (78.5) | 437 (82.6) | 1715 (84.1) | 2097 (87.5) | |
| Non-white | 955 | 239 (21.5) | 92 (17.4) | 324 (15.9) | 300 (12.5) | |
| Anatomical location of the tumor | <.001 | |||||
| All supratentorial brain, overlapping lesion of brain, and brain, not otherwise specified (NOS) | 1116 | 458 (41.2) | 140 (26.5) | 243 (11.9) | 275 (11.5) | |
| Spinal cord, spinal meninges, cerebral meninges, and cauda equina | 3323 | 106 (9.5) | 254 (48.0) | 1371 (67.2) | 1592 (66.4) | |
| Posterior fossa, cerebellum, and brainstem | 1083 | 391 (35.2) | 90 (17.0) | 266 (13.0) | 336 (14.0) | |
| Ventricles | 540 | 156 (14.0) | 44 (8.3) | 152 (7.5) | 188 (7.8) | |
| Cranial nerves and other | 14 | 0 (0.0) | 1 (0.2) | 7 (0.3) | 6 (0.3) | |
| Type of surgery | <.001 | |||||
| No surgery | 402 | 38 (3.4) | 23 (4.3) | 114 (5.6) | 227 (9.5) | |
| Biopsy | 2031 | 196 (17.6) | 164 (31.0) | 755 (37.0) | 916 (38.2) | |
| STR | 533 | 96 (8.6) | 48 (9.1) | 159 (7.8) | 230 (9.6) | |
| GTR | 1377 | 275 (24.8) | 124 (23.4) | 484 (23.7) | 494 (20.6) | |
| Surgery, NOS | 64 | 3 (0.3) | 9 (1.7) | 27 (1.3) | 25 (1.0) | |
| Unknown | 19 | 3 (0.3) | 1 (0.2) | 3 (0.1) | 12 (0.5) | |
| Radiation | <.001 | |||||
| No radiation | 3816 | 428 (38.5) | 261 (49.3) | 1382 (67.8) | 1745 (72.8) | |
| Radiation | 2260 | 683 (61.5) | 268 (50.7) | 657 (32.2) | 652 (27.2) | |
| All-cause mortality | <.001 | |||||
| Alive | 4462 | 696 (62.6) | 440 (83.2) | 1692 (83.0) | 1634 (68.2) | |
| Died | 1614 | 415 (37.4) | 89 (16.8) | 347 (17.0) | 763 (31.8) | |
| Grade | <.001 | |||||
| Well differentiated | 326 | 38 (3.4) | 28 (5.3) | 134 (6.6) | 126 (5.3) | |
| Moderately differentiated | 656 | 68 (6.1) | 50 (9.5) | 241 (11.8) | 297 (12.4) | |
| Poorly differentiated | 120 | 55 (5.0) | 9 (1.7) | 29 (1.4) | 27 (1.1) | |
| Undifferentiated | 579 | 309 (27.8) | 65 (12.3) | 105 (5.1) | 100 (4.2) | |
| Unknown | 4395 | 641 (57.7) | 377 (71.3) | 1530 (75.0) | 1847 (77.1) | |
| Histology | <.001 | |||||
| Ependymoma, NOS | 4126 | 671 (60.4) | 301 (56.9) | 1396 (68.5) | 1758 (73.3) | |
| Ependymoma, anaplastic | 766 | 395 (35.6) | 88 (16.6) | 141 (6.9) | 142 (5.9) | |
| Papillary ependymoma | 60 | 5 (0.5) | 6 (1.1) | 24 (1.2) | 25 (1.0) | |
| Myxopapillary ependymoma | 1124 | 40 (3.6) | 134 (25.3) | 478 (23.4) | 472 (19.7) |
Chi-square tests used for categorical variables.
*Statistical significants of P value < 0.05.
Fig. 1.
Characteristics of ependymoma patients, anatomical location, and treatment modality from 1975 to 2016 by various age groups (A) proportion of sex, (B) proportion of anatomical location, (C) proportion of radiation therapy, (D) proportion of the extent of surgical resection, (E) proportion of death.
Adjusted Relative Risks for All-Cause Mortality
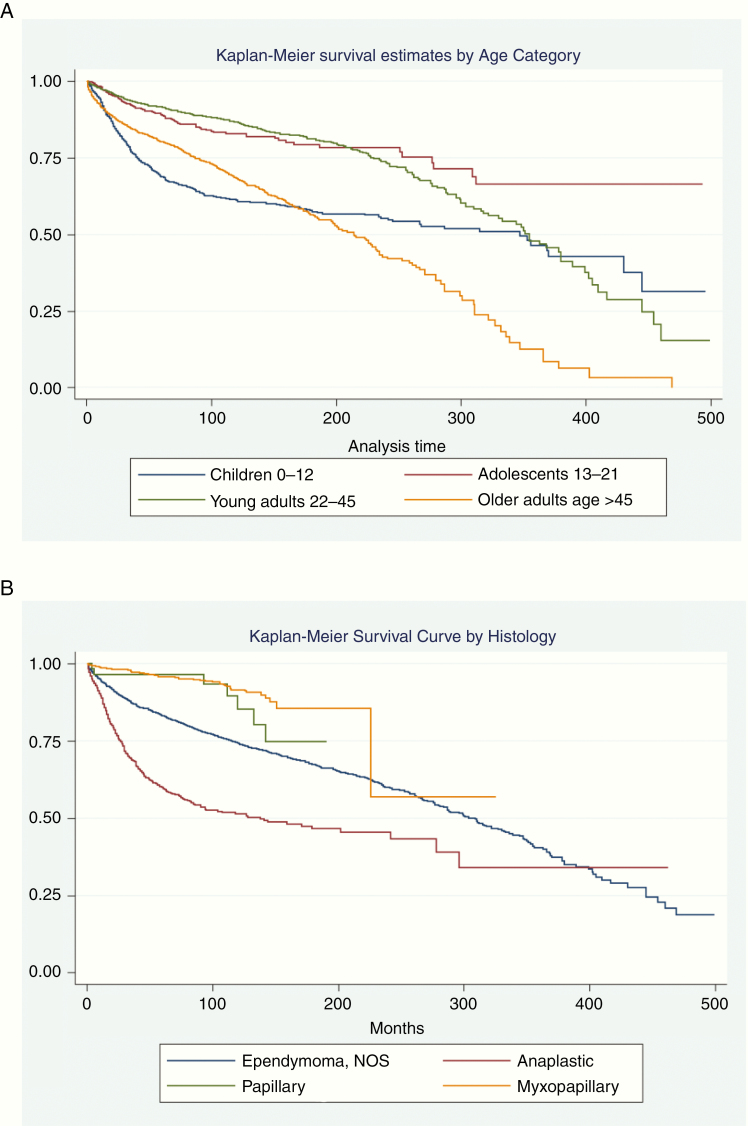
On multivariate regression analysis, male gender (RR: 1.24, 95% CI: 1.05–1.45, P = .010), age older than 45 years (RR: 1.61, 95% CI: 1.27–2.03, P < .001), and radiation therapy (RR: 1.43, 95% CI: 1.20 1.71, P < .001) were all independently associated with higher rates of all-cause mortality (Figure 2, Table 3). Age 13–21 years (RR: 0.5, 95% CI: 0.35–0.72, P < .001), 22–45 years (RR: 0.56, 95% CI: 0.44–0.73, P < .001), spinal cord location (RR: 0.23, 95% CI: 0.19–0.29, P < .001), ventricle location (RR: 0.63, 95% CI: 0.47–0.86, P = .004), biopsy only (RR: 0.34, 95% CI: 0.26–0.44, P < .001), and use of GTR (RR: 0.46, 95% CI: 0.35–0.6, P ≤ .001) were all independently associated with lower rates of all-cause mortality (Table 3). Non-white race (P = .222), posterior fossa tumor location (P = .051), cranial nerve tumor location (P = .548), STR (P = .267), NOS surgery (P = .359), and unknown surgical status (P = .351) were not found to have a statistically significant independent association with all-cause mortality (Table 3).
Fig. 2.
Kaplan–Meier survival curves for ependymoma patients by (A) age category and (B) histology. Thirty-nine patients with unknown survival months were excluded (n = 6042).
Table 3.
Multivariate Model for Factors Associated With All-Cause Mortality Among Ependymoma Patients, 1975–2016 (n = 6076)
| OR | 95% CI | P | |
|---|---|---|---|
| Sex | |||
| Female | ref | ||
| Male | 1.24 | 1.05–1.45 | .010 |
| Age category, years | |||
| Children 0–12 | ref | ||
| Adolescents 13–21 | 0.5 | 0.35–0.72 | <.001 |
| Young adults 22–45 | 0.56 | 0.44–0.73 | <.001 |
| Older adults >45 | 1.61 | 1.27–2.03 | <.001 |
| Race | |||
| White | ref | ||
| Non-white | 1.14 | 0.92–1.41 | .222 |
| Anatomical location of the tumor | |||
| All supratentorial brain, overlapping lesion of brain, and brain, NOS | ref | ||
| Spinal cord, spinal meninges, cerebral meninges | 0.23 | 0.19–0.29 | <.001 |
| Posterior fossa, cerebellum, and brainstem | 0.79 | 0.62–1 | .051 |
| Ventricles | 0.63 | 0.47–0.86 | .004 |
| Cranial nerves and other | 0.66 | 0.18–2.52 | .548 |
| Type of surgery | |||
| No surgery | ref | ||
| Biopsy | 0.34 | 0.26–0.44 | <.001 |
| STR | 0.84 | 0.62–1.14 | .267 |
| GTR | 0.46 | 0.35–0.6 | <.001 |
| Surgery, NOS | 0.73 | 0.38–1.42 | .359 |
| Unknown | 1.60 | 0.60–4.26 | .351 |
| Radiation | |||
| No radiation | ref | ||
| Radiation | 1.43 | 1.20–1.71 | <.001 |
Discussion
In this retrospective study of 6076 pediatric and adult ependymoma patients, we found significant differences between the cohorts with respect to race, anatomical location, type of resection, use of radiation, tumor grade, histological classification, and all-cause mortality. Additionally, in a multivariable regression analysis, factors associated with all-cause mortality rates included males (vs females), supratentorial tumors (vs spinal cord tumors), and radiation treatment (vs no radiation), and age older than 45 years (compared to younger than 45 years).
It has been widely established that ependymoma tumor locations commonly differ between children and adults. In a retrospective study of 1402 patients diagnosed with ependymoma, McGuire et al.6 demonstrated that most pediatric tumors are mostly intracranial while adult tumors are more frequent in the spinal cord. Similarly, in a retrospective study of 2802 patients diagnosed with ependymoma, Amirian et al.13 demonstrated that adult patients presented with spinal cord ependymomas at a rate nearly 8 times greater than pediatric patients. Furthermore, the author also found that intracranially, pediatric patients have twice as high a rate of supratentorial ependymomas as adult patients.13 Analogous to the aforementioned studies, our study found that adults had approximately 7 times greater rate of spinal cord tumors as children and 1.39 times of adolescents.
While there have been a few studies attempting to find associations in tumor location, only a few studies introduced demographic associations within the pediatric versus adult ependymoma cohorts. In the Amirian et al.13 study, the authors found in the pediatric cohort there were higher rates of males and nearly twice the rate of both blacks and Hispanics when compared to the adult population. Similarly, in a retrospective cohort studies of 998 adult ependymoma patients, Khalid et al.14 found 58.5% of patients to be male and 80.9% to be white.
In another retrospective cohort study of 482 pediatric ependymoma patients, Snider et al.15 found that 56.0% of patients were male and 82.2% were white. In contrast, in a retrospective study of 773 patients with myxopapillary ependymoma, Bates et al.16 showed there to be higher rates of ependymomas among males while blacks and Asians were seen to have lower rates of tumor incidence. While, in the study by Khalid et al.,14 the authors did not find an association between either race or gender and tumor incidence. Our study had a greater proportion of females and whites, compared to male and non-white patients. Further genetic studies are warranted to understand patient characteristics and demographics that have a predisposition to pediatric and adult-onset ependymomas.
At a molecular level, a growing body of literature suggests different pathogenic mechanisms in adults versus pediatric ependymomas. In an ependymoma tissue immunohistochemical analysis from 84 ependymomas resected between 1967 and 2002, Rajaram et al.17 reported that particular gene mutations (4.1B deletion and loss of 4.1R expression) were statistically more common in pediatric populations (P < .001). Of note, 4.1B deletions were also associated with a higher tumor grade (P < .001).17 Similarly, in the retrospective study by Amirian et al.,13 the authors found that children were proportionally 3 times more likely to have high-grade, anaplastic ependymoma than their adult counterparts (P < .001). There have been conflicting findings on the impact that tumor grade has on survival. In a retrospective study of 179 pediatric patients with intracranial ependymoma, Indelicato et al.18 actually showed no association between tumor grade and overall survival. Similarly, in a retrospective study of 482 pediatric patients with ependymoma, Snider et al.15 reported no significant association between tumor grade and overall survival. Contrary, in a retrospective study of 258 ependymoma patients, Korshunov et al.19 found higher tumor grade to be significantly and independently associated with inferior event-free survival as well as overall survival. Along the same lines, in a retrospective study of 1353 patients with spinal ependymoma, Lin et al.20 supported Korshunov et al. findings by reporting an association between lower grade ependymomas with higher rates of overall survival. Ongoing clinical trials are making efforts to integrate ependymoma molecular subgroups into the design of the trials to produce more focused treatment results.21 Due to the lack of consensus on tumor grading,22,23 a more complete understanding of the molecular and pathogenic mechanisms of ependymoma is necessary to allow pursuit toward individualized novel therapeutic approaches.
Although the characteristics of ependymomas differ among pediatric and adult populations, there remains a lack of clear consensus regarding differential management strategies by age group. In a review article that summarizes clinical aspects of ependymoma management in children and adolescents, Thorp et al.24 state that GTR and focal radiotherapy is the standardized care for localized disease in pediatrics, as in adults.25 In fact, in the retrospective study of 2802 ependymoma patients, Amiran et al. reported proportionally more children were treated with GTR (P = .02) and fewer were treated with radiation therapy (P < .001) than their adult counterparts.13,26 Additionally, the authors found that complete surgical resection conferred the most protection among both pediatric and adult patients compared to STR and biopsy.13 Similarly, in a retrospective study of 1353 patients with spinal ependymoma, Lin et al.20 demonstrated that complete surgical resection was associated with improved clinical outcomes compared to STR. Analogously, in a retrospective study of 482 pediatric ependymoma patients, Snider et al.15 demonstrated GTR and radiotherapy to be associated with improved clinical outcomes. Investigations to compare treatment outcomes for adult and pediatric ependymoma patients have been sparse. In a systematic review of 28 myxopapillary ependymoma articles describing 475 patients aimed to establish evidence-based guidelines for management, Feldman et al.27 reported higher recurrence rates for children relative to adults (P = .02) despite similar primary treatment (P = .34) and extent of resection (P = .74) utilized across all ages. In another retrospective study of 51 intradural and intramedullary ependymoma resections for 43 patients, Domazet et al.26 detected a trend between younger age and tumor recurrence. Establishment of guidelines delineating the appropriate treatment paradigm by age group are needed to improve clinical outcomes following management of ependymoma.
A number of studies have examined the effect of various factors on mortality rate in ependymoma patients. In a retrospective study of 635 children with diagnoses of ependymoma, McGuire et al.28 found much higher rates of survival for spinal tumors as compared to infratentorial and supratentorial tumors following multivariate regression. Furthermore, there was no differential survival based on race or gender, while higher rates of survival were associated with radiotherapy, although this latter finding was strictly for infratentorial tumors.28 Similarly, in a retrospective study of 2408 ependymoma patients, Rodriguez et al.29 found intracranial tumor locations were independent predictors of poor outcome. Moreover, Rodriguez et al.29 found neither race nor radiation to be associated with differences in survival, while female patients had higher survival rates than their male counterparts. In the Amirian et al.30 retrospective study of 2802 older adult patients (defined as over the age of 60), the authors showed that GTR had much lower rates of mortality than STR. Similarly, in a retrospective study of 179 pediatric patients with intracranial ependymoma, Indelicato et al.18 found both STR and male gender to be associated with poorer overall survival while race, age, and tumor location had no effect. In a retrospective study of 773 patients with myxopapillary ependymoma, Bates et al.16 demonstrated that neither male gender or race was statistically significant prognostic factors for overall survival while young age was significantly associated with more positive outcomes. In our study we found male gender, supratentorial location, and the use of radiation therapy to be independently associated with higher rates of mortality. Overall, further studies are necessary to identify risk factors associated with mortality in pediatric and adult patients diagnosed with an ependymoma. Furthermore, standardizing evidence-based treatment regiments focused on pediatrics and adults separately may provide avenues to reducing overall mortality rates and bettering outcomes.
This study has several inherent limitations, which has potential implications for its interpretation. First, the analysis is retrospective, with data only available by ICD-O-3 codes which may contain coding and reporting biases. Second, there is a possibility of misclassified or incomplete data, including tumor grade and histology. Third, there is a lack of information regarding radiation fields, doses, and intent in the SEER database, thus obfuscating the quality of radiation therapy. We are also unable to comment on the changes in the classification of grading for ependymomas, molecular subtypes, nor the similarities and differences between the treatments provided for different anatomical locations (eg, spinal vs intracranial). Furthermore, this study is limited by a lack of data on the WHO ependymoma grading system, general anatomical locations, and granularity of surgical operations. Finally, patient migration or losing the patient to follow-up may lead to skewed mortality rates. Regardless, this study demonstrates the demographic and treatment risk factors leading to increased rates of mortality in various patient subpopulations following the management of ependymoma.
Conclusions
Our study using the SEER database demonstrates the various demographic and treatment risk factors that are associated with increased rates of all-cause mortality between the pediatric and adult populations following a diagnosis of ependymoma. Further prospective analysis is warranted to identify optimal treatment paradigms for both pediatric and adult patient populations to better overall patient care and decrease all-cause mortality rates.
Funding
None to disclose.
Conflict of interest statement. None to disclose.
Authorship Statement
Conceived and designed the research: A.A.E., A.B.K., V.L., and C.K.Z. Acquired the data: A.A.E., A.B.K., and V.L. Analyzed and interpreted the data: A.A.E., A.B.K., V.L., and C.K.Z. Performed statistical analysis: A.B.K., V.L., and C.K.Z. Study supervision: K.T.K. and M.D. Drafted the manuscript: A.A.E., A.B.K., W.B.D., A.J.K., C.S.H., T.D., and B.R. Made critical revision of the manuscript for important intellectual content: all authors.
References
- 1. Dorfer C, Tonn J, Rutka JT. Ependymoma: a heterogeneous tumor of uncertain origin and limited therapeutic options. Handb Clin Neurol. 2016;134:417–431. [DOI] [PubMed] [Google Scholar]
- 2. Rudà R, Reifenberger G, Frappaz D, et al. . EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20(4):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Gittleman H, Xu J, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5. Villano JL, Parker CK, Dolecek TA. Descriptive epidemiology of ependymal tumours in the United States. Br J Cancer. 2013;108(11):2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg. 2009;110(4):725–729. [DOI] [PubMed] [Google Scholar]
- 7. Kleihues P, Louis DN, Scheithauer BW, et al. . The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225; discussion 226. [DOI] [PubMed] [Google Scholar]
- 8. Pajtler KW, Witt H, Sill M, et al. . Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: prognosis and the M. D. Anderson Cancer Center experience. Neuro Oncol. 2010;12(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010;10(3):240–247. [DOI] [PubMed] [Google Scholar]
- 12. Torre M, Alexandrescu S, Dubuc AM, Ligon AH, Hornick JL, Meredith DM. Characterization of molecular signatures of supratentorial ependymomas. Mod Pathol. 2020;33(1):47–56. [DOI] [PubMed] [Google Scholar]
- 13. Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology. 2012;39(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalid SI, Adogwa O, Kelly R, et al. . Adult spinal ependymomas: an epidemiologic study. World Neurosurg. 2018;111:e53–e61. [DOI] [PubMed] [Google Scholar]
- 15. Snider CA, Yang K, Mack SC, et al. . Impact of radiation therapy and extent of resection for ependymoma in young children: a population-based study. Pediatr Blood Cancer. 2018;65(3):e26880. [DOI] [PubMed] [Google Scholar]
- 16. Bates JE, Choi G, Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol. 2016;129(2):251–258. [DOI] [PubMed] [Google Scholar]
- 17. Rajaram V, Gutmann DH, Prasad SK, Mansur DB, Perry A. Alterations of protein 4.1 family members in ependymomas: a study of 84 cases. Mod Pathol. 2005;18(7):991–997. [DOI] [PubMed] [Google Scholar]
- 18. Indelicato DJ, Bradley JA, Rotondo RL, et al. . Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018;57(5):644–648. [DOI] [PubMed] [Google Scholar]
- 19. Korshunov A, Golanov A, Sycheva R, Timirgaz V. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer. 2004;100(6):1230–1237. [DOI] [PubMed] [Google Scholar]
- 20. Lin Y, Smith ZA, Wong AP, Melkonian S, Harris DA, Lam S. Predictors of survival in patients with spinal ependymoma. Neurol Res. 2015;37(7):650–655. [DOI] [PubMed] [Google Scholar]
- 21. Venneti S. Integrating ependymoma molecular subgroups into clinical trials. Neuro Oncol. 2019;21(10):1219–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouffet E, Perilongo G, Canete A, Massimino M. Intracranial ependymomas in children: a critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol. 1998;30(6):319–329; discussion 329. [DOI] [PubMed] [Google Scholar]
- 23. Ellison DW, Kocak M, Figarella-Branger D, et al. . Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thorp N, Gandola L. Management of ependymoma in children, adolescents and young adults. Clin Oncol (R Coll Radiol). 2019;31(3):162–170. [DOI] [PubMed] [Google Scholar]
- 25. Toescu SM, Aquilina K. Current and emerging methods of management of ependymoma. Curr Oncol Rep. 2019;21(9):78. [DOI] [PubMed] [Google Scholar]
- 26. Domazet I, Pašalić I, Nemir J, Peterković V, Vukić M. Predictors of functional outcome after spinal ependymoma resection. J Neurosci Rural Pract. 2018;9(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldman WB, Clark AJ, Safaee M, Ames CP, Parsa AT. Tumor control after surgery for spinal myxopapillary ependymomas: distinct outcomes in adults versus children: a systematic review. J Neurosurg Spine. 2013;19(4):471–476. [DOI] [PubMed] [Google Scholar]
- 28. McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52(1):65–69. [DOI] [PubMed] [Google Scholar]
- 29. Rodríguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005). J Surg Res. 2009;156(2):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amirian ES, Armstrong TS, Gilbert MR, Scheurer ME. Predictors of survival among older adults with ependymoma. J Neurooncol. 2012;107(1):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]