Abstract
Suicide gene therapy has represented an experimental cancer treatment modality for nearly 40 years. Among the various cancers experimentally treated by suicide gene therapy, high-grade gliomas have been the most prominent both in preclinical and clinical settings. Failure of a number of promising suicide gene therapy strategies in the clinic pointed toward a bleak future of this approach for the treatment of high-grade gliomas. Nevertheless, the development of new vectors and suicide genes, better prodrugs, more efficient delivery systems, and new combinatorial strategies represent active research areas that may eventually lead to better efficacy of suicide gene therapy. These trends are evident by the current increasing focus on suicide gene therapy for high-grade glioma treatment both in the laboratory and in the clinic. In this review, we give an overview of different suicide gene therapy approaches for glioma treatment and discuss clinical trials, delivery issues, and immune responses.
Keywords: glioma, immunotherapy, stem cells, suicide gene therapy, viral vectors
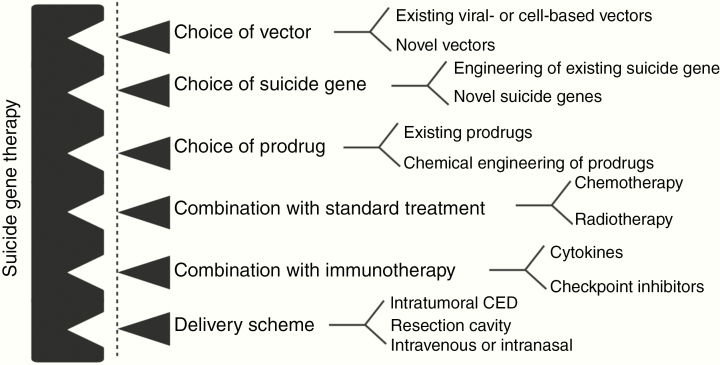
High-grade gliomas (HGGs), collectively known as WHO grade III and IV primary brain tumors, belong to the most deadly and incurable group of cancers.1 Lack of specificity of chemotherapeutic drugs, delivery issues to the central nervous system, and development of therapy resistance pose challenges in glioma management. The urgent need for novel and more efficient treatment strategies has produced various novel molecularly targeted therapeutic options that have been tested in clinical trials alongside the traditional chemotherapy/radiation approaches.2 However, the outcomes have mostly fallen short of expectations.2,3 Another experimental treatment modality that has persistently been tested in HGG patients is suicide gene therapy (SGT) or also known as gene-directed enzyme prodrug therapy. The initial successful proof of concept in animal models4 and small-scale clinical trials5 could not be replicated in a large-scale trial6 generating a negative wave against further development of SGT. However, continued research has promoted further improvements of this strategy resulting in a more tailored therapeutic avenue for HGG. SGT is a multi-componential approach (Figure 1) and thus offers unique possibilities of tailoring the therapy further by improving the individual components according to the new mechanistic insights into glioma biology, novel vector developments, and also the development of new delivery techniques such as convection-enhanced delivery (CED). Therefore, these new developments have been molding SGT to become potentially more effective for HGG treatment. As a result, several improved SGT systems are currently being tested in the laboratory and some have entered clinical trials. In this review, we discuss past and present developments in the SGT field and also critically review the clinical translation, delivery issues, and immune responses.
Fig. 1.
SGT consists of different modules where each single module can be subjected to improvement to enhance therapeutic efficacy. CED, convection-enhanced delivery.
The Concept of SGT
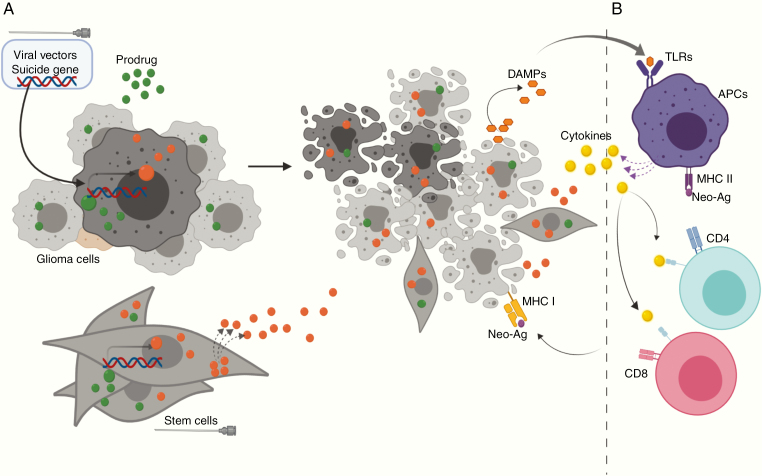
SGT represents the most common form of gene therapy used to treat HGGs in both preclinical and clinical settings. From a theoretical perspective, SGT is a two-step treatment modality for solid tumors (Figure 2A): the first step is the transduction of cancer cells by a vector encoding an enzyme (suicide gene) capable of catalyzing a prodrug into a toxic metabolite. The second step involves administration of the corresponding prodrug that upon catalysis by the prodrug-converting enzyme induces cell death. Ideally the prodrug should exhibit (1) features of an ideal substrate for the enzyme, (2) activation of cell death after catalysis with minimal or no off-target toxicity, (3) capability of crossing the blood–brain barrier efficiently, and (4) induction of the so-called bystander effect (BE). Since 100% transduction of all tumor cells is virtually impossible, the BE is an important feature of SGT. It facilitates collateral killing of non-transduced (“bystander”) cells caused by the transfer of intermediate or final metabolites of the prodrug. Thus, it is sufficient to transduce a certain fraction of the tumor to potentially achieve complete eradication of all malignant cells. Growing evidence indicates that the process of cell death induced by certain SGTs is immunogenic, which means that it can alert and stimulate an antitumor response adding to the treatment effect of SGT (Figure 2B).
Fig. 2.
The basic mechanism of SGT. (A) The suicide gene is delivered into glioma cells by viral vectors that convert the nontoxic prodrug (green) into a toxic metabolite (orange) that causes tumor cell death. Note that the toxic metabolite (or intermediate byproducts) can travel from the transduced tumor cells (dark) to the untransduced tumor cells (light) by either gap junctions or diffusion, finally leading to the death of both transduced and untransduced cells. This phenomenon is known as the bystander effect (BE). The precise mechanism of BE is dependent on the nature of the toxic drug. Suicide gene-modified stem cells kill the tumor cells via BE only. (B) Recent studies suggest that SGT can cause a release of damage-associated molecular pattern (DAMP) molecules and/or can induce a display of neo-antigens (neo-Ags) leading to immunogenic cell death. Both myeloid antigen-presenting cells (APCs) and lymphocytes are instrumental in the resulting antitumor immune response.
Development of Suicide Genes and Prodrugs
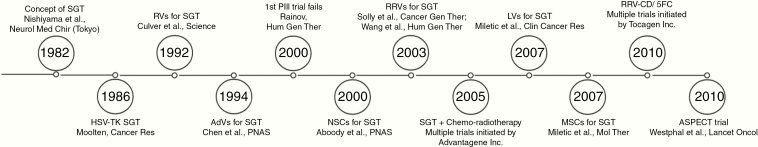
The possibility of using microbial enzymes and an antimicrobial compound to introduce selective cytotoxicity in mammalian cells was first reported in the 1980s demonstrating that transfer of genetic material of the Herpes simplex virus 1 (HSV), rendered the cells more sensitive to acyclovir (ACV)7 (Figure 3). Nishiyama et al.8,9 adopted this concept for cancer treatment by showing that delivery of cytosine deaminase (CD) from Escherichia coli followed by 5-fluorocytosine (5FC) administration leads to a significant reduction of tumor burden in a syngeneic EA285 rat glioma model. Moolten et al.10,11 contemporarily introduced SGT based on thymidine kinase (TK) from HSV for cancer treatment (Figure 3). CD and HSV-TKHSV-TK are the two most widely used suicide genes for cancer treatment including HGGs. However, a wide variety of other SGTs have been HSV-TK established (Table 1).
Fig. 3.
Timeline of major developments in SGT for HGG treatment.
Table 1.
List of Most Prominent Suicide Gene Therapy Systems Used for HGG Treatment
| Suicide Gene | Origin of Suicide Gene | Prodrug/Drug | PMID |
|---|---|---|---|
| HSV-TK | Viral | GCV/GCV-TP | 19617915 |
| VZV-TK | Viral | GCV/GCV-TP | 9231072 |
| Tomato-TK | Viral | AZT/AZT-TP | 20154339 |
| EHV4-TK | Viral | GCV/GCV-TP | 12489026 |
| Cytosine deaminase | Bacterial and yeast | 5-FC/5-FU | 23969884 |
| Purine nucleoside phosphorylase (PNP) | Bacterial | MeP-dR/MEP | 15374975 |
| Nitroreductase | Bacterial | CB1954/AHNB | 27840931 |
| Guanine phosphorybosyl transferase | Bacterial | 6TX/6GMP | 9414253 |
| Carboxylesterases (CE) | Mammalian | IRT/SN-38 | 24167321 |
| Cytochrome P450 | Mammalian, rodent | CPA/PM | 9354446 |
While some suicide genes are compatible with several prodrugs, only one representative prodrug along with the corresponding toxic drug is mentioned here.
GCV-TP, GCV triphosphate; MeP-dR, 9-β-d-[2–deoxyribofuranosyl]-6-methylpurine; MEP, 6-methylpurine; CB1954, 5-aziridinyl-2,4-dinitrobenzamide; AHNB, 5-(aziridinyl)-4-hidroxylamine-2-nitrobenzamide; IRT, irinotecan; 6TX, 6-thioxanthine; 6GMP, 6-thioguanine monophosphate; CPAC, cyclophosphamide; PM, phosphoramide mustard.
CD-Based SGT
CD, an enzyme found in bacteria and lower eukaryotes (eg, yeast), is involved in microbial pyrimidine metabolism and deaminates 5FC (and other analogs, namely, 6-azacytosine, isocytosine) into 5-fluorouracil (5FU). 5FU is a pyrimidine analog that directly inhibits nucleic acid synthesis due to misincorporation instead of uracil or thymine. 5FU can also be catalyzed by cellular enzymes into fluorodeoxyuridine monophosphate that can interfere with DNA metabolism by binding thymidylate synthase.12 Therefore, 5FU itself has been used as a potent anticancer chemotherapeutic agent for many years, but off-target toxicity has limited its direct applicability.8,9,13 Two different CD proteins have been adopted for developing the SGT system with CD/5FC: bacterial CD (bCD; source: E. coli) and yeast CD (yCD; source: Saccharomyces cerevisiae). Although bCD and yCD bear little homology, they catalyze cytosine and 5FC in a similar fashion, albeit with different efficiency. yCD has a significantly lower Km and a higher Vmax for 5FC than bCD13; however, yCD exhibits thermal instability. The enzyme works optimally at around 26°C and loses activity as temperature rises.8,13 This shortcoming has been mended by rational protein engineering that involves alteration of 3 amino acids in the yCD gene.14 Currently, both bCD and the recombinant yCD are being used for HGG treatment.15,16
HSV-TK-Based SGT
HSV encodes a TK gene that is evolutionarily and functionally different than the human thymidine kinases (hTKs).17 Compared to hTKs, HSV-TK more efficiently catalyzes various prodrugs (synthetic nucleoside analogs) producing mono-phosphorylated nucleoside analogs that are further phosphorylated by cellular kinases.17 The resulting triphosphorylated analogs are incorporated into DNA strands during replication and cause strand abrogation leading to cell death of actively proliferating cells. Importantly, the analogs (ie, prodrugs) are not efficiently recognized by the hTKs preventing toxicity for normal cells. As a result, HSV-TK acts as a suicide gene upon prodrug exposure without any major interference of the hTKs. Various purine and pyrimidine analogs are compatible with HSV-TK SGT such as ganciclovir (GCV), ACV, and brivudin (BVDU).18,19 BVDU is an efficient substrate of HSV-TK and a potent inducer of cell death,20 but exhibits poor BE.21 GCV is a better substrate for HSV-TK compared with ACV and exhibits a greater BE compared to either ACV or BVDU.21,22 Valganciclovir (valGCV), an oral analog of GCV, has recently been shown to be suitable for long-term treatment in a GBM xenograft model.23
The wild-type HSV-TK suffers from a few shortcomings: higher affinity toward its natural substrate endogenous thymidine (dT) compared to GCV24 and presence of cryptic sites leading to anomalous transcription25 or splicing.26 Such limitations can be overcome by optimizing sequences of the HSV-TK gene,27 and a novel mutant with superior functionality has been developed to be used for treatment of experimental HGG.17,23,28,29
Vector Systems for SGT
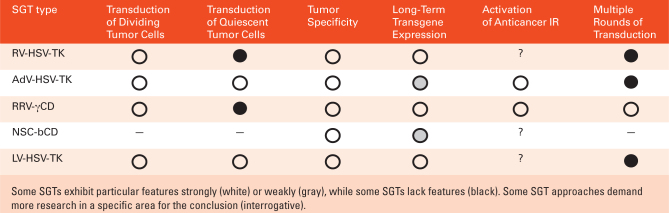
Soon after the emergence of SGT principles,9,10 γ-retroviral vectors (RVs) and later adenoviral vectors (AdVs) were employed to deliver suicide genes into tumors.4,30 Although these vectors were effective in glioma animal models and to some extent in early-phase clinical trials, results from a larger phase III trials were disappointing.6,31 Treatment failure was mostly attributed to various shortcomings of the viral vectors indicating that more efficient vector systems need to be developed to harness the power of SGT for cancer treatment. Thus, per today a wide variety of vectors derived from different viral backbones are used for SGT (Table 2). Apart from viral vectors, stem-cell-based vectors have been developed for SGT.
Table 2.
Key Features of an Efficient SGT System for HGG Treatment
AdV-Mediated SGT
The failure of RV-mediated SGT in a phase III clinical trial6 was attributed to low transduction efficiency which gave an impetus to the development of viral vectors with better transduction capability. Since AdVs can transduce non-dividing cells, it was anticipated that the problem of suboptimal transduction of RVs would be solved by using AdVs. Indeed, non-replicating AdVs have been demonstrated to deliver a transgene more efficiently than RVs in human gliomas.32 In line with this, AdV-mediated HSV-TK/GCV (AdV-TK/GCV) therapy demonstrated a significant therapeutic benefit in experimental glioma models by several independent investigators18,33,34 and also in small-scale HGG clinical trials.35–37 No serious adverse effects were observed, which is an important finding since AdVs had caused death of a patient in a trial for ornithine transcarbamylase gene therapy.38 Thus, a phase III clinical trial (known as ASPECT) was performed to evaluate the potential therapeutic benefit of AdV-TK/GCV therapy as an adjuvant treatment arm to standard of care (resection, radiotherapy, and temozolomide [TMZ] administration).31 While ASPECT showed significant improvement in “time-to-re-intervention” compared to standard care, unfortunately the study failed to show a significant effect on overall survival. Although there was a stronger treatment effect in patients with non-methylated MGMT (O-6-methylguanine-DNA methyltransferase; a DNA repair protein and a major prognostic factor for TMZ treatment), the results did not satisfy the European Medicines Agency (EMA) and thus the marketing request was denied. Several theoretical reasons could be attributed to the failure of the ASPECT trial. Firstly, it is possible that the interpretation of the therapeutic outcome of the SGT was compromised by the lack of universal standard care protocols (namely, differential TMZ administration) across all institutions.31,39 Secondly, the therapy itself might not have been effective enough. Despite improved transduction capability, AdVs suffer from a major drawback by the lack of long-term expression of transgenes. Unlike RVs, AdVs do not integrate into the host genome and thus transgenes are only expressed transiently for up to a few weeks.38,40 In this context, we have recently shown that short-term SGT can result in treatment-escape and that long-term suicide gene activity can improve treatment efficacy.23 While the problem of short-term gene expression can be somewhat mitigated in the CNS where the transgene expression may be detected for at least up to 3 months,18 the episomal maintenance of AdVs still causes the transgene expression to be reduced following cell divisions that would likely lead to compromised efficacy during tumor expansion.
Because of its excellent safety profile, high transduction efficiency in both dividing and non-dividing cells, standardized manufacturing process of clinical batches, and potential to immunogenic stimulation (discussed below), AdV-mediated SGT is still pursued in both laboratories and the clinic with different combinatorial HGG treatment strategies. In this context, concomitant radiotherapy with AdV-TK/ACV has recently been tested in clinical trials showing notable improvement in survival outcomes.19,41,42 Other combination strategies such as concomitant TMZ or Nivolumab (checkpoint inhibitor) are also being pursued in different clinical trials at this point (Table 3). Another combinatorial paradigm involves dual AdV therapy where the second vector delivers the immunostimulatory gene Flt3L that increases antitumor immune responses (Table 3).43,44
Table 3.
Clinical Trials Involving SGT for HGG Treatment
| Trial No. | Start– Completiona | Phase | Patients | Vector Used | Suicide Gene/ Prodrug | Combination | Result | Citation |
|---|---|---|---|---|---|---|---|---|
| NCT00751270 | 2005–2011 | Ib | Newly diagnosed HGG | AdV | HSV1-TK/ valACV | RT+TMZb | Safety assessed | 20 |
| NCT00589875 | 2007–2015 | Iia | Newly diagnosed HGG | AdV | HSV1-TK/ valACV | RT+TMZb | Safety assessed | 45 |
| NCT00870181 | 2008–2012 | II | Recurrent HGG | AdV | HSV1-TK/ GCV | N/A | Improved survival | 42 |
| NCT01172964 | 2010–2015 | I | Recurrent HGG | NSC(HB1. F3.CD) | bCD/5FC | Safety assessed | 46 | |
| NCT00634231 | 2010–2015 | I | Newly diagnosed HGG (pediatric) | AdV | HSV1-TK/ valACV | RT+TMZb | Safety assessed/ ongoing | 47 |
| NCT01156584 | 2010–2016 | I | Recurrent HGG | RRV | yCD/5FC | N/A | — | — |
| NCT01470794 | 2012–2016 | I | Recurrent HGG (undergoing surgery) | RRV | yCD/5FC | N/A | Safety + encouraging efficacy | 48,49 |
| NCT01985256 | 2014–2016 | I | Recurrent HGG (undergoing surgery) | RRVc | yCD/5FC | N/A | — | — |
| NCT02015819 | 2014–2019 | I | Recurrent HGG | NSC(HB1. F3.CD) | bCD/5FC+ Leucovorin | N/A | Ongoing | |
| NCT01811992 | 2014–2020 | I | Newly diagnosed HGG | AdV | HSV1-TK/ valACV | AdV-Flt3L | Ongoing | — |
| NCT02414165 | 2015–2019 | II-III | Recurrent HGG | RRV | yCD/5FC | — | — | 50 |
| NCT02192359 | 2016–2020 | I | Recurrent HGG | HB1. F3.CD21. hCE1m6 | hCE1m6/ irinotecan | — | Ongoing | — |
| NCT03596086 | 2017–2023 | I-II | Recurrent HGG | AdV | HSV1-TK/ valACV | RT+TMZ | Ongoing | — |
| NCT03603405 | 2018–2023 | I-II | Newly diagnosed HGG | AdV | HSV1-TK/ valACV | RT+TMZ | Ongoing | — |
| NCT03576612 | 2018–2021 | PI | Newly diagnosed HGG | AdV | HSV1-TK/ valACV | RT+ Nivolumab+TMZb | Ongoing | — |
| NCT02598011 | 2016–2022 | I | Newly diagnosed HGG | RRV | yCD/5FC | RT+TMZ | Planned | — |
Only the trials carried on/undertaken/planned since 2010 are mentioned here. See review from Kaufmann et al.36 where some of the trials before 2010 are discussed.
aPrimary completion.
bTMZ allowed after prodrug administration.
cIntravenous administration.
Replicating Retroviral Vector-Mediated SGT
The major drawbacks of RVs and AdVs, namely, low transduction rate and episomal nature may be circumvented by using replicating retroviral vectors (RRVs). RRVs of non-primate origin have been reported to efficiently transduce glioma cells and thus refocused the translational attention of SGT involving γ-RVs.51 Since RRVs exhibit a non-lytic life cycle, the therapy is mostly dependent on the suicide gene activity which could be achieved by using CD or viral TK.45,51 While RRVs in general transduce only dividing cells, the high-transduction ability is conferred by the replicative nature of the vectors. Most importantly, RRV replication is restricted to glioma cells in vivo due to the post-mitotic state of most normal cells within the CNS.51 By using a recombinant yCD gene,47 several clinical trials funded by Tocagen, Inc., were performed demonstrating safety as well as encouraging results compared to external lomustine-treated cohorts.51,52 These positive indications resulted in the designation of breakthrough and PRIME status for HGG treatment by FDA and EMA, respectively. The clinical studies took advantage of next-generation sequencing technologies revealing some interesting aspects of the therapy in relation to associated prognostic factors. For example, an important discovery was made by identifying a transcriptomic signature, termed survival-related neuronal subtype (SRNS), that is associated with Toca 511/5FC-mediated survival.51 Interestingly, the SRNS signature shows functional similarities with the TCGA neural subtype and thus patients who exhibited SRNS (and a TCGA neural subtype) benefitted most from Toca 511/5FC. Aside from SRNS, some other prognostic factors were also identified including a tentative identification of Isocitrate dehydrogenase 1 (IDH1) mutation as a positive prognostic factor.51,52 Furthermore, the study revealed that the activity of CD/5FC, similar to HSV-TK/GCV, is independent of patient MGMT status. The preliminary success warranted a phase III clinical trial (NCT02598011) which has been performed in 403 HGG patients. Recently Tocagen, Inc., made a press release announcing that the phase III trial unfortunately failed to meet the study endpoints.53 While detailed results are currently unavailable, the failure once again reveals the tremendous challenge of treating HGGs. Further improvement related to this treatment strategy may most likely depend on combinatorial approaches with other treatment modalities.
Lentiviral Vector-Mediated HSV-TK/GCV Therapy
Lentiviral vectors (LVs), developed as a spin-off from HIV research, serve as one of the most popular vector systems in gene therapy. In contrast to the aforementioned vectors, LVs offer 2 unique features that are very important for SGT toward HGGs: transduction capability in quiescent cells and long-term gene expression. Similar to all retroviruses, lentiviruses integrate the provirus into host-cell genomes. However, unlike γ-retroviruses, lentiviruses are equipped with active nuclear transport machinery leading to genome integration independent of mitosis. LVs, availing this mechanism, can transduce quiescent glioma cells much more efficiently than RVs, as shown in biopsy-based glioma spheroids,48 where a significant fraction of non-dividing tumor cells is present. These resting glioma cells could not be targeted by RVs neither in vitro nor in vivo. LVs are most frequently pseudotyped with the glycoprotein (GP) of vesicular stomatitis virus (VSV-G). However, GPs from other viruses can also be used. We have shown that LVs pseudotyped with the GPs of the lymphocytic choriomeningitis virus transduce glioma cells more specifically compared to VSV-G pseudotyped vectors which have a strong tropism for neurons.54 To investigate therapeutic efficacy, LV-based HSV-TK/GCV treatment was tested in a patient-derived GBM xenograft model and subsequently complete, albeit temporary, tumor remission after GCV administration was observed.48 In this study, a fraction of tumor cells from recurrent tumors still expressed the suicide gene indicating that short-term prodrug administration (2–3 weeks), which is currently standard in clinical trials, is not sufficient to achieve the maximum treatment effect. This hypothesis was confirmed in a follow-up study showing that long-term administration of valGCV, a prodrug tailored for oral application, increased the treatment effect compared to short-term GCV treatment.23 Due to unrestricted transducing potential, LVs, in particular those pseudotyped with VSV-G, can also transduce normal post-mitotic brain cells, however, without toxicity even when using HSV-TK/GCV.55 On the contrary, normal brain cells expressing HSV-TK contribute in eliminating glioma cells through BE.49 LV-based SGT has not yet been tested in clinical trials; however, the various auspicious features observed in the most clinically relevant GBM models strongly warrant clinical investigation.
Cell-Based SGT
Apart from viral vectors, different types of cells are also used as vectors for SGT. Stem cells such as neural stem cells (NSCs) and mesenchymal stem cells (MSCs) are the major sources for this strategy. Recently, olfactory ensheathing cells have also been shown to be an efficient vector for SGT.50 In general, these cells show an intrinsic migratory capacity and exhibit exceptional tropism toward pathological conditions including neoplastic lesions in the CNS.50,56 The tumor-tropic property of these cells has its origin in the bona fide regenerative and reparative roles in cellular homeostasis which also relates to the sensing of various pro-tumorigenic signals such as angiogenesis, hypoxia, inflammatory signals, etc..50,57–60 Furthermore, these cells survive in vivo engraftment (even in an allogeneic situation) for a certain time period due to low or undetectable MHC expression56 and do not form neoplastic lesions indicating a high safety profile.56 Cell-mediated tumor-killing activity solely depends on the BE and thus an SGT system with high bystander efficiency is a prerequisite.57,61 NSCs were the first type of cells used as a vector for SGT. The delivery potential of NSCs for SGT of glioma was first reported by using an immortalized murine NSC line which was retrovirally transduced with bCD. The engrafted NSCs migrated in a glioma-specific manner, both ipsilaterally and contralaterally, and were able to kill tumor cells upon 5FC administration.15 An important issue for clinical translation of NSC-mediated SGT (or any cell-based system for that matter) is to choose between an autologous and allogeneic source. Ideally an autologous source of NSC will be preferential based on immune escape. Although NSCs normally show low levels of MHC expression, there exists an immunogenic potential62 that would eventually promote the clearing of allogeneic NSCs within week(s).56 This issue can be partially circumvented by using immunosuppressive drugs; however, this may thwart anticancer immune responses and interfere with the overall treatment efficacy. Furthermore, the use of autologous NSCs is associated with several logistic shortcomings such as lack of adequate source as well as long-term culture for expansion to large cell numbers for clinical application. Cellular reprogramming technologies such as induced pluripotent stem cells are currently being pursued to obtain sufficient cell numbers of autologous NSCs.63,64 In contrast, the use of allogeneic NSCs offers several advantages over autologous NSCs in terms of time, cost, scalability, and standardization procedures.57 To date, two different NSC-mediated SGTs have been pursued in clinical studies and both are based on an immortalized allogeneic human NSC line known as HB1.F3.65,66 The first trial involved treatment with a bCD-modified HB1.F3 cell line in order to evaluate initial safety and feasibility.67 The NSCs were observed to migrate to distant tumor sites in the HGG patients and initial safety was demonstrated. As a result, a phase I trial with 18 patients has been started which will be completed soon (Table 3). The second trial involves the HB1.F3 line expressing the suicide gene human carboxylesterase (hCE1m6) (Table 3).
MSCs68 possess an intrinsic migratory capacity toward pathological lesions similar to NSCs and in this regard no substantial differences have been found between these two cell types.69 In addition, MSCs can be derived from various tissues and organs such as bone marrow, adipose tissue, umbilical cord blood, and placenta68 offering better accessibility for procurement compared to NSCs. Furthermore, MSCs can be easily expanded to high cell numbers for clinical application. Thus, the accessibility and also scalability of MSCs can provide advantages over NSCs for treatment application. The first SGT approach using MSCs for HGG treatment was reported by Miletic et al.70 in an orthotopic, syngeneic rat glioma model. The study showed a substantial treatment effect of intratumorally injected MSCs termed bone-marrow-derived tumor-infiltrating cells expressing HSV-TK following prodrug treatment. The direct tumor cell killing by MSCs was mediated through BE, while an immune response with infiltration of T cells and NK cells was detected in the treated tumors, which may have contributed to the treatment effect.70 Since then a number of preclinical studies have been published.46,71 However, no clinical trial has been performed yet for HGG treatment.
Delivery of SGT Vectors
Delivery of gene therapeutic products into brain tumors is an important issue that is critically discussed in the field, however still lacks optimal solutions. Systemic delivery of viral vectors is feasible72 but challenging due to potential off-target transduction in non-CNS tissues and/or insufficient bioavailability in the CNS. Intranasal delivery of cell-based vectors has also been pursued successfully in preclinical models.50,73 However, intracranial injection has been the most popular mode of administration and for the majority of SGT trials so far vectors have been injected directly into the resection cavity after surgery using multiple injections.6,31,51 This method of application is suboptimal as the tissue around the resection cavity is very heterogeneous containing either diffusely infiltrating tumor cells or reactive brain tissue. Thus, there is no control of how much tumor tissue is reached with this method, which might also partly explain the failure of clinical trials. There is a huge interpatient variation concerning injection efficacy and the amount of target tissue reached, which makes interpretation of clinical trial data extremely difficult. CED is a sophisticated delivery method into solid tissue that has been developed in particular for the brain and also brain tumors.74 CED is applied through stereotactically placed catheters that are connected to a micropump maintaining a continuous low-pressure flow into the tissue. This method has substantially increased the amount of tissue that can be targeted and thus is frequently used to inject vectors or drugs into the brain.74 Regarding the treatment of brain tumors, this method is optimized for application into solid tumor tissue, however not into a resection cavity after surgery. The problem that emerges from here is that primary tumors are usually treated by neurosurgery that is the standard of care. Thus, at this point, the only choice in order to implement CED into future clinical trials is to inject the vectors either into recurrent tumors or inoperable primary tumors.
Interaction of Glioma SGT With the Immune System and Combination With Immunotherapy
In general, potential antitumor immune responses mediated by SGT can originate from its different modules such as the type of vector, the cell death mechanism following suicide activity, the type of prodrug, the immune microenvironment of the tumor, and any additional treatment regimen. Different types of vector systems can have a variable impact on the immune system. While RRVs, LVs, and NSCs are not highly immunogenic in nature,16,55,57,58,75 AdVs are capable of eliciting an acute immune response including secretion of several proinflammatory cytokines.18 However, vector-induced immune responses can be a double-edged sword with either limiting transgene delivery and thereby impeding treatment efficacy or in contrast potentially breaking the immune tolerance of the glioma microenvironment76 and thereby enhancing the treatment effect of SGT.
The mechanism of HSV-TK/prodrug-mediated cell death can be variable involving necrotic and immunogenic cell death (ICD) in melanoma cells, but apoptotic and non-ICD in colorectal cancer cells.77 Glioma cells also undergo HSV-TK/prodrug-mediated cell death via apoptosis.78,79 The immunogenicity of apoptosis in general is controversial and not yet fully explored, in particular not in the context of SGT. Considering the plastic nature of leukocytes and their dichotomous role in antitumor IR,80,81 more studies are warranted to unravel the exact nature of HSV-TK/GCV-mediated cell death from an immunogenic point of view.
AdV-HSV-TK SGT has been shown to cause infiltration of various immune cells including macrophages and T cells in both rodent models18,30 and clinical settings.19,82 Still, in glioma, the antitumor IR elicited by AdV-HSV-TK/GCV therapy is often not strong enough without additional immunostimulatory strategies.43 When boosted by the co-expression of Flt3L or treatment with checkpoint inhibitors such as anti-PD1 antibodies, AdV-HSV-TK/GCV elicits a more robust antitumor IR.43,44,83–85 The co-expression of Flt3L has been shown to recruit dendritic cells to the tumor microenvironment and thereby increase antigen presentation and subsequently T-cell infiltration and activation in glioma animal models.43,44,83 This strategy is currently being investigated in phase I clinical trial (Table 3).
Toca 511 has been shown to activate an antitumor immune response in murine glioma cells where CD4+ T cells seemed to be crucial.16,86 While the precise nature of cell death due to yCD/5FC is not known, Toca 511 generated a strong antitumor immune response with an immunological memory that rejected a subsequent xenograft of the same tumor.16 This immune response was shown to be associated with reduced myeloid-derived suppressor cells and regulatory T cells in the tumor microenvironment.16,86 By conducting the adoptive transfer of splenocytes from cured mice, Mitchell et al.86 demonstrated that a T-cell-mediated antitumor immunity can be transferred to the host.
To conclude, a number of studies indicate that SGT can elicit an antitumor immune response (Figure 2B). While some of the underlying mechanisms of this causality have been identified, there remain several open questions highlighting the need for more fundamental research. Disappointing results of the clinical trials with AdV-HSV-TK/GCV31 and RRV-yCD/5FC53 further highlight the gravity of these issues.
Future Perspectives
Over the last decade(s), new developments in SGT have emerged, in particular new vectors and suicide genes with improved affinity to prodrugs (Figure 3). There are, however, some important issues that should be considered for further improvement of SGTs, which is urgently needed as larger phase III clinical trials have failed so far. For instance, the preclinical model systems should be revisited. The serum-culture-based patient-derived-xenografts that have been used so far to develop various SGTs, namely, AdVs and RRVs, neither share the genetics nor the invasive features of human gliomas in patients.87 Another crucial difference is the proliferative index. U87, one of the most popular serum-culture-based glioma cell lines, shows an extreme in vivo proliferative index of up to 80%.88 In contrast, the median proliferative index of human glioblastoma in patients is about 27%.89 Thus, quiescent glioma cells, which often have been associated with a stem cell and resistant phenotype, pose an enormous challenge to γ-RV-based SGTs which only transduce actively dividing cells. The RRVs have not been tested either in patient-derived primary spheroid models87,90 or glioma stem cell lines (GSCs) which contain such quiescent cells and are considered as the new standard for preclinical studies in gliomas.87,91 Another important issue is the delivery of the vectors as discussed above. CED could overcome the poor distribution of viral vectors; however, the presence of a solid tumor mass instead of a resection cavity is clearly preferred. Preclinical studies should be performed to test this hypothesis.
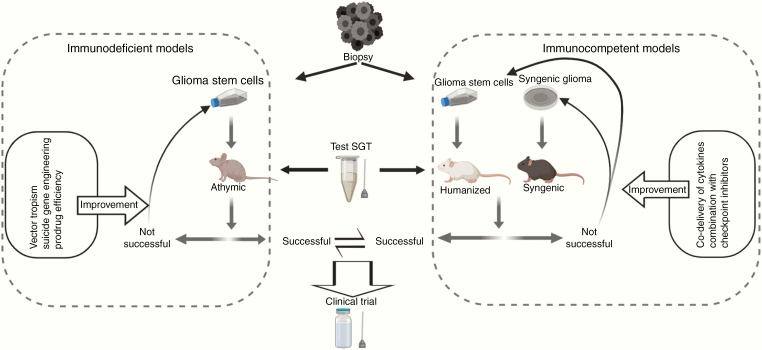
The immunosuppressive glioma microenvironment represents another clinical challenge. Although there are indications that the SGT-induced cell death is immunogenic, this has not been tested in detail, especially not in gliomas. Here, a lot can be learned from oncolytic viruses where an antitumor immune response is promoted by the vectors’ ability to induce ICD through oncolysis and thereby recruit an efficient antitumor IR.92,93 ICD might thus be a compulsory prerequisite for the engagement of an effective antitumor IR. More fundamental research is needed to identify the intrinsic nature of cell death, namely, immunogenic or immunosuppressive by the SGT systems in GSCs. If one SGT system fails to elicit strong ICD in GSCs (or in primary glioma cells), novel strategies or combinations could be designed to reroute the cell death mechanism. Unfortunately, the syngeneic animal models for glioma available today remain a serious drawback in this regard, because the immune microenvironment in these models differs substantially in composition from the one observed in HGG patients. The use of GSCs in humanized rodent models in this context may create valuable new information. Thus, a number of different preclinical models should be adopted to further develop SGT in the future and to achieve a better clinical translation procedure (Figure 4).
Fig. 4.
Outline of translational strategies to improve future SGTs. The choice of preclinical model systems is an important aspect for developing SGTs toward HGG treatment. GSCs are known to be the most clinically relevant preclinical models that recapitulate patient tumors very closely and thus a particular SGT should be tested in GSCs. If unsuccessful, the therapy should be subject to further improvements in different aspects as indicated. If successful, the therapy should be tested in immunocompetent models, for example, syngeneic glioma models and humanized models to analyze immune responses. If durable antitumor immune responses are detected, the particular SGT should be considered for clinical translation. Otherwise, additional treatments as combination with SGT can be considered to boost the immunostimulatory effect of a particular SGT. Combination with checkpoint inhibitors and co-delivery of immunostimulatory cytokines are important examples.
Combinatorial approaches using SGT with co-expression of immune-stimulating cytokines have been performed; however, these approaches might be too unspecific and are mostly directed toward T cells. Yet, T cell infiltration is scarce in glioblastoma, where immunosuppressive microglia and macrophages predominate. To improve future SGT approaches, the changes in the immune microenvironment under SGT should be analyzed more thoroughly. Based on this knowledge, specific shortcomings in the antitumor immune response during SGT could be detected and more targeted approaches could be developed.
Funding
This work was supported by Helse Vest [grant number 912151 and 912044].
Conflict of interest statement. The authors declare that they have no conflict of interests.
References
- 1. Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang H, Xu T, Jiang Y, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17(3):239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. [DOI] [PubMed] [Google Scholar]
- 5. Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3(12):1354–1361. [DOI] [PubMed] [Google Scholar]
- 6. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. [DOI] [PubMed] [Google Scholar]
- 7. Furman PA, McGuirt PV, Keller PM, Fyfe JA, Elion GB. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980;102(2):420–430. [DOI] [PubMed] [Google Scholar]
- 8. Nishiyama T, Kawamura Y, Kawamoto K, et al. Antineoplastic effects in rats of 5-fluorocytosine in combination with cytosine deaminase capsules. Cancer Res. 1985;45(4):1753–1761. [PubMed] [Google Scholar]
- 9. Nishiyama T, Kawamura Y, Kawamoto K, et al. [Antineoplastic effect of 5-fluorocytosine and cytosine deaminase on brain tumor (author’s transl)]. Neurol Med Chir (Tokyo). 1982;22(5):344–352. [DOI] [PubMed] [Google Scholar]
- 10. Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46(10):5276–5281. [PubMed] [Google Scholar]
- 11. Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990;82(4):297–300. [DOI] [PubMed] [Google Scholar]
- 12. Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13(8):1551–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kievit E, Bershad E, Ng E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59(7):1417–1421. [PubMed] [Google Scholar]
- 14. Korkegian A, Black ME, Baker D, Stoddard BL. Computational thermostabilization of an enzyme. Science. 2005;308(5723):857–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiraoka K, Inagaki A, Kato Y, et al. Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro Oncol. 2017;19(7):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hossain JA, Riecken K, Miletic H, Fehse B. Cancer suicide gene therapy with TK.007. Methods Mol Biol. 2019;1895:11–26. [DOI] [PubMed] [Google Scholar]
- 18. Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5(11):1256–1263. [DOI] [PubMed] [Google Scholar]
- 19. Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29(27):3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deville-Bonne D, El Amri C, Meyer P, Chen Y, Agrofoglio LA, Janin J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: structural and catalytic properties. Antiviral Res. 2010;86(1):101–120. [DOI] [PubMed] [Google Scholar]
- 21. Degrève B, De Clercq E, Balzarini J. Bystander effect of purine nucleoside analogues in HSV-1 tk suicide gene therapy is superior to that of pyrimidine nucleoside analogues. Gene Ther. 1999;6(2):162–170. [DOI] [PubMed] [Google Scholar]
- 22. Hlubinová K, Hlavatý J, Altaner C. Human glioma cells expressing herpes simplex virus thymidine kinase gene treated with acyclovir, ganciclovir and bromovinyldeoxyuridine. Evaluation of their activity in vitro and in nude mice. Neoplasma. 2001;48(5):398–406. [PubMed] [Google Scholar]
- 23. Hossain JA, Latif MA, Ystaas LAR, et al. Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro Oncol. 2019;21(7):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balzarini J, Liekens S, Solaroli N, El Omari K, Stammers DK, Karlsson A. Engineering of a single conserved amino acid residue of herpes simplex virus type 1 thymidine kinase allows a predominant shift from pyrimidine to purine nucleoside phosphorylation. J Biol Chem. 2006;281(28):19273–19279. [DOI] [PubMed] [Google Scholar]
- 25. Salomon B, Maury S, Loubière L, Caruso M, Onclercq R, Klatzmann D. A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol Cell Biol. 1995;15(10):5322–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garin MI, Garrett E, Tiberghien P, et al. Molecular mechanism for ganciclovir resistance in human T lymphocytes transduced with retroviral vectors carrying the herpes simplex virus thymidine kinase gene. Blood. 2001;97(1):122–129. [DOI] [PubMed] [Google Scholar]
- 27. Chalmers D, Ferrand C, Apperley JF, et al. Elimination of the truncated message from the herpes simplex virus thymidine kinase suicide gene. Mol Ther. 2001;4(2):146–148. [DOI] [PubMed] [Google Scholar]
- 28. Preuss E, Treschow A, Newrzela S, et al. TK.007: a novel, codon-optimized HSVtk(A168H) mutant for suicide gene therapy. Hum Gene Ther. 2010;21(8):929–941. [DOI] [PubMed] [Google Scholar]
- 29. Preuss E, Muik A, Weber K, Otte J, von Laer D, Fehse B. Cancer suicide gene therapy with TK.007: superior killing efficiency and bystander effect. J Mol Med (Berl). 2011;89(11):1113–1124. [DOI] [PubMed] [Google Scholar]
- 30. Perez-Cruet MJ, Trask TW, Chen SH, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39(4):506–511. [DOI] [PubMed] [Google Scholar]
- 31. Westphal M, Ylä-Herttuala S, Martin J, et al. ; ASPECT Study Group Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 32. Puumalainen AM, Vapalahti M, Agrawal RS, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9(12):1769–1774. [DOI] [PubMed] [Google Scholar]
- 33. Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994;91(8):3054–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tyynelä K, Sandmair AM, Turunen M, et al. Adenovirus-mediated herpes simplex virus thymidine kinase gene therapy in BT4C rat glioma model. Cancer Gene Ther. 2002;9(11):917–924. [DOI] [PubMed] [Google Scholar]
- 35. Immonen A, Vapalahti M, Tyynelä K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10(5):967–972. [DOI] [PubMed] [Google Scholar]
- 36. Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014;16(3):334–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ji N, Weng D, Liu C, et al. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget. 2016;7(4):4369–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. [DOI] [PubMed] [Google Scholar]
- 39. Castro MG, Lowenstein PR. Neuro-oncology: the long and winding road–gene therapy for glioma. Nat Rev Neurol. 2013;9(11):609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. 2014;25(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wheeler LA, Manzanera AG, Bell SD, et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kieran MW, Goumnerova L, Manley P, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol. 2019;21(4):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15(19):6113–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solly SK, Trajcevski S, Frisén C, et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003;10(1):30–39. [DOI] [PubMed] [Google Scholar]
- 46. Chang DY, Yoo SW, Hong Y, et al. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer. 2010;127(8): 1975–1983. [DOI] [PubMed] [Google Scholar]
- 47. Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huszthy PC, Giroglou T, Tsinkalovsky O, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One. 2009;4(7):e6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miletic H, Fischer YH, Giroglou T, et al. Normal brain cells contribute to the bystander effect in suicide gene therapy of malignant glioma. Clin Cancer Res. 2007;13(22 Pt 1):6761–6768. [DOI] [PubMed] [Google Scholar]
- 50. Carvalho LA, Teng J, Fleming RL, et al. Olfactory ensheathing cells: a Trojan horse for glioma gene therapy. J Natl Cancer Inst. 2019;111(3):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cloughesy TF, Landolfi J, Vogelbaum MA, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20(10):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inc. T. Press Release 12 September 2019; https://ir.tocagen.com/news-releases/news-release-details/tocagen-reports-results-toca-5-phase-3-trial-recurrent-brain/.
- 54. Miletic H, Fischer YH, Neumann H, et al. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther. 2004;15(11):1091–1100. [DOI] [PubMed] [Google Scholar]
- 55. Hossain JA, Ystaas LR, Mrdalj J, et al. Lentiviral HSV-Tk.007-mediated suicide gene therapy is not toxic for normal brain cells. J Gene Med. 2016;18(9):234–243. [DOI] [PubMed] [Google Scholar]
- 56. Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15(10):739–752. [DOI] [PubMed] [Google Scholar]
- 57. Mooney R, Abdul Majid A, Batalla J, Annala AJ, Aboody KS. Cell-mediated enzyme prodrug cancer therapies. Adv Drug Deliv Rev. 2017;118:35–51. [DOI] [PubMed] [Google Scholar]
- 58. Kim J, Hall RR, Lesniak MS, Ahmed AU. Stem cell-based cell carrier for targeted oncolytic virotherapy: translational opportunity and open questions. Viruses. 2015;7(12):6200–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shinojima N, Hossain A, Takezaki T, et al. TGF-β mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73(7):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heese O, Disko A, Zirkel D, Westphal M, Lamszus K. Neural stem cell migration toward gliomas in vitro. Neuro Oncol. 2005;7(4): 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Metz MZ, Gutova M, Lacey SF, et al. Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: implications for clinical use. Stem Cells Transl Med. 2013;2(12):983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ubiali F, Nava S, Nessi V, et al. Allorecognition of human neural stem cells by peripheral blood lymphocytes despite low expression of MHC molecules: role of TGF-beta in modulating proliferation. Int Immunol. 2007;19(9):1063–1074. [DOI] [PubMed] [Google Scholar]
- 63. Bago JR, Okolie O, Dumitru R, et al. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med. 2017;9(375). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bagó JR, Alfonso-Pecchio A, Okolie O, et al. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun. 2016;7:10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mooney R, Majid AA, Mota D, et al. Bcl-2 overexpression improves survival and efficacy of neural stem cell-mediated enzyme prodrug therapy. Stem Cells Int. 2018;2018:7047496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24(3):159–171. [DOI] [PubMed] [Google Scholar]
- 67. Portnow J, Synold TW, Badie B, et al. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23(12):2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Binello E, Germano IM. Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro Oncol. 2012;14(3):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm. 2011;8(5):1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miletic H, Fischer Y, Litwak S, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15(7):1373–1381. [DOI] [PubMed] [Google Scholar]
- 71. Altaner C, Altanerova V, Cihova M, et al. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer. 2014;134(6):1458–1465. [DOI] [PubMed] [Google Scholar]
- 72. Huang TT, Parab S, Burnett R, et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015;26(2):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li G, Bonamici N, Dey M, Lesniak MS, Balyasnikova IV. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert Opin Drug Deliv. 2018;15(2):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg. 2017;126(1):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Okada H, Thorne SH. Is the immune response a friend or foe for viral therapy of glioma? Neuro Oncol. 2017;19(7):882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4(5):581–587. [DOI] [PubMed] [Google Scholar]
- 78. Glaser T, Castro MG, Löwenstein PR, Weller M. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001;8(6):469–476. [DOI] [PubMed] [Google Scholar]
- 79. Fischer U, Steffens S, Frank S, Rainov NG, Schulze-Osthoff K, Kramm CM. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005;24(7):1231–1243. [DOI] [PubMed] [Google Scholar]
- 80. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164(6):1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. [DOI] [PubMed] [Google Scholar]
- 83. King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10(1):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Candolfi M, Yagiz K, Foulad D, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15(13):4401–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Speranza MC, Passaro C, Ricklefs F, et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mitchell LA, Lopez Espinoza F, Mendoza D, et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19(7):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huszthy PC, Daphu I, Niclou SP, et al. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol. 2012;14(8):979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Strojnik T, Kavalar R, Lah TT. Experimental model and immunohistochemical analyses of U87 human glioblastoma cell xenografts in immunosuppressed rat brains. Anticancer Res. 2006;26(4B): 2887–2900. [PubMed] [Google Scholar]
- 89. Alkhaibary A, Alassiri AH, AlSufiani F, Alharbi MA. Ki-67 labeling index in glioblastoma; does it really matter? Hematol Oncol Stem Cell Ther. 2019;12(2):82–88. [DOI] [PubMed] [Google Scholar]
- 90. Bjerkvig R, Tønnesen A, Laerum OD, Backlund EO. Multicellular tumor spheroids from human gliomas maintained in organ culture. J Neurosurg. 1990;72(3):463–475. [DOI] [PubMed] [Google Scholar]
- 91. Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. [DOI] [PubMed] [Google Scholar]
- 92. Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J. Immune conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front Immunol. 2019;10:1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Marchini A, Scott EM, Rommelaere J. Overcoming barriers in oncolytic virotherapy with HDAC inhibitors and immune checkpoint blockade. Viruses. 2016;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]